Abstract
Background:
Piper nigrum (PN) is well known for its cytotoxic and pharmacological benefits. However, there is minimal documented evidence about its cytotoxic efficacy against colorectal carcinoma. We therefore sought to procure a precisely quantitative and qualitative result, pertaining the efficacy of an ethanolic extract of PN (EEPN) against colorectal carcinoma.
Materials and Methods:
EEPN was prepared by subjecting dried PN seeds to gradient ethanol fractionation. The total phenol content (TPC), antioxidant activity (AOA), and anti-inflammatory activity (AIA) were determined using Folin–Ciocalteu assay, ferric reducing ability of plasma and 2, 2-diphenyl-1-picrylhydrazyl methods, and human red blood cells membrane stabilizing assay, respectively. Colorectal carcinoma cell lines (HCT-116, HCT-15, and HT-29) were procured from National Centre for Cell Science, Pune, and were cultured in Dulbecco's modified eagle media supplemented with 10% fetal bovine serum and 1 mM L-glutamine. Cells were seeded into a 96-well plate, followed by treatment with increasing concentrations of EEPN. The cytotoxic efficacy was evaluated based on percentage inhibition of cells, using sulforhodamine-B assay. The IC-50 values were calculated using Prism software (Prism from GraphPad software, Inc. CA, USA).
Results:
Biochemical analysis revealed that 50% EEPN exhibited higher TPC, AOA, and AIA when compared to 70% and 100% EEPN at any given concentration (P = 0.041). Cytotoxic analysis revealed a dose-dependent response with maximum cellular inhibition at TPC of 6 and 3 μg/ml, using 50% EEPN. However, 50% inhibition of cellular growth using 50% EEPN was seen with TPC of 3.2, 2.9, and 1.9 μg/ml at 24, 48, and 72 h, respectively, in HCT-15 cells. Hence, time- and dose-dependent increase in the cytotoxic efficacy of 50% EEPN against colorectal carcinoma cell lines were noted (P < 0.001).
Conclusion:
Given the significantly positive correlations exhibited between the biochemical and the cytotoxic properties evaluated in our study, we hereby conclude PN as a novel therapeutic spice for the treatment of colorectal carcinoma.
Key words: Anticancer activity, antioxidant activity, colorectal cancer, ethanolic extract of Piper nigrum
INTRODUCTION
The global burden of cancer continues to increase largely because of the aging and growth of the worldwide population alongside an increase in habituation of cancer-causing behavior, particularly smoking in economically developing countries. According to the International Agency for Research on Cancer's latest online database GLOBOCAN 2012, the global burden rises to 1.4 million new cases and 8.2 million cancer deaths in 2012 as compared to 12.7 and 7.6 million, respectively, in 2008. Projections, based on GLOBOCAN 2012 estimates, predict a substantial increase to 19.3 million new cases per year by 2025.[1]
Colorectal carcinoma is the third most commonly diagnosed cancer worldwide with a prevalence of 1.4 million (9.7%) preceded by lung cancer (13.0%) and breast cancer (11.9%), despite all the advanced therapeutic and surgical interventions available.[1,2] Hence, it is the need of the hour to plunge our fingers into the field of research to find out safer and more effective therapeutic modalities which not only would limit the spread of colorectal carcinoma but also would ensure patient's well-being by having minimal adverse effects unlike the current day chemotherapeutic regimens.
With all the recent advances in the field of phytomedicine and its beneficial immunomodulatory and anticancer effects, we need to emphasize on different plants and their phytochemical constituents. Of all the plants investigated over these long years, the one exhibiting tremendous potential through its biovaluable phytochemicals is Piper nigrum (PN) (black pepper aka king of spices) which belongs to the family Piperaceae.[3] Although promising results were also exhibited by Piper longum and Piper bettle, PN is the most commonly used and highly appraised spice in the world, especially in India.
