Abstract
Background:
Carbapenemase-producing Enterobacteriaceae (CPE) have increased in recent years leading to limitations of treatment options. The present study was undertaken to detect CPE, risk factors for acquiring them and their impact on clinical outcomes.
Methods:
This retrospective observational study included 111 clinically significant Enterobacteriaceae resistant to cephalosporins subclass III and exhibiting a positive modified Hodge test. Screening for carbapenemase production was done by phenotypic methods, and polymerase chain reaction was performed to detect genes encoding them. Retrospectively, the medical records of the patients were perused to assess risk factors for infections with CPE and their impact. The data collected were duration of hospital stay, Intensive Care Unit (ICU) stay, use of invasive devices, mechanical ventilation, the presence of comorbidities, and antimicrobial therapy. The outcome was followed up. Univariate and multivariate analysis of the data were performed using SPSS software.
Results:
Carbapenemase-encoding genes were detected in 67 isolates. The genes detected were New Delhi metallo-β-lactamase, Verona integron-encoded metallo-β-lactamase, and oxacillinase-181.Although univariate analysis identified risk factors associated with acquiring CPE infections as ICU stay (P = 0.021), mechanical ventilation (P = 0.013), indwelling device (P = 0.011), diabetes mellitus (P = 0.036), usage of multiple antimicrobial agents (P = 0.007), administration of carbapenems (P = 0.042), presence of focal infection or sepsis (P = 0.013), and surgical interventions (P = 0.016), multivariate analysis revealed that all these factors were insignificant. Mortality rate was 56.7% in patients with CPE infections. By both univariate and multivariate analysis of impact of the variables on mortality in these patients, the significant factors were mechanical ventilation (odds ratio [OR]: 0.141, 95% confidence interval [CI]: 0.024–0.812) and presence of indwelling invasive device (OR: 8.034; 95% CI: 2.060–31.335).
Conclusion:
In this study, no specific factor was identified as an independent risk for acquisition of CPE infection. However, as it is evident by multivariate analysis, there is an increased risk of mortality in patients with CPE infections when they are ventilated and are supported by indwelling devices.
Key words: Carbapenemase, Enterobacteriaceae, outcomes, risk factors
INTRODUCTION
Enterobacteriaceae, namely Escherichia coli and Klebsiella pneumoniae, are the most common human pathogens, causing infections that range from cystitis to pyelonephritis, septicemia, pneumonia, peritonitis, and meningitis.[1,2] The other Enterobacteriaceae causing infections in humans include Citrobacter species, Enterobacter species, Serratia marcescens, Proteus spp., and Providencia spp. These organisms persist and spread rapidly in the health care settings by hand carriage as well as contaminated food and water.[1]
The carbapenems are the main stay of therapy for treating serious and life-threatening infections caused by Enterobacteriaceae producing extended-spectrum beta-lactamase. However, the subsequent emergence of resistance to carbapenems has led to limited therapeutic options. This resistance is mediated by the production of β-lactamases (carbapenemases) that hydrolyze the carbapenems, changes in outer-membrane porins or by upregulation of efflux pumps. The most important carbapenemases in Enterobacteriaceae are Class A K. pneumoniae carbapenemase (KPC) enzymes, Class B metallo-beta-lactamases (MBL) such as New Delhi metallo-β-lactamase (NDM), Verona integron-encoded metallo-β-lactamase (VIM), Imipenemase (IMP), and Class D oxacillinase (OXA)-48 and its variants.[1,3]
Several factors have been reported to increase the risk of colonization and infection with carbapenemase-producing Enterobacteriaceae (CPE). Risk factors for infection with CPE include severe underlying illness, prolonged hospital stay, the presence of invasive medical devices, and antibiotic use.[4,5,6,7] CPE have been associated with adverse clinical and economic outcomes, including increased mortality, increased length of stay, delay in the institution of effective therapy, decreased functional status on discharge, and increased cost of health care.[8,9,10,11]
It is imperative that risk factors for infection with these organisms are clearly identified so that effective strategies can be developed to curtail the emergence and spread of these strains. This observational study was undertaken to detect carbapenemase production among Enterobacteriaceae to identify the risk factors for acquiring the infection with CPE and also the factors influencing mortality in infected patients.
