SUMMARY
Transcriptional regulation plays an important role in the control of gene expression during aging. However, translation efficiency likely plays an equally important role in determining protein abundance, but has been relatively under studied in this context. Here we used RNA-seq and ri-bosome profiling to investigate the role of translational regulation in lifespan extension by CAN1 gene deletion in yeast. Through comparison of the transcriptional and translational changes in cells lacking CAN1 with other long-lived mutants, we were able to identify critical regulatory factors, including transcription factors and mRNA-binding proteins, that coordinate transcriptional and translational responses. Together, our data support a model in which deletion of CAN1 extends replicative lifespan through increased translation of proteins that facilitate cellular response to stress. This study extends our understanding of the importance of translational control in regulating stress resistance and longevity.
Graphical abstract
Introduction
Transcriptional regulation plays an important role in determining gene expression during aging, but many genes are also regulated at the level of translation, which has been under studied in this context. Microarray and transcriptome analyses of different long-lived mutants have enabled identification of genome-wide changes in gene expression associated with increased longevity (Cheng et al., 2007; Dang et al., 2014; McElwee et al., 2003; Murphy et al., 2003; Wierman et al., 2015). In addition, several research groups have characterized age-dependent transcriptional changes across different species (Lin et al., 2001; Pletcher et al., 2002; Wood et al., 2013). Evidence supporting an equally important role for mRNA translation during aging is provided by reports documenting increased lifespan following inhibition of mRNA translation in yeast, worms, and flies (Kaeberlein and Kennedy, 2011). However, the mechanistic basis for these effects remains unclear, and relatively few studies have comprehensively investigated translational regulation and accompanying changes in protein synthesis during aging.
In eukaryotes, both global and mRNA-specific translational control can be regulated by various stresses and external stimuli. To restore cellular homeostasis in response to stress, cells can activate an integrated stress response (ISR) (Simpson and Ashe, 2012). This signaling pathway is initiated upon phosphorylation of the alpha subunit of eukaryotic initiation factor 2 (eIF2α) by diverse stress-sensing kinases, leading to attenuation of translation initiation and inhibition of overall protein synthesis (Harding et al., 2003). In addition, phosphorylated eIF2α allows for the preferential translation of specific mRNA transcripts that lead to alleviation of stress (Sidrauski et al., 2015). Among translationally activated genes in response to ISR is the bZIP transcription factor Gcn4, a homolog of mammalian ATF4. Gcn4 regulates the transcription of many genes involved in amino acid biosynthesis, metabolism and multiple stress responses, whose activation has been implicated in increased longevity in model organisms (Li et al., 2014; McCormick et al., 2015; Steffen et al., 2008).
One mechanism by which translational up-regulation of specific mRNAs can be achieved involves regulatory upstream open reading frames (uORFs) (Dever, 2002). In addition to cis-regulatory sequences, RNA-binding proteins (RBPs) have been implicated in regulation of protein translation. A number of RBPs are known to associate with specific mRNAs and adjust the production of proteins to cellular needs in a transcript-specific manner (Lackner and Bahler, 2008). Importantly, many of the RBPs have been shown to bind multiple functionally related transcripts or mRNAs, which share a common sub-cellular localization (Hogan et al., 2008). Conversely, most mRNAs can be targeted by several RBPs indicating that this class of regulatory proteins is well suited to linking multiple pathways. How the cell coordinates transcriptional and translational levels to fine-tune gene expression and how the different mechanisms of translational control are coordinated with each other are not well understood (Jackson et al., 2010). Although more than 600 RBPs have been identified in the yeast genome, the function of the majority of them and the contribution of translational regulation during the aging process remains elusive (Mitchell et al., 2013; Tsvetanova et al., 2010).
To elucidate the role of translational regulation in aging, we decided to investigate ge-nome-wide transcriptome (RNA-seq) and translatome (Ribo-seq) changes in the long-lived CAN1 gene deletion mutant, previously identified in a genetic screen for single gene deletion mutants with increased replicative lifespan (McCormick et al., 2015). CAN1 encodes an arginine amino acid transporter localized to the plasma membrane of yeast (Ahmad and Bussey, 1986). Given that amino acid restriction has been shown to increase lifespan in yeast (He et al., 2014; Jiang et al., 2000) and other model organisms (Brown-Borg and Buffenstein, 2016; Cabreiro et al., 2013; Grandison et al., 2009), understanding the mechanisms of translational regulation in this mutant may provide insight into lifespan extension during dietary restriction. We find that the lack of Can1 extends yeast replicative lifespan through activation of the integrated stress response. We also demonstrate that the increased longevity in can1Δ cells is dependent on Gcn4 and Hac1 transcription factors. Further, comparing protein translation changes in can1Δ with other long-lived yeast mutants allowed us to identify common and unique patterns of protein synthesis associated with increased longevity. Together, our analyses reveal an extensive regulatory network in which transcriptional and translational responses coordinately control aging genes and pathways.
Results
CAN1 Gene Deletion Extends Replicative Lifespan and Leads to Distinct Changes in Transcriptional and Translational Profiles
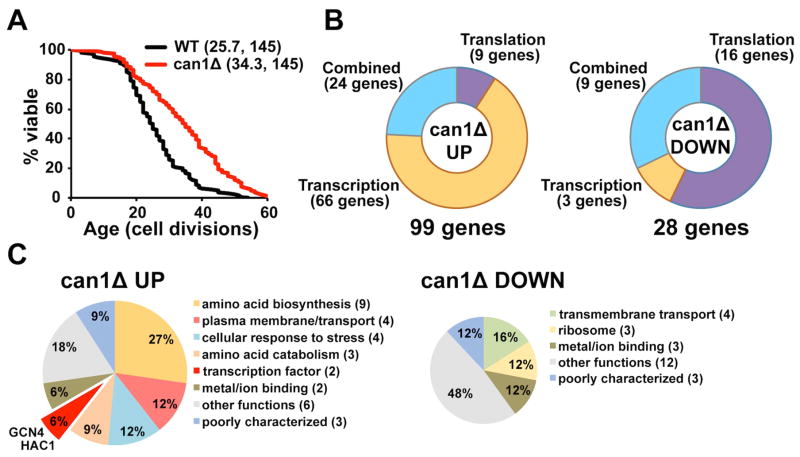
Here we used RNA sequencing (RNA-seq) and ribosome profiling (Ribo-seq) to investigate the role of translational regulation in lifespan extension by CAN1 gene deletion in yeast. We found that deletion of the CAN1 gene, which encodes an arginine transporter, extended replicative lifespan by about 30% (p < 0.0001) (Figure 1A and Figure S1A, related to Figure 1). To test whether intracellular levels of arginine are affected in can1Δ cells, we compared the levels of free amino acids in the can1Δ mutant with those in wild-type cells (Figure S1B, related to Figure 1). Our data demonstrate that arginine levels were decreased about 5-fold in cells lacking CAN1. In addition, can1Δ cells were more resistant to the toxic arginine analog canavanine, compared to wild-type cells, confirming inefficient arginine uptake in these cells (Figure S1C, related to Figure 1).
