Abstract
Ethanol is a biofuel used worldwide. However, the presence of excessive water either during the distillation process or by fraudulent adulteration is a major concern in the use of ethanol fuel. High water levels may cause engine malfunction, in addition to being considered illegal. Here, we describe the development of a simple, fast and accurate platform based on nanostructured sensors to evaluate ethanol samples. The device fabrication is facile, based on standard microfabrication and thin-film deposition methods. The sensor operation relies on capacitance measurements employing a parallel plate capacitor containing a conformational aluminum oxide (Al2O3) thin layer (15 nm). The sensor operates over the full range water concentration, i.e., from approximately 0% to 100% vol. of water in ethanol, with water traces being detectable down to 0.5% vol. These characteristics make the proposed device unique with respect to other platforms. Finally, the good agreement between the sensor response and analyses performed by gas chromatography of ethanol biofuel endorses the accuracy of the proposed method. Due to the full operation range, the reported sensor has the technological potential for use as a point-of-care analytical tool at gas stations or in the chemical, pharmaceutical, and beverage industries, to mention a few.
The limited amount of fossil fuels in the globe and their respective pollution concerns have led to the search for greener and efficient alternatives for energy production1,2. Among the possibilities, ethanol biofuel has been one of the main choices for combustion engines due to its suitable energy efficiency (about 70% of the gasoline power per gallon) along with lower greenhouse gas emission rates1,2. In addition, ethanol can be produced from renewable sources such as corn and sugarcane, which helps to reduce the emission of air pollutants by means of crop carbon sequestration2. In the United States, ethanol has been mainly used as a gasoline additive (usually at 10% vol.), while in Brazil flexible cars are able to run with either gasoline/ethanol mixture (about 25% vol. of anhydrous ethanol fuel - AEF) or simply ethanol (hydrated ethanol fuel - HEF). The main issue in ethanol as vehicle fuel, however, is its ease of adulteration by the addition of water above the standard values, which may cause engine malfunction3. The fraud is difficult to notice given the high water/ethanol miscibility and colorless aspect of the mixture. According to the Brazilian regulatory agency, the water content in AEF cannot exceed 0.5% vol., while the hydrated fuel must not contain more than 6.0% vol. of water4. In Brazil, the addition of water in ethanol fuel above the standards is considered illegal, resulting in the application of a fine and other administrative punishments. The contamination of ethanol by water is a major concern also during its distillation procedure, which is critical for fuel production as well as in chemical, pharmaceutical and beverage industries. In this scenario, the development of simple, accurate and fast methods for ethanol evaluation is of major importance.
Ethanol can be evaluated, for example, at gas stations, using a hydrometer. Although simple and fast, such method is subject to human errors during the reading of the density values. The standard laboratory method for the determination of water in ethanol is the Karl-Fischer titration (ASTM E203), which although accurate, is slow, destructive and requires skilled personnel5. In this sense, alternative methods for the evaluation of ethanol have been developed, such as optical sensors and spectroscopic methods6,7,8,9,10, mass-sensitive detectors11 and ultrasonic techniques12. Some of these, however, are complex, expensive, or cannot be made portable for point-of-care usage. Electrical/electrochemical techniques, on the other hand, have been proven to be suitable to monitor analytes in a simpler, cheaper and accurate way3,13,14. Among these, capacitive devices are particularly interesting, because the sensor response can be evaluated at different frequencies, depending on the analyte dielectric properties, and whether the event of interest occurs at the electrode surface or in the solution bulk15. Bueno and Paixão, for example, evaluated the water content in ethanol by means of capacitance measurements using an electronic tongue system14. The reported method, however, has shown to be restricted to water proportions of 5–25% vol., which is a very limited range of water percentage with a relatively high minimum concentration (5% vol.). In addition, their approach utilizes bare copper electrodes and ultrapure water as the contaminant. All these characteristics make the use of such devices limited for real applications. De Queiroz et al., on the other hand, utilized electronic tongue units coated with different materials to determine the content of tap water in ethanol, which is more realistic considering fuel adulteration, for instance3. From capacitance measurements, they were able to quantify the water content varying from 0% to 20% vol. in real ethanol biofuel samples.
