Abstract
The cornea is the tough, transparent tissue through which light first enters the eye and functions as a barrier to debris and infection as well as two-thirds of the refractive power of the eye. Corneal damage that is not promptly treated will often lead to scarring and vision impairment. Due to the limited options currently available to treat corneal scars, the identification and isolation of stem cells in the cornea has received much attention, as they may have potential for autologous, cell-based approaches to the treatment of damaged corneal tissue.
The cornea is the tough, transparent tissue through which light first enters the eye. Serving as a barrier to debris and infection as well as two-thirds of the refractive power of the eye, the cornea is imperative to proper vision. Corneal damage that is not promptly treated will often lead to scarring and vision impairment. In fact, millions of people around the world suffer from corneal scars resulting in the loss of vision.1
The cornea is composed of the three cellular layers: the epithelium, stroma, and endothelium. The corneal epithelium is the most anterior layer and the first cellular barrier between the eye and environment. Like the epidermis of the skin, superficial corneal epithelium is continually sloughed off and replaced as it shields the eye from external insults. The stroma comprises roughly 90% of the cornea and is made primarily of highly organized collagen, making it both tough and transparent.2 Damage to these layers by trauma or infection may result in corneal scarring, leading to visual impairment and often blindness. The corneal endothelium, the third and most posterior layer of the cornea, is a single-celled layer of epithelial cells responsible for maintaining deturgescence. The three cellular layers must function together to maintain transparency and, therefore, vision. Currently, the most common form of treatment for damage to any of these layers involves transplanting tissue, a procedure limited by the availability of donor tissue and complicated by the risk of immune-mediated rejection. In an attempt improve treatment options for corneal disorders and damage, research is being directed at bioprosthetics and stem cell biology.
Adult stem cells are characterized as slow-dividing cells with the ability to self-renew and give rise to differentiated progeny via mitosis. These adult stem cells are often found in specialized locations, or niches, in tissues throughout the body. When tissue is damaged (e.g., a flesh wound or blood loss), stem cell populations are often instrumental in replacing the lost cells to restore tissue function and integrity. Due to the devastating effects of corneal wounds and infections, and the limited options currently available to treat them, the identification and isolation of stem cells in the cornea has received much attention. The identification of stem cells in the cornea has the potential for autologous, cell-based approach to the treatment of damaged corneal tissue.
1. CORNEAL EPITHELIAL STEM CELLS
1.1 Anatomy
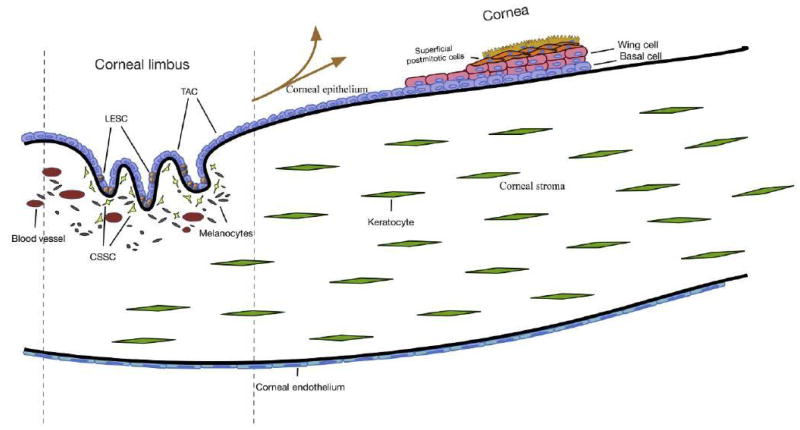
The corneal epithelium is a nonkeratinized, stratified squamous epithelium approximately 5–6 cells thick that covers the front of the cornea. The basal, columnar cell layer, is anchored to the basal lamina via hemidesmosomes and is covered by 2–3 layers of “wing” cells (Fig. 1). The outermost layer of cells is continuously sloughed off and replaced by the proliferation of wing and basal cells.3 There is high corneal epithelial cell turnover due to blinking and both physical and chemical environmental insults. As such, there must be a self-renewing source of corneal epithelial cells from which replacement cells can be drawn. It was suggested in 1971 that renewal of the corneal epithelium was maintained by the migration of epithelial cells in the basal layer of the epithelium.4 We now know that this source is in the Palisades of Vogt at the limbal region that marks the transition zone between cornea and conjunctiva. A steady movement of epithelial cells in both human and mouse corneas from the limbal region toward the central cornea has been documented in a number of studies.4–7 Located primarily at the superior and inferior corneal limbus, the Palisades are a vascularized series of crypts that provide a nutrient-rich, discrete, protected environment for limbal epithelial stem cells (LESCs) (Fig. 1). Cells here are protected from UV rays both by the upper and lower eyelids and by the presence of melanocytes. To support the hypothesis that this niche harbors LESC, the niche cells have been analyzed in a multitude of in vitro and in vivo studies for stem cell characteristics.
Figure 1.
The cornea is composed of three cellular layers: the epithelium, stroma, and endothelium. The vascular limbal region is located at the peripheral cornea and is bordered by the conjunctiva—this region is the proposed niche for stem cell populations in each layer. LESC, limbal epithelial stem cell; TAC, transit-amplifying cell; CSSC, corneal stromal stem cell.
1.2 Characterization
DNA labeling of basal cells in the limbal region revealed them to be “slow cycling,” a characteristic of stem cells. Basal limbal cells also show differences in protein expression when compared to basal cells of the central epithelium.8 Keratin expression is notably distinct in the limbal basal cells with a lack of cytokeratins CK3 and CK12, and expression of CK14/CK59–11 (Fig. 2). Additionally, basal limbal cells grow clonally as holoclones, whereas clones from epithelial cells isolated from the central cornea are rare and do not proliferate extensively.12 The adult stem cell marker ABCG2 has proven remarkably useful in the identification and isolation of stem cells due to its ability to efflux Hoechst 33342, a fluorescent dye that binds DNA. Expression of ABCG2 alters cellular fluorescence in the presence of Hoechst, allowing sorting of a cell population called the “side population” (SP) via fluorescence-activated cell sorting (FACS), separating the ABCG2+ cells (stem cells) from a heterogeneous population. SP sorting of the limbal epithelium has indeed isolated a population of ABCG2+ stem cells that have greater colony-forming efficiency and reduced K3/K12 expression compared to central corneal epithelium.13,14 ABCB5, a member of the ATP-binding cassette family of proteins, has also been identified as a definitive LESC marker. Experiments in both mouse and human revealed that the ABCB5+ limbal epithelium is necessary for proper wound healing, corneal development, and epithelial homeostasis.15 Additional stem cells genes are expressed by LESC, notable among them are C/EBP-delta and Notch1, proteins known to regulate stem cell self-renewal and differentiation.16,17
Figure 2.
