Abstract
Objective
To test whether infants randomized to a lower oxygen saturation (SpO2) target range while on supplemental oxygen from birth will have better growth velocity from birth to 36 weeks postmenstrual age (PMA), and less growth failure at 36 weeks PMA and 18–22 months corrected age.
Study design
We evaluated a subgroup of 810 preterm infants from the Surfactant, Positive Pressure, and Oxygenation Randomized Trial, randomized at birth to lower (85–89%, n=402, GA 26 ± 1wk, BW 839 ± 186 g) or higher (91–95%, n=408, GA 26 ± 1wk, BW 840 ± 191 g) SpO2 target ranges. Anthropometric measures were obtained at birth, postnatal days 7, 14, 21, and 28; then at 32 and 36 weeks PMA, and 18–22 months corrected age. Growth velocities were estimated using the exponential method and analyzed using linear mixed models. Poor growth outcome, defined as weight < 10th percentile at 36 weeks PMA and 18–22 months corrected age, was compared across the two treatment groups using robust Poisson regression.
Results
Growth outcomes including growth at 36 weeks PMA and 18–22 months corrected age, as well as growth velocity were similar in the lower and higher SpO2 target groups.
Conclusion
Targeting different oxygen saturation ranges between 85% and 95% from birth did not impact growth velocity or reduce growth failure in preterm infants.
The improved survival of extremely low gestational age infants highlights the significant incidence of growth restriction seen around the age of term equivalence (1), which persists into later childhood (2). The incidence of postnatal growth restriction (weight less than the tenth percentile for postmenstrual age at the time of hospital discharge) ranges anywhere from 79% to 99% (1, 3), when using fetal-infant growth curves (4). Poor postnatal growth is associated with poor neurodevelopmental outcome (2, 5) as well as increased risks in adulthood for metabolic syndrome and type 2 diabetes if there is subsequent catch-up growth (6).
The recent emphasis on early and improved nutritional support recognizes the association between nutrition and growth (7–9). However, a prospective study by Embleton et al was only able to attribute 45% of variance in weight gain to energy intake deficits (10), suggesting that postnatal growth is influenced by factors beyond caloric intake. Tissue oxygenation has been postulated to be among these factors (11–16). Animal studies investigating this possibility have shown species-specific outcomes, with rat pups raised in hypoxic conditions after birth showing reduced body mass, and hamster pups raised in similar conditions have unaffected growth (11). In humans, the relationship between oxygenation and postnatal growth is not well understood. Infants with bronchopulmonary dysplasia (BPD) have slower growth velocities when weaned from supplemental oxygen before discharge (12, 13); whereas those discharged on home oxygen have either better growth (14, 15) or no difference in growth (16). For preterm infants without BPD, assignment to different saturation targets starting several weeks after birth did not impact later growth (17, 18). However, a retrospective study of neonatal units in the U.K. with differing oxygen saturation targeting policies showed that infants cared for in units with lower saturation targets incidentally also had better inhospital growth (19). The design of the NICHD Neonatal Research Network (NRN) Surfactant, Positive Pressure and Pulse Oximetry Randomized Trial (SUPPORT) offered an opportunity to investigate this possibility in a randomized and controlled fashion from the time of birth (20, 21). Based on the U.K. data, we hypothesized that infants enrolled in SUPPORT assigned to the lower saturation target group would have better growth velocity and less growth failure inhospital and at 18–22 months corrected age.
Methods
Our sample is composed of a subgroup of infants enrolled in SUPPORT. This subgroup of infants was enrolled sequentially in participating centers after the secondary study was approved by individual center Institutional Review Boards (IRB) and enrollment in the main trial was underway. SUPPORT was a 2 × 2 factorial, randomized trial, in which the oxygen saturation targeting arm was designed to determine whether exposure to a lower saturation target soon after birth, within the accepted normal range at the time, 85–95%, was associated with a lower incidence of severe retinopathy of prematurity (ROP) or death before discharge from the hospital; the CPAP arm was designed to determine whether early CPAP use with limited ventilation was associated with increased survival without BPD at 36 weeks postmenstrual age (PMA) compared with surfactant and conventional ventilator strategy. Between February 2005 and February 2009, women at risk for delivering between 24 weeks 0 days and 27 weeks 6 days of gestation were asked to enroll in the study at participating centers. Infants were randomized to either lower (85–89%) or higher (91–95%) saturation targets within the accepted oxygen saturation range in the first two hours after birth. Pulse oximeters (Masimo Corp, Irvine, California), electronically altered for masking, were used for both groups until 36 weeks PMA or until the infant was breathing ambient air and off positive pressure support for more than 72 hours.
The protocol for the growth secondary study was approved by the IRB of all the participating centers, and written informed consent was obtained from each infant’s parent or guardian before any measurements were acquired for the study. In addition to the patient descriptors collected in the main trial (20), selected anthropometric measurements and nutrition snapshots were periodically collected by research nurses at each institution. Measurements were obtained at birth, weekly for the first 4 weeks, then at 32 and 36 weeks PMA and 18- 22 months corrected age. If an infant was deemed stable, weight was obtained using a bedside scale, length was measured using the Preemie Length Board (Ellard Instrumentation, Ltd, Monroe Washington), and head circumference was measured using a tape measure. Each measurement was obtained twice and then averaged. Detailed 24-hour nutritional data were collected weekly for the first 4 weeks and then at 32 and 36 weeks PMA by chart review. Type and volume of intravenous solutions, including composition of parenteral nutrition, and type and volume of enteral feedings, including modular additives, were recorded. Composition of milk formula and breast milk (mother’s own or donor) was based on the assumed average composition of breast milk and the manufacturer’s product information for the various milk formulas. Research nurses used standardized study forms while collecting information, and all data were subsequently transmitted to the central NRN datacoordinating center at RTI International.