In the following, we report the evaluation of ethanol employing a novel capacitive sensor concept based on a nanostructured solid-liquid interface. The determination of tap water in ethanol is described for the full range water concentration (approximately from 0 to 100% vol.). The reported method relies on the impedance measurements of devices containing Ni electrodes coated with a nanostructured and conformational aluminum oxide (Al2O3) film prepared by atomic layer deposition (ALD). Depending on the water/ethanol concentration, different frequencies can be used to precisely evaluate the sensor response. Such variable frequency operation provides different sensor sensitivities, which can be properly selected according to the user’s need. Here, capacitive devices have shown the capability to detect water traces in anhydrous ethanol down to 0.5%, with a limit of quantification (LoQ) of 3%. The sensor operation is simple, fast and non-destructive. Finally, we employed such sensor in the evaluation of ethanol biofuel acquired from different gas stations. The sensor response was contrasted with the results of gas chromatography (GC) analyses obtained from an accredited analytical laboratory, which confirm the accuracy of the reported method.
Results and Discussion
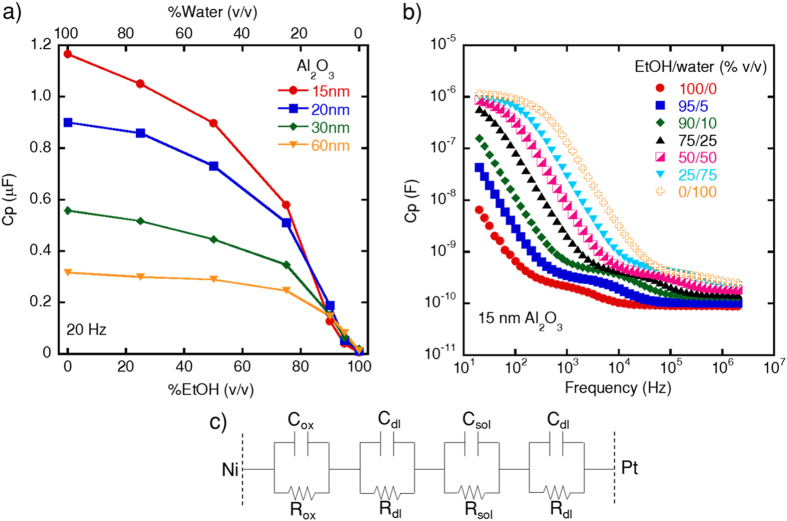
The capacitive device is composed of a parallel plate capacitor containing in one of the plates a thin film of oxide dielectric. Here, Al2O3 has been chosen as the dielectric material to coat electrodes because of its good chemical and thermal stability, large bandgap (~8.8 eV), dielectric permittivity (~9), and the possibility to obtain nanometric and conformational coatings by ALD16. The thickness of Al2O3 thin-films deposited by ALD has been reported as key for the sensitivity of capacitive sensors17. Thus, we evaluated the device response in different anhydrous ethanol (EtOH)/water mixtures varying the thickness of the Al2O3 film in the sensor active area. Solutions at different concentrations were prepared by mixing known contents of tap water and EtOH. The amount of ethanol (or water) is expressed in terms of the volume percent of total solution (water/ethanol mixture). Figure 1a exhibits the measured capacitance (Cp) at 20 Hz as a function of the water/EtOH concentration for 15, 20, 30 and 60 nm of Al2O3. At this particular frequency and for EtOH concentrations ≥90%, all samples presented the same capacitance values. The increase of the water content leads to distinct sensor response for each Al2O3 thickness. Thin films (e.g. 15 nm) have shown to be more susceptible to variations in the water/EtOH concentration than thicker layers (viz. 60 nm) where nearly constant Cp values are observed for a wide range of %EtOH. According to the literature, at low frequencies (<100 Hz), charged species in the water (OH−, H3O+ and ionic impurities) have enough time to drift and form electrical double layers at the interface of metal electrodes in solution18. For electrodes coated with ultrathin oxide films, the electric double layer capacitance (Cdl) has been reported to be non-negligible17. In addition, Cdl is known to be proportional to the solution ionic strength19. Thus, by increasing the content of tap water (8.6 kΩ cm) in the water/EtOH mixture, i.e., the concentration of ionic species in the sensing medium, Cdl is expected to increase. From Fig. 1a, such changes are observed with a more pronounced response in capacitors containing 15 nm of Al2O3. For thick Al2O3 films (e.g. 60 nm), changes in Cdl cannot be detected because of the oxide capacitance (Cox), shown in the equivalent circuit of Fig. 1c, prevails over Cdl. In this case, Cp becomes approximately Cox and a precise discrimination of the water content cannot be achieved. Thus, to detect small variations in the water/EtOH concentration, capacitive sensors containing