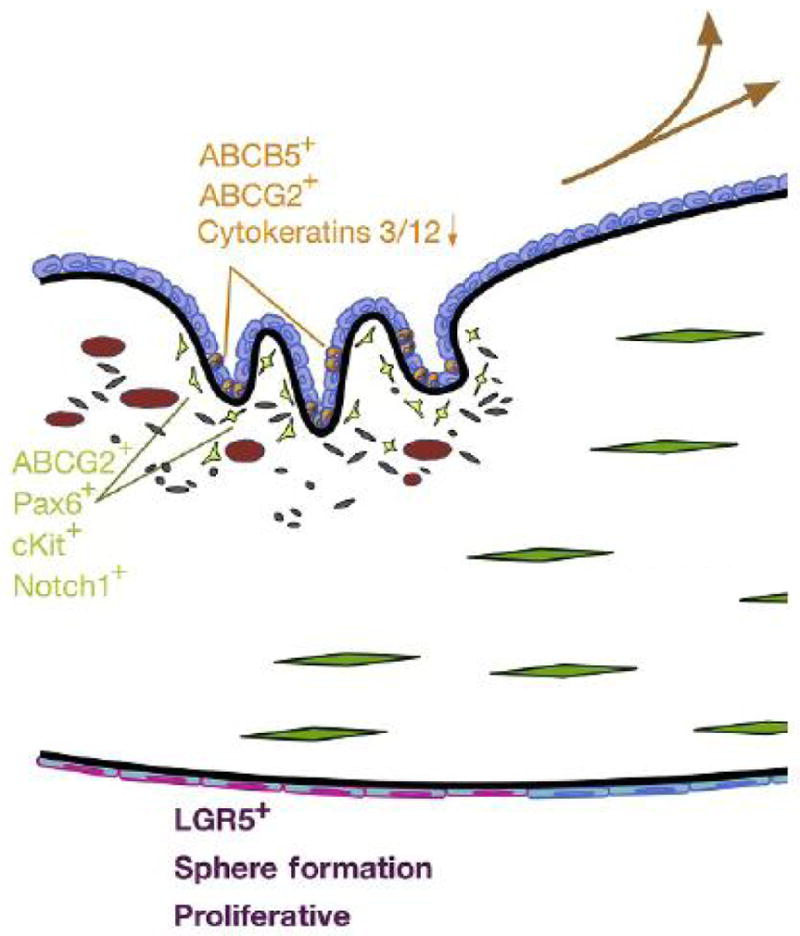
Stem cell markers/characteristics. Orange, limbal epithelial stem cell (LESC) markers, expression generally compared to central epithelial cells; green, human corneal stromal stem cell markers; and purple, proposed markers and characteristics corneal endothelial progenitor cells.
1.3 Wound Healing
In self-renewing epithelia, stem cells are essential for normal tissue maintenance; a second important characteristic is the ability of stem cells to regenerate tissue that has been damaged or lost.18–20 Additional studies in vivo support the importance of LESC after epithelial debridement, concluding that limbal epithelial cells are involved in the rapid, functional healing of the epithelium.21,22 Kruse et at. showed that when the basal limbal epithelium is completely removed before corneal wounding, vascularization, and conjunctivalization result. When the limbal cells were in place, however, wounds healed without vascularization and cells from the limbal region migrated into the central cornea to reform the epithelium.23 In addition to replenishing the mitotic basal cells of the central epithelium, cells in the limbal epithelium appear to provide a biological barrier preventing conjunctivalization and vascularization of the corneal epithelium after wounding.24,25
Expansion of limbal cells in vitro and transplantation to central cornea can restore epithelial function for >10 years in human eyes.26 In these corneas, there is no direct evidence that stem cells have repopulated a limbal niche, leaving open the question as to whether this tissue, like skin, can be supported by a distributed population of stem cells. The idea is supported by a study of destrin knockout mice, which show limited or no migration of epithelial cells.27 Indeed, Dua observed human eyes with limbal damage to contain patches of normal epithelium in the central cornea, suggesting maintenance of the epithelial layer by local stem cells. In a 2008 study, limbal tissue expressing beta-galactosidase (βGal) was transplanted into normal mouse eyes but showed no centripetal migration of the labeled cells unless the central epithelium was wounded.28 Both this and a more recent study found that cells from the central epithelium are capable of growing as holoclones.28,29 These studies suggest that rare stem cells may occur in the central epithelium and that, where they do occur, they can maintain a stable column of epithelium in a manner similar to that of epidermis. A more recent study reconfirmed limbal stem cell-driven migration using lineage-tracing expression of fluorescent proteins driven by a K14 promoter. This gene is limited to stem and progenitor cells in the limbus, thus all fluorescent cells after induction will represent descendants of those progenitors. After induction, fluorescent cells were observed in radial streaks of cells moving toward the central cornea at about 11 μm/day in normal, unwounded eyes.30 These experiments confirm the centripetal migration of cells observed in many earlier studies and support the idea that these cells in the central cornea are derived from limbal stem cells. The current state of our understanding leaves open the question as to whether stem cells in the central cornea can and do contribute to epithelial homeostasis on a long-term basis and whether the centripetal movement of progenitor-derived cells is a requirement for maintenance of healthy and stable corneal epithelium.
In spite of these unresolved questions, there remains no doubt in the clinical importance of LESC. This is manifest in the condition known as limbal stem cell deficiency (LSCD). In this relatively rare condition, loss of limbal cells, typically as a result of genetic disease or chemical burns, results in conjunctivalization of the cornea, inflammation, neovascularization, pain, and corneal opacity.31 Standard penetrating keratoplasty involves only central cornea and allogenic tissue grafts that do not include limbal tissue experience an increased rate of failure.32–34 In unilateral LSCD, limbal tissue can be harvested from the healthy eye without significant damage and cells transplanted on the affected cornea either directly or after expansion in culture.35–37 Use of autologous LESC provides long-term stable epithelium to 50–70% of recipient eyes and regrafting can improve that result.38,39 LESC allografts from cadaver eyes often fail40; however, use of biopsy tissue from living relatives and systemic antirejection drug therapy has led to survival rates for allogenic grafts similar to that of autologous tissue.41 LESC treatment has been carried out on >1000 individuals, becoming the most common and successful of nonhematopoietic stem cell transplantation procedures, restoring vision to individuals with no alternative.
