Abstract
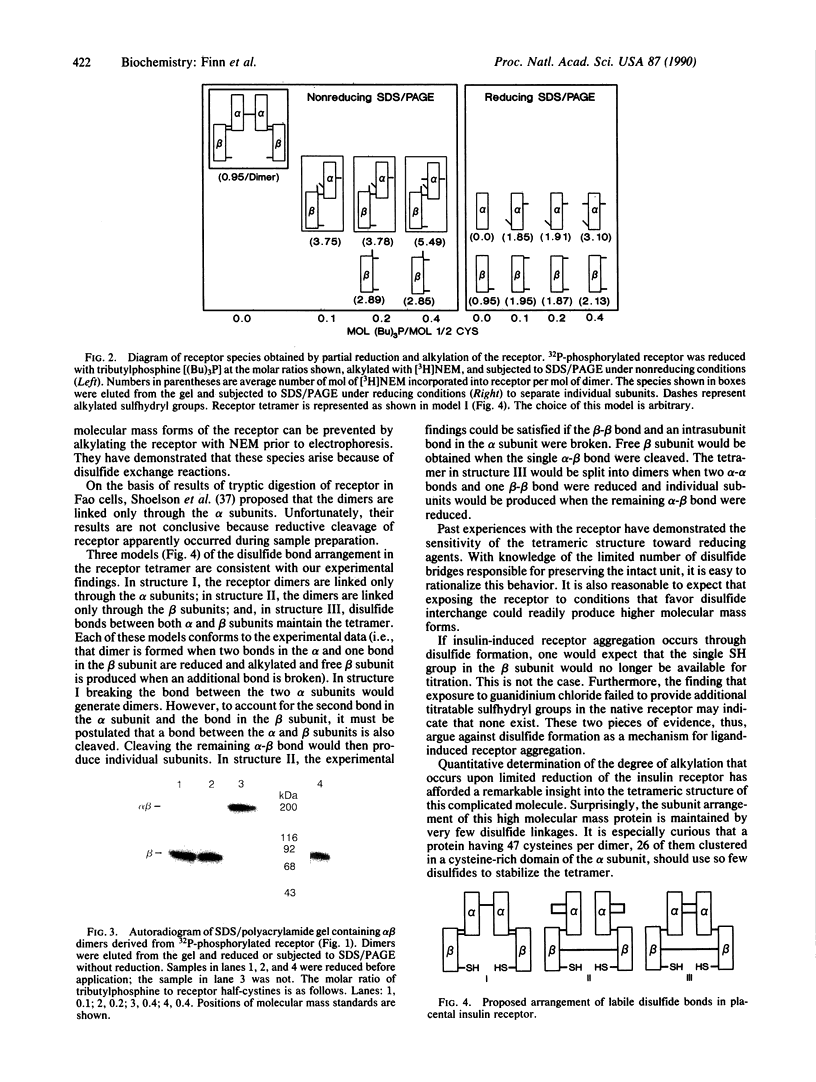
The disulfide crosslinking pattern of human placental insulin receptor was investigated using selective reduction with tributylphosphine followed by alkylation with N-[3H]ethylmaleimide. Insulin receptor contains a single sulfhydryl group in each beta subunit whose alkylation with N-[3H]ethylmaleimide inhibits receptor autophosphorylation. Alkylation is partially inhibited by ATP or the nonhydrolyzable substrate analog adenosine 5'-[beta,gamma-imido]triphosphate when the nucleotides are added as Mn2+ complexes. Neither insulin nor 6 M guanidinium chloride renders additional sulfhydryl groups accessible to alkylation. When the receptor is reduced under drastic conditions with tributylphosphine in guanidinium chloride, 32 of the 37 sulfhydryl groups in the receptor's alpha subunit can be alkylated with N-[3H]ethylmaleimide. Surprisingly only three of the 10 cysteines in the beta subunit become titratable under identical conditions. By using highly selective reducing conditions, we were able to determine quantitatively the maximum number of disulfide bridges that link the two alpha beta halves to form the tetrameric structure and those that couple the alpha to the beta subunits. Liberation of two sulfhydryl groups in the alpha and one in the beta subunit resulted in formation of alpha beta dimers. Free beta subunit was formed when an additional disulfide bond was reduced. It is remarkable that the tetrameric structure of this highly complex receptor molecule, which contains a large number of cysteine residues, is maintained by such a small number of disulfide bonds. Three models of the arrangement of the labile disulfide bonds, consistent with these findings, are proposed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolen J. B., Israel M. A. Inhibition of polyoma virus middle T antigen-associated tyrosyl kinase activity by N-ethylmaleimide. J Biol Chem. 1983 Dec 25;258(24):15135–15140. [PubMed] [Google Scholar]
- Boyle T. R., Campana J., Sweet L. J., Pessin J. E. Subunit structure of the purified human placental insulin receptor. Intramolecular subunit dissociation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1985 Jul 15;260(14):8593–8600. [PubMed] [Google Scholar]
- Brooker R. J., Slayman C. W. Inhibition of the plasma membrane [H+]-ATPase of Neurospora crassa by N-ethylmaleimide. Protection by nucleotides. J Biol Chem. 1982 Oct 25;257(20):12051–12055. [PubMed] [Google Scholar]
- Cuatrecasas P. Isolation of the insulin receptor of liver and fat-cell membranes (detergent-solubilized-( 125 I)insulin-polyethylene glycol precipitation-sephadex). Proc Natl Acad Sci U S A. 1972 Feb;69(2):318–322. doi: 10.1073/pnas.69.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- Finn F. M., Titus G., Horstman D., Hofmann K. Avidin-biotin affinity chromatography: application to the isolation of human placental insulin receptor. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7328–7332. doi: 10.1073/pnas.81.23.7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita-Yamaguchi Y. Characterization of purified insulin receptor subunits. J Biol Chem. 1984 Jan 25;259(2):1206–1211. [PubMed] [Google Scholar]
