Abstract
Objective
We studied the effect of resistance exercise (RE) on mRNA levels of atrogin-1, MuRF-1, and myostatin in the gastrocnemius muscle of arthritic rats after loss of ovarian function (LOF).
Material and methods
Thirty female Wistar rats (nine weeks old, 195.3 ±17.4 grams) were randomly allocated into five groups: control group (CT-Sham; n = 6); group with rheumatoid arthritis (RA; n = 6); group with rheumatoid arthritis subjected to RE (RAEX; n = 6); ovariectomy group with rheumatoid arthritis (RAOV; n = 6); and an ovariectomy group with rheumatoid arthritis subjected to RE (RAOVEX; n = 6). After 15 days of intra-articular injections with Met-BSA the animals were subjected to RE and six hours after workout were euthanised.
Results
The rheumatoid arthritis provoked reduction in the cross-sectional area (CSA) of muscle fibres, but the CSA was lower in the RAOV when compared to the RA groups. Skeletal muscle atrogin-1 mRNA level was increased in arthritic rats (RA and RAOV), but the atrogin-1 level was higher in RAOV group when compared to other arthritic groups. The Muscle MuRF-1 mRNA level was also increased in the RAOV group. The increased atrogin-1 and MuRF-1 mRNA levels were lower in the RAOVEX group than in the RAOV group. The myostatin mRNA level was similar in all groups, except for the RAOVEX group, in which it was lower than the other groups.
Conclusions
LOF results in increased loss of skeletal muscle-related ubiquitin ligases (atrogin-1 and MuRF-1). However, the RE reduces the atrogin-1, MuRF-1, and myostatin mRNA levels in muscle of arthritic rats affected by LOF.
Keywords: Cachexia, rheumatoid arthritis, E3 ubiquitin ligase, ovariectomy, resistance exercise
Introduction
Loss of skeletal muscle (LSM) has been postulated as an important contributor to increasing morbidity and mortality [1]. Thereby, LSM is considered a serious consequence of rheumatoid arthritis (RA) [1], especially in women [2]. RA-induced LSM is associated with chronic inflammation and reduced physical activity [3]. The complex nature of muscle wasting involves upregulation of E3 ubiquitin ligases of the ubiquitin–proteasome system genes (muscle ring finger 1 and muscle atrophy F-box protein) [4, 5], which cause proteins to be degraded by the 26S proteasome [6]. An increased mRNA level of myostatin has also been associated with joint inflammation [7]. Myostatin, a member of the transforming growth factor (TGF)-β family that negatively regulates skeletal muscle growth, has been associated with the activation of E3 ubiquitin ligases genes in muscle [8]. Notably, loss of ovarian function (LOF), such as menopause or ovariectomy, has been reported to be a direct contributor to LSM and also through interactions with inflammation [9–12]. We have shown that LOF results in increased RA-induced LSM associated with increased ubiquitin ligases atrogin-1 and MuRF-1 in arthritic rats [13]. Thus, since women naturally progress to menopause or can be subjected to ovariectomy, the identification of interventions against RA-induced LSM is necessary for women with loss of ovarian function.
Resistance exercise (RE) is known to increase the muscle mass and therefore is considered an important way to prevent the muscle mass loss [14]. The adaptive responses of skeletal muscle to RE have been reported to be the result of a cumulative effect of acute responses of a series of molecular signalling pathways of each successive exercise bout [15]. These molecular signalling pathways from RE modulate muscle protein breakdown and synthesis to promote hypertrophy. Specifically, RE has been reported to regulate those gene expressions associated with rheumatoid arthritis-induced LSM (i.e. atrogin-1, MuRF-1, and myostatin). For instance, while the MuRF-1 mRNA level is increased 1-4 hours post-exercise and then restored back to control, the atrogin-1 mRNA level is reduced 6-12 hours post-exercise [16, 17]. Moreover, myostatin mRNA level is also reduced during 24 hours post RE [16, 18]. Thus, RE appears to have great potential to restore the increased gene expressions associated with rheumatoid arthritis back to control. However, LOF may negatively influence the RE effects on gene expression of atrogin-1, MuRF-1, and myostatin. Dieli-Conwright et al. reported greater levels in expression of muscle mRNA of myostatin, E3 ubiquitin ligases (Atrogin-1/MAFbx and MuRF-1), and pro-inflammatory cytokine and muscular damage after RE in postmenopausal women compared to postmenopausal women taking hormone replacement [19, 20]. Therefore, it would seem reasonable to assume that LOF interferes with the effect of RE on rheumatoid arthritis-induced gene expressions in muscle.
To tackle this problem, we studied the effects of RE on mRNA levels of atrogin-1, MuRF-1, and myostatin in the gastrocnemius muscle of arthritic rats (an experimental model of rheumatoid arthritis – adjuvant-induced arthritis) after ovariectomy. This paper clarifies the molecular responses of the skeletal muscle to RE during rheumatoid arthritis and LOF.
Material and methods
Animal and experimental groups
This study was in accordance with the National Guide for Care and use of Laboratory Animals, and it obtained the approval of the University Ethics Committee (No. 274/2014). All procedures were performed in the Research Institute of Oncology (IPON) of the Federal University of Triangulo Mineiro (UFTM).
Nine-week-old female Wistar rats (195.3 ±17.4 g) were used in the present study. The rats were housed in plastic cages in standard conditions at 22°C, 12-hour light-dark cycles, and had free access (ad libitum) to water and standard food (Nuvilab-CR1, Curitiba, PR, Brazil). Three experimental groups were used in this study: the control group (CT-Sham; n = 6); a group with rheumatoid arthritis (RA; n = 12); and a group with rheumatoid arthritis and ovariectomy (RAOV; n = 12). Six rats of the RA group (RAEX) and six rats of the RAOV group (RAOVEX) were undergoing RE to study the effect of RE on gene expression. All rats were treated similarly in terms of daily manipulation. The ovariectomy (RAOV and RAOVEX) or Sham (CT-Sham or RA) procedures were performed at the same time, fifteen days before the rheumatoid arthritis induction. RA and RAOV groups were immunised, and then the groups were injected with methylated bovine albumin (Met-BSA) in the tibiotarsal joint [5]. Fifteen days after intra-articular injection, the RAEX and RAOVEX groups performed an acute RE session and after six hours of the end of workout were euthanised at the same time of day and time. The gastrocnemius muscle of the left hind paw was removed, weighed, cleaned, and their white portion was obtained and stored in TRIzol at –80°C for molecular analysis [13]. We investigated the gastrocnemius white portion because it possesses more fast-twitch fibres [12, 21].