METHODS
The study protocol was approved by the Institutional Ethics Committee. The study was conducted in a 1600-bedded University teaching hospital between April and October 2010. It was a retrospective observational study. The study included 111 clinically significant, nonrepetitive Enterobacteriaceae resistant to one of the cephalosporins subclass III, isolated from 96 patients admitted to Intensive Care Units (ICUs) and 15 patients in non-ICU settings of the health-care facility. It included K. pneumoniae (52), E. coli (25), Citrobacter freundii (16), Enterobacter cloacae (16), and Providencia rettgeri (2). Species identification was carried out by Microscan Walkaway 96 using Gram-negative panels (Siemens Health-care Diagnostics Inc., Sacramento CA, USA). The source of the isolates was blood (23), respiratory secretions (24), exudative specimens (19), and urine (45). Commensals were differentiated from pathogens for isolates obtained from nonsterile sites (respiratory tract, urinary tract, and wound swabs) by ascertaining their significance based on clinical history, the presence of the organism in the Gram-stain, presence of intracellular forms of the organism, and pure growth in culture with significant colony count.
Antimicrobial susceptibility testing was done by disc diffusion method. The antimicrobial agents tested were aztreonam (30 μg), cefepime (30 μg), piperacillin-tazobactam (100/10 μg), ciprofloxacin (5 μg), amikacin (30 μg), imipenem (10 μg), and meropenem (10 μg) (Hi-Media Laboratories, India). The results were interpreted as per Clinical and Laboratory Standards Institute (CLSI) 2014 guidelines.[12] Susceptibility to tigecycline was performed using 15 μg disc (BBL™ BD, USA) and interpretation of zone of inhibition was done using the United States Food and Drug Administration, tigecycline susceptibility breakpoints criteria.[13] Minimum inhibitory concentration (MIC) to imipenem and meropenem was determined by broth microdilution method and results interpreted according to CLSI document M100-S 24. MIC to colistin was determined by the E-test (Biomerieux, SA, France).[12]
Carbapenamase production was screened by the modified Hodge test (MHT) and MBL production by inhibitor potentiated disk diffusion test with ethylene diamine tetra acetic acid (EDTA). Screening for KPC was done using phenylboronic acid.[12,14] All study isolates were subjected to polymerase chain reaction (PCR) using primers targeting blaNDM-1, blaKPC, blaVIM, blaIMP, and blaOXA-181 irrespective of their susceptibility profile to carbapenems.[15,16,17,18] Multiplex PCR was done to detect all the MBL encoding genes.[19] To optimize PCR, strains previously confirmed by PCR and gene sequencing were used as positive controls and E. coli ATCC 25922 was used as negative control. PCR products of representative isolates were purified using PCR DNA purification kit (QIA quick Gel Extraction Kit, Qiagen, Valencia, CA, USA) and subjected to automated DNA sequencing (ABI 3100, Genetic Analyzer, Applied Biosystems, Foster City, CA, USA). The aligned sequences were analyzed with the Bioedit sequence program and similarities searches for the nucleotide sequences were performed with BLAST program (http://www.ncbi.nlm.nih.gov).
Data were sought retrospectively from medical records and clinical microbiology laboratory. Variables analyzed as risk factors included (1) demographics (age, gender), (2) presence of comorbid conditions such as chronic renal failure (CRF), chronic obstructive pulmonary disease (COPD), connective tissue disorders, malignancy, coronary artery disease, and multiple injuries due to road traffic accidents, (3) hospitalization history such as duration of hospital stay and ICU stay, (4) exposure to invasive interventions (central lines, urinary catheters, drainage devices, mechanical ventilation, dialysis, and procedures such as endoscopic procedures, invasive surgery), (5) receipt of immunosuppressive therapy (chemotherapy or immunosuppressive agents, corticosteroids), (6) diabetes mellitus, (7) antimicrobial agents used and their duration, and (8) presence of focal or generalized infections. The treatment instituted was recorded and the outcome was followed up. All the clinical data and microbiological results were tabulated.
The data were analyzed with the SPSS version 17.0 (Chicago: SPSS Inc.). Proportions were compared using Chi-square test/Mann-Whitney test. Differences were considered significant if P < 0.05. (if P < 0.05 [0.02–0.05] = 95%; P < 0.01 [0.002–0.01] = 99%; P < 0.001 [0.000–0.001] = 99.9%). In the risk factor analysis, multivariate logistic regression models were used to compare CPE and non-CPE infections. Significant variables identified in the univariate analysis were included in a stepwise selection multivariate logistic regression model if P < 0.05. Odds ratios (OR) and 95% confidence intervals (CI) were calculated to evaluate the strength of any association. In the outcomes analysis, both CPE and non-CPE infected patients who died were compared with those who survived to determine factors predicting mortality by univariate and subsequently multivariate logistic regression analysis.
