Abstract
Objective:
Globally, the number of people living with human immunodeficiency virus (PLHIV) particularly in sub-Saharan Africa is growing. This has been resulted in increased number of tuberculosis (TB) new cases. To control burden of TB among PLHIV, a number of collaborative TB/HIV activities were recommended. However, data about collaborative TB/HIV services in the study area is scarce. The objective of this study is to assess intensified TB case finding, implementation of isoniazid preventive therapy (IPT) and associated factors among PLHIV.
Methods:
A facility based cross-sectional study design was employed among 419 randomly selected PLHIV from public health facilities of Harari region. Systematic sampling method was used to obtain sample from each health facilities. Interviewer-administered questionnaire was used to collect data. Data were entered into EpiData and analyzed by SPSS statistical software. Multivariate logistic regression analysis was conducted to determine the presence of association between variables using odds ratio with 95% confidence interval and association was declared significant at P ≤ 0.05.
Results:
One hundred fifteen (75.2%) of the respondents reported that they offered screening for TB during their HIV chronic cares and 94 (29.8%) of them were found to be positive for active TB. Female sex [AOR 2.51; 95%CI (1.52, 6.14)], educated patients [AOR 0.52; 95%CI (0.21, 0.83)], CD4 count greater than 350 cells/dl3 [AOR 0.62; 95%CI(0.22,0.82)], Antiretroviral Therapy (ART) initiation [AOR 0.50; 95%CI (0.35, 0.88)] and missing dose of ART [AOR 2.57; 95%CI (1.21, 5.32)] were significantly associated with TB infection. Nearly four-fifth (78.7 %) of the study participants were provided IPT.
Conclusions:
Screening of TB among PLHIV and implementation of IPT in the region is lower when compared to the findings of other studies conducted in different parts of the country and needs to be improved through implementation of national and international guidelines.
Keywords: Intensified case finding, isoniazid preventive therapy, people living with human immunodeficiency virus, tuberculosis
Introduction
Globally, the number of people living with human immunodeficiency virus (PLHIV) is growing, particularly in sub-Saharan Africa including Ethiopia. This has been resulted in increased number of TB new cases among them.1 Worldwide, tuberculosis (TB) is the leading opportunistic infection (OI) among PLHIV.2 In 2013, an estimated 1.1 million (13%) of the 9.0 million people who developed TB worldwide were HIV-positive. The African region accounted for 78% of the estimated number of HIV-positive incident TB cases.3 In Ethiopia, routine data in the year 2014/15 showed that 96% newly enrolled clients in HIV pre-antiretroviral therapy (ART) care were screened for TB at initial visit and active TB was detected in 9.2% of them. However, only 18.2% of those with no clinical symptoms for TB received isoniazid (INH) preventive therapy (IPT).4 For these reasons, regular screening of all PLHIV for active TB and provision of either treatment for an active disease or preventive therapy are strongly recommended.5
HIV aggravates the development of TB disease among individuals with latent or new mycobacterium TB infection. The risk of acquiring TB is 20-37 times higher in PLHIV as compared with those who have not been infected with HIV.6 TB is the leading cause of death among PLHIV accounting for more than a quarter of deaths.7 The dual epidemics have a number of impacts on the health sector. It increases demand for care, deplete resources, make healthcare providers busy and worsen health-care delivery system. The World Health Organization (WHO) has recommended a number of collaborative TB/HIV activities as a response to the dual epidemics. These include interventions that reduce morbidity and mortality from TB in PLHIV. This interventions include, but not limited to, provision of ART and the three I’s for HIV/TB: Intensified case finding (ICF), IPT and infection control for TB including those on ART.8
ICF and early initiation of treatment of TB among PLHIV are among the activities recommended by WHO to interrupt TB transmission and reduces morbidity and mortality. Early screening of TB is the best opportunity to provide IPT for those who do not have symptoms and signs of TB to control TB among PLHIV.9 There should be a regular screening of TB for all PLHIV using a clinical algorithm at every visit to a health facility. Those who do not show any one of the symptoms of a current cough, fever, and weight loss or night sweats are unlikely to have active TB and should be offered IPT after ruling out any contraindication. For those PLHIV who show any one of the symptoms of TB, further diagnosis should be conducted and if TB is confirmed treatment for TB should be considered (Figure 1).10
Figure 1.