Ovariectomy
The rats were anesthetised with intraperitoneal injection solution containing ketamine (80 mg/kg) and xylazine (10 mg/kg) for ovariectomy procedures. Ovariectomy was preceded by a single ventral transverse incision of 0.4-0.6 cm at the middle abdominal region according to the method described by Khajuria et al. [22].
Rheumatoid arthritis protocol
The rats were anesthetised with intraperitoneal injection solution containing ketamine (40 mg/kg) and xylazine (5 mg/kg) for rheumatoid arthritis procedures. Initially, rats were immunised with two subcutaneous injections of 50 μl. Met-BSA (40 mg/ml) diluted in glucose 5% emulsified with Freund's Complete Adjuvant (FCA) (supplemented with 1 mg/ml of inactivated Mycobacterium tuberculosis) was injected into the tail base, with an interval of seven days between the injections. Seven days after the last injection, an intra articular injection of 25 μl was applied in the tibiotarsal joints [23].
Familiarisation to climb model
During three sessions of RE the rats were adapted to the act of climbing. Five consecutive climbs were made by RE session during one week. The animals were placed at the bottom of the ladder and motivated to climb by applying on the tail a manual stimulus to start, or every time they interrupted the movement. This familiarity was made to minimise possible interference that might exist during the acute bout of resistance exercise.
Acute resistance exercise protocol
The RE model used in this current study was chosen because the stress applied to the animal is lower than other models, such as swimming (water), the squat RE model (electric shock), and treadmill (electric shock and noise).
The progressive loading protocol proposed by Matheny et al. [24] was adapted for the research needs. The model consisted of animals climbing up a ladder (1.1 × 0.18 m, with 2 cm spacing between grid steps, 80º inclination grades) with a fixed load attached to the tail. The ladder’s length and space between grid steps forced the rats to perform 8-12 movements (in each paw) in each climb. The apparatus attached to the tail consisted of cylindrical tubes containing spherical lead weights inside. It was attached to the proximal part of the animal’s tail by a self-adhesive tape (1.5 cm, Tartan 3M). The acute RE initially consisted of no load and was progressively increased by an additional 25% of their own body weight every three climbs. For instance, the rats started doing three climbs with no load; next, three climbs with a load at 25% of their body weight (BW) added to the apparatus; then, three climbs with 50% of BW; followed by three climbs with 75% of BW; and finally three climbs with 100% of BW, totalling 15-climbs with a recovery period of 120 seconds between them. When the animal was unable to complete a climb with the stipulated load the load was decreased to complete the total of 15 climbs. The RE session was performed in the morning.
Clinical markers
Joint oedema, body weight, and food intake were examined weekly after the second subcutaneous injection. Evaluation of arthritis severity was performed by measuring the joint oedema of each animal. Joint oedema was assessed by size of latero-lateral thickness of the tarsal joint with analogic callipers (Starfer, São Paulo, SP, Brazil) [25]. The joint oedema was performed (calculated) with mean values (average arithmetic) of two hind paws. Data were collected at various time points.
Euthanasia
Animals were anaesthetised with intraperitoneal injection solution containing ketamine (80 mg/kg) and xylazine (10 mg/kg). Euthanasia was performed by cardiac puncture and hypovolemic shock.
Oestradiol, TNF-α and IL-6
The blood samples were collected by cardiac puncture in a vacuum-sealed system (Vacutainer, England) into a dry tube with a gel separator. The sample was centrifuged for 10 minutes (3000 rpm), and samples were separated and stocked (–20°C) for futures analysis. The serum oestradiol (MyBiosource, San Diego, CA, USA), TNF-α, and IL-6 (BD Biosciences, United States) were analysed by enzymatic immunoassay ELISA. All assays were in accordance with the manufacturer’s protocol.
RNA extraction and qPCR
Muscles samples were extracted (50-70 mg) from gastrocnemius white portion. Total RNA extraction was performed using TRIzol (SIGMA-ALDRICH, St Louis MO, USA), following the manufacturers protocol. After extraction, a dry pellet was resuspended in RNase-free water, treated with DNase I (Life Technologies, Carlsbad, CA, EUA) to remove any possible DNA presence in the sample. Total RNA was quantified using a high precision fluorometer QUBIT 2.0 (Life Technologies, Carlsbad, CA, EUA) and RNA BR Assay kit (Life Technologies, Carlsbad, CA, EUA). All samples had RNA concentrations between 20 and 100 ng/μl. When higher RNA values were found, samples were diluted in RNase-free water.
Quantification of mRNA was obtained by 7900HT Fast Real-Time PCR System (Life Technologies, Carlsbad, CA, USA) using Quantifast SYBR Green RT-PCR one-step kits (QIAGEN, Hilden, Germany). Thus, 1-μl RNA samples treated with DNAse were added to a mixture containing, 10 μl 2 × Quantifast SYBR GREEN RT-PCR Master Mix, 0.2 μl Quant Fast, 0.6 μl primer sense and anti-sense, and completed with RNAse-Free water to reach a volume of 20 μl. Annealing temperature and curve were used as measures of the quality. The qPCR reaction conditions and cycles performed in the 7900HT Fast Real-Time PCR System apparatus (Life Technologies, Carlsbad, CA, USA) were as follows: 50ºC per 10 minutes, 95ºC per 5 minutes for initial denaturation and amplification of 40 cycles (95ºC per 10 seconds for denaturation, 60ºC per 30 seconds for annealing and extension). Fluorescence values were obtained between annealing/extension stages, and threshold cycle numbers (CT) were determined using the software SDS version 1.2.3 (Applied Biosystems, USA). mRNA MuRF-1, atrogin-1, and myostatin were normalised for GAPDH values (reference gene) and calculated by Livak method (ΔΔCT). Primers for all genes (Table I) were obtained from other studies [5, 26] and constructed from sequences published in GenBank (www.pubmed.com) to ensure the specificity of target sequences, and to avoid generation of secondary structures of primers and dimerisation in each primer and between sense and antisense primers.
