Abstract
A previous study showed that long-chain n-3 polyunsaturated fatty acids (LCn-3PUFA; >18 carbons n-3) exert an anabolic effect on protein metabolism through the upregulation of insulin sensitivity and activation of the insulin signaling pathway. This study further delineates for the first time whether the anabolic effect of LCn-3PUFA on metabolism is dose responsive. Six steers were used to test three graded amounts of menhaden oil rich in LCn-3PUFA (0%, 2% and 4%; enteral infusions) according to a double 3 × 3 Latin square design. Treatment comparisons were made using iso-energetic substitutions of control oil for menhaden oil and using 6-week experimental periods. The LCn-3PUFA in muscle total membrane phospholipids increased from 8%, 14% to 20% as dietary menhaden oil increased. Feeding graded amounts of menhaden oil linearly decreased plasma insulin concentration (49, 35 and 25 μU/ml, P = 0.01). The insulin-stimulated amino acid disposal rates as assessed using hyperinsulinemic–euglycemic–euaminoacidemic clamps (20, 40 and 80 mU/kg per h) were linearly increased by the incremental administrations of menhaden oil from 169, 238 to 375 μmol/kg per h (P = 0.005) during the 40 mU/kg per h clamp, and from 295, 360 and 590 mmol/kg per h (P = 0.02) during the 80 mU/kg per h clamp. Glucose disposal rate responded according to a quadratic relationship with the incremental menhaden oil amounts (P < 0.05). A regression analysis showed that 47% of the amino acid disposal rates elicited during the hyperinsulinemic clamp was related to muscle membrane LCn-3PUFA content (P = 0.003). These results show for the first time that both protein and glucose metabolism respond in a dose-dependent manner to menhaden oil and to muscle membrane LCn-3PUFA.
Keywords: insulin sensitivity, glucose metabolism, protein metabolism, muscle membrane phospholipids, long-chain n-3 polyunsaturated fatty acids
Implications
The developmental physiology shows that the sensitivity of the musculature to insulin decays exponentially during the neonatal period and this decline continuously progresses throughout life. This suggests that there is a large ‘natural’ potential for improving insulin sensitivity as the animal matures. A previous study using a growing steer model showed that muscle membrane long-chain n-3 poly-unsaturated fatty acids (LCn-3PUFA) can augment the sensitivity of protein anabolism to insulin. This study delineates for the first time that the anabolic effects of LCn-3PUFA are dose responsive. The current results contribute to developing feeding approaches that promote sustainable agricultural practices.
Introduction
The prominent role of the insulin sensitivity of muscle in metabolic health and growth has generated numerous investigations to develop interventions that can improve insulin sensitivity (Palmquist, 2009). Certain studies have shown that when insulin resistance is induced through high-fat feeding, the sensitivity of glucose metabolism to insulin in muscle can be improved by increasing the LCn-3PUFA content in muscle membranes (Storlien et al., 1987; Liu et al., 1994). Certain mechanisms upregulated by LCn-3PUFA during the high-fat feeding-induced insulin resistance (Storlien et al., 1987; Liu et al., 1994) are in part similar to those downregulated with age (Eisemann et al., 1997; Davis et al., 2003; Yang et al., 2008). Therefore, we previously investigated the potential of LCn-3PUFA to blunt the age-regulated decline in insulin sensitivity of protein metabolism during the neonatal period using neonatal pigs (Bergeron et al., 2007) and growing steers (Gingras et al., 2007). An iso-energetic substitution of 4% of control oil (60% cotton : 40% olive oils) with menhaden oil rich in LCn-3PUFA enhanced the insulin sensitivity of both glucose disposal (+37%) and amino acid utilization (+108%), as measured using hyperinsulinemic–euglycemic–euaminoacidemic clamps (Gingras et al., 2007). The improvement in insulin sensitivity with menhaden oil feeding was further demonstrated by an increased whole-body (WB) protein retention (+41%; Bergeron et al., 2007). Reduced amino acid oxidation and a downregulation of WB protein breakdown (Bergeron et al., 2007; Gingras et al., 2007) with menhaden oil feeding were indicative of reduced catabolic conditions. The increased activity of the PKB-mTOR-S6K pathway and of the abundance of muscle GLUT4 (Gingras et al., 2007) likely contributed to a more anabolic environment.
In the light of these data, the question that this second study addressed was to establish whether the insulin-regulated metabolism would respond in a dose-dependent manner. Long-chain n-3 PUFA are known to be incorporated into membranes according to dynamic acylation and deacylation reactions for which the extents vary between phospholipid fractions (Voelker, 1991). These acylation and deacylation dynamics result in different fatty acid compositions between membrane phospholipid fractions, and between tissues (Stubbs and Smith, 1984). The membrane fatty acid composition is a regulated characteristic, which impacts on cell metabolism potentially through changes in membrane fluidity, hydrocarbon and protein transmembrane motions and organelle reorganization. These changes may impact ATPase, adenylate cyclase, cellular interaction, insulin action and transmembrane transport (Stubbs and Smith, 1984; Else and Hulbert, 2003). This study directly examined for the first time the regulation of a dose–response to menhaden oil on both insulin-regulated protein and glucose metabolism in parallel to muscle membrane LCn-3PUFA composition using growing steers.
Material and methods
Animals, treatments and feeding
Six Red Angus × Simmental crossbred steers were divided into two groups based on body weight (BW) and were used to compare the effects of three graded amounts of menhaden oil rich in LCn-3PUFA on insulin-regulated metabolism over three experimental periods of 42 days according to a double 3 × 3 Latin square design. Steers weighed 272 kg (s.e. = 11 kg) at the onset of the study and 458 kg (s.e. = 20 kg) at the end of the study. They aged from 8 to 12.5 months on average during the study. Their physiological state can be compared to an adolescent stage. Steers were maintained in air-conditioned rooms at 16°C, in tie-stalls equipped with rubber mats and bedded with wood shavings. Steers were not implanted with growth promoters. They were fed a basal total mixed ration typical of the growing stage, programmed for 1.38 kg/day of BW gain (Table 1). The latter was composed of 58% concentrates; net energy for gain and crude protein were fed at 103% and 113%, respectively, of requirements to avoid net energy and protein limitations in response to treatments Protein was mainly supplied through soybean meal. The basal diet provided more than 97% of daily requirements of essential amino acids with the exception of histidine that was supplied at 92% (Fox et al., 1992); total histidine requirements were met by injecting 2 g of histidine (feeding grade; ACP Chemicals Inc., Quebec, Canada) into the abomasum daily during morning meals throughout the experiment to avoid essential amino acid limitation. Steers were fed ad libitum twice daily, allowing a minimum of 10% refusals. Refusals were weighed daily and sampled twice a week throughout the experiment. A constant ratio of ingredients was maintained by determining weekly dry matter of silages and pelleted concentrates. From days 28 to 42 of each experimental period, steers were restricted to 98% of the previous average 7-day ad libitum intake and fed every 2 h with automated feeders. The use of 2% restriction allowed avoiding refusal and helped maintain a similar nutritional steady state throughout the measurement days. Steers were equipped with chronic catheters implanted into the abomasum for oil infusions and histidine administration and into a mesenteric artery for sampling as described previously (Thivierge et al., 2002). Four to five weeks were allocated for surgical recovery of intake and BW gain before the study onset. The patency of the abomasal catheter failed for two steers during the experiment. Those animals were fitted with a rumen fistula. Oil infusions were then performed into the abomasum through the sulcus omasi, allowing for a 4-week period of post-surgical recovery.