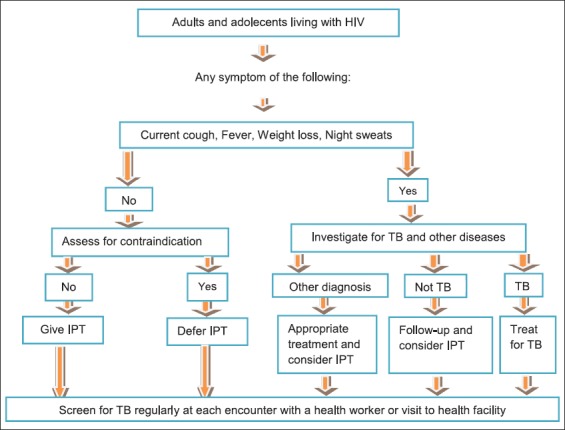
Algorism for tuberculosis screening in adults and adolescents living with human immunodeficiency virus in high prevalence and resource constraint settings (Adapted from WHO guideline, 2011)
IPT plays a major role in the prevention of TB among PLHIV. It has been recommended since 1998 by WHO and the Joint United Nations Programme on HIV/acquired immune deficiency virus (AIDS) as part of a comprehensive HIV and AIDS prevention strategy. However, its implementation has been very poor, particularly in the highest risk populations. Implementation of IPT has been impeded by several barriers such as weak active TB screening and low access to INH for fear of drug resistance.11 Reluctance of healthcare providers in advising the patients to cure or control TB disease is often resulted in passage of incorrect message, misunderstood, forgotten, or even completely ignored.8 The WHO recommended regimen for TB preventive therapy in adolescents and adults PLHIV is INH 300 mg daily for at least 6 months.12 IPT can be safely given to PLHIV who have no TB, reducing the risk of developing TB by 33-67% for up to 48 months.10
Despite the significant progress made in targeting people living with TB, the implementation of interventions to reduce the impact of TB among PLHIV is far below the targets of global plan to stop TB.13 Intensified case finding or screening for TB among PLHIV remains very low, only a limited amount of the global target of screening PLHIV by 2015 received the service.14 Information about the status of TB/HIV collaborative activities in general and reducing the burden of TB among adult PLHIV in particular in Ethiopia is limited. This is due to the absence of well-standardized information flow at all levels and scarcity of research-based evidence.15 Therefore, the main aim of this research is to assess ICF, implementation of IPT and its associated factors among adult PLHIV at public health facilities of Harari region.
Methods
Study design and setting
A facility-based cross-sectional study was conducted among 419 PLHIV and following their HIV/AIDS care and support at public health institutions in Harari region. Harari region is one of the nine regional states found in Ethiopia. Harari is the capital city of the Harari region and is found at 526 km far from Addis Ababa, the capital city of Ethiopia, to the east. The region has nine woredas (district) administration structures. Three of the weredas are rural and six are urban. The urban weredas are subdivided into 19 kebeles (smallest administrative structure), whereas the rural weredas are subdivided into 17 peasant associations. According to the population projection from 2007 census, currently, the region has a total population of 205,000 of which 54.8% were urban dwellers.16 The study was conducted from January to March 2015 among PLHIV attending their HIV/AIDS care and support at six public health facilities, two hospitals and four health centers found in the region.
Study participants
The participants of this study were randomly selected adult PLHIV who attended HIV/AIDS care and support at public health facilities. PLHIV with mental problem and seriously ill who cannot provide appropriate information was excluded from the study.
Sample size and sampling technique
Sample size was calculated using a single population proportion formula, (n = [Z α/2]2 p (1−p)/d2]. Proportion of TB infection among PLHIV (39%) was taken from the result of the previous study conducted in Ethiopia17 and 95% confidence level, 5% margin of error, and 15% nonresponse rate were considered. Therefore, the final sample size was calculated to be 421.
To collect sample from each health facility, proportional sample size allocation was used depending on HIV patient load in each health facility. The cumulative patient load in each health facility was obtained from HIV registration books (ART and pre-ART registers). Then using systematic random sampling, samples were selected from logbooks of PLHIV. The most next person on the registration book was interviewed if the selected person was died or defaulted. The samples obtained from the six health facilities were not similar as the commutative loads of patients among different health facilities were not similar.