Figure 1. Deletion of CAN1 Increases Replicative Lifespan in Yeast.
(A) Survival curves for can1Δ and corresponding experiment-matched wild-type cells. Data obtained for the MATa and MATα deletion strains are pooled. Mean lifespans and the number of cells assayed are shown in parentheses.
(B) Transcriptional and translational changes in the can1Δ mutant. Significantly up-regulated and down-regulated genes in can1Δ are grouped in accordance to whether they are affected by a change in mRNA transcription (as quantified by RNA-seq), translation efficiency or by a combined effect.
(C) Pathway enrichment analysis. Top hits down-regulated or up-regulated in can1Δ cells were analyzed using DAVID.
To determine translational changes in can1Δ cells, we used a Ribo-seq approach, which provides information about the extent of translation of individual genes at the genome-wide level (Ingolia et al., 2009). By detecting ribosome occupancy, this technique reports on the combined changes in mRNA levels and “translation efficiency”. In addition, we separately quantified the levels of mRNA abundance by RNA-seq. Analysis of the can1Δ mutant revealed 99 up-regulated and 28 down-regulated genes that were changed more than 1.5-fold at either the transcriptional or translational level (Figure 1B; Table S1 and S2, related to Figure 1). Among genes that were up-regulated in can1Δ cells, 66 (67%) displayed changes exclusively at the mRNA abundance level, whereas 9 (9%) were specifically changed at the translation level. Among down-regulated genes, the majority of the transcripts (16 genes or 57%) were translationally regulated, but were not changed at the transcriptional level. Only 3 genes (11%) were down-regulated at the transcriptional level.
Pathway enrichment analysis using DAVID (Huang da et al., 2009) revealed that the majority of the genes translationally up-regulated in can1Δ encoded proteins involved in amino acid biosynthesis and nitrogen metabolism (Figure 1C and Table S1, related to Figure 1). In addition, the top hits identified in the can1Δ mutant formed several clusters enriched in genes that function in arginine biosynthesis, transmembrane transport, cellular response to stress, as well as genes encoding proteins involved in metal/ion binding. We also observed that levels of footprints (ribosome-protected mRNA fragments) corresponding to Gcn4 and Hac1 transcription factors were elevated in the can1Δ mutant, suggesting that the lifespan extension may be partially mediated by these transcription factors and their downstream targets.
Deletion of CAN1 Translationally Activates the Gcn4 Transcription Factor
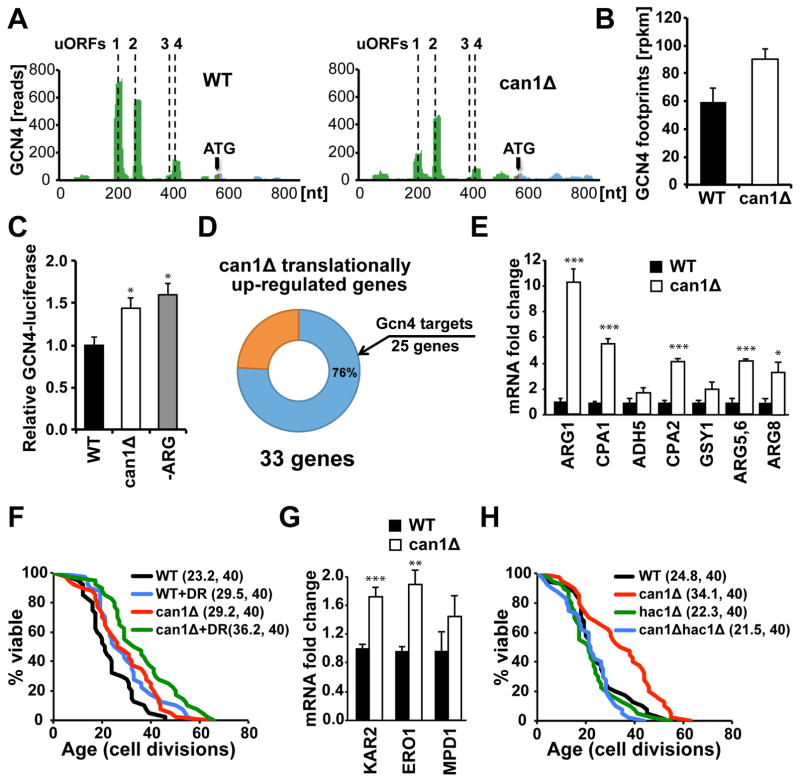
The Gcn4 transcription factor is translationally activated in response to dietary restriction (DR) or depletion of 60S ribosomal subunits, both of which extend replicative lifespan (Steffen et al., 2008). Consistent with the well-established presence of four regulatory upstream open reading frames (uORFs) in the GCN4 5′-untranslated region (UTR) (Grant et al., 1995), our analysis of the can1Δ mutant identified GCN4 mRNA among those that showed significant translational up-regulation. We found that the first two uORFs located upstream of the GCN4 coding sequence were effectively translated in wild-type cells under nutrient replete conditions leading to inhibition of the main ORF translation (Figure 2A). In contrast, in can1Δ cells translation of uORF1 and uORF2 was inhibited, allowing reinitiation at the main ORF and leading to an approximately 1.5-fold increase in GCN4 footprint levels (Figure 2B). To confirm translational up-regulation of GCN4 in the can1Δ strain, we monitored GCN4 expression using a GCN4-luciferase reporter (Steffen et al., 2012). The can1Δ cells exhibited an increase in luciferase activity due to GCN4-luciferase translational up-regulation similar to the levels induced by arginine amino acid starvation in wild-type cells (Figure 2C). We also found that many of the genes up-regulated in the can1Δ mutant were previously annotated as Gcn4 targets. Our analysis using the YEASTRACT database (Teixeira et al., 2014) revealed that out of 33 genes translationally up-regulated in can1Δ cells, 25 (76%) genes are subject to transcriptional regulation by Gcn4 (Figure 2D). The up-regulation of Gcn4 target genes in the can1Δ mutant was confirmed by quantitative PCR (qPCR) (Figure 2E).