Table I.
Primers for qPCR
| Genes | Number (GenBank) | Sequence (5’-3’) |
|---|---|---|
| MuRF-1 | NM_080903.1 | S: TGACCAAGGAAAACAGCCAC CAG |
| A: TCACT CCTTCTTCTCGTCCAGGATGG | ||
| Atrogin-1 | NM_133521.1 | S: TACTAAGGAGCGCCATGGATACT |
| A: GTTGAATCTTCTGGAATCCAGGAT | ||
| Myostatin | NM_019151.1 | S: CTACCACGGAAACAATCATTACCA |
| A: AGCAAC ATTTGGGCTTTCCAT | ||
| GAPDH | NM_001034034 | S: AGATGGTGAAGGTCGGAGTG |
| A: GAAGGTCAATGAAGGGGTCA |
S – sense; A – anti-sense
Muscle morphometric analysis
Morphological analyses were performed from gastrocnemius histological sections (6 μm thickness). The gastrocnemius histological sections were obtained in a microtome (Leica Biosystems, Nussloch, Germany) and stained by haematoxylin and eosin (HE) method. The stained sections were used for photographic documentation of six random histological fields (20 × lens) (Nikon Evolucion MP 5.0). Image analysis software (Image J 1.46r) was used to determine cross-sectional area (CSA) of 200 fibres per muscle.
Statistical analysis
The data were tested for normal distribution using the Shapiro–Wilk test and for variance homogeneity using the Levene test. The intergroup comparison was done by the Kruskal-Wallis or Mann-Whitney tests (nonparametric data). When appropriate (Kruskal-Wallis, p < 0.05), a post hoc comparison test of subgroups was made. Spearman's coefficient of rank correlation was used to assess associations among variables. Data are expressed as median ± 25-75th percentiles. Statistical procedures were performed with a statistical software MedCalc (version 11.1.1.0). Significance was set at p < 0.05.
Results
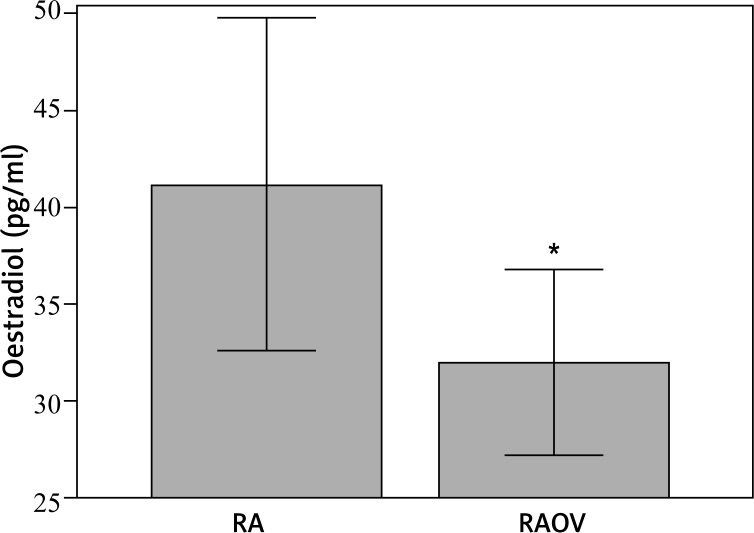
Circulating oestradiol, IL-6, and TNF-α were measured at the end of the study, after euthanasia, to ensure the ovariectomy and also assess the inflammatory condition. As expected, the ovariectomised rats displayed a lower level of circulating oestradiol when compared to non-ovariectomised rats (Fig. 1). Also, there was no change in circulating IL-6 and TNF-α, probably because of the long recovery time (15 days) after rheumatoid arthritis induction (Table II).
Fig. 1.
Oestradiol concentration in RA group (CT-sham + RA + RAEX) (n = 18) – [CT-Sham – control group (n = 6); RA – rheumatoid arthritis (n = 6); RAEX – rheumatoid arthritis who held an acute session of resistance exercise (n = 6)]; RAOV group (RAOV + RAOVEX) (n = 12) – [RAOV – ovariectomy with rheumatoid arthritis (n = 6); RAOVEX – ovariectomy with rheumatoid arthritis who held an acute session of resistance exercise (n = 6)], *p < 0.05
Table II.
mRNA of genes and serum cytokines
| CT-Sham | RA | RAEX | RAOV | RAOVEX | p | |
|---|---|---|---|---|---|---|
| Median 25-75 P | Median 25-75 P | Median 25-75 P | Median 25-75 P | Median 25-75 P | ||
| Atrogin-1 (Fold change) | 0.999a (0.979-1.011) | 3.355b (3.070-11.590) | 3.230b (1.650-6.620) | 10.320c (8.278-20.257) | 5.125b (3.660-8.080) | 0.001 |
| Myostatin (Fold change) | 1.004a (0.987-1.012) | 0.715a,b (0.490-1.160) | 0.840a (0.508-1.030) | 1.460a (1.000-2.895) | 0.405b (0.210-0.680) | 0.022 |
| MuRF-1 (Fold change) | 0.995a,c (0.989-1.010) | 1.040a,c (0.720-1.530) | 3.810a,b (0.992-5.170) | 5.780b (2.715-8.348) | 0.340c (0.310-1.660) | 0.028 |
| TNF-α (pg/ml) | 54.5a (45.7-93.1) | 56.5a (44.5-66.6) | 59.0a (55.6-76.9) | 54.6a (48.3-72.7) | 40.5a (40.0-58.3) | 0.437 |
| IL-6 (pg/ml) | 103.5a (85.7-125.1) | 90.2a (78.5-119.4) | 91.9a (89.4-103.4) | 93.1a (85.9-143.1) | 120.5a (96.6-133.3) | 0.662 |
CT-Sham group – control group (n = 6); RA group – rheumatoid arthritis group (n = 6); RAEX group – rheumatoid arthritis, who held an acute session of resistance exercise (n = 6); RAOV group – ovariectomy with rheumatoid arthritis (n = 6); RAOVEX group – ovariectomy with rheumatoid arthritis who held an acute session of resistance exercise (n = 6). Data are expressed as median and 25-75th percentiles. ANOVA – Kruskal-Wallis test. Different letters = P < 0.05 (e.g. a is difference of b and c; a,c is difference of b; a,c is not difference of c)
The body weight, joint thickness, food intake, and muscle atrophy were measured as clinical signs of rheumatoid arthritis during the current study. Because these variables were not affected by acute exercise, we pooled the exercised groups with the group not exercised to perform the statistical comparisons (Table III).
