Abstract
Abstract: Tissue plasminogen activator (t-PA) is the only FDA-approved drug for acute ischemic stroke treatment, but its clinical use is limited due to the narrow therapeutic time window and severe adverse effects, including hemorrhagic transformation (HT) and neurotoxicity. One of the potential resolutions is to use adjunct therapies to reduce the side effects and extend t-PA's therapeutic time window. However, therapies modulating single target seem not to be satisfied, and a multi-target strategy is warranted to resolve such complex disease. Recently, large amount of efforts have been made to explore the active compounds from herbal supplements to treat ischemic stroke. Some natural compounds revealed both neuro- and blood-brain-barrier (BBB)-protective effects by concurrently targeting multiple cellular signaling pathways in cerebral ischemia-reperfusion injury. Thus, those compounds are potential to be one-drug-multi-target agents as combined therapy with t-PA for ischemic stroke.
In this review article, we summarize current progress about molecular targets involving in t-PA-mediated HT and neurotoxicity in ischemic brain injury. Based on these targets, we select 23 promising compounds from currently available literature with the bioactivities simultaneously targeting several important molecular targets. We propose that those compounds merit further investigation as combined therapy with t-PA. Finally, we discuss the potential drawbacks of the natural compounds' studies and raise several important issues to be addressed in the future for the development of natural compound as an adjunct therapy.
Keywords: Combination therapy, hemorrhagic transformation, ischemic stroke, multi-target, natural compounds, neurotoxicity, tissue plasminogen activator (t-PA)
1. INTRODUCTION
Stroke is one of the most prevalent diseases with high mortality and disability, and ischemic stroke accounts for more than 80% stroke incidences [1, 2]. Up-to-date, tissue plasminogen activator (t-PA) is the only FDA-approved thrombolytic drug for ischemic stroke [3]. Early recanalization with t-PA infusion indeed improves the outcomes of ischemic stroke [4, 5], but t-PA has a restrictive time window within 4.5 hours. Beyond this time window, t-PA treatment increases the 10-fold risk of hemorrhagic transformation (HT), which accounts for 10-40% of ischemic stroke cases with increased morbidity and mortality [6]. Therefore, developing approaches to reduce HT is critical to extend t-PA therapeutic window and increase t-PA’s eligibility for ischemic stroke.
Recently, developing an adjunct therapeutic strategy for extension of the therapeutic window and prevention of t-PA’s complications is gaining great interests [7]. Until now, there is no drug available for reducing t-PA’s side effects and improving therapeutic outcomes. Multiple molecular targets involved in the process of t-PA-mediated HT and neurotoxicity [8], making drug developed from one-drug-one-target paradigm unlikely achieve satisfactory outcomes. In fact, the one-molecule-one-target paradigm has been recently criticized for Alzheimer's Diseases (AD) [9], and a multi-target-directed ligands (MTDLs) strategy is proposed for AD and Parkinson’s disease (PD) [9, 10]. There are two possible ways for MTDLs strategy: one is to synthesize molecules endowed with multiple motifs hitting those targets, and the other is to elect the potential MTDLs from natural compounds with multiple targets [9]. Stroke is also a complex pathological process, and the process of ischemia-initiated neurodegeneration and post-stroke rehabilitation involves multiple signaling pathways. Chinese herbal medicine has been used in China for thousand years. Recent studies indicate that there are many promising drug candidates from herbal medicine with the potentials of modulating multiple cellular signaling pathways for reducing HT and neurotoxicity [11, 12]. Many natural compounds could be important sources of MTDLs since they concurrently can act on multiple targets for neuroprotection or brain repair. In this article, we first briefly introduce current molecular targets contributing to HT and neurotoxicity in thrombolytic therapy and then review bioactive compounds from herbal sources for reducing t-PA-mediated HT and neurotoxicity. Finally, we discuss several important issues which should be considered in future drug development.
2. CURRENT MOLECULAR TARGETS CONTRI-BUTING TO BBB DAMAGE, HT AND NEURO-TOXICITY IN ISCHEMIC STROKE WITH t-PA TREATMENT
Blood-brain barrier (BBB) damage is a vital process of HT in ischemic stroke with delayed t-PA treatment [13]. HT occurs in the BBB compromised region of ischemic brains [14, 15]. BBB is defined as a complex unit composed of brain microvascular endothelial cells, tight junction proteins, extracellular matrix (ECM), astrocytes and pericyte endfeet [16]. Delayed t-PA treatment in ischemic stroke can further disrupt the BBB and mediate HT via multiple signaling pathways. Fig. (1) summarizes several important molecular targets identified to be inducers for the BBB damage, HT and neurotoxicity, including LRP-1, NF-κB, MMP-9, HMGB-1, VEGF, PDGF-CC, NMDAR, APC, ROS, and RNS. We briefly discuss these molecular targets in the following sections.
Fig. (1).
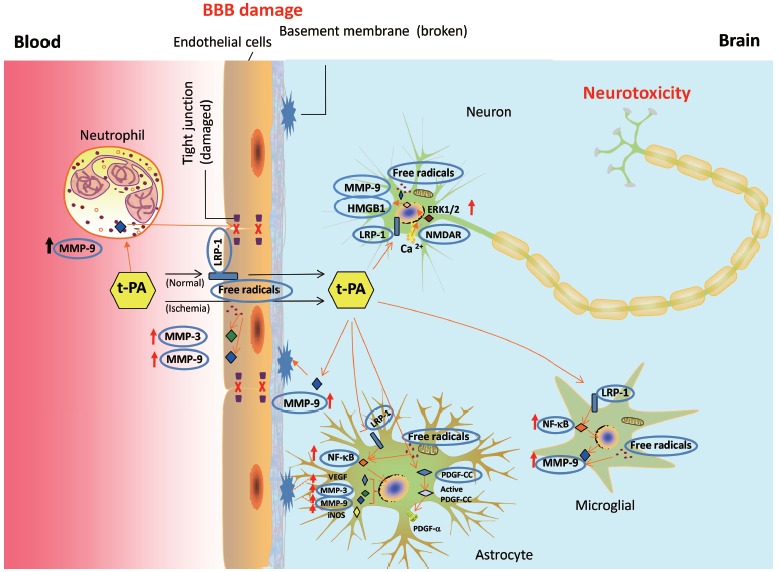
Mechanisms and molecular targets participate in tissue plasminogen activator (t-PA)-mediated BBB damage and neurotoxicity. T-PA acts on neutrophil, endothelial cells, astrocytes, microglials, and neurons to induce BBB damage and neurotoxicity. T-PA crosses BBB through LRP-1 dependent and independent pathways. LRP-1, NF-κB, MMPs, VEGF, HMGB1, and PDGF-CC are important targets involved in t-PA induced BBB damage and HT. LRP-1 and NMDAR are involved in t-PA induced neurotoxicity, mediating calcium influx and activation of ERK1/2. Free radicals are involved both in BBB damage and neurotoxicity possibly by upregulating MMPs. LRP-1: Low density lipoprotein receptor-related protein 1; MMP-9/3: Matrix metallopeptidase 9/3; NMDAR: N-methyl-D-aspartate receptor; PDGF-CC: Platelet-derived growth factor CC; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells.
2.1. Low-Density Lipoprotein Receptor-related Protein 1 (LRP-1)
LRP-1 is a member of the low-density-lipoprotein-receptor family for regulating extracellular matrix protease and mediating the activities of t-PA and matrix metalloproteinases [17]. Previous review articles have extensively discussed the roles of LRP-1 in mediating BBB damage and HT [18, 19]. As shown in Fig. (1), t-PA could penetrate the BBB in both LRP-1 dependent and independent pathways [20, 21]. By binding to LRP-1 in astrocytes or brain microvascular endothelial cells, t-PA could activate NF-κB, Akt and matrix metalloproteinase-9 (MMP-9), subsequently leading to the BBB damage and HT in ischemic brains [22-24]. Similarly, the interaction of t-PA and LRP-1 was also found in microglial cells, contributing to MMP-9/-3 production and tight junction disruptions [25, 26]. Ablation of LRP-1 or inhibition of the downstream signaling completely abolished t-PA induced MMP-9 overproduction [27]. Anti-LRP-1 IgG and LRP-1 antagonist receptor-associated protein (RAP) reduced t-PA-mediated BBB permeability [28]. LRP-1 assists the interaction between t-PA and NMDAR, contributing to t-PA induced neurotoxicity [29]. Thus, targeting LRP-1 could be a promising strategy to prevent BBB disruption, HT, and neurotoxicity during thrombolytic treatment in ischemic stroke.
2.2. NF-κB Pathway
NF-κB is a transcriptional factor and a player in t-PA-mediated HT. NF-κB not only serves as an LRP-1 downstream pathway but also interacts with t-PA to induce the MMP-9 expression and activity, leading to BBB hyperpermeability and neuronal cell death [30]. The roles of MMPs in BBB disruption and HT will be discussed later. At the ischemic onset, the activation and translocation of NF-κB induced the expressions of iNOS and MMP-9 in ischemic mice brains [24, 30]. Exogenous t-PA triggered activation of NF-κB signaling in astrocytes of ischemic brains, leading to MMP-9 activation and iNOS overexpression [24, 31, 32]. Inhibition of NF-κB signaling attenuated t-PA-mediated MMP-3 activation in brain microvascular endothelial cells [22]. Thus, targeting NF-κB signaling pathway has beneficial effects on reducing BBB permeability and HT during thrombolytic treatment for ischemic stroke.
2.3. Matrix Metalloproteinase-9 (MMP-9)
MMPs play an important role in mediating HT during t-PA treatment for ischemic stroke [33, 34]. Over-production of MMP-9 mainly comes from leukocytes at the early phase of ischemic brain injury [35, 36] but from brain endothelial cells, neurons and microglials in the late phase [37, 38]. Activation of MMP-9 greatly contributes to BBB damage during cerebral ischemia [39, 40]. Delayed t-PA treatment up-regulated the expression and activity of MMP-9 in leukocytes, endothelial cells and glial cells [41-43], and disrupted tight junction proteins [44, 45]. Treatment of broad-spectrum MMP inhibitor significantly reduced the incidence and severity of HT in experimental ischemic stroke animal models with delayed thrombolysis [46-48] and reduced the mortality rate in rodent ischemic stroke models [49]. MMP-9 activity is considered as one of the critical targets for reducing HT in thrombolytic treatment for stroke [31, 50, 51]. Clinical observation yielded the similar results in the association of MMP-9 activity and t-PA treatment in the serum samples of stroke patients [52]. High baseline in plasma MMP-9 level could be a biomarker for predicting the appearance of HT in ischemic stroke patients with t-PA treatment [53, 54]. A recent meta-analysis on 22 clinical trials revealed that serum MMP-9 level was highly correlated with stroke severity, cerebral infarct volume, intracerebral hemorrhage and functional outcomes [55]. Therefore, MMP-9 could be a critical molecular target in preventing HT for the ischemic brain with delayed t-PA treatment.
2.4. High Mobility Group Box Protein 1 (HMGB1)
High mobility group box protein 1 (HMGB1), a non-histone DNA-binding protein, is also an important mediator in cerebral ischemia-reperfusion injury [56]. Upon stroke onset, HMGB1 release was found at neurons in the ischemic core within a half hour [57]. Released HMGB1 can target glial cells and initiate production of inflammatory cytokines, such as TNF-α, iNOS, IL-6, and IL-1β, etc. [57, 58]. HMGB1 activates MMP-9 in astrocytes and neurons via binding toll-like receptor 4 (TLR4), contributing to BBB disruption in ischemic brains [59]. Inhibition of HMGB1 signaling ameliorates cerebral ischemic injury [60-65]. Anti-HMGB1 antibodies suppressed MMP-9 activity, TNF-α and iNOS expression, subsequently attenuated BBB disruption, infarct volume, and brain edema, and improved neurological outcomes in rodent ischemic stroke models [60, 61]. HMGB1 shRNA significantly reduced brain infarct volume, ameliorated microglial activation and suppressed the expression of iNOS, COX-2, IL-1β and TNF-α [58, 62]. HMGB-1 binding heptamer dose-dependently reduced infarct volume and improved neurological outcomes in a rat MCAO-reperfusion model [63]. HMGB1 A box, an antagonist of HMGB1, significantly decreased brain infarct size and attenuated neurons death in ischemic stroke models [64, 65]. A clinical study showed a strong association of HMGB1 with MMP-9 in the serum of stroke patients [66]. Serum HMGB1 level could predict mortality and other unfavorable outcomes of stroke patients [67]. Those studies strongly support that HMGB1 is a critical factor in ischemic brain injury.
2.5. Vascular Endothelial Growth Factor (VEGF)
VEGF exerts angiogenesis-promoting effects at the late phase of post-stroke brains, but VEGF overproduction is deleterious to mediate BBB damage at the early phase [68, 69]. VEGF activation in astrocytes increased endothelial barrier permeability whereas knockdown of VEGF expression abolished this process [70]. Exogenous VEGF greatly aggravated the BBB damage and HT in ischemic rat brains, possibly through regulating NOS and MMP-9 activity [68, 71, 72]. Anti-VEGF neutralizing antibody significantly reduced the BBB damage in rodent cerebral ischemic model [73] and reduced HT in ischemic stroke with t-PA treatment possibly via down-regulating MMP-9 activity [74]. Thus, VEGF could be an important target for reducing t-PA-mediated HT in the post-ischemic brain.
2.6. Platelet-derived Growth Factor-CC (PDGF-CC)
PDGF-CC is a member of PGDF family. A previous review article summarized the role of PDGF-CC in HT during thrombolytic treatment [75]. t-PA is a potent activator of PDGF-CC, which cleaves latent PDGF-CC into active PDGF-CC [76]. Active PDGF-CC subsequently binds to a PDGF-α receptor in astrocytes, leading to impairment of BBB integrity [77]. PDGF-CC neutralizing antibody and PDGF-α receptor antagonist revealed to reduce BBB damage and t-PA mediated HT [77]. The increase of plasma PDGF-CC level appears to be a predictor for HT in ischemic stroke patients with t-PA treatment [78]. Thus, targeting PDGF-CC could be a potential biomarker for HT in thrombolytic treatment.
2.7. N-methyl-D-aspartic Receptor (NMDAR)
NMDAR is an ion-gated glutamate receptor composed of two GluN1 subunits and two GluN2 subunits. NMDAR plays an important role in t-PA induced neurotoxicity [34, 79]. By interacting with NMDAR, t-PA induces calcium influx and ERK1/2 activation and produce neurotoxicity [29]. Several subunits of NMDAR were found to mediate t-PA induced NMDAR activation. Specifically, NR2B subunit appears to be a critical target of t-PA with the interaction site at arginine 67 [80]. GluN2D participates in t-PA/NMDAR induced neurotoxicity as well [81]. Besides, blocking NR1 subunit reduced neurotoxicity and improved spatial memory, indicating that NR1 subunit also participates in t-PA-induced neurotoxicity [82, 83]. By adopting point mutation analysis, a previous study revealed that the arginine 260 on NR1 subunit could be critical for t-PA-induced NMDAR signaling [84]. Therefore, NMDAR is a promising therapeutic target in reducing the t-PA-mediated neuro-toxicity.
2.8. Activated Protein C (APC)
Evidence is accumulated to support that APC is a critical player in different pathophysiological processes of ischemic brain injury, including apoptosis [85], inflammation [86], neurogenesis, neuro-vascularization [87] and neurological recovery [88]. APC protein could counteract the toxic effect of t-PA via reducing neuro-apoptosis. In ischemic stroke, t-PA treatment could shift the intrinsic apoptotic pathway (casepase-9 dependent) to extrinsic pathway (caspase-8 dependent) bypassing mitochondrial amplification [89]. APC and its analog 3K3A-APC significantly reduced the t-PA-mediated HT via down-regulating NF-κB-MMP-9 pathway [31, 90, 91]. Thus, APC could be a therapeutic target for preventing HT and neurotoxicity during t-PA treatment. Surprisingly, in a clinical study, high level of APC in plasma was associated with the increased HT rates during t-PA treatment. A possible explanation is that elevated APC could be insufficient to attenuate the brain damage during ischemic stroke with t-PA treatment [92].
2.9. Reactive Nitrogen Species (RNS) and Reactive Oxygen Species (ROS)
It is well-known that free radicals are important players in cerebral ischemia/reperfusion injury. Recanalization by t-PA infusion provides oxygen as a substrate for the generation of free radicals [93]. Free radicals, including ROS, RNS, could mediate the BBB damage, neurotoxicity and cerebral inflammation, contributing to brain damage and HT [94].
2.9.1. ROS
ROS includes superoxide, hydroxyl radical, single oxygen, hydrogen peroxide, etc. ROS production is remarkably increased and subsequently causes neurotoxicity, BBB damage, inflammation as well as brain edema during cerebral ischemia-reperfusion injury [95]. ROS can directly modify lipids, DNA, and proteins, and trigger apoptotic signal pathways [93]. As an antioxidant, Edaravone has been approved in Japan clinically for ischemic stroke treatment [96]. A review article covering studies for the period of 1993-2008 indicates that Edaravone is effective for ischemic stroke treatment [97]. Edaravone treatment for 14 days significantly down-regulated the serum MMP-9 level in ischemic stroke patients [98]. Co-treatment of Edaravone with t-PA enhanced the recanalization efficacy, reduced the rates of HT and improved functional outcomes in ischemic stroke patients [99-101]. Similar results were also found in animal experiments [102]. A systematic review analyzing 49 experiments with 519 animals showed that Edaravone treatment improved both structural and functional outcomes in experimental ischemic stroke models [103]. However, contrary evidence revealed that Edaravone increased the incidence of HT in stroke patients with cardiogenic embolism [104]. Reasons for such discrepancy are not yet understood, and further research is needed for evaluating edaravone as potential adjunct therapy with t-PA.
2.9.2. RNS
RNS has gained great interests in the field of cerebral ischemia-reperfusion injury. Nitric oxide (NO) and peroxynitrite (ONOO-) are two representatives of RNS in mediating cerebral ischemia-reperfusion injury. Thus, we discuss the roles of NO and peroxynitrite in ischemic brain injury in the following section.
2.9.2.1. Nitric Oxide
Nitric oxide was first identified as an endothelium-derived relaxing factor (EDRF) in the 1980s [105, 106]. Three nitric oxide synthase (NOS) isoforms could produce nitric oxide from L-arginine with molecular oxygen, namely, neural NOS (nNOS), endothelial NOS (eNOS) and inducible NOS (iNOS) [94]. In brain, nNOS is majorly expressed in neurons, while eNOS is majorly expressed in the vascular endothelial cells. Both eNOS and nNOS are calcium-dependent and constitutively expressed in normal condition to produce NO at nanomole level with physiological regulating functions [107]. However, activation of iNOS from glial cells and macrophages produces a large amount of NO at micromole level in a calcium-independent way [108]. Upon stroke onset, NO level is remarkably increased within half hour from less than 10 nM to more than 1 µM [109], which is mainly derived from nNOS and eNOS [110-112]. In brain microvessels, iNOS activity was induced, and a large amount of NO was produced within 4 hours after cerebral ischemia [113]. Increased iNOS activity lasted for at least 24 hours, continuously over-producing NO [114, 115]. NO derived from different isoforms of NOS has different roles in cerebral ischemia-reperfusion injury. NO derived from iNOS is deleterious to ischemic brain injury [116-119]. For instances, treatment of iNOS inhibitor 1400W blocked glutamate release [118], attenuated infarct size and improved neurological outcomes in an experimental ischemic stroke animal model [119]. NO precursor L-arginine treatment worsened ischemic brain injury in wild-type mice but not in iNOS knockout mice [120]. NO derived from nNOS is also detrimental. It was reported that nNOS inhibitor 7-nitroindazole attenuated brain damages [121] and BBB disruption [122]. Consistently, nNOS knockout mice had less brain infarct volume in an ischemic stroke model [123]. NO directly interacts with multiple proteins, inducing reversible protein nitrosylation [124], S-glutathiolation [125] or nitrosothiols formation [126]. In contrast, eNOS knockout mice had enlarged infarct size and brain damages in both focal cerebral ischemia and global cerebral ischemia model, indicating that NO derived from eNOS could be neuroprotective [123]. NO derived from endothelial NOS is essential for maintaining or restoring the cerebral blood flow with the evidence that eNOS knockout mice had less cerebral blood flow volume [127]. Therefore, NO production has double edge effects on ischemic brains during ischemic stroke, dependent on the source, amount and its microenvironment.