The primary outcome measures were growth failure, defined as weight less than 10th percentile, for survivors to 36 weeks PMA and at 18–22 months corrected age, and in-hospital growth velocities. The reference growth standards used were the sex-specific intrauterine growth curves of Olsen (22) for in-hospital growth and the WHO Growth Curves (23) for growth at 18–22 months corrected age.
Clinical characteristics and outcomes for infants in the higher and lower oxygen saturation target groups were compared using linear mixed models for continuous variables and robust Poisson regression for binary outcomes, adjusting for multiple birth clustering and trial stratification variables: gestational age (24–25 and 26–27 weeks) and center. An unadjusted Wilcoxon rank sum test was used for skewed continuous variables. In-hospital growth velocity was calculated using the exponential method (24). In a post-hoc analysis, mortality and select primary growth outcomes were analyzed by identifying the quartile of actual median saturations while on supplemental oxygen. Adjusted results for these analyses were obtained using robust Poisson regression and expressed as relative risks and 95% confidence intervals. The proportion of infants with severe illness (defined as FiO2 > 0.4 and mechanical ventilation for more than 8 hours in the first 14 days) was analyzed by quartile of actual median saturation using a Mantel- Haenszel Chi-square test. All analyses were performed at RTI International using SAS version 9.3 (SAS, Cary, North Carolina).
Results
A total of 1316 infants were enrolled in SUPPORT; of these, the parents or caregivers of 810 infants provided consent for the Growth Secondary Study. Of the enrolled infants, 681 infants (84%) survived to 36 weeks PMA or discharge (whichever came first) and 609 infants (89%) returned for follow-up at 18–22 months corrected age (Figure 1; available at www.jpeds.com).
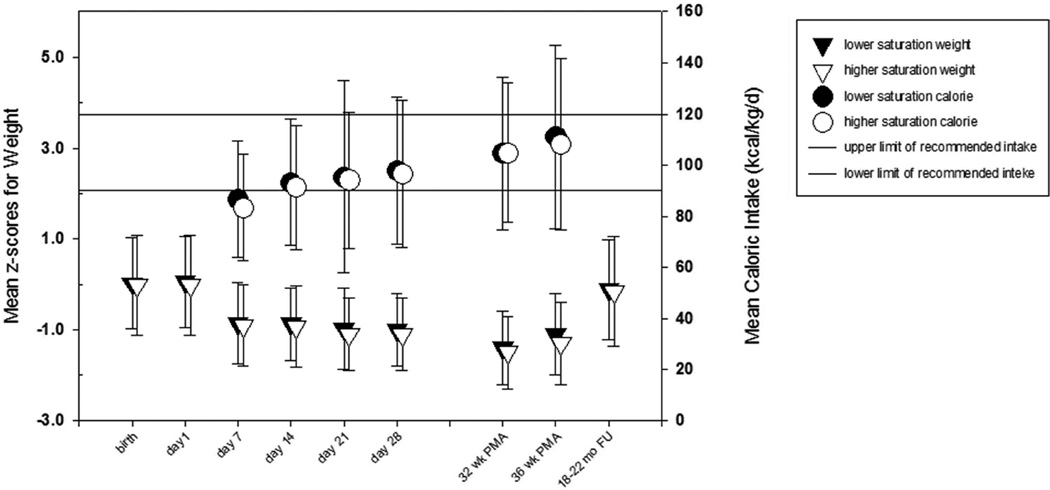
The baseline characteristics of the lower and the higher saturation groups, including the anthropometric measures, were similar for all enrollees (Table I) and for the subset of survivors to 36 weeks (not shown). The rate of growth failure (weight < 10th percentile) for survivors at 36 weeks PMA (or at discharge if earlier) did not differ significantly between the lower and higher saturation groups (46.4 and 50.3%, respectively; relative risk [RR] with lower oxygen saturation 0.92; 95% confidence interval [CI] 0.8 to 1.1, p = 0.28; Table II). The means and proportion of infants <10th percentile (Table III; available at www.jpeds.com) for individual anthropometric measures (weight, length and head circumference) and mean z-scores for weight (Figure 2), at different study time points, were not significantly different between groups. The proportion of infants with weight and head circumference less than 10th percentile in both groups increased by day 7 and was highest at 32 weeks PMA, whereas for length the less than 10th percentile proportion increased progressively until 36 weeks PMA (Table III). In-hospital growth velocity did not differ between the groups (Table II).
Table 1.
