Abstract
Convection-enhanced delivery (CED) is a technique designed to deliver drugs directly into the brain or tumors. Its ability to bypass the blood-brain barrier (BBB), one of the major hurdles in delivering drugs to the brain, has made it a promising drug delivery method for the treatment of primary brain tumors. A number of clinical trials utilizing CED of various therapeutic agents have been conducted to treat patients with supratentorial high-grade gliomas. Significant responses have been observed in certain patients in all of these trials. However, the insufficient ability to monitor drug distribution and pharmacokinetics hampers CED from achieving its potentials on a larger scale. Brainstem CED for diffuse intrinsic pontine glioma (DIPG) treatment is appealing because this tumor is compact and has no definitive treatment. The safety of brainstem CED has been established in small and large animals, and recently in early stage clinical trials. There are a few current clinical trials of brainstem CED in treating DIPG patients using targeted macromolecules such as antibodies and immunotoxins. Future advances for CED in DIPG treatment will come from several directions including: choosing the right agents for infusion; developing better agents and regimen for DIPG infusion; improving instruments and technique for easier and accurate surgical targeting and for allowing multisession or prolonged infusion to implement optimal time sequence; and better understanding and control of drug distribution, clearance and time sequence. CED-based therapies for DIPG will continue to evolve with new understanding of the technique and the disease.
Keywords: Catheter design, convection-enhanced delivery, diffuse intrinsic pontine glioma, drug distribution
1. Introduction
Diffuse intrinsic pontine glioma (DIPG) is a group of highly infiltrative brainstem gliomas that occur mainly in children. In these tumors, cancer tissue cannot be distinguished from normal brain tissue macroscopically. This infiltrative nature makes effective therapy extremely difficult, if not impossible. An effective therapy would have to selectively remove or kill tumor cells without causing significant damage to the normal brain tissue. Therefore, surgical resection and stereotactic radiosurgery are not considered treatment options given their risk to cause serious injury in this situation. Conventional radiation therapy is currently employed routinely as a palliative approach, which gives most patients several months of symptom relief. Newer antineoplastic agents’ improved tumor selectivity and the development of targeted therapeutic agents in recent decades raise hopes that improved chemotherapy will lead to improved outcome. Paralleling this development has been the advances in drug delivery to the central nervous system via local delivery to overcome the blood-brain barrier (BBB), one of the major hurdles in delivering drugs to the brain.
In this review, we will be discussing the application of convection-enhanced delivery (CED) in DIPG treatment. CED is a technique designed to deliver drugs directly into the brain or tumors at high concentrations. This bypasses the BBB and avoids or at least greatly reduces systemic exposure to the drug. Drugs being studied for delivery through CED include conventional antineoplastic drugs, novel small molecule agents and macromolecules such as therapeutic antibodies, immunotoxins and viral vectors, some of which would otherwise never gain access to the brain.
1.1. Blood-Brain Barrier
An important limitation of systemic chemotherapy in primary brain tumor treatment is the existence of the blood-brain barrier (BBB). The BBB is a barrier that isolates the circulating blood from the cerebrospinal fluid (CSF) and the interstitial fluid in the central nervous system (CNS). It occurs along cerebral capillaries and consists of tight junctions (zona occludens) that do not exist in vasculatures in other organs. Endothelial cells restrict the diffusion of microscopic objects (e.g., bacteria) and large or hydrophilic molecules from the brain vasculature, while allowing the diffusion of small hydrophobic molecules (e.g., O2, CO2 and certain hormones). Typically, molecules larger than ~40kD are unlikely to penetrate the intact barrier. For the brain’s supply of nutrients and removal of metabolites, cells of the brain vasculature actively transport glucose and metabolic products across the barrier with transporters.
The BBB acts effectively to protect the brain from many common bacterial infections and some toxic substances. Yet it presents a major challenge in delivering therapeutic agents to specific regions of the brain for the treatment of brain tumors and certain other disorders. Most cancer drugs are not able to permeate the BBB because they are polar in structure or too large in molecular weight. Even for drugs that are able to cross the cerebral capillary bed, it is difficult for them to achieve optimal concentrations in the brain due to limitations posed by systemic toxicity.
Another difficulty in the delivery of drugs for the treatment of primary brain tumors and certain other CNS diseases is how to direct those agents to the specific anatomic region or tumor mass and tumor cell-infiltrated parenchyma to reduce the disturbance of normal neurological functions.
Several strategies have been developed in an attempt to overcome this barrier, including temporary disruption of the BBB, modification of drugs to enhance their ability to permeate the BBB and local delivery methods such as intra-tumoral/intra-cavitary embedding of drug-containing polymers or microchips, intra-arterial injection, direct injection of drugs into the tissue or CSF in the ventricles or subarachnoid space, and convection-enhanced delivery (CED) to deliver drugs directly into the interstitial space.
1.2. Local Delivery
Direct injection into the tumor or CSF is one of the earliest local delivery methods attempted. When injected into the tumor, a drug relies on diffusion to reach cancer cells not directly adjacent to the injection site. Therefore, the drug has an uneven distribution and can only reach tumor tissue that is close to the injection site, which is usually only several millimeters with small molecules, with an exponential decay in concentration from the point source. Thus, the distribution of the drug is limited to a small volume of tissue around the injection site, often with very high and sometimes toxic concentration at the center. When a drug is injected directly into the CSF, the drug is only able to reach a shallow layer of the brain, therefore it is typically used for leptomeningeal diseases, which is often seen in CNS lymphoma and some metastatic carcinomas.
Drug-containing polymers and microchips are a more recent development and they can be embedded at the time of surgical resection of brain tumors. As in the case of direct injection, this delivery method relies on diffusion for the drug to spread past the embedding site and has similar limited and uneven distribution.
2. Convection-Enhanced Delivery
Convection-enhanced delivery (CED) is a novel drug delivery method first developed in the early 1990s [1]. In this method, a drug-containing solution is distributed into the interstitial space driven by a small, persistent hydraulic pressure (i.e., forced convection). In contrast to diffusion that depends on a concentration gradient to distribute the molecules, the use of hydraulic pressure in CED allows for a homogeneous distribution of small and large molecules over large distances by displacing interstitial fluid with the infusate. In practice, the agent is delivered into the parenchyma or tumor driven by a pump through a microcatheter, or multiple microcatheters, inserted into the tissue. Infusion rates typically range from 0.1-10µl/min.
2.1. Drug Distribution and Some Basic Principles
In CED, the distribution from a single point source results in an elliptical to spherical distribution, and spatial distribution is in some degree dependent on the tissue type (i.e., gray versus white matter). In a given tissue type, distribution volume is roughly linear to infusion volume.
CED into brain parenchyma, both white and gray matter, has shown reproducible large volumes of distribution with homogeneous drug concentration. Early work showed that the concentration fall-off at the border is steep [1], resulting in a potentially large benefit in cancer drug delivery when reducing toxicity to surrounding normal brain tissue is desired.
Several factors influence the distribution volume. One key factor to achieve a large volume of distribution is the stability of the agent in the interstitial space. Lipophilic agents may be exported transvascularly through blood vessels leading to a high efflux of the drug and limited distribution. Some other drugs may be prone to enzymatic degradation in the interstitial space. Another important determinant for distribution of macromolecules is the surface characteristics of the molecule and the extracellular matrix. Binding of the molecule to the extracellular matrix or surface receptors may limit distribution [2]. Although binding to cell surface receptors may be overcome by saturating receptor binding, adherence to heparin sulfate proteoglycan (HSP) in the extracellular matrix limits distribution of macromolecules such as growth factors [3]. Co-infusion of heparin overcomes this limitation and allows reproducible large volumes of distribution of large particles such as adeno-associated virus serotype 2 (AAV2) [4].
Size of the molecules also affects volume of distribution. Early CED studies suggested that 180kD, approximately the size of immunoglobulin G (IgG), appeared to be the largest size that could pass through the interstitial space without the need of surface modification to the extracellular matrix. Recently, with the help of surface modification, adeno-associated virus (AAV, 40nm) [4] and liposomes (50-200nm) [5] have been distributed to large volumes of brain tissue. Surface modifications used were pegylation with liposomes and heparin co-infusion to saturate HSP binding with AAV. Another interesting observation is the effect of infusate viscosity on the volume of distribution. Counter-intuitively, some evidence suggests that increasing the viscosity of infusates actually improves the volume of distribution significantly [6, 7]. The cause might be that low-viscosity infusate tends to reflux, or is easier to be taken up by cells. This demonstrates the importance of infusate formulation in CED, which requires further study.
The volume of distribution is also affected by the retrograde movement of fluid along the outside of the catheter (backflow or reflux). Reflux is determined by catheter material, catheter diameter, infusion rate and tissue density among other factors. The larger the catheter diameter, the greater is the chance of backflow along its outer wall. If reflux reaches a low-pressure zone (necrosis or CSF space), the fluid will inadvertently be lost into these spaces. This leads to the accumulation of drug in these regions that may cause toxicity. Finally, increasing the infusion rate can increase the overall volume of distribution; however, this will also increase the chance of reflux, potentially shunting fluid away from the target region.
Ideally, agents delivered via CED should be contained within the target region of brain parenchyma or tumor mass. However, there are low-pressure regions in some tumors along which infusate will flow, sometimes into ventricles or subarachnoid space. This phenomenon is usually referred to as leakage and has often been observed in both humans and experimental animals. One study indicates that this can happen in 20% of CED procedures [8]. This obvious waste of therapeutic agent will consequently reduce volume of distribution and drug concentration in the planned target region. It may also cause untoward effects on normal brain tissue. It is therefore critical to follow the flow of infused agents. When this happens, it might be helpful to adjust catheter placement to move the opening away from the low-pressure region. It is also unknown yet whether this leakage is reversible. If reversible, pausing infusion for a period of time and subsequently restarting the infusion could eliminate leakage.
2.2. Catheter Design for CED
Metal needles have been used as the infusion tool since the early studies of CED in laboratory animals. Most of the recent clinical trials of CED in the treatment of malignant gliomas have used ventricular catheters made of Silastic® rubber. Ideally, a catheter for CED should be reflux-free; does not adsorb therapeutic agents to its wall, especially when expensive novel targeted agents are used; and should have tip configurations that direct the drug to desired regions. In certain instances, it may be required to confirm catheter placement with magnetic resonance imaging (MRI) before drug infusion where MRI-compatible catheters are needed.