Figure 2. Lifespan Extension in can1Δ Mutant is Dependent on Gcn4 and Hac1 Transcription Factors, and Deletion of CAN1 Increases Lifespan Additively with DR.
(A) Deletion of CAN1 translationally activates Gcn4 through upstream translated uORFs.
(B) GCN4 footprint levels (rpkm) in wild-type and can1Δ cells. Results are represented as means ± SEM from three independent experiments.
(C) GCN4-luciferase translation is increased in can1Δ cells similar to the levels induced by argi-nine starvation in wild-type cells (-ARG). Results are represented as means ± SEM from three independent experiments, *p<0.05.
(D) 76% of genes up-regulated in can1Δ mutant are annotated as Gcn4 targets on YEASTRACT.
(E) The up-regulation of Gcn4 target genes in can1Δ cells was verified by qPCR. Results are represented as means ± SEM from three independent experiments; *p<0.05, ***p<0.001. The sequences of the primers used for qPCR are listed in Table S4, related to Figure 2.
(F) Replicative lifespan in can1Δ mutant is further extended (p < 0.05) in response to DR by reducing the concentration of glucose in the media from 2% to 0.05%.
(G) The up-regulation of Hac1 target genes in can1Δ cells was verified by qPCR. Results are represented as means ± SEM from three independent experiments; **p<0.01, ***p<0.001.
(H) Extended lifespan in can1Δ is dependent on the Hac1 transcription factor. Mean lifespans and the number of cells assayed are shown in parentheses.
Lifespan Extension in the can1Δ Mutant is Dependent on Gcn4 and Hac1 Transcription Factors
We then tested whether Gcn4 is required for lifespan extension in the can1Δ mutant. Although both CAN1 and GCN4 are non-essential genes, CAN1 demonstrated synthetic lethality with GCN4 (data not shown). This observation indicates that the can1Δ mutant is strictly dependent on the Gcn4 transcription factor for expression of genes involved in arginine biosynthesis. A previous study also demonstrated that Gcn4 is required for full lifespan extension by DR (Steffen et al., 2008). DR is among the most studied interventions to delay aging across evolutionarily divergent species (Fontana et al., 2010), and in yeast it leads to a 30–40% increase in lifespan (Lin et al., 2000). To test whether Can1 and DR modulate replicative lifespan by a similar mechanism, we examined the effect of DR on lifespan in a can1Δ background. Under DR conditions, which involve reducing the concentration of glucose in the growth media from 2% to 0.05% (Kaeberlein et al., 2004), the lifespan of cells lacking CAN1 was increased 24% (p<0.05) relative to control conditions (Figure 2F).
Next, we asked if Hac1 transcription factor, which was also activated in the can1Δ mutant, is required for lifespan extension similar to Gcn4. The Hac1 transcription factor is the central regulator of the unfolded protein response (UPR) in budding yeast, which activates transcription of genes and protective mechanisms that enhance protein-folding capacity of the endoplasmic reticulum (ER) and lead to alleviation of ER stress (Cox and Walter, 1996). Consistent with activation of Hac1, we found that expression of Hac1 target genes was up-regulated in the can1Δ mutant (Figure 2G), and deletion of HAC1 significantly decreased the lifespan of can1Δ cells (p < 0.001) (Figure 2H). Taken together, these data indicate that lifespan extension in the can1Δ mutant is dependent on Gcn4 and Hac1 transcription factors, and that Can1 modulates longevity by a mechanism that is at least partially distinct from DR.
Loss of CAN1 Increases Lifespan by a Mechanism Distinct From that of Reduced mTOR Signaling
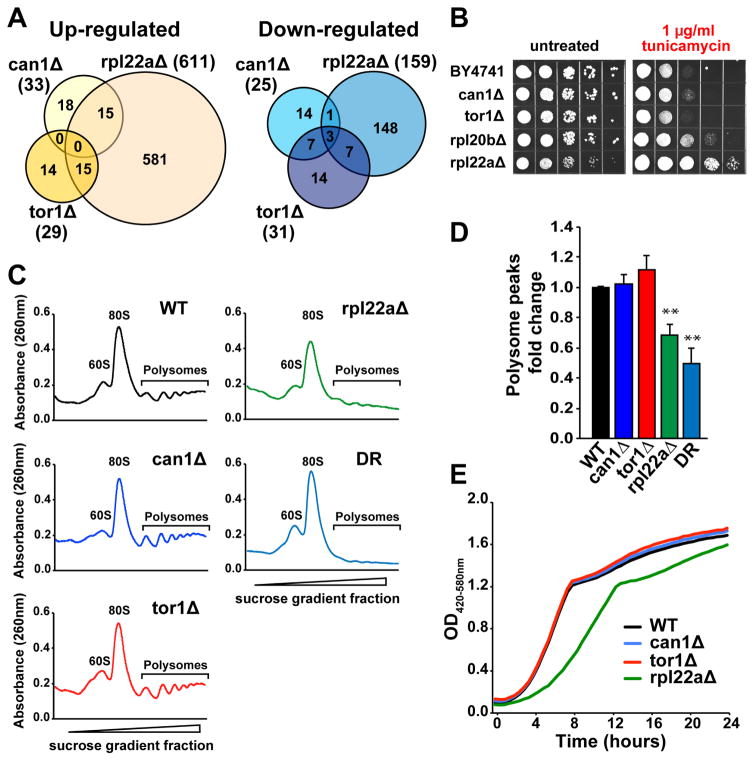
Like DR, deletion of TOR1 increases replicative lifespan in a partially Gcn4-dependent manner. Also reduction of 60S ribosomal subunits through large subunit ribosomal protein (RPL) gene deletions extends lifespan in a manner that is largely Gcn4-dependent (Steffen et al., 2012). Given that the can1Δ mutant has increased Gcn4 translation and expression of Gcn4 target genes, we attempted to test whether Can1 regulates lifespan by a mechanism similar to that of RPL gene deletions or reduced mTOR signaling. For this, we performed Ribo-seq analysis in long-lived strains carrying deletion of RPL22A, a gene encoding a component of the large ribosomal subunit, and TOR1, a component of mTOR complex 1 (Figure S2A and S2B, related to Figure 3). Our data indicate that genes translationally up- or down-regulated in the can1Δ mutant have only limited overlap with the genes whose expression is changed in response to deletion of TOR1 and RPL22A (Figure 3A and Table S1, related to Figure 1). We found that genes whose footprint coverage was increased in can1Δ cells were distinct from those up-regulated in tor1Δ. However, when comparing the footprint data obtained for can1Δ and rpl22aΔ, we observed 15 genes that increase expression in both gene deletion mutants (Table S1, related to Figure 1). Among the genes activated in both can1Δ and rpl22aΔ are GCN4 and known Gcn4 target genes. Expression of the Gcn4 transcriptional targets in rpl22aΔ was further validated by qPCR (Figure S2C, related to Figure 3). As opposed to can1Δ, deletion of RPL22A does not increase expression of the Hac1 transcription factor and its downstream targets (Figure S2D, related to Figure 3). Together, these data suggest that loss of Can1 increases lifespan in a Gcn4-dpenendent manner, similar to rpl22aΔ, through a mechanism that is independent from reduced mTOR signaling.