ultrathin Al2O3 films are more suitable. Among the investigated thicknesses, devices containing 15 nm of Al2O3 exhibited the best response (Fig. 1a). Oxide layers thinner than 15 nm were not used to avoid possible film degradation20. No significant differences in Cp among the tested thicknesses have been observed at higher frequencies (Fig. S1). Finally, for devices absent of the nanostructured Al2O3 coating, small additions of water in highly concentrated EtOH solutions do not provide significant capacitance changes as those found using Al2O3-coated electrodes (Fig. S2). For variations from 0% to 5% vol. of water, for example, capacitance changes (∆Cp) have been found to be 15 times greater for Al2O3-coated sensors in respect to uncoated ones at 20 Hz (Fig. S2).
Figure 1. Electrical characterization of the device in ethanol.
(a) Device capacitance (at 20 Hz) as a function of the water/EtOH concentration for different oxide thicknesses. (b) Capacitance as a function of frequency for different EtOH/water concentrations using a 15 nm-thick Al2O3-coated electrode. (c) The device equivalent circuit. The indexes ox, dl and sol correspond, respectively, to the capacitive and resistive contributions of the oxide, the electrical double layer and the solution bulk to measured impedance.
As the Al2O3 conformational coating plays an import role in the evaluation of water/EtOH mixtures, we assessed the frequency-dependent response of the sensing unit employing a 15 nm-thick Al2O3. Figure 1b shows Cp as a function of frequency (20 Hz–2 MHz) for different water/EtOH mixtures. For samples absent of EtOH, Cp exhibits a plateau at low frequencies (<100 Hz), which according to the equivalent circuit in Fig. 1c can be ascribed to the series sum of Cox and Cdl. Such capacitance plateau occurs at lower frequencies (100 mHz) for the different EtOH concentrations, including 100%EtOH (Fig. S2). This suggests that Cp is approximately Cox at very low frequencies. Thus, from the capacitance plateau (1.1 μF) we can retrieve the Al2O3 relative permittivity (εox) using Eq. 1, where ε0 is the vacuum permittivity, d is the oxide layer thickness (15 nm) and A the device active area (2.05 × 10−4 m2). From Eq. (1), we obtain εox = 9.1, which is in agreement with the literature for Al2O3 films21,22. The possibility to retrieve the oxide relativity permittivity from the capacitance curves corroborates the data interpretation method.
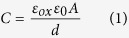 |
The increase of EtOH concentration, i.e., reduction in the tap water content, shifts the capacitance curves towards lower frequencies (Fig. 1b). This is considered a direct consequence of the increase in the solution resistance, as the concentration of ions in the medium reduces (Fig. S3). Such evaluation can be further confirmed by measuring different water/EtOH mixtures using resistive (18 MΩ cm) deionized water (Fig. S4). From Fig. 1b, capacitance changes are particularly evident in the 20 Hz–2 kHz frequency range. At high frequencies (106 Hz), ions in solution are not able to follow the electric field oscillations and Cp becomes the device geometric capacitance with the water/EtOH solution acting as the dielectric medium (Csol). From Fig. 1b, the sum of Cox and Csol in a series association is approximately Csol, given the respective thicknesses (15 nm for the Al2O3 layer and 620 μm for the plate interspace) and relative dielectric permittivities. Therefore, from the capacitance curves at 106 Hz for both 100% EtOH and 100% water samples, we can retrieve the relative permittivity of EtOH (εEtOH) and water (εH2O), respectively. Replacing in Eq. (1) εox by the relative permittivity of the respective solutions, and d by the plate spacing (620 μm), we find εEtOH = 29 and εH2O = 76. These values are in reasonable agreement with the literature, where εEtOH = 25 and εH2O = 79 are found23.