Table 1.
Feed and chemical composition of the basal diet onadry matter basis
| Ingredient | Percentage of DM |
|---|---|
| Corn silage | 30 |
| Grass silage | 12 |
| Pelleted concentrates | 58 |
| Wheat | 34 |
| Cracked corn | 31 |
| Soybean meal | 13 |
| Soybean hulls | 16 |
| Dried molasses | 1.2 |
| Urea | 0.87 |
| Vitamins and minerals | 3.32 |
| Lignosol | 0.61 |
| Rumensin® 1 (p.p.m.) | 28 |
| Chemical composition | % |
| DM (%, as fed basis) | 54 |
| CP2 | 15 |
| Ether extract | 2.7 |
| Acid detergent fibers | 17 |
| Neutral detergent fibers | 31 |
DM = dry matter.
Elanco Animal Health Division, Eli Lilly, Ontario, Canada Inc.
In all, 66% rumen-degradable and 34% rumen-undegradable proteins.
Three oil treatments were compared using iso-energetic substitutions of oils on a dry matter basis (Table 2): (i) 0% menhaden oil + 4% control oil; (ii) 2% menhaden oil + 2% control oil; and (iii) 4% menhaden oil + 0% control oil. In a pilot study, the maximal oil infusion rate was initially tested at 6% of dry matter intake, and than decreased to 5% and finally set to 4%. Infusion rates greater than 4% significantly reduced intake, which would interfere with the objectives of this study. To test a dose–response curve to menhaden oil in this study, an intermediate dose between 0% and 4% was set at 2%. The control oil was a mixture composed of 60% cotton seed oil (Cedar Vale Natural Health Products; Cedar Vale, KS, USA) and 40% extra virgin olive oil (first-cold press, Olivia; Imperial Foods Inc., Québec, QC, Canada). The control oil had a similar fatty acid profile to beef to allow not to denature the animal receiving the control treatment, but was less saturated in order to assist liquefaction for infusion, as used previously (Gingras et al., 2007). Menhaden oil was alkali refined, bleached and pressed, and was supplemented with 500 p.p.m. of ethoxyquin (Omega Protein Inc., Reedville, VA, USA). Dietary oils were continuously infused into the abomasum via an abomasal catheter at a constant proportion of 4% dry matter intake using peristaltic pumps (Patrol Enteral Pump; Abbott Laboratories, Chicago, IL, USA) over the 42 consecutive days of each experimental period. Because of the fundamental nature of the current research, abomasal infusion approach was selected to administrate the oil treatments instead of using a dietary incorporation to bypass the rumen metabolism of oils and to minimize the variation in response to treatments. The oil infusion rate was individually adjusted on days 1, 14 and 28 of each experimental period according to actual dry matter intake (National Research Council, 2000). If an animal ate more than the predicted dry matter intake (National Research Council, 2000), this adjustment was set according to the predicted rather than actual dry matter intake in order to minimize fattening. Menhaden and control oils were kept in N-flushed amber bottles. Peroxide index (amount of hydrogen peroxide produced through oxidation) was determined weekly to control the quality of both dietary oil treatments administrated to the animals (Chapman and Mackay, 1949), and the latter were kept lower than 13 mEq O2/kg throughout the experiment.
Table 2.
Fatty acid composition of experimental oils
| Treatment | |||
|---|---|---|---|
|
|
|||
| Control oil (%) | 4 | 2 | 0 |
| Menhaden oil (%) | 0 | 2 | 4 |
| Fatty acid | % total | ||
| C14:0 | 0.9 | 4.2 | 9.0 |
| C14:1 | 0.0 | 0.2 | 0.3 |
| C16:0 | 18.9 | 18.7 | 16.8 |
| C16:1n-7 | 1.5 | 6.0 | 13.0 |
| C18:0 | 2.8 | 3.2 | 3.2 |
| C18:1 | 38.4 | 27.1 | 11.5 |
| C18:2n-6 | 34.4 | 19.2 | 1.5 |
| C18:3n-3 | 0.6 | 1.2 | 1.6 |
| C18:4n-3 | 0.2 | 1.8 | 2.9 |
| C20:3n-6 | nd | nd | 0.3 |
| C20:3n-3 | nd | 0.2 | 0.5 |
| C20:4n-6 | nd | 0.1 | 1.0 |
| C20:5n-3 | 0.8 | 6.2 | 15.6 |
| C22:0 | nd | nd | nd |
| C22:5n-3 | nd | 1.2 | 2.5 |
| C22:6n-3 | nd | 7.4 | 14.6 |
| SAT | 22.9 | 26.4 | 29.2 |
| PUFA | 36.9 | 38.9 | 43.7 |
| PUFA/SAT | 1.6 | 1.5 | 1.5 |
| n-3 | 2.5 | 19.1 | 39.9 |
| n-6 | 34.4 | 19.8 | 3.8 |
| n3/n6 | 0.1 | 1.0 | 10.5 |
| LCn-3PUFA | 1.6 | 14.8 | 32.6 |
SAT = total saturated fatty acids; PUFA = total polyunsaturated fatty acids; n-3 = total n-3 fatty acids; n-6 = total n-6 fatty acids; n-3/n-6 = ratio of total n-3/total n-6 fatty acids; LCn-3PUFA = 20:5n-3 + 22:5n-3 + 22:6n-3; nd = not detected.
Each 42-day experimental period allocated days 1 to 14 to enrich muscle membrane phospholipids in LCn-3PUFA, days 15 to 27 to monitor performance, and days 28 to 42 to perform measurements such as a dose–response to insulin and WB phenylalanine kinetics with muscle biopsies. Five weeks of enteral administration of LCn-3PUFA were sufficient to achieve large response to treatments, as shown previously (Gingras et al., 2007). That study has highlighted that a 5-week experimental period on the control treatment following a 5-week experimental period on the menhaden oil treatment did not allow to reduce the LCn-3PUFA amount in muscle membranes to the baseline value (i.e. 3.9%, s.e. = 0.7, A. A. Gingras, unpublished data). The experimental period of this study was extended to 6 weeks to assist substitution of LCn-3PUFA for control dietary fatty acids in muscle phospholipids. In this connection, the animals were transferred directly from one treatment to another between experimental periods to rapidly start modifying the fatty acid profile of membrane phospholipids, as managed previously (Gingras et al., 2007). To this end, the first 14 days of each experimental period were allocated to an adaptation to treatments. The phenylalanine kinetics and muscle biopsies were performed on day 31 (following a 3-day period where steers were fed every 2 h). A 20 mU/kg per h clamp was performed between days 35 and 36; a 40 mU/kg per h clamp was performed between days 37 and 38, and an 80 mU/kg per h clamp was performed between days 41 and 42 of each experimental period. This schedule respected a minimum of 36 h separating the 20 and 40 mU/kg per h clamps, and a minimum of 72 to 96 h, separating the 40 and 80 mU/kg per h clamps. This study was designed to investigate basic regulations of sensitivity of protein and glucose metabolism to insulin using growing steers. In this study, the performances are presented for informative purpose. Larger number of animals would be required to assess performance due to the elevated variability of these measures. The performances were not assessed during the in vivo measure period conducted over days 29 to 42, because the normal behavior of the animal would be altered over the course of these measures.