It is loaded with numerous pharmacological activities as it is proved to behave like an antihypertensive,[4] antiasthmatic,[5] antimicrobial,[6] antioxidant,[7] anticancer,[8] anti-inflammatory,[9] and immunomodulatory activities.[10] Several phytochemicals have been isolated from PN among which phenolic acids are considered to be the most potent compound exhibiting medicinal activities against several diseases including cancer.
Despite all this information and research over these long years, a definite attempt has not been made to find out a way to formulate an effective phytomedicinal agent against colorectal carcinoma, using well-established protocols. Thus, we in our study have made an attempt to unveil the whole spectrum in regard to in vitro anticancer activity along with effective dose of 50% ethanolic extract of PN (EEPN) against colorectal carcinoma cell lines (HT-29, HCT-116, and HCT-15).
MATERIALS AND METHODS
The plant material, i.e., dried unripe fruits of black pepper were collected from local farm of Sakhula Pura Taluk, Hassan District, Karnataka, and were authenticated by the Department of Biology, JSS College of Pharmacy, Mysore. The plant belonged to family Piperaceae, Genus – Piper, species – nigrum.
Black pepper fruits were shade dried and powdered. Powder sample (100 g) was subjected to maceration (using magnetic stirrer), allowed to undergo percolation completely, and then filtered using Whatman filter paper. Sequential and gradient extractions were done using 50%, 70%, 100% ethanol resulting in three different fractions, which were preserved in stoppered brown bottle at –20°C. The respective filtrates were concentrated by rotary flash evaporator separately. Finally, the concentrated extracts were divided further into two sets, one for biochemical analysis and the other for cytotoxic analysis, after being lyophilized and reconstituted in 100% dimethyl sulfoxide (DMSO).
The Folin–Ciocalteu method was carried out as reported by Ainsworth and Gillespie.[11] A hundred microliters of 50%, 70%, and 100% EEPN (EEPN) was taken and made up to 1 ml with absolute ethanol. One milliliter of Folin-Ciocalteu (FC) reagent and 0.8 ml of 4% NaHCO3 were added followed by mixing thoroughly and incubating for 30 min in dark at room temperature (RT), wherein absorbance maxima was recorded at 760 nm. Using gallic acid (GA) (1 μg/ml) as standard, total phenol content (TPC) was determined as GA equivalents (GAE) based on FC calibration curve. The TPC was expressed as mg GAE and % total phenol (TP) as gram weight (%w/w).
The working ferric reducing ability of plasma (FRAP) reagent and working stock standard (WSS) with a working range of 200–1600 μM were prepared following standard procedure and methodology from Benzie and Strain.[12] 50%, 70%, and 100% EEPN were taken in different concentrations. A volume of 2800μl of FRAP reagent was added to all the samples and standards and incubated for 30 min in dark at RT, where in absorbance maxima was recorded at 593 nm. The antioxidant activity (AOA) was estimated from the linear calibration curve (constructed by using four different concentrations of FeSO4 – 200 μM, 600 μM, 1000 μM, 1600 μM) and expressed in mmol of FeSO4 equivalents (FRAP units).
The 2, 2-diphenyl-1-picrylhydrazyl (DPPH) method was carried out following the method of Blois[13] and Brand-Williams et al.[14] Working DPPH reagent and WSS ranging in 5, 25, 75, and 100 μg/ml were obtained. A volume of 200μl of WSS from respective concentrations and 50%, 70%, 100% EEPN in different concentrations were taken as samples. A volume of 1400μl of DPPH reagent was added to all standards and samples and incubated for 30 min in dark at RT, and absorbance maxima was recorded at 536 nm, against that of the blank. Free radical scavenging activity of each fraction was determined from the linear calibration curve (by comparing its absorbance with that of blank) using the formula: Percentage inhibition I% = (Ab − As/Ab) ×100, where Ab is the absorbance of the blank and As is the absorbance of the sample. The IC-50 values were calculated from the plot of I% against the concentration of extracts.