2.9.2.2. Peroxynitrite
In ischemic brain, superoxide (O2.-) is simultaneously produced with NO and subsequently react each other to generate ONOO- in an extremely fast rate [128]. Upon reperfusion, a large amount of O2.- is produced from neurons, endothelial cells, microglial cells and leukocytes, etc. In an ischemic stroke rat model with 2 h ischemia plus reperfusion for 4-70 h, nitrotyrosine was used as a biomarker of ONOO- and concurrent formation of ONOO- was associated with the expression of iNOS in the ischemia-reperfused brain [129]. Nitrotyrosine level in the peri-infarct area is much higher (about 0.95%) than that in the core area (about 0.5%) at one hour after reperfusion [130]. Peroxynitrite is much more toxic than NO [131], with permeability coefficient at about 400 fold faster than superoxide [131, 132]. Peroxynitrite could easily pass through the cell membrane and inhibit mitochondrial respiration [133, 134], trigger protein nitration [135, 136] and lipid peroxidation [137], induce DNA damage [138], and inactivate multiple ion channels and enzymes [139, 140]. Peroxynitrite donor 3-morpholino sydnonimine (SIN-1) decreased tight junction protein ZO-1 expression and exacerbated BBB damage [141]. FeTMPyP, a peroxynitrite decomposition catalyst, significantly attenuated the neurons loss and improved the functional outcomes in a global cerebral ischemia-reperfusion model [142]. Consistently, FeTMPyP treatment at 3 mg/kg remarkably decreased cerebral infarct volume in a rat MCAO model, with an extension of the therapeutic time window up to 6 hours [143]. In our recent study, t-PA treatment at 2 hours after cerebral ischemia remarkably inhibited ONOO- generation, protected the brains from ischemia-reperfusion injury and improved neurological deficit scores whereas delayed treatment of t-PA at 4.5 hours worsened the brain damages and neurological deficit scores. What’s more, delayed t-PA treatment led to high incidence of HT in the ischemia-reperfusion rat brains. Interestingly, co-treatment of FeTMPyP significantly reduced HT and improved neurological outcomes in the animal models of cerebral ischemia-reperfusion injury with the delayed thrombolysis [41]. The underlying mechanisms could be related to attenuating ONOO--mediated MMP-9/-2 expression and activation in ischemic rat brains [41]. Uric acid, an ONOO- scavenger, is capable of preventing ONOO--mediated nitration [144]. Pre- or post-treatment of uric acid significantly reduced the cortex and striatum infarct volume in a rat stroke model [145]. The combination of uric acid with t-PA further reduced cerebral infarct size and improved neurological outcomes in a rodent thromboembolic model [146]. A recent meta-analysis involving 10 studies and 8131 patients showed that high serum uric acid was associated with good neurological outcome in ischemic stroke [147]. Taken together, these works suggest that scavenging RNS, particularly ONOO-, would be a promising therapeutic strategy in reducing t-PA associated HT and improving the overall outcomes.
In summary, we have briefly discussed current knowledge about the molecular targets in t-PA-mediated HT and neurotoxicity. Notably, some targets are involved both in HT and neurotoxicity. With the profound studies, we believe that more and more molecular targets could be found in future. Those signaling molecules could independently or synergistically contribute to HT and neurotoxicity at different stages of cerebral ischemia-reperfusion injury during thrombolytic treatment. With complex molecular network systems, strategies targeting one molecular target might not be sufficient to reverse the toxic effects of t-PA practically. Therefore, we anticipate that compounds hitting multi-targets may serve as strong candidates in combating t-PA’s complications.
3. REPRESENTATIVE NATURAL COMPOUND CANDIDATES AS COMBINATION THERAPIES WITH T-PA IN THE TREATMENT OF ACUTE ISCHEMIC STROKE
Based on those molecular targets, we have reviewed the current research progress of the natural compounds for ischemic stroke within recent 10 years. We selected compounds reported to target more than two targets in ischemic stroke models. As a result, totally 23 suitable compounds were selected and summarized in Tables 1, 2. Those compounds have multimodal interactions with the mentioned targets involved in t-PA induced brain injury in experimental cerebral ischemia-reperfusion models. Details of studies on those compounds are summarized in Table 1, and will be discussed in the following section.
Table 1.
Representative studies of selected natural compounds.
| Compound |
Representative
Source |
Experimental
Model |
Treatment Time Point and Path | Dosage | Targets | Major Results | Refs. | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baicalin | Scutellaria baicalensis Georgi | tMCAO | 2 hour after MCAO, I.V. | 10, 25, 50 mg/kg |
ONOO- | Infarct volume, neuron death |
[148] | |||||||||||||||||||||||
| pMCAO | 2 hour and 12 hour after MCAO, I.P. | 100 mg/kg | TLR2/4, NF-κB, TNF-α, IL-1β, COX-2 |
Infarct volume | [149] | |||||||||||||||||||||||||
| tMCAO | at MCAO onset, I.V. | 50,100, 200 mg/kg |
NF-κB | Infarct volume, neurological deficit |
[150] | |||||||||||||||||||||||||
| pMCAO | 2 hours and 12 hours after MCAO, I.P. | 100 mg/kg | MMP-9, Occludin | Infarct volume, BBB damage, brain edema, neuronal damage |
[152] | |||||||||||||||||||||||||
| OGD | 30 min before OGD | 1, 5 uM | 5-LOX, NMDAR | Neurotoxicity | [151] | |||||||||||||||||||||||||
| Tanshinone IIA | Salvia miltiorrhiza | tMCAO | Daily, for 7 or 15 days, I.P. | 5 or 10 mg/kg I.P. | HMGB1, NF-κB |
Infarct volume, cell apoptosis | [157] | |||||||||||||||||||||||
| tMCAO | 10 min after MCAO, I.P. | 25 mg/kg | MIF, NF-κB | Infarct volume, brain edema, neurological deficit, neuro-inflammation | [155] | |||||||||||||||||||||||||
| tMCAO | 10 min after MCAO, I.P. | 25 mg/kg, 40 mg/kg |
Bcl-2, caspase 3 | Infarct volume, brain edema, neurological deficit, apoptosis | [160] | |||||||||||||||||||||||||
| tMCAO | 5 min after MCAO. I.P. | 10 mg/kg, 20 mg/kg, 30 mg/kg |
MMP-9 | Infarct volume, brain edema, BBB damage, | [158] | |||||||||||||||||||||||||
| tMCAO | 15 min after MCAO. I.P. | 20 mg/kg | TORC1, pCREB, BDNF | Infarct volume, brain edema, neurological deficit | [161] | |||||||||||||||||||||||||
| pMCAO | Immediately after MCAO, I.P. | 10 mg/kg, 20 mg/kg |
HMGB1, TLR4, RAGE, NF-κB |
Infarct volume, brain edema, neurological deficit, BBB damage |
[159] | |||||||||||||||||||||||||
| pMCAO | One day and 30 min before MCAO, 4 hour after MCAO, I.P. | 5, 10, 20 mg/kg | NF-κB, SOD, MDA, iNOS, NO | Infarct volume, neurological deficit |
[156] | |||||||||||||||||||||||||
| Huperzine A | Huperzia Serrata | tMCAO | At MCAO onset and 6 hour after MCAO, I.P. | 0.1 mg/kg | NF-κB, nAChR | CBF, infarct volume, neurological deficit, neuro-inflammation | [166] | |||||||||||||||||||||||
| tMCAO | 2 hour before MCAO and 4 hour after reperfusion, I.G. | 0.1 mg/kg | ROS | Infarct volume, neurological deficit, mitochondrial swelling | [165] | |||||||||||||||||||||||||
| tMCAO | 2 day before MCAO, for 9 days, I.G. | 0.2 mg/kg | NGF, BDNF and TGF-β1 | Memory deficit | [168] | |||||||||||||||||||||||||
| OGD | 2 hour before OGD | 1 uM | NF-κB and RNS | Cell death | [167] | |||||||||||||||||||||||||
| NMDA treatment | 45 min pretreatment | 10 nM to 1 uM | NMDAR | Neurotoxicity | [169] | |||||||||||||||||||||||||
| Honokiol | Magnolia grandiflora | tMCAO, LPS treatment |
15 min after ischemia and 15 min after reperfusion, I.P. |
0.7-70 mug/kg 0.1-10 muM |
NF-κB, NO, TNF-α | Neuro-inflammation, BBB damage, brain edema |
[171] | |||||||||||||||||||||||
| tMCAO, OGD or NMDA treatment | 0, 1 or 3 hour after reperfusion, I.V. | 50 mug/kg | PSD95/nNOS, NO | Brain edema, infarct volume, neurological deficit, | [174] | |||||||||||||||||||||||||
| tMCAO | 15 min before and 60 min after ischemia, I.P. | 10 μg/kg | ROS | Infarct volume, mitochondrial function |
[172] | |||||||||||||||||||||||||
| NMDA treatment in vivo |
3 days before NMDA treatment, I.G. |
1-100 mg/kg | GSH | Neurotoxicity, mortality | [172] | |||||||||||||||||||||||||
| tMCAO | 15 min before and 60 min after ischemia, I.V. | 0.1, 1, 10 ug/kg | ROS | Neutrophil infiltration | [173] | |||||||||||||||||||||||||
| Puerarin | Radix puerariae | tMCAO | 10 min before MCAO, I.P. | 25, 50 mg/kg | iNOS, caspase-3, TNF-α |
Infarct volume | [183] | |||||||||||||||||||||||
| tMCAO | 24 hour after MCAO, repeated daily, for 14 days, I.P. | 50, 100, 200 mg/kg | Not mentioned | Infarct volume, neurological deficit, vascular genesis | [179] | |||||||||||||||||||||||||
| tMCAO | 1 hour after MCAO, I.G. | 2.62, 7.86 and 23.59 mg/kg | BDNF, caspase-3 |
Infarct volume, neurological deficit, astrocyte apoptosis | [180] | |||||||||||||||||||||||||
| tMCAO | At the onset of MCAO, I.P. | 50, 100 mg/kg | Active caspase-3 |
Infarct volume, neurological deficit, neuronal apoptosis | [181] | |||||||||||||||||||||||||
| tMCAO | 1 hour before MCAO, I.P. | 60 mg/kg | NF-κB | Infarct volume | [184] | |||||||||||||||||||||||||
| Curculigoside | Curculigo orchioides Gaertn | NMDA treatment | Co-treatment with NMDA | 1, 10 uM | ROS, bcl-2, bax, caspase-3 | Neurotoxicity | [185] | |||||||||||||||||||||||
| tMCAO, OGD | 1, 3, 5 or 7 hour after MCAO, I.V. | 10 mg/kg, 20 mg/kg |
NF-κB, HMGB1 | Neurotoxicity, BBB damage | [186] | |||||||||||||||||||||||||
| Shikonin | Lithospermum erythrorhizon | tMCAO | 3 day before MCAO and once after MCAO, I.G. | 10 mg/kg, 25 mg/kg |
NF-κB, MMP-9, p38MAPK, TLR4, TNF-α | Infarct volume, BBB damage, brain edema, neurological deficit, neuro-inflammation | [189] | |||||||||||||||||||||||
| tMCAO | 3 times before MCAO, one time after MCAO, I.G. | 12.5, 25, 50 mg/kg |
GSH, MAD, ROS | Infarct volume, neurological deficit, oxidative stress | [188] | |||||||||||||||||||||||||
| Glycyrrhizin |
Glycyrhizae glabra |
tMCAO | 1, 24 and 48 hour after MCAO, I.P. | 10 mg/mouse | HMGB1, TLR4, IL-17A |
Infarct volume, neuronal apoptosis |
[195] | |||||||||||||||||||||||
| tMCAO | 30 min after MCAO, I.V. | 2, 4, 10 mg/kg | HMGB1 | Infarct volume, oxidative stress, apoptosis, neuron-inflammation, | [193] | |||||||||||||||||||||||||
| tMCAO | 30 min before and 24 hour after MCAO, I.G. | 30 mg/kg | TLR2/4, NF-κB | Infarct volume, neurological deficit, neuro-inflammation | [196] | |||||||||||||||||||||||||
| Glycyrrhizin |
Glycyrhizae glabra |
MCAO + hyperglycemia | 20 min post MCAO, I.P. |
50 mg/kg | HMGB1 | Infarct volume, BBB damage, neurological deficit, brain edema | [194] | |||||||||||||||||||||||
| tMCAO | 3 hour or 6 hour after MCAO, I.V. | 10 mg/kg | HMGB1, iNOS | Neuro-inflammation, Neuro-excitation, oxidative stress, neurotoxicity | [192] | |||||||||||||||||||||||||
| Gastrodin |
Gastrodia elata |
tMCAO | 1 hour after MCAO, I.P. | 10, 50, 100 mg/kg | SOD, MDA, HO-1, Bcl-2, Bax, TNF-α, IL-1β, Nrf-2, AKT | Infarct volume, neurological deficit, neuronal damage and apoptosis, oxidative stress, brain inflammation | [197] | |||||||||||||||||||||||
| Hypoxia; NMDA treatment |
pretreatment | 100, 200 microg/mL | Glutamate, NMDA | Neurotoxicity | [198] | |||||||||||||||||||||||||
|
Ginsenoside Rg1 |
Panax notoginseng |
tMCAO | For 2 days or 7 days after MCAO, I.P. | 20, 40, 80 mg/kg, twice daily | MDA, Trx-1, SOD, PKB/Akt, NF-κB | Infarct volume, neurological deficit | [199] | |||||||||||||||||||||||
| tMCAO, OGD | 1 hour before MCAO; repeated every 12 hour, I.P.; 10 min before OGD | 20mg/kg (In vivo); 10 nM -100 uM (In vitro) |
NMDAR, L-type voltage-dependent Ca2+ channels, Ca2+ | Infarct volume, neurological deficit, Cell viability, Neurotoxicity |
[200] | |||||||||||||||||||||||||
| Paeonol | Paeonia suffruticosa Andrews | pMCAO | Pre-treatment, 3 days, 30 min before MCAO, I.G. |
30,60 mg/kg | MDA, SOD, Nrf-2, HO-1, P-AKT, SOD | Infarct volume, neurological deficit, brain edema, | [201] | |||||||||||||||||||||||
| OGD | Co-treatment with OGD | 2- 50 uM | NMDAR, Ca2+ | Neurotoxicity | [202] | |||||||||||||||||||||||||
| Apocynin |
Picrorhiza kurroa |
tMCAO | 1 hour before MCAO, I.P. | 50 mg/kg | NADPH Oxidase, O2- | Infarct volume | [235] | |||||||||||||||||||||||
| tMCAO | Not mentioned | 30 mg/kg | NADPH Oxidase, MMP-9 |
Infarct volume | [204] | |||||||||||||||||||||||||
| Astragaloside | Astragalus membranaceus | tMCAO | Immediately, 24 hours and 48 hours after MCAO, I.P. | 20, 40 mg/kg | MDA, GSH-PX, SOD | Infarct volume, | [205] | |||||||||||||||||||||||
| tMCAO | Immediately and 12 hour after reperfusion, I.P. | 10, 20 mg/kg | MMP-9, AQP4 | Brain edema, neurological deficit, BBB damage | [206] | |||||||||||||||||||||||||
| Osthole |
Cnidium monnieri (L.) Cusson |
tMCAO | 30 min before MCAO and immediately after reperfusion, I.P. | 100 mg/kg | MMP-9 | Infarct volume | [207] | |||||||||||||||||||||||
| tMCAO | 30 min before MCAO, I.P. | 10, 20 40 mg/kg | MDA, GSH, MPO, IL-1β, IL-8 | Neurological deficit, infarct volume, brain edema | [208] | |||||||||||||||||||||||||
| Caffeic acid |
Phaseolus vulgaris L. |
Global stroke | 30 min prior to ischemia, I.P. | 10, 30, 50 mg/kg | SOD, MDA, NF-κB, 5-LOX | Spatial learning and memory, neuron damage | [209] | |||||||||||||||||||||||
| Magnolol | Magnolia officinalis | Global stroke | 90 min after ischemia, I.P. | 10, 30 mg/kg | IL-1β, TNF-α, IL-6, iNOS, AKT, NF-κB, | Infarct volume | [210] | |||||||||||||||||||||||
| Tetrandrine |
Stephania tetrandra
S. Moore |
tMCAO | Immediately after reperfusion, I.P. | 5, 10, 20 mg/kg | ICAM-1, NF-κB, | Brain edema, neutrophilic recruitment | [211] | |||||||||||||||||||||||
| Paeoniflorin | Paeoniae Radix | tMCAO | 14 day after MCAO, twice per day, I.P. | 5 mg/kg | TNF-α, IL-1β, iNOS, COX-2, 5-LOX, JNK, p38 MAPK, NF-κB |
Astrocyte and microglial activation, neuron loss, | [213] | |||||||||||||||||||||||
| Scutellarin | Erigeron breviscapus | tMCAO | 2 hour before MCAO, 12, 24, 36 and 48 hour after MCAO, I.P. | 50, 100 mg/kg | NF-κB, Notch-1, NICD, Hes-1, MCP-1 | Microglial activation | [214] | |||||||||||||||||||||||
| tMCAO, OGD | 30 min before MCAO and immediately after reperfusion | 20, 60 mg/kg, 25, 50, 100 uM |
SOD, CAT, GSH, ROS | Infarct volume and neurological deficit | [215] | |||||||||||||||||||||||||
| Silymarin | Silybum marianum | tMCAO | Once daily for 15 days, I.G. | 200 mg/kg | GSH, GR, GPx, SOD, p53, caspase-9/3 | Neurological deficit, | [216] | |||||||||||||||||||||||
| Transient cerebral ischemia | 15 min before or 60 min after ischemia, I.V. | 1, 5, 10 ug/kg | COX-2, iNOS, MPO, STAT-1, NF-κB, IL-1β, TNF-α, O2-, 3-NT |
Infarct volume, neurological deficit | [217] | |||||||||||||||||||||||||
| Berberin |
Coptidis Rhizoma |
pMCAO | Immediately after MCAO | 10, 40 mg/kg | p-Akt, p-GSK3β, NF-κB, p-CREB, | Infarct volume, brain edema, neurological deficit, | [218] | |||||||||||||||||||||||
| tMCAO | 30 min before and 1 day after MCAO | 20 mg/kg | ROS, cytochrome C, AIF | Infarct volume, neurological deficit, neurotoxicity, | [219] | |||||||||||||||||||||||||
| Ferulic acid | Angelica sinensis | tMCAO | Immediately after MCAO, I.V. | 80, 100 mg/kg | ICAM-1, O2-, MPO, NF-κB, | Infarct volume, neurological deficit, | [220] | |||||||||||||||||||||||
| Tetramethylpy-razine | Ligusticum wallichii Rhizomes | pMCAO | 30 min before and 60 min after MCAO, I.P. | 20 mg/kg | NO, HMGB1, TLR4, Nrf2, HO-1, ERK, iNOS |
Neuronal loss, microglial/macrophage activation, neutrophil activation, |
[221] | |||||||||||||||||||||||
Table 2.
Targets of selected natural compounds.
| Natural Compound | Targets | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| LRP-1 | PDGF-CC | MMP-9 | NF-κB | VEGF | HMGB1 | RNS and ROS | NMDAR | APC | |
| Baicalin | Y | Y | Y | Y | |||||
| Tanshinone IIA | Y | Y | Y | Y | |||||
| Huperzine A | Y | Y | Y | ||||||
| Honokiol | Y | Y | Y | ||||||
| Puerarin | Y | Y | Y | ||||||
| Curculigoside | Y | Y | Y | ||||||
| Shikonin | Y | Y | Y | ||||||
| Glycyrrhizin | Y | Y | |||||||
| Gastrodin | Y | Y | |||||||
| Ginsenoside Rg1 | Y | Y | |||||||
| Paeonol | Y | Y | |||||||
| Apocynin | Y | Y | |||||||
| Astragaloside IV | Y | Y | |||||||
| Osthole | Y | Y | |||||||
| Caffeic acid | Y | Y | |||||||
| Magnolol | Y | Y | |||||||
| Tetrandrine | Y | Y | |||||||
| Paeoniflorin | Y | Y | |||||||
| Scutellarin | Y | Y | |||||||
| Silymarin | Y | Y | |||||||
| Berberine | Y | Y | |||||||
| Ferulic acid | Y | Y | |||||||
| Tetramethylpyrazine | Y | Y | |||||||
3.1. Baicalin
Baicalin is a promising neuroprotective drug candidate with the extensive studies on ischemic stroke animal models. Baicalin regulates many molecular targets including free radicals, NMDA receptor, NF-κB, and MMP-9 in various experimental stroke models. Baicalin is a flavonoid compound isolated from Radix of Scutellaria baicalensis Georgi. Our work and others showed that baicalin treatment reduced cerebral infarct volume, attenuated neurotoxicity and neuroinflammation in both transient and permanent rodent cerebral ischemia models [148-150]. We found that its neuroprotective mechanisms could be at least partially attributed to scavenging ONOO- [148]. Mass spectrometry revealed baicalin could directly scavenge ONOO- and ameliorate endogenous and exogenous ONOO- mediated neurotoxicity in cultured neuronal cells in vitro. Animal experiments further confirmed that baicalin inhibited 3-nitrotyrosine formation and decreased the infarct size in a rat model of cerebral ischemic brain injury [148]. In addition, baicalin could inhibit NMDAR mediated calcium influx and 5-lipoxygenase translocation and activation, and reduced primary cortical neurons death in vitro [151]. Baicalin revealed its anti-inflammatory properties of inhibiting NF-κB expression up to 70% and down-regulating the downstream signaling molecules including iNOS, COX-2, TNF-α, and IL-1β in the ischemic brains under both permanent and transient cerebral ischemia animal models [149, 150]. Baicalin treatment also reduced BBB damage and brain edema by substantially suppressing the MMP-9 expression and protecting tight junction proteins in ischemic brains [152]. The underlying mechanisms of inhibiting MMP-9 activity could be related to down-regulating NF-κB signaling [153]. Importantly, baicalin is capable of penetrating the BBB [154]. Therefore, baicalin could be a promising candidate for ischemic stroke treatment as well as the adjunct therapy for t-PA, and further study is warranted to verify this idea with clinical evidence.