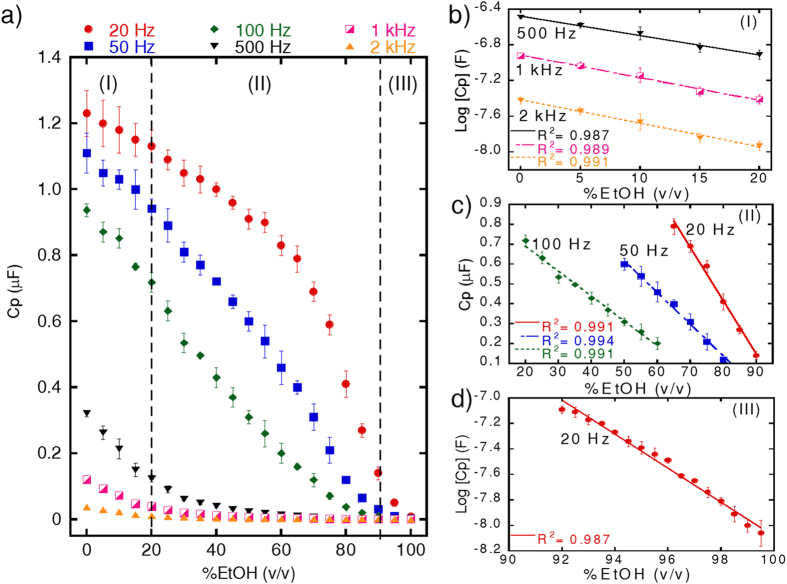
After the device electrical characterization, we evaluated the sensor response in EtOH/water mixtures at different frequencies, as shown in Fig. 2. Six frequency points (20 Hz, 50 Hz, 100 Hz, 500 Hz, 1 kHz and 2 kHz) have been considered to determine the best device operation range. Here, we observe that the device behaves differently at each frequency depending on the water/EtOH concentration. From Fig. 2a, we notice the device response sectored in three operation regions according to the water/EtOH concentration. Region I accounts for EtOH concentrations ranging from 0% to 20% vol.; region II is related to %EtOH varying from 20% to 90% vol.; and region III accounts for %EtOH higher than approximately 90% vol. From the device’s response, we can obtain calibration curves for each of the above-mentioned regions, as shown in Fig. 2b–d. The error bars in the curves correspond to the standard deviation values obtained using six similar devices.
Figure 2. Sensor response for different ethanol/water mixtures.
(a) Cp as a function of the EtOH content (from 0% to 100% vol.). The device response can be sectored in three regions: region I for %EtOH from 0% to 20% vol., region II from 20% to 90% vol. and region III for EtOH concentrations higher than 90% vol. Calibration curves for the device response in the respective regions (b–d). The error bars correspond to the standard deviation values obtained using six similar devices.