The human red blood cell (HRBC) membrane stabilizing activity assay was carried out as reported by Rsnakk et al., 2001;[15] Chippada et al., 2011,[16] using 10% (v/v) HRBC suspension while diclofenac solution (1 mg/10 ml) was used as standard drug. The assay mixtures consisted of 2 ml of hyposaline (0.25% w/v sodium chloride), 1.0 ml of 0.15 M sodium phosphate buffer, pH 7.4, 0.5 ml of 10% (v/v) HRBC suspension, and 1.0 ml of compound solution in hyposaline (0.25% NaCl) (standard and extracts), and final reaction mixtures were made up to 4.5 ml with isosaline. Drugs were omitted in the blood control while the drug control did not contain the erythrocyte suspension. The reaction mixtures were incubated at 37°C for 30 min and centrifuged at 3000 rpm for 20 min. The absorbance of the supernatant solution was measured spectrophotometrically at 560 nm. Each experiment was carried out in triplicates and was averaged. The percentage inhibition of hemolysis or membrane stabilization was calculated using the following equation: Percentage inhibition of hemolysis = (A1 – A2 /A1) × 100 where A1 = absorbance of hypotonic buffered saline and A2 = absorbance of test sample in hyposaline and was compared with the percentage inhibition of positive control.
Colorectal cancer cell lines (HT-29, HCT-116, and HCT-15) procured from National Centre For Cell Science, Pune, India, were maintained in laboratory in Dulbecco's modified eagle media (DMEMs) supplemented with 10% fetal bovine serum, 1% glutamine, and 1% penicillin-streptomycin in adherent tissue culture flasks and allowed to grow to achieve 80% confluency. A 96-well microtiter plate was seeded with 5 × 103 cells per well and incubated in a humidified atmosphere with 5% CO2 at 37°C. After 48 h of incubation, once seeded plates achieved 80% confluency, the cells were subjected to treatment with plant extracts.
Stock sample for evaluation of anticancer activity was prepared by dissolving 25 mg of 50% lyophilized PN extract in 1 ml of 100% DMSO which is equivalent to 300 μg/ml concentration of TPC. Working sample extract was prepared in 4% DMSO to avoid cell death caused by 100% DMSO. Concentration of TP in 4% DMSO was calculated. Serial dilution was done to get concentrations as 12, 6, 3, 1.5, 0.75, and 0.37 μg/ml. Diallyl disulfides (DADS) – 1 mM dissolved in 4% DMSO was run as positive control, 2% DMSO was run as a vehicle control, DMEMs without cells as a media blank, and DMEMs with cells as cell blank. Treated microtiter plates were incubated for 24 h, 48 h, and 72 h in a humidified atmosphere with 5% CO2 at 37°C. All treatments were prepared in triplicates. Sulforhodamine-B (SRB) assay was done for evaluation of anticancer activity.
Fixing of viable cells in the sample treated microtiter plate was done using 50% trichloroacetic acid (TCA). Fixation was done at 4°C for 1 h. After fixation, plates were washed thrice with distilled water to remove excessive fixative and dead cells. A volume of 100μl of 0.4% SRB was added and plates were incubated for 30 min at RT. After incubation, plates were again washed with 1% acetic acid for 3 times to remove unbound SRB dye. Viable cells take up SRB dye and stain pink. Plates were allowed for air drying and finally added 100 μl of 10 mM – tris base (tris [hydroxymethyl] aminomethane). Plates were kept over rotor for 5 min for complete mixing of bound dye with tris base and absorbance was recorded at 490 nm using microtiter plate reader from BioRad Laboratories Inc. Decrease in absorbance signifies the increase in cytotoxic effect indicating decrease in the number of viable cells. Percentage inhibition (I%) was determined by the equation: I (%) = (A control – A sample/A control) ×100, where A control is the absorbance of the positive control with 1 mM – DADS and A sample is the absorbance of the plant extract in different concentrations. The IC-50 value for all the three different cell lines was calculated from the plot of inhibition (%) in dose- and time-dependent manner.