- Fujita-Yamaguchi Y., Kathuria S. The monomeric alpha beta form of the insulin receptor exhibits much higher insulin-dependent tyrosine-specific protein kinase activity than the intact alpha 2 beta 2 form of the receptor. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6095–6099. doi: 10.1073/pnas.82.18.6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Covalent binding of lipid to protein. Diglyceride and amide-linked fatty acid at the N-terminal end of the murein-lipoprotein of the Escherichia coli outer membrane. Eur J Biochem. 1973 Apr;34(2):284–296. doi: 10.1111/j.1432-1033.1973.tb02757.x. [DOI] [PubMed] [Google Scholar]
- Hedo J. A., Collier E., Watkinson A. Myristyl and palmityl acylation of the insulin receptor. J Biol Chem. 1987 Jan 25;262(3):954–957. [PubMed] [Google Scholar]
- Hempel J., Kaiser R., Jörnvall H. Mitochondrial aldehyde dehydrogenase from human liver. Primary structure, differences in relation to the cytosolic enzyme, and functional correlations. Eur J Biochem. 1985 Nov 15;153(1):13–28. doi: 10.1111/j.1432-1033.1985.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Kirley T. L. Determination of three disulfide bonds and one free sulfhydryl in the beta subunit of (Na,K)-ATPase. J Biol Chem. 1989 May 5;264(13):7185–7192. [PubMed] [Google Scholar]
- Kwok Y. C., Nemenoff R. A., Powers A. C., Avruch J. Kinetic properties of the insulin receptor tyrosine protein kinase: activation through an insulin-stimulated tyrosine-specific, intramolecular autophosphorylation. Arch Biochem Biophys. 1986 Jan;244(1):102–113. doi: 10.1016/0003-9861(86)90098-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Massey D. M., Lenard J. Inactivation of the RNA polymerase of vesicular stomatitis virus by N-ethylmaleimide and protection by nucleoside triphosphates. Evidence for a second ATP binding site on L protein. J Biol Chem. 1987 Jun 25;262(18):8734–8737. [PubMed] [Google Scholar]
- O'Connell P. B., Brady C. J. Polyacrylamide gels with modified cross-linkages. Anal Biochem. 1976 Nov;76(50):63–73. doi: 10.1016/0003-2697(76)90264-5. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Abdulaev N. G., Bogachuk A. S. Two adjacent cysteine residues in the C-terminal cytoplasmic fragment of bovine rhodopsin are palmitylated. FEBS Lett. 1988 Mar 28;230(1-2):1–5. doi: 10.1016/0014-5793(88)80628-8. [DOI] [PubMed] [Google Scholar]
- Perumal N. B., Minkley E. G., Jr The product of the F sex factor traT surface exclusion gene is a lipoprotein. J Biol Chem. 1984 May 10;259(9):5357–5360. [PubMed] [Google Scholar]