From Fig. 2b, we observe that three distinct frequencies, namely 500 Hz, 1 kHz and 2 kHz, provide a similar sensor response for the %EtOH varying from 0% to 20% vol. The three curves present approximately the same slope, i.e., the same sensor sensitivity, which is defined by the amplitude of the output signal per percent of EtOH. From Fig. 2b, we find a mean sensitivity of −0.02 F/%EtOH (in the semi-log scale) for the analyzed frequencies. For %EtOH varying from 20% to 90% vol., a linear response (R2 > 0.99) can be obtained in 20 Hz, 50 Hz, and 100 Hz, depending on the EtOH concentration range (Fig. 2c). At such frequencies, the sensor sensitivity is −2.8 × 10−8 F/%EtOH (20 Hz), −1.7 × 10−8 F/%EtOH (50 Hz) and −1.2 × 10−8 F/%EtOH (100 Hz). Each calibration curve was determined in frequencies where the calculated sensitivity is the highest found for the respective EtOH concentration range. Such variable frequency operation allows one to choose the desired frequency according to the EtOH/water composition in the envisioned application. For an unknown water content, a first screening at the high-frequency region is suggested, followed by the water determination using one of the calibration curves shown. For %EtOH > 90% vol. (Fig. 2d), which is of interest in the evaluation of fuel samples, a sensitivity of −0.13 F/%EtOH (in the semi-log scale) is obtained. Given the sensor sensitivity and the capability to easily to select the operation region, the developed platform may be used as a universal tool to monitor EtOH/water mixtures. From Fig. 2d, we determined the sensor limit of detection (LoD) for water in EtOH as being 0.5% and the LoQ as 3%. In the literature, just a few sensors present similar characteristics that combine operation over full range concentration with low detectable amounts of water. Such devices include electrical sensors based on ZnO nanorod arrays13 and optical fiber-based sensors7,8,9,10. Our device, however, is simpler than the reported methods, in addition to being responsive to tap water, which is more realistic considering fuel adulteration. Finally, we evaluated the sensor response in the presence of methanol to emulate real fuel samples. According to the regulation4, the maximum amount of methanol allowed in bioethanol fuel is 0.5% vol. Thus, we have carried out measurements in highly concentrated ethanol solutions (94.5–95%) having 0.5% vol. of methanol and 4.5–5.0% vol. of water. No differences in the sensor response have been found among samples where 0.5% vol. of methanol is present or absent (Fig. S5). This indicates that methanol, at low concentrations, is not an interferent in the operation of the reported sensor. The proposed method, therefore, possesses the capabilities necessary to evaluate HEF samples, as discussed in the following.
The capacitive sensor was employed in the evaluation of real ethanol biofuel samples acquired from different gas stations. Three identical devices were employed in the analysis of HEF. The sensor response was compared with the results obtained from GC (two measurements). Finally, two HEF samples were also intentionally adulterated with water and further analysed by GC. The results are expressed in terms of the ethanol content, as shown in Table 1.
Table 1. Comparison of responses between the proposed device and GC analyses.
| Sample | Ethanol content (% vol.) |
Deviation | |
|---|---|---|---|
| Sensor response | GC response | ||
| HEF#1 | 94.6 (±0.5) | 95.6 (±0.1) | 1% |
| HEF#2 | 94.1 (±0.4) | 96.4 (±0.1) | 2.4% |
| HEF#3 | 94.4 (±0.5) | 96.1 (±0.1) | 1.8% |
| adulterated HEF#1 | 93.1 (±0.3) | 94.1 (±0.1) | 1.1% |
| adulterated HEF#2 | 83.5 (±0.8) | 85.8 (±0.1) | 2.7% |
From Table 1, the proposed platform has shown to be very precise, presenting low standard deviation values (in average 0.5%). The capacitive devices are also accurate, exhibiting deviations from 1% to 2.7% with respect to the results obtained by GC. Such discrepancies can be attributed to the presence of adventitious contaminants in the fuel (e.g., additives) rather than simply water, in which the reported sensor is able to detect at concentrations down to 0.5% vol. The method described here, however, is simpler and cheaper than GC. In contrast to GC, it does not need skilled personnel for the evaluation of samples, it is a non-destructive technique and it can be made portable for point-of-care analysis of fuel, for example, at gas stations. Regarding the literature for the evaluation of real fuel samples, our sensor is more accurate than the device reported by Bueno and Paixão14. In addition, their lowest quantifiable amount of water is 10% vol., which is higher than the minimum concentration allowed in HEF according to the Brazilian regulation (viz. 6% vol.)4. Our device, on the other hand, is able to detect water concentrations below such a value. De Queiroz et al.3 have also shown to be able to detect such low amounts of water; however, no details about their sensor accuracy have been reported.
The time required for the evaluation of ethanol samples is short, varying from 2 to 15 min. The first analysis requires the sensor to remain immersed in solution for stabilization prior to the electrical measurements, i.e. for a complete frequency sweep (20 to 2 MHz). The whole procedure takes approximately 15 min. Further analyses are faster, taking 2 min to perform the measurements. The sensor has shown to be robust, enduring at least ca. 50 consecutive measurements with the same precision and accuracy reported in Table 1. In addition, the devices have lasted for a minimum of 5 months, with no special care during storage. In the case of eventual failure, the Al2O3 conformational layer can be easily regenerated without compromising neither the substrate nor the metallic parts. Since the whole device fabrication is based on standard microfabrication techniques and thin-film deposition, the sensors can be produced in large scale at low cost. Finally, we believe the reported sensor has the technological potential for the evaluation of ethanol biofuel and other applications utilizing ethanol in the chemical, pharmaceutical and beverage industries.