As briefly discussed above, reflux negates the bulk flow of infusate in the interstitial space produced by CED. In the presence of reflux, an increase in infusion volume does not produce an increase in distribution volume accordingly. Reflux causes the drug to flow into ventricular or subarachnoid space where it may cause toxicity. While reduction in infusion rate may reduce the chance of reflux, it would be ideal to have the option of infusing at various flow rates, i.e., up to 10µl/min or more if possible, to achieve desired volume of distribution in a reasonable period of time.
Simple infusion tools such as metal needles have high rates of reflux. Several groups, including ours, observed that a step-design cannula significantly reduces, or even effectively prevents, reflux. We used a 22 gauge guide cannula with a 28 gauge internal cannula, both of fused silica. The internal cannula extended beyond the end of the guide cannula by 5 mm. The cannula set was left in place for 5 minutes before infusion started. At flow rates as high as 8µl/min of an 124I-labeled monoclonal antibody, no reflux was observed on positron emission tomography (PET) imaging. Presumably the tissue surrounding the extended internal cannula sealed off the entry tract. There might be a threshold that this design can withstand the pressure. Whereas fused silica seals well with brain tissue, the material is not strong enough by itself for clinical use and needs to be reinforced with other materials. Nevertheless this design offers an attractive improvement over the cannula design previously used. MRI-compatible step-design cannulas and catheters have become commercially available lately and are being tested in a few current CED clinical trials. Preliminary results showed that they have lower reflux rates compared to ventricular catheters used in earlier clinical trials.
Another aspect of catheter design is the tip configuration. Standard cannulas only have opening at their tips. In certain instances, such as after radiation therapy where scars may have formed inside the tumor, this simple tip opening may not allow for sufficient infusate flow. Considering infusates will follow the path of least resistance, a multi-tipped cannula may provide better pressure output, and therefore, achieve a better volume of distribution. The effectiveness of multi-opening configuration has been questioned by studies showing that a multi-port catheter delivered most of the infusate through the proximal port and thus behaved like catheters with only one port [9].
One research group constructed a 3-mm long porous hollow fiber catheter to increase the surface area of the brain in immediate contact with the drug releasing area [10]. The hollow fiber has innumerous pores of 0.45µm along its walls. The hollow fiber catheter offered up to a threefold increase in distribution volume into the normal mouse brain under test conditions when compared to a needle that has a single macroscopic pore. The tiny microscopic pores do not have the same pressure-shunting properties as the macroscopic pores do, therefore a long length of the porous wall is effective in delivering drugs. In large animal and human applications, it is more reasonable to have this porous hollow fiber configuration at the tip for a few millimeters rather than the entire catheter being porous. The porous wall and step design could be combined to reduce reflux during drug administration.
In certain other instances, it may be desirable to direct the infusate preferentially in a specific direction. Due to the pressure-shunting properties of the proximal port on regular multi-port cannulas, it may not be effective to direct infusate distribution via such a tip configuration. One potential design is to construct a catheter with independent cannulas inside. Each cannula has an opening at a predetermined location and direction with its pressure being independently controlled. This design will require additional engineering and testing to determine its feasibility.
2.3. Monitoring Drug Distribution
Monitoring the distribution and concentration of an infused drug is critical for numerous reasons. In addition to its biological effectiveness, a drug would need to be distributed within the tumor in therapeutic concentrations to be effective. Exposure of normal tissue to the drug should be controlled to reduce the probability of toxicity. It is also highly desirable to monitor for possible reflux and leakage so that cannula placement can be adjusted to correct any problems that may arise. In the brainstem, the transverse and longitudinal fiber bundles may direct infusate flow, which also needs to be monitored. The importance of monitoring in vivo distribution and concentration is highlighted by the difficulty in achieving optimal therapeutic efficacy in recent clinical trials. In the recent TGFα-PE38 study and the phase III PRECISE trial for glioblastoma, poor drug distribution was cited as one of the reasons for the unsatisfactory efficacy results [11, 12].
Monitoring the distribution and concentration of CED infusate in humans is difficult due to the fact that the majority of therapeutic agents cannot be seen on any of the clinical imaging methods. Nevertheless, distribution can be visualized under certain circumstances. T2-weighted MR images are helpful in identifying infusate distribution in regions of relatively normal intensity, but identifying distribution is more difficult when infused into already hyperintense regions, such as in DIPG [13]. Another choice is to use surrogate tracers. Gd-DTPA and 123I-albumin have been co-infused as surrogate tracers, viewable on T1-weighted imaging and single photon emission computed tomography (SPECT) images, respectively, in clinical studies [11, 13-16]. The shortcomings of surrogate markers are that they are only able to estimate the initial distribution accurately. Differences in biological activities and clearance confound their ability to follow the distribution of the therapeutic agent over time. Moreover, neither T2-weighted signals nor surrogate tracers are able to provide information on the concentration of the infused therapeutic agent. The ideal scenario would be to directly image the therapeutic compound. With calibration, the concentration of the drug can be determined as well as the distribution. Utilizing serial imaging, clearance can be followed over time. In an ongoing clinical trial at Memorial Sloan-Kettering Cancer Center and Weill Cornell Medical College (NCT01502917), a therapeutic monoclonal antibody is labeled with 124I to treat DIPG. 124I is a positron emitter that can be used for PET imaging at a high resolution. 124I PET has a significantly higher spatial resolution than 123I SPECT. 124I has an intrinsic spatial resolution loss of only 2.3 mm [17]. It is expected that this more detailed information regarding the distribution and concentration of CED infusate will give us a better understanding of CED. The approach of labeling a therapeutic agent with imageable radionuclide can be applied to some other agents and applications. For certain therapeutic agents, novel tags such as paramagnetic particles may prove useful in labeling drugs for quantitative in vivo imaging.
2.4. Predicting and Planning CED Distribution
It is critical to define the relationship between the volume of infusion (Vi) and the volume of distribution (Vd) to understand the expected distribution of an agent delivered into the brain via CED. This relationship is approximately linear in short session infusions and has variable slopes depending on the anatomical site of administration as well as the therapeutic compound. For instance, the Vd/Vi ratio is 8.2 in the non-human primate (NHP) striatum [18] compared to a ratio of 4.1 in cerebral white matter [19] for small molecules. A ratio of 8.7 was observed in the NHP brainstem for Gd-albumin (72kD) [20]. This ratio can serve as an estimate to match tumor volume in clinical trials.
BrainLAB AG (Feldkirchen, Germany) has developed a software package called iPlan Flow specifically for use in planning CED. The software takes data obtained via MRI regarding brain tissue characteristics of individual patients as input. Then the software helps in determining cannula placement, calculating the infusion parameters and predicting distribution. The plan for treatment can be visualized in three dimensions, including the number and position of catheters. One study retrospectively tested the ability of this software using MR diffusion tensor imaging (DTI) to predict patient-specific drug distributions by CED [21]. 123I-labeled albumin was co-infused as a surrogate tracer with the targeted recombinant cytotoxin IL13-PE38QQR in patients with recurrent malignant gliomas. The spatial distribution of 123I-albumin was then compared with a drug distribution simulation provided by iPlan Flow. The algorithm had a high sensitivity and specificity in identifying catheter trajectories that resulted in reflux or leakage. The mean concordance of the volume of distribution between the actual 123I-albumin distribution and the simulation was 65.75% and the mean maximal inplane deviation was less than 8.5 mm. The use of this simulation algorithm was considered clinically useful in 85% of the catheters. Even though albumin does not have a specific affinity towards malignant tissue compared to targeted agents, this simulation showed that software with the ability to take into account characteristics of an individual patient’s anatomy and pathophysiology is helpful in the planning of CED. iPlan Flow has yet to be tested in CED in the brainstem. Even though iPlan Flow takes fiber tracts into account, it remains to be seen how well it performs with the compact transverse and longitudinal fiber bundles in the brainstem that may direct infusate flow. An ongoing clinical trial of DIPG at Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College (NCT01502917) will attempt to assess how well this software performs in brainstem CED.
2.5. Safety of CED in the Brainstem
The concept of using CED for DIPG treatment is appealing given that this particular tumor is relatively compact, has growth patterns simulating white matter tracts, seldom metastasizes before local relapse and no surgical resection is performed. Our group first established the feasibility of this delivery route in the brainstem in small animals for potential clinical application in 2002 [22]. Subsequently, the safety of inert agents, characteristics of distribution and toxicity of potential therapeutic agents in the brainstem of small animals and non-human primates have been studied [23-28]. These studies showed that CED does not cause clinically relevant mechanical injury to the brainstem and this approach has a promising therapeutic application in humans. In clinical practice, image-guided frameless stereotaxy can be utilized to target the brainstem in children for biopsy or cannula insertion with high accuracy and low risks of temporary or permanent morbidity [29-31]. These will help establish CED as an accepted drug delivery method in the treatment of DIPG.
Recently, brainstem CED has been used safely on a limited clinical basis in a few children with brainstem diseases outside of clinical trials [16, 32]. In addition to our clinical trial, there is another one mainly treating DIPG using CED technique that has reported partial results [33]. A small number of DIPG patients were also treated on a clinical trial not specifically for pediatric patients or brainstem gliomas [34]. These two trials reported reasonably good safety and tolerance. In our phase I clinical trial (NCT01502917), more than 20 patients with DIPG have been treated without any dose-limiting toxicities. These results directly demonstrated the safety of CED in human brainstem.
2.6. Therapeutic Efficacy of CED
Although the physical parameters influencing drug distribution in CED have not been thoroughly clarified, the ability of CED to achieve high concentrations of a therapeutic agent over large volumes of brain tissue has led to several clinical trials in patients with neurodegenerative disorders and malignant gliomas. These CED therapeutic studies for malignant gliomas have focused on delivering targeted macromolecules (e.g., monoclonal antibodies and recombinant toxins) or currently available small molecule drugs. CED of antineoplastic agents has shown considerable promise in phase I and phase II clinical trials in patients with recurrent malignant gliomas. However, phase III results are less encouraging. CED in the treatment of DIPG has produced encouraging results in preclinical studies. A few phase I trials of CED in DIPG are ongoing or in the planning stage.
Several factors that are critical in achieving good therapeutic efficacy require further elucidation. The convective force used in CED facilitates drug distribution to larger volumes of brain tissue. However, malignant gliomas may contain areas of gliosis and necrosis, especially after receiving external beam radiation therapy, which is currently the standard of care. CED, as an investigational therapy, usually is not started until the completion of radiation therapy. The gliosis and necrosis may cause chaotic pressure gradients within the tumor and therefore an unpredictable distribution of the drug. Even within the peri-tumoral margins, targeting infiltrating tumor cells may be limited by the normal anisotropy of the brain tissue resulting in preferential flow of fluid away from the intended target. Furthermore, the presence of areas of disrupted BBB either by the pathological changes or by previous treatment such as radiation therapy may increase efflux of the drug out of the CNS. A better understanding of drug distribution will become a critical part of evaluating future studies involving CED.