Figure 3. Deletion of CAN1 Promotes ER Stress Resistance and Activates Gcn4 without Reducing Global mRNA Translation.
(A) Genes translationally up- or down-regulated in can1Δ only partially overlap with rpl22aΔ and tor1Δ.
(B) Cells lacking CAN1 are resistant to tunicamycin. 10× serial dilutions of logarithmically growing cells were spotted on agar plates with indicated concentrations of tunicamycin and incubated for 48 h at 30°C.
(C–D) Polysome profiles of can1Δ, tor1Δ and rpl22aΔ mutants and cells under DR conditions indicate that neither CAN1 nor TOR1 deletion leads to inhibition of global mRNA translation. Representative polysome profiles (C) from three independent experiments are shown, and the area under polysome peaks was quantified using ImageJ software (D); **p<0.01. Error bars denote the standard error of the mean (SEM).
(E) Representative growth curves of can1Δ, tor1Δ and rpl22aΔ mutants. The doubling time for each strain is provided in Figure S2F, related to Figure 3.
Deletion of CAN1 Promotes ER Stress Resistance and Activates the Gcn4 Transcription Factor without Reducing Global mRNA Translation
Studies in model organisms demonstrate that improved ER stress resistance is often associated with increased lifespan (Delaney et al., 2013; Rion and Kawecki, 2007), and a subset of long-lived ribosomal protein gene deletion strains are resistant to ER stress pharmacologically induced by tunicamycin (Steffen et al., 2012). To test whether increased lifespan in can1Δ may be partially attributed to ER stress resistance, we tested sensitivity of these cells to tunicamycin. Our data demonstrate that cells lacking CAN1 are resistant to tunicamycin-induced ER stress (Figure 3B), and this resistance is dependent upon HAC1 (Figure S2E, related to Figure 3). The ribosomal protein gene deletion mutants rpl20bΔ and rpl22aΔ that extend lifespan through a Gcn4-dependent mechanism are also resistant to tunicamycin, whereas tor1Δ cells are not (Figure 3B).
To further characterize the mechanism by which deletion of CAN1 regulates replicative lifespan, we analyzed global mRNA translation. We hypothesized that loss of CAN1 may extend replicative lifespan by inhibiting overall protein synthesis, similar to rpl22aΔ. However, we did not observe any changes in global protein synthesis in this mutant as measured by polysome analysis (Figure 3C and 3D). A similar lack of suppression of mRNA translation was also observed in tor1Δ cells, whereas global protein synthesis was significantly decreased in rpl22aΔ and cells under DR conditions. In addition, we did not observe any changes in growth rate in cells lacking CAN1 (Figure 3E and Figure S2F, related to Figure 3). These data suggest that Gcn4 is activated by deletion of CAN1 through a mechanism distinct from the reduced global mRNA translation observed in rpl22aΔ cells.
RBPs Mediate Translational Changes in Response to Genetic Alterations that Increase Lifespan
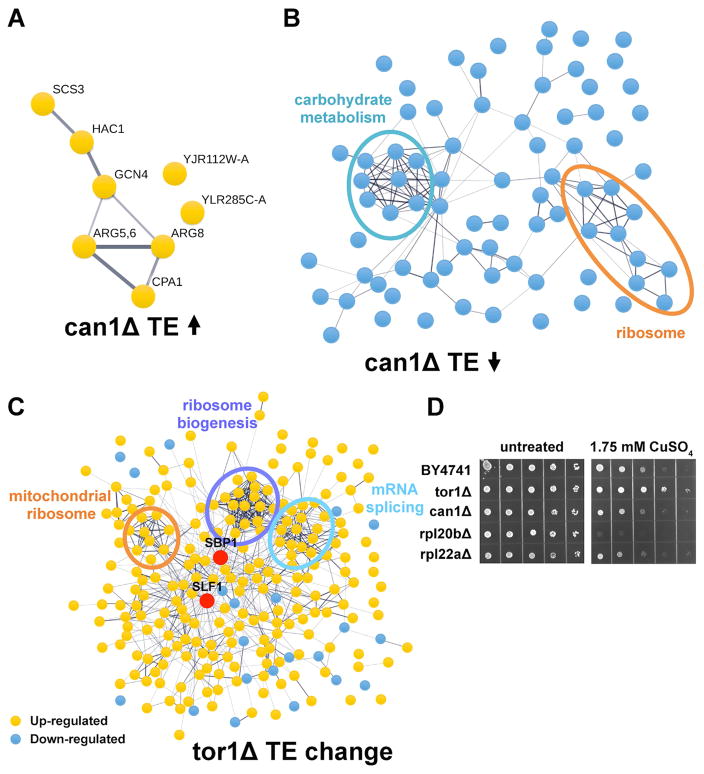
To test if any of the footprint changes observed in the long-lived mutants can be attributed to translational regulation, we calculated translation efficiency (TE) for each mRNA, which represents the number of footprint reads divided by mRNA abundance measurements. We found that among genes whose TE was changed in can1Δ mutant more than 1.5-fold, a majority were down-regulated (Figure 4A and 4B; Table S3, related to Figure 4). These data indicate that deletion of CAN1 is associated with an overall decrease in TE, consistent with activation of the integrated stress response. Specifically, we found that three genes involved in arginine biosynthesis (ARG5,6, ARG8, and CPA1) as well as genes encoding the Gcn4 and Hac1 transcription factors are activated at the level of translation. In addition, we identified two functional clusters among translationally down-regulated genes including genes involved in carbohydrate metabolism (ALD4, CIT1, GPD1, GLK1, HXK1, PNC1, TPS1, and YDL124W) and mitochondrial and cytoplasmic ribosomal protein genes (MRPL25, MRPS8, RPL29, RPL39, RPS29A, RPS30A, RRF1, YDR115W, YNL122C). In contrast to can1Δ cells, a number of genes were activated at the level of translation in cells lacking TOR1, whereas very few genes were down-regulated (Figure 4C). Genes whose TE was significantly increased in tor1Δ were enriched for mitochondrial ribosomal proteins, ribosome biogenesis, and mRNA splicing functions. We also found a significant number of genes that were either up- or down-regulated specifically at the level of translation in the rpl22aΔ mutant (Figure S3, related to Figure 4).