RESULTS
All the study isolates were resistant to cefotaxime, ceftazidime, aztreonam, cefepime, piperacillin-tazobactam, and ciprofloxacin. Resistance to amikacin was 76.6% (85). Resistance to imipenem and/or meropenem was detected 45% (50) of the isolates both by disc diffusion testing and MIC determination. Susceptibility to tigecycline and colistin was universal. All the study isolated exhibited a positive MHT. MBL and KPC screen tests were positive in 54 and 36 isolates, respectively [Table 1].
Table 1.
Results of phenotypic tests and polymerase chain reaction
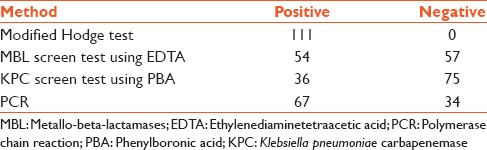
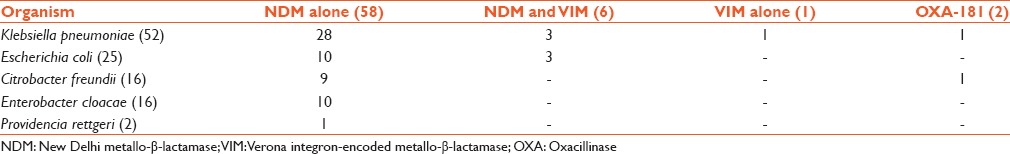
Carbapenemase-encoding genes were detected in 67 isolates [Table 2]. Of the 111 Enterobacteriaceae included in the study, 58 isolates produced NDM, 6 isolates produced both VIM and NDM. OXA-181 was produced by 2 isolates and VIM by one isolate. All the VIM and OXA-181 producers exhibited resistance to carbapenems but 27 NDM producers were susceptible to carbapenems as per CLSI 2014 interpretation with the MIC to imipenem and meropenem ranging from 0.03 to 1 mg/L. BlaKPC and the other MBLs such as blaIMP, blaGIM, blaSIM, and blaSPM were not present in any of the study isolate. Of the 67 CPE, 62 were from patients in ICU and 5 from postsurgical ward. Mortality rate was 56.7% in patients infected with CPE. The mortality rate was 47.7% in patients with carbapenemase-negative Enterobacteriaceae infections.
Table 2.
Distribution of carbapenemase encoding genes in Enterobacteriaceae
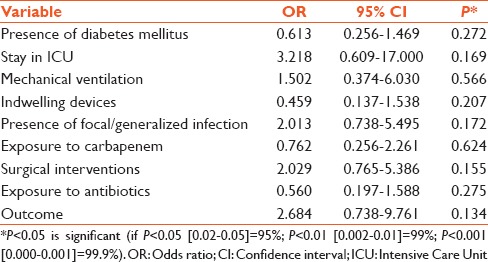
Univariate analysis revealed that male gender (P = 0.050), stay in ICU (P = 0.021), mechanical ventilation (P = 0.013), presence of multiple indwelling device (P = 0.011) including drains and central lines, presence of diabetes mellitus (P = 0.036), presence of focal infection or sepsis (P = 0.013), surgical interventions (P = 0.016), and usage of multiple antimicrobial agents (P = 0.007) and carbapenems (P = 0.042) were significant risk factors influencing the acquisition of CPE. The mean duration of hospital stay was 26.4 days in patients with carbapenemase-positive Enterobacteriaceae infections and 22.98 in those with carbapenemase-negative Enterobacteriaceae infections. The duration of hospital stay (P = 0.939) was prolonged in both groups, but it was not a significant risk factor. The presence of other comorbid disease (P = 0.636) and the outcome (P = 0.353) of the infection were not significant [Table 3]. The variables were subjected to multivariate analysis which revealed that none of the factors assessed were significantly associated with acquiring CPE [Table 4].
Table 3.