Baseline Population Characteristics
| Lower Saturation (402) |
Higher Saturation (408) |
p-value1 | |
|---|---|---|---|
| Gestational age (wk), mean (SD) | 26.2 (1.1) | 26.2 (1.1) | 0.65 |
| Birth weight (g), mean (SD) | 838.6 (186) | 839.9 (191) | 0.87 |
| Birth weight < 10th %ile2 (SGA), n/N (%) | 40/402 (10.0) | 53/408 (13.0) | 0.19 |
| HC at birth (cm), mean (SD) | 23.5 (1.8) | 23.6 (1.9) | 0.74 |
| HC at birth < 10th %ile2, n/N (%) | 41/396 (10.4) | 53/398 (13.3) | 0.19 |
| Length at birth (cm), mean (SD) | 33.4 (2.9) | 33.3 (2.9) | 0.22 |
| Length at birth <10th %ile2, n/N (%) | 50/396 (12.6) | 57/400 (14.3) | 0.48 |
| Non-Hispanic black, n/N (%) | 179/402 (44.5) | 159/408 (39.0) | 0.10 |
| Multiple birth, n/N (%) | 90/402 (22.4) | 112/408 (27.5) | 0.14 |
| Antenatal steroids, n/N (%) | 390/402 (97.0) | 389/407 (95.6) | 0.29 |
| Vaginal delivery, n/N (%) | 138/402 (34.3) | 147/408 (36.0) | 0.56 |
| Mother’s education: HS grad, n/N (%) | 69/311 (22.2) | 90/313 (28.8) | 0.07 |
| Male, n/N (%) | 211/402 (52.5) | 236/408 (57.8) | 0.19 |
adjusted for multiple-birth clustering and SUPPORT stratification variables, GA group and center, using linear mixed models for continuous variables and robust Poisson regression for categorical variables, where appropriate;
based on 10th percentile weight for GA, by sex, from Olsen growth tables
Table 2.
Primary Outcomes – Survivors to 36 weeks3
| Outcome1 All infants |
Lower Saturation (333) |
Higher Saturation (348) |
p- value2 |
|---|---|---|---|
| 36 weeks PMA3: | |||
| Wt < 10th %ile, n/N (%) | 154/332 (46.4) | 172/342 (50.3) | 0.28 |
| Growth velocity (gm/kg/d)4, mean (SD), n | 13.7 (2.3), 259 | 13.4 (2.6), 274 | 0.49 |
| 18–22 mos corrected age follow-up: | |||
| Wt < 10th %ile, n/N (%) | 48/296 (16.2) | 45/313 (14.4) | 0.49 |
| GA 24-25 weeks | Lower Saturation (130) |
Higher Saturation (135) |
|
| 36 weeks PMA3: | |||
| Wt < 10th %ile, n/N (%) | 70/130 (53.9) | 85/133 (63.9) | 0.16 |
| Growth velocity (gm/kg/d) 4, mean (SD), n | 13.9 (2.0), 97 | 13.2 (2.8), 109 | 0.24 |
| 18–22 mos corrected age follow-up: | |||
| Wt < 10th %ile, n/N (%) | 24/112 (21.4) | 29/121 (24.0) | 0.48 |
| GA 26–27 weeks | Lower Saturation (203) |
Higher Saturation (213) |
|
| 36 weeks PMA3: | |||
| Wt < 10th %ile, n/N (%) | 84/202 (41.6) | 87/209 (41.6) | 0.76 |
| Growth velocity (gm/kg/d) 4, mean (SD), n | 13.5 (2.5), 162 | 13.6 (2.5), 165 | 0.70 |
| 18–22 months corrected age follow-up: | |||
| Wt < 10th %ile, n/N (%) | 24/184 (13.0) | 16/192 (8.3) | 0.12 |
36 week outcomes based on Olsen growth tables; follow-up outcomes based on WHO growth tables;
p-values are from robust Poisson regression models and linear mixed models (growth velocity); adjusted for multiple birth clustering, SUPPORT stratification variables center, and gestational age group (models for ‘All infants’) and multiple birth clustering and center (models for GA subgroups);
include infants discharged prior to 36 weeks PMA;
calculated for the subset of survivors to 36 weeks PMA or discharge/transfer, whichever came first using the exponential method (Patel 2005, 2009) with available growth study data at Day 1 and 36 weeks
Figure 2.
Mean z-scores for Weight and Caloric Intake over Time
The time from birth to initiation of feeds and time to achieve full feeds were similar between groups (Table IV). The mean 24-hour energy intake on the pre-specified study days was not different between groups and was 85 kcal/kg/day on day 7, advancing progressively until 36 weeks PMA to 110 kcal/kg/day (Figure 2). The estimated macronutrient composition of energy source was also not different between groups (Table IV). The estimated proportions of protein and carbohydrate intakes were higher early and declined over time, whereas the estimated proportion of fat intake increased over time. The leading energy source was carbohydrate early on and became fat later. On day 7, 47% of energy intake was from carbohydrate (38% fat, 15% protein), and by 36 weeks PMA, 55% of energy intake was from fat (35% carbohydrate, 10% protein).
Table 4.