Table III.
Characteristic of the sample
| CT-Sham (n = 6) | RA (n = 12) | RAOV (n = 12) | p | |
|---|---|---|---|---|
| Median 25-75 P | Median 25-75 P | Median 25-75 P | ||
| Body weight (g) | ||||
| Pre | 226.5a (218.0-257.0) | 239.0a (226.5-253.0) | 277.0c (268.5-284.5) | < 0.001 |
| 7 days | 235.0a (219.0-263.0) | 227.0a (221.0-235.0) | 244.0a (236.5-269.0) | 0.051 |
| 15 days | 244.0a (230.0-277.0) | 230.5a (218.0-245.0) | 262.5c (247.5-280.0) | 0.007 |
| Food intake (g) | ||||
| Pre | 129.0a (129.0-129.0) | 118.5b (115.0-122.0) | 132.0c (128.0-136.0) | < 0.001 |
| 7 days | 121.0a (121.0-121.0) | 87.5b (80.0-95.0) | 86.0b (81.0-91.0) | < 0.001 |
| 15 days | 176.0a (176.0-176.0) | 94.0b (93.0-95.0) | 153.0c (151.0-155.0) | < 0.001 |
| Food intake / body weight | ||||
| Pre | 0.570a (0.502-0.592) | 0.496a (0.467-0.514) | 0.478a (0.462-0.496) | 0.058 |
| 7 days | 0.515a (0.460-0.553) | 0.384b (0.357-0.414) | 0.336c (0.322-0.375) | < 0.001 |
| 15 days | 0.721a (0.635-0.765) | 0.410b (0.384-0.431) | 0.575c (0.554-0.618) | < 0.001 |
| Joint oedema (mm) | ||||
| Pre | 6.0a (6.0-6.0) | 6.0a (6.0-6.0) | 6.0a (6.0-6.0) | 0.114 |
| 1 day | 6.0a (6.0-6.0) | 9.0b (9.0-10.0) | 10.0c (10.0-10.0) | < 0.001 |
| 7 days | 6.0a (6.0-6.0) | 11.0b (10.0-12.0) | 10.0c (9.0-10.0) | < 0.001 |
| 15 days | 6.0a (6.0-6.0) | 9.0b (9.0-10.0) | 9.0b (8.0-10.0) | < 0.001 |
| Gastrocnemius weight (g) | 1.4a (1.3-1.5) | 1.2b (1.1-1.3) | 1.2b (0.9-1.3) | 0.022 |
| Gastrocnemius / body weight (%) | 0.56a (0.53-0.64) | 0.52a (0.491-0.558) | 0.44c (0.391-0.496) | 0.003 |
CT-Sham group – control group (n = 6), RA Group – RA + RAEX (n = 12), RAOV Group – RAOV + RAOVEX (n = 12), RA – rheumatoid arthritis group (n = 6), RAEX – rheumatoid arthritis who held a resistance exercise session (n = 6), RAOV – ovariectomy with rheumatoid arthritis (n = 6), RAOVEX – ovariectomy with rheumatoid arthritis who held a resistance exercise session (n = 6), Pre-immediately before intra-articular injection, 1 day – one day after intra-articular injection, 7 days – seven days after intra-articular injection, 15 days – fifteen days after intra-articular injection, (g) – gram, (mm) – millimeter, (%) – percentage value. Data are expressed as median and 25-75th percentiles. ANOVA – Kruskal-Wallis test. Different letters = P < 0.05 (e.g. a is difference of b and c; a,c is difference of b; a,c is not difference of c).
At the beginning of the study (Pre), after ovariectomy and before intra-articular injection, the body weight of ovariectomised rats was greater (22.2%) than in other groups. At the middle of the study (seven days after before intra-articular injection), the body weight did not differ between groups. At the end of the study (15 days after intra-articular injection) body weight of ovariectomised rats was greater (7%) than in rats in other groups (Table III).
The food intake was significantly different between groups at baseline (pre) and higher (2%) in the RAOV group. On the seventh day, the RA and RAOV groups showed lower intake (~30%) compared to CT-Sham group. At the end of the study (day 15) the feed intake was also significantly different between groups, but was higher (62%) in the RAOV group compared to the RA group (Table III).
The food intake corrected by body weight did not differ between the groups at the beginning of the study (Pre). However, at day seven after intra-articular injection, food intake was different between CT-Sham, RA, and RAOV groups (0.515 [0.460-0.553]; 0.384 [0.357-0.414]; 0.336 [0.322-0.375], respectively) being lower in the RAOV group. At end of the study (day 15) the food intake was also different between groups, but it was higher in the RAOV group (0.575 [0.554-0.618]) compared to the RA group (0.410 [0.384-0.431]).
Joint thickness changes were measured throughout the progression of rheumatoid arthritis. The RA and RAOV groups greatly increased the joint thickness (9.0 [9.0-10.0] and 10.0 [10.0-10.0] mm, respectively) at day one after intra-articular Met-BSA injection compared to CT-Sham (6.0 [6.0-6.0] mm). These increases in joint thickness were maintained up to the end of the study (15 days). The ovariectomy affected the magnitude of increased of joint thickness at day one after intra-articular Met-BSA injection. The RAOV showed a greater joint thickness (10.0 [10.0-10.0] mm) when compared to RA (9.0 [9.0-10.0] mm) at day one. This increased joint thickness in the RAOV group disappeared at days seven and 15 after intra-articular Met-BSA injection (Table III).