Data collection
A pretested structured questionnaire was used to collect data via interviewer-administered approach. The tool was adapted from standardized WHO guideline, prepared for monitoring and evaluation of TB/HIV activities.10 The tool was then translated to local languages: Amharic and Afaan Oromoo for data collection. After completion of data collection, the questionnaire was again translated back to English language for analysis. The questionnaires were developed after completion of a literature review and pretested on 5% of the sample size of PLHIV attending their HIV care and support at health facilities other than the selected health facilities. Feedback obtained from pretest was incorporated, and the survey tools were finalized with some modifications. Six bachelor degree nurses (data collectors) and three public health professionals (supervisors) were recruited to facilitate data collection process. They were given training before commencement of data collection.
Data analysis
Data were entered into EpiData version 3.1 and exported to SPSS version 17 statistical packages for analysis. Descriptive statistics was used to summarize data, and the results were presented using frequency tables and percentages. Multivariate logistic regression analysis was used to determine the presence of associations between explanatory variables and dependent variable. Crude odds ratio with 95% confidence interval (CI) was used to determine the presence of association between independent variables and dependent variable. The degree of association between variables was measured using adjusted odds ratio (AOR) with 95% CI at significance level of ≤0.05.
Measurements
Dependent variables for this study were TB infection among PLHIV and treatment status with IPT. Explanatory variables include sociodemographic variables such as age, sex, residence, monthly income, educational status, religion and ethnicity, length of stay with HIV, ART treatment, length of stay on ART, ART missed dose, and hospitalization.
Data quality control
The questionnaire was pretested and feedback was used to make modifications to the questionnaires. Members of field staff (data collectors and supervisors) were selected based on their experience in the field of data collection and supervision. They were given extensive training before data collection was commenced. During training, the objective of the study, method of data collection and supervision were discussed. Furthermore, each question included in the questionnaire was discussed in detail and pretested to check its applicability. Each day, collected data were checked for its completeness and consistence by supervisors and investigators. Data were also cleaned and rechecked after double data entry was performed.
Ethical consideration
The study protocol was approved by the Institutional Health Research Ethics Review Committee of Haramaya University, College of Health and Medical Science (CHMS). Official letter of cooperation was written to Harari Regional Health Bureau and each health facilities that were included in the study from CHMS, Haramaya University. A letter explaining about the purpose, method and anticipated benefit and risk of the study was attached to each questionnaire and read to the participants. Since the participants were adults, it was explained for the respondents that participation in this study was voluntary and private information would be protected. Written and signed informed consent was obtained from each participant and patients’ identifiers were recorded.
Results
A total of 419 PLHIV were included into the study making response rate of 99.5%. 272 (64.9%) of the respondents were females. The mean age ± standard deviation (SD) of the study participants was 38 ± 10 years. 337 (80.4%) of the participants were in the age group 25-49 years. 184 (43.9%) were Amhara by ethnicity and 201 (48.0%) were followers of Christian Orthodox by religion. From total participants, 38.7% have no formal education while the remaining 61.3% attended some formal education of primary, secondary, and tertiary education. 237 (56.6%) of the respondents were currently married. The majority of the participants, 344 (82.1%), were urban dwellers. Regarding the occupation of the respondents, 121 (28.9%) were government employees while 111 (26.5%) and 56 (13.4%) were nongovernmental employee and merchant, respectively. The average household monthly income is 1402 Ethiopian birr. The participants’ household family size ranges from 1 to 9 with mean ± SD – 3 ± 1.6 (Table 1).
Table 1.
Sociodemographic characteristics of the respondents (N=419) Harari Region, 2015

HIV-related health services
A total of 359 (85.7%) of the study participants have already started highly active antiretroviral treatment (HAART) treatment while the remaining 14.3% were on pre ART care service. The CD4 level of the study participants ranged from 50 to 1242 with mean ± SD - 476.6 ± 214.6 cells/ml3. The mean period ± SD since diagnosed positive for HIV and length of stay ± SD on HAART was 6.5 ± 3.2 and 5.6 ± 2.6 years, respectively. 39 (9.3%) of those who started HAAR have missed at least a dose of their medications in the last 4 days of data collection period. 177 (42.2%) of the respondents were admitted to hospital at least once due to HIV-related illness (Table 2).