Conclusion
In this work, we demonstrated a novel platform to evaluate ethanol samples based on nanostructured capacitive devices. The sensor fabrication is simple, relying solely on photolithographic and thin film deposition processes. The sensor active region is composed of a conformational Al2O3 film deposited by ALD. We evaluated the effect of different Al2O3 thickness on the detection of the water content in anhydrous ethanol samples. The evaluation of ethanol is carried out by impedance spectroscopy measurements and the results expressed in terms of the capacitance values. Sensing units containing a 15 nm-thick Al2O3 film allows the monitoring of water content in ethanol over its full range of concentration, i.e., from 0% to 100% vol. of water. To the best of our knowledge, this is the widest range of operation for the water content in ethanol reported using capacitive devices. The proposed sensor exhibited a limit of detection of 0.5% vol. of water in anhydrous ethanol, with a limit of quantification of 3%. In the evaluation of ethanol fuel, the reported device shows an excellent agreement with the results obtained from gas chromatography analyses. The method described here, however, is simpler and cheaper than gas chromatography for the analysis of ethanol. In addition, the reported platform can be incorporated in portable point-of-care systems, for example, to evaluate ethanol fuel along the whole supply chain. We envisage that by combining such platform with proper electronics, the very same sensor could be used to monitor the fuel quality from the biorefinery to the final customer, i.e., from its production to transportation, storage, and commercialization at gas stations. Such a possibility would allow the continuous control of the fuel integrity by real-time data reading and uploading to cloud severs. Other possibilities include the monitoring of ethanol quality, for example, in the chemical, pharmaceutical and beverage industries.
Materials and Methods
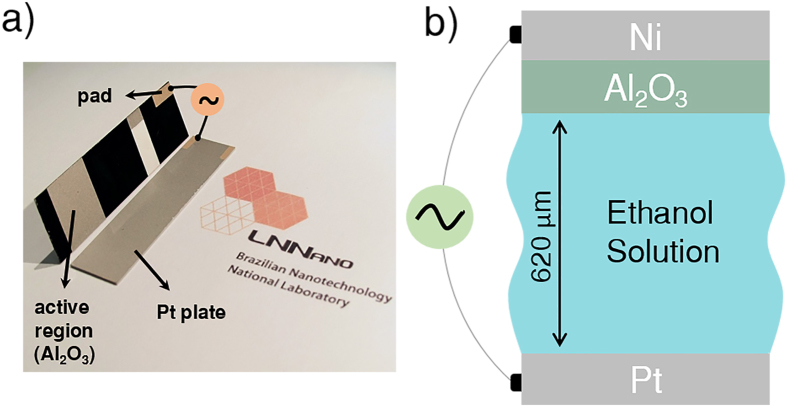
The capacitive sensors (Fig. 3a) were fabricated by photolithography and thin-film deposition on clean alumina substrates (5.5 cm × 1.3 cm). The cleaning procedure starts with rinsing the substrates sequentially in acetone p.a., acetone VLSI (very-large-scale-integration) and in isopropanol VLSI, for 20 min each in ultrasonic bath. Following, the substrates were cleaned in piranha solution 2:1 v/v (H2SO4:H2O2) and oxygen plasma for 3 min (90 W, pressure 0.4 mbar). In one of the plates, thin films of 50 nm Cr (2 Å/s) and 200 nm Ni (1 Å/s) were deposited by e-beam evaporation using a shadow mask to pattern the metallic parts. Contact pads consisting of Cr (20 nm, 2 Å/s) and Au (50 nm, 1 Å/s) were thermally deposited in the region indicated in Fig. 3a. A 25 μm-thick film of AZ nLof 2070 photoresist was spun cast on the surface, followed by a hot plate baking (100 °C, 90 s) and UV exposure (480 mJ/cm2 dose) to define the sensor active area. In this region, Al2O3 films at different thicknesses (15, 20, 30 and 60 nm) were deposited by ALD at 150 °C. Al2O3 films were produced by pulsing alternately the precursor trimethylaluminum (TMA) 97%, acquired from Sigma-Aldrich, and water in the reaction chamber (Cambridge Nanotech Savannah 100 ALD System). The TMA and water reservoirs were kept at room temperature, while all other heating stages, including the sample stage, were kept at 150 °C. The pulse duration was 20 ms, with a waiting time of 15 s. The chamber pressure was set between 10−1–10−2 Torr and Argon was used as the carrier/purging gas at a flow of 5 sccm. Such