2. CORNEAL STROMAL STEM CELLS
2.1 Anatomy
The corneal stroma is a tough, collagenous tissue derived from the embryonic neural crest. Corneal collagen is arranged in sheets of fibrils called lamellae that are arranged orthogonally throughout the stroma. The precise spacing and arrangement of the fibrils and lamellae are essential to stromal transparency.42 The stroma is sparsely populated by quiescent, mesenchymal cells called keratocytes which are responsible for collagen production and turnover.43,44 Stromal scarring can occur as a result of ocular trauma, surgery, or infection. Loss of visual acuity due to stromal opacity affects more than 23 million individuals worldwide and 4.6 million are estimated to suffer bilateral corneal blindness. Although comprehensive data are limited, stromal opacity is the source of most corneal blindness, greatly exceeding the numbers of individuals affected by LSCD.45–47 Although fully prosthetic corneal replacements are in limited use and acellular prosthetic tissue replacements are in clinical trials, replacement of scarred stromal tissue with cadaveric human stromal tissue is the current clinical method of choice.48,49 Corneal scarring results from trauma or infectious keratitis that initiates an inflammatory response. Upon wounding, the normally quiescent keratocytes adjacent to the affected area become mitotically active fibroblasts, migrate to the wounded area, and subsequently differentiate to myofibroblasts. These cells are responsible for the production of fibrotic extracellular matrix components that contribute to light scatter by stromal scarring.43,44,50,51
2.2 Characterization
Over the past 15–20 years, a large number of reports have identified and characterized stem cells from various mesenchymal tissues. These stem cell populations are identified by self-renewal ability, differentiation into multiple cell types, and their ability to grow clonally. In an initial study, a small population of cells exhibiting clonal growth from bovine corneal stroma was found to express genes associated with mesenchymal stem cells.52 Successful stem cell isolation from the bovine cornea led to investigation of human corneal tissue for a similar population. To achieve this, the well-documented adult stem cell marker ABCG2 was used.53 ABCG2 is an ABC cassette membrane transporter, which has the ability to efflux the DNA-binding dye Hoechst 33342, allowing the ABCG2(+) cells to be sorted via FACS. ABCG2+ cells from the limbal region of human cornea were found to represent less than 1% of the total cell population. These SP cells were shown to grow clonally and exhibited a multipotent differentiation potential, unlike ABCG2(−) cells isolated from the same region.54 SP cells also expressed the stem cell markers Bmi1, Notch1, cKit, Six2, and Pax6 (Fig. 2). When cultured in serum-free medium supplemented with ascorbic acid and insulin, the human corneal SP cells upregulated keratocyte-specific markers, including the corneal stroma-specific proteoglycan keratocan.54 Since the original report, cells isolated from corneal stroma with characteristics of mesenchymal stem cells have been described in a number of publications.55–62 Similar properties and the location of these reported stem cells support the idea that each study is describing the same population of mesenchymal stem cells largely localized to the anterior limbal stroma.63
2.3 Niche Function of Stromal Stem Cells
Several studies have shown limbal mesenchymal cells with stem cell properties to be closely associated in vivo with limbal epithelial cells.59,61,63–68 The two cell types are also coisolated in collagenase digests of the limbal tissue.61,64 In vivo and ex vivo, the two associated cell types, exhibit different protein phenotypes, and both express stem cell genes.61,64 A recent three-dimensional electron microscopic analysis of the limbal region found the epithelial basement membrane to be fenestrated, providing direct cell–cell contact between basal epithelial cells and elongated stromal cells in the limbus.68 Melanocytes were also associated with the niche complex. Both LESC and the limbal mesenchymal stem cell express N-cadherin, suggesting this cell–cell junction protein provides interaction between these cell populations.59,65,69,70 In vitro, coculture of LESC with coisolated stromal stem cells improved LESC expansion and clonogenicity.59 Similarly, culture of limbal epithelial cells shows improved expansion, if it is carried out in the presence of limbal mesenchymal cells but less so in the presence of mesenchymal cells from the central stroma.64 These findings lend credence to the idea that some or all of the mesenchymal stem cells in the stroma exist in vivo as a part of a multicellular limbal niche complex, and their presence supports the stem cell character of the LESC population.
2.4 Bioengineering Corneal Tissue with Stromal Stem Cells
Limited access to donated cadaveric tissue reduces the number of individuals with corneal blindness who benefit from corneal grafts. This shortage has led to widespread interest in development of alternative to penetrating keratoplasty. Alternatives include fully synthetic corneal prostheses as well as acellular polymeric replacements for scarred regions of the stroma.48,49 Other investigators have examined the potential for bioengineering stromal tissue in vitro using a variety of cell types and scaffolding to generate stroma-like tissue.32–34,38,40,41,71–103 Corneal stromal stem cells (CSSC) have also been shown to produce a collagenous matrix similar to that seen in the corneal stroma. When cultured on parallel, aligned nanofibers, CSSC secrete layers of organized collagen nearly identical in fibril diameter and spacing to that of the corneal stroma. This tissue construct also contains the unique corneal proteoglycans which are known to be required for corneal transparency.74,75,88,94,95,100 Although the stem cell-based bioengineered constructs have yet to be tested in vivo, the stromal stem cells appear to be highly suited for generating tissue in vitro that may be useful for replacing scarred stroma.
2.5 Anti-Inflammatory Properties
In addition to their ability to generate corneal tissues in vitro, CSSC like many mesenchymal stem cells exhibit a potential to mediate immune response. In the lumican knockout mouse model of corneal haze, CSSC were shown to restore transparency after being injected directly into the stroma.104 Even more striking, CSSC were shown to completely prevent stromal scarring in a mouse wound model.105 Prevention of scarring appears to be the result of paracrine signaling, as tissue regeneration occurs both in the anterior stroma where the cells were present and the posterior where no cells were seen.105 Importantly, in neither in vivo study was T-cell-mediated tissue rejection observed. This points to both the immune-privilege and immunomodulatory characteristics of CSSC.