3.2. Tanshinone IIA
Tanshinone IIA is a representative active ingredient of Salvia miltiorrhiza root, a commonly used herbal medicine for cardiovascular and cerebral vascular diseases. Accumulating evidences indicate that Tanshinone IIA could target on NF-κB, MMP-9, HMGB1 and RNS synergistically in different experimental stroke models [155-159] Tanshinone IIA is effective to reduce infarct volume, brain edema, and improve neurological deficits, and its underlying mechanisms are involved in multiple molecular targets in current literature including apoptosis-related factors [160, 161], NF-κB and neuro-inflammation factors [155], RNS [156] as well as HMGB1 [157]. Tanshinone IIA down-regulated the expression of MMP-9, HMGB1, RAGE, TLR4, NF-κB, ameliorated BBB permeability and endothelial cell dysfunction function, and protected rat brains against focal ischemia in transient and permanent middle cerebral artery occlusion (MCAO) models [158, 159]. Thus, tanshinone IIA is a promising candidate compound from a natural source with multiple targets for reducing neurotoxicity and BBB damage for stroke treatment.
3.3. Huperzine A, Honokiol, Puerarin
Huperzine A, honokiol and puerarin are natural compounds regulating the same targets including NF-κB, free radicals, and NMDAR. Huperzine A is an alkaloid isolated from Huperzia serrata, which is regarded as a selective acetylcholinesterase (AChE) inhibitor and a potential drug for treatment of Alzheimer's disease [162, 163]. Huperzine A was nontoxic in both human and animal safety tests [163]. A recent meta-analysis revealed that huperzine A improved the cognitive performance of patients with AD and vascular dementia (VD) [164]. In a focal cerebral ischemia model, huperzine A treatment at 0.1 mg/kg reduced brain infarct volume and improved the neurological functions. Pre-treatment of huperzine A preserved the activities of mitochondrial respiratory chain enzymes (complex I, complex II-III, and complex IV), inhibited ROS generation, attenuated mitochondrial dysfunction and mitochondrial swelling [165] and ameliorated brain inflammation in a focal cerebral ischemia animal model [166]. Huperzine A inhibited NF-κB expression and attenuated glial activation in ischemic brains [166]. In vitro oxygen and glucose deprivation (OGD) experiments showed that huperzine A reduced NF-κB translocation and activation, downregulated iNOS, COX-2, inhibited NO production, and prevented cell death [167]. Oral administration of huperzine A at 0.2 mg/kg for 9 days up-regulated neurotrophic factors like BDNF, NGF, and TGF-β1 and improved memory in a mouse model of focal cerebral ischemia [168]. Moreover, huperzine A could interact with the NMDA receptor and prevent glutamate-induced calcium influx and neurotoxicity in vitro [163, 169]. Huperzine A is also capable of penetrating BBB, which makes it more competitive in preventing t-PA-mediated HT [163]. Thus, huperzine A is also a good drug candidate as adjunct therapy with t-PA for ischemic stroke treatment.
Honokiol is a natural compound derived from Magnolia grandiflora. Honokiol has pleiotropic effects, including chemotherapeutic, neuroprotective and anesthetic effects [170]. Honokiol treatment revealed to inhibit inflammation and reduce brain edema as well as the BBB leakage [171-173]. Honokiol blocked translocation and activation of NF-κB, down-regulated TNF-α expression and inhibited NO production in glial cells [171]. Honokiol interrupted the interaction of nNOS with postsynaptic density protein 95 (PSD95) [174]. In a mouse cerebral ischemia model, Honokiol was found to reduce ROS production through preserving Na+/K+-ATPase activity and maintaining the mitochondrial membrane potentials [172]. Moreover, honokiol attenuated neutrophil infiltration and reduced calcium overload [173], inhibited NMDA neurotoxicity [175]. Honokiol is also a BBB-permeable active compound [176, 177]. It is notable that honokiol is a potent inhibitor of arterial thrombosis [178] and has the value for thrombotic stroke. Given that the promising evidence has been obtained, honokiol could be considered as a good candidate for further study.
Puerarin is an isoflavonoid derived from medicinal plant Radix puerariae and has great potentials for stroke treatment. Even delayed treatment of puerarin at 24 hours after ischemic stroke successfully reduced brain infarct volume and improved neurological outcome [179]. Puerarin treatment for 7 days was effective in reducing infarct size and improving neurological outcome, as well as reducing astrocyte apoptosis [180]. In addition, puerarin inhibited caspase-3 and NMDAR expression, reducing neuronal damage in cerebral ischemia [181, 182]. Puerarin ameliorated neuroinflammation and infarct size and its underlying mechanisms could be associated with inhibiting NF-κB activity and scavenging hydroxyl radical in rat models of MCAO cerebral ischemia [183, 184].
3.4. Curculigoside
Curculigoside is a major bioactive compound isolated from the rhizome of Curculigo orchioides Gaertn. Curculigoside was reported to inhibit ROS, NF-κB, and HMGB1 in previous studies [185-187]. Curculigoside reduced NMDA-induced neurotoxicity via scavenging ROS and modulating apoptotic-related proteins in cultured cortical neurons [185]. Curculigoside treatment reduced the BBB damage and neuro-toxicity by concurrently inhibiting NF-κB phosphorylation and reducing HMGB1 expression in vivo and in vitro [186]. Liquid chromatography–mass spectrometry analysis revealed that curculigoside was detectable in rat brain tissues after oral intake or intravenous injection, indicating that curculigoside could cross the BBB. [187]. Therefore, further studies on curculigoside are worth for evaluating its potentials for preventing t-PA induced HT in stroke treatment.
3.5. Shikonin
Shikonin is a naphthoquinone pigment isolated from the roots of Lithospermum erythrorhizon. Shikonin was reported to inhibit ROS production, alleviate brain infarct size and improve the neurological outcome in a mouse MCAO model [188]. Shikonin inhibited the expression of MMP-9, TLR-4 and the activity of NF-κB, attenuated neuroinflammation and the BBB damage in a mouse transient MCAO model [189]. Shikonin down-regulated the activation of NF-κB and the expression of iNOS, COX-2 and TNF-α, and reduced ROS and NO production in LPS mediated microglial cells activation experiments [190]. In addition, Shikonin down-regulated HMGB1 and NF-κB signaling, and attenuated inflammation in an LPS-induced macrophage activation model [191]. The effects of shikonin on t-PA mediated HT and neurotoxicity remain to be addressed.
3.6. Glycyrrhizin
Glycyrrhizin is the main constitute of herbal medicine Glycyrrhiza glabra, and it has inhibitory effects on NF-κB and HMGB1. Glycyrrhizin reduced HMGB1 secretion, down-regulated many inflammatory factors including TNF-α, iNOS, IL-1β, and IL-6 in ischemic brains [192, 193]. Glycyrrhizin inhibited the interaction between HMGB1 and PKC or calcium/calmodulin-dependent protein kinase IV (CaMKIV), reducing HMGB1 phosphorylation and its subsequent release [192]. Glycyrrhizin treatment ameliorated infarct volume and improved neurological outcomes in both transient ischemic stroke model and acute hyperglycemia stroke model [192-194]. Moreover, glycyrrhizin exhibited anti-apoptotic effects via inhibiting IL-17 A mediated reduction of bcl-2/bax ratio and cytochrome C release in ischemic brains [193, 195]. Glycyrrhizin reduced oxidative stress and NF-κB related brain inflammation in rodent ischemic stroke model [192, 193, 196]. Thus, glycyrrhizin is a potent multi-target compound in alleviating cerebral ischemia-reperfusion injury. The effects of glycyrrhizin on the BBB permeability and t-PA’-mediated HT remain to be addressed in future.
3.7. Gastrodin, Ginsenoside Rg1, and Paeonol
These compounds could target on scavenging free radicals and inhibiting NMDAR activation and have therapeutic potentials for cerebral ischemia-reperfusion injury. Gastrodin, the main constitute of Gastrodia elata, exhibited anti-inflammation and antioxidant effects through down-regulating TNF-α, IL-1β, and increasing SOD and Nrf2 in rodent ischemic brains [197]. Gastrodin also reduced NMDA or glutamate-induced neurotoxicity in cortical neurons in vitro [197, 198]. Ginsenoside Rg1 reduced brain infarct volume and alleviated neurological deficit through reducing MDA level and NF-κB translocation in an experimental stroke model [199]. In an in vitro study, Ginsenoside Rg1 reduced neurotoxicity via inhibiting NMDAR induced calcium influx [200]. Similarly, paeonol, an active compound from Paeonia suffruticosa Andrews and Paeonia lactiflora Pall, revealed to reduce malondialdehyde (MDA), an index of lipid peroxidation, and upregulate SOD activity, increase the HO-1 and Nrf2 level and attenuate infarct volume in experimental ischemic brain model [201]. Paeonol also reduced neurotoxicity via targeting on NMDAR in cultured neurons exposed to OGD condition [202]. The values of Gastrodin, Ginsenoside Rg1, and Paeonol for reducing t-PA’s side effects remain to be addressed.
3.8. Apocynin, Astragaloside IV, Osthole
Apocynin is an NADPH oxidase inhibitor. By inhibiting NADPH oxidase activity, apocynin reduced superoxide production and down-regulated MMP-9 expression in an experimental cerebral ischemia model [203, 204]. Astragaloside IV treatment at 20 mg/kg down-regulated MMP-9 expression and upregulated SOD activity in ischemic brain tissues [205, 206]. Osthole was reported to have similar effects on inhibiting MMP-9 activity and oxidative stress in rat MCAO cerebral ischemia models [207, 208]. However, with current evidence, it is still premature to draw a conclusion about the neuroprotective effects of those compounds for ischemic stroke with or without t-PA treatment.
3.9. Other Compounds
Compounds mainly targeting on NF-κB and free radicals include caffeic acid [209], magnolol [210], tetrandrine [211, 212], paeoniflorin [213], scutellarin [214, 215], silymarin [216, 217], berberine [218, 219], ferulic acid [220] and tetramethylpyrazine [221], which are listed in Table 2. Due to the limited space, we will not discuss in details about those compounds but simply summarize their pharmacological functions in Table 1. Those compounds also merit further investigation in regarding to reducing t-PA-induced HT and neurotoxicity.
4. DISCUSSION
In this comprehensive review article, we have summarized current progress on the molecular targets involved in t-PA-mediated HT and neurotoxicity and selected several active compounds potentially for t-PA as the combined therapies for ischemic stroke. Total 23 compounds with the functions of targeting at least two mentioned targets are included in the study. Those compounds merit further studies toward combined therapy with t-PA for ischemic stroke treatment. Nevertheless, with limited literature evidence, we should remark that it is still premature to argue which compound enjoy the highest priority for further development of an adjunct agent to reduce the side effects of t-PA and enhance therapeutic outcome. We specifically address several important issues in the interpretation of research results, which should be paid great attentions in future studies.
4.1. Multi-Target and Their Interaction
Current evidence indicates the involvement of multiple molecular targets in mediating t-PA-induced HT and neurotoxicity during stroke treatment. As shown in Fig. (1), LRP-1 can activate NF-κB and induce MMP-9 expression in glial cells, and mediate NMDAR activation in neurons in the presence of t-PA. HMGB-1 could bind to its receptors including TLR4 and RAGE, inducing NF-κB activation and MMP-9 production. Free radicals participate in NF-κB activation, MMP-9 production, and activation. We believe that more and more targets will be found in further studies. For example, NURR1 was recently reported to participate in the process of HT in t-PA treated ischemic brains [222]. Under such complex network systems involving different signaling pathways, these targets themselves may have independent and/or synergic effects on certain pathological processes in ischemic stroke. Modulating one target may affect other targets consequently. For example, the antioxidant effects might contribute to the modulation of other targets like MMP-9, NF-κB and so on. In data interpretation, the pharmacological effects on the different targets could be due to direct interactions, or simply because of the indirect responses of molecules to the regulation of upstream signaling and/or the synergic effects of the network signaling systems. On the other hand, current studies mainly focus on the effects of natural compounds for scavenging free radicals or their antioxidant properties and regulating NF-κB signaling, but much less attention has been paid to other molecular targets such as LRP-1, PDGF-CC, VEGF, and APC (Table 2). Thus, further study is desirable for addressing to the specific interactions or direct binding effects of natural compounds on their molecular targets.
4.2. One-Drug-Multi-Target: Potential of Natural Compounds
With multiple targets involved in t-PA mediated HT and neurotoxicity, strategy for modulating one target seems to be insufficient to achieve satisfactory outcomes [223]. Multiple target modulating strategy should be considered to intervene with the complex pathological process. There are two possible ways to modulate multiple targets simultaneously: one is to use multiple drugs for different targets at the same time; the other is to use one compound interfering with multiple targets, which is so called “one-drug-multi-target.” The combination of t-PA with natural compounds fits in the concept of targeting thrombolysis and achieving neuro-protection at the same time. Herein, using multi-target natural compounds could be a promising combined therapy with t-PA to reduce HT and neurotoxicity and extend the therapeutic time windows during stroke treatment.
One-drug-multi-target paradigm strategy has been proposed for AD treatment [224]. Although the strategy seems to be challenging for stroke treatment but it should be feasible. Many compounds from natural source have good profiles in both safety and efficacy. For examples, Flavocoxid, containing baicalin and catechin, has been approved by FDA as a medicinal food for osteoarthritis. Flavocoxid has neuro-protective effects against global cerebral ischemic injury [225]. Tanshinone IIA is approved for treating cardiovascular diseases in China [226, 227]. With its neuroprotective property, Tanshinone IIA is also promising for stroke treatment with multiple targets involved. Huperzine A is a nonprescription diary supplement. Although huperzine A is not yet approved by FDA [228], it has been approved for AD in China since 1994. A recent meta-analysis suggests that huperzine A has good safety [164]. Glycyrrhizin is a clinically used drug in Japan for treating viral hepatitis. A recent study indicates that glycyrrhizin has well-tolerance in patients and healthy volunteers without severe adverse effects [229]. Thus, those healthy food supplements, if their neuroprotection effects are proved, have the priority for the translation into clinical use for stroke.
Among 23 selected natural compounds, 7 compounds including baicalin, huperzine A, tanshinone IIA, honokiol, puerarin, curculigoside, and shikonin could act on at least 3 mentioned targets and have neuroprotective effects in various experimental stroke models, while others affect at least 2 molecular targets. Some of the natural compounds have been verified by several independent studies, with consistent protective effects and pleiotropic effects against ischemic brain injury in different experimental stroke models. For example, baicalin has been proved to maintain the BBB integrity, reduce infarct volume and brain edema, and improve the neurological outcomes in transient and permanent MCAO models in vivo as well as OGD model in vitro. Similarly, Tanshinone IIA protects against ischemic brain damages in various animal models of both transient ischemia and permanent ischemia. Honokiol protects neurons and maintains BBB integrity with in vivo and in vitro experimental evidence. Glycyrrhizin inhibits HMGB1 and NF-κB and has anti-inflammatory effects, subsequently reducing infarct volume, BBB damage and neurological deficit in transient MCAO cerebral ischemic models. The pleiotropic effects on multi-targets have been tested in at least 5 independent studies (Tables 1 and 2). Thus, those compounds should have high priority for further investigation and have potentials to develop into new drugs for stroke [230]. Notably, due to the diversity of research qualities and methodology, high-quality investigations are needed to further evaluate their pharmacological efficacy, bioavailability, pharmacokinetics/pharmacodynamics and toxicity for drug development.
4.3. Development of Natural Compounds: Issues to be Considered
For developing drug candidates, we should also consider following aspects.
4.3.1. Optimal Dosage
Although most of the studies provided the data of dose-dependent responses, we should note the same compound has a diversity of dosages used in different studies. One study used the lowest dosage of honokiol at 0.1 µg/kg but the other employed the highest dosage up to 100 mg/kg (Table 1). With huge difference in the dosage, it is tough to interpret the results when they are put together. Hence, the optimal dosages for the combined treatment with t-PA are important. We note that some compounds revealed their neuroprotective effects even at relatively low dosages. For example, even at the very low dosage (0.1 mg/kg), huperzine still presented its neuroprotective effects [166]. Based on the critical consideration, we recommend giving high priority to huperzine A, curculigoside, glycyrrhizin, and honokiol for further studies.
4.3.2. Therapeutic Time Window
The therapeutic time window is another important issue. As shown in Table 1, in most of the studies, the compounds were administrated within 2 hours after MCAO, which is at the benefit time window of t-PA for neuroprotection. We should be cautious whether the protective effects of those compounds are still existed when treated beyond 4.5 hours after ischemic stroke. By considering this critical issue, puerarin, curculigoside and glycyrrhizin are good drug candidates. Puerarin treated at 24 hours after ischemic onset and continuously for 14 days revealed to improve the neurological outcome in a rat MCAO model [179]. Curculigoside treated at 5 hours after MCAO effectively down-regulated the expression of NF-κB and HMGB1, reduced brain damage and BBB breakdown [186]. Similarly, treatment of glycyrrhizin at 6 hours after MCAO significantly inhibited HMGB1 secretion, reduced infarct volume and suppressed inflammation [192]. Those studies are valuable, as they demonstrated the protective effects of those compounds administrated beyond the t-PA’s treatment time window.
Stroke has three pathological phases [231]. The first phase occurs within hours after stroke onset. In this period, infarct volume develops quickly at the penumbra area of ischemic brains. Strategies for rapid recanalization or neuroprotection are necessary to attenuate brain damage at this phage. The second phase is from days to weeks after stroke onset. In this period, the brain begins to remodel with neurogenesis and angiogenesis. Post-stroke inflammation could exacerbate the brain damage and block neurogenesis. Promoting brain repair and anti-inflammation should be the promising strategy at that period for stroke treatment. Puerarin and glycyrrhizin treatment could achieve the goals to anti-inflammation and promoting neurogenesis and angiogenesis. The third phase is the chronic progress of post-stroke recovery in which brain condition gets relatively stable, but therapeutic modification is still possible. Glycyrrhizin and puerarin appear to be good candidates. Hence, more attentions should be given to the compounds with long term effects even administrated at late phase for translational study. For the development of combined treatment with thrombolytic therapy, the optimal time point should be taken into consideration to evaluate the application potentials of the candidate drugs.
4.3.3. Long-term Efficacy
The long-term efficacy is also an important issue for drug development. Compounds exert their protective effects at the acute phase but may not necessarily possess long-term efficacy, eventually failing to provide therapeutic benefits in a long-term way. Previous studies seldom paid attention to evaluating the long-term effectiveness of those natural compounds. Notably, treatment of Tanshinone IIA for 7 days or 15 days inhibited the expression of HMGB1 and NF-κB signaling and attenuated infarct volume in an experimental stroke model [157]. Puerarin also has long-term neuro-protective effects. Treatment for 14 days enhanced neuro-vascular remodeling, attenuated brain infarct volume and improved the neurological outcome [179]. Paeoniflorin treatment for 14 days significantly suppressed activation of glial cells and reduced the cytokines expression in a rat MCAO cerebral ischemia model [213]. Those compounds are valuable for their long-term neuroprotective effects.
4.3.4. Outcome Measurement
Outcome measurement is another important issue. Many natural compounds were reported to reduce the infarct volume, attenuate BBB damage and brain edema, inhibit inflammation and improve neurological functions (Table 1). The compounds have high priority for further investigations because of achieving multiple outcomes to modulate different pathological aspects. For drug discovery, we should evaluate multiple aspects in protection against ischemic brain damages with the outcomes at molecular, histological and neurological function levels. In data interpretation, we should emphasize to differentiate the therapeutic outcome from the observed compound itself or the synergic effects of the compound with t-PA or other drugs when combination therapy is applied.
4.3.5. Safety and Drug-drug Interaction
Safety issues and drug-drug interactions are other critical issues when those compounds are simultaneously administrated with t-PA, particularly for the compounds with the function of thrombolysis [232]. Honokiol is one of the representative thrombolytic compounds [178]. The combination of natural compounds with t-PA may yield other potential problems such as complex drug-drug interaction, potential side-effects, uneven absorption and distribution [233]. Selection of suitable animal models is another important issue for drug development. Embolic stroke model should be adopted for thrombolytic treatment because suture occlusion of MCAO could not tell the recanalization rate induced by t-PA. All of those aspects should be carefully addressed for the development of combination therapy. We also remark that the combination of two compounds may be not necessary to generate a better therapeutic outcome. For example, erythropoietin was shown neuroprotective; however, when combined with t-PA, it could exacerbate HT [234]. Hence, the concept of combining t-PA with multi-target compounds needs to be carefully tested, and multiple issues as stated above should be considered for developing the adjunct therapy for reducing t-PA’s side effects.