RESULTS
The percentage of TP in 50%, 70%, and 100% EEPN was 0.21%, 0.08%, and 0.02%, respectively [Figure 1], revealing that 50% extract has the highest percentage of TP followed with 70% and 100% extract.
Figure 1.
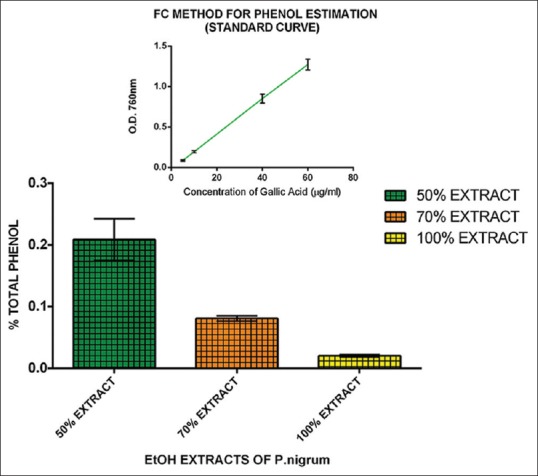
Percentage total phenol in 50%, 70%, 100% ethanolic extract of Piper nigrum using Folin-Ciocalteu method. The inset shows the standard curve of gallic acid
The AOA was found to be higher in 50% EEPN (in terms of FRAP units) at any given concentration of TP, when compared to 70% and 100% extracts, by FRAP method [Figure 2a].
Figure 2.
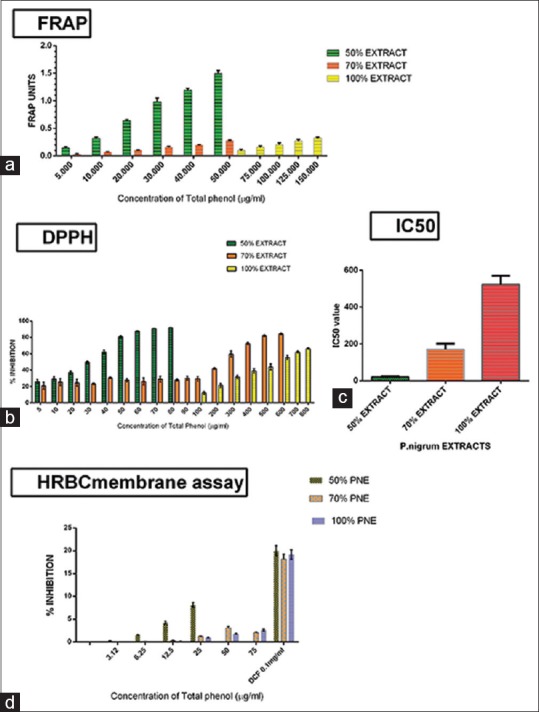
(a) Ferric reducing antioxidant power units for 50%, 70%, and 100% ethanolic extract of Piper nigrum using ferric reducing antioxidant power method. (b) Percentage inhibition for 50%, 70%, and 100% ethanolic extract of Piper nigrum using 2, 2-diphenyl-1-picrylhydrazyl method. (c) IC50 value (2, 2-diphenyl-1-picrylhydrazyl) of 50%, 70%, and 100% ethanolic extract of Piper nigrum. (d) Percentage inhibition of 50%, 70%, and 100% ethanolic extract of Piper nigrum using human red blood cell membrane stabilization assay
The radical scavenging activity was established to be higher in 50% EEPN (in terms of percentage inhibition) at any given concentration of TP, when compared to 70% and 100% ethanolic extracts, using DPPH method [Figure 2b]. The IC-50 value of 50% EEPN was estimated to be lesser than that of 70% and 100% ethanolic extracts clearly, indicating its higher radical scavenging activity [Figure 2c].