Table 2.
Collaborative TB/HIV care service delivery among PLHIV in Harari Region, 2015

Prevalence of TB among PLHIV
Nearly three-fourth, 315 (75.2%) of the study participants reported that they were asked for cardinal signs of TB during their clinic visit. In other words, only 75.2% of the respondents were offered TB screening service out of which 94 (29.8%) were found to be positive for active TB since they started HIV care and support follow-up. 36 (38.3%) of the coinfected clients responded that they were infected with TB before they knew their HIV serostatus (Table 2).
Provision of IPT
Even though, IPT is one of the packages of HIV/AIDS care and support, only 291 (69.5%) of the study participants have awareness about IPT service. 288 (68.7%) of them knew that PLHIV who do not have active TB should be given IPT service as a package to prevent TB infection. Among 221 (52.7%) eligible patients for IPT (negative result for TB screening) only 174 (78.7%) were provided IPT service. From the study participants who provided IPT services 125 (71.8%) participants received INH from HIV/AIDS care and support clinic, while 43 (24.7%) received the drug from TB clinic and the remaining were given the prescription and collected the drug from other health facilities (Table 2).
Factors associated with TB infection among PLHIV
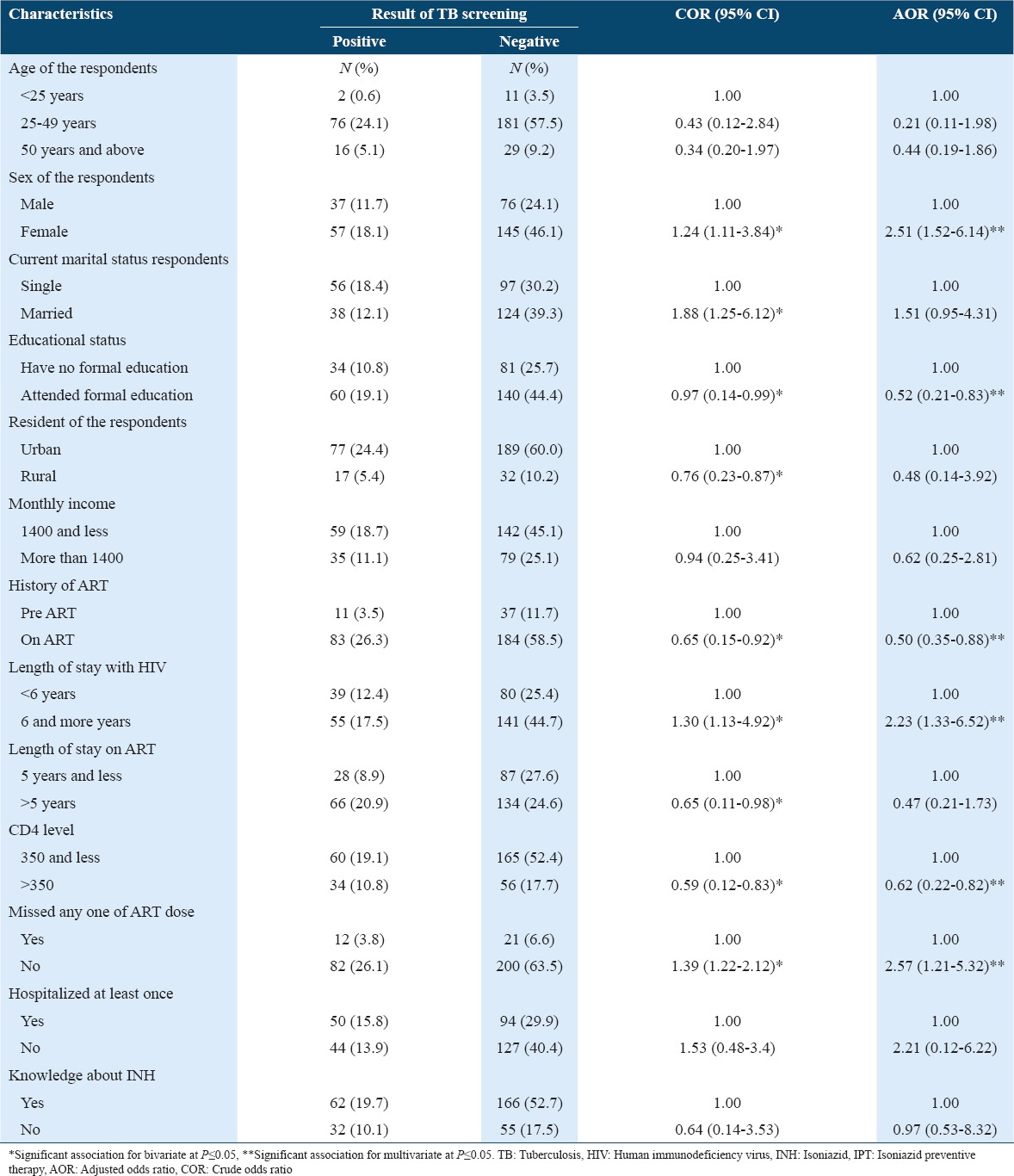
Multivariate logistic regression analysis showed that sex, educational status, ART status, duration of stay with HIV, CD4 level, and missing dose of ART medications were some of the factors that were significantly associated with TB infection among PLHIV after possible confounders were controlled. Female clients were 2.5 times more likely to be positive for TB than males (AOR: 2.51; 95% CI: 1.52-6.14) and those who attended formal education were less likely to have TB infection than those had never attended formal education (AOR: 0.52; 95% CI: 0.21-0.83). It was observed that being on ART treatment is safe as those clients on ART were less likely to have TB infection than those on pre ART (AOR: 0.50; 95% CI: 0.35-0.88). HIV clients who stayed with HIV for 6 years and more were 2.23 times more likely to acquire TB infection as compared with those stayed with HIV for <6 years (AOR: 2.23; 95% CI: 1.33-6.52). HIV/AIDS patients whose CD4 count is >350 cells/dl3 were 38% less likely to be infected with TB than those for which CD 4 count is 350 cells/dl3 and less (AOR: 0.62; 95% CI: 0.22-0.82). The study participants who missed their ART dose at least once were 2.57 more likely to acquire TB infection than those who did not miss dose (AOR: 2.57; 95% CI: 1.21-5.32) (Table 3).
Table 3.
Factors association with TB infection among people living with HIV (N=315) in Harari Region, Eastern Ethiopia, 2015
Discussion