Another factor to consider is that CED, in its current form, is a surgery and typically performed as a single session. It is unknown how long the infused drugs remain at therapeutic concentrations after a single session of CED. One concern is that they may maintain their therapeutic concentration for a period too short to be effective before being cleared out of the target region. Indeed, our studies showed rapid concentration decrease within a few hours after single infusions of Gd-DTPA into the rat striatum via CED (Zhou et al., unpublished data). Once we have a better understanding of drug distribution and clearance, other unsolved questions including optimal catheter design and placement, rate and duration of infusion, and the benefit of repeat infusions can be better addressed.
The use of targeted macromolecules allows for either intra-tumoral or peri-tumoral treatment in malignant gliomas. Some of these agents may not be specific enough, potentially leading to injury to normal tissue. This was seen with IL4-PE, which initially started at a concentration of 2µg/ml in a clinical trial [35]. The potential benefit of targeting multiple molecules by combining recombinant toxins, or combining these agents with other chemotherapies, remains unknown. Despite these limitations and uncertainties, significant responses have been observed in certain patients in all of the CED clinical trials.
3. CED Clinical Trials for the Treatment of Brain Tumors
Theoretically, both small molecule drugs and novel macromolecules, such as monoclonal antibodies and viral vectors, can be delivered through CED for the treatment of brain tumors. For a variety of reasons, most small molecule drugs, including standard antineoplastic agents and targeted agents, do not cross the BBB in sufficient amounts to have a significant effect on the cancer. CED of such small molecules showed that these agents have observable antitumor responses. However, more neurological complications have been observed when these agents were delivered via CED compared to systemic chemotherapy [36]. There are efforts to improve formulations of these agents for local delivery to reduce neurotoxicity and enhance therapeutic response [37]. These efforts, if successful, will make CED of small chemotherapeutic molecules applicable on a larger scale.
More effort is focused on recombinant toxins delivered via CED in the treatment of brain tumors. These toxins are recombinant proteins and have two components, a targeting moiety, typically a monoclonal antibody or a ligand to an over-expressed cell membrane receptor, and a toxin, which can be bacterial toxins. Bacterial toxins frequently utilized in recombinant toxins are Pseudomonas exotoxin (PE) and Diphtheria toxin (DT). These polypeptide toxins have strong cytotoxicity against mammalian cells by inhibiting protein synthesis. They do not show selectivity in killing cancer cells over normal cells. But by attaching them to a targeting moiety directed to cancer cells, the recombinant toxins can become highly selective in killing cancer cells while sparing normal cells. For this purpose, these bacterial toxins have been genetically modified to make them easier to attach to targeting moieties. Genetic modification also reduces the activity of these toxins to give a wider therapeutic window. One targeting moiety widely studied for adult malignant brain tumors is interleukin-13 (IL-13), because the IL-13 receptor is known to be over-expressed in high percentage of these tumors [38, 39]. Binding of a recombinant toxin on the cell surface triggers internalization of the toxin, which enzymatically arrests protein synthesis and ultimately causes cell death. Several recombinant toxins have been utilized in clinical trials for adult malignant brain tumors delivered via CED. These toxins are attractive in that they have strong cell-killing capabilities and resistance rarely develops.
3.1. Transferrin-CRM107
Several recombinant toxins have reached the stage of clinical study. The first cytotoxin that was used in brain cancer therapy via CED was Transferrin-CRM107, a thioether conjugate of human transferrin and CRM107, a mutant form of Diphtheria toxin [40]. Transferrin-CRM107 (commercially as TransMID™) targets tumor cells by binding to the transferrin receptor, which is over-expressed on rapidly dividing cells.
In a multicenter, open label phase II clinical trial, the cytotoxin was delivered directly into the tumor bed by CED at 0.67µg/ml [41]. Numerous significant clinical responses were observed. 44 patients received intra-tumoral CED of Transferrin-CRM107. Of the 34 evaluable patients, five had a complete response and seven a partial response. The median survival for all 44 patients was 37 weeks. However, the tumor-selectivity of this recombinant toxin is not high, shown by the toxicity to normal tissue. In eight of the patients, increased cerebral edema was noticed. Those with clinical neurotoxicity also had MRI changes suggestive of microvascular injury, perhaps related to the higher levels of transferrin receptors on normal blood vessel walls. A phase III multicenter, randomized study in recurrent, nonresectable glioblastoma multiforme (GBM) was opened but withdrawn prior to patient enrollment due to the toxicity data from the phase II trial.
3.2. IL4-PE
Another recombinant toxin clinically examined is IL4-PE (commercially as NBI-3001 and PRX321). More accurately called IL-4(38-37)-PE38KDEL, the agent uses a mutant interleukin-4 (IL-4) as the targeting moiety and a truncated and modified Pseudomonas exotoxin as the cytotoxic effector.
A phase I study of intra-tumoral CED of IL4-PE started at a concentration of 2µg/ml and was dose escalated to determine the maximum tolerated dose [35]. Drug-related grade 3 or 4 CNS toxicity was seen in a total of 39% of patients in all groups, and no systemic toxicity was seen. A phase II, multicenter randomized study of intra-tumoral IL4-PE followed by tumor resection between 2 and 7 days after the completion of toxin infusion enrolled a total of 30 adult patients. The accrual was completed in 2003 and the objective clinical responses were not as good as Transferin-CRM107. A phase II trial of CED of IL4-PE with real-time imaging for therapy of recurrent glioblastoma (CLARITY-1) was approved but not recruiting patients as of November 2008, the last time the status of the trial was reported. There are no plans for a phase III study.
3.3. TGF-α-PE38
TGF-α-PE38 (commercially as TP-38) is another recombinant toxin that entered clinical phase. It is composed of transforming growth factor-α (TGF-α), a native epidermal growth factor receptor (EGFR) ligand, and a 38kD fragment of the Pseudomonas exotoxin. TGF-α-PE38 binds to the EGFR, which is over-expressed in the majority of GBM and is naturally present in many normal organs [42].
Moderate responses were recorded in several patients in clinical trials. A phase I study of intra-tumoral and peri-tumoral infusion of TGF-α-PE38 was performed in 20 patients with recurrent malignant glioma with a concentration escalation of 0.025 to 0.1µg/ml [11]. Two catheters were initially placed during tumor resection and then a total volume of 40 ml was infused. TGF-α-PE38 was well tolerated and a maximum tolerated dose was not established. At the completion of the study, four patients had no recurrence of tumor over 55 weeks after treatment. The median survival for all patients after treatment was 28 weeks. For those without radiographic evidence of residual disease at the time of therapy, the median survival was 33 weeks. One patient with GBM remains alive and without progression >211 weeks after CED therapy, and another with GBM went 198 weeks without progressive disease after a nearly complete response to TGF-α-PE38 and remains alive >260 weeks after CED therapy. In the majority of patients imaged using SPECT, infusate distributions were significantly influenced by leakage and failed to produce any significant intra-parenchymal distribution. This highlights the importance of accurate catheter placement and drug distribution monitoring.
A phase II multicenter randomized study was conducted in adults with recurrent GBM. Patients were randomized into two groups treated with peri-tumoral CED of 0.05 or 0.1µg/ml of TGF-α-PE38. The total volume infused was ~40 ml. Post-infusion MRI changes were seen 1 to 4 months after treatment, geographically associated with the site of catheter placement. These changes usually resolved by 20 weeks post-treatment. There were no grade 3 or 4 toxicities related to TGF-α-PE38. Only 20% of patients retained the cytotoxin within the tumors by imaging. A phase I/II clinical trial evaluating TGF-α-PE38 in treating young patients with recurrent or progressive supratentorial high-grade glioma was terminated prematurely. Further clinical trials are pending resolution of issues encountered in the phase I and II trials, with catheter placement and infusate leakage as the most important concerns.
3.4. IL13-PE38
IL13-PE38 (commercially as Cintredekin Besudotox) was developed in the mid-1990s [43]. It is a recombinant toxin consisting of human IL-13 with PE38QQR, a 38kD fragment of the Pseudomonas exotoxin. High levels of the IL-13 receptor have been found in more than 90% of glioblastoma, whereas expression of the receptor in the normal brain is not present or at low levels [38, 39]. This toxin demonstrated efficacy in several preclinical GBM models before moving into clinical study.
Intra-tumoral and peri-tumoral CED of IL13-PE38 has been investigated in four separate phase I studies. In the largest peri-tumoral phase I study, a maximum tolerated concentration of 0.5µg/ml was observed [44]. In this four-stage study, histological efficacy, maximum tolerated concentration and maximum infusion time were assessed. The final stage explored the stereotactic placement of catheters after tumor resection to improve targeting the peri-tumoral brain tissue. A total of 51 patients with malignant gliomas were treated including 46 patients with GBM. IL13-PE38 and procedure-related adverse events were primarily limited to the CNS, including those associated with increased edema. With the administration of steroids, all patients tolerated infusions of 40ml through 2 to 3 catheters lasting up to 6 days. The maximum tolerated concentration was 0.5µg/ml and tumor necrosis was observed at this concentration. There were no grade 3 or 4 adverse events associated with drug infusion at concentrations lower than 0.5µg/ml, and no systemic toxicities were observed. Delayed radiographic changes were observed in some patients 2 to 4 months after therapy, which responded to steroids and may represent an inflammatory response or nonspecific activity. Median survival for GBM patients was 42.7 weeks. Catheter placement was variable in the early portion of the study, with some catheter tips placed in CSF spaces. Catheter placement was correlated with survival. The 27 GBM patients with two or more catheters placed optimally without loss of drug into the CSF compartments had a median survival of 55.6 weeks with follow-up extending beyond 5 years, and 5 of these patients (18.5%) survived beyond two years after a single treatment. These trials showed that most of the effective drug deliveries were achieved into the parenchyma surrounding the gross total resection cavities rather than into the remaining tumors in situ. They also demonstrated that the chance of successful delivery without reflux or leakage was enhanced if the catheter tip was at least 2cm deep from the last traverse pial surface and 5mm from the nearest non-traverse pial or ependymal surface.