Univariate analysis of factors influencing the acquisition of carbapenemase-producing Enterobacteriaceae
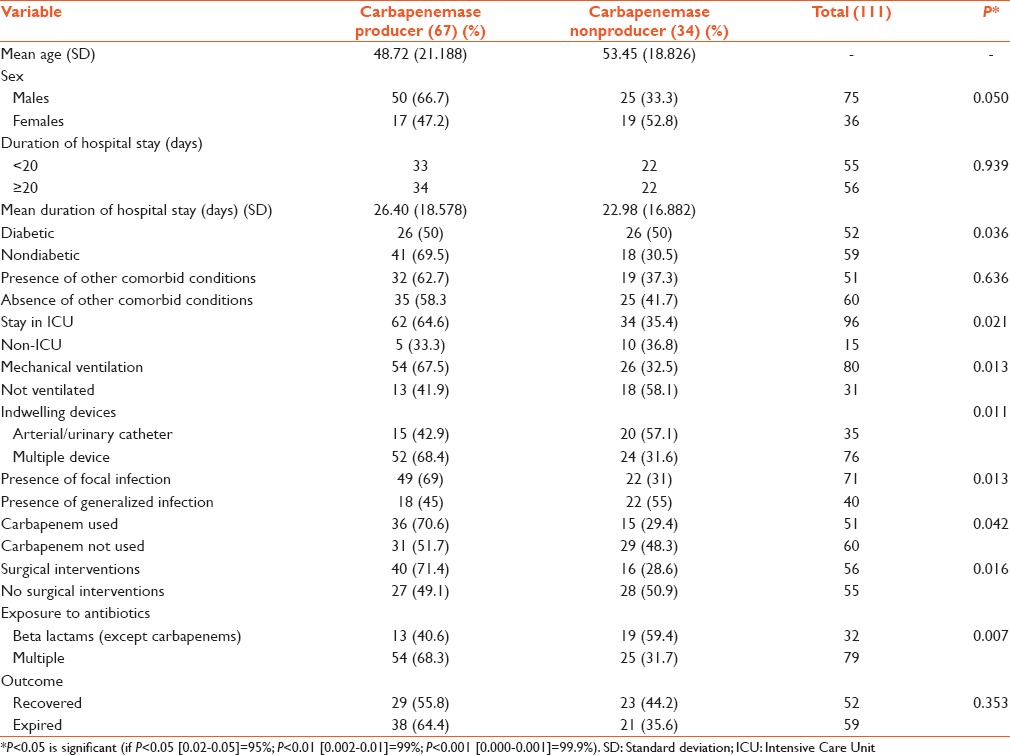
Table 4.
Multivariate analysis of factors influencing the acquisition of carbapenemase producing Enterobacteriaceae
Univariate analysis revealed that male gender (P = 0.004), stay in ICU (P = 0.000), mechanical ventilation (P = 0.000), presence of indwelling device (P = 0.000), presence of comorbid conditions (P = 0.009), presence of focal infection or sepsis (P = 0.000), usage of multiple antimicrobial agents (P = 0.012), and carbapenem (P = 0.000) were all factors influencing mortality in patients infected with CPE. Surgical intervention (P = 0.639), duration of hospital stay (P = 0.502), and presence of diabetes mellitus (P = 0.314) were not significant factors influencing mortality. Table 5 shows the univariate analysis of factor influencing the mortality in infections with CPE.
Table 5.
Univariate analysis of impact of variables on mortality in patients infected with carbapenem-resistant Enterobacteriaceae
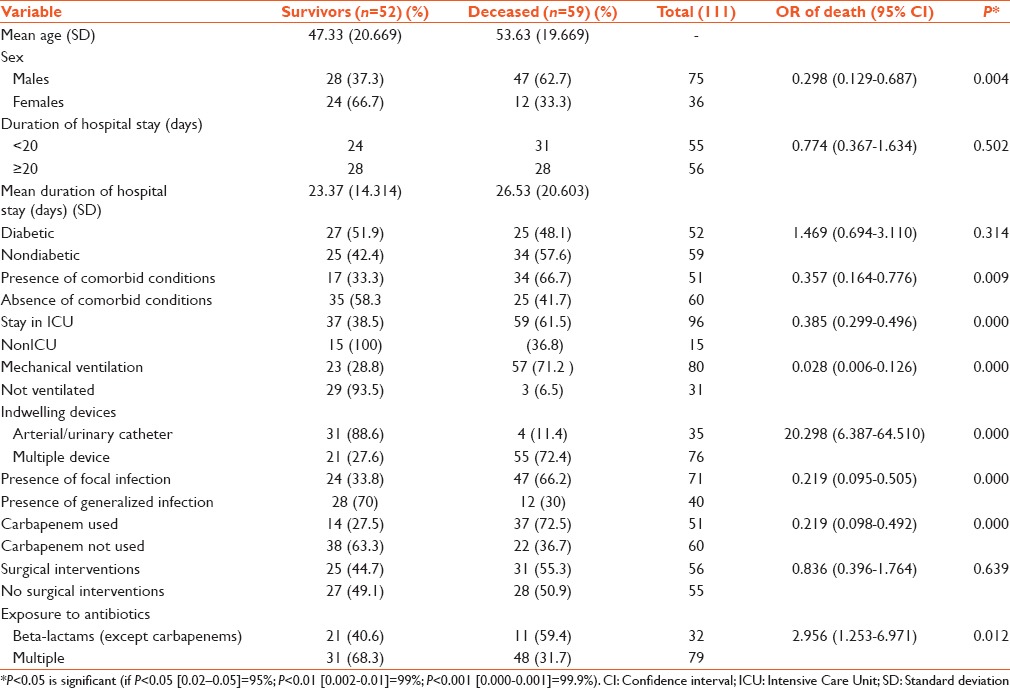
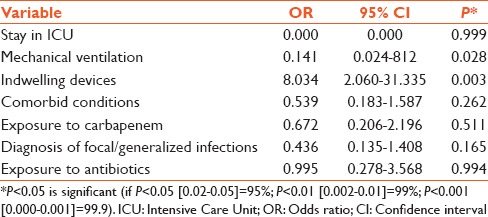
Multivariate logistic regression analysis demonstrated that mechanical ventilation (OR: 0.141, 95% CI: 0.024–0.812) and presence of indwelling invasive device (OR: 8.034; 95% CI: 2.060–31.335) were the only significant factors influencing mortality. Table 6 shows the results of the multivariate logistic regression analysis of impact of variables on mortality in patients infected with carbapenem-resistant Enterobacteriaceae
Table 6.