Nutritional Intake
| Combined parenteral and enteral intake1 | Lower Saturation (333) | Higher Saturation (348) | p-value2 |
|---|---|---|---|
| Total caloric intake (kcal/kg/day): | |||
| Day 7 | 86.8 (22.8) | 83.4 (20.8) | 0.07 |
| Day 14 | 93.3 (24.7) | 91.3 (24.3) | 0.37 |
| Day 21 | 95.4 (37.6) | 94.2 (26.6) | 0.69 |
| Day 28 | 98.0 (28.8) | 96.6 (28.6) | 0.59 |
| 32 weeks PMA | 104.7 (29.9) | 104.8 (27.3) | 0.94 |
| 36 weeks PMA | 111 (35.9) | 108.1 (33.5) | 0.38 |
| Discharge or 36 weeks PMA | 109 (36.7) | 107.2 (34) | 0.61 |
| Protein intake (kcal/kg/day): | |||
| Day 7 | 13.3 (3.2) | 13.1 (3.2) | 0.81 |
| Day 14 | 12.4 (4.9) | 12.0 (4.9) | 0.52 |
| Day 21 | 10.8 (5.4) | 10.8 (5.0) | 0.98 |
| Day 28 | 9.9 (5.0) | 10.3 (4.9) | 0.47 |
| 32 weeks PMA | 9.9 (5.3) | 9.9 (4.9) | 0.85 |
| 36 weeks PMA | 10.5 (5.0) | 10.3 (4.9) | 0.98 |
| Discharge or 36 weeks PMA | 10.4 (5.1) | 10.2 (4.8) | 0.97 |
| Carbohydrate intake (kcal/kg/day): | |||
| Day 7 | 41.6 (13.5) | 39.6 (11.2) | 0.08 |
| Day 14 | 39.6 (14.7) | 39.0 (11.8) | 0.47 |
| Day 21 | 39.3 (16.6) | 38.2 (12.3) | 0.26 |
| Day 28 | 39.0 (12.9) | 37.4 (11.6) | 0.08 |
| 32 weeks PMA | 37.9 (12.1) | 38.3 (11.4) | 0.92 |
| 36 weeks PMA | 38.4 (11.9) | 37.8 (11.9) | 0.61 |
| Discharge or 36 weeks PMA | 38.0 (12.0) | 37.7 (12.0) | 0.76 |
| Fat intake (kcal/kg/day): | |||
| Day 7 | 33.8 (15.8) | 32.0 (15.2) | 0.19 |
| Day 14 | 44.9 (20.2) | 44.4 (20.7) | 0.92 |
| Day 21 | 51.0 (27.1) | 50.4 (21.9) | 1.0 |
| Day 28 | 54.5 (22.6) | 53.0 (21.7) | 0.56 |
| 32 weeks PMA | 59.5 (20.2) | 57.8 (20.2) | 0.49 |
| 36 weeks PMA | 60.9 (20.4) | 60.1 (19.9) | 0.81 |
| Discharge or 36 weeks PMA | 60.4 (20.2) | 59.9 (20.4) | 0.88 |
| Age at first enteral feed (days) n, median, IQR | 332, 4, 3 to 7 | 347, 4, 3 to 7 | 0.53 |
| Age at first full enteral feed (days) n, median, IQR | 323, 23,16 to 34 | 339, 24, 16 to 34 | 0.78 |
presented as mean (SD) for continuous variables, except where noted;
adjusted for multiple-birth clustering and SUPPORT stratification variables GA group and center, using linear mixed models for continuous variables; unadjusted rank sum test for age at first enteral feed and age at first full enteral feed
Catch-up growth occurred post-discharge, with the proportion of growth failure (weight < 10th percentile) declining in both lower and higher saturation groups to 16.2% and 14.4%, respectively by 18–22 months corrected age (RR 1.1, 95% CI 0.8 to 1.7, p = 0.49). Similar results were observed when subgroup analysis was performed by gestational age strata (Table II).
The percentage with length and head circumference < 10th percentile at 36 weeks PMA was not different between groups (Table III). At 18–22 months corrected age, the percentage of infants with length and head circumference <10th percentile was lower than observed at the 36 weeks PMA measure; the amount of catch-up was less for length than for head circumference.
In-hospital growth velocity to 36 weeks PMA was determinable for 533 infants with all measurements available, and this was not different between the lower and higher saturation groups (13.7 ± 2.3 vs. 13.4 ± 2.6 g/kg/d, p = 0.49). Growth velocity between saturation groups was not influenced by gestational age stratum, 24–25 vs. 26–27 weeks (Table II). The degree of growth restriction at 36 weeks PMA was more pronounced for length (z-score −1.7) than for weight (−1.2) or head circumference (−1.0).
As was intended by the protocol, the median levels of oxygen saturation while on supplemental oxygen differed between randomization groups (Table V; available at www.jpeds.com). The number of days on oxygen supplementation was also greater in the higher saturation group. However, as in the main trial, there was substantial overlap, and the actual median levels of saturation were higher than the target levels (20). Due to this overlap, a post hoc analysis was done by quartile of the actual median oxygen saturation of the cohort as a whole. Infants with actual median saturation in the lowest quartile had a higher incidence of weight below the 10 percentile at 36 weeks PMA when compared with the highest quartile (56.1 vs. 39.9%, RR 1.4, 95% CI 1.1–1.8, p < 0.05). This was also seen at 18–22 months corrected age follow-up (19.2 vs. 9.9%, RR 1.9, 95% CI 1.0–3.4, p < 0.05). Actual median saturation was also associated with severity of illness, with the proportion of severely ill infants, by our definition, increasing significantly within quartiles from highest to lowest (14, 29, 44 and 51%, respectively; p <.0001). Other than severe ROP being higher in the higher saturation group, other clinical outcomes were not different between groups in this cohort.
Discussion
In this large, multicenter trial that randomized extremely premature infants from birth to lower or higher oxygen saturation targets while on supplemental oxygen, we found no difference in the primary outcome of growth failure (weight less than 10th percentile) at 36 weeks PMA or at 18–22 months corrected age, by saturation target group assignment. We also found no difference in the in-hospital growth velocity between the two groups. Our outcomes differ from the observational, non-randomized, study of Tin et al, which studied different saturation targets from birth by virtue of differing unit policies (19), and concluded that infants cared for with lower saturation targets were less likely to have growth restriction at discharge and decreased risk for ROP and BPD. Other studies of targeted oxygen saturation in different populations have not found a difference in growth. With saturation targeting in the BOOST (Benefit of Oxygen Saturation Targeting) trial (17), started at 32 weeks PMA for infants still requiring oxygen supplementation, Askie et al found no difference in growth at 36 weeks PMA or at 12 months corrected age. More recently, the Canadian Oxygen Trial, with a trial design similar to SUPPORT, reported no difference in growth variables at 18 months follow-up (25). A meta-analysis of the growth outcome of the three trials is planned (25, 26).