3. CORNEAL ENDOTHELIUM STEM/PROGENITOR CELLS
3.1 Anatomy
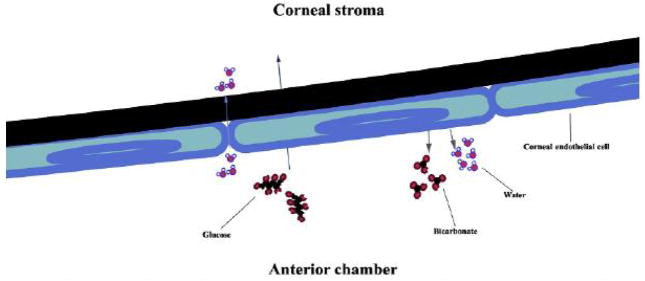
The corneal endothelium is a simple, squamous epithelial sheet on the posterior side of the cornea. Serving as a “leaky” barrier, the corneal endothelium is responsible for pumping fluid out of the corneal stroma to prevent the development of edematous haze. The barrier “leaks” to let water and nutrients into the stroma from the anterior chamber, thus establishing two opposing forces that must function properly in order to maintain corneal transparency (Fig. 3).
Figure 3.
The corneal endothelium serves as a leaky barrier between the corneal stroma (anterior) and anterior chamber (posterior). Water passively moves from the ac into the stroma, while protein and other nutrients (such as glucose) are actively transported by corneal endothelial cells. To pump water from the stroma into the anterior chamber, bicarbonate is actively pumped out of the endothelial cells (along with other ions) and water follows.
The corneal endothelium is notable for its lack of mitotic activity after birth and inability to regenerate after damage.106 Upon cell loss during aging, surrounding cells spread out and change shape. If enough cells are lost, the pump function is compromised and the cornea becomes edematous and cloudy. Perhaps, the most common disorder of this nature is Fuch’s Dystrophy, leading to the thickening of Descemet’s membrane and corneal edema causing corneal haze. Currently, the only treatment option to restore vision requires transplantation of donor tissue. While this is largely successful, it does not avoid the problems often encountered with transplant operations, namely immune rejection and the declining availability of donor tissue.
3.2 Characterization
Many laboratories are searching for corneal endothelial stem cells for their potential in regenerative medicine. It has been shown that cells with clonogenic potential exist in the normal adult corneal endothelium by sphere formation.107 While these cells did not express markers that would be indicative of a stem cell population, clonogenic sphere formation and their ability to form a hexagonal monolayer of cells with pump function may indicate that they are corneal endothelial progenitor cells.56 As is seen in other ocular tissues, notably, the corneal epithelium, stroma, and retina, proposed stem cell niches are often found at the periphery of the tissue.12,54,108,109 Further characterization of the sphere forming corneal endothelial cells revealed that the cells at the periphery of the endothelium have a greater propensity to form spheres than do those in the center, as has previously been described in rabbits.110,111
Recently, a progenitor cell population in human corneal endothelium was identified using the established stem cell marker LGR5.112 LGR5+ endothelial cells were shown to have greater proliferation capacity than LGR5− cells and were located at the periphery of the cornea, both expected characteristics of a stem/progenitor cell population. Another laboratory identified a progenitor cell population based on expression of the neural crest cell markers p75, SOX9, and FOXC2.113 It was further shown that the progenitor cells had high proliferative capacity and demonstrated pump function. While there was no statistical difference between the number of p75+ cells between the periphery and central corneal endothelium, the cells in the center were dispersed and those at the periphery much more concentrated, suggesting the presence of a niche.
3.3 Conclusion
Corneal wounding and disease often have the devastating consequence of visual impairment or blindness. While there are treatment options available when this occurs, tissue rejection and graft availability limit the ability to treat the numerous patients in need. Stem cells isolated from the cornea may offer an alternative to the current treatment options in that they can be used in an autologous fashion. This is already being used for trauma and corneal epithelial disorders such as LSCD and is widely successful.39,114 CSSC have successfully been isolated from human tissue and animal studies show encouraging results that these cells may soon be used to treat stromal scarring. The corneal endothelium appears to have a progenitor population that may provide a source of cells for regeneration, though more studies need to be completed to demonstrate this. Taken together, the progress made thus far in the isolation and use of corneal stem cells for use in the clinic is promising. Though much work still needs to be done, an alternative, cell-based treatment for corneal pathologies is achievable.
References
- 1.Johnson GJ. Vision 2020: the right to sight: report on the sixth general assembly of the international agency for the prevention of blindness (IAPB) Commun Eye Health. 1999;12(32):59–60. [PMC free article] [PubMed] [Google Scholar]
- 2.Hassell JR, Birk DE. The molecular basis of corneal transparency. Exp Eye Res. 2010;91(3):326–335. doi: 10.1016/j.exer.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983;24(10):1442–1443. [PubMed] [Google Scholar]
- 4.Davanger M, Evensen A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature. 1971;229(5286):560–561. doi: 10.1038/229560a0. [DOI] [PubMed] [Google Scholar]
- 5.Nagasaki T, Zhao J. Centripetal movement of corneal epithelial cells in the normal adult mouse. Invest Ophthalmol Vis Sci. 2003;44(2):558–566. doi: 10.1167/iovs.02-0705. [DOI] [PubMed] [Google Scholar]
- 6.Collinson JM, Morris L, Reid AI, et al. Clonal analysis of patterns of growth, stem cell activity, and cell movement during the development and maintenance of the murine corneal epithelium. Dev Dyn. 2002;224(4):432–440. doi: 10.1002/dvdy.10124. [DOI] [PubMed] [Google Scholar]
- 7.Buck RC. Measurement of centripetal migration of normal corneal epithelial cells in the mouse. Invest Ophthalmol Vis Sci. 1985;26(9):1296–1299. [PubMed] [Google Scholar]