Figure 4. Translational Regulation of Gene Expression in the Long-Lived Mutants.
(A–B) Genes, whose TE was increased (A) or decreased (B) more than 1.5-fold in can1Δ, were visualized using STRING.
(C) Protein-protein interaction network of the proteins whose TE was changed more than 1.5-fold in tor1Δ. Genes whose TE was increased are indicated in yellow, and genes whose TE was decreased are shown in blue. Up-regulated mRNA-binding proteins are highlighted in red.
(D) Deletion of TOR1 confers increased resistance to Cu.
How could the changes in translation in the long-lived mutants be explained? We hypothesized that functionally related transcripts are regulated through a set of mRNA-binding proteins (RBPs) that bind specific sequences or structural elements in mRNAs and coordinately regulate their translation. Intriguingly, we found that several known RBPs previously implicated in translational regulation were activated in the long-lived mutants (Figure 4C and Figure 5A). Among RBPs up-regulated in tor1Δ cells were Slf1 and Sbp1, which represented the most highly connected nodes in the protein interaction network (Figure 4C). Slf1 has been previously shown to be involved in mRNA-specific regulation of translation in response to oxidative stress and to facilitate the translation of stress-responsive mRNAs (Kershaw et al., 2015), whereas Sbp1 is an abundant RBP found in stress granules and P-bodies implicated in translational repression (Mitchell et al., 2013; Segal et al., 2006). It has been also shown that overexpression of Slf1 increases mitochondrial respiration and leads to yeast chronological lifespan extension (Chatenay-Lapointe and Shadel, 2011), and in addition protects cells from copper-induced oxidative stress (Schenk et al., 2012). Consistent with activation of Slf1 in the tor1Δ mutant, cells lacking TOR1 demonstrated increased copper resistance compared to wild-type cells (Figure 4D).
Figure 5. Translational Control by mRNA-Binding Proteins is Closely Interlinked with the Regulation of Gene Expression by Transcription Factors.
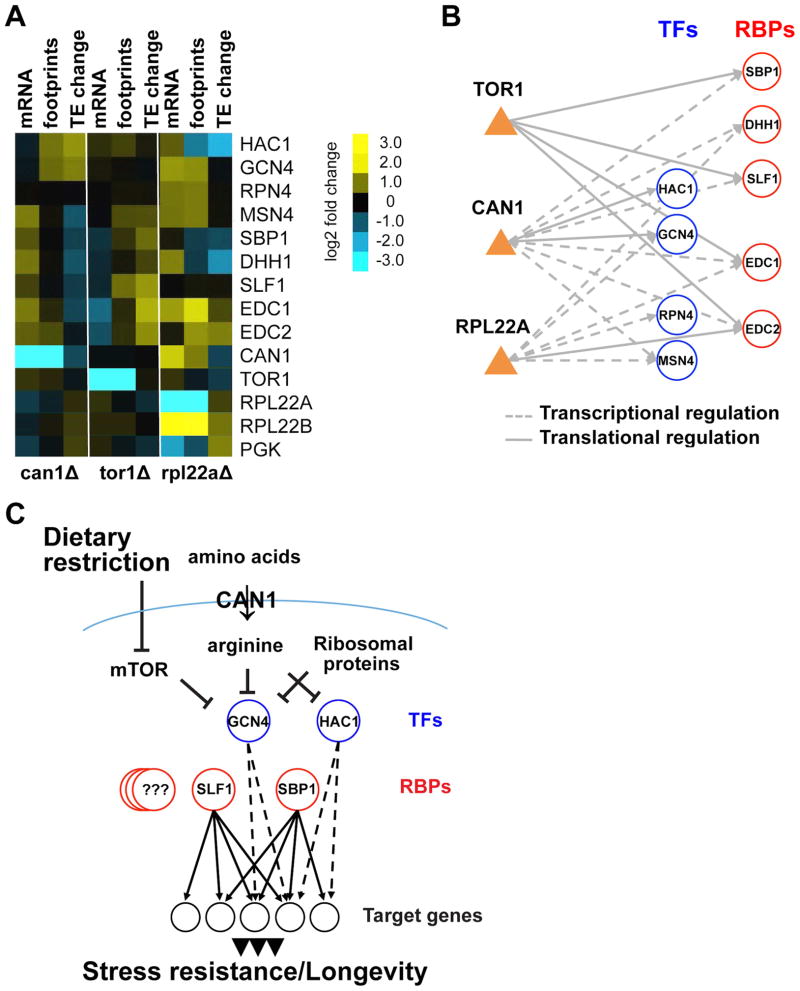
(A) Heatmap displaying transcription factors and mRNA-binding proteins that show significant change in the long-lived mutants either at transcriptional or translational levels. Changes in log2 mRNA and footprints rpkm, and log2 TE change are shown.
(B) The regulatory network showing interactions between transcriptional and translational regulators in the long-lived mutants. Genetic alterations (CAN1, TOR1, RPL22A) are represented as orange triangles. Aging-associated transcription factors (TFs) and mRNA-binding proteins (RBPs) are shown as blue and red nodes respectively.
(C) A model for the role of post-transcriptional gene regulation in aging. Post-transcriptional control by RBPs is closely interlinked with the regulation of transcription by TFs allowing coordinated changes in protein translation.