Even at low concentrations of TP, 50% EEPN demonstrated an increased anti-inflammatory activity (AIA) when compared to 70% and 100% ethanolic extracts which demonstrated a significantly lower activity by HRBC membrane assay [Figure 2d]. Hence, clearly, it indicated that 50% EEPN has a greater and more potent AIA.
A dose-dependent response was observed of which, TP concentration of 6 and 3 μg/ml showed maximum cell inhibition compared to lower concentrations on all the three cell lines [Figure 3a–c].
Figure 3.
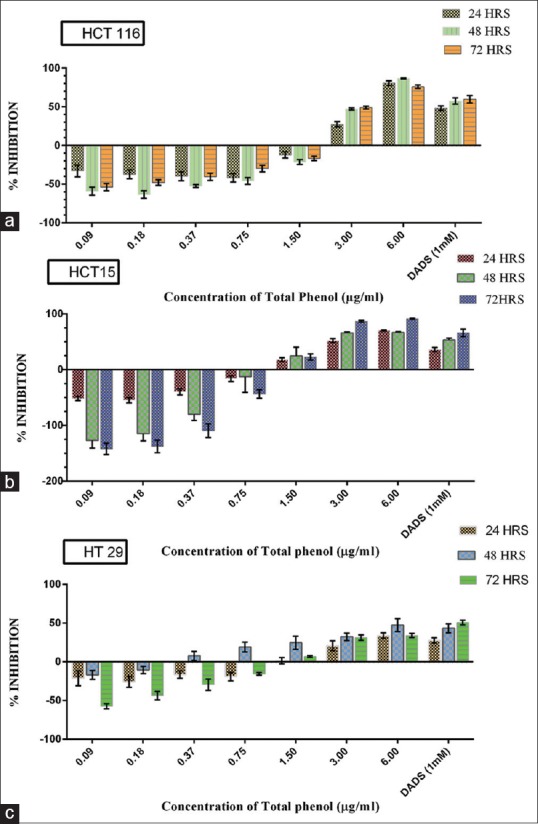
Cytotoxic analysis of 50% ethanolic extract of Piper nigrum on HCT-116 (a), HCT-15 (b), and HT-29 (c) colon cancer cell lines at 3 time-points
Fifty percent of inhibition using 50% EEPN was seen with TP concentration of 3.2, 2.9, and 1.9 μg/ml in HCT-15 cells; 4.0, 3.1, and 3.4 μg/ml in HCT-116 cells; and 7.9, 6.1, and 7.4 μg/ml in HT-29 cells at 24, 48, and 72 h, respectively [Figure 4].
Figure 4.
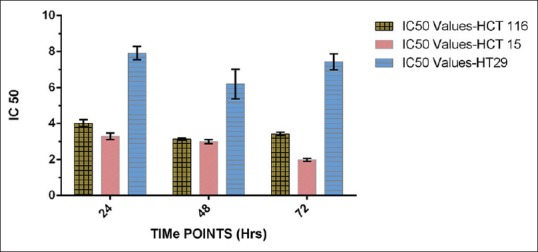
IC50 values of 50% ethanolic extract of Piper nigrum on HCT-116, HCT-15, and HT-29 colon cancer cell lines at 3 time-points
DISCUSSION
The reactivity of phenolic compounds with FC reagent is proportional to polarity of the medium which in turn is the availability of hydroxyl groups present on the aromatic (phenyl) ring.[17] With increase in concentration of ethanol, reactivity also decreases proportionally, exhibiting the minimal reactivity with 100% ethanol soluble fractions of phenol contents.
Determination of antioxidant property of any plant extract through FRAP method depends on its ability to reduce the colorless ferric-2,4,6-tri-pyridyl-s-triazine (Fe[III]-TPTZ) complex to intense blue-colored ferrous-2,4,6-tri-pyridyl-s-triazine (Fe[II]-TPTZ) complex at low pH, and the change in absorbance was recorded at 593nm.[18] Antioxidants are well known for hydrogen donating property, thereby reducing active free radicals into stable nonradical forms and thus, blocking the chain of lipid peroxidation. Fifty percent ethanolic extract is rich in water soluble fraction of phenolic acids which are more reactive toward Fe (III)-TPTZ and reduce it into intense blue-colored Fe (II)-TPTZ, indicating relatively higher reducing power in a dose-dependent manner when compared to 70% and 100% ethanolic extracts.