- 8.Schlötzer-Schrehardt U, Kruse FE. Identification and characterization of limbal stem cells. Exp Eye Res. 2005;81(3):247–264. doi: 10.1016/j.exer.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 9.Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57(2):201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- 10.Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103(1):49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodrigues M, Ben-Zvi A, Krachmer J, Schermer A, Sun TT. Suprabasal expression of a 64-kilodalton keratin (no. 3) in developing human corneal epithelium. Differentiation. 1987;34(1):60–67. doi: 10.1111/j.1432-0436.1987.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 12.Lavker RM, Tseng SC, Sun TT. Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Exp Eye Res. 2004;78(3):433–446. doi: 10.1016/j.exer.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Chen Z, de Paiva CS, Luo L, Kretzer FL, Pflugfelder SC, Li DQ. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22(3):355–366. doi: 10.1634/stemcells.22-3-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Paiva CS, Chen Z, Corrales RM, Pflugfelder SC, Li DQ. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells. 2005;23(1):63–73. doi: 10.1634/stemcells.2004-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ksander BR, Kolovou PE, Wilson BJ, et al. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature. 2014;511(7509):353–357. doi: 10.1038/nature13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbaro V, Testa A, Di Iorio E, Mavilio F, Pellegrini G, De Luca M. C/EBPdelta regulates cell cycle and self-renewal of human limbal stem cells. J Cell Biol. 2007;177(6):1037–1049. doi: 10.1083/jcb.200703003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas PB, Liu YH, Zhuang FF, et al. Identification of Notch-1 expression in the limbal basal epithelium. Mol Vis. 2007;13:337–344. [PMC free article] [PubMed] [Google Scholar]
- 18.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15(1):19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 19.Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414(6859):98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 20.Tadeu AM, Horsley V. Epithelial stem cells in adult skin. Curr Top Dev Biol. 2014;107:109–131. doi: 10.1016/B978-0-12-416022-4.00004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen JJ, Tseng SC. Corneal epithelial wound healing in partial limbal deficiency. Invest Ophthalmol Vis Sci. 1990;31(7):1301–1314. [PubMed] [Google Scholar]
- 22.Huang AJ, Tseng SC. Corneal epithelial wound healing in the absence of limbal epithelium. Invest Ophthalmol Vis Sci. 1991;32(1):96–105. [PubMed] [Google Scholar]
- 23.Kruse FE, Chen JJ, Tsai RJ, Tseng SC. Conjunctival transdifferentiation is due to the incomplete removal of limbal basal epithelium. Invest Ophthalmol Vis Sci. 1990;31(9):1903–1913. [PubMed] [Google Scholar]
- 24.Ahmad S. Concise review: limbal stem cell deficiency, dysfunction, and distress. Stem Cells Transl Med. 2012;1(2):110–115. doi: 10.5966/sctm.2011-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sangwan VS. Limbal stem cells in health and disease. Biosci Rep. 2001;21(4):385–405. doi: 10.1023/a:1017935624867. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein RS, Pomp O, Brokhman I, Ziegler L. Generation of neural crest cells and peripheral sensory neurons from human embryonic stem cells. Methods Mol Biol. 2010;584:283–300. doi: 10.1007/978-1-60761-369-5_15. [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, Zhao J, Chen L, Urbanowicz MM, Nagasaki T. Abnormal epithelial homeostasis in the cornea of mice with a destrin deletion. Mol Vis. 2008;14:1929–1939. [PMC free article] [PubMed] [Google Scholar]
- 28.Majo F, Rochat A, Nicolas M, Jaoude GA, Barrandon Y. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature. 2008;456(7219):250–254. doi: 10.1038/nature07406. [DOI] [PubMed] [Google Scholar]
- 29.Chang C-YA, McGhee JJ, Green CR, Sherwin T. Comparison of stem cell properties in cell populations isolated from human central and limbal corneal epithelium. Cornea. 2011;30(10):1155–1162. doi: 10.1097/ICO.0b013e318213796b. [DOI] [PubMed] [Google Scholar]
- 30.Di Girolamo N, Bobba S, Raviraj V, et al. Tracing the fate of limbal epithelial progenitor cells in the murine cornea. Stem Cells. 2015;33(1):157–169. doi: 10.1002/stem.1769. [DOI] [PubMed] [Google Scholar]
- 31.Dua HS, Gomes JA, Singh A. Corneal epithelial wound healing. Br J Ophthalmol. 1994;78(5):401–408. doi: 10.1136/bjo.78.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reinhard T, Spelsberg H, Henke L, et al. Long-term results of allogeneic penetrating limbo-keratoplasty in total limbal stem cell deficiency. Ophthalmology. 2004;111(4):775–782. doi: 10.1016/j.ophtha.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 33.Miri A, Al-Deiri B, Dua HS. Long-term outcomes of autolimbal and allolimbal transplants. Ophthalmology. 2010;117(6):1207–1213. doi: 10.1016/j.ophtha.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 34.Eberwein P, Böhringer D, Schwartzkopff J, Birnbaum F, Reinhard T. Allogenic limbo-keratoplasty with conjunctivoplasty, mitomycin C, and amniotic membrane for bilateral limbal stem cell deficiency. Ophthalmology. 2012;119(5):930–937. doi: 10.1016/j.ophtha.2011.10.039. [DOI] [PubMed] [Google Scholar]
- 35.Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349(9057):990–993. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 36.Tsai RJ, Tseng SC. Human allograft limbal transplantation for corneal surface reconstruction. Cornea. 1994;13(5):389–400. doi: 10.1097/00003226-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Koizumi N, Inatomi T, Suzuki T, Sotozono C, Kinoshita S. Cultivated corneal epithelial stem cell transplantation in ocular surface disorders. Ophthalmology. 2001;108(9):1569–1574. doi: 10.1016/s0161-6420(01)00694-7. [DOI] [PubMed] [Google Scholar]