The experimental proposal and procedures were approved by the Animal Care and Use Committee of UniversitéLaval and were conducted in accordance with the Canadian Council on Animal Care guidelines (Canadian Council on Animal Care, 1993).
In vivo assays
Hyperinsulinemic–euglycemic–euaminoacidemic clamp procedure
Clamps were performed during steady-state feeding conditions achieved through 98% restricted 2-h feeding as used previously (Gingras et al., 2007). Hyper-insulinemic–euglycemic–euaminoacidemic clamps were conducted according to the procedures described previously (Gingras et al., 2007) based on the approach of Wray-Cahen et al. (1997). Insulin sensitivity was assessed using a dose–response curve to insulin by conducting three hyperinsulinemic clamps at 20, 40, 80 mU/kg per h. Individual basal concentrations of glucose and amino acids, using branched-chain amino acids (BCAA) as an index, were established early in the morning using four blood samples harvested every 10 min and immediately analyzed. Blood glucose was quantified by peroxidase reaction (YSI 2300 STAT Plus analyzer; Yellow Springs Instruments, Yellow Springs, OH, USA). Plasma concentration of total BCAA was measured by analysis of leucine, isoleucine and valine deamination by leucine dehydrogenase with stoichiometric reduction of NAD measured by spectrophotometry (Genesis 10 UV; Thermo Scientific, Ottawa, Ontario, Canada). Dextrose (50% sterile; CDMV, St-Hyacinthe, Quebec, Canada) and a complete sterile solution of L-amino acids (Gingras et al., 2007) were infused into a jugular vein to maintain circulating glucose and branched-chain amino acid concentrations at ±10% baseline values. An amino acid profile similar to muscle-mixed proteins was selected to match to a certain extent the requirement in amino acids during the insulin stimulation, because muscle is the main tissue using amino acids, as induced through the clamp technique. This approach previously allowed to maintain euaminoacidemia (Gingras et al., 2007). Clamps were conducted over a 180-min period on average; steady-state disposal rate of glucose and amino acids were monitored during the last 60 min of the 3-h period. During the last 60 min of each clamp, four blood samples were collected at 20-min intervals, analyzed for glucose and centrifuged. Plasma was harvested and frozen at −20°C until later determination of insulin and amino acid concentrations. In this study, three insulin doses were tested (20, 40 and 80 mU/kg per h) in combination with the three concentrations of menhaden oil (0%, 2% and 4%) to obtain a complete overview of the regulation of graded amounts of menhaden oil on components of insulin sensitivity and responsiveness (nine combinations). The clamp data for the 40 mU/kg per h insulin dose performed for the menhaden oil amounts of 0% and 4% (two out of nine combinations) have been reported previously (Gingras et al., 2007).
Fed steady-state WB amino acid tracer kinetics followed by muscle biopsies
l-[1-13C]phenylalanine kinetics were conducted during steady intake of nutrients achieved through 98% restriction feedings at 2-h intervals. The kinetic data are independent from those of the clamp and expand the response to LCn-3PUFA to a feeding-induced stimulation; the latter were performed at least 4 days before clamps. Tracer infusion onset was preceded by four background samplings of blood to determine the natural abundance of isotope. WB irreversible loss rate (WB ILR) of phenylalanine was measured using a continuous 8-h infusion of l-[1-13C]phenylalanine (2.00 μmol/kg per h; 98% mol percent excess (MPE); Cambridge Isotopes Laboratories, Andover, MA, USA), preceded by a pulse-dose injection (2.00 μmol/kg). During 6 to 8 h of the tracer-infusion period, five blood samples were taken at 30-min intervals. Blood samples were centrifuged at 4°C and plasma kept frozen at −20°C until further analyses for amino acids and phenylalanine isotopic enrichments. At 8 h of the tracer infusion, steers were sedated with a mixture of acepromazine (AtravetQR 0.1 mg/kg; Ayerst Veterinary Laboratories, Guelph, Ontario, Canada) and Butorphanol (TorbugesicQR 0.05 mg/kg; Ayerst Veterinary Laboratories). After 30 min, a biopsy of the longissimus dorsi was performed under local anesthesia (8 ml Lidocaïne HCl 5%; Bioniche Pharma Canada Ltd, Belleville, Ontario, Canada), and the tracer infusion was then stopped. Muscle samples were rapidly weighed and immediately frozen in liquid nitrogen. They were kept at −80°C until analyzed for membrane phospholipid and intramuscular triglyceride fatty acid profiles and insulin signaling. Sedatives used were chosen to minimize hyperglycemia and alteration of metabolism after their administration (Adams, 2001). This sedation protocol has been previously shown not to alter metabolism (Gingras et al., 2007).
Laboratory assays
Feeds were analyzed for total nitrogen, ether extract, acid detergent fibers and neutral detergent fibers using standard procedures (Association of Official Analytical Chemists, 1990). Plasma insulin was analyzed by radioimmuno assay using 125I-labeled porcine insulin and a guinea pig antibovine insulin serum (intra-assay coefficient of variation (CV) 6%; inter-assay CV 3%) as performed previously (Gingras et al., 2007). Plasma amino acid concentrations were determined by HPLC (Waters, Alliance system, Mississauga, Ontario, Canada) according to the Pico-Tag procedure as detailed previously (Thivierge et al., 2005). Isotopic enrichment of phenylalanine in plasma was determined after the conversion of phenylalanine into the n-propyl ester heptafluorobutyramide derivative and analyzed by gas chromatography mass spectrometry as described previously (Gingras et al., 2007).
The fatty acid composition of the total phospholipids was measured according to the method Counil et al. (2009). The composition of total phospholipids instead of individual phospholipid fractions was analyzed because we previously observed that the effects of LCn-3PUFA on insulin action were not specific to either phosphatidylcholine, phosphatidyethanolamine, phosphatidylinositol or phosphatidylserine (Gingras et al., 2007). The total phospholipids include plasma and organelle membranes; enrichment dynamic in LCn-3PUFA varies among these structures, but the fatty acid composition of total phospholipids relates to insulin sensitivity (Liu et al., 1994; Pan et al., 1995). Approximately 70 mg of frozen longissimus dorsi sample was analyzed. Briefly, total lipids were extracted along with internal standards for total phospholipids (C:15, Avanti Polar Lipids, Alabaster, AL, USA) and triglycerides (C:17, Sigma, St Louis, MO, USA) with a chloroform–methanol mixture (2 : 1) (Counil et al., 2009). Extracted lipids were separated by thin layer chromatography and fatty acid methyl esters of phospholipids and triglycerides were analyzed by capillary gas chromatography using an HP-88 column (100 m × 0.25 mm, internal diameter × 0.20 μm thickness; Agilent Technologies, Oakville, Ontario, Canada) in a Hewlett-Packard 5890 GC chromatograph (Hewlett Packard, Toronto, Ontario, Canada) coupled with a flame ionization detector; the carrier gas used was He. Fatty acids were identified according to their retention time using standard individual or mixtures of fatty acids as a basis for comparison (FAME 37 mix: Supelco Inc., Bellefonte, PA, USA; GLC-411 fatty acid mix: NuChek Prep Inc., Elysian, MN, USA; 22:5n-6: Larodan AB, Malmô, Sweden; 22:5n-3: Supelco Inc.) (Counil et al., 2009). Fatty acids are expressed in milligrams of fatty acids per 100 g of wet tissue or in percentage of total fatty acids. Fatty acid methyl esters of enteral oils were prepared by base-catalysed transmethylation (Chouinard et al., 1999), and presented as percentage of peak areas.