Multivariate analysis of impact of variables on mortality in patients infected with carbapenemase-producing Enterobacteriaceae
DISCUSSION
In our study, the most common carbapenemase among Enterobacteriaceae is NDM, followed by VIM. OXA-48/OXA-181 was detected in two isolates only. Other MBL types namely IMP, SIM, SPM, and GIM were not produced by any of the study isolates. While the OXA-48/OXA-181 and the VIM producers exhibited resistance to carbapenems, notably 27 NDM producers remained susceptible to carbapenems. Most CPE were from patients in ICU (62/67). Five patients with CPE infections were from the postsurgical ward.
While several Indian studies have focused on the laboratory detection methods and molecular characterization of carbapenemase-producing bacteria, data on analysis of the risk factors for acquiring the CPE infections and their clinical outcomes are seldom published.[15,18] Identification of risk factors associated with carbapenem-resistant infection assists in the empiric therapeutic decision-making process and also allows for early implementation of appropriate infection prevention measures.[4,5] In various studies from different countries, stay in ICU, mechanical ventilation, prolonged hospital stay, multiple indwelling device, severity of underlying illness, organ/stem-cell transplantation, the presence of comorbid conditions, and recent surgical procedures were identified as risk factors for acquiring CPE infection.[4,5,6,7,20,21] Compared with infections caused by susceptible strains of the same species, infections caused by multiple antibiotic-resistant bacteria have been associated with worse outcomes, including longer hospitalizations and high rates of morbidity and mortality.[9,21,22] Considering these facts, this study attempted to retrospectively analyze the factors influencing the acquisition of CPE and their impact on mortality.
By univariate analysis, our study identified several risk factors for infection with CPE which have also been highlighted by other investigators such as stay in the ICU (P = 0.021), mechanical ventilation (P = 0.013), presence of multiple indwelling device (P = 0.011), and presence of focal or generalized infection (P = 0.013). These factors portray a severely ill patient who receives intensive nursing and for whom, the disease treatment and the invasive devices compromise the protective barriers. ICU stay had been found in previous studies to be an important risk factor for acquisition of resistant organisms and also more than half of the patients hospitalized in ICU acquire a nosocomial infection.[5,23,24] In this study, most patients with CPE infection were in ICU (62/67), had various comorbid conditions, required mechanical ventilation, and were exposed to broad-spectrum antibiotics. Even though stay in ICU (P = 0.021) seemed to be a risk factor for acquiring CPE infection in univariate analysis, it was insignificant by multivariate analysis. The two groups of patients (ICU and non-ICU) could not be analyzed separately since the majority of the study isolates were from ICU patients. A matched case-control study would have helped to analyze this factor more accurately.
In our study, comorbid conditions such as CRF, COPD, and road traffic accidents with multiple injuries were not statistically significant for infection with CPE. Since the medical records were perused retrospectively, assessment of the severity of the illness during admission by the Acute Physiology and Chronic Health Evaluation (APACHE) scoring was not done for the study patients. The duration of hospital stay was prolonged in both the groups, but it was not a significant risk factor. The mean duration of hospital stay was 26.4 days in patients with CPE infections and 22.98 in those with carbapenemase-negative Enterobacteriaceae infections.