Comparing transcriptional and translational profiles among mutants allowed us to identify common and unique transcription factors and RBPs that might mediate increased lifespan in each mutant (Figure 5A). Since footprint levels report on the combined changes in transcript levels and translation efficiency, we also could differentiate whether these factors are regulated transcriptionally or translationally (Figure 5B). Our analysis revealed that Gcn4 and Hac1 transcription factors were specifically activated at the translational level in can1Δ. On the other hand, we found that the Msn4 transcription factor and several RBPs, including Sbp1, Dhh1, Slf1, Edc1, and Edc2, were transcriptionally up-regulated in cells lacking CAN1, but were not changed at the translational level. In turn, inhibition of mTOR signaling in tor1Δ cells led to increased translation efficiency of these RBPs. In the case of rpl22aΔ, we found activation of Gcn4, Rpn4, and Msn4 transcription factors, which were transcriptionally up-regulated. However, Sbp1 and Slf1 mRNA-binding proteins were not activated in this mutant. Together, our data indicate that genetic alterations that extend lifespan lead to the activation of several common regulatory proteins (i.e. transcription factors and RBPs) that coordinate transcriptional and translational outputs and enhance translation of specific mRNAs that facilitate response to stress.
Discussion
Aging is a complex process that involves multiple metabolic and regulatory pathways. In order to elucidate mechanisms underlying aging, it is important to understand how different aging pathways are interconnected and coordinately regulated. To investigate the complex interaction between different aging pathways, we used systems biology approaches to develop a regulatory interaction network linking factors involved in translational regulation with aging-associated genes. Specifically, we used Ribo-seq to identify global translational changes in three single-gene deletion mutants previously identified in genome-wide yeast replicative lifespan screens, including can1Δ, tor1Δ and rpl22aΔ. We further compared changes in translational level and mRNA abundance to identify groups of genes that are regulated in these mutants by changing translation efficiency. This analysis allowed us to (i) identify genes that are translationally regulated in the long-lived mutants, (ii) identify common and unique patterns of protein synthesis associated with increased longevity, and (iii) uncover regulatory factors that link gene expression profiles with aging.
We found that CAN1 amino acid permease deficiency leads to global changes in transcriptional and translational profiles. Analysis of genes activated by CAN1 gene deletion specifically at the translation level demonstrates a narrow and specific translational activation of transcription factors Gcn4 and Hac1, in addition to genes involved in arginine biosynthesis. In contrast, deletion of CAN1 leads to reduced translation of a larger set of proteins. Enhanced translation of Gcn4 and Hac1 in can1Δ suggests that these transcription factors might mediate the lifespan extension effect in this mutant. Consistent with this model, we found that deletion of both CAN1 and GCN4 is synthetically lethal, whereas deletion of HAC1 significantly reduced lifespan in can1Δ cells. Consistent with increased Gcn4 translation, we found that 76% of the genes up-regulated in can1Δ were previously annotated as Gcn4 targets on YEASTRACT (Teixeira et al., 2014). Together our data indicate that deletion of CAN1 extends replicative lifespan by activating an integrated stress response program leading to translational activation of genes, including the transcription factors Gcn4 and Hac1, whose products mediate adaptation to stress. Importantly, the lifespan of cells lacking CAN1 was further increased upon DR compared to non-restricted conditions, leaving open the possibility that Can1 extends lifespan by a mechanism that is at least partially distinct from DR.
Intriguingly, we identified several RBPs among genes that were changed in the long-lived mutants either at a transcriptional or translational level. In agreement with the potential role of RBPs in translational regulation, we observed multiple targets of RBPs that changed their level of translation and/or translation efficiency. One such example includes Slf1, which is activated in tor1Δ cells. Slf1 has been previously implicated in translational regulation of genes involved in oxidative stress response (Kershaw et al., 2015). During stress, Slf1 interacts with translating ribosomes and mediates preferential translation of its target mRNAs including oxidative stress response genes, ribosomal protein genes, as well as genes involved in ribosome biogenesis (Kershaw et al., 2015; Schenk et al., 2012). Notably, inhibition of mTOR signaling leads to translational activation of multiple Slf1 targets and oxidative stress resistance (Figure 4C and D).
Previous studies have shown that many of the yeast deletion mutants that extend lifespan also demonstrate resistance to multiple stresses (Delaney et al., 2013). We recently found that deletion of ALG12 and BST1 extends yeast replicative lifespan, in part, by activating multiple cytoprotective pathways that confer resistance to stress (Labunskyy et al., 2014). Here, we show that the can1Δ mutant is resistant to ER stress. One well-studied mechanism that is required for restoring homeostasis in response to ER stress involves the Hac1-dependent transcriptional response, which facilitates protein folding in the ER (Walter and Ron, 2011). Increased ER stress resistance in cells lacking CAN1 is consistent with the activation of Hac1 in this mutant. Interestingly, rpl20bΔ and rpl22aΔ mutants are also resistant to ER stress. However, increased ER stress resistance in these mutants is independent of Hac1, as deletion of HAC1 in these cells does not prevent ER stress resistance (Steffen et al., 2012).
Our data also provide an explanation for the variation in phenotypes between can1Δ, tor1Δ, and rpl22aΔ mutants. Whereas deletion of RPL22A leads to global inhibition of translation, deletion of either CAN1 or TOR1 does not alter overall levels of translation. In addition, can1Δ and rpl22aΔ cells were more resistant to tunicamycin compared to wild-type cells. However, tor1Δ cells did not show increased ER stress resistance and instead were more resistant to oxidative stress. Our Ribo-seq analysis revealed that post-transcriptional changes might be responsible for divergence in phenotypes in the long-lived mutants. As noted earlier, while both can1Δ and rpl22aΔ are resistant to ER stress, the lifespan extension in the can1Δ mutant is dependent on the Hac1 transcription factor. But in the case of rpl22aΔ, deletion of Hac1 did not completely prevent resistance to ER stress. Similarly, inhibition of mTOR signaling activated downstream post-transcriptional reprograming through activation of Slf1, a RBP responsible for the activation of oxidative stress response genes.
Together, our experiments revealed that gene deletions extending lifespan in yeast lead to activation of several common transcription factors and RBPs that coordinately regulate gene expression programs and enhance translation of transcripts that facilitate cellular response to stress (Figure 5C). On the other hand, the long-lived strains also have specific adaptations that are not shared with other mutants, suggesting that there might be multiple ways to extend lifespan. Importantly, our data demonstrate that translational control plays a key role in regulation of stress signaling pathways and longevity. Given that there are more than 600 RBPs present in the yeast genome, it is expected that additional RBPs involved in translational regulation during aging will be uncovered by analyzing other long-lived mutants, adding additional levels of complexity.