Calculations
WB ILR of phenyl alanine was calculated by isotopic dilution of the tracer corrected for the tracer infusion rate:
where IEpp represents phenylalanine isotopic enrichment in the arterial plasma precursor pool. Isotopic enrichments are presented as MPE.
Statistical analyses
Mean effect of graded amounts of menhaden oil on the different parameters were analyzed according to a double 3 × 3 Latin square design using the mixed procedure of SAS (SAS Institute Inc., 2000). The model included square, period and treatment as fixed effects and steer tested within square as a random effect. One steer died after the first experimental period, his data for periods 2 and 3 were estimated using predicted values of the model. Polynomial contrasts were used to compare treatment effects. Nonlinear regression modeling was performed using the regression procedure of SAS. Least square means with standard error of means are presented in tables and figures. Statistical difference is declared at P < 0.05.
Results
Fatty acids of membranes and intramuscular triglycerides
The substitution of control oil for menhaden oil increased the concentration of LCn-3PUFA in the infusate from 2% to 15% and to 33% of total fatty acids (Table 2). The total long-chain n-3 fraction (>18 carbons n-3) in muscle total membrane phospholipids was quite responsive to menhaden oil amounts: C20:5n-3 (+208%) and C22:6n-3 (+302%) were linearly increased (P = 0.001) as LCn-3PUFA in the enteral administration increased (Table 3). Less noticeable changes were observed within the long-chain n-6 fraction: C20:4n-6 content remained unaltered, but C20:3n-6 was decreased in a quadratic manner by 13% (P < 0.05). Changes in unsaturated fatty acids of the 18-carbon series were mainly led by alteration in C18:2n-6 that were linearly decreased by 36% (P < 0.001), whereas other fatty acids such as C18:1n-9 and C18:3n-3 remained unaltered. Graded menhaden oil administration did not impact muscle membrane content in saturated fatty acids such as C16:0 and C18:0, but did linearly increase C16:1n-7 from 0.81% and 0.88% to 1.27%.
Table 3.
Fatty acid composition of total membrane phospholipids of the longissimus dorsi (% total) of steers infused for 42 days with enteral-graded amounts of menhaden oil rich in LCn-3PUFA1
| Treatment | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Control oil (%) | 4 | 2 | 0 | Contrast P-value | ||
|
|
||||||
| Menhaden oil (%) | 0 | 2 | 4 | s.e. | Lin | Quad |
| 16:0 | 19.8 | 19.7 | 19.8 | 0.4 | 0.99 | 0.77 |
| 16:1n-7 | 0.81 | 0.88 | 1.27 | 0.12 | 0.02 | 0.27 |
| 18:0 | 16.6 | 16.3 | 17.1 | 0.4 | 0.36 | 0.26 |
| 18:1 | 11.5 | 9.9 | 10.4 | 0.9 | 0.24 | 0.19 |
| 18:2n-6 | 34.2 | 31.1 | 21.8 | 1.5 | <0.001 | 0.06 |
| 18:3n-3 | 0.48 | 0.48 | 1.05 | 0.16 | 0.07 | 0.22 |
| 20:3n-6 | 1.36 | 1.12 | 1.18 | 0.07 | 0.04 | 0.05 |
| 20:4n-6 | 5.5 | 5.3 | 5.6 | 0.5 | 0.5 | 0.37 |
| 20:5n-3 | 4.4 | 8.3 | 13.6 | 1.1 | 0.0003 | 0.62 |
| 22:0 | 0.42 | 0.41 | 0.41 | 0.02 | 0.79 | 0.93 |
| 22:5n-3 | 2.8 | 2.7 | 2.6 | 0.1 | 0.35 | 0.83 |
| 22:6n-3 | 0.96 | 2.9 | 3.9 | 0.4 | 0.0003 | 0.3 |
| LCn-3PUFA2 | 8.1 | 13.9 | 20.0 | 1.4 | 0.0003 | 0.93 |
| Total n-3 | 8.6 | 14.4 | 21.1 | 1.5 | 0.0004 | 0.82 |
| Total n-6 | 41.4 | 37.9 | 29.1 | 1.6 | <0.0001 | 0.11 |
| Saturated | 37.4 | 37.1 | 37.9 | 0.7 | 0.72 | 0.58 |
Lin = linear effect of Menhaden oil amounts; Quad = quadratic effect of Menhaden oil amounts; LCn-3PUFA = long-chain n-3 polyunsaturated fatty acids; LS = least square.
LS means ± s.e.; n = 6.
LCn-3PUFA = 20:5n-3 + 22:5n-3 + 22:6n-3.
When the fatty acids are summed, the percentage of total n-3 fatty acids linearly increased (P < 0.001; Table 3), but this increment was mainly driven by the LCn-3PUFA. Total n-6 fatty acids were linearly decreased by 30% (P < 0.001). The saturated fatty acid fraction was not altered by the menhaden oil feeding. When the sums of fatty acids were compared on a quantitative basis, and further comparisons were made between phospholipid and intramuscular triglyceride compositions, large differences in lipid dynamics are noted (Table 4). On an absolute basis, the phospholipids contained about 10 times the amount of LCn-3PUFA found in intramuscular triglycerides. The triglyceride n-6 fraction was not altered by the graded menhaden oil administration, whereas membrane phospholipids exhibited a simultaneous depletion in the latter. As for phospholipids, triglyceride saturated fatty acid content remained unaltered, but their content was four- to five-fold greater than the amount found in phospholipids. The total amount of fatty acids in intramuscular triglycerides was calculated for each treatment as an estimate of the compartment size of intramuscular fat (i.e. marbling; Table 4). Total fatty acids in intramuscular fat increased over the first, second and third experimental periods, along with the maturity of the animals, from 515 to 1211 and to 1435 mg/100 g muscle, respectively.
Table 4.
Sums of fatty acids in total membrane phospholipids and in intramuscular triglycerides of the longissimus dorsimuscle expressed on a quantitative basis (mg/100gfresh tissue) of steers infused for 42 days with enteral-graded amounts of menhaden oil rich in LCn-3PUFA1
| Treatment | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Control oil % | 4 | 2 | 0 | Contrast P-value | ||
|
|
||||||
| Menhaden oil % | 0 | 2 | 4 | s.e. | Lin | Quad |
| Total phospholipids | ||||||
| LCn-3PUFA2 | 22.8 | 37.9 | 56.2 | 3.6 | 0.0001 | 0.71 |
| Total n-3 | 23.9 | 39.0 | 59.2 | 3.8 | 0.0002 | 0.60 |
| Total n-6 | 116.2 | 104.1 | 79.3 | 6.4 | 0.002 | 0.39 |
| Saturated | 105.1 | 100.1 | 104.7 | 2.9 | 0.93 | 0.16 |
| Intramuscular triglycerides | ||||||
| LCn-3PUFA2 | 0.44 | 1.5 | 3.9 | 1.0 | 0.03 | 0.56 |
| Total n-3 | 2.2 | 3.6 | 6.9 | 1.4 | 0.02 | 0.52 |
| Total n-6 | 48.3 | 51.6 | 46.2 | 9.3 | 0.83 | 0.6 |
| Saturated | 406 | 464 | 506 | 66 | 0.16 | 0.9 |
| Period effect on total fatty acid amount in intramuscular triglycerides (index of marbling) mg/100 g fresh tissue3 | ||||||
| Period | 1 | 2 | 3 | s.e. | P | |
|
| ||||||
| 515 | 1211 | 1435 | 210 | 0.02 | ||
Lin = linear effect of Menhaden oil amounts; Quad = quadratic effect of Menhaden oil amounts; LCn-3PUFA = long-chain n-3 polyunsaturated fatty acids; LS = least square.