- 38.Sangwan VS, Jain R, Basu S, et al. Transforming ocular surface stem cell research into successful clinical practice. Indian J Ophthalmol. 2014;62(1):29–40. doi: 10.4103/0301-4738.126173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363(2):147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 40.Shortt AJ, Bunce C, Levis HJ, et al. Three-year outcomes of cultured limbal epithelial allografts in aniridia and Stevens-Johnson syndrome evaluated using the Clinical Outcome Assessment in Surgical Trials assessment tool. Stem Cells Transl Med. 2014;3(2):265–275. doi: 10.5966/sctm.2013-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basu S, Fernandez MM, Das S, Gaddipati S, Vemuganti GK, Sangwan VS. Clinical outcomes of xeno-free allogeneic cultivated limbal epithelial transplantation for bilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96(12):1504–1509. doi: 10.1136/bjophthalmol-2012-301869. [DOI] [PubMed] [Google Scholar]
- 42.Knupp C, Pinali C, Lewis PN, et al. The architecture of the cornea and structural basis of its transparency. Adv Protein Chem Struct Biol. 2009;78:25–49. doi: 10.1016/S1876-1623(08)78002-7. [DOI] [PubMed] [Google Scholar]
- 43.Funderburgh JL, Funderburgh ML, Mann MM, Corpuz L, Roth MR. Proteoglycan expression during transforming growth factor beta-induced keratocyte-myofibroblast transdifferentiation. J Biol chem. 2001;276(47):44173–44178. doi: 10.1074/jbc.M107596200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Funderburgh JL, Mann MM, Funderburgh ML. Keratocyte phenotype mediates proteoglycan structure: a role for fibroblasts in corneal fibrosis. J Biol Chem. 2003;278(46):45629–45637. doi: 10.1074/jbc.M303292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oliva MS, Schottman T, Gulati M. Turning the tide of corneal blindness. Indian J Ophthalmol. 2012;60(5):423–427. doi: 10.4103/0301-4738.100540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shortt AJ, Tuft SJ, Daniels JT. Ex vivo cultured limbal epithelial transplantation. A clinical perspective. Ocul Surf. 2010;8(2):80–90. doi: 10.1016/s1542-0124(12)70072-1. [DOI] [PubMed] [Google Scholar]
- 47.Ontario HQ. Limbal stem cell transplantation: an evidence-based analysis. Ont Health Technol Assess Ser. 2008;8(7):1–58. [PMC free article] [PubMed] [Google Scholar]
- 48.Tan DTH, Dart JKG, Holland EJ, Kinoshita S. Corneal transplantation. Lancet. 2012;379(9827):1749–1761. doi: 10.1016/S0140-6736(12)60437-1. [DOI] [PubMed] [Google Scholar]
- 49.Lagali N, Fagerholm P, Griffith M. Biosynthetic corneas: prospects for supplementing the human donor cornea supply. Expert Rev Med Devices. 2011;8(2):127–130. doi: 10.1586/erd.10.89. [DOI] [PubMed] [Google Scholar]
- 50.Fini ME. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res. 1999;18(4):529–551. doi: 10.1016/s1350-9462(98)00033-0. [DOI] [PubMed] [Google Scholar]
- 51.Cintron C, Schneider H, Kublin C. Corneal scar formation. Exp Eye Res. 1973;17(3):251–259. doi: 10.1016/0014-4835(73)90176-0. [DOI] [PubMed] [Google Scholar]
- 52.Funderburgh ML, Du Y, Mann MM, Sundar Raj N, Funderburgh JL. PAX6 expression identifies progenitor cells for corneal keratocytes. FASEB J. 2005;19(10):1371–1373. doi: 10.1096/fj.04-2770fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Golebiewska A, Brons NH, Bjerkvig R, Niclou SP. Critical appraisal of the side population assay in stem cell and cancer stem cell research. Cell Stem Cell. 2011;8(2):136–147. doi: 10.1016/j.stem.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 54.Du Y, Funderburgh ML, Mann MM, SundarRaj N, Funderburgh JL. Multipotent stem cells in human corneal stroma. Stem Cells. 2005;23(9):1266–1275. doi: 10.1634/stemcells.2004-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoshida S, Shimmura S, Nagoshi N, et al. Isolation of multipotent neural crest-derived stem cells from the adult mouse cornea. Stem Cells. 2006;24(12):2714–2722. doi: 10.1634/stemcells.2006-0156. [DOI] [PubMed] [Google Scholar]
- 56.Amano S, Yamagami S, Mimura T, Uchida S, Yokoo S. Corneal stromal and endothelial cell precursors. Cornea. 2006;25(10 suppl 1):S73–S77. doi: 10.1097/01.ico.0000247218.10672.7e. [DOI] [PubMed] [Google Scholar]
- 57.Polisetty N, Fatima A, Madhira SL, Sangwan VS, Vemuganti GK. Mesenchymal cells from limbal stroma of human eye. Mol Vis. 2008;14:431–442. [PMC free article] [PubMed] [Google Scholar]
- 58.Lu J-M, Zhou Z-Y, Zhang X-R, Li X-L, Wang H-F, Song X-J. A preliminary study of mesenchymal stem cell-like cells derived from murine corneal stroma. Graefes Arch Clin Exp Ophthalmol. 2010;248(9):1279–1285. doi: 10.1007/s00417-010-1367-0. [DOI] [PubMed] [Google Scholar]
- 59.Xie H-T, Chen S-Y, Li G-G, Tseng SCG. Isolation and expansion of human limbal stromal niche cells. Invest Ophthalmol Vis Sci. 2012;53(1):279–286. doi: 10.1167/iovs.11-8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Branch MJ, Hashmani K, Dhillon P, Jones DRE, Dua HS, Hopkinson A. Mesenchymal stem cells in the human corneal limbal stroma. Invest Ophthalmol Vis Sci. 2012;53(9):5109–5116. doi: 10.1167/iovs.11-8673. [DOI] [PubMed] [Google Scholar]
- 61.Li G-G, Zhu Y-T, Xie H-T, Chen S-Y, Tseng SCG. Mesenchymal stem cells derived from human limbal niche cells. Invest Ophthalmol Vis Sci. 2012;53(9):5686–5697. doi: 10.1167/iovs.12-10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garfias Y, Nieves-Hernandez J, Garcia-Mejia M, Estrada-Reyes C, Jimenez-Martinez-MC Stem cells isolated from the human stromal limbus possess immunosuppressant properties. Mol Vis. 2012;18:2087–2095. [PMC free article] [PubMed] [Google Scholar]
- 63.Pinnamaneni N, Funderburgh JL. Concise review: stem cells in the corneal stroma. Stem Cells. 2012;30(6):1059–1063. doi: 10.1002/stem.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen S-Y, Hayashida Y, Chen M-Y, Xie HT, Tseng SCG. A new isolation method of human limbal progenitor cells by maintaining close association with their niche cells. Tissue Eng Part C Methods. 2011;17(5):537–548. doi: 10.1089/ten.tec.2010.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hayashi R, Yamato M, Sugiyama H, et al. N-Cadherin is expressed by putative stem/progenitor cells and melanocytes in the human limbal epithelial stem cell niche. Stem Cells. 2007;25(2):289–296. doi: 10.1634/stemcells.2006-0167. [DOI] [PubMed] [Google Scholar]