SUMMARY AND CONCLUSION
In this article, we reviewed recent developments in the molecular targets contributing to the pathogenesis of t-PA-induced HT and neurotoxicity. Based on the multi-targets involved in HT and neurotoxicity, we then selected 23 representative natural compounds modulating multi-targets and evaluated the potentials of those compounds as combination therapies with t-PA for acute ischemic stroke. Seven of them including baicalin, puerarin, huperzine A, honokiol, curculigoside, shikonin, tanshinone IIA are reported to modulate at least three molecular targets and protect ischemic brains. Those compounds are valuable as drug candidates for reducing t-PA-induced HT and neurotoxicity. Nevertheless, it is still unclear whether those bioactivities are achieved directly or indirectly. Due to the diversity of experimental designs and methods, we are far beyond the point to draw a conclusion and remark which compound is valuable for clinical investigation and developing into an adjunct therapy. Information about the possible drug-drug interaction of those compounds with t-PA is still lacking. The experiments on the pharmacological activities, pharmacokinetics/pharmacodynamics and toxicity are also necessary for developing those drug candidates. In conclusion, natural compounds are essential sources for developing a new therapeutic strategy for stroke treatment. Developing adjunct therapeutic agents from natural compounds would bring novel idea to reduce the t-PA’s side-effects and finally save many stroke patients with a better outcome.
ACKNOWLEDGEMENTs
This work was supported by Hong Kong General Research Fund (GRF No 17102915 and No. 776512M), Research Grant Council, Hong Kong SAR; and Grant from National Natural Science Foundation of China (No. 31270902 and No. 81171075); Seed Funding Programme for Basic Research, University of Hong Kong (No. 201411159208) and Natural Science Foundation of the Jiangsu Higher Education Institutions of China (No. 13KJA310005).
lIST OF ABBREVIATIONS
- AD
Alzheimer’s disease
- AKT
protein kinase B
- AIF
Apoptosis Inducing Factor
- AQP4
Aquaporin 4
- APC
activated protein C
- Bax
Bcl-2-Like Protein 4
- BBB
blood-brain barrier
- Bcl-2
B-cell lymphoma 2
- BDNF
Brain-derived neurotrophic factor
- CAT
Catalase
- CBF
cerebral blood flow
- COX-2
Cyclooxygenase-2
- CREB
cAMP response element-binding protein
- DNA
deoxyribonucleic acid
- eNOS
endothelial nitric oxide synthase
- ERK1/2
extracellular-signal-regulated kinases
- FeTMPyP
Fe(III) tetrakis (1-methyl-4-pyridyl) porphyrin pentachlorideporphyrin pentachloride
- GPx
Glutathione peroxidase
- GR
glutathione reductase
- GSH
Glutathione
- GSK-3
Glycogen synthase kinase 3
- HES-1
hairy and enhancer of split-1
- HMGB1
High-mobility group protein B1
- HO-1
heme oxygenase 1
- HT
hemorrhagic transformation
- ICAM-1
Intercellular Adhesion Molecule 1
- IL-1β
Interleukin-1 beta
- IL-6
Interleukin 6
- IL-8
Interleukin 8
- IL-17A
Interleukin 17A
- iNOS
inducible nitric oxide synthase
- I.P.
Intraperitoneal
- I.V.
intravenous
- I.G.
intragastric
- JNK
c-Jun N-terminal kinase
- LPS
Lipopolysaccharide
- LRP-1
Low density lipoprotein receptor-related protein 1
- MCAO
middle cerebral artery occlusion
- MCP-1
monocyte chemotactic protein 1
- MDA
malondialdehyde
- MIF
Migration inhibitory factor
- MMP-9/-3
Matrix metallopeptidase 9/3
- MPO
Myeloperoxidase
- MTDLs
multi-target directed ligands
- nAChR
Nicotinic acetylcholine receptor
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NGF
nerve growth factor
- NMDA
N-methyl-D-aspartate
- NMDAR
N-methyl-D-aspartate receptor
- NICD
Notch intracellular domain
- NURR1
Nuclear receptor related 1 protein
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- NOS
nitric oxide synthase
- Nrf2
nuclear factor erythroid 2–related factor 2
- OGD
oxygen and glucose deprivation
- ONOO-
peroxynitrite
- PD
Parkinson’s disease
- PDGF-CC
Platelet-derived growth factor-CC
- PDGF-α
platelet-derived growth factor alpha
- pMCAO
permanent MCAO
- PSD95
postsynaptic density protein 95
- p38 MAPK
p38 mitogen-activated protein kinases
- RAGE
receptor for advanced glycation end-products
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- shRNA
small heparin RNA
- siRNA
small interfering RNA
- SOD
Superoxide Dismutase
- STAT-1
Signal Transducers and Activators of Transcription-1
- TGF-β1
Transforming growth factor beta 1
- TLR2/4
Toll-like receptor 2/4
- tMCAO
transient MCAO
- TNF-α
Tumor necrosis factor alpha
- 5-LOX
5-lipoxygenase
- TORC1
TOR Complex 1
- t-PA
tissue plasminogen activator
- Trx-1
thioredoxin-1
- VEGF
vascular endothelial growth factor
- ZO-1
zona occuldens protein 1
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Esenwa C., Gutierrez J. Secondary stroke prevention: challenges and solutions. Vasc. Health Risk Manag. 2015;11:437–450. doi: 10.2147/VHRM.S63791. [PMID: 26300647]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roger V.L., Go A.S., Lloyd-Jones D.M., Adams R.J., Berry J.D., Brown T.M., Carnethon M.R., Dai S., de Simone G., Ford E.S., Fox C.S., Fullerton H.J., Gillespie C., Greenlund K.J., Hailpern S.M., Heit J.A., Ho P.M., Howard V.J., Kissela B.M., Kittner S.J., Lackland D.T., Lichtman J.H., Lisabeth L.D., Makuc D.M., Marcus G.M., Marelli A., Matchar D.B., McDermott M.M., Meigs J.B., Moy C.S., Mozaffarian D., Mussolino M.E., Nichol G., Paynter N.P., Rosamond W.D., Sorlie P.D., Stafford R.S., Turan T.N., Turner M.B., Wong N.D., Wylie-Rosett J. Heart disease and stroke statistics2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [http://dx.doi.org/10.1161/CIR.0b013 e3182009701]. [PMID: 21160056]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raphaeli G., Mazighi M., Pereira V.M., Turjman F., Striefler J. State-of-the-art endovascular treatment of acute ischemic stroke. Adv. Tech. Stand. Neurosurg. 2015;42:33–68. doi: 10.1007/978-3-319-09066-5_3. [http://dx.doi.org/10.1007/978-3-319-09066-5_3]. [PMID: 25411144]. [DOI] [PubMed] [Google Scholar]
- 4.Lansberg M.G., Bluhmki E., Thijs V.N. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke: a metaanalysis. Stroke. 2009;40(7):2438–2441. doi: 10.1161/STROKEAHA.109.552547. [http://dx. doi.org/10.1161/STROKEAHA.109.552547]. [PMID: 19478213]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hacke W., Donnan G., Fieschi C., Kaste M., von Kummer R., Broderick J.P., Brott T., Frankel M., Grotta J.C., Haley E.C., Jr, Kwiatkowski T., Levine S.R., Lewandowski C., Lu M., Lyden P., Marler J.R., Patel S., Tilley B.C., Albers G., Bluhmki E., Wilhelm M., Hamilton S., Investigators A.T., Investigators E.T. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363(9411):768–774. doi: 10.1016/S0140-6736(04)15692-4. [http://dx.doi.org/10.1016/ S0140-6736(04)15692-4]. [PMID: 15016487]. [DOI] [PubMed] [Google Scholar]
- 6.Balami J.S., Sutherland B.A., Buchan A.M. Complications associated with recombinant tissue plasminogen activator therapy for acute ischaemic stroke. CNS Neurol. Disord. Drug Targets. 2013;12(2):155–169. doi: 10.2174/18715273112119990050. [http://dx.doi.org/10.2174/18715273112119990050]. [PMID: 23394532]. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L., Zhang Z.G., Chopp M. The neurovascular unit and combination treatment strategies for stroke. Trends Pharmacol. Sci. 2012;33(8):415–422. doi: 10.1016/j.tips.2012.04.006. [http://dx.doi.org/10.1016/j.tips.2012. 04.006]. [PMID: 22595494]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishrat T., Soliman S., Guan W., Saler M., Fagan S.C. Vascular protection to increase the safety of tissue plasminogen activator for stroke. Curr. Pharm. Des. 2012;18(25):3677–3684. doi: 10.2174/138161212802002779. [http://dx.doi.org/10.2174/138161212802002779]. [PMID: 22574982]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agis-Torres A., Sölhuber M., Fernandez M., Sanchez-Montero J.M. Multi-Target-Directed Ligands and other Therapeutic Strategies in the Search of a Real Solution for Alzheimers Disease. Curr. Neuropharmacol. 2014;12(1):2–36. doi: 10.2174/1570159X113116660047. [http://dx.doi.org/10. 2174/1570159X113116660047]. [PMID: 24533013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geldenhuys W.J., Youdim M.B., Carroll R.T., Van der Schyf C.J. The emergence of designed multiple ligands for neuro- degenerative disorders. Prog. Neurobiol. 2011;94(4):347–359. doi: 10.1016/j.pneurobio.2011.04.010. [http://dx.doi.org/10.1016/j.pneurobio.2011.04.010]. [PMID: 21536094]. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh N., Ghosh R., Bhat Z.A., Mandal V., Bachar S.C., Nima N.D., Sunday O.O., Mandal S.C. Advances in herbal medicine for treatment of ischemic brain injury. Nat. Prod. Commun. 2014;9(7):1045–1055. [PMID: 25230523]. [PubMed] [Google Scholar]
- 12.Gu Y., Chen J., Shen J. Herbal medicines for ischemic stroke: combating inflammation as therapeutic targets. J. Neuroimmune Pharmacol. 2014;9(3):313–339. doi: 10.1007/s11481-014-9525-5. [http://dx.doi.org/10.1007/ s11481-014-9525-5]. [PMID: 24562591]. [DOI] [PubMed] [Google Scholar]
- 13.Hamann G.F., del Zoppo G.J., von Kummer R. Hemorrhagic transformation of cerebral infarctionpossible mechanisms. Thromb. Haemost. 1999;82(Suppl. 1):92–94. [PMID: 10695495]. [PubMed] [Google Scholar]
- 14.Bang O.Y., Buck B.H., Saver J.L., Alger J.R., Yoon S.R., Starkman S., Ovbiagele B., Kim D., Ali L.K., Sanossian N., Jahan R., Duckwiler G.R., Viñuela F., Salamon N., Villablanca J.P., Liebeskind D.S. Prediction of hemorrhagic transformation after recanalization therapy using T2*-permeability magnetic resonance imaging. Ann. Neurol. 2007;62(2):170–176. doi: 10.1002/ana.21174. [http://dx. doi.org/10.1002/ana.21174]. [PMID: 17683090]. [DOI] [PubMed] [Google Scholar]
- 15.Hemorrhagic P.E. MRI Detection of Early Blood-Brain Barrier Disruption. Brain. 2008;39:1025–1028. doi: 10.1161/STROKEAHA.107.497719. [DOI] [PubMed] [Google Scholar]
- 16.Lo E.H., Dalkara T., Moskowitz M.A. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 2003;4(5):399–415. doi: 10.1038/nrn1106. [http://dx.doi.org/10.1038/nrn1106]. [PMID: 12728267]. [DOI] [PubMed] [Google Scholar]
- 17.Etique N., Verzeaux L., Dedieu S., Emonard H. 2013. [DOI] [PMC free article] [PubMed]
- 18.Ortolano S., Spuch C. tPA in the central nervous system: relations between tPA and cell surface LRPs. Recent Pat. Endocr. Metab. Immune Drug Discov. 2013;7(1):65–76. [http://dx.doi.org/10. 2174/187221413804660962]. [PMID: 23231415]. [PubMed] [Google Scholar]
- 19.Herz J. LRP: a bright beacon at the blood-brain barrier. J. Clin. Invest. 2003;112(10):1483–1485. doi: 10.1172/JCI20337. [http://dx.doi.org/10.1172/ JCI20337]. [PMID: 14617749]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benchenane K., Ali C., Brillault J., Berezowski V., Fernandez-Monreal M., Lopez-Attalaya J.P., Chuquet J., Nouvelot A., MacKenzie E.T., Dehouck M-P. Tissue-type plasminogen activator crosses the intact blood-brain barrier by LRP-mediated transcytosis: A phenomenon that is switched during oxygen glucose deprivation to an increased and LRP-independent process. J. Cereb. Blood Flow Metab. 2005;25:S140–S140. [http://dx.doi.org/10.1038/sj.jcbfm.9591524.0140]. [Google Scholar]
- 21.Benchenane K., Berezowski V., Fernández-Monreal M., Brillault J., Valable S., Dehouck M.P., Cecchelli R., Vivien D., Touzani O., Ali C. Oxygen glucose deprivation switches the transport of tPA across the blood-brain barrier from an LRP-dependent to an increased LRP-independent process. Stroke. 2005;36(5):1065–1070. doi: 10.1161/01.STR.0000163050.39122.4f. [http://dx.doi.org/10.1161/01.STR.0000163050. 39122.4f]. [PMID: 15817895]. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki Y., Nagai N., Yamakawa K., Kawakami J., Lijnen H.R., Umemura K. Tissue-type plasminogen activator (t-PA) induces stromelysin-1 (MMP-3) in endothelial cells through activation of lipoprotein receptor-related protein. Blood. 2009;114(15):3352–3358. doi: 10.1182/blood-2009-02-203919. [http://dx.doi.org/10.1182/blood-2009-02-203919]. [PMID: 19608750]. [DOI] [PubMed] [Google Scholar]
- 23.An J., Zhang C., Polavarapu R., Zhang X., Zhang X., Yepes M. Tissue-type plasminogen activator and the low-density lipoprotein receptor-related protein induce Akt phosphorylation in the ischemic brain. Blood. 2008;112(7):2787–2794. doi: 10.1182/blood-2008-02-141630. [http://dx.doi.org/10.1182/ blood-2008-02-141630]. [PMID: 18628488]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X., Polavarapu R., She H., Mao Z., Yepes M. Tissue-type plasminogen activator and the low-density lipoprotein receptor-related protein mediate cerebral ischemia-induced nuclear factor-kappaB pathway activation. Am. J. Pathol. 2007;171(4):1281–1290. doi: 10.2353/ajpath.2007.070472. [http://dx.doi.org/10.2353/ajpath.2007.070472]. [PMID: 17717150]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang C., An J., Strickland D.K., Yepes M. The low-density lipoprotein receptor-related protein 1 mediates tissue-type plasminogen activator-induced microglial activation in the ischemic brain. Am. J. Pathol. 2009;174(2):586–594. doi: 10.2353/ajpath.2009.080661. [http://dx. doi.org/10.2353/ajpath.2009.080661]. [PMID: 19147818]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang C., An J., Haile W.B., Echeverry R., Strickland D.K., Yepes M. Microglial low-density lipoprotein receptor-related protein 1 mediates the effect of tissue-type plasminogen activator on matrix metalloproteinase-9 activity in the ischemic brain. J. Cereb. Blood Flow Metab. 2009;29(12):1946–1954. doi: 10.1038/jcbfm.2009.174. [http://dx. doi.org/10.1038/jcbfm.2009.174]. [PMID: 19672275]. [DOI] [PubMed] [Google Scholar]
- 27.Hu K., Yang J., Tanaka S., Gonias S.L., Mars W.M., Liu Y. Tissue-type plasminogen activator acts as a cytokine that triggers intracellular signal transduction and induces matrix metallo- proteinase-9 gene expression. J. Biol. Chem. 2006;281(4):2120–2127. doi: 10.1074/jbc.M504988200. [http://dx.doi.org/10.1074/jbc.M504988200]. [PMID: 16303771]. [DOI] [PubMed] [Google Scholar]
- 28.Yepes M., Sandkvist M., Moore E.G., Bugge T.H., Strickland D.K., Lawrence D.A. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J. Clin. Invest. 2003;112(10):1533–1540. doi: 10.1172/JCI19212. [http://dx.doi.org/10.1172/JCI200319212]. [PMID: 14617754]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin A.M., Kuhlmann C., Trossbach S., Jaeger S., Waldron E., Roebroek A., Luhmann H.J., Laatsch A., Weggen S., Lessmann V., Pietrzik C.U. The functional role of the second NPXY motif of the LRP1 beta-chain in tissue-type plasminogen activator-mediated activation of N-methyl-D-aspartate receptors. J. Biol. Chem. 2008;283(18):12004–12013. doi: 10.1074/jbc.M707607200. [http://dx.doi.org/10.1074/jbc.M707607200]. [PMID: 18321860]. [DOI] [PubMed] [Google Scholar]
- 30.Yepes M., Roussel B.D., Ali C., Vivien D. Tissue-type plasminogen activator in the ischemic brain: more than a thrombolytic. Trends Neurosci. 2009;32(1):48–55. doi: 10.1016/j.tins.2008.09.006. [http://dx.doi.org/10.1016/j.tins.2008.09.006]. [PMID: 18963068]. [DOI] [PubMed] [Google Scholar]
- 31.Cheng T., Petraglia A.L., Li Z., Thiyagarajan M., Zhong Z., Wu Z., Liu D., Maggirwar S.B., Deane R., Fernández J.A., LaRue B., Griffin J.H., Chopp M., Zlokovic B.V. Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nat. Med. 2006;12(11):1278–1285. doi: 10.1038/nm1498. [http://dx.doi.org/10.1038/nm1498]. [PMID: 17072311]. [DOI] [PubMed] [Google Scholar]
- 32.Lin L., Wu C., Hu K. Tissue plasminogen activator activates NF-κB through a pathway involving annexin A2/CD11b and integrin-linked kinase. J. Am. Soc. Nephrol. 2012;23(8):1329–1338. doi: 10.1681/ASN.2011111123. [http://dx.doi.org/10.1681/ASN.2011111123]. [PMID: 22677557]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jickling G.C., Liu D., Stamova B., Ander B.P., Zhan X., Lu A., Sharp F.R. Hemorrhagic transformation after ischemic stroke in animals and humans. J. Cereb. Blood Flow Metab. 2014;34(2):185–199. doi: 10.1038/jcbfm.2013.203. [http://dx.doi.org/10.1038/jcbfm.2013.203]. [PMID: 24281743]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin R., Yang G., Li G. Molecular insights and therapeutic targets for blood-brain barrier disruption in ischemic stroke: critical role of matrix metalloproteinases and tissue-type plasminogen activator. Neurobiol. Dis. 2010;38(3):376–385. doi: 10.1016/j.nbd.2010.03.008. [http://dx.doi.org/10.1016/ j.nbd.2010.03.008]. [PMID: 20302940]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gidday J.M., Gasche Y.G., Copin J.C., Shah A.R., Perez R.S., Shapiro S.D., Chan P.H., Park T.S. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am. J. Physiol. Heart Circ. Physiol. 2005;289(2):H558–H568. doi: 10.1152/ajpheart.01275.2004. [http://dx. doi.org/10.1152/ajpheart.01275.2004]. [PMID: 15764676]. [DOI] [PubMed] [Google Scholar]
- 36.Justicia C., Panés J., Solé S., Cervera A., Deulofeu R., Chamorro A., Planas A.M. Neutrophil infiltration increases matrix metalloproteinase-9 in the ischemic brain after occlusion/ reperfusion of the middle cerebral artery in rats. J. Cereb. Blood Flow Metab. 2003;23(12):1430–1440. doi: 10.1097/01.WCB.0000090680.07515.C8. [http://dx.doi.org/10.1097/ 01.WCB.0000090680.07515.C8]. [PMID: 14663338]. [DOI] [PubMed] [Google Scholar]