fabrication conditions provided a film coverage of 1.1 Å/cycle of operation. This method ensures a conformational coating of the Ni electrode. As a result, the ethanol solution will not be in direct contact with the capacitor metallic parts, preserving its integrity. To complete the capacitor architecture, we coupled this unit to a 200 nm-thick Pt electrode in a parallel plate configuration, with a plate separation of approximately 620 μm. The fabrication steps are shown in detail in the Supplementary Information (Fig. S6). Finally, the capacitor is immersed in solution, as shown in Fig. 3b, for the evaluation of samples.
Figure 3. Device layout and equivalent circuit model.
(a) Picture of the sensing device showing the two capacitor plates, (b) the sketch of the capacitor configuration for the evaluation of ethanol samples (not to scale).
The evaluation of ethanol was carried out by electrical impedance measurements using an Agilent E4980A LCR meter. We recorded the device parallel capacitance and resistance values in the 20 Hz–2 MHz frequency range using a sine-wave voltage signal amplitude of 10 mV. All electrical measurements were performed in laboratory ambient with controlled temperature (20 ± 2 °C) and humidity (50%). The device response was analyzed in different frequencies varying the amount of tap water, from 0% to 100% vol., in anhydrous ethanol absolute (EtOH), purity ≥99.9%, acquired from Merck (Germany). The device response was modeled using the equivalent circuit shown in Fig. 1c.
The proposed platform was used to evaluate ethanol biofuel (viz. HEF) acquired from different gas stations. The sensor response was contrasted with the results of GC analyses obtained from an accredited ISO17025 analytical service laboratory. We also performed blind tests, where HEF samples were intentionally adulterated with water by a third party and the ethanol content confirmed by using the proposed device.
Additional Information
How to cite this article: Vello, T. P. et al. A simple capacitive method to evaluate ethanol fuel samples. Sci. Rep. 7, 43432; doi: 10.1038/srep43432 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
The authors acknowledge the Brazilian funding agencies CAPES, CNPq (Project 483550/2013-2) and FAPESP (Projects 2013/22127-2 and 2014/25979-2) for their support and Group of Physics of Nanosystems and Nanostructured Materials at UNICAMP (Brazil) for the use of some facilities.
Footnotes
The authors declare no competing financial interests.
Author Contributions T.P.V. and D.H.S.C. performed the experiments and analyzed the data, R.F.O. discussed results and wrote the manuscript, G.O.S. and D.H.S.C. fabricated the devices, and C.C.B.B. supervised the experiments, discussed the results and lead the work. All authors reviewed and approved the final manuscript.
References
- Hill J., Nelson E., Tilman D., Polasky S. & Tiffany D. Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc. Natl. Acad. Sci. 103, 11206–11210 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A. K. Biofuels (alcohols and biodiesel) applications as fuels for internal combustion engines. Prog. Energy Combust. Sci. 33, 233–271 (2007). [Google Scholar]
- De Queiroz D. P. et al. The use of an e-tongue for discriminating ethanol/water mixtures and determination of their water content. Sens. Actuators B Chem. 230, 566–570 (2016). [Google Scholar]
- Resolution ANP N19 (in Portuguese), available at www.anp.gov.br accessed 06.10.2016 (2015).
- ASTM E203-16, Standard Test Method for Water Using Volumetric Karl Fischer Titration, ASTM International, West Conshohocken, PA, 2016 www.astm.org.