Intermittent 'snapshot' determinations of 24-hour nutritional intake showed that the caloric intake and dietary composition were similar between the lower and higher saturation groups. However, the entire population suffered from suboptimal early intake. The earliest time point for collection of nutritional information was age 7 days, the protein intake at this time was about 3.2 g/kg/d in both groups. The importance of improved early protein intake and the association with better growth outcomes is achieving greater recognition (8); however, achieving suggested early protein targets during clinical practice may still be challenging. The generally recommended energy intake for healthy low birth weight infants of 90–120 kcal/kg/d (26) was only achieved by 2 weeks of age, potentially contributing to significant accumulated deficits. Distribution of energy sources varied over time and transformed from predominantly carbohydrates to predominantly fats, different from the nutrient supplies that normally growing fetuses receive (high fraction of amino acids and glucose) (27). Although a growth pattern similar to fetal growth is considered ideal for extremely low gestational age neonates, the environment and metabolic demands are markedly different after birth, and adequate nutrient transfer is hindered by the immature gastrointestinal tract. The clinician’s perception of illness may also unduly limit the provision of optimal nutritional intake (28). This shortfall in early nutritional intake can translate into profound cumulative nutritional intake deficits, which contribute to the significant growth restriction often seen in this population (46–50%) (10). Growth restriction in the SUPPORT cohort was less than reported in older studies, but caution is needed in interpretation, as the reference growth curves used in this study underestimate growth failure as compared with the older growth curves. The updated intrauterine growth curves represent a contemporary, large, racially diverse U.S. cohort. Compared with the older, widely-used, Lubchenco growth curves (29), the Olsen curves (22) are slightly shifted rightward especially at the higher gestational ages. The use of fetal growth reference standards as the ideal for postnatal growth may be another limitation. Comparing the growth of an infant born preterm and a fetus of the same gestational age is inherently disadvantageous to the preterm infant; hence, the inevitable “excessive” incidence of postnatal growth restriction in preterm infants (30). Plotting the actual postnatal growth measures of a recent cohort of VLBW infants (including early physiologic weight loss) against fetal growth curves showed that they were consistently below the 10th percentile by 36 weeks PMA or discharge (30).
Consistent with other masked, randomized trials of targeting different oxygen saturation ranges (17, 18), the attained oxygen saturation levels overlapped, presumably because of the dynamic nature of preterm infant oxygenation. The frequent episodes of decreased oxygen saturation in preterm infants require adjustment of the fraction of inspired oxygen (FiO2) and lead to wide fluctuation in SpO2 (31). In the absence of an automated FiO2 delivery system or one-to-one dedicated nursing, regulating oxygen delivery to keep infants tightly within a target saturation range is difficult (32, 33). The absence of any difference in growth outcome may have been influenced by the overlapping of attained oxygen saturations in the two groups. Anticipating this limitation, a post hoc analysis of the entire cohort irrespective of group assignment, was done comparing the extreme quartiles of attained median oxygen saturations. It showed that spending more time in the lowest quartile (median saturation between 69–91%) was associated with increased risk for death and/or growth failure. An additional analysis looking at the association of severity of illness (defined as FiO2 > 0.4 and mechanical ventilation for more than 8 hours in the first 14 days) with quartile of actual median SpO2 found a significant link between illness severity and quartile of attained SpO2. This may mean that lower oxygen saturation is simply an indicator of increased disease severity. We speculate that the infants whose actual median saturations were in the lower quartiles were more ill and therefore experiencing more episodes of decreased oxygen saturation (34).
The strength of our data lies in the large group of preterm infants studied and randomized from birth to two target oxygen saturation ranges within the accepted limits at the time. The nutritional dataset allows weekly snapshots of nutritional intake, and although these measures of nutritional intake demonstrate the inadequate early non-protein caloric intake and its relationship with growth, we are unable to calculate cumulative nutritional deficits. With the targeted number of participating subjects, there was more than 80% power to detect a weight difference of as little as 40 g between groups.
In the SUPPORT trial, oxygen saturation targeting from birth did not lead to differences on growth outcomes between groups. However, when evaluated against actual attained SpO2 values, the greatest degree of growth restriction was seen in infants with the lowest attained median oxygen saturation levels. A high incidence of postnatal growth restriction persists despite use of an updated growth reference standard, and insufficient caloric provision remains an issue for infants less than 28 weeks gestation.
Supplementary Material
Acknowledgments
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study.
The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute provided grant support for the Neonatal Research Network’s SUPPORT Trial. Additional funding information is available at www.jpeds.com (Appendix). Participating sites collected data and transmitted it to RTI International, the data coordinating center for the network, which stored, managed, and analyzed the data for this study.
Appendix
Additional members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network include:
Abhik Das (DCC Principal Investigator) and Marie Gantz (DCC Statistician) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
Neonatal Research Network Steering Committee Chairs: Alan H. Jobe, MD PhD, University of Cincinnati (2003–2006); Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine (2006–2011).