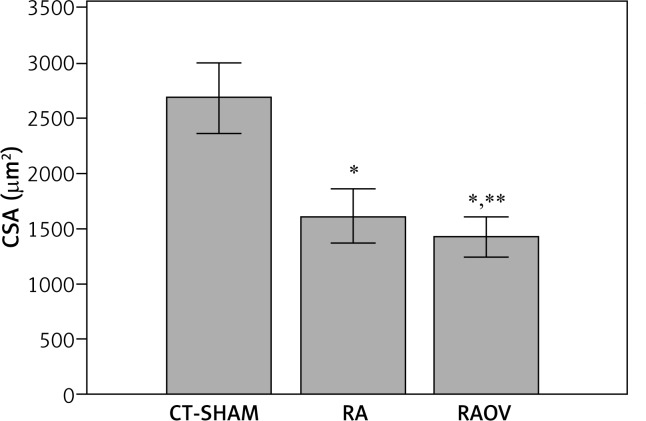
To determine the muscle atrophy we evaluated the gastrocnemius weight (g), gastrocnemius weight corrected by body weight (%) (Table III) and cross-sectional area (CSA) of gastrocnemius muscle fibres (μm2) (Fig. 2). The RA and RAOV groups showed reduction in CSA of fibres (43.6% and 49.7%, respectively) and gastrocnemius weight (14.2%). The RAOV group showed lower values of CSA when compared to the RA group (1367.5 [1290.8-1587.9] μm2 and 1533.2 [1424.9-1718.3] μm2, respectively). Only the RAOV group showed reduction (21.5%) in relative gastrocnemius weight (gastrocnemius weight corrected by body weight).
Fig. 2.
Cross section area of fibers of gastrocnemius muscle. CT-Sham – control group (n = 6); RA group – RA + RAEX (n = 12), RA – rheumatoid arthritis group (n = 6); RAEX – rheumatoid arthritis who held an acute session of resistance exercise (n = 6)]; RAOV – ovariectomy with rheumatoid arthritis (n = 6); RAOVEX – ovariectomy with rheumatoid arthritis who held an acute session of resistance exercise (n = 6)], *p < 0.05, **p < 0.05 (difference from RA group)
The atrogin-1 mRNA level was higher in gastrocnemius muscle of arthritic groups (RA, RAEX, RAOV and RAOVEX) when compared to control group. However, the increased atrogin-1 mRNA level was higher (307.6%; 319.5%; 201.3%) in the RAOV group when compared to other arthritic groups (RA; RAEX; RAOVEX, respectively). The myostatin mRNA level did not change in gastrocnemius muscle of arthritic rats, except for RAOVEX, which was lower by 72.2%. The MuRF-1 mRNA level was higher (580.9%) in gastrocnemius muscle of the RAOV group, but it was lower (65.8%) in the RAOVEX group muscle (Table II).
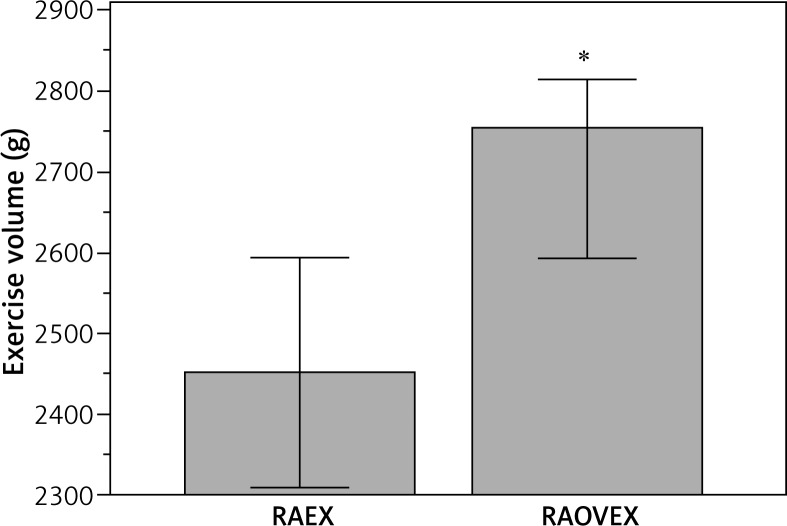
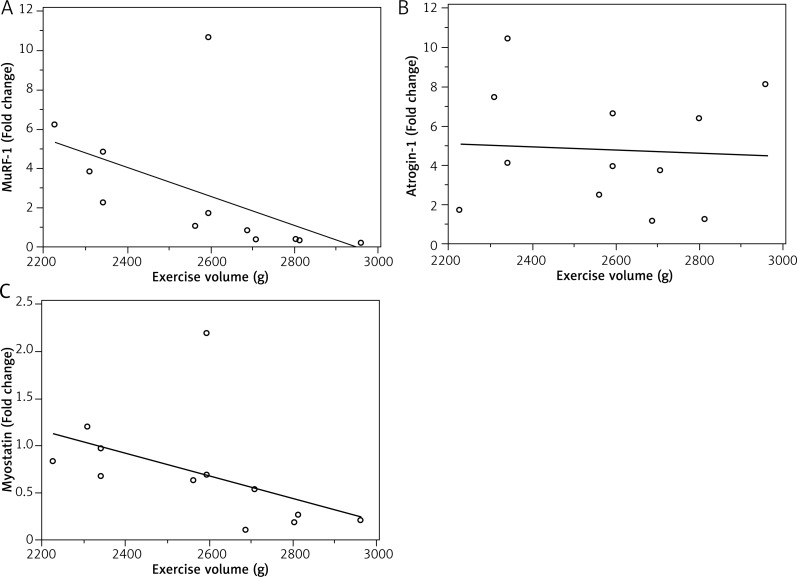
The exercise volume was greater (12.4%) in the RAOVEX group when compared to the RAEX group (Fig. 3). There were significant negative correlations of exercise volume with MuRF-1 (r = 0.846; p = 0.0050) and myostatin (r = 0.726; p = 0.0160), but not between exercise volume and atrogin-1 (r = 0.0912; p = 0.7622) (Fig. 4).
Fig. 3.