LS means ± s; n = 6.
LCn-3PUFA = 20:5n-3 + 22:5n-3 + 22:6n-3.
LS means and P-value of the period term of the mixed procedure model.
Growth performance
This study was planned to assess specific mechanistic aspects of LCn-3PUFA regulation of metabolism, and was not designed to evaluate animal performance, as the latter requires a large number of animals due to measurement variability. Feed intake tended to decrease (−10%; P = 0.10) with the graded menhaden oil amounts while BW gain remained similar (1.22 kg/d) (Table 5). The conversion of dietary crude protein into body protein gain were not different between treatments.
Table 5.
Performance of steers infused for 42 days with enteral-graded amounts of menhaden oil rich in LCn-3PUFA1
| Treatment | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Control oil % | 4 | 2 | 0 | Contrast P-value | ||
|
|
||||||
| Menhaden oil % | 0 | 2 | 4 | s.e. | Lin | Quad |
| BW (kg) | 406 | 392 | 399 | 19 | 0.62 | 0.46 |
| DM intake (kg) | 7.8 | 7.5 | 7.0 | 0.3 | 0.10 | 0.90 |
| CP intake (kg/day) | 1.18 | 1.13 | 1.05 | 0.56 | 0.20 | 0.87 |
| Daily gain (kg/day) | 1.34 | 1.19 | 1.13 | 0.23 | 0.40 | 0.83 |
| CP conversion ratio2 | 8.8 | 9.5 | 9.3 | 1.0 | 0.80 | 0.75 |
Lin = linear effect of menhaden oil amounts; Quad = quadratic effect of menhaden oil amounts; DM = dry matter; LS = least square.
LS ± s.e.; n = 6.
CP conversion = daily crude protein intake (kg/day)/daily weight protein gain (kg/day) using 10% body protein gain for similar steers and performance according to the National Research Council (2000).
Hyperinsulinemic–euglycemic–euaminoacidemic clamps
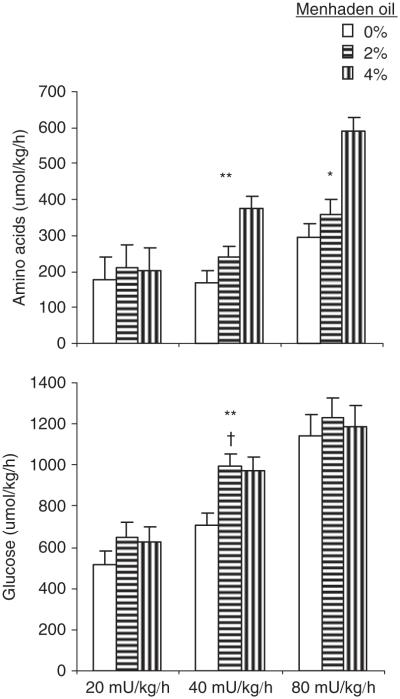
The sensitivity of glucose and protein metabolism to insulin was assessed during graded insulin infusions of 20, 40 and 80 mU/kg per h. The average baseline insulin concentration was linearly decreased from 49 to 25 μU/ml (P = 0.01; Table 6) with the incremental amounts of menhaden oil, suggesting improvement in insulin sensitivity. The insulin concentrations obtained during the clamps were linearly reduced as menhaden oil administration increased; however, this reduction was mainly driven by the insulin baselines, as the resulting insulinemia corrected for background concentration was similar during the clamp. Insulin concentrations during clamps were increased above baseline 1.6-fold between clamps 20- to 40- and 2.1-fold between clamps 40 to 80 mU insulin/kg per h. Graded amounts of menhaden oil linearly increased the amino acid disposal rate during the 40 mU/kg per h clamp, whereas plasma insulin concentration was elevated to 144 μU/ml (P < 0.01; Figure 1). Similarly, graded amounts of menhaden oil linearly increased amino acid disposal rate during the 80 mU/kg per h clamp, whereas plasma insulin concentration was elevated to 267 μU/ml. The insulin dose of 20 mU/kg per h, which produced an insulin concentration of 103 μU/ml was insufficient to generate difference between menhaden oil treatments. Finally, when amino acid infusion rates are regarded within one treatment but across insulin doses, a linear increase in amino acid disposal is seen with the increase in the insulin dose. Similarly to the amino acid disposal rate, treatment effect on glucose disposal rate was not detected at twice the baseline insulin concentration generated with the 20 mU/kg per h clamp. However, at 144 μU insulin/ml, there was a quadratic response in glucose disposal rate with the graded amounts of menhaden oil (P < 0.05), with a maximal disposal rate achieved with 2% menhaden oil. Greater insulin stimulation to 267 μU insulin/ml induced through a 80 mU/kg per h clamp apparently induced a maximal glucose disposal rate and steers receiving the control, 2% or 4% menhaden oil showed similar glucose disposal rates.
Table 6.
Insulin concentrations at baseline and during hyperinsulinemic clamps and insulin stimulation during clamps corrected for baseline in steers enterally infused for 42 days with graded amounts of menhaden oil rich in LCn-3PUFA1
| Treatment (μU/ml) | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Control oil % | 4 | 2 | 0 | Contrast P-value | ||
|
|
||||||
| Menhaden oil % | 0 | 2 | 4 | s.e. | Lin | Quad |
| Overall baseline average | 49 | 35 | 25 | 6 | 0.01 | 0.70 |
| Individual insulin baseline | ||||||
| 20 mU/kg per h | 50 | 29 | 25 | 6 | 0.01 | 0.24 |
| 40 mU/kg per h | 44 | 32 | 28 | 6 | 0.07 | 0.54 |
| 80 mU/kg per h | 53 | 42 | 21 | 10 | 0.03 | 0.66 |
| Clamp insulin | ||||||
| 20 mU/kg per h | 115 | 108 | 87 | 6 | 0.01 | 0.37 |
| 40 mU/kg per h | 147 | 148 | 139 | 6 | 0.26 | 0.45 |
| 80 mU/kg per h | 285 | 277 | 240 | 9 | 0.01 | 0.25 |
| Clamp insulin corrected for baseline | ||||||
| 20 mU/kg per h | 65 | 79 | 62 | 6 | 0.80 | 0.11 |
| 40 mU/kg per h | 101 | 116 | 109 | 7 | 0.54 | 0.30 |
| 80 mU/kg per h | 231 | 235 | 218 | 16 | 0.55 | 0.56 |
Lin = linear effect of menhaden oil amounts; Quad = quadratic effect of menhaden oil amounts; LS = least square.
LS means ± s.e.; n = 6.
Figure 1.