HIV is the strongest risk factor for developing TB. The risk of developing TB is between 20 and 37 times greater in PLHIV than among those who do not have HIV infection. In response to the dual epidemics of HIV and TB, WHO has recommended a number of collaborative TB/HIV activities as part of core HIV and TB prevention among which ICF and IPT are the main one.18
In this study, 75.2% of the respondents reported that they had been screened for typical signs and symptoms of TB using clinical algorism by their healthcare providers. This result is slightly lower than that of an earlier study conducted in Addis Ababa by Wesen and Mitike which reported 89.7% and Denegetu and Dolamo in which 92.8% of PLHIV were screened for TB.19,20 Our finding is also lower than the study conducted in Ethiopia in which record of 7411 newly enrolled clients in HIV care was reviewed and 7113 (96%) of them were screened for TB at initial visit.4 The finding of our study is also lower than the study conducted in northeast Ethiopia in which 98.2% was screened but found to be higher than the latest national data which reported 71% PLHIV were screened for TB.21,22 This might indicate that TB/HIV care service in peripheral part of the country still requires special attention. The finding of this research is higher than findings from the previous studies in Sub-Saharan African countries. A study on integration of TB and HIV services in Sub-Saharan Africa in 2010 showed that only 64% of newly enrolled persons with HIV infection or AIDS were screened for TB.12
The results of this study showed that 94 (29.8%) of PLHIV developed active TB during HIV follow-up cares. This finding is higher than the finding from a study conducted in Addis Ababa in 2008 in which prevalence of TB among PLHIV was reported to be 15.6%19 and in 2011 which reported 10.4% of PLHIV were coinfected with TB.20 Another study conducted in Addis Ababa also indicated lower coinfection rate in which 32 (7%) HIV positive clients were diagnosed with confirmed TB disease.23 Results of a study from northeast Ethiopia indicated lower TB/HIV coinfection 24.3%.21 The results of this study indicated significantly higher proportions of PLHIV were infected with TB when compared with the case of developed countries. A study conducted in German among cohort of 11,693 patients, 233 (~2%) were diagnosed with TB, 62 at enrollment and 171 during follow-up, respectively.24 A study conducted in Georgia in 2008 also showed a lower prevalence of TB among PLHIV where 22% of HIV positive peoples have active TB.25 It is also higher than the result of meta-analysis study, which reported 23.51% TB/HIV coinfection rate.26 However, the finding of this study reported lower prevalence of TB among PLHIV when compared with the study findings of Ethiopia, 39.0%,17 Hong Kong 39%,27 and Pakistan 30.2%.28 This difference could be attributed to the weak TB screening among PLHIV which can affect early implementation of IPT that could reduce the occurrence TB.