- 37.del Zoppo G.J., Frankowski H., Gu Y.H., Osada T., Kanazawa M., Milner R., Wang X., Hosomi N., Mabuchi T., Koziol J.A. Microglial cell activation is a source of metalloproteinase generation during hemorrhagic transformation. J. Cereb. Blood Flow Metab. 2012;32(5):919–932. doi: 10.1038/jcbfm.2012.11. [http://dx.doi.org/10.1038/ jcbfm.2012.11]. [PMID: 22354151]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao B.Q., Wang S., Kim H.Y., Storrie H., Rosen B.R., Mooney D.J., Wang X., Lo E.H. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat. Med. 2006;12(4):441–445. doi: 10.1038/nm1387. [http://dx.doi.org/10.1038/nm1387]. [PMID: 16565723]. [DOI] [PubMed] [Google Scholar]
- 39.Chaturvedi M., Kaczmarek L. Mmp-9 inhibition: a therapeutic strategy in ischemic stroke. Mol. Neurobiol. 2014;49(1):563–573. doi: 10.1007/s12035-013-8538-z. [http://dx.doi.org/10.1007/s12035-013-8538-z]. [PMID: 24026771]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Y., Rosenberg G.A. Matrix metalloproteinases as therapeutic targets for stroke. Brain Res. 2015;1623:30–38. doi: 10.1016/j.brainres.2015.04.024. [http://dx.doi.org/10.1016/j.brainres.2015.04.024]. [PMID: 25916577]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen H.S., Chen X.M., Feng J.H., Liu K.J., Qi S.H., Shen J.G. Peroxynitrite Decomposition Catalyst Reduces Delayed Thrombolysis-induced Hemorrhagic Transformation in Ischemia-reperfused Rat Brains. CNS Neurosci. Ther. 2015;21(7):585–590. doi: 10.1111/cns.12406. [http://dx.doi.org/10.1111/cns.12406]. [PMID: 25996167]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Golab P., Kielbus M., Bielewicz J., Kurzepa J. The effect of recombinant tissue plasminogen activator on MMP-2 and MMP-9 activities in vitro. Neurol. Res. 2015;37(1):9–13. doi: 10.1179/1743132814Y.0000000412. [http://dx.doi.org/10.1179/1743132814Y.0000000412]. [PMID: 24963695]. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki Y. Role of tissue-type plasminogen activator in ischemic stroke. J. Pharmacol. Sci. 2010;113(3):203–207. doi: 10.1254/jphs.10r01cp. [http://dx.doi.org/10.1254/jphs.10R01CP]. [PMID: 20595786]. [DOI] [PubMed] [Google Scholar]
- 44.Shen J., Gu Y. Systems Biology of Free Radicals and Antioxidants. Springer-Verlag Berlin Heidelberg; 2014. Insights into mechanisms of blood-brain barrier permeability - Roles of free radicals, matrix metalloproteinsases, and caveolin-1. pp. 2049–2067. [http://dx.doi.org/10.1007/978-3-642-30018-9_92] [Google Scholar]
- 45.Yang Y., Estrada E.Y., Thompson J.F., Liu W., Rosenberg G.A. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J. Cereb. Blood Flow Metab. 2007;27(4):697–709. doi: 10.1038/sj.jcbfm.9600375. [PMID: 16850029]. [DOI] [PubMed] [Google Scholar]
- 46.Lapchak P.A., Chapman D.F., Zivin J.A. Metalloproteinase inhibition reduces thrombolytic (tissue plasminogen activator)-induced hemorrhage after thromboembolic stroke. Stroke. 2000;31(12):3034–3040. doi: 10.1161/01.str.31.12.3034. [http://dx.doi.org/10.1161/01.STR.31.12.3034]. [PMID: 11108768]. [DOI] [PubMed] [Google Scholar]
- 47.Sumii T., Lo E.H. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke. 2002;33(3):831–836. doi: 10.1161/hs0302.104542. [http://dx. doi.org/10.1161/hs0302.104542]. [PMID: 11872911]. [DOI] [PubMed] [Google Scholar]
- 48.Mishiro K., Ishiguro M., Suzuki Y., Tsuruma K., Shimazawa M., Hara H. A broad-spectrum matrix metalloproteinase inhibitor prevents hemorrhagic complications induced by tissue plasminogen activator in mice. Neuroscience. 2012;205:39–48. doi: 10.1016/j.neuroscience.2011.12.042. [http://dx.doi.org/10.1016/j.neuroscience.2011.12.042]. [PMID: 22244977]. [DOI] [PubMed] [Google Scholar]
- 49.Pfefferkorn T., Rosenberg G.A. Closure of the blood-brain barrier by matrix metalloproteinase inhibition reduces rtPA-mediated mortality in cerebral ischemia with delayed reperfusion. Stroke. 2003;34(8):2025–2030. doi: 10.1161/01.STR.0000083051.93319.28. [http://dx.doi.org/10.1161/01.STR. 0000083051.93319.28]. [PMID: 12855824]. [DOI] [PubMed] [Google Scholar]
- 50.Fan X., Lo E.H., Wang X. Effects of minocycline plus tissue plasminogen activator combination therapy after focal embolic stroke in type 1 diabetic rats. Stroke. 2013;44(3):745–752. doi: 10.1161/STROKEAHA.111.000309. [http://dx.doi.org/10.1161/STROKEAHA.111.000309]. [PMID: 23422086]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kasahara Y., Nakagomi T., Matsuyama T., Stern D., Taguchi A. Cilostazol reduces the risk of hemorrhagic infarction after administration of tissue-type plasminogen activator in a murine stroke model. Stroke. 2012;43(2):499–506. doi: 10.1161/STROKEAHA.111.635417. [http://dx.doi.org/10.1161/STROKEAHA.111.635417]. [PMID: 22033992]. [DOI] [PubMed] [Google Scholar]
- 52.Gołąb P., Boguszewska-Czubara A., Kiełbus M., Kurzepa J. The rtPA increases MMP-9 activity in serum during ischaemic stroke. Neurol. Neurochir. Pol. 2014;48(5):309–314. doi: 10.1016/j.pjnns.2014.07.012. [http://dx.doi.org/10.1016/j.pjnns.2014.07.012]. [PMID: 25440008]. [DOI] [PubMed] [Google Scholar]
- 53.Castellanos M., Leira R., Serena J., Pumar J.M., Lizasoain I., Castillo J., Dávalos A. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke. 2003;34(1):40–46. [http://dx.doi.org/10.1161/01.STR. 0000046764.57344.31]. [PMID: 12511748]. [PubMed] [Google Scholar]
- 54.Montaner J., Molina C.A., Monasterio J., Abilleira S., Arenillas J.F., Ribó M., Quintana M., Alvarez-Sabín J. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation. 2003;107(4):598–603. doi: 10.1161/01.cir.0000046451.38849.90. [http://dx.doi.org/10.1161/ 01.CIR.0000046451.38849.90]. [PMID: 12566373]. [DOI] [PubMed] [Google Scholar]
- 55.Ramos-Fernandez M., Bellolio M.F., Stead L.G. Matrix metalloproteinase-9 as a marker for acute ischemic stroke: a systematic review. J. Stroke Cerebrovasc. Dis. 2011;20(1):47–54. doi: 10.1016/j.jstrokecerebrovasdis.2009.10.008. [http://dx.doi.org/10.1016/j.jstrokecerebrovasdis.2009.10.008]. [PMID: 21044610]. [DOI] [PubMed] [Google Scholar]
- 56.Yang Q.W., Wang J.Z., Li J.C., Zhou Y., Zhong Q., Lu F.L., Xiang J. High-mobility group protein box-1 and its relevance to cerebral ischemia. J. Cereb. Blood Flow Metab. 2010;30(2):243–254. doi: 10.1038/jcbfm.2009.202. [http://dx.doi.org/10.1038/jcbfm.2009.202]. [PMID: 19794402]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qiu J., Nishimura M., Wang Y., Sims J.R., Qiu S., Savitz S.I., Salomone S., Moskowitz M.A. Early release of HMGB-1 from neurons after the onset of brain ischemia. J. Cereb. Blood Flow Metab. 2008;28(5):927–938. doi: 10.1038/sj.jcbfm.9600582. [http://dx.doi.org/10.1038/sj.jcbfm. 9600582]. [PMID: 18000511]. [DOI] [PubMed] [Google Scholar]
- 58.Kim J.B., Sig Choi J., Yu Y.M., Nam K., Piao C.S., Kim S.W., Lee M.H., Han P.L., Park J.S., Lee J.K. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J. Neurosci. 2006;26(24):6413–6421. doi: 10.1523/JNEUROSCI.3815-05.2006. [http://dx.doi.org/10.1523/JNEUROSCI.3815-05.2006]. [PMID: 16775128]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qiu J., Xu J., Zheng Y., Wei Y., Zhu X., Lo E.H., Moskowitz M.A., Sims J.R. High-mobility group box 1 promotes metalloproteinase-9 upregulation through Toll-like receptor 4 after cerebral ischemia. Stroke. 2010;41(9):2077–2082. doi: 10.1161/STROKEAHA.110.590463. [http://dx.doi.org/10.1161/STROKEAHA.110.590463]. [PMID: 20671243]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J., Takahashi H.K., Liu K., Wake H., Liu R., Maruo T., Date I., Yoshino T., Ohtsuka A., Mori S., Nishibori M. Anti-high mobility group box-1 monoclonal antibody protects the blood-brain barrier from ischemia-induced disruption in rats. Stroke. 2011;42(5):1420–1428. doi: 10.1161/STROKEAHA.110.598334. [http://dx.doi.org/10.1161/STROKEAHA. 110.598334]. [PMID: 21474801]. [DOI] [PubMed] [Google Scholar]
- 61.Liu K., Mori S., Takahashi H.K., Tomono Y., Wake H., Kanke T., Sato Y., Hiraga N., Adachi N., Yoshino T., Nishibori M. Anti-high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rats. FASEB J. 2007;21(14):3904–3916. doi: 10.1096/fj.07-8770com. [http://dx.doi.org/10.1096/fj.07-8770com]. [PMID: 17628015]. [DOI] [PubMed] [Google Scholar]
- 62.Kim I.D., Shin J.H., Kim S.W., Choi S., Ahn J., Han P.L., Park J.S., Lee J.K. Intranasal delivery of HMGB1 siRNA confers target gene knockdown and robust neuroprotection in the postischemic brain. Mol. Ther. 2012;20(4):829–839. doi: 10.1038/mt.2011.291. [http://dx.doi.org/10.1038/ mt.2011.291]. [PMID: 22252450]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim I.D., Shin J.H., Lee H.K., Jin Y.C., Lee J.K. Intranasal delivery of HMGB1-binding heptamer peptide confers a robust neuroprotection in the postischemic brain. Neurosci. Lett. 2012;525(2):179–183. doi: 10.1016/j.neulet.2012.07.040. [http://dx.doi.org/10.1016/j.neulet.2012.07.040]. [PMID: 22877697]. [DOI] [PubMed] [Google Scholar]
- 64.Kim S.W., Lim C.M., Kim J.B., Shin J.H., Lee S., Lee M., Lee J.K. Extracellular HMGB1 released by NMDA treatment confers neuronal apoptosis via RAGE-p38 MAPK/ERK signaling pathway. Neurotox. Res. 2011;20(2):159–169. doi: 10.1007/s12640-010-9231-x. [http://dx.doi.org/10.1007/ s12640-010-9231-x]. [PMID: 21116767]. [DOI] [PubMed] [Google Scholar]
- 65.Muhammad S., Barakat W., Stoyanov S., Murikinati S., Yang H., Tracey K.J., Bendszus M., Rossetti G., Nawroth P.P., Bierhaus A., Schwaninger M. The HMGB1 receptor RAGE mediates ischemic brain damage. J. Neurosci. 2008;28(46):12023–12031. doi: 10.1523/JNEUROSCI.2435-08.2008. [http://dx.doi.org/10.1523/JNEUROSCI.2435-08.2008]. [PMID: 19005067]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sapojnikova N., Kartvelishvili T., Asatiani N., Zinkevich V., Kalandadze I., Gugutsidze D., Shakarishvili R., Tsiskaridze A. Correlation between MMP-9 and extracellular cytokine HMGB1 in prediction of human ischemic stroke outcome. Biochim. Biophys. Acta. 2014;1842(9):1379–1384. doi: 10.1016/j.bbadis.2014.04.031. [http://dx.doi.org/10.1016/j.bbadis. 2014.04.031]. [PMID: 24815357]. [DOI] [PubMed] [Google Scholar]
- 67.Huang J.M., Hu J., Chen N., Hu M.L. Relationship between plasma high-mobility group box-1 levels and clinical outcomes of ischemic stroke. J. Crit. Care. 2013;28(5):792–797. doi: 10.1016/j.jcrc.2012.10.003. [http://dx.doi.org/10.1016/j.jcrc.2012.10.003]. [PMID: 23137435]. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Z.G., Zhang L., Jiang Q., Zhang R., Davies K., Powers C., Bruggen Nv., Chopp M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J. Clin. Invest. 2000;106(7):829–838. doi: 10.1172/JCI9369. [http://dx.doi.org/10.1172/JCI9369]. [PMID: 11018070]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Z.G., Zhang L., Tsang W., Soltanian-Zadeh H., Morris D., Zhang R., Goussev A., Powers C., Yeich T., Chopp M. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2002;22(4):379–392. doi: 10.1097/00004647-200204000-00002. [http://dx.doi.org/10.1097/00004647-200204000-00002]. [PMID: 11919509]. [DOI] [PubMed] [Google Scholar]
- 70.Li Y.N., Pan R., Qin X.J., Yang W.L., Qi Z., Liu W., Liu K.J. Ischemic neurons activate astrocytes to disrupt endothelial barrier via increasing VEGF expression. J. Neurochem. 2014;129(1):120–129. doi: 10.1111/jnc.12611. [http://dx.doi.org/10.1111/jnc.12611]. [PMID: 24251624]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chi O.Z., Hunter C., Liu X., Weiss H.R. Effects of VEGF and nitric oxide synthase inhibition on blood-brain barrier disruption in the ischemic and non-ischemic cerebral cortex. Neurol. Res. 2005;27(8):864–868. doi: 10.1179/016164105X49418. [http://dx.doi.org/10.1179/016164105X49418]. [PMID: 16354548]. [DOI] [PubMed] [Google Scholar]
- 72.Valable S., Montaner J., Bellail A., Berezowski V., Brillault J., Cecchelli R., Divoux D., Mackenzie E.T., Bernaudin M., Roussel S., Petit E. VEGF-induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: both effects decreased by Ang-1. J. Cereb. Blood Flow Metab. 2005;25(11):1491–1504. doi: 10.1038/sj.jcbfm.9600148. [http://dx.doi.org/10.1038/sj.jcbfm.9600148]. [PMID: 15902195]. [DOI] [PubMed] [Google Scholar]
- 73.Chi O.Z., Hunter C., Liu X., Weiss H.R. Effects of anti-VEGF antibody on blood-brain barrier disruption in focal cerebral ischemia. Exp. Neurol. 2007;204(1):283–287. doi: 10.1016/j.expneurol.2006.11.001. [http://dx.doi.org/10.1016/j.expneurol.2006.11.001]. [PMID: 17188266]. [DOI] [PubMed] [Google Scholar]
- 74.Kanazawa M., Igarashi H., Kawamura K., Takahashi T., Kakita A., Takahashi H., Nakada T., Nishizawa M., Shimohata T. Inhibition of VEGF signaling pathway attenuates hemorrhage after tPA treatment. J. Cereb. Blood Flow Metab. 2011;31(6):1461–1474. doi: 10.1038/jcbfm.2011.9. [http://dx.doi.org/10.1038/jcbfm.2011.9]. [PMID: 21304556]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Su E.J., Fredriksson L., Schielke G.P., Eriksson U., Lawrence D.A. Tissue plasminogen activator-mediated PDGF signaling and neurovascular coupling in stroke. J. Thromb. Haemost. 2009;7(Suppl. 1):155–158. doi: 10.1111/j.1538-7836.2009.03402.x. [http://dx.doi.org/10.1111/j.1538-7836.2009. 03402.x]. [PMID: 19630790]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fredriksson L., Li H., Fieber C., Li X., Eriksson U. Tissue plasminogen activator is a potent activator of PDGF-CC. EMBO J. 2004;23(19):3793–3802. doi: 10.1038/sj.emboj.7600397. [http://dx.doi.org/10.1038/sj.emboj.7600397]. [PMID: 15372073]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Su E.J., Fredriksson L., Geyer M., Folestad E., Cale J., Andrae J., Gao Y., Pietras K., Mann K., Yepes M., Strickland D.K., Betsholtz C., Eriksson U., Lawrence D.A. Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat. Med. 2008;14(7):731–737. doi: 10.1038/nm1787. [http://dx.doi.org/10.1038/nm1787]. [PMID: 18568034]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodríguez-González R., Blanco M., Rodríguez-Yáñez M., Moldes O., Castillo J., Sobrino T. Platelet derived growth factor-CC isoform is associated with hemorrhagic transformation in ischemic stroke patients treated with tissue plasminogen activator. Atherosclerosis. 2013;226(1):165–171. doi: 10.1016/j.atherosclerosis.2012.10.072. [http://dx.doi.org/10.1016/ j.atherosclerosis.2012.10.072]. [PMID: 23218119]. [DOI] [PubMed] [Google Scholar]
- 79.Wang X., Tsuji K., Lee S.R., Ning M., Furie K.L., Buchan A.M., Lo E.H. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004;35(11) Suppl. 1:2726–2730. doi: 10.1161/01.STR.0000143219.16695.af. [http://dx.doi.org/10.1161/01.STR.0000143219.16695.af]. [PMID: 15459442]. [DOI] [PubMed] [Google Scholar]
- 80.Ng K.S., Leung H.W., Wong P.T., Low C.M. Cleavage of the NR2B subunit amino terminus of N-methyl-D-aspartate (NMDA) receptor by tissue plasminogen activator: identification of the cleavage site and characterization of ifenprodil and glycine affinities on truncated NMDA receptor. J. Biol. Chem. 2012;287(30):25520–25529. doi: 10.1074/jbc.M112.374397. [http://dx.doi.org/10.1074/jbc.M112.374397]. [PMID: 22610100]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jullienne A., Montagne A., Orset C., Lesept F., Jane D.E., Monaghan D.T., Maubert E., Vivien D., Ali C. Selective inhibition of GluN2D-containing N-methyl-D-aspartate receptors prevents tissue plasminogen activator-promoted neurotoxicity both in vitro and in vivo. Mol. Neurodegener. 2011;6:68. doi: 10.1186/1750-1326-6-68. [http://dx. doi.org/10.1186/1750-1326-6-68]. [PMID: 21975018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Benchenane K., Castel H., Boulouard M., Bluthé R., Fernandez-Monreal M., Roussel B.D., Lopez-Atalaya J.P., Butt-Gueulle S., Agin V., Maubert E., Dantzer R., Touzani O., Dauphin F., Vivien D., Ali C. Anti-NR1 N-terminal-domain vaccination unmasks the crucial action of tPA on NMDA-receptor-mediated toxicity and spatial memory. J. Cell Sci. 2007;120(Pt 4):578–585. doi: 10.1242/jcs.03354. [http://dx.doi.org/10.1242/jcs.03354]. [PMID: 17244650]. [DOI] [PubMed] [Google Scholar]
- 83.Nicole O., Docagne F., Ali C., Margaill I., Carmeliet P., MacKenzie E.T., Vivien D., Buisson A. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat. Med. 2001;7(1):59–64. doi: 10.1038/83358. [http://dx.doi.org/10.1038/ 83358]. [PMID: 11135617]. [DOI] [PubMed] [Google Scholar]
- 84.Fernández-Monreal M., López-Atalaya J.P., Benchenane K., Cacquevel M., Dulin F., Le Caer J.P., Rossier J., Jarrige A.C., Mackenzie E.T., Colloch N., Ali C., Vivien D. Arginine 260 of the amino-terminal domain of NR1 subunit is critical for tissue-type plasminogen activator-mediated enhancement of N-methyl-D-aspartate receptor signaling. J. Biol. Chem. 2004;279(49):50850–50856. doi: 10.1074/jbc.M407069200. [http://dx.doi.org/10.1074/jbc.M407069200]. [PMID: 15448144]. [DOI] [PubMed] [Google Scholar]
- 85.Cheng T., Liu D., Griffin J.H., Fernández J.A., Castellino F., Rosen E.D., Fukudome K., Zlokovic B.V. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat. Med. 2003;9(3):338–342. doi: 10.1038/nm826. [http://dx.doi.org/10.1038/nm826]. [PMID: 12563316]. [DOI] [PubMed] [Google Scholar]