Exercise volume in groups who held an acute session of resistance exercise. RAEX – rheumatoid arthritis who held an acute session of resistance exercise (n = 6)]; RAOVEX – ovariectomy with rheumatoid arthritis who held an acute session of resistance exercise (n = 6)], *p < 0.05
Fig. 4.
A) Spearman’s coefficient of rank correlation of MuRF-1 mRNA with exercise volume (r = 0.846; p = 0.0050); B) Spearman’s coefficient of rank correlation of Atrogin-1 mRNA with exercise volume (r = 0.0912; p = 0.7622); C) Spearman’s coefficient of rank correlation of Myostatin mRNA with exercise volume (r = 0.726; p = 0.0160)
Discussion
The LSM has been postulated to be an important contributor to increasing morbidity and mortality in rheumatoid arthritis [1], especially in women [2]. The molecular signalling pathway of muscle wasting involves upregulation of E3 ubiquitin ligases of the ubiquitin–proteasome system (i.e. Atrogin-1 and MuRF-1) [4, 5, 19, 20] and myostatin [7] genes. RE has been reported to modulate E3 ubiquitin ligases of the ubiquitin–proteasome system and myostatin genes in muscle [16, 17]. However, the effect of RE on E3 ubiquitin ligases has not been tested in rheumatoid arthritis. Moreover, LOF (menopause or ovariectomy) has been reported to be a contributor to LSM directly and also through interactions with inflammation [9–12]. It has been reported that LOF may negatively influence the effects of RE on muscle [19, 20]. Thus, it seems reasonable to assume that LOF intensifies the expression of myostatin and E3 ubiquitin ligases (Atrogin-1 and MuRF-1) mRNA and also negatively affects the “anti-catabolic” effect of RE on skeletal muscle during rheumatoid arthritis. Hence, we investigated the effects of RE on mRNA levels of atrogin-1, MuRF-1, and myostatin in gastrocnemius muscle of arthritic rats after ovariectomy. In the current study, rheumatoid arthritis induced LSM and also increased atrogin-1 mRNA levels, but not MuRF-1 mRNA and myostatin mRNA, in skeletal muscle. The LOF intensified the LSM and increased atrogin-1 gene expression in rheumatoid arthritis. The LOF increased MuRF-1 and myostatin mRNA levels in skeletal muscle of arthritics rats. Thus, the identification of regulatory pathways of LOF-induced LSM during rheumatoid arthritis, such as atrogin-1 and MuRF-1, may suggest a new therapeutic target during rheumatoid arthritis with LOF. Hence, we studied the effect of RE on atrogin-1, MuRF-1, and myostatin mRNA levels in arthritic rats affected by LOF. Our study showed that RE reduces atrogin-1, MuRF-1, and myostatin mRNA levels in arthritic rats affected by LOF. This data suggests the RE would to be an important therapeutic means against LOF-induced LSM during rheumatoid arthritis. So far, to the best of our knowledge, this is the first study that has investigated the effects of acute RE on molecular signalling pathways associated with LSM in rheumatoid arthritis with LOF. Moreover, we observed that the RE effect on MuRF-1 and myostatin, but not atrogin-1, seems to be dependent on exercise volume/load. Thus, future works should include clinical studies designed to evaluate whether this therapeutic approach (RE) will be effective in avoiding or preventing LSM in arthritic women affected by LOF.
In the current study, the oestradiol concentration (Fig. 1) and external signs of the illness measured by joint thickness showed success in ovariectomy and Met-BSA-induced arthritis in the current study (Table III). Arthritic rats (RA and RAOV) showed reduction in fibres CSA of gastrocnemius muscle when compared to rats without arthritis (CT-Sham). Indeed, rheumatoid arthritis-induced LSM has been consistently demonstrated in animal [5, 13, 25] and human studies [27]. Additionally, we observed that the rheumatoid arthritis induced a substantial increase in the atrogin-1 gene expression in skeletal muscle. Because the complex nature of LSM involves upregulation of E3 ubiquitin ligases of the ubiquitin–proteasome system (UPS) genes [4, 5, 19, 20], the increased E3 mRNA level in muscle has also been observed in different atrophy models [5, 7, 28]. However, there was no increase in MuRF-1 mRNA levels in arthritic rats. These data suggest that the atrogin-1 and MuRF-1 genes might be differently regulated in rheumatoid arthritis. Previous studies have suggested that atrogin-1 causes the ubiquitination of myogenic regulatory factors, such as MyoD [29] and myogenin [30], which leads to its degradation in the proteasome, while other proteins are actually degraded by MuRF1-mediated UPS [31].
Although the increased myostatin mRNA level has been associated with muscle wasting in different atrophy models [7, 8, 32], including joint inflammation [7], our study did not support increased myostatin mRNA in muscle in rheumatoid arthritis. Indeed, increased myostatin mRNA in muscle has not been consistent in rheumatoid arthritis models [7, 33]. For example, while Castillero et al. did not find increased myostatin mRNA in muscle 15 days after administration of adjuvant injection [33], Ramires et al. found increased myostatin mRNA in muscle after two days of I-carrageenan injection [7]. These two studies suggest that after induction of rheumatoid arthritis there is an early increase of the myostatin mRNA level, which returns to basal levels after a few days. Thus, in the current study, the myostatin mRNA may have already peaked before the time of our muscle excision. However, we may only speculate this, and future research is needed to address this issue.
The current study findings showed that LOF results in increased loss of skeletal muscle. Also, LOF increased the mRNA levels of atrogin-1 and MuRF-1 in skeletal muscle of arthritic rats. Indeed, increased mRNA levels of atrogin-1 and MuRF-1 have been associated with LOF in women [19]. Dieli-Conwright et al. investigated proteolytic gene expression in postmenopausal women taking and not taking hormone replacement therapy, and they reported increased gene expression of atrogin-1 and MuRF-1 in postmenopausal women not taking hormone replacement therapy. Thus, our data support previous findings that have suggested that oestrogen acts as an anti-catabolic agent based on higher gene expression levels of atrogin-1 and MuRF-1 in lowered muscle of ovariectomised rats and postmenopausal women [12, 19, 34].