These encouraging results led to a phase III multicenter, randomized study (known as the PRECISE study) in patients with first recurrent GBM. The patients were randomized 2:1 to surgery followed by peri-tumoral infusion of IL13-PE38 versus surgery and Gliadel wafer implant. Gliadel wafer contains carmustine (bis-chloroethyl-nitrosourea [BCNU]) and is approved by the Food and Drug Administration (FDA) as a standard therapy for GBM following surgical resection. 52 medical centers participated in this trial worldwide. Total enrollment was targeted at 300 patients to demonstrate a 50% improvement in median survival in the experimental arm [45]. Enrollment was completed in December 2005. Analysis of follow-up data showed that this goal was not achieved. The median survival of the 184 patients in the CED arm was 36.4 weeks compared to 35.3 weeks for the 92 patients in the control arm (p = 0.476). When the dataset was restricted to sites having enrolled more than six patients progressing to drug delivery, the results are more encouraging. In this case, the CED arm had a median survival of 46.8 weeks versus 41.6 in the control arm (p = 0.288) and a hazard ratio of 0.77 (p = 0.163). Most significant was the finding that progression-free survival was 17.7 versus 11.4 weeks in favor of CED (p = 0.008). The investigators believe poor drug distribution in some patients is a major factor that adversely affected the therapeutic response [12]. The trial implies that a uniform method must be applied in participating centers to ensure exact and reproducible drug delivery. This trial was also hampered by its unrealistically high statistical expectation, which required a 50% increase in median survival over the Gliadel control arm, ultimately making the trial being declared unsuccessful. Future trials will probably benefit from improved catheter placement, drug distribution monitoring and screening of expression level of IL-13 receptor chain α2 (IL-13Rα2). IL-13Rα2 is expressed specifically by glioma cells [46, 47]. The next generation toxin has been developed to bind the tumor-specific IL-13Rα2 [48] rather than the IL-13 physiological receptor, and should be studied clinically.
3.5. 131I-chTNT-1/B mAb
131I-chTNT-1/B mAb (commercially as Cotara) is an 131I-labeled humanized murine monoclonal antibody (mAb). It binds to a universal intracellular antigen, histone H1. Histone H1 is in the assembled double strand DNA and is exposed and accessible for antibody binding in the necrotic core of solid tumors. This antigen provides an abundant insoluble anchor for the mAb. 131I emits γ rays with sufficiently high energy to penetrate and kill adjacent tumor cells. From the principle of how the drug was designed, 131I-chTNT-1/B mAb is not as specific as those targeting specific receptors (e.g., the EGFR or IL-13 receptors) expressed by tumor cells, but rather deliver cytotoxic radiation to the tumor mass as well as to tumor cells invading the surrounding tissue. “TNT” in the name of the agent stands for “tumor necrosis therapy.”
The effect of 131I-chTNT-1/B mAb in patients with malignant gliomas was investigated in several clinical studies. The results of two non-randomized, open-label studies have been published [49]: a phase I study in 12 patients with recurrent anaplastic astrocytoma (AA) and GBM, and a phase II study in 39 patients with newly diagnosed or recurrent malignant gliomas.
The 51 patients enrolled in the two studies include 37 recurrent GBM, 8 newly diagnosed GBM and 6 recurrent AA. All patients had previously undergone radiation therapy, 42 had previously undergone at least one surgery and 31 had a chemotherapy regimen. More than half of the patients (53%) had a tumor volume of ≥ 30 cm3 (mean 36 ± 27.6 cm3). One or two catheters with slit openings near the closed distal end were placed with tips at or near the center of the enhancing tumor. 131I-chTNT-1/B mAb was infused using CED over 1 to 2 days at a rate of 0.18ml/h. In the first 6 patients, 1.5mCi/cm3 clinical target volume (CTV) was prescribed, which was calculated to deliver a dose of 137 Gy. For subsequent patients, the dose was based on tumor size and the prescribed activity was 0.5 – 3.0mCi/cm3 administered in 1 or 2 infusions.
The phase I study showed that more than 130 Gy could be delivered to the tumor with 34 ± 9% dose retention at 24h and a biological half-life of 46 ± 16h. Imaging and dosimetry studies on a subset of 6 malignant glioma patients in the phase II study showed that infusion of 13.2 – 71.1mCi of activity produced a calculated absorbed dose of 55 - 135Gy.
Treatment-emergent, drug-related CNS adverse events included brain edema (16%), hemiparesis (14%) and headache (14%). Most of these were reversed by corticosteroids. Systemic adverse events were mild.
Treatment with 131I-chTNT-1/B mAb in the phase I study resulted in 3 of 9 GBM patients having stable disease at 60 days, and all 9 patients with progressive disease at 90 days. The median time to progression (MTTP) and median survival time (MST) were 8.7 and 27.3 weeks, respectively. Of the three patients with AA, one achieved a partial response and the other two had stable disease 90 days after treatment. Recurrent GBM patients in the phase II study (n = 28) had a MTTP of 8.4 weeks (historical control 8 weeks) and a MST of 23 weeks (historical control 24 weeks).
The phase II study contained patients with more diverse conditions. In an effort to “normalize” findings in this study, efficacy data from a subset of 12 recurrent GBM patients who received a total activity between 1.25 and 2.5mCi/cm3, which was considered a therapeutic window based on efficacy versus toxicity, were examined. The median survival for these patients was 37.9 weeks. In addition, 7 of the 28 recurrent GBM patients and 1 of the 3 recurrent AA patients survived for > 1 year. Further research is required to determine the value of 131I-chTNT-1/B CED in these patients.
Two other phase I trials of 131I-chTNT-1/B CED in patients with recurrent or relapsed GBM has been completed recently and the results have not been published. A dose confirmation and dosimetry phase II study for GBM patients at first relapse is ongoing. The dose is a single 25-hour infusion of 2.5mCi/cm3 CTV. Brief interim results for a subset of 14 patients were reported in October 2010 and the median survival was 86 weeks.
4. CED Clinical Trials for DIPG Treatment
There are no completed CED clinical trials for DIPG, and only a small number of CED trials for DIPG are under way or in the planning stage. This is in contrast to the application of CED in the treatment of adult malignant gliomas, where a number of clinical trials have been completed as summarized above. Institutions sponsoring CED trials for DIPG have spent significant effort in studying the safety of CED into the brainstem in small and large animals, including non-human primates. A small number of DIPG patients were also treated on a clinical trial not specifically for pediatric patients or brainstem gliomas [34].
4.1. CED of IL13-PE38 for DIPG
The NINDS is sponsoring a phase I clinical trial led by Dr. Russell Lonser, using CED to deliver IL13-PE38QQR in treating DIPG and childhood supratentorial high-grade glioma. This study started recruiting patients in 2009. It is an open label dose escalation safety study. About 90% malignant gliomas have high levels of IL-13 receptors while the normal brain tissue has only a low level of these receptors [38, 39]. Like in adult malignant gliomas, the IL-13 receptor subtype IL-13Rα2 is highly expressed in DIPG [50, 51]. The experimental drug, IL13-PE38QQR, which combines the modified PE with human IL-13, has been discussed above.
This study recruits patients 3-17 years of age with DIPG or supratentorial high-grade glioma that have not responded well to standard radiation therapy. 28 patients are expected to enroll in this study. The planned doses are 0.125, 0.25 and 0.5µg/ml. Safety and tolerability are the primary endpoints with secondary endpoints including imaging changes and treatment responses.
4.2. CED of 124I-8H9 for DIPG
Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College are sponsoring a phase I clinical trial led by Dr. Mark Souweidane, using CED to deliver 124I-8H9 for DIPG treatment. This study has been open since late 2011. This is an open label dose escalation safety study. 124I is a radionuclide with a half-life of 4.18 days. It emits equal amounts of 1540 and 2150keV positrons and also 603 and 1691keV γ rays. Annihilation of each positron in biological tissue results in two 511keV γ ray photons, which are detected in PET imaging. 124I has an intrinsic spatial resolution loss of 2.3 mm [17]. Both the positron and γ emissions have energies sufficiently high for therapy. 8H9 is a monoclonal antibody that binds to membrane protein B7-H3, which is expressed in over 90% of high-grade pontine glioma but not by normal brain tissue [52]. In principle, this antibody conjugated to 124I potentiates the anti-neoplastic effects of the radionuclide by directing therapeutic irradiation preferentially to cancer cells.
This study recruits patients with DIPG ages 3-21 years old. The enrolled patients will have undergone standard external beam radiation therapy but have not shown signs of progression. Safety and tolerability are the primary endpoints. Uniquely, this study uses PET to image drug distribution and calculate absorbed dose, which provide invaluable information to correlate with tolerability and therapeutic response. The usefulness of BrainLab’s iPlan Flow software package in CED planning in the brainstem is also being assessed as a secondary objective.
The originally planned dose levels of 0.25, 0.5 and 1.0mCi of 124I-8H9 were completed in 2014. The trial has been extended to dose levels of 2.5, 3.25 and 4.0mCi. As of summer 2015, the trial was at the 4.0mCi dose level, and none of the 20 treated patient experienced dose-limiting toxicities.
Determining Vd is important in assessing the efficacy of CED treatment. In addition to PET imaging, this study also explores Vd determination using MRI and various image processing methods. PET and intraoperative MRI data from 10 patients showed that the Vd/Vi ratio in this situation is in the range of 2.5 to 3.0.
5. Future Directions
CED of therapeutic agents in the treatment of malignant brain tumors has shown considerable promise in preclinical and some clinical studies. Future advances in CED for DIPG treatment will occur on two fronts: the selection or development of therapeutic agents for delivery via CED, and the improvement of the technique of CED.
Even though CED is considered by many as a passive mechanical process, this does not appear to be true considering that the neural tissue will certainly respond to the hydraulic pressure as well as the substance being infused. Indeed, our study showed that when infusion was performed into the rat striatum, cells take up the infusion solution and proteins in the solution (Zhou et al. unpublished data presented at the 82nd AANS Annual Scientific Meeting, 2014), indicating that CED is a cellularly active process. This process may impact a drug’s specificity and toxicity when used in CED, and should be taken into consideration when choosing a drug for delivery using CED.
In choosing an antineoplastic agent for CED in the brainstem, one must consider the delicate structures of the brainstem. Not only potential toxicities to the pontine gray, but also to the transverse and longitudinal fiber bundles should be considered. Some toxicities are on-target effects of an antineoplastic drug, which may be predicted, whereas others are off-target effects, which can only be discovered by careful testing. Vinca alkaloids, especially vincristine, cause massive CNS demyelination at therapeutic concentrations, and should be avoided for infusion into the brainstem. Taxane-family microtubule inhibitors and platinum-based antineoplastic drugs are the other two classes of drugs that have high risks in causing severe demyelination. Most members in the classes of antimetabolites, antibiotics, alkylating agents and topoisomerase inhibitors are less likely to cause severe neurotoxicity. In short, any agents that are planned for brainstem infusion in patients should be carefully studied for safety in animals.