Absolute net disposal rates of amino acids and glucose in response to 20, 40 and 80 mU/kg per h insulin clamp. *Linear P < 0.05; **linear P < 0.01; †quadratic P = 0.06. Least square means ± s.e.; n = 6.
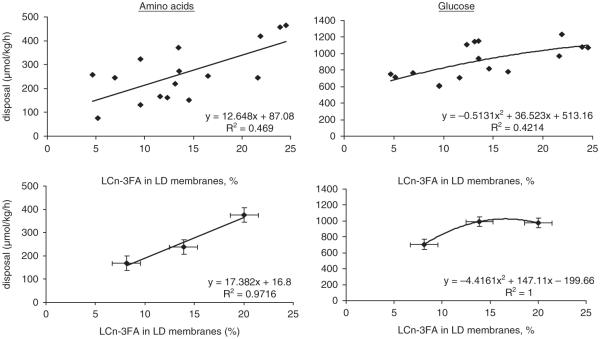
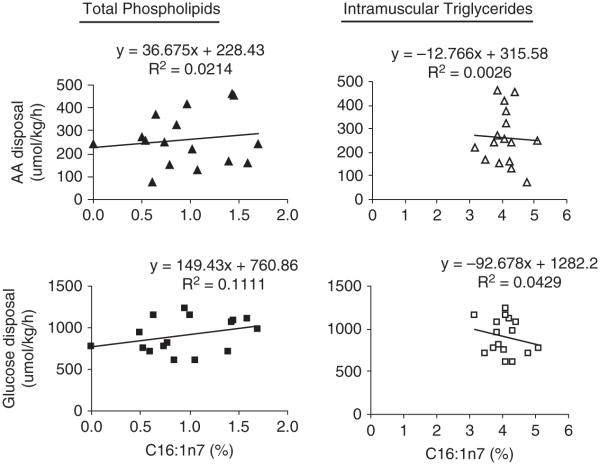
The net disposal of amino acids and glucose were regressed against the amount of LCn-3PUFA in muscle membrane phospholipids to further delineate the role of membrane fatty acid composition on the insulin-regulated metabolism (Figure 2). The net amino acid disposal can be explained using a linear model. The use of individual data shows that the net amino acid disposal relates to LCn-3PUFA with an R2 value of 0.50 (P = 0.0003). When treatment data are averaged, this relationship explains 0.97 of the variation. Glucose disposal appears to be regulated by membrane LCn-3PUFA according to a quadratic relationship. The regression model using individual data shows that membrane LCn-3PUFA explains 42% of glucose disposal (P = 0.007). A similar regression analysis was made using C16:1n-7 fatty acid (Figure 3). Its amount varied marginally from 0.81% to 1.27% in muscle membranes across the three treatments. The regression models show that the R2 values ranged from 0.02 to 0.11 using this fatty acid. When intramuscular triglycerides were used in the model, although the later lipid depot is not recognized to play a role in the insulin-regulated metabolism, no relationship was established with R2 values varying between 0.003 and 0.04. Similarly, when the quantitative amounts of C16:1n-7 (mg/100 g) in phospholipids and triglycerides were assessed using regression approach, similar low R2 values to those observed for C16:1n-7 presented as percentage of total were observed (data not presented).
Figure 2.
Regression analyses between net disposal rates of amino acids and glucose measured during a 40 mU/kg per h clamp and long-chain n-3 polyunsaturated fatty acids (LCn-3PUFA) content (% total) in muscle total membrane phospholipids. Panel a presents individual amino acid disposal rates, P = 0.003; Panel b presents average treatment effects on amino acid disposal rate as a function of membrane LCn-3PUFA (% total), error bars represent standard errors of mean; Panel c presents individual glucose disposal rates as a function of membrane LCn-3PUFA content, P = 0.007. Panel d presents average treatment effects on glucose disposal rates as a function of LCn-3PUFA in membranes, error bars represent standard errors of mean. LCn-3PUFA, C20:5n-3 + C22:5n-3 + C22:6n-3.
Figure 3.
Regression analyses between net disposal rates of amino acids and glucose measured during a 40 mU/kg per h clamp and the C16:1n-7 content (% total) in muscle total membrane phospholipids and triglycerides.
WB amino acid kinetics and plasma amino acids
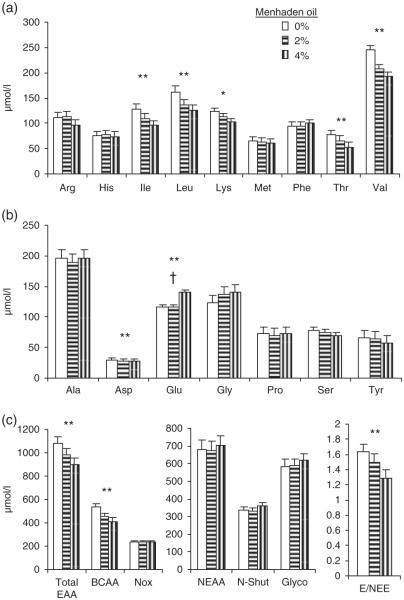
Kinetics were measured at the WB level during a fed steady state achieved through feeding at 2-h intervals while labeled phenylalanine was continuously infused (Figure 4). Under those conditions, the graded amounts of menhaden oil rich in LCn-3PUFA were associated with linear reductions in the plasma concentrations of isoleucine (−25%; P < 0.01), leucine (−22%; P < 0.01), lysine (−17%; P < 0.05), threonine (−31%; P < 0.01), valine (−21%; P < 0.01) (Figure 4a) and aspartic acid (−7%; P < 0.01; Figure 4b). Glutamic acid, which is mainly produced by the liver and used by peripheral tissues in the fed state was increased in a quadratic manner (+21%; P < 0.05) with menhaden oil feeding. The summation of the amino acids provides additional information highlighting that the arterial essential amino acids were reduced by 17% (P < 0.01; Figure 4c) and the BCAA were in part responsible for this observation with a 23% reduction (P < 0.01) in their arterial concentration. Amino acids involved in the inter-organ nitrogen movements were not different between graded amounts of enteral oils. Amino acids with lower metabolism and that are oxidized at low rates in cattle, such as phenylalanine, histidine and methionine (Black et al., 1990), remained unaltered with menhaden oil feeding. Similarly, phenylalanine isotopic enrichments were unaltered (3.4 MPE; Figure 5). In this study, WB total flux of phenylalanine was numerically reduced from 60 to 58 μmol/kg per h, but did not reach significance.
Figure 4.
Plasma concentrations of amino acids as measured during steady-fed state and whole-body phenylalanine kinetics. Panel a presents individual essential amino acid concentrations; Panel b presents individual non-essential amino acid concentrations; Panel c presents different sums of amino acids. Total EAA, total essential amino acids; BCAA, branched-chain amino acids; Nox, amino acids the most preserved from oxidation in the bovine, i.e. His, Met, Phe; NEAA, non-essential amino acids; N-Shut, amino acids shuttling N between tissues of the body, i.e. Ala, Asp, Glu; Glyco, glycogenic non-essential amino acids, i.e. Ala, Asp, Glu, Gly, Pro, Ser. *Linear P < 0.05; **linear P < 0.01; †quadratic P < 0.05. Least square means ± s.e.; n = 6.
Figure 5.