Case Western Reserve University, Rainbow Babies & Children's Hospital (U10 HD21364, M01 RR80) – Avroy A. Fanaroff, MD; Deanne E. Wilson-Costello, MD; Bonnie S. Siner, RN; Arlene Zadell RN; Julie DiFiore, BS; Monika Bhola, MD; Harriet G. Friedman, MA; Gulgun Yalcinkaya, MD.
Cincinnati Children's Hospital Medical Center, University of Cincinnati Medical Center, and Good Samaritan Hospital, Cincinnati, OH (U10 HD27853, M01 RR8084) – Edward F. Donovan, MD; Vivek Narendran, MD MRCP; Kimberly Yolton, PhD; Kate Bridges, MD; Barbara Alexander, RN; Cathy Grisby, BSN CCRC; Marcia Worley Mersmann, RN CCRC; Holly L. Mincey, RN BSN; Jody Hessling, RN; Teresa L. Gratton, PA.
Duke University School of Medicine, University Hospital, Alamance Regional Medical Center, and Durham Regional Hospital (U10 HD40492, M01 RR30) – Ronald N. Goldberg, MD; Ricki F. Goldstein, MD; Patricia Ashley, MD; Kathy J. Auten, MSHS; Kimberley A. Fisher, PhD FNP-BC IBCLC; Katherine A. Foy, RN; Sharon F. Freedman, MD; Kathryn E. Gustafson, PhD; Melody B. Lohmeyer, RN MSN; William F. Malcolm, MD; David K. Wallace, MD MPH.
Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory Crawford Long Hospital (U10 HD27851, UL1 TR454, M01 RR39) – Barbara J. Stoll, MD; Ira Adams-Chapman, MD; Susie Buchter, MD; David P. Carlton, MD; Sheena Carter, PhD; Sobha Fritz, PhD; Ellen C. Hale, RN BS CCRC; Amy K. Hutchinson, MD; Maureen Mulligan LaRossa, RN; Gloria V. Smikle, PNP MSN.
Eunice Kennedy Shriver National Institute of Child Health and Human Development – Stephanie Wilson Archer, MA.
Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (U10 HD27856, M01 RR750) – Anna M. Dusick, MD FAAP; James A. Lemons, MD; Gary J. Myers, MD; Leslie D. Wilson, BSN CCRC; Faithe Hamer, BS; Ann B. Cook, MS; Dianne E. Herron, RN; Carolyn Lytle, MD MPH; Heike M. Minnich, PsyD HSPP.
National Heart, Lung, and Blood Institute – Mary Anne Berberich, PhD; Carol J. Blaisdell, MD; Dorothy B. Gail, PhD; James P. Kiley, PhD.
RTI International (U10 HD36790) – W. Kenneth Poole, PhD; Jamie E. Newman, PhD MPH; Betty K. Hastings; Jeanette O’Donnell Auman, BS; Carolyn Petrie Huitema, MS; James W. Pickett II, BS; Dennis Wallace, PhD; Kristin M. Zaterka-Baxter, RN BSN.
Stanford University and Lucile Packard Children's Hospital (U10 HD27880, UL1 TR1085, M01 RR70) – David K. Stevenson, MD; Susan R. Hintz, MD MS Epi; M. Bethany Ball, BS CCRC; Barbara Bentley, PsychD MSEd; Elizabeth F. Bruno, PhD; Alexis S. Davis, MD MS; Maria Elena DeAnda, PhD; Anne M. DeBattista, RN, PNP; Lynne C. Huffman, MD; Jean G. Kohn, MD MPH; Melinda S. Proud, RCP; Renee P. Pyle, PhD; Nicholas H. St. John, PhD; Hali E. Weiss, MD.
Tufts Medical Center, Floating Hospital for Children (U10 HD53119, M01 RR54) – John M. Fiascone, MD; Elisabeth C. McGowan, MD; Anne Furey, MPH; Brenda L. MacKinnon, RNC; Ellen Nylen, RN BSN; Ana Brussa, MS OTR/L; Cecelia Sibley, PT MHA.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (U10 HD34216, M01 RR32) – Namasivayam Ambalavanan, MD; Myriam Peralta-Carcelen, MD MPH; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN. Vivien A. Phillips, RN BSN; Kirstin J. Bailey, PhD; Fred J. Biasini, PhD; Maria Hopkins, PhD; Kristen C. Johnston, MSN CRNP; Sara Krzywanski, MS; Kathleen G. Nelson, MD; Cryshelle S. Patterson, PhD; Richard V. Rector, PhD; Leslie Rodriguez, PhD; Amanda Soong, MD; Sally Whitley, MA OTR-L FAOTA; Sheree York, PT DPT MS PCS.
University of Iowa (U10 HD53109, UL1 TR442, M01 RR59) – John A. Widness, MD; Michael J. Acarregui, MD MBA; Jonathan M. Klein, MD; Tarah T. Colaizy, MD MPH; Karen J. Johnson, RN BSN; Diane L. Eastman, RN CPNP MA.
University of Miami, Holtz Children's Hospital (U10 HD21397, M01 RR16587) – Charles R. Bauer, MD; Ruth Everett-Thomas, RN MSN; Maria Calejo, MEd; Alexis N. Diaz, BA; Silvia M. Frade Eguaras, BA; Andrea Garcia, MA; Kasey Hamlin-Smith, PhD; Michelle Harwood Berkowits, PhD; Sylvia Hiriart- Fajardo, MD; Helina Pierre, BA; Arielle Rigaud, MD; Alexandra Stroerger, BA.