One promising advance in the development of novel therapeutic agents for the treatment of DIPG is the recent molecular characterization of this tumor. DIPGs are genetically complex and distinct from both adult and childhood supratentorial high-grade gliomas. Recent evidence points to platelet-derived growth factor (PDGF) and its receptors (PDGFR) as among the major driving forces of tumori- genesis in the majority of cases [53-57]. Another growth factor receptor, epidermal growth factor receptor (EGFR), shows strong immunohistochemistry staining in about 27% of cases [54], and amplification of the gene at a rate of 7-9% [54, 56]. Unlike the case in childhood supratentorial high-grade gliomas, CDKN2A deletion is non-existent [56, 58] or only occurs at a low rate (3%) [55] in DIPG. Amplification of CDK4 and CDK6 in DIPG occurs at a rate of 7% and 11.6%, respectively [56]. Approximately 50% of DIPG have TP53 mutations [59, 60] and three groups report loss of a region of 17p containing the TP53 gene in 31%, 57% and 64% of cases, respectively [54, 58, 61]. In approximately 50% of DIPG patients, allelic loss of a region of 10q where the PTEN gene is located is observed [61-63].
Histone H3, which forms part of the nucleosome core, plays an essential role in the epigenetic regulation of DNA replication and gene transcription. Recent studies of histone mutations indicate DIPGs are also epigenetically distinct from pediatric supratentorial HGG. Recurrent adenine-to-thymine transversions in the H3F3A gene, encoding a lysine-to-methionine missense at position 27 (K27M) of histone H3.3, is seen in 60-75% of DIPGs [64, 65], significantly higher than that in pediatric supratentorial glioblastoma multiforme (GBM) (14-19%) [64, 66]. The H3F3A mutation is not present in the matching germline DNA samples [64], suggesting its somatic nature. The K27M mutation is found in 66-77% of pretreatment DIPG samples [64, 65], indicating that it is not the result of a selection or mutation process secondary to therapies.
In contrast to the H3.3K27M mutation profiles, a guanine-to-adenine transition in H3F3A, resulting in a glycine-to-arginine missense at position 34 (G34R) of H3.3, is identified in 10-14% of pediatric supratentorial GBMs [64, 66] but not in any of the 90 DIPG samples analyzed by two groups [64, 65].
The presence of mutations in the HIST1H3B gene, which encodes histone H3.1, is less conclusive. One study found that the adenine-to-thymine transversion that encodes the K27M missense was present in 18% (9/50) of DIPGs [64], whereas another group did not detect the mutation in any of their DIPG samples (0/27) [65].
It is thought that inhibition of the histone methyltransferase Polycomb repressive complex 2 (PRC2) and hypomethylation of H3K27 play an important role in the effects of H3.3K27M mutation on tumorigenesis of DIPG [67]. However, there is also evidence that activation of PRC2 and hypermethylation of H3K27 may be driving the initiation of medulloblastoma [68], ependymoma [69] and lymphoma [70], suggesting that rebalancing this pathway for therapeutic purposes may not be an easy task.
Some of the mutations observed in DIPG are targetable, such as the PDGFR pathway, and many others are not yet currently. As long as a molecule is differentially expressed between tumor cells and normal tissue, it does not need to be growth-promoting to be considered a therapeutic target. One example is IL-13Rα2. Like in adult malignant gliomas, IL-13Rα2 is highly expressed in DIPG [50, 51], therefore recombinant toxins using IL-13 as a targeting moiety are also potentially effective therapeutic agents for DIPG. The safety of CED of IL13-PE38QQR in the brainstem has been investigated by our group in preclinical studies [23] and a clinical trial sponsored by the NINDS is studying this agent in DIPG patients.
Increasing evidence shows that each individual tumor harbors multiple mutations. For instance, there are on average 60 mutations per glioblastoma [71]. There is no reason to believe DIPG contains a much smaller number of mutations than other malignant gliomas. Targeting one therapeutic target rarely causes death to 100% of the cancer cells. Receptor tyrosine kinases, downstream and parallel signal transduction pathways may be regulated in a compensatory fashion that reduce the chance of cell death when the tumor is treated aimed at only one therapeutic target. Therefore, it is not surprising that drug resistance has been inevitable in almost all single-drug targeted therapies. We believe it is worthwhile to characterize parallel and downstream signal transduction pathways in DIPG and devise multi-targeting therapeutic regimens based on such characterization.
Even though biopsy of DIPG is far from being routine, when these targeted therapies based on molecular profiling of tumors come to clinical use, it would be ideal for the tumor to be pre-screened for the targets that the drugs are designed for.
On the technical front of the delivery method, there is a need for better designed cannulas and easily implementable accurate stereotactic placement of cannulas into the tumor to achieve optimal drug distribution. The use of computer algorithms may help in planning the cannula placement and infusion parameters, taking into account anatomical structures and structural changes induced by the disease and prior treatment. Perhaps more important, imaging should accompany CED to ensure effective drug distribution and concentration as well as to determine how long the therapeutic agents are retained in the tumor and tumor-infiltrated brain tissue in individual patients. This requires the improvement of current imaging techniques or the development of new imaging methods. As discussed above, the current single session CED may not be sufficient for DIPG treatment. Clinically feasible methods to deliver multiple sessions of CED or continuous CED lasting up to several weeks is desired to allow the implementation of optimal time sequence. This will require the development and engineering of catheters suitable for these purposes, and desirably also pumps that can be embedded to allow patients to remain ambulatory while undergoing continuous CED.
CED-based therapies for DIPG will continue to evolve with new understanding of the technique and the disease, and additional preclinical and clinical research is needed to address the current insufficiency.
Fig. (1).
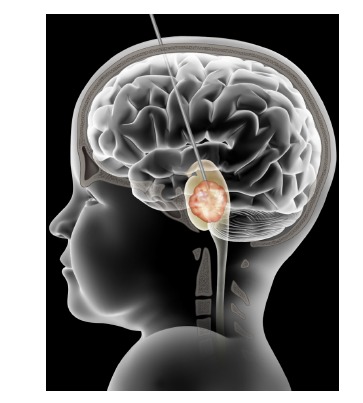
Illustration demonstrating convection-enhanced delivery (CED) in the treatment of diffuse intrinsic pontine glioma (DIPG). An infusion cannula is inserted through the transfrontal extraventricular approach into the pons. The tip of the cannula will be at or near the center of the tumor. This is achieved by image-guided high-precision stereotaxy. With the cannula in place, drugs are infused into the pons driven by a precision pump. Ideally, the drug infused area encompasses the tumor and the surrounding infiltrated area.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Bobo R.H., Laske D.W., Akbasak A., Morrison P.F., Dedrick R.L., Oldfield E.H. Convection-enhanced delivery of macro- molecules in the brain. Proc. Natl. Acad. Sci. USA. 1994;91(6):2076–2080. doi: 10.1073/pnas.91.6.2076. [http://dx.doi.org/10.1073/pnas.91.6.2076]. [PMID: 8134351]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen M.Y., Hoffer A., Morrison P.F., Hamilton J.F., Hughes J., Schlageter K.S., Lee J., Kelly B.R., Oldfield E.H. Surface properties, more than size, limiting convective distribution of virus-sized particles and viruses in the central nervous system. J. Neurosurg. 2005;103(2):311–319. doi: 10.3171/jns.2005.103.2.0311. [http://dx.doi.org/10.3171/ jns.2005.103.2.0311]. [PMID: 16175862]. [DOI] [PubMed] [Google Scholar]
- 3.Saito R., Krauze M.T., Noble C.O., Tamas M., Drummond D.C., Kirpotin D.B., Berger M.S., Park J.W., Bankiewicz K.S. Tissue affinity of the infusate affects the distribution volume during convection-enhanced delivery into rodent brains: implications for local drug delivery. J. Neurosci. Methods. 2006;154(1-2):225–232. doi: 10.1016/j.jneumeth.2005.12.027. [http://dx.doi.org/10.1016/j.jneumeth.2005.12.027]. [PMID: 16472868]. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen J.B., Sanchez-Pernaute R., Cunningham J., Bankiewicz K.S. Convection-enhanced delivery of AAV-2 combined with heparin increases TK gene transfer in the rat brain. Neuroreport. 2001;12(9):1961–1964. doi: 10.1097/00001756-200107030-00037. [http://dx.doi.org/10.1097/00001756-200107030-00037]. [PMID: 11435930]. [DOI] [PubMed] [Google Scholar]
- 5.Saito R., Bringas J.R., McKnight T.R., Wendland M.F., Mamot C., Drummond D.C., Kirpotin D.B., Park J.W., Berger M.S., Bankiewicz K.S. Distribution of liposomes into brain and rat brain tumor models by convection-enhanced delivery monitored with magnetic resonance imaging. Cancer Res. 2004;64(7):2572–2579. doi: 10.1158/0008-5472.can-03-3631. [http://dx.doi.org/10.1158/0008-5472.CAN-03-3631]. [PMID: 15059914]. [DOI] [PubMed] [Google Scholar]