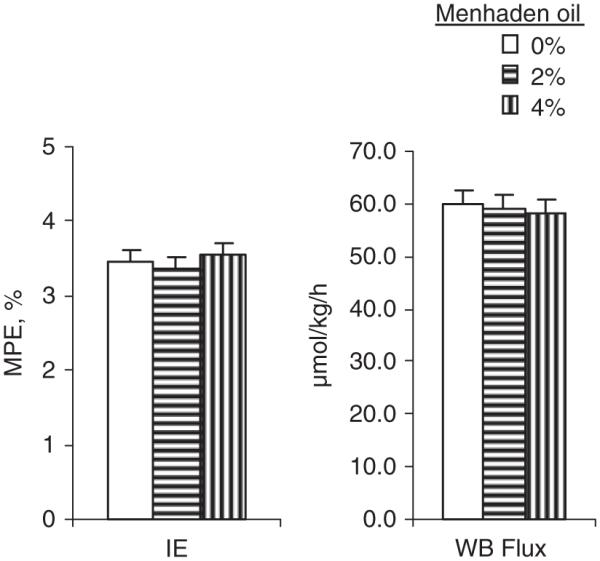
Isotopic enrichments of arterial plasma phenylalanine as assessed during steady-fed state and whole-body phenylalanine kinetics. MPE = mol percent excess, IE = isotopic enrichment, WB flux = whole body flux. Least square means ± s.e.; n = 6.
Discussion
A previous study using growing steers showed the regulatory role of LCn-3PUFA on protein anabolism through improvement of insulin sensitivity and activation of the insulin signaling pathway (Gingras et al., 2007). This study further investigated whether the regulation of the sensitivity of both protein and glucose metabolism to insulin are altered in a dose-dependent manner by the membrane content in LCn-3PUFA when graded amounts of menhaden oil are infused enterally over 6 weeks according to a Latin square design. The sensitivity and the responsiveness to insulin were investigated by performing hyperinsulinemic–euglycemic–euaminoacidemic clamps at three different insulin doses (20, 40 and 80 mU/kg per h). The use of healthy growing steers provided a model to examine whether the LCn-3PUFA upregulate protein anabolism during a period when muscle insulin sensitivity continues to decline due to the progression of maturity (Eisemann et al., 1997).
Skeletal muscle membranes are known to be sensitive to dietary LCn-3PUFA alterations along with heart and liver membranes (MacDonald and Sprecher, 1991; Jones et al., 1995). The regulated enzymatic process that alters the fatty acyl composition of phospholipids once these are transported to organelles and plasma membrane after their synthesis in the endoplasmic reticulum is a multistep mechanism not fully understood (Stubbs and Smith, 1984). However, this study contributes to provide evidences on how finely can be regulated the fatty acid composition of membranes. A linear increase in the enteral administration of LCn-3PUFA administrated over 42 days resulted in a linear increase in the content of muscle membrane LCn-3PUFA, with a parallel decrease in n-6 fatty acids. This suggests that LCn-3PUFA could exert a functional role in membrane function and cellular metabolism regulation through this substitution and through their content in membrane phospholipids (Stubbs and Smith, 1984; MacDonald and Sprecher, 1991; Voelker, 1991; Daveloose et al., 1993; Liu et al., 1994; Abel et al., 1997). To our knowledge, this study is the first investigation studying a dose–response effect of menhaden oil simultaneously on muscle total phospholipids and intramuscular triglycerides. The polar property of phospholipids potentially plays a role on the large affinity of phospholipids for highly unsaturated n-3 fatty acids that is highlighted through the quantitative comparison with non-polar triglycerides. LCn-3PUFA were incorporated into intramuscular triglycerides only at 2%, 4% and 7% of their inclusions into the phospholipid fraction. This preferential inclusion of LCn-3PUFA in phospholipids on a quantitative basis occurred even if the intramuscular triglyceride compartment size was increased over time in the steers (marbling), which is a normal physiological process that accompanies ageing along with a change in body composition in these animals (Dudouet, 1999). In parallel, the degree of saturation of membrane phospholipids being not sensitive to dietary oils in this study, which is consistent with observations for non-muscle tissues in other species (MacDonald and Sprecher, 1991; Simopoulos, 1991) excludes the role of saturated fatty acids in the current metabolic responses. However, the amount of saturated fatty acids in muscle membranes appears to be highly regulated as it represented 1/5 of the amount found in intramuscular triglycerides. The regulation of membrane phospholipid composition would occur in accordance with membrane fluidity, carbohydrate and protein transmembrane motions and organelle reorganization, and these have been shown to impact ATPase, adenylate cyclase, cellular interaction, transmembrane transport and insulin action (Stubbs and Smith, 1984; Else and Hulbert, 2003). The arginine-activated nitric-oxide pathway in muscle could also be part of the basic regulation (Jobgen et al., 2006; Li et al., 2008a and 2008b). Previous studies showed a maximal LCn-3PUFA threshold of 22% in muscle membranes in newborn piglets (Bergeron et al., 2007) and in rat liver plasma membranes (Abel et al., 1997). In this study, a maximal LCn-3PUFA threshold was not detected in non-neonate skeletal muscle membranes at the inclusion levels and for the time of supplementation investigated. The changes in membrane composition induced through enteral oil administration were reversible, as observed previously (Innis and Clandinin, 1981; Gingras et al., 2007). Although these changes in membrane composition are reversible, 6-week experimental periods did not fully impoverish the muscle phospholipids in LCn-3PUFA to the initial baseline value (4.9%; average control treatment period 1, M. Fortin, unpublished data). The phospholipids are recognized for their affinity for highly unsaturated fatty acids (Stubbs and Smith, 1984; MacDonald and Sprecher, 1991). The LCn-3PUFA turnover involves their recycling within the phospholipid structure and they are exchanged between the phospholipid fractions within the membrane structures (Stubbs and Smith, 1984; Voelker, 1991). This recycling of specific fatty acids within the membrane phospholipid structure would play a functional role in regulating membrane fluidity and functions of the cell (Stubbs and Smith, 1984).
Feeding increasing amounts of menhaden oil resulted in a linear reduction in basal insulin concentrations indicating a dose-dependent effect. In a similar manner, a linear improvement in insulin sensitivity of protein metabolism occurred. The linear increase in the amino acid infusion rates measured with an average insulin concentration of 267 μU/ml (four-fold above baseline; clamp 80 mU/kg per h) may be explained by two distinct phenomenon – the increased amino acid infusion rate may result from a reduced endogenous amino acid entry into plasma as a result of a marked reduction in protein breakdown and absorption; and/or an increase in amino acids exiting plasma as a result of an increase in the amino acid disposal through protein deposition and amino acid oxidation. This study does not allow differentiating between entries and via the plasma pool; however, phenylalanine oxidation was largely decreased by LCn-3PUFA feeding in steers studied in similar conditions (Gingras et al., 2007). Because the steers used in this study were insulin resistant due to the progression of maturity, the current observations support the hypothesis that LCn-3PUFA feeding enhances the sensitivity of proteolysis and/or protein synthesis to insulin, both of which are downregulated with development and age in different animal models including the human (Eisemann et al., 1997; Volpi et al., 1998; Combaret et al., 2005; Bergeron et al., 2007). During hyperinsulinemic clamp, muscle uptake of amino acids accounts for about half of the whole body disposal rate in human (Gelfand et al., 1988), so we can speculate that the amino acid disposal rates measured in this study were mainly driven by the muscle insulin sensitivity. When the insulin stimulation was increased seven-fold above baseline, the linear effect of enteral menhaden oil on the net amino acid disposal rate was still largely effective. As the sensitivity of muscle and responsiveness to insulin decrease with maturity and age across species (Fink et al., 1983; Eisemann et al., 1997; Davis et al., 1998), the current results highlight two key age-related aspects of the regulation of protein metabolism: first, insulin sensitivity of protein metabolism is improved through the increase in net amino acid disposal rate during hyperinsulinemia (i.e. right shift of the insulin sensitivity curve); second, this increase in amino acid disposal had not reached a maximal rate at an insulin level of seven-fold above the baseline concentration. The improvement in the responsiveness of protein metabolism to insulin may then potentially occur with menhaden oil feeding; however, this is in part speculative as supraphysiological insulin doses have not been tested. We previously published that the improvement in the sensitivity of muscle protein metabolism to insulin with menhaden oil is regulated at the post-receptor level with phosphorylation and activation of the Akt-mTOR-S6K pathway (Gingras et al., 2007). The latter observation is likely to relate to the improvement in insulin responsiveness (Fink et al., 1983).