A proton radical scavenging action is known to be one of the various mechanisms for measuring AOA.[12] DPPH is a stable free radical. As DPPH picks up one electron in the presence of a free radical scavenger, the absorption decreases and the resulting discoloration from intense purple to yellow are stoichiometrically related to the number of electrons gained.[19] DPPH stable radical contains an odd electron, which is responsible for the absorbance at 515 nm; however, as there is an increase in the level of radical scavenger, gradually, absorbance decreases indicating that these radical scavengers can be used against oxidative damage caused by free radical species in the body. Antioxidants possess free radical scavenging activity due to its ability to donate electron which decreases the reactivity of DPPH stable free radical by combining with its odd electron, and hence, the absorbance decreases. Since 50% ethanolic extract showed rich TPC as compared to 70% and 100% extracts, the similar pattern was observed for their radical scavenging activity. The activity is maximum in 50% extract and the scavenging ability decreases as there is a decrease in TPCs. A 100% ethanolic plant extract showed the least radical scavenging activity indicating insignificant AOA.
HRBC membrane is analogous to the lysosomal membrane and its stabilization by test compound is indicative of stabilizing the lysosomal membrane. Exposure of human red blood cells to injurious substances such as hypotonic medium, heat, methyl salicylate, or phenyl hydrogen results in the lysis of the membrane, accompanied by releasing of hemoglobin contents and its hemolysis and oxidation.[20] The in vitro AIA of ethanolic extracts of stem and leaves of Pergularia daemia and Solanum xanthocarpum was evaluated by Vijaya et al. 2013.[21] Hypotonicity-induced HRBC membrane stabilization assay was carried out along with standard hydrocortisone. The result showed good AIA having maximum protection of HRBC at 12.0 mg/ml concentration when compared with standard showing more than 92% HRBC protection.[22] Anosike et al., 2012, performed HRBC membrane stabilization assay to evaluate AIA of methanolic extract of garden egg, and the results were compared with standard indomethacin. Hypotonicity-induced membrane stabilization was performed, and maximum protection was observed at concentration 800 μg/ml (50.8 ± 3.75) when compared with standard indomethacin having 61.47% HRBC protection.[20] In both assays, the anti-inflammatory response showed dose dependence. In our study, even at lower concentration of TP, 50% ethanolic extract showed increased AIA when compared to 70% and 100% ethanolic extract; hence, it is clear that 50% EEPN has more potent AIA.
Cell culture is an indispensable technique and one of the major tools to understand the structure and function of the cells. The cell-based assay provides mechanistic data pertaining to various investigations on cancer studies. To evaluate anticancer activity of PN against colorectal cancer, HCT-15, HCT-116, and HT-29 cancer cell lines were used in our study. Several methods have been employed for the assessment of cell proliferation assays in vitro based on the principle of colorimetry or fluorometry. The more commonly used are SRB assay and MTT(3-4 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide)-assay. Piperine is a principle alkaloid present in pepper such as PN, and it has been reported to show cytotoxic activity on cancer cell lines at high concentrations.[23,24] Piperine exhibited cytotoxic effect against human leukemia (CEM and HL-60), murine melanoma (B-16), and human colon (HCT-8) cell lines at concentration higher than 17 μg/ml.[24] The methanolic extracts of P. longum showed cytotoxic effect on Dalton's lymphoma ascites and Ehrlich ascites carcinoma (EAC) cells with IC50 value of 250 and 100 μg/ml, respectively.[23] Furthermore, in other studies, the crude extract of PN was separated by column chromatography, in which the fraction D showed activity against both MCF-7 and MDA-MB-468 cells. Fraction DE that was isolated from D demonstrated a highly cytotoxic effect with IC50 values of 8.33 ± 1.27 and 7.48 ± 0.57μg/ml on MCF-7 and MDA-MB-468 cells, respectively. Furthermore, fraction DF exhibited a strong cytotoxic effect only on MCF-7 with IC50 value of 6.51 ± 0.39 μg/ml on breast cancer cell lines. Both fractions also promoted DNA fragmentation which is related to the induction of cell death through apoptosis.[25] In other study of human cells (HeLa and Raji cells) and murine cell lines (EAC and melanoma B-16), the IC50 values for EAC and Raji cells have greater sensitivity and these cells exhibited “dose and time” response of PN treatment.