- 6.Perlstein B., Ram Z., Daniels D., Ocherashvilli A., Roth Y., Margel S., Mardor Y. Convection-enhanced delivery of maghemite nanoparticles: Increased efficacy and MRI monitoring. Neuro-oncol. 2008;10(2):153–161. doi: 10.1215/15228517-2008-002. [http://dx.doi.org/10.1215/15228517-2008-002]. [PMID: 18316474]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mardor Y., Rahav O., Zauberman Y., Lidar Z., Ocherashvilli A., Daniels D., Roth Y., Maier S.E., Orenstein A., Ram Z. Convection-enhanced drug delivery: increased efficacy and magnetic resonance image monitoring. Cancer Res. 2005;65(15):6858–6863. doi: 10.1158/0008-5472.CAN-05-0161. [http://dx.doi.org/10.1158/0008-5472.CAN-05-0161]. [PMID: 16061669]. [DOI] [PubMed] [Google Scholar]
- 8.Varenika V., Dickinson P., Bringas J., LeCouteur R., Higgins R., Park J., Fiandaca M., Berger M., Sampson J., Bankiewicz K. Detection of infusate leakage in the brain using real-time imaging of convection-enhanced delivery. J. Neurosurg. 2008;109(5):874–880. doi: 10.3171/JNS/2008/109/11/0874. [http://dx.doi.org/10.3171/JNS/2008/109/11/0874]. [PMID: 18976077]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raghavan R., Brady M.L., Rodríguez-Ponce M.I., Hartlep A., Pedain C., Sampson J.H. Convection-enhanced delivery of therapeutics for brain disease, and its optimization. Neurosurg. Focus. 2006;20(4):E12. doi: 10.3171/foc.2006.20.4.7. [http://dx.doi.org/10.3171/foc.2006. 20.4.7]. [PMID: 16709017]. [DOI] [PubMed] [Google Scholar]
- 10.Oh S., Odland R., Wilson S.R., Kroeger K.M., Liu C., Lowenstein P.R., Castro M.G., Hall W.A., Ohlfest J.R. Improved distribution of small molecules and viral vectors in the murine brain using a hollow fiber catheter. J. Neurosurg. 2007;107(3):568–577. doi: 10.3171/JNS-07/09/0568. [http://dx.doi.org/10.3171/JNS-07/09/0568]. [PMID: 17886557]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sampson J.H., Akabani G., Archer G.E., Berger M.S., Coleman R.E., Friedman A.H., Friedman H.S., Greer K., Herndon J.E., II, Kunwar S., McLendon R.E., Paolino A., Petry N.A., Provenzale J.M., Reardon D.A., Wong T.Z., Zalutsky M.R., Pastan I., Bigner D.D. Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neuro-oncol. 2008;10(3):320–329. doi: 10.1215/15228517-2008-012. [http://dx.doi.org/10.1215/15228517-2008-012]. [PMID: 18403491]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sampson J.H., Archer G., Pedain C., Wembacher-Schröder E., Westphal M., Kunwar S., Vogelbaum M.A., Coan A., Herndon J.E., Raghavan R., Brady M.L., Reardon D.A., Friedman A.H., Friedman H.S., Rodríguez-Ponce M.I., Chang S.M., Mittermeyer S., Croteau D., Puri R.K. Poor drug distribution as a possible explanation for the results of the PRECISE trial. J. Neurosurg. 2010;113(2):301–309. doi: 10.3171/2009.11.JNS091052. [http://dx.doi.org/10.3171/2009.11.JNS091052]. [PMID: 20020841]. [DOI] [PubMed] [Google Scholar]
- 13.Sampson J.H., Raghavan R., Provenzale J.M., Croteau D., Reardon D.A., Coleman R.E., Rodríguez Ponce I., Pastan I., Puri R.K., Pedain C. Induction of hyperintense signal on T2-weighted MR images correlates with infusion distribution from intracerebral convection-enhanced delivery of a tumor-targeted cytotoxin. AJR Am. J. Roentgenol. 2007;188(3):703–709. doi: 10.2214/AJR.06.0428. [http:// dx.doi.org/10.2214/AJR.06.0428]. [PMID: 17312057]. [DOI] [PubMed] [Google Scholar]
- 14.Sampson J.H., Akabani G., Friedman A.H., Bigner D., Kunwar S., Berger M.S., Bankiewicz K.S. Comparison of intratumoral bolus injection and convection-enhanced delivery of radiolabeled antitenascin monoclonal antibodies. Neurosurg. Focus. 2006;20(4):E14. doi: 10.3171/foc.2006.20.4.9. [http://dx.doi.org/10.3171/foc.2006.20.4.9]. [PMID: 16709019]. [DOI] [PubMed] [Google Scholar]
- 15.Sampson J. H., Brady M. L., Petry N. A., Croteau D., Friedman A. H., Friedman H. S., Wong T., Bigner D. D., Pastan I., Puri R. K., Pedain C. Intracerebral infusate distribution by convection-enhanced delivery in humans with malignant gliomas: descriptive effects of target anatomy and catheter positioning. 2007. [DOI] [PubMed]
- 16.Lonser R.R., Warren K.E., Butman J.A., Quezado Z., Robison R.A., Walbridge S., Schiffman R., Merrill M., Walker M.L., Park D.M., Croteau D., Brady R.O., Oldfield E.H. Real-time image-guided direct convective perfusion of intrinsic brainstem lesions. Technical note. J. Neurosurg. 2007;107(1):190–197. doi: 10.3171/JNS-07/07/0190. [http://dx.doi.org/10.3171/JNS-07/07/0190]. [PMID: 17639894]. [DOI] [PubMed] [Google Scholar]
- 17.Pagani M., Stone-Elander S., Larsson S.A. Alternative positron emission tomography with non-conventional positron emitters: effects of their physical properties on image quality and potential clinical applications. Eur. J. Nucl. Med. 1997;24(10):1301–1327. doi: 10.1007/s002590050156. [http://dx.doi.org/10.1007/s002590050156]. [PMID: 9323273]. [DOI] [PubMed] [Google Scholar]
- 18.Heiss J.D., Walbridge S., Asthagiri A.R., Lonser R.R. Image-guided convection-enhanced delivery of muscimol to the primate brain. J. Neurosurg. 2010;112(4):790–795. doi: 10.3171/2009.7.JNS09652. [http://dx.doi.org/ 10.3171/2009.7.JNS09652]. [PMID: 19715424]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Croteau D., Walbridge S., Morrison P.F., Butman J.A., Vortmeyer A.O., Johnson D., Oldfield E.H., Lonser R.R. Real-time in vivo imaging of the convective distribution of a low-molecular-weight tracer. J. Neurosurg. 2005;102(1):90–97. doi: 10.3171/jns.2005.102.1.0090. [http://dx.doi.org/10.3171/jns.2005.102.1.0090]. [PMID: 15658101]. [DOI] [PubMed] [Google Scholar]
- 20.Lonser R.R., Walbridge S., Garmestani K., Butman J.A., Walters H.A., Vortmeyer A.O., Morrison P.F., Brechbiel M.W., Oldfield E.H. Successful and safe perfusion of the primate brainstem: in vivo magnetic resonance imaging of macromolecular distribution during infusion. J. Neurosurg. 2002;97(4):905–913. doi: 10.3171/jns.2002.97.4.0905. [http://dx.doi.org/10.3171/jns.2002.97.4.0905]. [PMID: 12405380]. [DOI] [PubMed] [Google Scholar]
- 21.Sampson J.H., Raghavan R., Brady M.L., Provenzale J.M., Herndon J.E., II, Croteau D., Friedman A.H., Reardon D.A., Coleman R.E., Wong T., Bigner D.D., Pastan I., Rodríguez-Ponce M.I., Tanner P., Puri R., Pedain C. Clinical utility of a patient-specific algorithm for simulating intracerebral drug infusions. Neuro-oncol. 2007;9(3):343–353. doi: 10.1215/15228517-2007-007. [http://dx.doi.org/ 10.1215/15228517-2007-007]. [PMID: 17435179]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandberg D.I., Edgar M.A., Souweidane M.M. Convection-enhanced delivery into the rat brainstem. J. Neurosurg. 2002;96(5):885–891. doi: 10.3171/jns.2002.96.5.0885. [http://dx.doi.org/10.3171/jns.2002.96.5.0885]. [PMID: 12005396]. [DOI] [PubMed] [Google Scholar]
- 23.Souweidane M.M., Occhiogrosso G., Mark E.B., Edgar M.A. Interstitial infusion of IL13-PE38QQR in the rat brain stem. J. Neurooncol. 2004;67(3):287–293. doi: 10.1023/b:neon.0000024219.47447.91. [http://dx.doi.org/10.1023/B: NEON.0000024219.47447.91]. [PMID: 15164984]. [DOI] [PubMed] [Google Scholar]
- 24.Souweidane M.M., Occhiogrosso G., Mark E.B., Edgar M.A., Dunkel I.J. Interstitial infusion of carmustine in the rat brain stem with systemic administration of O6-benzylguanine. J. Neurooncol. 2004;67(3):319–326. doi: 10.1023/b:neon.0000024242.59770.7a. [http://dx.doi.org/10.1023/B:NEON.0000024242. 59770.7a]. [PMID: 15164987]. [DOI] [PubMed] [Google Scholar]
- 25.Luther N., Cheung N.K., Dunkel I.J., Fraser J.F., Edgar M.A., Gutin P.H., Souweidane M.M. Intraparenchymal and intratumoral interstitial infusion of anti-glioma monoclonal antibody 8H9. Neurosurgery. 2008;63(6):1166–1174. doi: 10.1227/01.NEU.0000334052.60634.84. [http://dx.doi.org/10.1227/ 01.NEU.0000334052.60634.84]. [PMID: 19057330]. [DOI] [PubMed] [Google Scholar]
- 26.Luther N., Cheung N.K., Souliopoulos E.P., Karampelas I., Bassiri D., Edgar M.A., Guo H.F., Pastan I., Gutin P.H., Souweidane M.M. Interstitial infusion of glioma-targeted recombinant immunotoxin 8H9scFv-PE38. Mol. Cancer Ther. 2010;9(4):1039–1046. doi: 10.1158/1535-7163.MCT-09-0996. [http://dx.doi.org/10.1158/1535-7163.MCT-09-0996]. [PMID: 20371725]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luther N., Zhou Z., Zanzonico P., Cheung N.K., Humm J., Edgar M.A., Souweidane M.M. The potential of theragnostic 124I-8H9 convection-enhanced delivery in diffuse intrinsic pontine glioma. Neuro-oncol. 2014;16(6):800–806. doi: 10.1093/neuonc/not298. [http://dx.doi.org/10. 1093/neuonc/not298]. [PMID: 24526309]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho S.L., Singh R., Zhou Z., Lavi E., Souweidane M.M. Toxicity evaluation of prolonged convection-enhanced delivery of small-molecule kinase inhibitors in naive rat brainstem. Childs Nerv. Syst. 2014;31(2):221–226. doi: 10.1007/s00381-014-2568-3. [PMID: 25269544]. [DOI] [PubMed] [Google Scholar]