University of New Mexico Health Sciences Center (U10 HD53089, M01 RR997) – Robin K. Ohls, MD; Janell Fuller, MD; Julie Rohr, MSN RNC CNS; Conra Backstrom Lacy, RN; Jean Lowe, PhD; Rebecca Montman, BSN.
University of Texas Southwestern Medical Center at Dallas, Parkland Health & Hospital System, and Children's Medical Center Dallas (U10 HD40689, M01 RR633) – Luc Brion, MD; Charles R. Rosenfeld, MD; Walid A. Salhab, MD; Roy J. Heyne, MD; Sally S. Adams, MS RN CPNP; James Allen, RRT; Lijun Chen, RN, PhD; Laura Grau, RN; Alicia Guzman; Gaynelle Hensley, RN; Elizabeth T. Heyne, PsyD PA-C; Jackie Hickman, RN; Melissa H. Lepps, RN; Linda A. Madden, RN CPNP; Nancy A. Miller, RN; Janet S. Morgan, RN; Araceli Solis, RRT; Lizette E. Torres, RN; Catherine Twell Boatman, MS CIMI; Diana M Vasil, RNC-NIC.
University of Texas Health Science Center at Houston Medical School and Children's Memorial Hermann Hospital (U10 HD21373) – Kathleen A. Kennedy, MD MPH; Jon E. Tyson, MD MPH; Esther G. Akpa, RN BSN; Nora I. Alaniz, BS; Susan Dieterich, PhD; Patricia W. Evans, MD; Charles Green, PhD; Beverly Foley Harris, RN BSN; Margarita Jiminez, MD MPH; Anna E. Lis, RN, BSN; Karen Martin, RN; Sarah Martin, RN BSN; Georgia E. McDavid, RN; Brenda H. Morris, MD; M. Layne Poundstone, RN BSN; Stacey Reddoch, BA; Saba Siddiki, MD; Maegan C. Simmons, RN; Patti L. Pierce Tate, RCP; Sharon L. Wright, MT (ASCP).
Wayne State University, Hutzel Women’s Hospital, and Children’s Hospital of Michigan (U10 HD21385) – Beena G. Sood, MD MS; Athina Pappas, MD; Rebecca Bara, RN BSN; Elizabeth Billian, RN MBA; Laura A. Goldston, MA; Mary Johnson, RN BSN.
Yale University, Yale-New Haven Children’s Hospital, and Bridgeport Hospital (U10 HD27871, UL1 TR142, M01 RR125) – Vineet Bhandari, MD DM; Harris C. Jacobs, MD; Pat Cervone, RN; Patricia Gettner, RN; Monica Konstantino, RN BSN; JoAnn Poulsen, RN; Janet Taft, RN BSN; Christine G. Butler, MD; Nancy Close, PhD; Walter Gilliam, PhD; Sheila Greisman, RN; Elaine Romano, MSN; Joanne Williams, RN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dusick AM, Poindexter BB, Ehrenkranz RA, Lemons JA. Growth failure in the preterm infant: can we catch up? Semin Perinatol. 2003;27:302–310. doi: 10.1016/s0146-0005(03)00044-2. [DOI] [PubMed] [Google Scholar]
- 3.Lemons JA, Bauer CR, Oh W, Korones SB, Papile LA, Stoll BJ, et al. Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics. 2001;107:E1. doi: 10.1542/peds.107.1.e1. [DOI] [PubMed] [Google Scholar]
- 4.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstetrics and gynecology. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 5.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117:1253–1261. doi: 10.1542/peds.2005-1368. [DOI] [PubMed] [Google Scholar]
- 6.Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303:1019–1022. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valentine CJ, Fernandez S, Rogers LK, Gulati P, Hayes J, Lore P, et al. Early amino-acid administration improves preterm infant weight. J Perinatol. 2009;29:428–432. doi: 10.1038/jp.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poindexter BB, Langer JC, Dusick AM, Ehrenkranz RA. Early provision of parenteral amino acids in extremely low birth weight infants: relation to growth and neurodevelopmental outcome. J Pediatr. 2006;148:300–305. doi: 10.1016/j.jpeds.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 9.Clark RH, Chace DH, Spitzer AR. Effects of two different doses of amino acid supplementation on growth and blood amino acid levels in premature neonates admitted to the neonatal intensive care unit: a randomized, controlled trial. Pediatrics. 2007;120:1286–1296. doi: 10.1542/peds.2007-0545. [DOI] [PubMed] [Google Scholar]
- 10.Embleton NE, Pang N, Cooke RJ. Postnatal malnutrition and growth retardation: an inevitable consequence of current recommendations in preterm infants? Pediatrics. 2001;107:270–273. doi: 10.1542/peds.107.2.270. [DOI] [PubMed] [Google Scholar]
- 11.Frappell PB, Mortola JP. Hamsters vs. rats: metabolic and ventilatory response to development in chronic hypoxia. J Appl Physiol. 1994;77:2748–2752. doi: 10.1152/jappl.1994.77.6.2748. [DOI] [PubMed] [Google Scholar]
- 12.Markestad T, Fitzhardinge PM. Growth and development in children recovering from bronchopulmonary dysplasia. J Pediatr. 1981;98:597–602. doi: 10.1016/s0022-3476(81)80774-3. [DOI] [PubMed] [Google Scholar]
- 13.Moyer-Mileur LJ, Nielson DW, Pfeffer KD, Witte MK, Chapman DL. Eliminating sleep-associated hypoxemia improves growth in infants with bronchopulmonary dysplasia. Pediatrics. 1996;98:779–783. [PubMed] [Google Scholar]