IPT is one of the key interventions recommended by WHO to reduce the burden of TB in PLHIV.29 The result of a meta-analysis showed that the provision of IPT to persons with HIV infection reduced the incidence of TB by 33%.9 The finding of our study indicated that among 221 eligible patients for IPT, only 174 (78.7%) were provided with IPT service during their HIV chronic follow-up cares. The finding of this study is higher than the previous study findings conducted in Addis Ababa where 28.7% were provided with IPT20 and in Ethiopia where IPT had been initiated for 39% of eligible patients.30 Our finding is also higher than previous findings in Ethiopia and other countries. For instance, according to the 2011 global TB report, sampled data revealed IPT coverage of 15.1% for Ethiopia, only 5.4% for Bangladesh, for Myanmar 8.0%, and only 3% for Nigeria.18 The possible explanation for higher IPT coverage in our study may be improved TB/HIV collaborative activity in the country over time.
In this study sex, educational status, ART initiation status, duration of stay with HIV, CD4 level, and missing dose of ART medications were some of the factors that were significantly associated with TB infection among PLHIV. The same factors were reported from the study conducted in Addis Ababa and another part of Ethiopia where length of stay with HIV, length of stay on HAART and diagnosed for TB before HIV were found to be factors affecting TB infection among PLHIV.20,30,31 Being female sex of the respondent is one of the factors that have a significant association with TB infection. This may be due to the higher number of female participants than male in our study. Duration of stay with HIV was found to be a factor that is associated to TB infection; this may be attributed to that as time elapses CD4 count is diminished and reduced CD4 count is a risk for acquiring OIs including TB. Missing ART medication could also reduce drug sensitivity of the virus and increase the probability of acquiring OIs.32
Limitation of the study
This study used only quantitative data and did not include exploratory qualitative data and might be affected with recall bias as findings were dependent on patients’ self-report. The study suffered from the usual limitation of a cross-sectional nature of the design and the limited numbers of similar studies that were conducted in the study area to compare our findings with.
Conclusion
It can be concluded that screening for TB among PLHIV in Harari region is lower when compared to other studies conducted in different parts of the country. However, the prevalence of TB among PLHIV seems to be higher compared to previous findings both in Ethiopia and in other countries. In addition, provision of IPT is still low, though better than the previous studies. ICF for TB and IPT among PLHIV in Harari region need to be strengthened by adopting the available national and international guidelines. In addition, continuous support of health-care providers through ongoing trainings and experience sharing should be used as a tool for improving the implementation of ICF and IPT.
Acknowledgment
We would like to thank Haramaya University for funding our research. Our gratitude also goes to HRHB, all participated health facilities and study participants for their cooperation during data collection period. We would also like to express our appreciation to all individuals who supported and encouraged us during this research work.