Increased proteolytic genes (i.e. E3 ubiquitin ligase) are indicative of protein degradation [6]. Hence, a lot of effort has been made to block the increase in E3 ubiquitin ligase mRNA level during rheumatoid arthritis [33, 35, 36] and therefore avoid LSM. In other models of muscle atrophy, such as dexamethasone and unload, the RE has downregulated mRNA levels of E3 ubiquitin ligase and myostatin [28, 37, 38]. Notably, in the current study, RE downregulated the mRNA levels of atrogin-1, Murf-1, and myostatin solely in arthritic rats with ovariectomy (Table III). However, we observed significantly negative correlations of exercise volume with Murf-1 and myostatin mRNA levels (Fig. 4). These data suggest that downregulated mRNA levels of MuRF-1 and myostatin to RE are dependent on the amount of exercise, such as exercise volume (load [weight on tail] × sets [number of climb]) or tension applied in muscle. Indeed, the role of exercise-induced mechanosignalling and skeletal muscle remodelling have been demonstrated. Titin (a protein with elastic I-band domains and filament-like integration in the half-sarcomere) seem to act as a central mediator that controls protein quality control, integrating with proteins such as MuRF-1 [39]. The RE model used in this study consisted of the animal climbing up a ladder with a fixed load attached to the tail [24]. We were unable to determine the maximum muscle strength (i.e. one repetition maximum) in arthritic rats because of the physical limitations of the animal. Thus, the RE model was progressively increased, adding 25% of rat body weight every three climbs. 25% of the rat’s body weight was used because this percentage load was the most tolerable by arthritic rats during load progression. As the ovariectomised rats were heavier than non-ovariectomised rats (Table II), the RAOVEX groups performed a greater exercise volume than RAEX (Fig. 3). Thus, in the present study the lack of response in the RAEX groups might be related to the low volume performed.
Conclusions
A single RE session was able to reduce atrogin-1, MuRF-1, and myostatin mRNA levels in arthritic rats affected by LOF. However, the effects of RE on MuRF-1 and myostatin mRNA levels, but not atrogin-1, seem to be dependent on exercise volume/load. Thus, future works should include clinical studies designed to evaluate whether this therapeutic approach (RE) would be effective to avoid or prevent LSM in arthritic women affected by LOF.
Acknowledgements
This investigation was supported by Fundação de Amparo à Pesquisa do Estado de Minas Gerais – FAPEMIG and by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES.
Disclosure
The authors report no conflict of interest.
References
- 1.von Haehling S, Anker SD. Prevalence, incidence and clinical impact of cachexia: facts and numbers-update. J Cachexia Sarcopenia Muscle. 2014;5:261–263. doi: 10.1007/s13539-014-0164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alamanos Y, Drosos AA. Epidemiology of adult rheumatoid arthritis. Autoimmun Rev. 2005;4:130–136. doi: 10.1016/j.autrev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Rajbhandary R, Khezri A, Panush RS. Rheumatoid cachexia: what is it and why is it important? J Rheumatol. 2011;38:406–408. doi: 10.3899/jrheum.101036. [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Menduina M, Martin AI, Castillero E, et al. Short-term growth hormone or IGF-I administration improves the IGF-IGFBP system in arthritic rats. Growth Horm IGF Res. 2012;22:22–29. doi: 10.1016/j.ghir.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Castillero E, Martin AI, Lopez-Menduina M, et al. IGF-I system, atrogenes and myogenic regulatory factors in arthritis induced muscle wasting. Mol Cell Endocrinol. 2009;309:8–16. doi: 10.1016/j.mce.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Bodine SC, Latres E, Baumhueter S, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 7.Ramírez C, Russo TL, Sandoval MC, et al. Joint inflammation alters gene and protein expression and leads to atrophy in the tibialis anterior muscle in rats. Am J Phys Med Rehabil. 2011;90:930–939. doi: 10.1097/PHM.0b013e31822dea3c. [DOI] [PubMed] [Google Scholar]
- 8.McFarlane C, Plummer E, Thomas M, et al. Myostatin induces cachexia by activating the ubiquitin proteolytic system through an NF-kappaB-independent, FoxO1-dependent mechanism. J Cell Physiol. 2006;209:501–514. doi: 10.1002/jcp.20757. [DOI] [PubMed] [Google Scholar]
- 9.Milanesi L, Vasconsuelo A, de Boland AR, Boland R. Expression and subcellular distribution of native estrogen receptor beta in murine C2C12 cells and skeletal muscle tissue. Steroids. 2009;74:489–497. doi: 10.1016/j.steroids.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Velders M, Schleipen B, Fritzemeier KH, Zierau O, Diel P. Selective estrogen receptor-beta activation stimulates skeletal muscle growth and regeneration. FASEB J. 2012;26:1909–1920. doi: 10.1096/fj.11-194779. [DOI] [PubMed] [Google Scholar]
- 11.Sitnick M, Foley AM, Brown M, Spangenburg EE. Ovariectomy prevents the recovery of atrophied gastrocnemius skeletal muscle mass. J Appl Physiol (1985) 2006;100:286–293. doi: 10.1152/japplphysiol.00869.2005. [DOI] [PubMed] [Google Scholar]
- 12.Tiidus PM, Lowe DA. Estrogen replacement and skeletal muscle: mechanisms and population health. J Appl Physiol (1985) 2013;115:569–578. doi: 10.1152/japplphysiol.00629.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furlanetto Júnior R, Martins FM, Oliveira AA, et al. Loss of ovarian function results in increased loss of skeletal muscle in arthritic rats. Rev Bras Ginecol Obstet. 2016;38:56–64. doi: 10.1055/s-0035-1571265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American College of Sports Medicine. Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. American College of Sports Medicine position stand. Med Sci Sports Exerc. 2009;41:1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 15.Hawley JA, Hargreaves M, Joyner MJ, Zierath JR. Integrative biology of exercise. Cell. 2014;159:738–749. doi: 10.1016/j.cell.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 16.Louis E, Raue U, Yang Y, et al. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol (1985) 2007;103:1744–1751. doi: 10.1152/japplphysiol.00679.2007. [DOI] [PubMed] [Google Scholar]