In our present study, SRB assay has shown that 50% ethanolic extract has cytotoxic effect even at lower concentrations of TP, i.e., 6 and 3 μg/ml on HCT-15, HCT-116, and HT-29 colon cancer cell lines, whereas at that particular concentrations, 70% and 100% extract showed no significant activity. Hence, 50% EEPN has more potent anticancer activity.
In our study, we used three different concentrations of EEPN, i.e., 50%, 70%, and 100%, for biochemical and cytotoxic analysis of the phytochemicals present in the plant. The results revealed that 50% EEPN has the maximum TPC when compared to 70% and 100% EEPN. There was significantly positive correlation noted between TPC and AOA, AIA, cytotoxic activities, which revealed that 50% EEPN has greater efficacy in terms of all the above-mentioned parameters, thereby attributing its biochemical and cytotoxic properties to the secondary metabolites, such as phenols, present in it. Further studies should be carried out to establish the molecular mechanism of action of EEPN against colorectal carcinoma.
Given the significantly positive correlations exhibited between the biochemical and the cytotoxic properties evaluated in our study, we hereby conclude PN as a novel therapeutic spice for the treatment of colorectal carcinoma.
Financial support and sponsorship
This project was funded by Vision Group on Science and Technology, Government of Karnataka (GRD-103) and Department of Science and Technology – Funds for improvement of Science and Technology (DST-FIST 2012).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.WHO; IARC (International Agency for Research on Cancer); Latest World Cancer Statistics, Global Cancer Burden Rises to 14.1 Million New Cases in 2012, 12th December 2013 Press Release No. 233. [Last accessed on 2015 Jun 17]. Available from: http://www.iarc.fr/en/media-centre/pr/2013/pdfs/pr223_E.pdf .
- 2.GLOBOCAN 2012 - Estimated Cancer Incidence, Mortality and Prevalence About Colorectal Carcinoma, Worldwide in 2012. [Last accessed on 2015 Jun 19]. Available from: http://www.globocan.iarc.fr/pages/fact_sheets_cancer.aspx?cancer=colorectal .