References
- 1.The Global Fund to Fight AIDS, TB and Malaria (GFATM): Joint Tuberculosis and HIV Programming Information Note. 2014. [Last accessed on 2014 May]. Available from: http://www.theglobalfund.org/documents/core/infonotes/Core_TB-HIV_Infonote_en/
- 2.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in Sub-Saharan Africa. AIDS. 2008;22:1897–908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Global Tuberculosis Report, 2014, WHO/HTM/TB/2014.08. Geneva, Switzerland: WHO; 2014. [Google Scholar]
- 4.Ethiopian Public Health Institute (EPHI), Ministry of Health (MOH) National TB/HIV Sentinel Surveillance Annual Report, (July 2014 - June 2015) [Last accessed on 2015 Apr]. Available from: http://www.ephi.gov.et/images/pictures/Final_National_TB-HIV_Sentinel_Surveillance__OCT_20_FE2015_(1)%20(1).pdf .
- 5.World Health Organization. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for A Public Health Approach. Geneva: WHO; 2010. [PubMed] [Google Scholar]
- 6.Ayles H, Schaap A, Nota A, Sismanidis C, Tembwe R, De Haas P, et al. Prevalence of tuberculosis, HIV and respiratory symptoms in two Zambian communities: Implications for tuberculosis control in the era of HIV. PLoS One. 2009;4:e5602. doi: 10.1371/journal.pone.0005602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cain KP, McCarthy KD, Heilig CM, Monkongdee P, Tasaneeyapan T, Kanara N, et al. An algorithm for tuberculosis screening and diagnosis in people with HIV. N Engl J Med. 2010;362:707–16. doi: 10.1056/NEJMoa0907488. [DOI] [PubMed] [Google Scholar]
- 8.Chheng P, Tamhane A, Natpratan C, Tan V, Lay V, Sar B, et al. Pulmonary tuberculosis among patients visiting a voluntary confidential counseling and testing center, Cambodia. Int J Tuberc Lung Dis. 2008;12(3 Suppl 1):54–62. [PubMed] [Google Scholar]
- 9.Corbett EL, Bandason T, Cheung YB, Munyati S, Godfrey-Faussett P, Hayes R, et al. Epidemiology of tuberculosis in a high HIV prevalence population provided with enhanced diagnosis of symptomatic disease. PLoS Med. 2007;4:e22. doi: 10.1371/journal.pmed.0040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. Guidelines for Intensified Tuberculosis Case-Finding and Isoniazid Preventive Therapy for People Living with HIV in Resource Constrained Settings. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 11.Gordin FM, Matts JP, Miller C, Brown LS, Hafner R, John SL, et al. Acontrolled trial of isoniazid in persons with anergy and human immunodeficiency virus infection who are at high risk for tuberculosis. Terry Beirn Community Programs for Clinical Research on AIDS. N Engl J Med. 1997;337:315–20. doi: 10.1056/NEJM199707313370505. [DOI] [PubMed] [Google Scholar]
- 12.United Nations for AIDS Program, (UNAIDS): Global Fund Information Note: TB/HIV Collaborative Activities. [Last accessed on 2013 Jan 02]. Available from: http://www.stoptb.org/assets/2012/02/21/global/TB/HIV Collaborative Activities .
- 13.World Health Organization. The Global Plan to Stop TB 2006-2015. Geneva: Stop TB Partnership; 2006. [Google Scholar]
- 14.WHO, UNAIDS, UNICEF. Towards Universal Access: Scaling Up Priority HIV/AIDS Interventions in the Health Sector. Progress Report. Geneva: World Health Organization; 2008. [Google Scholar]
- 15.Ethiopian HIV/AIDS Prevention, Control Office (HAPCO), Global HIV/AIDS Monitoring and Evaluation Team, (GAMET). HIV/AIDS in Ethiopia: An Epidemiological Synthesis. The Global HIV/AIDS Program. Washington, DC: The World Bank; 2008. [Google Scholar]
- 16.Harari Regional Health Bureau. Activity Report of HIV Prevention and Control Office. 2014 [Google Scholar]
- 17.Adelman MW, Tsegaye M, Kempker RR, Alebachew T, Haile K, Tesfaye A, et al. Intensified tuberculosis case finding among HIV-infected persons using a WHO symptom screen and Xpert(®) MTB/RIF. Int J Tuberc Lung Dis. 2015;19:1197–203. doi: 10.5588/ijtld.15.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization, (WHO): Global Tuberculosis Control 2011 Report. Available from: http://www.apps.who.int/iris/bitstream/10665/44728/1/9789241564380_eng.pdf .