- 66.Higa K, Kato N, Yoshida S, et al. Aquaporin 1-positive stromal niche-like cells directly interact with N-cadherin-positive clusters in the basal limbal epithelium. Stem Cell Res. 2013;10(2):147–155. doi: 10.1016/j.scr.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 67.Massie I, Dziasko M, Kureshi A, et al. Advanced imaging and tissue engineering of the human limbal epithelial stem cell niche. Methods Mol Biol. 2015;1235:179–202. doi: 10.1007/978-1-4939-1785-3_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dziasko MA, Armer HE, Levis HJ, Shortt AJ, Tuft S, Daniels JT. Localisation of epithelial cells capable of holoclone formation in vitro and direct interaction with stromal cells in the native human limbal crypt. PLoS One. 2014;9(4):e94283. doi: 10.1371/journal.pone.0094283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Higa K, Shimmura S, Miyashita H, et al. N-cadherin in the maintenance of human corneal limbal epithelial progenitor cells in vitro. Invest Ophthalmol Vis Sci. 2009;50(10):4640–4645. doi: 10.1167/iovs.09-3503. [DOI] [PubMed] [Google Scholar]
- 70.Omoto M, Miyashita H, Shimmura S, et al. The use of human mesenchymal stem cell-derived feeder cells for the cultivation of transplantable epithelial sheets. Invest Ophthalmol Vis Sci. 2009;50(5):2109–2115. doi: 10.1167/iovs.08-2262. [DOI] [PubMed] [Google Scholar]
- 71.Guan L, Ge H, Tang X, et al. Use of a silk fibroin-chitosan scaffold to construct a tissue-engineered corneal stroma. Cells Tissues Organs. 2013;198(3):190–197. doi: 10.1159/000355944. [DOI] [PubMed] [Google Scholar]
- 72.Karamichos D, Hutcheon AEK, Zieske JD. Transforming growth factor-β3 regulates assembly of a non-fibrotic matrix in a 3D corneal model. J Tissue Eng Regen Med. 2011;5(8):e228–e238. doi: 10.1002/term.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Karamichos D, Rich CB, Hutcheon AEK, et al. Self-assembled matrix by umbilical cord stem cells. J Funct Biomater. 2011;2(3):213–229. doi: 10.3390/jfb2030213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Du Y, Sundarraj N, Funderburgh ML, Harvey SA, Birk DE, Funderburgh JL. Secretion and organization of a cornea-like tissue in vitro by stem cells from human corneal stroma. Invest Ophthalmol Vis Sci. 2007;48(11):5038–5045. doi: 10.1167/iovs.07-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karamichos D, Funderburgh ML, Hutcheon AE, et al. A role for topographic cues in the organization of collagenous matrix by corneal fibroblasts and stem cells. PLoS One. 2014;9(1):e86260. doi: 10.1371/journal.pone.0086260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karamichos D, Hutcheon AEK, Zieske JD. Reversal of fibrosis by TGF-β3 in a 3D in vitro model. Exp Eye Res. 2014;124:31–36. doi: 10.1016/j.exer.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoeruek E, Bayyoud T, Maurus C, et al. Reconstruction of corneal stroma with decellularized porcine xenografts in a rabbit model. Acta Ophthalmol. 2012;90(3):e206–e210. doi: 10.1111/j.1755-3768.2011.02300.x. [DOI] [PubMed] [Google Scholar]
- 78.Karamichos D, Zareian R, Guo X, Hutcheon AEK, Ruberti JW, Zieske JD. Novel in vitro model for keratoconus disease. J Funct Biomater. 2012;3(4):760–775. doi: 10.3390/jfb3040760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lai J-Y, Li Y-T, Cho C-H, Yu T-C. Nanoscale modification of porous gelatin scaffolds with chondroitin sulfate for corneal stromal tissue engineering. Int J Nanomedicine. 2012;7:1101–1114. doi: 10.2147/IJN.S28753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo X, Hutcheon AEK, Melotti SA, Zieske JD, Trinkaus-Randall V, Ruberti JW. Morphologic characterization of organized extracellular matrix deposition by ascorbic acid-stimulated human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2007;48(9):4050–4060. doi: 10.1167/iovs.06-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tanaka Y, Shi D, Kubota A, et al. Irreversible optical clearing of rabbit dermis for autogenic corneal stroma transplantation. Biomaterials. 2011;32(28):6764–6772. doi: 10.1016/j.biomaterials.2011.05.081. [DOI] [PubMed] [Google Scholar]
- 82.Ionescu A-M, Alaminos M, de la Cruz Cardona J, et al. Investigating a novel nanostructured fibrin-agarose biomaterial for human cornea tissue engineering: rheological properties. J Mech Behav Biomed Mater. 2011;4(8):1963–1973. doi: 10.1016/j.jmbbm.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 83.Karamichos D, Hutcheon AEK, Rich CB, Trinkaus-Randall V, Asara JM, Zieske JD. In vitro model suggests oxidative stress involved in keratoconus disease. Sci Rep. 2014;4:4608. doi: 10.1038/srep04608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ren R, Hutcheon AEK, Guo XQ, et al. Human primary corneal fibroblasts synthesize and deposit proteoglycans in long-term 3-D cultures. Dev Dyn. 2008;237(10):2705–2715. doi: 10.1002/dvdy.21606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakatsu MN, González S, Mei H, Deng SX. Human limbal mesenchymal cells support the growth of human corneal epithelial stem/progenitor cells. Invest Ophthalmol Vis Sci. 2014;55(10):6953–6959. doi: 10.1167/iovs.14-14999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Karamichos D, Guo XQ, Hutcheon AEK, Zieske JD. Human corneal fibrosis: an in vitro model. Invest Ophthalmol Vis Sci. 2010;51(3):1382–1388. doi: 10.1167/iovs.09-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garzón I, Martín-Piedra MA, Alfonso-Rodríguez C, et al. Generation of a biomimetic human artificial cornea model using Wharton’s jelly mesenchymal stem cells. Invest Ophthalmol Vis Sci. 2014;55(7):4073–4083. doi: 10.1167/iovs.14-14304. [DOI] [PubMed] [Google Scholar]
- 88.Wu J, Du Y, Watkins SC, Funderburgh JL, Wagner WR. The engineering of organized human corneal tissue through the spatial guidance of corneal stromal stem cells. Biomaterials. 2012;33(5):1343–1352. doi: 10.1016/j.biomaterials.2011.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Then KY, Yang Y, Ahearne M, El Haj AJ. Effect of microtopographical cues on human keratocyte orientation and gene expression. Curr Eye Res. 2011;36(2):88–93. doi: 10.3109/02713683.2010.512407. [DOI] [PubMed] [Google Scholar]