- Petruzzelli L., Herrera R., Rosen O. M. Insulin receptor is an insulin-dependent tyrosine protein kinase: copurification of insulin-binding activity and protein kinase activity to homogeneity from human placenta. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3327–3331. doi: 10.1073/pnas.81.11.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilch P. F., Czech M. P. Interaction of cross-linking agents with the insulin effector system of isolated fat cells. Covalent linkage of 125I-insulin to a plasma membrane receptor protein of 140,000 daltons. J Biol Chem. 1979 May 10;254(9):3375–3381. [PubMed] [Google Scholar]
- Pilch P. F., Czech M. P. The subunit structure of the high affinity insulin receptor. Evidence for a disulfide-linked receptor complex in fat cell and liver plasma membranes. J Biol Chem. 1980 Feb 25;255(4):1722–1731. [PubMed] [Google Scholar]
- Ridge K. D., Hofmann K., Finn F. M. ATP sensitizes the insulin receptor to insulin. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9489–9493. doi: 10.1073/pnas.85.24.9489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüegg U. T., Rudinger J. Reductive cleavage of cystine disulfides with tributylphosphine. Methods Enzymol. 1977;47:111–116. doi: 10.1016/0076-6879(77)47012-5. [DOI] [PubMed] [Google Scholar]
- Shia M. A., Rubin J. B., Pilch P. F. The insulin receptor protein kinase. Physicochemical requirements for activity. J Biol Chem. 1983 Dec 10;258(23):14450–14455. [PubMed] [Google Scholar]
- Shoelson S. E., White M. F., Kahn C. R. Tryptic activation of the insulin receptor. Proteolytic truncation of the alpha-subunit releases the beta-subunit from inhibitory control. J Biol Chem. 1988 Apr 5;263(10):4852–4860. [PubMed] [Google Scholar]
- Sugden P. H., Holladay L. A., Reimann E. M., Corbin J. D. Purification and characterization of the catalytic subunit of adenosine 3':5'-cyclic monophosphate-dependent protein kinase from bovine liver. Biochem J. 1976 Nov;159(2):409–422. doi: 10.1042/bj1590409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Wessel D., Flügge U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984 Apr;138(1):141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- White M. F., Haring H. U., Kasuga M., Kahn C. R. Kinetic properties and sites of autophosphorylation of the partially purified insulin receptor from hepatoma cells. J Biol Chem. 1984 Jan 10;259(1):255–264. [PubMed] [Google Scholar]
- Wilden P. A., Boyle T. R., Swanson M. L., Sweet L. J., Pessin J. E. Alteration of intramolecular disulfides in insulin receptor/kinase by insulin and dithiothreitol: insulin potentiates the apparent dithiothreitol-dependent subunit reduction of insulin receptor. Biochemistry. 1986 Jul 29;25(15):4381–4388. doi: 10.1021/bi00363a031. [DOI] [PubMed] [Google Scholar]
- Wilden P. A., Pessin J. E. Differential sensitivity of the insulin-receptor kinase to thiol and oxidizing agents in the absence and presence of insulin. Biochem J. 1987 Jul 15;245(2):325–331. doi: 10.1042/bj2450325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Molecular analysis of signal transduction by growth factors. Biochemistry. 1988 May 3;27(9):3113–3119. doi: 10.1021/bi00409a001. [DOI] [PubMed] [Google Scholar]