- 14.Hudak BB, Allen MC, Hudak ML, Loughlin GM. Home oxygen therapy for chronic lung disease in extremely low-birth-weight infants. Am J Dis Child. 1989;143:357–360. doi: 10.1001/archpedi.1989.02150150115028. [DOI] [PubMed] [Google Scholar]
- 15.Groothuis JR, Rosenberg AA. Home oxygen promotes weight gain in infants with bronchopulmonary dysplasia. Am J Dis Child. 1987;141:992–995. doi: 10.1001/archpedi.1987.04460090069028. [DOI] [PubMed] [Google Scholar]
- 16.Baraldi E, Carra S, Vencato F, Filippone M, Trevisanuto D, Milanesi O, et al. Home oxygen therapy in infants with bronchopulmonary dysplasia: a prospective study. Eur J Pediatr. 1997;156:878–882. doi: 10.1007/s004310050735. [DOI] [PubMed] [Google Scholar]
- 17.Askie LM, Henderson-Smart DJ, Irwig L, Simpson JM. Oxygen-saturation targets and outcomes in extremely preterm infants. N Engl J Med. 2003;349:959–967. doi: 10.1056/NEJMoa023080. [DOI] [PubMed] [Google Scholar]
- 18.Supplemental Therapeutic Oxygen for Prethreshold Retinopathy Of Prematurity (STOPROP), a randomized, controlled trial. I: primary outcomes. Pediatrics. 2000;105:295–310. doi: 10.1542/peds.105.2.295. [DOI] [PubMed] [Google Scholar]
- 19.Tin W, Milligan DW, Pennefather P, Hey E. Pulse oximetry, severe retinopathy, and outcome at one year in babies of less than 28 weeks gestation. Arch Dis Child Fetal Neonatal Ed. 2001;84:F106–F110. doi: 10.1136/fn.84.2.F106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlo WA, Finer NN, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010;362:1959–1969. doi: 10.1056/NEJMoa0911781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010;362:1970–1979. doi: 10.1056/NEJMoa0911783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125:e214–e224. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 23. http://www.cdc.gov/growthcharts/who_charts.htm
- 24.Patel AL, Engstrom JL, Meier PP, Kimura RE. Accuracy of methods for calculating postnatal growth velocity for extremely low birth weight infants. Pediatrics. 2005;116:1466–1473. doi: 10.1542/peds.2004-1699. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt B, Whyte RK, Asztalos EV, Moddemann D, Poets C, Rabi Y, et al. Effects of targeting higher vs lower arterial oxygen saturations on death or disability in extremely preterm infants: a randomized clinical trial. JAMA. 2013;309:2111–2120. doi: 10.1001/jama.2013.5555. [DOI] [PubMed] [Google Scholar]
- 26.AAP. Nutritional Needs of the Preterm Infant. Pediatric Nutrition Handbook. (6th) 2009:79–112. [Google Scholar]
- 27.Thureen PJ, Hay WW. Nutritional Requirements of the Very Low Birth Weight Infant. In: Neu J, editor. Gastroenterology and Nutrition Neonatology Questions and Controversies. 2008. pp. 206–222. [Google Scholar]
- 28.Ehrenkranz RA, Das A, Wrage LA, Poindexter BB, Higgins RD, Stoll BJ, et al. Early nutrition mediates the influence of severity of illness on extremely LBW infants. Pediatr Res. 2011;69:522–529. doi: 10.1203/PDR.0b013e318217f4f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lubchenco LO, Hansman C, Dressler M, Boyd E. Intrauterine Growth as Estimated from Liveborn Birth-Weight Data at 24 to 42 Weeks of Gestation. Pediatrics. 1963;32:793–800. [PubMed] [Google Scholar]
- 30.Ehrenkranz RA, Younes N, Lemons JA, Fanaroff AA, Donovan EF, Wright LL, et al. Longitudinal growth of hospitalized very low birth weight infants. Pediatrics. 1999;104:280–289. doi: 10.1542/peds.104.2.280. [DOI] [PubMed] [Google Scholar]
- 31.Laptook AR, Salhab W, Allen J, Saha S, Walsh M. Pulse oximetry in very low birth weight infants: can oxygen saturation be maintained in the desired range? J Perinatol. 2006;26:337–341. doi: 10.1038/sj.jp.7211500. [DOI] [PubMed] [Google Scholar]
- 32.Claure N, Bancalari E, D'Ugard C, Nelin L, Stein M, Ramanathan R, et al. Multicenter crossover study of automated control of inspired oxygen in ventilated preterm infants. Pediatrics. 2011;127:e76–e83. doi: 10.1542/peds.2010-0939. [DOI] [PubMed] [Google Scholar]
- 33.Sink DW, Hope SA, Hagadorn JI. Nurse:patient ratio and achievement of oxygen saturation goals in premature infants. Arch Dis Child Fetal Neonatal Ed. 2011;96:F93–F98. doi: 10.1136/adc.2009.178616. [DOI] [PubMed] [Google Scholar]
- 34.Di Fiore JM, Walsh M, Wrage L, Rich W, Finer N, Carlo WA, et al. Low oxygen saturation target range is associated with increased incidence of intermittent hypoxemia. J Pediatr. 2012;161:1047–1052. doi: 10.1016/j.jpeds.2012.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.