- 86.Shibata M., Kumar S.R., Amar A., Fernandez J.A., Hofman F., Griffin J.H., Zlokovic B.V. Anti-inflammatory, antithrombotic, and neuroprotective effects of activated protein C in a murine model of focal ischemic stroke. Circulation. 2001;103(13):1799–1805. doi: 10.1161/01.cir.103.13.1799. [http://dx.doi.org/10.1161/01.CIR.103.13.1799]. [PMID: 11282913]. [DOI] [PubMed] [Google Scholar]
- 87.Thiyagarajan M., Fernández J.A., Lane S.M., Griffin J.H., Zlokovic B.V. Activated protein C promotes neovascularization and neurogenesis in postischemic brain via protease-activated receptor 1. J. Neurosci. 2008;28(48):12788–12797. doi: 10.1523/JNEUROSCI.3485-08.2008. [http://dx.doi.org/10.1523/JNEUROSCI.3485-08.2008]. [PMID: 19036971]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zlokovic B.V., Zhang C., Liu D., Fernandez J., Griffin J.H., Chopp M. Functional recovery after embolic stroke in rodents by activated protein C. Ann. Neurol. 2005;58(3):474–477. doi: 10.1002/ana.20602. [http://dx. doi.org/10.1002/ana.20602]. [PMID: 16130108]. [DOI] [PubMed] [Google Scholar]
- 89.Liu D., Cheng T., Guo H., Fernández J.A., Griffin J.H., Song X., Zlokovic B.V. Tissue plasminogen activator neurovascular toxicity is controlled by activated protein C. Nat. Med. 2004;10(12):1379–1383. doi: 10.1038/nm1122. [http://dx.doi.org/10.1038/nm1122]. [PMID: 15516929]. [DOI] [PubMed] [Google Scholar]
- 90.Wang Y., Zhao Z., Chow N., Rajput P.S., Griffin J.H., Lyden P.D., Zlokovic B.V. Activated protein C analog protects from ischemic stroke and extends the therapeutic window of tissue- type plasminogen activator in aged female mice and hypertensive rats. Stroke. 2013;44(12):3529–3536. doi: 10.1161/STROKEAHA.113.003350. [http://dx.doi.org/10.1161/ STROKEAHA.113.003350]. [PMID: 24159062]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Y., Zhang Z., Chow N., Davis T.P., Griffin J.H., Chopp M., Zlokovic B.V. An activated protein C analog with reduced anticoagulant activity extends the therapeutic window of tissue plasminogen activator for ischemic stroke in rodents. Stroke. 2012;43(9):2444–2449. doi: 10.1161/STROKEAHA.112.658997. [http://dx.doi.org/10.1161/STROKEAHA.112. 658997]. [PMID: 22811462]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mendioroz M., Fernández-Cadenas I., Alvarez-Sabín J., Rosell A., Quiroga D., Cuadrado E., Delgado P., Rubiera M., Ribó M., Molina C., Montaner J. Endogenous activated protein C predicts hemorrhagic transformation and mortality after tissue plasminogen activator treatment in stroke patients. Cerebrovasc. Dis. 2009;28(2):143–150. doi: 10.1159/000225907. [http://dx.doi.org/10.1159/000225907]. [PMID: 19546541]. [DOI] [PubMed] [Google Scholar]
- 93.Chan P.H. Reactive oxygen radicals in signaling and damage in the ischemic brain. J. Cereb. Blood Flow Metab. 2001;21(1):2–14. doi: 10.1097/00004647-200101000-00002. [http://dx.doi.org/10.1097/00004647-200101000-00002]. [PMID: 11149664]. [DOI] [PubMed] [Google Scholar]
- 94.Chen X.M., Chen H.S., Xu M.J., Shen J.G. Targeting reactive nitrogen species: a promising therapeutic strategy for cerebral ischemia-reperfusion injury. Acta Pharmacol. Sin. 2013;34(1):67–77. doi: 10.1038/aps.2012.82. [http://dx.doi.org/10.1038/aps.2012.82]. [PMID: 22842734]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heo J.H., Han S.W., Lee S.K. Free radicals as triggers of brain edema formation after stroke. Free Radic. Biol. Med. 2005;39(1):51–70. doi: 10.1016/j.freeradbiomed.2005.03.035. [http://dx.doi.org/10.1016/j.freeradbiomed.2005.03.035]. [PMID: 15925278]. [DOI] [PubMed] [Google Scholar]
- 96.Ren Y., Wei B., Song X., An N., Zhou Y., Jin X., Zhang Y. Edaravones free radical scavenging mechanisms of neuroprotection against cerebral ischemia: review of the literature. Int. J. Neurosci. 2015;125(8):555–565. doi: 10.3109/00207454.2014.959121. [http://dx.doi.org/10.3109/00207454.2014. 959121]. [PMID: 25171224]. [DOI] [PubMed] [Google Scholar]
- 97.Lapchak P.A. A critical assessment of edaravone acute ischemic stroke efficacy trials: is edaravone an effective neuroprotective therapy? Expert Opin. Pharmacother. 2010;11(10):1753–1763. doi: 10.1517/14656566.2010.493558. [http://dx.doi.org/10.1517/14656566.2010.493558]. [PMID: 20491547]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Isahaya K., Yamada K., Yamatoku M., Sakurai K., Takaishi S., Kato B., Hirayama T., Hasegawa Y. Effects of edaravone, a free radical scavenger, on serum levels of inflammatory biomarkers in acute brain infarction. J. Stroke Cerebrovasc. Dis. 2012;21(2):102–107. doi: 10.1016/j.jstrokecerebrovasdis.2010.05.009. [http://dx.doi.org/10.1016/j.jstrokecerebrovasdis.2010.05. 009]. [PMID: 21215657]. [DOI] [PubMed] [Google Scholar]
- 99.Takenaka K., Kato M., Yamauti K., Hayashi K. Simultaneous administration of recombinant tissue plasminogen activator and edaravone in acute cerebral ischemic stroke patients. J. Stroke Cerebrovasc. Dis. 2014;23(10):2748–2752. doi: 10.1016/j.jstrokecerebrovasdis.2014.06.016. [http://dx.doi.org/10. 1016/j.jstrokecerebrovasdis.2014.06.016]. [PMID: 25307430]. [DOI] [PubMed] [Google Scholar]
- 100.Wada T., Yasunaga H., Inokuchi R., Horiguchi H., Fushimi K., Matsubara T., Nakajima S., Yahagi N. Effects of edaravone on early outcomes in acute ischemic stroke patients treated with recombinant tissue plasminogen activator. J. Neurol. Sci. 2014;345(1-2):106–111. doi: 10.1016/j.jns.2014.07.018. [http://dx.doi.org/10.1016/j.jns.2014.07.018]. [PMID: 25085762]. [DOI] [PubMed] [Google Scholar]
- 101.Miyaji Y., Yoshimura S., Sakai N., Yamagami H., Egashira Y., Shirakawa M., Uchida K., Kageyama H., Tomogane Y. Effect of edaravone on favorable outcome in patients with acute cerebral large vessel occlusion: subanalysis of RESCUE-Japan Registry. Neurol. Med. Chir. (Tokyo) 2015;55(3):241–247. doi: 10.2176/nmc.ra.2014-0219. [http://dx.doi.org/10.2176/nmc.ra.2014-0219]. [PMID: 25739433]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yamashita T., Sato T., Sakamoto K., Ishii H., Yamamoto J. The free-radical scavenger edaravone accelerates thrombolysis with alteplase in an experimental thrombosis model. Thromb. Res. 2015;135(6):1209–1213. doi: 10.1016/j.thromres.2015.04.011. [http://dx.doi.org/10.1016/j.thromres. 2015.04.011]. [PMID: 25908261]. [DOI] [PubMed] [Google Scholar]
- 103.Wu S., Sena E., Egan K., Macleod M., Mead G. Edaravone improves functional and structural outcomes in animal models of focal cerebral ischemia: a systematic review. Int. J. Stroke. 2014;9(1):101–106. doi: 10.1111/ijs.12163. [http://dx.doi.org/10.1111/ijs.12163]. [PMID: 24148907]. [DOI] [PubMed] [Google Scholar]
- 104.Mishina M., Komaba Y., Kobayashi S., Kominami S., Fukuchi T., Mizunari T., Teramoto A., Katayama Y. Administration of free radical scavenger edaravone associated with higher frequency of hemorrhagic transformation in patients with cardiogenic embolism. Neurol. Med. Chir. (Tokyo) 2008;48(7):292–297. doi: 10.2176/nmc.48.292. [http://dx.doi.org/10.2176/nmc.48.292]. [PMID: 18654047]. [DOI] [PubMed] [Google Scholar]
- 105.Furchgott R.F., Carvalho M.H., Khan M.T., Matsunaga K. Evidence for endothelium-dependent vasodilation of resistance vessels by acetylcholine. Blood Vessels. 1987;24(3):145–149. doi: 10.1159/000158689. [PMID: 3036283]. [DOI] [PubMed] [Google Scholar]
- 106.Ignarro L.J., Buga G.M., Wood K.S., Byrns R.E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. USA. 1987;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [http://dx.doi.org/10.1073/pnas.84.24.9265]. [PMID: 2827174]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Knowles R.G., Palacios M., Palmer R.M., Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc. Natl. Acad. Sci. USA. 1989;86(13):5159–5162. doi: 10.1073/pnas.86.13.5159. [http://dx.doi.org/10.1073/pnas.86.13.5159]. [PMID: 2567995]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cho H.J., Xie Q.W., Calaycay J., Mumford R.A., Swiderek K.M., Lee T.D., Nathan C. Calmodulin is a subunit of nitric oxide synthase from macrophages. J. Exp. Med. 1992;176(2):599–604. doi: 10.1084/jem.176.2.599. [http://dx.doi.org/10.1084/jem.176.2.599]. [PMID: 1380065]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Malinski T., Bailey F., Zhang Z.G., Chopp M. Nitric oxide measured by a porphyrinic microsensor in rat brain after transient middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 1993;13(3):355–358. doi: 10.1038/jcbfm.1993.48. [http://dx.doi.org/10.1038/jcbfm.1993.48]. [PMID: 8478395]. [DOI] [PubMed] [Google Scholar]
- 110.Moro M.A., Almeida A., Bolaños J.P., Lizasoain I. Mitochondrial respiratory chain and free radical generation in stroke. Free Radic. Biol. Med. 2005;39(10):1291–1304. doi: 10.1016/j.freeradbiomed.2005.07.010. [http://dx. doi.org/10.1016/j.freeradbiomed.2005.07.010]. [PMID: 16257638]. [DOI] [PubMed] [Google Scholar]
- 111.Bolaños J.P., Almeida A. Roles of nitric oxide in brain hypoxia-ischemia. Biochim. Biophys. Acta. 1999;1411(2-3):415–436. doi: 10.1016/s0005-2728(99)00030-4. [http://dx.doi.org/10.1016/S0005-2728(99)00030-4]. [PMID: 10320673]. [DOI] [PubMed] [Google Scholar]
- 112.Liu K., Li Q., Zhang L., Zheng X. The dynamic detection of NO during stroke and reperfusion in vivo. Brain Inj. 2009;23(5):450–458. doi: 10.1080/02699050902838173. [http://dx.doi.org/10.1080/02699050902838173]. [PMID: 19408167]. [DOI] [PubMed] [Google Scholar]
- 113.Iadecola C., Zhang F., Casey R., Clark H.B., Ross M.E. Inducible nitric oxide synthase gene expression in vascular cells after transient focal cerebral ischemia. Stroke. 1996;27(8):1373–1380. doi: 10.1161/01.str.27.8.1373. [http://dx.doi.org/10.1161/01.STR.27.8.1373]. [PMID: 8711805]. [DOI] [PubMed] [Google Scholar]
- 114.Nagafuji T., Sugiyama M., Matsui T. Temporal profiles of Ca2+/calmodulin-dependent and -independent nitric oxide synthase activity in the rat brain microvessels following cerebral ischemia. Acta Neurochir. Suppl. (Wien) 1994;60:285–288. doi: 10.1007/978-3-7091-9334-1_76. [PMID: 7526623]. [DOI] [PubMed] [Google Scholar]
- 115.MacMicking J., Xie Q.W., Nathan C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [http://dx.doi.org/10.1146/annurev.immunol.15.1.323]. [PMID: 9143691]. [DOI] [PubMed] [Google Scholar]
- 116.Liu H., Li J., Zhao F., Wang H., Qu Y., Mu D. Nitric oxide synthase in hypoxic or ischemic brain injury. Rev. Neurosci. 2015;26(1):105–117. doi: 10.1515/revneuro-2014-0041. [http://dx.doi.org/10.1515/revneuro-2014-0041]. [PMID: 25720056]. [DOI] [PubMed] [Google Scholar]
- 117.Heeba G.H., El-Hanafy A.A. Nebivolol regulates eNOS and iNOS expressions and alleviates oxidative stress in cerebral ischemia/ reperfusion injury in rats. Life Sci. 2012;90(11-12):388–395. doi: 10.1016/j.lfs.2011.12.001. [http://dx.doi.org/10.1016/j.lfs.2011.12.001]. [PMID: 22226906]. [DOI] [PubMed] [Google Scholar]
- 118.Pérez-Asensio F.J., Hurtado O., Burguete M.C., Moro M.A., Salom J.B., Lizasoain I., Torregrosa G., Leza J.C., Alborch E., Castillo J., Knowles R.G., Lorenzo P. Inhibition of iNOS activity by 1400W decreases glutamate release and ameliorates stroke outcome after experimental ischemia. Neurobiol. Dis. 2005;18(2):375–384. doi: 10.1016/j.nbd.2004.10.018. [http://dx.doi.org/10.1016/j.nbd.2004.10.018]. [PMID: 15686966]. [DOI] [PubMed] [Google Scholar]
- 119.Parmentier S., Böhme G.A., Lerouet D., Damour D., Stutzmann J.M., Margaill I., Plotkine M. Selective inhibition of inducible nitric oxide synthase prevents ischaemic brain injury. Br. J. Pharmacol. 1999;127(2):546–552. doi: 10.1038/sj.bjp.0702549. [http://dx.doi.org/10.1038/ sj.bjp.0702549]. [PMID: 10385257]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhao X., Ross M.E., Iadecola C. L-Arginine increases ischemic injury in wild-type mice but not in iNOS-deficient mice. Brain Res. 2003;966(2):308–311. doi: 10.1016/s0006-8993(02)04223-3. [http://dx.doi.org/10.1016/S0006-8993(02)04223-3]. [PMID: 12618354]. [DOI] [PubMed] [Google Scholar]
- 121.Khan M., Dhammu T.S., Matsuda F., Singh A.K., Singh I. Blocking a vicious cycle nNOS/peroxynitrite/AMPK by S-nitrosoglutathione: implication for stroke therapy. BMC Neurosci. 2015;16:42. doi: 10.1186/s12868-015-0179-x. [http://dx.doi.org/10.1186/s12868-015-0179-x]. [PMID: 26174015]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jiang Z., Li C., Arrick D.M., Yang S., Baluna A.E., Sun H. Role of nitric oxide synthases in early blood-brain barrier disruption following transient focal cerebral ischemia. PLoS One. 2014;9(3):e93134. doi: 10.1371/journal.pone.0093134. [http://dx.doi.org/10.1371/journal.pone.0093134]. [PMID: 24671193]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huang P.L. Mouse models of nitric oxide synthase deficiency. J. Am. Soc. Nephrol. 2000;11(Suppl. 16):S120–S123. [PMID: 11065342]. [PubMed] [Google Scholar]
- 124.Matsushita K., Morrell C.N., Cambien B., Yang S-X., Yamakuchi M., Bao C., Hara M.R., Quick R.A., Cao W. ORourke, B.; Lowenstein, J.M.; Pevsner, J.; Wagner, D.D.; Lowenstein, C.J. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell. 2003;115(2):139–150. doi: 10.1016/s0092-8674(03)00803-1. [http://dx.doi.org/10.1016/S0092-8674(03)00803-1]. [PMID: 14567912]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cohen R.A., Adachi T. Nitric-oxide-induced vasodilatation: regulation by physiologic s-glutathiolation and pathologic oxidation of the sarcoplasmic endoplasmic reticulum calcium ATPase. Trends Cardiovasc. Med. 2006;16(4):109–114. doi: 10.1016/j.tcm.2006.02.001. [http://dx.doi.org/10.1016/ j.tcm.2006.02.001]. [PMID: 16713532]. [DOI] [PubMed] [Google Scholar]
- 126.Stamler J.S. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell. 1994;78(6):931–936. doi: 10.1016/0092-8674(94)90269-0. [http://dx. doi.org/10.1016/0092-8674(94)90269-0]. [PMID: 7923362]. [DOI] [PubMed] [Google Scholar]
- 127.Lo E.H., Hara H., Rogowska J., Trocha M., Pierce A.R., Huang P.L., Fishman M.C., Wolf G.L., Moskowitz M.A. Temporal correlation mapping analysis of the hemodynamic penumbra in mutant mice deficient in endothelial nitric oxide synthase gene expression. Stroke. 1996;27(8):1381–1385. doi: 10.1161/01.str.27.8.1381. [http://dx.doi.org/10. 1161/01.STR.27.8.1381]. [PMID: 8711806]. [DOI] [PubMed] [Google Scholar]
- 128.Suzuki M., Tabuchi M., Ikeda M., Tomita T. Concurrent formation of peroxynitrite with the expression of inducible nitric oxide synthase in the brain during middle cerebral artery occlusion and reperfusion in rats. Brain Res. 2002;951(1):113–120. doi: 10.1016/s0006-8993(02)03145-1. [http://dx.doi.org/10.1016/S0006-8993(02)03145-1]. [PMID: 12231464]. [DOI] [PubMed] [Google Scholar]
- 129.Suzuki M., Tabuchi M., Ikeda M., Tomita T. Concurrent formation of peroxynitrite with the expression of inducible nitric oxide synthase in the brain during middle cerebral artery occlusion and reperfusion in rats. Brain Res. 2002;951(1):113–120. doi: 10.1016/s0006-8993(02)03145-1. [http://dx.doi.org/10.1016/S0006-8993(02)03145-1]. [PMID: 12231464]. [DOI] [PubMed] [Google Scholar]
- 130.Takizawa S., Fukuyama N., Hirabayashi H., Nakazawa H., Shinohara Y. Dynamics of nitrotyrosine formation and decay in rat brain during focal ischemia-reperfusion. J. Cereb. Blood Flow Metab. 1999;19(6):667–672. doi: 10.1097/00004647-199906000-00010. [http://dx.doi.org/10.1097/ 00004647-199906000-00010]. [PMID: 10366197]. [DOI] [PubMed] [Google Scholar]
- 131.Crow J.P., Beckman J.S. The role of peroxynitrite in nitric oxide-mediated toxicity. Curr. Top. Microbiol. Immunol. 1995;196:57–73. doi: 10.1007/978-3-642-79130-7_7. [http://dx.doi.org/10.1007/978-3-642-79130-7_7]. [PMID: 7634825]. [DOI] [PubMed] [Google Scholar]
- 132.Groves J.T. Peroxynitrite: reactive, invasive and enigmatic. Curr. Opin. Chem. Biol. 1999;3(2):226–235. doi: 10.1016/S1367-5931(99)80036-2. [http://dx.doi.org/10.1016/ S1367-5931(99)80036-2]. [PMID: 10226050]. [DOI] [PubMed] [Google Scholar]
- 133.Lizasoain I., Moro M.A., Knowles R.G., Darley-Usmar V., Moncada S. Nitric oxide and peroxynitrite exert distinct effects on mitochondrial respiration which are differentially blocked by glutathione or glucose. Biochem. J. 1996;314(Pt 3):877–880. doi: 10.1042/bj3140877. [http://dx.doi.org/10.1042/bj3140877]. [PMID: 8615783]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vieira H.L., Belzacq A.S., Haouzi D., Bernassola F., Cohen I., Jacotot E., Ferri K.F., El Hamel C., Bartle L.M., Melino G., Brenner C., Goldmacher V., Kroemer G. The adenine nucleotide translocator: a target of nitric oxide, peroxynitrite, and 4-hydroxynonenal. Oncogene. 2001;20(32):4305–4316. doi: 10.1038/sj.onc.1204575. [http://dx. doi.org/10.1038/sj.onc.1204575]. [PMID: 11466611]. [DOI] [PubMed] [Google Scholar]
- 135.Salgo M.G., Squadrito G.L., Pryor W.A. Peroxynitrite causes apoptosis in rat thymocytes. Biochem. Biophys. Res. Commun. 1995;215(3):1111–1118. doi: 10.1006/bbrc.1995.2578. [http://dx.doi.org/10.1006/bbrc.1995.2578]. [PMID: 7488038]. [DOI] [PubMed] [Google Scholar]
- 136.Tajes M., Ill-Raga G., Palomer E., Ramos-Fernandez E., Guix F.X., Bosch-Morato M., Guivernau B., Jimenez-Conde J., Ois A., Perez-Asensio F., Reyes-Navarro M., Caballo C., Galan A.M., Alameda F., Escolar G., Opazo C., Planas A., Roquer J., Valverde M.A., Munoz F.J. Nitro-oxidative stress after neuronal ischemia induces protein nitrotyrosination and cell death. 2013. [DOI] [PMC free article] [PubMed]