- Xiong F. B. & Sisler D. Determination of low-level water content in ethanol by fiber-optic evanescent absorption sensor. Opt. Commun. 283, 1326–1330 (2010). [Google Scholar]
- Srivastava S. K., Verma R. & Gupta B. D. Surface plasmon resonance based fiber optic sensor for the detection of low water content in ethanol. Sens. Actuators B Chem. 153, 194–198 (2011). [Google Scholar]
- Coradin F. K., Possetti G. R. C., Kamikawachi R. C., Muller M. & Fabris J. L. Etched fiber bragg gratings sensors for water-ethanol mixtures: a comparative study. J. Microw. Optoelectron. Electromagn. Appl. 9, 131–143 (2010). [Google Scholar]
- Possetti G. R. C. et al. Application of a long-period fibre grating-based transducer in the fuel industry. Meas. Sci. Technol. 20, 034012 (2009). [Google Scholar]
- Fujiwara E. et al. Real-time optical fibre sensor for hydro-alcoholic solutions. Meas. Sci. Technol. 21, 094035 (2010). [Google Scholar]
- Kim B., Yamamoto T. & Kim Y. In-Line Measurement of Water Content in Ethanol Using a PVA-Coated Quartz Crystal Microbalance. Sensors 14, 1564–1575 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo M. K.-K., Costa-Felix R. P. B., Maggi L. E., Alvarenga A. V. & Romeiro G. A. Biofuel ethanol adulteration detection using an ultrasonic measurement method. Fuel 91, 209–212 (2012). [Google Scholar]
- Lee Y.-M., Huang C.-M., Chen H.-W. & Yang H.-W. Low temperature solution-processed ZnO nanorod arrays with application to liquid ethanol sensors. Sens. Actuators Phys. 189, 307–312 (2013). [Google Scholar]
- Bueno L. & Paixão T. R. L. C. A copper interdigitated electrode and chemometrical tools used for the discrimination of the adulteration of ethanol fuel with water. Talanta 87, 210–215 (2011). [DOI] [PubMed] [Google Scholar]
- Taylor D. M. & Macdonald A. G. AC admittance of the metal/insulator/electrolyte interface. J. Phys. Appl. Phys. 20, 1277–1283 (1987). [Google Scholar]
- Ding S.-J., Zhang D. W. & Wang L.-K. Atomic-layer-deposited Al2O3-HfO2 laminated and sandwiched dielectrics for metal–insulator–metal capacitors. J. Phys. Appl. Phys. 40, 1072–1076 (2007). [Google Scholar]
- Vello T. P. et al. Hybrid organic/inorganic interfaces as reversible label-free platform for direct monitoring of biochemical interactions. Biosens. Bioelectron. 87, 209–215 (2017). [DOI] [PubMed] [Google Scholar]
- De Oliveira R. F., Merces L., Vello T. P. & Bof Bufon C. C. Water-gated phthalocyanine transistors: Operation and transduction of the peptide–enzyme interaction. Org. Electron. 31, 217–226 (2016). [Google Scholar]
- Israelachvili J. N. In Intermolecular and Surface Forces 291–338 (2011) (Academic Press). [Google Scholar]
- Correa G. C., Bao B. & Strandwitz N. C. Chemical Stability of Titania and Alumina Thin Films Formed by Atomic Layer Deposition. ACS Appl. Mater. Interfaces 7, 14816–14821 (2015). [DOI] [PubMed] [Google Scholar]
- Bof Bufon C. C. et al. Self-Assembled Ultra-Compact Energy Storage Elements Based on Hybrid Nanomembranes. Nano Lett. 10, 2506–2510 (2010). [DOI] [PubMed] [Google Scholar]
- Sharma R. et al. Large-Area Rolled-Up Nanomembrane Capacitor Arrays for Electrostatic Energy Storage. Adv. Energy Mater. 4, 1301631 (2014). [Google Scholar]
- Mohsen-Nia M., Amiri H. & Jazi B. Dielectric Constants of Water, Methanol, Ethanol, Butanol and Acetone: Measurement and Computational Study. J. Solut. Chem. 39, 701–708 (2010). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.