- 29.Giese H., Hoffmann K.T., Winkelmann A., Stockhammer F., Jallo G.I., Thomale U.W. Precision of navigated stereotactic probe implantation into the brainstem. J. Neurosurg. Pediatr. 2010;5(4):350–359. doi: 10.3171/2009.10.PEDS09292. [http://dx.doi.org/10.3171/2009.10.PEDS09292]. [PMID: 20367339]. [DOI] [PubMed] [Google Scholar]
- 30.Pincus D.W., Richter E.O., Yachnis A.T., Bennett J., Bhatti M.T., Smith A. Brainstem stereotactic biopsy sampling in children. J. Neurosurg. 2006;104(2) Suppl.:108–114. doi: 10.3171/ped.2006.104.2.108. [PMID: 16506498]. [DOI] [PubMed] [Google Scholar]
- 31.Roujeau T., Machado G., Garnett M.R., Miquel C., Puget S., Geoerger B., Grill J., Boddaert N., Di Rocco F., Zerah M., Sainte-Rose C. Stereotactic biopsy of diffuse pontine lesions in children. J. Neurosurg. 2007;107(1) Suppl.:1–4. doi: 10.3171/PED-07/07/001. [PMID: 17647306]. [DOI] [PubMed] [Google Scholar]
- 32.Barua N.U., Lowis S.P., Woolley M. OSullivan, S.; Harrison, R.; Gill, S.S. Robot-guided convection-enhanced delivery of carboplatin for advanced brainstem glioma. Acta Neurochir. (Wien) 2013;155(8):1459–1465. doi: 10.1007/s00701-013-1700-6. [http://dx.doi.org/10.1007/s00701-013-1700-6]. [PMID: 23595829]. [DOI] [PubMed] [Google Scholar]
- 33.Chittiboina P., Heiss J.D., Warren K.E., Lonser R.R. Magnetic resonance imaging properties of convective delivery in diffuse intrinsic pontine gliomas. J. Neurosurg. Pediatr. 2014;13(3):276–282. doi: 10.3171/2013.11.PEDS136. [http://dx.doi.org/10.3171/2013.11.PEDS136]. [PMID: 24410126]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson R.C., Kennedy B., Yanes C.L., Garvin J., Needle M., Canoll P., Feldstein N.A., Bruce J.N. Convection-enhanced delivery of topotecan into diffuse intrinsic brainstem tumors in children. J. Neurosurg. Pediatr. 2013;11(3):289–295. doi: 10.3171/2012.10.PEDS12142. [http://dx.doi.org/10. 3171/2012.10.PEDS12142]. [PMID: 23240851]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weber F., Asher A., Bucholz R., Berger M., Prados M., Chang S., Bruce J., Hall W., Rainov N.G., Westphal M., Warnick R.E., Rand R.W., Floeth F., Rommel F., Pan H., Hingorani V.N., Puri R.K. Safety, tolerability, and tumor response of IL4-Pseudomonas exotoxin (NBI-3001) in patients with recurrent malignant glioma. J. Neurooncol. 2003;64(1-2):125–137. doi: 10.1007/BF02700027. [http://dx.doi.org/10.1007/BF02700027]. [PMID: 12952293]. [DOI] [PubMed] [Google Scholar]
- 36.Lidar Z., Mardor Y., Jonas T., Pfeffer R., Faibel M., Nass D., Hadani M., Ram Z. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a phase I/II clinical study. J. Neurosurg. 2004;100(3):472–479. doi: 10.3171/jns.2004.100.3.0472. [http://dx.doi.org/ 10.3171/jns.2004.100.3.0472]. [PMID: 15035283]. [DOI] [PubMed] [Google Scholar]
- 37.Krauze M.T., Noble C.O., Kawaguchi T., Drummond D., Kirpotin D.B., Yamashita Y., Kullberg E., Forsayeth J., Park J.W., Bankiewicz K.S. Convection-enhanced delivery of nanoliposomal CPT-11 (irinotecan) and PEGylated liposomal doxorubicin (Doxil) in rodent intracranial brain tumor xenografts. Neuro-oncol. 2007;9(4):393–403. doi: 10.1215/15228517-2007-019. [http://dx.doi.org/10.1215/ 15228517-2007-019]. [PMID: 17652269]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Debinski W., Gibo D.M., Hulet S.W., Connor J.R., Gillespie G.Y. Receptor for interleukin 13 is a marker and therapeutic target for human high-grade gliomas. Clin. Cancer Res. 1999;5(5):985–990. [PMID: 10353730]. [PubMed] [Google Scholar]
- 39.Debinski W., Gibo D.M., Slagle B., Powers S.K., Gillespie G.Y. Receptor for interleukin 13 is abundantly and specifically over-expressed in patients with glioblastoma multiforme. Int. J. Oncol. 1999;15(3):481–486. doi: 10.3892/ijo.15.3.481. [PMID: 10427128]. [DOI] [PubMed] [Google Scholar]
- 40.Johnson V.G., Wilson D., Greenfield L., Youle R.J. The role of the diphtheria toxin receptor in cytosol translocation. J. Biol. Chem. 1988;263(3):1295–1300. [PMID: 3257214]. [PubMed] [Google Scholar]
- 41.Weaver M., Laske D.W. Transferrin receptor ligand-targeted toxin conjugate (Tf-CRM107) for therapy of malignant gliomas. J. Neurooncol. 2003;65(1):3–13. doi: 10.1023/a:1026246500788. [http://dx.doi.org/10.1023/ A:1026246500788]. [PMID: 14649881]. [DOI] [PubMed] [Google Scholar]
- 42.Torp S.H., Helseth E., Dalen A., Unsgaard G. Epidermal growth factor receptor expression in human gliomas. Cancer Immunol. Immunother. 1991;33(1):61–64. doi: 10.1007/BF01742530. [http://dx.doi.org/10.1007/ BF01742530]. [PMID: 2021959]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Debinski W., Obiri N.I., Pastan I., Puri R.K. A novel chimeric protein composed of interleukin 13 and Pseudomonas exotoxin is highly cytotoxic to human carcinoma cells expressing receptors for interleukin 13 and interleukin 4. J. Biol. Chem. 1995;270(28):16775–16780. doi: 10.1074/jbc.270.28.16775. [http://dx.doi.org/10.1074/jbc.270.28.16775]. [PMID: 7622490]. [DOI] [PubMed] [Google Scholar]
- 44.Kunwar S., Prados M.D., Chang S.M., Berger M.S., Lang F.F., Piepmeier J.M., Sampson J.H., Ram Z., Gutin P.H., Gibbons R.D., Aldape K.D., Croteau D.J., Sherman J.W., Puri R.K. Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J. Clin. Oncol. 2007;25(7):837–844. doi: 10.1200/JCO.2006.08.1117. [http://dx.doi.org/10.1200/JCO. 2006.08.1117]. [PMID: 17327604]. [DOI] [PubMed] [Google Scholar]
- 45.Kunwar S., Chang S., Westphal M., Vogelbaum M., Sampson J., Barnett G., Shaffrey M., Ram Z., Piepmeier J., Prados M., Croteau D., Pedain C., Leland P., Husain S.R., Joshi B.H., Puri R.K., Group P.S. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro-oncol. 2010;12(8):871–881. doi: 10.1093/neuonc/nop054. [http://dx.doi.org/10.1093/neuonc/ nop054]. [PMID: 20511192]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu H., Jacobs B.S., Liu J., Prayson R.A., Estes M.L., Barnett G.H., Barna B.P. Interleukin-13 sensitivity and receptor phenotypes of human glial cell lines: non-neoplastic glia and low-grade astrocytoma differ from malignant glioma. Cancer Immunol. Immunother. 2000;49(6):319–324. doi: 10.1007/s002620000110. [http://dx.doi.org/10.1007/ s002620000110]. [PMID: 10946814]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joshi B.H., Plautz G.E., Puri R.K. Interleukin-13 receptor alpha chain: a novel tumor-associated transmembrane protein in primary explants of human malignant gliomas. Cancer Res. 2000;60(5):1168–1172. [PMID: 10728667]. [PubMed] [Google Scholar]
- 48.Madhankumar A.B., Mintz A., Debinski W. Interleukin 13 mutants of enhanced avidity toward the glioma-associated receptor, IL13Ralpha2. Neoplasia. 2004;6(1):15–22. doi: 10.1016/s1476-5586(04)80049-6. [http://dx.doi.org/ 10.1016/S1476-5586(04)80049-6]. [PMID: 15068667]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel S.J., Shapiro W.R., Laske D.W., Jensen R.L., Asher A.L., Wessels B.W., Carpenter S.P., Shan J.S. Safety and feasibility of convection-enhanced delivery of Cotara for the treatment of malignant glioma: initial experience in 51 patients. Neurosurgery. 2005;56(6):1243–1252. doi: 10.1227/01.neu.0000159649.71890.30. [http://dx.doi.org/10.1227/01.NEU. 0000159649.71890.30]. [PMID: 15918940]. [DOI] [PubMed] [Google Scholar]
- 50.Joshi B.H., Puri R.A., Leland P., Varricchio F., Gupta G., Kocak M., Gilbertson R.J., Puri R.K. Identification of interleukin-13 receptor alpha2 chain overexpression in situ in high-grade diffusely infiltrative pediatric brainstem glioma. Neuro-oncol. 2008;10(3):265–274. doi: 10.1215/15228517-2007-066. [http://dx.doi.org/10.1215/15228517-2007-066]. [PMID: 18430795]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okada H., Low K.L., Kohanbash G., McDonald H.A., Hamilton R.L., Pollack I.F. Expression of glioma-associated antigens in pediatric brain stem and non-brain stem gliomas. J. Neurooncol. 2008;88(3):245–250. doi: 10.1007/s11060-008-9566-9. [http://dx.doi.org/10.1007/s11060-008-9566-9]. [PMID: 18324354]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou Z., Luther N., Ibrahim G.M., Hawkins C., Vibhakar R., Handler M.H., Souweidane M.M. B7-H3, a potential therapeutic target, is expressed in diffuse intrinsic pontine glioma. J. Neurooncol. 2013;111(3):257–264. doi: 10.1007/s11060-012-1021-2. [http://dx.doi.org/10.1007/ s11060-012-1021-2]. [PMID: 23232807]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Becher O.J., Hambardzumyan D., Walker T.R., Helmy K., Nazarian J., Albrecht S., Hiner R.L., Gall S., Huse J.T., Jabado N., MacDonald T.J., Holland E.C. Preclinical evaluation of radiation and perifosine in a genetically and histologically accurate model of brainstem glioma. Cancer Res. 2010;70(6):2548–2557. doi: 10.1158/0008-5472.CAN-09-2503. [http://dx.doi.org/10.1158/0008-5472.CAN-09-2503]. [PMID: 20197468]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zarghooni M., Bartels U., Lee E., Buczkowicz P., Morrison A., Huang A., Bouffet E., Hawkins C. Whole-genome profiling of pediatric diffuse intrinsic pontine gliomas highlights platelet-derived growth factor receptor alpha and poly (ADP-ribose) polymerase as potential therapeutic targets. J. Clin. Oncol. 2010;28(8):1337–1344. doi: 10.1200/JCO.2009.25.5463. [http://dx.doi.org/10.1200/JCO.2009.25.5463]. [PMID: 20142589]. [DOI] [PubMed] [Google Scholar]