- 3.Ahmad N, Fazal H, Abbasi BH, Farooq S, Ali M, Khan MA. Biological role of Piper nigrum L (Black pepper): A review. Asian Pac J Trop Biomed. 2012;2:S1945–53. [Google Scholar]
- 4.Taqvi SI, Shah AJ, Gilani AH. Blood pressure lowering and vasomodulator effects of piperine. J Cardiovasc Pharmacol. 2008;52:452–8. doi: 10.1097/FJC.0b013e31818d07c0. [DOI] [PubMed] [Google Scholar]
- 5.Parganiha R, Verma S, Chandrakar S, Pal S, Sawarkar HA, Kashyap P. In vitro anti-asthmatic activity of fruit extract of Piper nigrum (Piperaceae) Int J Herbal Drug Res. 2001;1:15–8. [Google Scholar]
- 6.Khan M, Siddiqui M. Antimicrobial activity of Piper fruits. Nat Prod Radiance. 2007;6:111–3. [Google Scholar]
- 7.Vijayakumar RS, Surya D, Nalini N. Antioxidant efficacy of black pepper (Piper nigrum L.) and piperine in rats with high fat diet induced oxidative stress. Redox Rep. 2004;9:105–10. doi: 10.1179/135100004225004742. [DOI] [PubMed] [Google Scholar]
- 8.Selvendiran K, Sakthisekaran D. Chemopreventive effect of piperine on modulating lipid peroxidation and membrane bound enzymes in benzo (a) pyrene induced lung carcinogenesis. Biomed Pharmacother. 2004;58:264–7. doi: 10.1016/j.biopha.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 9.Bang JS, Oh da H, Choi HM, Sur BJ, Lim SJ, Kim JY, et al. Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1β-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res Ther. 2009;11:R49. doi: 10.1186/ar2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sunila ES, Kuttan G. Immunomodulatory and antitumor activity of Piper longum Linn. and piperine. J Ethnopharmacol. 2004;90:339–46. doi: 10.1016/j.jep.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc. 2007;2:875–7. doi: 10.1038/nprot.2007.102. [DOI] [PubMed] [Google Scholar]
- 12.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 13.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–200. [Google Scholar]
- 14.Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. [Google Scholar]
- 15.Rsnakk C, Sandhya S, Banji D, Vinod KR, Murali S. HRBC membrane stabilizing property of root, stem and leaf of Glochidion velutinum. Int J Res Pharm Biomed Sci. 2001;1:256–9. [Google Scholar]
- 16.Chippada SC, Volluri SS, Bammidi SR, Vangalapati M. In vitro anti-inflammatory activity of methanolic extract of Centella asiatica by HRBC membrane stabilization. Rasayan Journal of Chemistry. 2011;4:457–60. [Google Scholar]
- 17.Duh PD, Du PC, Yen GC. Action of methanolic extract of mung bean hulls as inhibitors of lipid peroxidation and non-lipid oxidative damage. Food Chem Toxicol. 1999;37:1055–61. doi: 10.1016/s0278-6915(99)00096-4. [DOI] [PubMed] [Google Scholar]
- 18.Bhoyar MS, Mishra GP, Naik PK, Srivastava RB. Estimation of antioxidant activity and total phenolics among natural populations of caper (‘Capparis spinosa’) leaves collected from cold arid desert of trans-Himalayas. Aust J Crop Sci. 2011;15:912–9. [Google Scholar]
- 19.Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med. 2012;12:221. doi: 10.1186/1472-6882-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anosike CA, Obidoa O, Ezeanyika LU. Membrane stabilization as a mechanism of the anti-inflammatory activity of methanol extract of garden egg (Solanum aethiopicum) Daru. 2012;20:76. doi: 10.1186/2008-2231-20-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vijaya PP, Wilson D, Ali AA, Yogananth N, Ali MS, Anuradha V, et al. Original research article evaluation of in vitro anti-inflammatory and antimicrobial properties of Pergularia daemia and Solanum xanthocarpum. Int J Curr Microbiol Appl Sci. 2013;2:94–9. [Google Scholar]
- 22.Singh A, Duggal S. Piperine-review of advances in pharmacology. Int J Pharm Sci Nanotechnol. 2009;2:615–20. [Google Scholar]
- 23.Bezerra DP, Pessoa C, de Moraes MO, Silveira ER, Lima MA, Elmiro FJ, et al. Antiproliferative effects of two amides, piperine and piplartine, from Piper species. Z Naturforsch C. 2005;60:539–43. doi: 10.1515/znc-2005-7-805. [DOI] [PubMed] [Google Scholar]
- 24.Sriwiriyajan S, Ninpesh T, Sukpondma Y, Nasomyon T, Graidist P. Cytotoxicity screening of plants of genus piper in breast cancer cell lines. Trop J Pharm Re. 2014;13:921–8. [Google Scholar]
- 25.Roy UB, Vijayalaxmi KK. Evaluation of in vitro antitumor property of EEPN seeds. Int J Innov Res Stud. 2013;2:282–302. [Google Scholar]