EXPERIMENTAL PROCEDURES
Strains and media
All yeast strains (Table S5, related to Experimental Procedures) were derived from the parent strains of the haploid yeast ORF deletion collection (Winzeler et al., 1999). The double mutant strain combining can1Δ with hac1Δ was prepared by a standard PCR-based gene disruption method. Cells were grown at 30°C in standard YPD medium (1.0% yeast extract, 2.0% peptone, and 2.0% glucose) unless otherwise stated. For DR experiments, the concentration of glucose in the growth medium was reduced to 0.05%.
Replicative lifespan and growth rate analyses
Lifespan assays were carried out as described previously (Kaeberlein et al., 2004). In short, newborn daughter cells were isolated from each strain and then were allowed to grow on agar plates. Cells were monitored for cell divisions every 90 min, and subsequent daughters were microdissected and counted, until cells stopped dividing. Yeast growth rates were analyzed using the Bi-oscreen C automated microbiology growth curve analysis system (Growth Curves USA).
Ribosome profiling and mRNA sequencing
Yeast cultures were inoculated into 500 ml of standard YPD medium at the initial OD600 < 0.1 and incubated at 30°C with shaking until the OD600 reached 0.5. Cells were collected by filtering through 0.45 μm filter (Millipore) with glass holder. Pellets were scraped with spatula, flash frozen in liquid nitrogen and stored at −80°C. Yeast extracts were prepared by cryogrinding the cell paste with BioSpec cryomill. Aliquots of cell lysates were used for extraction of footprints (short ribosome-protected mRNA fragments) and isolation of total RNA. The RNA-seq and Ribo-seq libraries were prepared using ARTseq Ribosome Profiling kit (Illumina) as described previously (Labunskyy et al., 2014) and sequenced using the Illumina HiSeq 2000 platform.
Statistical methods
Statistical analysis of the lifespan data was performed by calculating p values using the Wilcox-on Rank-Sum test (Wilcoxon, 1946). Statistical significance of the GCN4 quantification, poly-some analysis, and qPCR data was determined using two-tailed Student’s t test. Results are represented as means ± SEM of three independent experiments.
Amino acid analysis, spot assays, bioinformatics analyses, Gcn4-luciferase assay, qPCR, and polysome analysis are described in detail in Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health grant AG040191 and the UW Nathan Shock Center or Excellence in the Basic Biology of Aging P30AG013280.
Footnotes
AUTHOR CONTRIBUTIONS
Conceptualization, V.M.L.; Investigation, C.B., B.W., J.L. and V.M.L.; Formal Analysis, C.B., B.W., J.L., B.K.K., M.K., and V.M.L; Writing – Original Draft, C.B. and V.M.L.; Writing – Review & Editing, C.B., B.W., J.L., B.K.K., M.K., and V.M.L; Resources, B.K.K. and M.K.; Supervision, V.M.L.
ACCESSION NUMBERS
High-throughput sequence data have been uploaded to GEO database under accession number GSE85198.
Supplemental Information includes three figures, five tables, and Extended Supplemental Procedures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad M, Bussey H. Yeast arginine permease: nucleotide sequence of the CAN1 gene. Curr Genet. 1986;10:587–592. doi: 10.1007/BF00418125. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Buffenstein R. Cutting back on the essentials: Can manipulating intake of specific amino acids modulate health and lifespan? Ageing Res Rev. 2016 doi: 10.1016/j.arr.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabreiro F, Au C, Leung KY, Vergara-Irigaray N, Cocheme HM, Noori T, Weinkove D, Schuster E, Greene ND, Gems D. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 2013;153:228–239. doi: 10.1016/j.cell.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatenay-Lapointe M, Shadel GS. Repression of mitochondrial translation, respiration and a metabolic cycle-regulated gene, SLF1, by the yeast Pumilio-family protein Puf3p. PloS One. 2011;6:e20441. doi: 10.1371/journal.pone.0020441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Fabrizio P, Ge H, Longo VD, Li LM. Inference of transcription modification in long-live yeast strains from their expression profiles. BMC Genomics. 2007;8:219. doi: 10.1186/1471-2164-8-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JS, Walter P. A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell. 1996;87:391–404. doi: 10.1016/s0092-8674(00)81360-4. [DOI] [PubMed] [Google Scholar]
- Dang W, Sutphin GL, Dorsey JA, Otte GL, Cao K, Perry RM, Wanat JJ, Saviolaki D, Murakami CJ, Tsuchiyama S, et al. Inactivation of Yeast Isw2 Chromatin Remodeling Enzyme Mimics Longevity Effect of Calorie Restriction via Induction of Genotoxic Stress Response. Cell Metab. 2014;19:952–966. doi: 10.1016/j.cmet.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney JR, Ahmed U, Chou A, Sim S, Carr D, Murakami CJ, Schleit J, Sutphin GL, An EH, Castanza A, et al. Stress profiling of longevity mutants identifies Afg3 as a mitochondrial determinant of cytoplasmic mRNA translation and aging. Aging Cell. 2013;12:156–166. doi: 10.1111/acel.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever TE. Gene-specific regulation by general translation factors. Cell. 2002;108:545–556. doi: 10.1016/s0092-8674(02)00642-6. [DOI] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandison RC, Piper MD, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant CM, Miller PF, Hinnebusch AG. Sequences 5′ of the first upstream open reading frame in GCN4 mRNA are required for efficient translational reinitiation. Nucleic Acids Res. 1995;23:3980–3988. doi: 10.1093/nar/23.19.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- He C, Tsuchiyama SK, Nguyen QT, Plyusnina EN, Terrill SR, Sahibzada S, Patel B, Faulkner AR, Shaposhnikov MV, Tian R, et al. Enhanced longevity by ibuprofen, conserved in multiple species, occurs in yeast through inhibition of tryptophan import. PLoS Genet. 2014;10:e1004860. doi: 10.1371/journal.pgen.1004860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan DJ, Riordan DP, Gerber AP, Herschlag D, Brown PO. Diverse RNA-binding proteins interact with functionally related sets of RNAs, suggesting an extensive regulatory system. PLoS Biol. 2008;6:e255. doi: 10.1371/journal.pbio.0060255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. 