- 90.Bray LJ, George KA, Hutmacher DW, Chirila TV, Harkin DG. A dual-layer silk fibroin scaffold for reconstructing the human corneal limbus. Biomaterials. 2012;33(13):3529–3538. doi: 10.1016/j.biomaterials.2012.01.045. [DOI] [PubMed] [Google Scholar]
- 91.Saeidi N, Guo X, Hutcheon AEK, et al. Disorganized collagen scaffold interferes with fibroblast mediated deposition of organized extracellular matrix in vitro. Biotechnol Bio-eng. 2012;109(10):2683–2698. doi: 10.1002/bit.24533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Katikireddy KR, Dana R, Jurkunas UV. Differentiation potential of limbal fibroblasts and bone marrow mesenchymal stem cells to corneal epithelial cells. Stem Cells. 2014;32(3):717–729. doi: 10.1002/stem.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Du L, Wu X. Development and characterization of a full-thickness acellular porcine cornea matrix for tissue engineering. Artif Organs. 2011;35(7):691–705. doi: 10.1111/j.1525-1594.2010.01174.x. [DOI] [PubMed] [Google Scholar]
- 94.Wu J, Du Y, Mann MM, Funderburgh JL, Wagner WR. Corneal stromal stem cells versus corneal fibroblasts in generating structurally appropriate corneal stromal tissue. Exp Eye Res. 2014;120:71–81. doi: 10.1016/j.exer.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu J, Rnjak-Kovacina J, Du Y, Funderburgh ML, Kaplan DL, Funderburgh JL. Corneal stromal bioequivalents secreted on patterned silk substrates. Biomaterials. 2014;35(12):3744–3755. doi: 10.1016/j.biomaterials.2013.12.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arjamaa O. Corneal reconstruction by stem cells and bioengineering. Clin Ophthalmol. 2012;6:1407–1409. doi: 10.2147/OPTH.S33826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xiao J, Duan H, Liu Z, et al. Construction of the recellularized corneal stroma using porous acellular corneal scaffold. Biomaterials. 2011;32(29):6962–6971. doi: 10.1016/j.biomaterials.2011.05.084. [DOI] [PubMed] [Google Scholar]
- 98.Ramachandran C, Basu S, Sangwan VS, Balasubramanian D. Concise review: the coming of age of stem cell treatment for corneal surface damage. Stem Cells Transl Med. 2014;3(10):1160–1168. doi: 10.5966/sctm.2014-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grobe GM, Reichl S. Characterization of vitamin C-induced cell sheets formed from primary and immortalized human corneal stromal cells for tissue engineering applications. Cells Tissues Organs. 2013;197(4):283–297. doi: 10.1159/000346172. [DOI] [PubMed] [Google Scholar]
- 100.Wu J, Du Y, Mann MM, Yang E, Funderburgh JL, Wagner WR. Bioengineering organized, multilamellar human corneal stromal tissue by growth factor supplementation on highly aligned synthetic substrates. Tissue Eng A. 2013;19(17–18):2063–2075. doi: 10.1089/ten.tea.2012.0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boulze Pankert M, Goyer B, Zaguia F, et al. Biocompatibility and functionality of a tissue-engineered living corneal stroma transplanted in the feline eye. Invest Ophthalmol Vis Sci. 2014;55(10):6908–6920. doi: 10.1167/iovs.14-14720. [DOI] [PubMed] [Google Scholar]
- 102.Espandar L, Bunnell B, Wang GY, Gregory P, McBride C, Moshirfar M. Adipose- derived stem cells on hyaluronic acid-derived scaffold: a new horizon in bioengineered cornea. Arch Ophthalmol. 2012;130(2):202–208. doi: 10.1001/archopthalmol.2011.1398. [DOI] [PubMed] [Google Scholar]
- 103.Giasson CJ, Deschambeault A, Carrier P, Germain L. Adherens junction proteins are expressed in collagen corneal equivalents produced in vitro with human cells. Mol Vis. 2014;20:386–394. [PMC free article] [PubMed] [Google Scholar]
- 104.Du Y, Carlson EC, Funderburgh ML, et al. Stem cell therapy restores transparency to defective murine corneas. Stem Cells. 2009;27(7):1635–1642. doi: 10.1002/stem.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Basu S, Hertsenberg AJ, Funderburgh ML, et al. Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Sci Transl Med. 2014;6(266):266ra172. doi: 10.1126/scitranslmed.3009644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mishima S. Clinical investigations on the corneal endothelium. Ophthalmology. 1982;89(6):525–530. doi: 10.1016/s0161-6420(82)34755-7. [DOI] [PubMed] [Google Scholar]
- 107.Yokoo S, Yamagami S, Yanagi Y, et al. Human corneal endothelial cell precursors isolated by sphere-forming assay. Invest Ophthalmol Vis Sci. 2005;46(5):1626–1631. doi: 10.1167/iovs.04-1263. [DOI] [PubMed] [Google Scholar]
- 108.Coles BL, Angenieux B, Inoue T, et al. Facile isolation and the characterization of human retinal stem cells. Proc Natl Acad Sci USA. 2004;101(44):15772–15777. doi: 10.1073/pnas.0401596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tropepe V, Coles BL, Chiasson BJ, et al. Retinal stem cells in the adult mammalian eye. Science. 2000;287(5460):2032–2036. doi: 10.1126/science.287.5460.2032. [DOI] [PubMed] [Google Scholar]
- 110.Mimura T, Yamagami S, Yokoo S, Araie M, Amano S. Comparison of rabbit corneal endothelial cell precursors in the central and peripheral cornea. Invest Ophthalmol Vis Sci. 2005;46(10):3645–3648. doi: 10.1167/iovs.05-0630. [DOI] [PubMed] [Google Scholar]
- 111.Yamagami S, Yokoo S, Mimura T, Takato T, Araie M, Amano S. Distribution of precursors in human corneal stromal cells and endothelial cells. Ophthalmology. 2007;114(3):433–439. doi: 10.1016/j.ophtha.2006.07.042. [DOI] [PubMed] [Google Scholar]
- 112.Hirata-Tominaga K, Nakamura T, Okumura N, et al. Corneal endothelial cell fate is maintained by LGR5 through the regulation of hedgehog and Wnt pathway. Stem Cells. 2013;31(7):1396–1407. doi: 10.1002/stem.1390. [DOI] [PubMed] [Google Scholar]
- 113.Hara S, Hayashi R, Soma T, et al. Identification and potential application of human corneal endothelial progenitor cells. Stem Cells Dev. 2014;23(18):2190–2201. doi: 10.1089/scd.2013.0387. [DOI] [PubMed] [Google Scholar]
- 114.Satake Y, Yamaguchi T, Hirayama M, et al. Ocular surface reconstruction by cultivated epithelial sheet transplantation. Cornea. 2014;33(suppl 11):S42–S46. doi: 10.1097/ICO.0000000000000242. [DOI] [PubMed] [Google Scholar]