We found an association between CPE infection and the usage of multiple antibiotics including beta lactams (P = 0.007). A relationship between CPE infection and administration of carbapenem either alone or in combination therapy (P = 0.042) was also observed. These results of our study are consistent with many studies.[5,10,23,24] It is known that broad spectrum antibiotics such as carbapenems can destroy the susceptible proportion of strains which is part of the normal flora, so infection could be accomplished by the resistant one. It has been documented that other classes of antimicrobials namely cephalosporins, fluoroquinolones, vancomycin, metronidazole, and antipseudomonal penicillins contribute significantly to the development of resistance to carbapenems. Exposure to multiple antibiotics leads to antibiotic selection pressure and hence the emergence of resistant strains.[5,20,25] In our study, every individual class of antibiotic class was not analyzed separately since most of the study patients were treated with multiple combinations of antibiotics. Therefore, we compared exposure to beta-lactams alone or their combination with other classes such as aminoglycoside or fluoroquinolones.
All these factors that were significant by univariate analysis were subjected to multivariate analysis to adjust the confounding factors. However, none of these variables remained significant when introduced into the multivariate model.
In our study, the mortality rate was 56.7% in patients infected with CPE and 47.7% in patients infected with carbapenemase nonproducers. This was statistically not significant. Moreover, it is difficult to assess the attributable mortality when both groups had high overall mortality. The impact of antibiotic resistance on the outcome of patients with nosocomial infections is controversial. Although it is generally accepted that drug resistance is associated with increased morbidity and mortality, some studies found no such relationship.[11,20]
We attempted to determine the predictors of mortality in both CPE and non-CPE infected patients by comparing the factors among those who died with those who survived. When compared to the other CPE and non-CPE studies, our study also highlighted some key factors in patient outcome whereby ICU stay (P = 0.000), mechanical ventilation (P = 0.000), invasive devices (P = 0.000), presence of focal or generalized infections (P = 0.000), and exposure to multiple antibiotics (P = 0.012) including carbapenem (P = 0.000) were identified as predictors.
In our study, presence of severe comorbid chronic conditions (P = 0.009) such as CRF, COPD was significant risk factors for mortality by univariate analysis. Since underlying comorbidities may be important confounders and appropriate adjustment for these confounding factors is essential to determine the true impact of antimicrobial resistance, we subjected this variable to multivariate analysis.[26,27] Comorbid conditions were insignificant by multivariate analysis. This may be explained by the recurring admissions to the hospital as well as a relatively longer length of stay in this patient population exposing them to greater risk than the general patient population. It may also be assumed that greater disease severity and poor patient condition contributed to the poor outcomes, not necessarily the infection itself.[5,28] Severity scores at the time of admission to the hospital could have been used for control of these characteristics. The present study being a retrospective one, we did not assess the severity of the underlying illness by APACHE scoring on admission and therefore it was not possible to attribute the mortality in relation to the infection or the comorbid conditions.
The presence of focal or generalized infections (P = 0.000) was a predictor of mortality in the study patients. Previous studies have suggested that removal of the focus of infection, such as catheter, debridement, or drainage, is an effective way of improving survival among patients with carbapenem-resistant K. pneumoniae infections.[25] However, these adjunctive therapies were not evaluated in the present study. This factor was insignificant in multivariate analysis.
In univariate analysis, stay in ICU, mechanical ventilation, and indwelling device were found to influence mortality in these patients. The multivariate analysis also revealed ventilation (OR: 0.141, 95% CI: 0.024–0.812) and presence of indwelling invasive device (OR: 8.034; 95% CI: 2.060–31.335) as significant factors for adverse outcome. The presence of these invasive devices interrupt the physiologic defense barriers favoring the development of nosocomial infection in ICU. The importance of these indwelling devices has been documented by several authors as risk factors for CPE infections as well as predictors of mortality.[5,20,23,24] It is notable that in CPE infection, even though stay in ICU was a risk for mortality in univariate analysis (P = 0.000), was not an independent factor for mortality as revealed by the multivariate analysis. A plausible explanation for this might be that ICU mortality is multifactorial, and several more factors play a contributing role.[20]
Administration of multiple antibiotics (P = 0.012) and carbapenems (P = 0.000) were both contributing factors in the univariate analysis. However, they were insignificant by multivariate analysis. These findings may be due to the fact that the profiles of study organisms were different in terms of site of infections and species of infecting organisms. The resulting characteristics and resistance mechanisms of study organisms were hence diverse. In our study, all the isolates were carbapenemase producers by phenotypic testing, but only 67/111 carried the gene encoding resistance. In the remaining 44 patients, the resistance to carbapenems could be due to novel carbapenemase genes or noncarbapenamases mediated mechanisms or even a combination of both. This diversity in carbapenem resistance mechanisms may be associated with distinct clinical risk factors, in particular, prior antibiotic exposure.[4,10]
A more extensive study with a larger sample size would have to be undertaken to better characterize the clinical outcomes. The present study being retrospective, severity scoring on admission, and active surveillance of rectal carriage of CPE was not incorporated. Prospective matched case-controlled studies are needed to have a better understanding of the risk factors for infection and the outcome of such infections to have a clear understanding of this problem.