- 137.Radi R., Beckman J.S., Bush K.M., Freeman B.A. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch. Biochem. Biophys. 1991;288(2):481–487. doi: 10.1016/0003-9861(91)90224-7. [http://dx.doi.org/10.1016/0003-9861(91)90224-7]. [PMID: 1654835]. [DOI] [PubMed] [Google Scholar]
- 138.Virág L., Szabó E., Gergely P., Szabó C. Peroxynitrite-induced cytotoxicity: mechanism and opportunities for intervention. Toxicol. Lett. 2003;140-141:113–124. doi: 10.1016/s0378-4274(02)00508-8. [http://dx.doi.org/10.1016/ S0378-4274(02)00508-8]. [PMID: 12676457]. [DOI] [PubMed] [Google Scholar]
- 139.Brzezinska A.K., Gebremedhin D., Chilian W.M., Kalyanaraman B., Elliott S.J. Peroxynitrite reversibly inhibits Ca(2+)-activated K(+) channels in rat cerebral artery smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2000;278(6):H1883–H1890. doi: 10.1152/ajpheart.2000.278.6.H1883. [PMID: 10843885]. [DOI] [PubMed] [Google Scholar]
- 140.Zanelli S.A., Ashraf Q.M., Delivoria-Papadopoulos M., Mishra O.P. Peroxynitrite-induced modification of the N-methyl-D-aspartate receptor in the cerebral cortex of the guinea pig fetus at term. Neurosci. Lett. 2000;296(1):5–8. doi: 10.1016/s0304-3940(00)01608-6. [http://dx.doi.org/10.1016/ S0304-3940(00)01608-6]. [PMID: 11099820]. [DOI] [PubMed] [Google Scholar]
- 141.Khan M., Dhammu T.S., Sakakima H., Shunmugavel A., Gilg A.G., Singh A.K., Singh I. The inhibitory effect of S-nitrosoglutathione on blood-brain barrier disruption and peroxynitrite formation in a rat model of experimental stroke. J. Neurochem. 2012;123(Suppl. 2):86–97. doi: 10.1111/j.1471-4159.2012.07947.x. [http://dx.doi.org/10.1111/j.1471-4159.2012.07947.x]. [PMID: 23050646]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dhar A., Kaundal R.K., Sharma S.S. Neuroprotective effects of FeTMPyP: a peroxynitrite decomposition catalyst in global cerebral ischemia model in gerbils. Pharmacol. Res. 2006;54(4):311–316. doi: 10.1016/j.phrs.2006.06.009. [http://dx.doi.org/10.1016/j.phrs.2006.06.009]. [PMID: 16877004]. [DOI] [PubMed] [Google Scholar]
- 143.Thiyagarajan M., Kaul C.L., Sharma S.S. Neuroprotective efficacy and therapeutic time window of peroxynitrite decomposition catalysts in focal cerebral ischemia in rats. Br. J. Pharmacol. 2004;142(5):899–911. doi: 10.1038/sj.bjp.0705811. [http://dx.doi.org/10.1038/ sj.bjp.0705811]. [PMID: 15197101]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li R., Huang C., Chen J., Guo Y., Tan S. The role of uric acid as a potential neuroprotectant in acute ischemic stroke: a review of literature. Neurol. Sci. 2015;36(7):1097–1103. doi: 10.1007/s10072-015-2151-z. [http://dx.doi.org/10.1007/s10072-015-2151-z]. [PMID: 25772077]. [DOI] [PubMed] [Google Scholar]
- 145.Yu Z.F., Bruce-Keller A.J., Goodman Y., Mattson M.P. Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J. Neurosci. Res. 1998;53(5):613–625. doi: 10.1002/(SICI)1097-4547(19980901)53:5<613::AID-JNR11>3.0.CO;2-1. [http://dx.doi.org/10.1002/ (SICI)1097-4547(19980901)53:5<613:AID-JNR11>3.0.CO;2-1]. [PMID: 9726432]. [DOI] [PubMed] [Google Scholar]
- 146.Romanos E., Planas A.M., Amaro S., Chamorro A. Uric acid reduces brain damage and improves the benefits of rt-PA in a rat model of thromboembolic stroke. J. Cereb. Blood Flow Metab. 2007;27(1):14–20. doi: 10.1038/sj.jcbfm.9600312. [http://dx.doi.org/10.1038/sj.jcbfm.9600312]. [PMID: 16596120]. [DOI] [PubMed] [Google Scholar]
- 147.Wang Z., Lin Y., Liu Y., Chen Y., Wang B., Li C., Yan S., Wang Y., Zhao W. Serum Uric Acid Levels and Outcomes After Acute Ischemic Stroke. Mol. Neurobiol. 2015 doi: 10.1007/s12035-015-9134-1. [PMID: 25744569]. [DOI] [PubMed] [Google Scholar]
- 148.Xu M., Chen X., Gu Y., Peng T., Yang D., Chang R.C., So K.F., Liu K., Shen J. Baicalin can scavenge peroxynitrite and ameliorate endogenous peroxynitrite-mediated neurotoxicity in cerebral ischemia-reperfusion injury. J. Ethnopharmacol. 2013;150(1):116–124. doi: 10.1016/j.jep.2013.08.020. [http://dx.doi.org/10.1016/j.jep.2013.08.020]. [PMID: 23973788]. [DOI] [PubMed] [Google Scholar]
- 149.Tu X.K., Yang W.Z., Shi S.S., Chen Y., Wang C.H., Chen C.M., Chen Z. Baicalin inhibits TLR2/4 signaling pathway in rat brain following permanent cerebral ischemia. Inflammation. 2011;34(5):463–470. doi: 10.1007/s10753-010-9254-8. [http://dx.doi.org/10.1007/s10753-010-9254-8]. [PMID: 20859668]. [DOI] [PubMed] [Google Scholar]
- 150.Xue X., Qu X.J., Yang Y., Sheng X.H., Cheng F., Jiang E.N., Wang J.H., Bu W., Liu Z.P. Baicalin attenuates focal cerebral ischemic reperfusion injury through inhibition of nuclear factor κB p65 activation. Biochem. Biophys. Res. Commun. 2010;403(3-4):398–404. doi: 10.1016/j.bbrc.2010.11.042. [http://dx.doi.org/10.1016/j.bbrc.2010.11.042]. [PMID: 21093411]. [DOI] [PubMed] [Google Scholar]
- 151.Ge Q.F., Hu X., Ma Z.Q., Liu J.R., Zhang W.P., Chen Z., Wei E.Q. Baicalin attenuates oxygen-glucose deprivation-induced injury via inhibiting NMDA receptor-mediated 5-lipoxygenase activation in rat cortical neurons. Pharmacol. Res. 2007;55(2):148–157. doi: 10.1016/j.phrs.2006.11.007. [http://dx.doi.org/10.1016/j.phrs.2006.11.007]. [PMID: 17187986]. [DOI] [PubMed] [Google Scholar]
- 152.Tu X.K., Yang W.Z., Liang R.S., Shi S.S., Chen J.P., Chen C.M., Wang C.H., Xie H.S., Chen Y., Ouyang L.Q. Effect of baicalin on matrix metalloproteinase-9 expression and blood-brain barrier permeability following focal cerebral ischemia in rats. Neurochem. Res. 2011;36(11):2022–2028. doi: 10.1007/s11064-011-0526-y. [http://dx.doi.org/10.1007/s11064-011-0526-y]. [PMID: 21678122]. [DOI] [PubMed] [Google Scholar]
- 153.Zhou Q.B., Jin Y.L., Jia Q., Zhang Y., Li L.Y., Liu P., Liu Y.T. Baicalin attenuates brain edema in a rat model of intracerebral hemorrhage. Inflammation. 2014;37(1):107–115. doi: 10.1007/s10753-013-9717-9. [http://dx.doi.org/10.1007/s10753-013-9717-9]. [PMID: 23974988]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Huang H., Zhang Y., Yang R., Tang X. Determination of baicalin in rat cerebrospinal fluid and blood using microdialysis coupled with ultra-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008;874(1-2):77–83. doi: 10.1016/j.jchromb.2008.09.005. [http://dx.doi.org/10.1016/j.jchromb. 2008.09.005]. [PMID: 18805743]. [DOI] [PubMed] [Google Scholar]
- 155.Chen Y., Wu X., Yu S., Lin X., Wu J., Li L., Zhao J., Zhao Y. Neuroprotection of tanshinone IIA against cerebral ischemia/ reperfusion injury through inhibition of macrophage migration inhibitory factor in rats. PLoS One. 2012;7(6):e40165. doi: 10.1371/journal.pone.0040165. [http://dx. doi.org/10.1371/journal.pone.0040165]. [PMID: 22768247]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dong K., Xu W., Yang J., Qiao H., Wu L. Neuroprotective effects of Tanshinone IIA on permanent focal cerebral ischemia in mice. Phytother. Res. 2009;23(5):608–613. doi: 10.1002/ptr.2615. [http://dx.doi.org/10.1002/ptr.2615]. [PMID: 18844253]. [DOI] [PubMed] [Google Scholar]
- 157.Wang J.G., Bondy S.C., Zhou L., Yang F.Z., Ding Z.G., Hu Y., Tian Y., Wen P.Y., Luo H., Wang F., Li W.W., Zhou J. Protective effect of Tanshinone IIA against infarct size and increased HMGB1, NFκB, GFAP and apoptosis consequent to transient middle cerebral artery occlusion. Neurochem. Res. 2014;39(2):295–304. doi: 10.1007/s11064-013-1221-y. [http://dx.doi.org/10.1007/s11064-013-1221-y]. [PMID: 24362639]. [DOI] [PubMed] [Google Scholar]
- 158.Tang C., Xue H., Bai C., Fu R., Wu A. The effects of Tanshinone IIA on blood-brain barrier and brain edema after transient middle cerebral artery occlusion in rats. Phytomedicine. 2010;17(14):1145–1149. doi: 10.1016/j.phymed.2010.03.017. [http://dx.doi.org/10.1016/j.phymed. 2010.03.017]. [PMID: 20570121]. [DOI] [PubMed] [Google Scholar]
- 159.Wang L., Zhang X., Liu L., Cui L., Yang R., Li M., Du W. Tanshinone II A down-regulates HMGB1, RAGE, TLR4, NF-kappaB expression, ameliorates BBB permeability and endothelial cell function, and protects rat brains against focal ischemia. Brain Res. 2010;1321:143–151. doi: 10.1016/j.brainres.2009.12.046. [http://dx.doi.org/10.1016/j.brainres. 2009.12.046]. [PMID: 20043889]. [DOI] [PubMed] [Google Scholar]
- 160.Chen Y., Wu X., Yu S., Fauzee N.J., Wu J., Li L., Zhao J., Zhao Y. Neuroprotective capabilities of Tanshinone IIA against cerebral ischemia/reperfusion injury via anti-apoptotic pathway in rats. Biol. Pharm. Bull. 2012;35(2):164–170. doi: 10.1248/bpb.35.164. [http://dx.doi.org/10.1248/bpb.35.164]. [PMID: 22293345]. [DOI] [PubMed] [Google Scholar]
- 161.Liu L., Zhang X., Wang L., Yang R., Cui L., Li M., Du W., Wang S. The neuroprotective effects of Tanshinone IIA are associated with induced nuclear translocation of TORC1 and upregulated expression of TORC1, pCREB and BDNF in the acute stage of ischemic stroke. Brain Res. Bull. 2010;82(3-4):228–233. doi: 10.1016/j.brainresbull.2010.04.005. [http://dx.doi.org/10.1016/j.brainresbull.2010.04.005]. [PMID: 20417695]. [DOI] [PubMed] [Google Scholar]
- 162.Li J., Wu H.M., Zhou R.L., Liu G.J., Dong B.R. Huperzine A for Alzheimers disease. Cochrane Database Syst. Rev. 2008;(2):CD005592. doi: 10.1002/14651858.CD005592.pub2. [PMID: 18425924]. [DOI] [PubMed] [Google Scholar]
- 163.Wang R., Yan H., Tang X.C. Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine. Acta Pharmacol. Sin. 2006;27(1):1–26. doi: 10.1111/j.1745-7254.2006.00255.x. [http://dx.doi.org/10. 1111/j.1745-7254.2006.00255.x]. [PMID: 16364207]. [DOI] [PubMed] [Google Scholar]
- 164.Xing S.H., Zhu C.X., Zhang R., An L. Huperzine a in the treatment of Alzheimer's disease and vascular dementia: a meta-analysis. 2014. [DOI] [PMC free article] [PubMed]
- 165.Zheng C.Y., Zhang H.Y., Tang X.C. Huperzine A attenuates mitochondrial dysfunction after middle cerebral artery occlusion in rats. J. Neurosci. Res. 2008;86(11):2432–2440. doi: 10.1002/jnr.21681. [http://dx.doi.org/10.1002/jnr.21681]. [PMID: 18438924]. [DOI] [PubMed] [Google Scholar]
- 166.Wang Z.F., Wang J., Zhang H.Y., Tang X.C. Huperzine A exhibits anti-inflammatory and neuroprotective effects in a rat model of transient focal cerebral ischemia. J. Neurochem. 2008;106(4):1594–1603. doi: 10.1111/j.1471-4159.2008.05504.x. [http://dx.doi.org/10.1111/j.1471-4159.2008. 05504.x]. [PMID: 18513368]. [DOI] [PubMed] [Google Scholar]
- 167.Wang Z.F., Tang X.C. Huperzine A protects C6 rat glioma cells against oxygen-glucose deprivation-induced injury. FEBS Lett. 2007;581(4):596–602. doi: 10.1016/j.febslet.2007.01.016. [http://dx.doi.org/10.1016/j.febslet.2007.01. 016]. [PMID: 17257593]. [DOI] [PubMed] [Google Scholar]
- 168.Wang Z.F., Tang L.L., Yan H., Wang Y.J., Tang X.C. Effects of huperzine A on memory deficits and neurotrophic factors production after transient cerebral ischemia and reperfusion in mice. Pharmacol. Biochem. Behav. 2006;83(4):603–611. doi: 10.1016/j.pbb.2006.03.027. [http://dx.doi.org/10.1016/j.pbb.2006.03.027]. [PMID: 16687166]. [DOI] [PubMed] [Google Scholar]
- 169.Gordon R.K., Nigam S.V., Weitz J.A., Dave J.R., Doctor B.P., Ved H.S. The NMDA receptor ion channel: a site for binding of Huperzine A. J. Appl. Toxicol. 2001;21(Suppl. 1):S47–S51. doi: 10.1002/jat.805. [http://dx.doi.org/10.1002/jat.805]. [PMID: 11920920]. [DOI] [PubMed] [Google Scholar]
- 170.Woodbury A., Yu S.P., Wei L., García P. Neuro-modulating effects of honokiol: a review. Front. Neurol. 2013;4:130. doi: 10.3389/fneur.2013.00130. [http://dx.doi.org/10.3389/fneur.2013.00130]. [PMID: 24062717]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang P., Liu X., Zhu Y., Chen S., Zhou D., Wang Y. Honokiol inhibits the inflammatory reaction during cerebral ischemia reperfusion by suppressing NF-κB activation and cytokine production of glial cells. Neurosci. Lett. 2013;534:123–127. doi: 10.1016/j.neulet.2012.11.052. [http://dx.doi.org/10.1016/j.neulet.2012.11.052]. [PMID: 23262090]. [DOI] [PubMed] [Google Scholar]
- 172.Chen C.M., Liu S.H., Lin-Shiau S.Y. Honokiol, a neuroprotectant against mouse cerebral ischaemia, mediated by preserving Na+, K+-ATPase activity and mitochondrial functions. Basic Clin. Pharmacol. Toxicol. 2007;101(2):108–116. doi: 10.1111/j.1742-7843.2007.00082.x. [http://dx.doi.org/10.1111/j.1742-7843.2007.00082.x]. [PMID: 17651312]. [DOI] [PubMed] [Google Scholar]
- 173.Liou K.T., Shen Y.C., Chen C.F., Tsao C.M., Tsai S.K. Honokiol protects rat brain from focal cerebral ischemia-reperfusion injury by inhibiting neutrophil infiltration and reactive oxygen species production. Brain Res. 2003;992(2):159–166. doi: 10.1016/j.brainres.2003.08.026. [http://dx.doi.org/10.1016/j.brainres.2003.08.026]. [PMID: 14625055]. [DOI] [PubMed] [Google Scholar]
- 174.Hu Z., Bian X., Liu X., Zhu Y., Zhang X., Chen S., Wang K., Wang Y. Honokiol protects brain against ischemia-reperfusion injury in rats through disrupting PSD95-nNOS interaction. Brain Res. 2013;1491:204–212. doi: 10.1016/j.brainres.2012.11.004. [http://dx.doi.org/10.1016/j.brainres. 2012.11.004]. [PMID: 23148950]. [DOI] [PubMed] [Google Scholar]
- 175.Cui H.S., Huang L.S., Sok D.E., Shin J., Kwon B.M., Youn U.J., Bae K. Protective action of honokiol, administered orally, against oxidative stress in brain of mice challenged with NMDA. Phytomedicine. 2007;14(10):696–700. doi: 10.1016/j.phymed.2007.03.005. [http://dx.doi.org/10.1016/ j.phymed.2007.03.005]. [PMID: 17470388]. [DOI] [PubMed] [Google Scholar]
- 176.Wang X., Duan X., Yang G., Zhang X., Deng L., Zheng H., Deng C., Wen J., Wang N., Peng C., Zhao X., Wei Y., Chen L. Honokiol crosses BBB and BCSFB, and inhibits brain tumor growth in rat 9L intracerebral gliosarcoma model and human U251 xenograft glioma model. PLoS One. 2011;6(4):e18490. doi: 10.1371/journal.pone.0018490. [http://dx.doi.org/10.1371/journal.pone.0018490]. [PMID: 21559510]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lin J-W., Chen J-T., Hong C-Y., Lin Y-L., Wang K-T., Yao C-J., Lai G-M., Chen R-M. Honokiol traverses the blood-brain barrier and induces apoptosis of neuroblastoma cells via an intrinsic bax-mitochondrion-cytochrome c-caspase protease pathway. Neuro-oncol. 2012;14(3):302–314. doi: 10.1093/neuonc/nor217. [http://dx.doi.org/10.1093/neuonc/ nor217]. [PMID: 22259050]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hu H., Zhang X.X., Wang Y.Y., Chen S.Z. Honokiol inhibits arterial thrombosis through endothelial cell protection and stimulation of prostacyclin. Acta Pharmacol. Sin. 2005;26(9):1063–1068. doi: 10.1111/j.1745-7254.2005.00164.x. [http://dx.doi.org/10.1111/j.1745-7254.2005.00164.x]. [PMID: 16115372]. [DOI] [PubMed] [Google Scholar]
- 179.Wu M., Liang S., Ma L., Han Y., Zhang X., Xu C. Effects of delayed puerarin treatment in long-term neurological outcomes of focal ischemic stroke in rats. Indian J. Pharmacol. 2014;46(2):157–160. doi: 10.4103/0253-7613.129305. [http://dx.doi.org/10.4103/0253-7613.129305]. [PMID: 24741185]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang N., Zhang Y., Wu L., Wang Y., Cao Y., He L., Li X., Zhao J. Puerarin protected the brain from cerebral ischemia injury via astrocyte apoptosis inhibition. Neuropharmacology. 2014;79:282–289. doi: 10.1016/j.neuropharm.2013.12.004. [http://dx.doi.org/10.1016/j.neuropharm.2013.12.004]. [PMID: 24333675]. [DOI] [PubMed] [Google Scholar]
- 181.Xu X., Zhang S., Zhang L., Yan W., Zheng X. The Neuro- protection of puerarin against cerebral ischemia is associated with the prevention of apoptosis in rats. Planta Med. 2005;71(7):585–591. doi: 10.1055/s-2005-871261. [http://dx.doi.org/10.1055/s-2005-871261]. [PMID: 16041641]. [DOI] [PubMed] [Google Scholar]
- 182.Zhang R., Guo H.N., Wu H.Q., Cheng H.X., Wang H.Q. Sichuan Da Xue Xue Bao Yi Xue Ban. 2011;42(1):52–55. [Effect of puerarin on the expression of NMDA receptor in the hippocampus CA1 region after focal cerebral ischemia in rats]. [Effect of puerarin on the expression of NMDA receptor in the hippocampus CA1 region after focal cerebral ischemia in rats]. [PMID: 21355301]. [PubMed] [Google Scholar]
- 183.Chang Y., Hsieh C.Y., Peng Z.A., Yen T.L., Hsiao G., Chou D.S., Chen C.M., Sheu J.R. Neuroprotective mechanisms of puerarin in middle cerebral artery occlusion-induced brain infarction in rats. J. Biomed. Sci. 2009;16:9. doi: 10.1186/1423-0127-16-9. [http://dx.doi.org/10.1186/ 1423-0127-16-9]. [PMID: 19272172]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ding M.P., Feng F., Hu H.T. Zhongguo Zhong Yao Za Zhi. 2007;32(23):2515–2518. [Effects of puerarin on expression of nuclear factor kappaB after cerebral ischemia/reperfusion in rats]. [Effects of puerarin on expression of nuclear factor kappaB after cerebral ischemia/reperfusion in rats]. [PMID: 18330247]. [PubMed] [Google Scholar]
- 185.Tian Z., Yu W., Liu H.B., Zhang N., Li X.B., Zhao M.G., Liu S.B. Neuroprotective effects of curculigoside against NMDA-induced neuronal excitoxicity in vitro. Food Chem. Toxicol. 2012;50(11):4010–4015. doi: 10.1016/j.fct.2012.08.006. [http://dx.doi.org/10.1016/j.fct.2012.08.006]. [PMID: 22902827]. [DOI] [PubMed] [Google Scholar]
- 186.Jiang W., Fu F., Tian J., Zhu H., Hou J. Curculigoside A attenuates experimental cerebral ischemia injury in vitro and vivo. Neuroscience. 2011;192:572–579. doi: 10.1016/j.neuroscience.2011.06.079. [http://dx.doi.org/10.1016/j. neuroscience.2011.06.079]. [PMID: 21756977]. [DOI] [PubMed] [Google Scholar]