- 55.Puget S., Philippe C., Bax D.A., Job B., Varlet P., Junier M.P., Andreiuolo F., Carvalho D., Reis R., Guerrini-Rousseau L., Roujeau T., Dessen P., Richon C., Lazar V., Le Teuff G., Sainte-Rose C., Geoerger B., Vassal G., Jones C., Grill J. Mesenchymal transition and PDGFRA amplification/mutation are key distinct oncogenic events in pediatric diffuse intrinsic pontine gliomas. PLoS One. 2012;7(2):e30313. doi: 10.1371/journal.pone.0030313. [http://dx.doi.org/10.1371/ journal.pone.0030313]. [PMID: 22389665]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paugh B.S., Broniscer A., Qu C., Miller C.P., Zhang J., Tatevossian R.G., Olson J.M., Geyer J.R., Chi S.N., da Silva N.S., Onar-Thomas A., Baker J.N., Gajjar A., Ellison D.W., Baker S.J. Genome-wide analyses identify recurrent amplifications of receptor tyrosine kinases and cell-cycle regulatory genes in diffuse intrinsic pontine glioma. J. Clin. Oncol. 2011;29(30):3999–4006. doi: 10.1200/JCO.2011.35.5677. [http://dx.doi.org/10.1200/JCO.2011.35.5677]. [PMID: 21931021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paugh B.S., Qu C., Jones C., Liu Z., Adamowicz-Brice M., Zhang J., Bax D.A., Coyle B., Barrow J., Hargrave D., Lowe J., Gajjar A., Zhao W., Broniscer A., Ellison D.W., Grundy R.G., Baker S.J. Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J. Clin. Oncol. 2010;28(18):3061–3068. doi: 10.1200/JCO.2009.26.7252. [http://dx.doi.org/10. 1200/JCO.2009.26.7252]. [PMID: 20479398]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barrow J., Adamowicz-Brice M., Cartmill M., MacArthur D., Lowe J., Robson K., Brundler M.A., Walker D.A., Coyle B., Grundy R. Homozygous loss of ADAM3A revealed by genome-wide analysis of pediatric high-grade glioma and diffuse intrinsic pontine gliomas. Neuro-oncol. 2011;13(2):212–222. doi: 10.1093/neuonc/noq158. [http://dx. doi.org/10.1093/neuonc/noq158]. [PMID: 21138945]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Badhe P.B., Chauhan P.P., Mehta N.K. Brainstem gliomasa clinicopathological study of 45 cases with p53 immunohisto- chemistry. Indian J. Cancer. 2004;41(4):170–174. [PMID: 15659871]. [PubMed] [Google Scholar]
- 60.Zhang S., Feng X., Koga H., Ichikawa T., Abe S., Kumanishi T. p53 gene mutations in pontine gliomas of juvenile onset. Biochem. Biophys. Res. Commun. 1993;196(2):851–857. doi: 10.1006/bbrc.1993.2327. [http://dx.doi.org/ 10.1006/bbrc.1993.2327]. [PMID: 8240361]. [DOI] [PubMed] [Google Scholar]
- 61.Louis D.N., Rubio M.P., Correa K.M., Gusella J.F., von Deimling A. Molecular genetics of pediatric brain stem gliomas. Application of PCR techniques to small and archival brain tumor specimens. J. Neuropathol. Exp. Neurol. 1993;52(5):507–515. doi: 10.1097/00005072-199309000-00009. [http://dx.doi.org/10.1097/00005072-199309000-00009]. [PMID: 8103086]. [DOI] [PubMed] [Google Scholar]
- 62.Cheng Y., Wu H. Zhonghua Bing Li Xue Za Zhi. 1999;28(3):165–168. [Recent advances on molecular biology of diffuse astrocytoma]. [Recent advances on molecular biology of diffuse astrocytoma]. [PMID: 12790137]. [PubMed] [Google Scholar]
- 63.Cheng Y., Ng H.K., Zhang S.F., Ding M., Pang J.C., Zheng J., Poon W.S. Genetic alterations in pediatric high-grade astrocytomas. Hum. Pathol. 1999;30(11):1284–1290. doi: 10.1016/s0046-8177(99)90057-6. [http://dx.doi.org/10.1016/ S0046-8177(99)90057-6]. [PMID: 10571506]. [DOI] [PubMed] [Google Scholar]
- 64.Wu G., Broniscer A., McEachron T.A., Lu C., Paugh B.S., Becksfort J., Qu C., Ding L., Huether R., Parker M., Zhang J., Gajjar A., Dyer M.A., Mullighan C.G., Gilbertson R.J., Mardis E.R., Wilson R.K., Downing J.R., Ellison D.W., Zhang J., Baker S.J. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat. Genet. 2012;44(3):251–253. doi: 10.1038/ng.1102. [http://dx.doi.org/10.1038/ng.1102]. [PMID: 22286216]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khuong-Quang D.A., Buczkowicz P., Rakopoulos P., Liu X.Y., Fontebasso A.M., Bouffet E., Bartels U., Albrecht S., Schwartzentruber J., Letourneau L., Bourgey M., Bourque G., Montpetit A., Bourret G., Lepage P., Fleming A., Lichter P., Kool M., von Deimling A., Sturm D., Korshunov A., Faury D., Jones D.T., Majewski J., Pfister S.M., Jabado N., Hawkins C. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012;124(3):439–447. doi: 10.1007/s00401-012-0998-0. [http://dx.doi.org/ 10.1007/s00401-012-0998-0]. [PMID: 22661320]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schwartzentruber J., Korshunov A., Liu X.Y., Jones D.T., Pfaff E., Jacob K., Sturm D., Fontebasso A.M., Quang D.A., Tönjes M., Hovestadt V., Albrecht S., Kool M., Nantel A., Konermann C., Lindroth A., Jäger N., Rausch T., Ryzhova M., Korbel J.O., Hielscher T., Hauser P., Garami M., Klekner A., Bognar L., Ebinger M., Schuhmann M.U., Scheurlen W., Pekrun A., Frühwald M.C., Roggendorf W., Kramm C., Dürken M., Atkinson J., Lepage P., Montpetit A., Zakrzewska M., Zakrzewski K., Liberski P.P., Dong Z., Siegel P., Kulozik A.E., Zapatka M., Guha A., Malkin D., Felsberg J., Reifenberger G., von Deimling A., Ichimura K., Collins V.P., Witt H., Milde T., Witt O., Zhang C., Castelo-Branco P., Lichter P., Faury D., Tabori U., Plass C., Majewski J., Pfister S.M., Jabado N. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482(7384):226–231. doi: 10.1038/nature10833. [http://dx.doi.org/10.1038/nature10833]. [PMID: 22286061]. [DOI] [PubMed] [Google Scholar]
- 67.Lewis P.W., Müller M.M., Koletsky M.S., Cordero F., Lin S., Banaszynski L.A., Garcia B.A., Muir T.W., Becher O.J., Allis C.D. Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science. 2013;340(6134):857–861. doi: 10.1126/science.1232245. [http://dx.doi.org/10.1126/science.1232245]. [PMID: 23539183]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Northcott P.A., Jones D.T., Kool M., Robinson G.W., Gilbertson R.J., Cho Y.J., Pomeroy S.L., Korshunov A., Lichter P., Taylor M.D., Pfister S.M. Medulloblastomics: the end of the beginning. Nat. Rev. Cancer. 2012;12(12):818–834. doi: 10.1038/nrc3410. [http://dx.doi.org/10. 1038/nrc3410]. [PMID: 23175120]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mack S.C., Witt H., Piro R.M., Gu L., Zuyderduyn S., Stütz A.M., Wang X., Gallo M., Garzia L., Zayne K., Zhang X., Ramaswamy V., Jäger N., Jones D.T., Sill M., Pugh T.J., Ryzhova M., Wani K.M., Shih D.J., Head R., Remke M., Bailey S.D., Zichner T., Faria C.C., Barszczyk M., Stark S., Seker-Cin H., Hutter S., Johann P., Bender S., Hovestadt V., Tzaridis T., Dubuc A.M., Northcott P.A., Peacock J., Bertrand K.C., Agnihotri S., Cavalli F.M., Clarke I., Nethery-Brokx K., Creasy C.L., Verma S.K., Koster J., Wu X., Yao Y., Milde T., Sin-Chan P., Zuccaro J., Lau L., Pereira S., Castelo-Branco P., Hirst M., Marra M.A., Roberts S.S., Fults D., Massimi L., Cho Y.J., Van Meter T., Grajkowska W., Lach B., Kulozik A.E., von Deimling A., Witt O., Scherer S.W., Fan X., Muraszko K.M., Kool M., Pomeroy S.L., Gupta N., Phillips J., Huang A., Tabori U., Hawkins C., Malkin D., Kongkham P.N., Weiss W.A., Jabado N., Rutka J.T., Bouffet E., Korbel J.O., Lupien M., Aldape K.D., Bader G.D., Eils R., Lichter P., Dirks P.B., Pfister S.M., Korshunov A., Taylor M.D. Epigenomic alterations define lethal CIMP-positive ependymomas of infancy. Nature. 2014;506(7489):445–450. doi: 10.1038/nature13108. [http://dx.doi.org/10.1038/nature13108]. [PMID: 24553142]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morin R.D., Johnson N.A., Severson T.M., Mungall A.J., An J., Goya R., Paul J.E., Boyle M., Woolcock B.W., Kuchenbauer F., Yap D., Humphries R.K., Griffith O.L., Shah S., Zhu H., Kimbara M., Shashkin P., Charlot J.F., Tcherpakov M., Corbett R., Tam A., Varhol R., Smailus D., Moksa M., Zhao Y., Delaney A., Qian H., Birol I., Schein J., Moore R., Holt R., Horsman D.E., Connors J.M., Jones S., Aparicio S., Hirst M., Gascoyne R.D., Marra M.A. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat. Genet. 2010;42(2):181–185. doi: 10.1038/ng.518. [http:// dx.doi.org/10.1038/ng.518]. [PMID: 20081860]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parsons D.W., Jones S., Zhang X., Lin J.C., Leary R.J., Angenendt P., Mankoo P., Carter H., Siu I.M., Gallia G.L., Olivi A., McLendon R., Rasheed B.A., Keir S., Nikolskaya T., Nikolsky Y., Busam D.A., Tekleab H., Diaz L.A., Jr, Hartigan J., Smith D.R., Strausberg R.L., Marie S.K., Shinjo S.M., Yan H., Riggins G.J., Bigner D.D., Karchin R., Papadopoulos N., Parmigiani G., Vogelstein B., Velculescu V.E., Kinzler K.W. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. doi: 10.1126/science.1164382. [http://dx.doi.org/10.1126/ science.1164382]. [PMID: 18772396]. [DOI] [PMC free article] [PubMed] [Google Scholar]