CONCLUSION
In this study, no specific factor was identified as an independent risk for acquisition of CPE infection. However, as it is evident by multivariate analysis, there is an increased risk of mortality in patients with CPE infections when they are ventilated and are supported by indwelling devices. CPE infections represent a major clinical and infection control challenge. Further investigations on mechanisms of resistance and clonal spread, as well as a more detailed analysis of clinical outcomes are warranted for a better understanding.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Nordmann P, Dortet L, Poirel L. Carbapenem resistance in Enterobacteriaceae: Here is the storm! Trends Mol Med. 2012;18:263–72. doi: 10.1016/j.molmed.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: An evolving crisis of global dimensions. Clin Microbiol Rev. 2012;25:682–707. doi: 10.1128/CMR.05035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nordmann P, Cornaglia G. Carbapenemase-producing Enterobacteriaceae: A call for action! Clin Microbiol Infect. 2012;18:411–2. doi: 10.1111/j.1469-0691.2012.03795.x. [DOI] [PubMed] [Google Scholar]
- 4.Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: Epidemiology and prevention. Clin Infect Dis. 2011;53:60–7. doi: 10.1093/cid/cir202. [DOI] [PubMed] [Google Scholar]
- 5.Ling ML, Tee YM, Tan SG, Amin IM, How KB, Tan KY, et al. Risk factors for acquisition of carbapenem resistant Enterobacteriaceae in an acute tertiary care hospital in Singapore. Antimicrob Resist Infect Control. 2015;4:26. doi: 10.1186/s13756-015-0066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel N, Harrington S, Dihmess A, Woo B, Masoud R, Martis P, et al. Clinical epidemiology of carbapenem-intermediate or -resistant Enterobacteriaceae. J Antimicrob Chemother. 2011;66:1600–8. doi: 10.1093/jac/dkr156. [DOI] [PubMed] [Google Scholar]
- 7.Swaminathan M, Sharma S, Poliansky Blash S, Patel G, Banach DB, Phillips M, et al. Prevalence and risk factors for acquisition of carbapenem-resistant Enterobacteriaceae in the setting of endemicity. Infect Control Hosp Epidemiol. 2013;34:809–17. doi: 10.1086/671270. [DOI] [PubMed] [Google Scholar]
- 8.Borer A, Saidel-Odes L, Riesenberg K, Eskira S, Peled N, Nativ R, et al. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol. 2009;30:972–6. doi: 10.1086/605922. [DOI] [PubMed] [Google Scholar]
- 9.Gasink LB, Edelstein PH, Lautenbach E, Synnestvedt M, Fishman NO. Risk factors and clinical impact of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Infect Control Hosp Epidemiol. 2009;30:1180–5. doi: 10.1086/648451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teo J, Cai Y, Tang S, Lee W, Tan TY, Tan TT, et al. Risk factors, molecular epidemiology and outcomes of ertapenem-resistant, carbapenem-susceptible Enterobacteriaceae: A case-case-control study. PLoS One. 2012;7:e34254. doi: 10.1371/journal.pone.0034254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bleumin D, Cohen MJ, Moranne O, Esnault VL, Benenson S, Paltiel O, et al. Carbapenem-resistant Klebsiella pneumoniae is associated with poor outcome in hemodialysis patients. J Infect. 2012;65:318–25. doi: 10.1016/j.jinf.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 12.CLSI. CLSI Document M100-S24. Wayne, PA: Clinical and Laboratory Standards Institute; 2014. Performance Standards for Antimicrobial Susceptibility Testing: 24 th Informational Supplement. [Google Scholar]
- 13.Behera B, Das A, Mathur P, Kapil A, Gadepalli R, Dhawan B. Tigecycline susceptibility report from an Indian tertiary care hospital. Indian J Med Res. 2009;129:446–50. [PubMed] [Google Scholar]
- 14.Miriagou V, Cornaglia G, Edelstein M, Galani I, Giske CG, Gniadkowski M, et al. Acquired carbapenemases in Gram-negative bacterial pathogens: Detection and surveillance issues. Clin Microbiol Infect. 2010;16:112–22. doi: 10.1111/j.1469-0691.2009.03116.x. [DOI] [PubMed] [Google Scholar]