- 187.Yuan T.T., Xu H.T., Zhao L., Lv L., He Y.J., Zhang N.D., Qin L.P., Han T., Zhang Q.Y. Pharmacokinetic and tissue distribution profile of curculigoside after oral and intravenously injection administration in rats by liquid chromatography-mass spectrometry. Fitoterapia. 2015;101:64–72. doi: 10.1016/j.fitote.2014.12.012. [http://dx.doi.org/10.1016/j.fitote. 2014.12.012]. [PMID: 25549926]. [DOI] [PubMed] [Google Scholar]
- 188.Wang Z., Liu T., Gan L., Wang T., Yuan X., Zhang B., Chen H., Zheng Q. Shikonin protects mouse brain against cerebral ischemia/reperfusion injury through its antioxidant activity. Eur. J. Pharmacol. 2010;643(2-3):211–217. doi: 10.1016/j.ejphar.2010.06.027. [http://dx.doi.org/10.1016/ j.ejphar.2010.06.027]. [PMID: 20599918]. [DOI] [PubMed] [Google Scholar]
- 189.Wang L., Li Z., Zhang X., Wang S., Zhu C., Miao J., Chen L., Cui L., Qiao H. Protective effect of shikonin in experimental ischemic stroke: attenuated TLR4, p-p38MAPK, NF-κB, TNF-α and MMP-9 expression, up-regulated claudin-5 expression, ameliorated BBB permeability. Neurochem. Res. 2014;39(1):97–106. doi: 10.1007/s11064-013-1194-x. [http://dx.doi.org/10.1007/s11064-013-1194-x]. [PMID: 24248858]. [DOI] [PubMed] [Google Scholar]
- 190.Prasad R.G., Choi Y.H., Kim G.Y. Shikonin isolated from Lithospermum erythrorhizon downregulates proinflammatory mediators in lipopolysaccharide-stimulated BV2 microglial cells by suppressing crosstalk between reactive oxygen species and NF-κB. Biomol. Ther. (Seoul) 2015;23(2):110–118. doi: 10.4062/biomolther.2015.006. [http://dx.doi.org/10.4062/biomolther.2015.006]. [PMID: 25767678]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yang Y., Wang J., Yang Q., Wu S., Yang Z., Zhu H., Zheng M., Liu W., Wu W., He J., Chen Z. Shikonin inhibits the lipopolysaccharide-induced release of HMGB1 in RAW264.7 cells via IFN and NF-κB signaling pathways. Int. Immunopharmacol. 2014;19(1):81–87. doi: 10.1016/j.intimp.2014.01.003. [http://dx.doi.org/10.1016/j.intimp.2014.01. 003]. [PMID: 24447680]. [DOI] [PubMed] [Google Scholar]
- 192.Kim S.W., Jin Y., Shin J.H., Kim I.D., Lee H.K., Park S., Han P.L., Lee J.K. Glycyrrhizic acid affords robust neuroprotection in the postischemic brain via anti-inflammatory effect by inhibiting HMGB1 phosphorylation and secretion. Neurobiol. Dis. 2012;46(1):147–156. doi: 10.1016/j.nbd.2011.12.056. [http://dx.doi.org/10.1016/j.nbd.2011.12.056]. [PMID: 22266336]. [DOI] [PubMed] [Google Scholar]
- 193.Gong G., Xiang L., Yuan L., Hu L., Wu W., Cai L., Yin L., Dong H. Protective effect of glycyrrhizin, a direct HMGB1 inhibitor, on focal cerebral ischemia/reperfusion-induced inflammation, oxidative stress, and apoptosis in rats. PLoS One. 2014;9(3):e89450. doi: 10.1371/journal.pone.0089450. [http://dx.doi.org/10.1371/journal.pone.0089450]. [PMID: 24594628]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Huang J., Liu B., Yang C., Chen H., Eunice D., Yuan Z. Acute hyperglycemia worsens ischemic stroke-induced brain damage via high mobility group box-1 in rats. Brain Res. 2013;1535:148–155. doi: 10.1016/j.brainres.2013.08.057. [http://dx.doi.org/10.1016/j.brainres.2013.08.057]. [PMID: 24012767]. [DOI] [PubMed] [Google Scholar]
- 195.Zhang J., Wu Y., Weng Z., Zhou T., Feng T., Lin Y. Glycyrrhizin protects brain against ischemia-reperfusion injury in mice through HMGB1-TLR4-IL-17A signaling pathway. Brain Res. 2014;1582:176–186. doi: 10.1016/j.brainres.2014.07.002. [http://dx.doi.org/10.1016/j.brainres. 2014.07.002]. [PMID: 25111887]. [DOI] [PubMed] [Google Scholar]
- 196.Barakat W., Safwet N., El-Maraghy N.N., Zakaria M.N. Candesartan and glycyrrhizin ameliorate ischemic brain damage through downregulation of the TLR signaling cascade. Eur. J. Pharmacol. 2014;724:43–50. doi: 10.1016/j.ejphar.2013.12.032. [http://dx.doi.org/10.1016/j.ejphar. 2013.12.032]. [PMID: 24378346]. [DOI] [PubMed] [Google Scholar]
- 197.Peng Z., Wang S., Chen G., Cai M., Liu R., Deng J., Liu J., Zhang T., Tan Q., Hai C. Gastrodin alleviates cerebral ischemic damage in mice by improving anti-oxidant and anti-inflammation activities and inhibiting apoptosis pathway. Neurochem. Res. 2015;40(4):661–673. doi: 10.1007/s11064-015-1513-5. [http://dx.doi.org/10.1007/s11064-015-1513-5]. [PMID: 25582916]. [DOI] [PubMed] [Google Scholar]
- 198.Xu X., Lu Y., Bie X. Protective effects of gastrodin on hypoxia-induced toxicity in primary cultures of rat cortical neurons. Planta Med. 2007;73(7):650–654. doi: 10.1055/s-2007-981523. [http://dx.doi.org/10.1055/s-2007-981523]. [PMID: 17583824]. [DOI] [PubMed] [Google Scholar]
- 199.Zeng X.S., Zhou X.S., Luo F.C., Jia J.J., Qi L., Yang Z.X., Zhang W., Bai J. Comparative analysis of the neuroprotective effects of ginsenosides Rg1 and Rb1 extracted from Panax notoginseng against cerebral ischemia. Can. J. Physiol. Pharmacol. 2014;92(2):102–108. doi: 10.1139/cjpp-2013-0274. [http://dx.doi.org/10.1139/cjpp-2013-0274]. [PMID: 24502632]. [DOI] [PubMed] [Google Scholar]
- 200.Zhang Y.F., Fan X.J., Li X., Peng L.L., Wang G.H., Ke K.F., Jiang Z.L. Ginsenoside Rg1 protects neurons from hypoxic-ischemic injury possibly by inhibiting Ca2+ influx through NMDA receptors and L-type voltage-dependent Ca2+ channels. Eur. J. Pharmacol. 2008;586(1-3):90–99. doi: 10.1016/j.ejphar.2007.12.037. [http://dx.doi.org/10.1016/ j.ejphar.2007.12.037]. [PMID: 18430419]. [DOI] [PubMed] [Google Scholar]
- 201.Zhao Y., Fu B., Zhang X., Zhao T., Chen L., Zhang J., Wang X. Paeonol pretreatment attenuates cerebral ischemic injury via upregulating expression of pAkt, Nrf2, HO-1 and ameliorating BBB permeability in mice. Brain Res. Bull. 2014;109:61–67. doi: 10.1016/j.brainresbull.2014.09.008. [http://dx.doi.org/10.1016/j.brainresbull.2014.09.008]. [PMID: 25286445]. [DOI] [PubMed] [Google Scholar]
- 202.Wu J.B., Song N.N., Wei X.B., Guan H.S., Zhang X.M. Protective effects of paeonol on cultured rat hippocampal neurons against oxygen-glucose deprivation-induced injury. J. Neurol. Sci. 2008;264(1-2):50–55. doi: 10.1016/j.jns.2007.06.057. [http://dx.doi.org/10.1016/j.jns.2007.06. 057]. [PMID: 17942121]. [DOI] [PubMed] [Google Scholar]
- 203.Denny-Brown D. Pathophysiology of focal cerebral ischemia. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 1966;41:196–204. [PMID: 5334398]. [PubMed] [Google Scholar]
- 204.Tang X., Zhong W., Tu Q., Ding B. NADPH oxidase mediates the expression of MMP-9 in cerebral tissue after ischemia-reperfusion damage. Neurol. Res. 2014;36(2):118–125. doi: 10.1179/1743132813Y.0000000266. [http://dx. doi.org/10.1179/1743132813Y.0000000266]. [PMID: 24131725]. [DOI] [PubMed] [Google Scholar]
- 205.Luo Y., Qin Z., Hong Z., Zhang X., Ding D., Fu J.H., Zhang W.D., Chen J. Astragaloside IV protects against ischemic brain injury in a murine model of transient focal ischemia. Neurosci. Lett. 2004;363(3):218–223. doi: 10.1016/j.neulet.2004.03.036. [http://dx.doi.org/10.1016/j.neulet. 2004.03.036]. [PMID: 15182947]. [DOI] [PubMed] [Google Scholar]
- 206.Li M., Ma R.N., Li L.H., Qu Y.Z., Gao G.D. Astragaloside IV reduces cerebral edema post-ischemia/reperfusion correlating the suppression of MMP-9 and AQP4. Eur. J. Pharmacol. 2013;715(1-3):189–195. doi: 10.1016/j.ejphar.2013.05.022. [http://dx.doi.org/10.1016/j.ejphar.2013.05.022]. [PMID: 23747593]. [DOI] [PubMed] [Google Scholar]
- 207.Mao X., Yin W., Liu M., Ye M., Liu P., Liu J., Lian Q., Xu S., Pi R. Osthole, a natural coumarin, improves neurobehavioral functions and reduces infarct volume and matrix metalloproteinase-9 activity after transient focal cerebral ischemia in rats. Brain Res. 2011;1385:275–280. doi: 10.1016/j.brainres.2011.02.015. [http://dx.doi.org/10.1016/j.brainres.2011. 02.015]. [PMID: 21316348]. [DOI] [PubMed] [Google Scholar]
- 208.Chao X., Zhou J., Chen T., Liu W., Dong W., Qu Y., Jiang X., Ji X., Zhen H., Fei Z. Neuroprotective effect of osthole against acute ischemic stroke on middle cerebral ischemia occlusion in rats. Brain Res. 2010;1363:206–211. doi: 10.1016/j.brainres.2010.09.052. [http://dx.doi.org/10.1016/ j.brainres.2010.09.052]. [PMID: 20869955]. [DOI] [PubMed] [Google Scholar]
- 209.Liang G., Shi B., Luo W., Yang J. The protective effect of caffeic acid on global cerebral ischemia-reperfusion injury in rats. Behav. Brain Funct. 2015;11:18. doi: 10.1186/s12993-015-0064-x. [http://dx.doi.org/10.1186/ s12993-015-0064-x]. [PMID: 25907417]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chen J.H., Kuo H.C., Lee K.F., Tsai T.H. Magnolol protects neurons against ischemia injury via the downregulation of p38/MAPK, CHOP and nitrotyrosine. Toxicol. Appl. Pharmacol. 2014;279(3):294–302. doi: 10.1016/j.taap.2014.07.005. [http://dx.doi.org/10.1016/j.taap.2014.07. 005]. [PMID: 25038313]. [DOI] [PubMed] [Google Scholar]
- 211.Chen Y., Tsai Y.H., Tseng S.H. The potential of tetrandrine as a protective agent for ischemic stroke. Molecules. 2011;16(9):8020–8032. doi: 10.3390/molecules16098020. [PMID: 21926947]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Liu S.J., Zhou S.W., Xue C.S. Effect of tetrandrine on neutrophilic recruitment response to brain ischemia/reperfusion. Acta Pharmacol. Sin. 2001;22(11):971–975. [PMID: 11749785]. [PubMed] [Google Scholar]
- 213.Guo R.B., Wang G.F., Zhao A.P., Gu J., Sun X.L., Hu G. Paeoniflorin protects against ischemia-induced brain damages in rats via inhibiting MAPKs/NF-κB-mediated inflammatory responses. PLoS One. 2012;7(11):e49701. doi: 10.1371/journal.pone.0049701. [http://dx.doi.org/10.1371/journal. pone.0049701]. [PMID: 23166749]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yuan Y., Rangarajan P., Kan E.M., Wu Y., Wu C., Ling E.A. Scutellarin regulates the Notch pathway and affects the migration and morphological transformation of activated microglia in experimentally induced cerebral ischemia in rats and in activated BV-2 microglia. J. Neuroinflammation. 2015;12(1):11. doi: 10.1186/s12974-014-0226-z. [http://dx.doi.org/10.1186/s12974-014-0226-z]. [PMID: 25600517]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Guo H., Hu L.M., Wang S.X., Wang Y.L., Shi F., Li H., Liu Y., Kang L.Y., Gao X.M. Neuroprotective effects of scutellarin against hypoxic-ischemic-induced cerebral injury via augmentation of antioxidant defense capacity. Chin. J. Physiol. 2011;54(6):399–405. doi: 10.4077/CJP.2011.AMM059. [PMID: 22229507]. [DOI] [PubMed] [Google Scholar]
- 216.Raza S.S., Khan M.M., Ashafaq M., Ahmad A., Khuwaja G., Khan A., Siddiqui M.S., Safhi M.M., Islam F. Silymarin protects neurons from oxidative stress associated damages in focal cerebral ischemia: a behavioral, biochemical and immunohistological study in Wistar rats. J. Neurol. Sci. 2011;309(1-2):45–54. doi: 10.1016/j.jns.2011.07.035. [http://dx. doi.org/10.1016/j.jns.2011.07.035]. [PMID: 21840019]. [DOI] [PubMed] [Google Scholar]
- 217.Hou Y.C., Liou K.T., Chern C.M., Wang Y.H., Liao J.F., Chang S., Chou Y.H., Shen Y.C. Preventive effect of silymarin in cerebral ischemia-reperfusion-induced brain injury in rats possibly through impairing NF-κB and STAT-1 activation. Phytomedicine. 2010;17(12):963–973. doi: 10.1016/j.phymed.2010.03.012. [http://dx.doi.org/10.1016/j.phymed.2010. 03.012]. [PMID: 20833521]. [DOI] [PubMed] [Google Scholar]
- 218.Zhang X., Zhang X., Wang C., Li Y., Dong L., Cui L., Wang L., Liu Z., Qiao H., Zhu C., Xing Y., Cao X., Ji Y., Zhao K. Neuroprotection of early and short-time applying berberine in the acute phase of cerebral ischemia: up-regulated pAkt, pGSK and pCREB, down-regulated NF-κB expression, ameliorated BBB permeability. Brain Res. 2012;1459:61–70. doi: 10.1016/j.brainres.2012.03.065. [http://dx.doi.org/10.1016/j.brainres.2012.03.065]. [PMID: 22560097]. [DOI] [PubMed] [Google Scholar]
- 219.Zhou X.Q., Zeng X.N., Kong H., Sun X.L. Neuroprotective effects of berberine on stroke models in vitro and in vivo. Neurosci. Lett. 2008;447(1):31–36. doi: 10.1016/j.neulet.2008.09.064. [http://dx.doi.org/10.1016/j.neulet. 2008.09.064]. [PMID: 18838103]. [DOI] [PubMed] [Google Scholar]
- 220.Cheng C.Y., Ho T.Y., Lee E.J., Su S.Y., Tang N.Y., Hsieh C.L. Ferulic acid reduces cerebral infarct through its antioxidative and anti-inflammatory effects following transient focal cerebral ischemia in rats. Am. J. Chin. Med. 2008;36(6):1105–1119. doi: 10.1142/S0192415X08006570. [http://dx.doi.org/10.1142/S0192415X08006570]. [PMID: 19051339]. [DOI] [PubMed] [Google Scholar]
- 221.Chang C.Y., Kao T.K., Chen W.Y., Ou Y.C., Li J.R., Liao S.L., Raung S.L., Chen C.J. Tetramethylpyrazine inhibits neutrophil activation following permanent cerebral ischemia in rats. Biochem. Biophys. Res. Commun. 2015;463(3):421–427. doi: 10.1016/j.bbrc.2015.05.088. [http://dx.doi.org/10.1016/j.bbrc.2015.05.088]. [PMID: 26043690]. [DOI] [PubMed] [Google Scholar]
- 222.Merino-Zamorano C., Hernández-Guillamon M., Jullienne A., Le Béhot A., Bardou I., Parés M., Fernández-Cadenas I., Giralt D., Carrera C., Ribó M., Vivien D., Ali C., Rosell A., Montaner J. NURR1 involvement in recombinant tissue-type plasminogen activator treatment complications after ischemic stroke. Stroke. 2015;46(2):477–484. doi: 10.1161/STROKEAHA.114.006826. [http://dx.doi.org/10.1161/STROKEAHA. 114.006826]. [PMID: 25503547]. [DOI] [PubMed] [Google Scholar]
- 223.Rogalewski A., Schneider A., Ringelstein E.B., Schäbitz W.R. Toward a multimodal neuroprotective treatment of stroke. Stroke. 2006;37(4):1129–1136. doi: 10.1161/01.STR.0000209330.73175.34. [http://dx.doi.org/10.1161/01.STR. 0000209330.73175.34]. [PMID: 16527996]. [DOI] [PubMed] [Google Scholar]
- 224.Li W., Fu H., Youdim M.B., Pang Y., Han Y. Brain Protection in Schizophrenia, Mood and Cognitive Disorders. Springer Netherlands; 2010. One-compound-multi-targets at amyloid β cascade offered by Bis(7)-Cognitin, a novel anti-Alzheimer's dimer. pp. 165–183. [http://dx.doi.org/10.1007/978-90-481-8553-5_7] [Google Scholar]
- 225.Singh D.P., Chopra K. Flavocoxid, dual inhibitor of cyclooxygenase-2 and 5-lipoxygenase, exhibits neuroprotection in rat model of ischaemic stroke. Pharmacol. Biochem. Behav. 2014;120:33–42. doi: 10.1016/j.pbb.2014.02.006. [http://dx.doi.org/10.1016/j.pbb.2014.02.006]. [PMID: 24561313]. [DOI] [PubMed] [Google Scholar]
- 226.Xu S., Liu P. Tanshinone II-A: new perspectives for old remedies. Expert Opin. Ther. Pat. 2013;23(2):149–153. doi: 10.1517/13543776.2013.743995. [http://dx.doi.org/10.1517/13543776.2013.743995]. [PMID: 23231009]. [DOI] [PubMed] [Google Scholar]
- 227.Tian X.H., Wu J.H. Tanshinone derivatives: a patent review (January 2006 - September 2012). Expert Opin. Ther. Pat. 2013;23(1):19–29. doi: 10.1517/13543776.2013.736494. [http://dx.doi.org/10.1517/13543776.2013.736494]. [PMID: 23094864]. [DOI] [PubMed] [Google Scholar]
- 228.Murphy R.A., Sarpong R. Heathcock-inspired strategies for the synthesis of fawcettimine-type Lycopodium alkaloids. Chemistry. 2014;20(1):42–56. doi: 10.1002/chem.201303975. [http://dx.doi.org/10.1002/chem.201303975]. [PMID: 24311383]. [DOI] [PubMed] [Google Scholar]
- 229.Mencucci R., Favuzza E., Menchini U. Assessment of the tolerability profile of an ophthalmic solution of 5% glycyrrhizin and copolymer PEG/PPG on healthy volunteers and evaluation of its efficacy in the treatment of moderate to severe blepharitis. Clin. Ophthalmol. 2013;7:1403–1410. doi: 10.2147/OPTH.S47657. [PMID: 23874081]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Petrelli A., Giordano S. From single- to multi-target drugs in cancer therapy: when aspecificity becomes an advantage. Curr. Med. Chem. 2008;15(5):422–432. doi: 10.2174/092986708783503212. [http://dx.doi.org/10.2174/ 092986708783503212]. [PMID: 18288997]. [DOI] [PubMed] [Google Scholar]
- 231.Cassidy J.M., Cramer S.C. Spontaneous and Therapeutic-Induced Mechanisms of Functional Recovery After Stroke. Transl. Stroke Res. 2016 doi: 10.1007/s12975-016-0467-5. [http://dx.doi.org/10.1007/s12975-016-0467-5]. [PMID: 27109642]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Fagan S.C., Lapchak P.A., Liebeskind D.S., Ishrat T., Ergul A. Recommendations for preclinical research in hemorrhagic transformation. Transl. Stroke Res. 2013;4(3):322–327. doi: 10.1007/s12975-012-0222-5. [http://dx. doi.org/10.1007/s12975-012-0222-5]. [PMID: 23730351]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Youdim M.B., Buccafusco J.J. Multi-functional drugs for various CNS targets in the treatment of neurodegenerative disorders. Trends Pharmacol. Sci. 2005;26(1):27–35. doi: 10.1016/j.tips.2004.11.007. [http://dx.doi.org/10.1016/j.tips.2004.11.007]. [PMID: 15629202]. [DOI] [PubMed] [Google Scholar]
- 234.Jia L., Chopp M., Zhang L., Lu M., Zhang Z. Erythropoietin in combination of tissue plasminogen activator exacerbates brain hemorrhage when treatment is initiated 6 hours after stroke. Stroke. 2010;41(9):2071–2076. doi: 10.1161/STROKEAHA.110.586198. [http://dx.doi.org/10.1161/STROKEAHA. 110.586198]. [PMID: 20671252]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Tang L.L., Ye K., Yang X.F., Zheng J.S. Apocynin attenuates cerebral infarction after transient focal ischaemia in rats. J. Int. Med. Res. 2007;35(4):517–522. doi: 10.1177/147323000703500411. [http://dx.doi.org/10.1177/ 147323000703500411]. [PMID: 17697529]. [DOI] [PubMed] [Google Scholar]