- 19.Wesen A, Mitike G. Screening and case detection for tuberculosis among people living with HIV in Addis Ababa, Ethiopia. Ethiop Med J. 2009;47:109–15. [PubMed] [Google Scholar]
- 20.Denegetu AW, Dolamo BL. Tuberculosis case finding and Isoniazid preventive therapy among people living with HIV at public health facilities of Addis Ababa, Ethiopia: A cross-sectional facility based study. BMC Public Health. 2014;14:52. doi: 10.1186/1471-2458-14-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniel M, Awoke D, Endalkachew D. TB/HIV co-infections and associated factors among patients on directly observed treatment short course in Northeastern Ethiopia: A 4 years retrospective study. BMC Res Notes. 2015;8:666. doi: 10.1186/s13104-015-1664-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO Country Office for Ethiopia. Tuberculosis Update in Ethiopia. Progress in 2014. Addis Ababa. 2015. [Last accessed on 2015 Sep 30]. Available from: http://www.afro.who.int/en/ethiopia/who-country-office-ethiopia.html .
- 23.Shah S, Demissie M, Lambert L, Ahmed J, Leulseged S, Kebede T, et al. Intensified tuberculosis case finding among HIV-Infected persons from a voluntary counseling and testing center in Addis Ababa, Ethiopia. J Acquir Immune Defic Syndr. 2009;50:537–45. doi: 10.1097/QAI.0b013e318196761c. [DOI] [PubMed] [Google Scholar]
- 24.Karo B, Haas W, Kollan C, Gunsenheimer-Bartmeyer B, Hamouda O, Fiebig L, et al. Tuberculosis among people living with HIV/AIDS in the German ClinSurv HIV Cohort: Long-term incidence and risk factors. BMC Infect Dis. 2014;14:148. doi: 10.1186/1471-2334-14-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gabunia P, Salakaia A, Kiria N, Kandelaki G, Tsertsvadze T. TB/HIV co infection in Georgia. Georgian Med News. 2008:7–10. [PubMed] [Google Scholar]
- 26.Gao J, Zheng P, Fu H. Prevalence of TB/HIV co-infection in countries except China: A systematic review and meta-analysis. PLoS One. 2013;8:e64915. doi: 10.1371/journal.pone.0064915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan CK, Alvarez Bognar F, Wong KH, Leung CC, Tam CM, Chan KC, et al. The epidemiology and clinical manifestations of human immunodeficiency virus-associated tuberculosis in Hong Kong. 2010;16:192–8. [PubMed] [Google Scholar]
- 28.Abdul RM, Muhammad AM, Arshad A, Sharaf AS, Bader FZ, Rashid Q, et al. Frequency of dual tuberculosis/human immunodeficiency virus infection in patients presenting at tertiary care centers at Karachi. JCPSP. 2007;17:591–3. doi: 10.2007/JCPSP.591593. [DOI] [PubMed] [Google Scholar]
- 29.WHO. Three Is Meeting: Intensified Case Finding (ICF), Isoniazid Preventive Therapy (IPT) and TB Infection Control (IC), for People Living with HIV. Geneva, Switzerland: WHO; 2008. [Last accessed on 2008 Apr]. Available from: http://www.who.int/hiv/pub/meetingreports/WHO_3Is_meeting_report.pdf . [Google Scholar]
- 30.Yirdaw KD, Jerene D, Gashu Z, Edginton ME, Kumar AM, Letamo Y, et al. Beneficial effect of isoniazid preventive therapy and antiretroviral therapy on the incidence of tuberculosis in people living with HIV in Ethiopia. PLoS One. 2014;9:e104557. doi: 10.1371/journal.pone.0104557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alemu YM, Awoke W, Wilder-Smith A. Determinants for tuberculosis in HIV-infected adults in Northwest Ethiopia: A multicentre case-control study. BMJ Open. 2016;6:e009058. doi: 10.1136/bmjopen-2015-009058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Assebe LF, Reda HL, Wubeneh AD, Lerebo WT, Lambert SM. The effect of isoniazid preventive therapy on incidence of tuberculosis among HIV-infected clients under pre-ART care, Jimma, Ethiopia: A retrospective cohort study. BMC Public Health. 2015;15:346. doi: 10.1186/s12889-015-1719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