- 15.Deshpande P, Rodrigues C, Shetty A, Kapadia F, Hedge A, Soman R. New Delhi metallo-beta lactamase (NDM-1) in Enterobacteriaceae: Treatment options with carbapenems compromised. J Assoc Physicians India. 2010;58:147–9. [PubMed] [Google Scholar]
- 16.Queenan AM, Bush K. Carbapenemases: The versatile beta-lactamases. Clin Microbiol Rev. 2007;20:440–58. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hujer KM, Hujer AM, Hulten EA, Bajaksouzian S, Adams JM, Donskey CJ, et al. Analysis of antibiotic resistance genes in multidrug-resistant Acinetobacter sp. isolates from military and civilian patients treated at the Walter Reed Army Medical Center. Antimicrob Agents Chemother. 2006;50:4114–23. doi: 10.1128/AAC.00778-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castanheira M, Deshpande LM, Mathai D, Bell JM, Jones RN, Mendes RE. Early dissemination of NDM-1- and OXA-181-producing Enterobacteriaceae in Indian hospitals: Report from the SENTRY antimicrobial surveillance program, 2006-2007. Antimicrob Agents Chemother. 2011;55:1274–8. doi: 10.1128/AAC.01497-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellington MJ, Kistler J, Livermore DM, Woodford N. Multiplex PCR for rapid detection of genes encoding acquired metallo-beta-lactamases. J Antimicrob Chemother. 2007;59:321–2. doi: 10.1093/jac/dkl481. [DOI] [PubMed] [Google Scholar]
- 20.Mantzarlis K, Makris D, Manoulakas E, Karvouniaris M, Zakynthinos E. Risk factors for the first episode of Klebsiella pneumoniae resistant to carbapenems infection in critically ill patients: A prospective study. Biomed Res Int. 2013;2013:850547. doi: 10.1155/2013/850547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borer A, Saidel-Odes L, Eskira S, Nativ R, Riesenberg K, Livshiz-Riven I, et al. Risk factors for developing clinical infection with carbapenem-resistant Klebsiella pneumoniae in hospital patients initially only colonized with carbapenem-resistant K. pneumoniae. Am J Infect Control. 2012;40:421–5. doi: 10.1016/j.ajic.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 22.Liu SW, Chang HJ, Chia JH, Kuo AJ, Wu TL, Lee MH. Outcomes and characteristics of ertapenem-nonsusceptible Klebsiella pneumoniae bacteremia at a university hospital in Northern Taiwan: A matched case-control study. J Microbiol Immunol Infect. 2012;45:113–9. doi: 10.1016/j.jmii.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 23.Falagas ME, Rafailidis PI, Kofteridis D, Virtzili S, Chelvatzoglou FC, Papaioannou V, et al. Risk factors of carbapenem-resistant Klebsiella pneumoniae infections: A matched case control study. J Antimicrob Chemother. 2007;60:1124–30. doi: 10.1093/jac/dkm356. [DOI] [PubMed] [Google Scholar]
- 24.Chang HJ, Hsu PC, Yang CC, Kuo AJ, Chia JH, Wu TL, et al. Risk factors and outcomes of carbapenem-nonsusceptible Escherichia coli bacteremia: A matched case-control study. J Microbiol Immunol Infect. 2011;44:125–30. doi: 10.1016/j.jmii.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol. 2008;29:1099–106. doi: 10.1086/592412. [DOI] [PubMed] [Google Scholar]
- 26.Correa L, Martino MD, Siqueira I, Pasternak J, Gales AC, Silva CV, et al. A hospital-based matched case-control study to identify clinical outcome and risk factors associated with carbapenem-resistant Klebsiella pneumoniae infection. BMC Infect Dis. 2013;13:80. doi: 10.1186/1471-2334-13-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ben-David D, Kordevani R, Keller N, Tal I, Marzel A, Gal-Mor O, et al. Outcome of carbapenem resistant Klebsiella pneumoniae bloodstream infections. Clin Microbiol Infect. 2012;18:54–60. doi: 10.1111/j.1469-0691.2011.03478.x. [DOI] [PubMed] [Google Scholar]
- 28.Daikos GL, Petrikkos P, Psichogiou M, Kosmidis C, Vryonis E, Skoutelis A, et al. Prospective observational study of the impact of VIM-1 metallo-beta-lactamase on the outcome of patients with Klebsiella pneumoniae bloodstream infections. Antimicrob Agents Chemother. 2009;53:1868–73. doi: 10.1128/AAC.00782-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