2009;324:218–223. doi: 10.1126/science.1168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol. 2010;11:113–127. doi: 10.1038/nrm2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JC, Jaruga E, Repnevskaya MV, Jazwinski SM. An intervention resembling caloric restriction prolongs life span and retards aging in yeast. FASEB J. 2000;14:2135–2137. doi: 10.1096/fj.00-0242fje. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Kennedy BK. Hot topics in aging research: protein translation and TOR signaling, 2010. Aging Cell. 2011;10:185–190. doi: 10.1111/j.1474-9726.2010.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Kirkland KT, Fields S, Kennedy BK. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2004;2:e296. doi: 10.1371/journal.pbio.0020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw CJ, Costello JL, Castelli LM, Talavera D, Rowe W, Sims PF, Ashe MP, Hubbard SJ, Pavitt GD, Grant CM. The yeast La related protein Slf1p is a key activator of translation during the oxidative stress response. PLoS Genet. 2015;11:e1004903. doi: 10.1371/journal.pgen.1004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labunskyy VM, Gerashchenko MV, Delaney JR, Kaya A, Kennedy BK, Kaeberlein M, Gladyshev VN. Lifespan extension conferred by endoplasmic reticulum secretory pathway deficiency requires induction of the unfolded protein response. PLoS Genet. 2014;10:e1004019. doi: 10.1371/journal.pgen.1004019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner DH, Bahler J. Translational control of gene expression from transcripts to transcriptomes. Int Rev Cell Mol Biol. 2008;271:199–251. doi: 10.1016/S1937-6448(08)01205-7. [DOI] [PubMed] [Google Scholar]
- Li W, Li X, Miller RA. ATF4 activity: a common feature shared by many kinds of slow-aging mice. Aging Cell. 2014;13:1012–1018. doi: 10.1111/acel.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Lin SS, Manchester JK, Gordon JI. Enhanced gluconeogenesis and increased energy storage as hallmarks of aging in Saccharomyces cerevisiae. J Biol Chem. 2001;276:36000–36007. doi: 10.1074/jbc.M103509200. [DOI] [PubMed] [Google Scholar]
- McCormick MA, Delaney JR, Tsuchiya M, Tsuchiyama S, Shemorry A, Sim S, Chou AC, Ahmed U, Carr D, Murakami CJ, et al. A Comprehensive Analysis of Replicative Lifespan in 4,698 Single-Gene Deletion Strains Uncovers Conserved Mechanisms of Aging. Cell Metab. 2015;22:895–906. doi: 10.1016/j.cmet.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwee J, Bubb K, Thomas JH. Transcriptional outputs of the Caenorhabditis elegans forkhead protein DAF-16. Aging Cell. 2003;2:111–121. doi: 10.1046/j.1474-9728.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- Mitchell SF, Jain S, She M, Parker R. Global analysis of yeast mRNPs. Nat Struct Mol Biol. 2013;20:127–133. doi: 10.1038/nsmb.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Pletcher SD, Macdonald SJ, Marguerie R, Certa U, Stearns SC, Goldstein DB, Partridge L. Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Curr Biol. 2002;12:712–723. doi: 10.1016/s0960-9822(02)00808-4. [DOI] [PubMed] [Google Scholar]
- Rion S, Kawecki TJ. Evolutionary biology of starvation resistance: what we have learned from Drosophila. J Evol Biol. 2007;20:1655–1664. doi: 10.1111/j.1420-9101.2007.01405.x. [DOI] [PubMed] [Google Scholar]
- Schenk L, Meinel DM, Strasser K, Gerber AP. La-motif-dependent mRNA association with Slf1 promotes copper detoxification in yeast. RNA. 2012;18:449–461. doi: 10.1261/rna.028506.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal SP, Dunckley T, Parker R. Sbp1p affects translational repression and decapping in Saccharomyces cerevisiae. Mol Cell Biol. 2006;26:5120–5130. doi: 10.1128/MCB.01913-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidrauski C, McGeachy AM, Ingolia NT, Walter P. The small molecule ISRIB reverses the effects of eIF2alpha phosphorylation on translation and stress granule assembly. eLife. 2015;4:e05033. doi: 10.7554/eLife.05033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson CE, Ashe MP. Adaptation to stress in yeast: to translate or not? Biochem Soc Trans. 2012;40:794–799. doi: 10.1042/BST20120078. [DOI] [PubMed] [Google Scholar]
- Steffen KK, MacKay VL, Kerr EO, Tsuchiya M, Hu D, Fox LA, Dang N, Johnston ED, Oakes JA, Tchao BN, et al. Yeast life span extension by depletion of 60s ribosomal subunits is mediated by Gcn4. Cell. 2008;133:292–302. doi: 10.1016/j.cell.2008.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen KK, McCormick MA, Pham KM, MacKay VL, Delaney JR, Murakami CJ, Kaeberlein M, Kennedy BK. Ribosome deficiency protects against ER stress in Saccharomyces cerevisiae. Genetics. 2012;191:107–118. doi: 10.1534/genetics.111.136549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira MC, Monteiro PT, Guerreiro JF, Goncalves JP, Mira NP, dos Santos SC, Cabrito TR, Palma M, Costa C, Francisco AP, et al. The YEASTRACT database: an upgraded information system for the analysis of gene and genomic transcription regulation in Saccharomyces cerevisiae. Nucleic Acids Res. 2014;42:D161–166. doi: 10.1093/nar/gkt1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetanova NG, Klass DM, Salzman J, Brown PO. Proteome-wide search reveals unexpected RNA-binding proteins in Saccharomyces cerevisiae. PloS One. 2010;5:e12671. doi: 10.1371/journal.pone.0012671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- Wierman MB, Matecic M, Valsakumar V, Li M, Smith DL, Jr, Bekiranov S, Smith JS. Functional genomic analysis reveals overlapping and distinct features of chronologically long-lived yeast populations. Aging. 2015;7:177–194. doi: 10.18632/aging.100729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcoxon F. Individual comparisons of grouped data by ranking methods. J Econ Entomol. 1946;39:269. doi: 10.1093/jee/39.2.269. [DOI] [PubMed] [Google Scholar]
- Winzeler EA, Shoemaker DD, Astromoff A, Liang H, Anderson K, Andre B, Bangham R, Benito R, Boeke JD, Bussey H, et al. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science. 1999;285:901–906. doi: 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]
- Wood SH, Craig T, Li Y, Merry B, de Magalhaes JP. Whole transcriptome sequencing of the aging rat brain reveals dynamic RNA changes in the dark matter of the genome. Age. 2013;35:763–776. doi: 10.1007/s11357-012-9410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.