- 17.Stefanetti RJ, Lamon S, Wallace M, et al. Regulation of ubiquitin proteasome pathway molecular markers in response to endurance and resistance exercise and training. Pflugers Arch. 2015;467:1523–1537. doi: 10.1007/s00424-014-1587-y. [DOI] [PubMed] [Google Scholar]
- 18.MacKenzie MG, Hamilton DL, Pepin M, et al. Inhibition of myostatin signaling through Notch activation following acute resistance exercise. PLoS One. 2013;8(7):e68743. doi: 10.1371/journal.pone.0068743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dieli-Conwright CM, Spektor TM, Rice JC, et al. Influence of hormone replacement therapy on eccentric exercise induced myogenic gene expression in postmenopausal women. J Appl Physiol (1985) 2009;107:1381–1388. doi: 10.1152/japplphysiol.00590.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dieli-Conwright CM, Spektor TM, Rice JC, et al. Hormone therapy and maximal eccentric exercise alters myostatin-related gene expression in postmenopausal women Journal of strength and conditioning research. J Strength Cond Res. 2012;26:1374–1382. doi: 10.1519/JSC.0b013e318251083f. [DOI] [PubMed] [Google Scholar]
- 21.Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol (1985) 1996;80:261–270. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- 22.Khajuria DK, Razdan R, Mahapatra DR. Description of a new method of ovariectomy in female rats. Rev Bras Reumatol. 2012;52:462–470. [PubMed] [Google Scholar]
- 23.Bär KJ, Schaible HG, Bräuer R, et al. The proportion of TRPV1 protein-positive lumbar DRG neurones does not increase in the course of acute and chronic antigen-induced arthritis in the knee joint of the rat. Neurosci Lett. 2004;361:172–175. doi: 10.1016/j.neulet.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 24.Matheny RW, Merritt E, Zannikos SV, et al. Serum IGF-I-deficiency does not prevent compensatory skeletal muscle hypertrophy in resistance exercise. Exp Biol Med (Maywood) 2009;234:164–170. doi: 10.3181/0808-RM-251. [DOI] [PubMed] [Google Scholar]
- 25.Filippin LI, Teixeira VN, Viacava PR, et al. Temporal development of muscle atrophy in murine model of arthritis is related to disease severity. J Cachexia Sarcopenia Muscle. 2013;4:231–238. doi: 10.1007/s13539-013-0102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aguiar AF, Vechetti-Júnior IJ, Alves de Souza RW, et al. Myogenin, MyoD and IGF-I regulate muscle mass but not fiber-type conversion during resistance training in rats. Int J Sports Med. 2013;34:293–301. doi: 10.1055/s-0032-1321895. [DOI] [PubMed] [Google Scholar]
- 27.Rall LC, Roubenoff R. Rheumatoid cachexia: metabolic abnormalities, mechanisms and interventions. Rheumatology (Oxford) 2004;43:1219–1223. doi: 10.1093/rheumatology/keh321. [DOI] [PubMed] [Google Scholar]
- 28.Macedo AG, Krug AL, Herrera NA, et al. Low-intensity resistance training attenuates dexamethasone-induced atrophy in the flexor hallucis longus muscle. J Steroid Biochem Mol Biol. 2014;143:357–364. doi: 10.1016/j.jsbmb.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Tintignac LA, Lagirand J, Batonnet S, et al. Degradation of MyoD mediated by the SCF (MAFbx) ubiquitin ligase. J Biol Chem. 2005;280:2847–2856. doi: 10.1074/jbc.M411346200. [DOI] [PubMed] [Google Scholar]
- 30.Jogo M, Shiraishi S, Tamura TA. Identification of MAFbx as a myogenin-engaged F-box protein in SCF ubiquitin ligase. FEBS Lett. 2009;583:2715–2719. doi: 10.1016/j.febslet.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 31.Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol. 2005;6:9–20. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- 32.McCroskery S, Thomas M, Maxwell L, et al. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol. 2003;162:1135–1147. doi: 10.1083/jcb.200207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castillero E, Nieto-Bona MP, Fernández-Galaz C, et al. Fenofibrate, a PPAR{alpha} agonist, decreases atrogenes and myostatin expression and improves arthritis-induced skeletal muscle atrophy. Am J Physiol Endocrinol Metab. 2011;300:E790–E799. doi: 10.1152/ajpendo.00590.2010. [DOI] [PubMed] [Google Scholar]
- 34.Dieli-Conwright CM, Spektor TM, Rice JC, et al. Hormone therapy attenuates exercise-induced skeletal muscle damage in postmenopausal women. J Appl Physiol (1985) 2009;107:853–858. doi: 10.1152/japplphysiol.00404.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin AI, Nieto-Bona MP, Castillero E, et al. Effect of cyclooxygenase-2 inhibition by meloxicam, on atrogin-1 and myogenic regulatory factors in skeletal muscle of rats injected with endotoxin. J Physiol Pharmacol. 2012;63:649–659. [PubMed] [Google Scholar]
- 36.Granado M, Priego T, Martín AI, et al. Anti-inflammatory effect of the ghrelin agonist growth hormone-releasing peptide-2 (GHRP-2) in arthritic rats. Am J Physiol Endocrinol Metab. 2005;288:E486–E492. doi: 10.1152/ajpendo.00196.2004. [DOI] [PubMed] [Google Scholar]
- 37.Lang CH, Pruznak A, Navaratnarajah M, et al. Chronic alpha-hydroxyisocaproic acid treatment improves muscle recovery after immobilization-induced atrophy. Am J Physiol Endocrinol Metab. 2013;305:E416–E428. doi: 10.1152/ajpendo.00618.2012. [DOI] [PubMed] [Google Scholar]
- 38.Haddad F, Adams GR, Bodell PW, Baldwin KM. Isometric resistance exercise fails to counteract skeletal muscle atrophy processes during the initial stages of unloading. J Appl Physiol (1985) 2006;100:433–441. doi: 10.1152/japplphysiol.01203.2005. [DOI] [PubMed] [Google Scholar]
- 39.Krüger M, Kötter S. Titin, a Central Mediator for Hypertrophic Signaling, Exercise-Induced Mechanosignaling and Skeletal Muscle Remodeling. Front Physiol. 2016;7:76. doi: 10.3389/fphys.2016.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]