The regulation of the sensitivity of glucose metabolism to insulin in response to the linear increase in enteral menhaden oil differs from that of protein metabolism. Glucose net disposal rate responded quadratically to the menhaden oil feeding. The maximal glucose disposal rate was achieved with the administration of 2% of menhaden oil, which suggests that the steers exhibited a greater sensitivity to improve the insulin-mediated glucose metabolism with the graded amounts of menhaden oil. A greater sensitivity of insulin-mediated glucose metabolism to LCn-3PUFA feeding could be consistent with the improvement in insulin resistance of non-lactating and non-gestational dairy cows enterally infused linseed oil (C18:3n-3) over a short period of 5 days, as assessed using glucose tolerance test (Pires et al., 2007). In this study, the lack of further increment in glucose disposal rate at 80 mU insulin/kg per h between the 2% and 4% menhaden oil administrations suggests that the elevated insulin stimulation at seven-fold above baseline was sufficient to achieve maximum glucose disposal rate in the control animals and no further increment occurred between 2% and 4% menhaden oil feeding. A maximal stimulation of glucose utilization during elevated insulin stimulation was also observed when performing supraphysiological insulin clamp in ageing humans (Fink et al., 1983). Insulin sensitivity of muscle glucose metabolism through LCn-3PUFA feeding is known to be maintained during high-fat feeding through an increase in insulin receptor content, augmentation in phosphorylation of the IRS-1 and PI3 kinase pathways and an increase in GLUT4 abundance in muscle (Taouis et al., 2002). Increased insulin receptor number and affinity were also previously shown (Liu et al., 1994) with LCn-3PUFA feeding. One or both of these regulatory pathways were likely maximally stimulated during the 80 mU/kg per h insulin clamp in this study.
Regression analyses between amino acid or glucose infusion rates monitored during the 40 mU/kg per h clamp and LCn-3PUFA in muscle total phospholipids, using individual data and average treatment data, provide an additional support to the hypothesis that membrane LCn-3PUFA regulate the insulin-regulated metabolism of both amino acids and glucose. Protein metabolism is linearly regulated by the LCn-3PUFA amount in muscle membranes as shown by the plot of individual data against LCn-3PUFA in muscle total membranes. The linear model shows that for an increment of one unit percentage of LCn-3PUFA in muscle membranes, the amino acid disposal increases by 13 μmol/kg per h. When treatment data are averaged, this relationship explains 0.97 of the variation. Glucose metabolism appears to be regulated by membrane LCn-3PUFA according to a quadratic relationship. In this regard, glucose metabolism is more sensitive to membrane LCn-3PUFA content than protein metabolism and achieved a maximal stimulation at 14% LCn-3PUFA in membranes with no further improvement in glucose disposal achieved at 20% LCn-3PUFA in muscle membranes. The current data does not allow delineating whether the improvement in insulin sensitivity of glucose metabolism involves changes in sensitivity of liver, muscle or a combination of both. However, the current data set complements the observations made previously using high-fat-fed rats, which showed that quadriceps membrane LCn-3PUFA linearly relate to glucose insulin sensitivity within the interval of 1% to 14% of LCn-3PUFA in membranes (Storlien et al., 1991), i.e., a higher content of these fatty acids in membranes in this study does not further improve glucose metabolism sensitivity to insulin.
C16:1n-7 was recently identified as a functional fatty acid with a lipokine activity that stimulates insulin action in dietary-induced obese mice (Cao et al., 2008; Olefsky, 2008). The impact of this fatty acid on the net amino acid and glucose disposal rates was investigated to delineate whether the improvement in insulin sensitivity through LCn3PUFA could be explained in part through the presence of C16:1n-7 in membrane phospholipids and/or muscle intramuscular triglycerides. The current data highlight that the modest changes in C16:1n-7 in muscle membranes and intramuscular fat that arise from supplementing 2% and 4% of menhaden oil in healthy normal (lean) steers and from endogenous synthesis do not explain the changes in amino acid and glucose disposal rates. Menhaden oil is not a suitable oil to test whether C16:1n-7 do exert any effect on insulin sensitivity as there is that large confounding effect with the LCn-3PUFA. Furthermore, the discrepancy between this study and the previous one (Cao et al., 2008) may arise from the differential regulation of lipid metabolism of mice as stated by the authors. Furthermore, the model in that study used dietary-induced obese mice, which involve further alteration of metabolic health than simply a reduction in insulin sensitivity due to age, such as elevated inflammation and oxidative stress, which are absent in our young and normal lean healthy steers.
Under the conditions of a stable insulinemia obtained through a steady-fed state, arterial concentrations of many essential amino acids were linearly decreased with increasing amounts of enteral LCn-3PUFA, accompanied by a lower ratio of essential to non-essential amino acids. As suggested for the increased amino acid disposal rates along with menhaden oil feeding monitored during the clamps, the reduction in amino acid arterial concentrations potentially mirrors an increased sensitivity of protein metabolism to insulin with the menhaden oil feeding. Increased amino acid utilization rates for protein synthesis and oxidation, combined or not with reduced appearance from breakdown and absorption could allow for these decreased arterial concentrations. The mild 10% decrease in intake would have a minor contribution to the decreased appearance rate of amino acids in plasma as the latter represents only 30% of inflow from protein breakdown (Harris et al., 1992). As the isotopic enrichments of phenylalanine were not increased along with the menhaden oil feeding as observed previously, reduced appearance from breakdown may not be the main pathway responsible for the current observation. A more anabolic environment and increased exit from the central plasma pool could be sustained through activation of protein synthetic apparatus, which was shown for the current animals through activated PKB-mTOR-S6K pathway with the 4% menhaden oil feeding (Gingras et al., 2007). WB ILR of phenylalanine was marginally decreased in this study, but not significantly, while it was reduced by 23% previously (Gingras et al., 2007). The reason for unaltered WB ILR of phenylalanine in this study is unknown. Phenylalanine arterial concentration remained unaltered as previously measured, but increases in isotopic enrichments were not seen in this study. We speculate that BCAA, such as leucine, may be a more sensitive marker to consistently measure changes in WB protein metabolism in response to LCn-3PUFA feeding. This statement is supported by the large variation in branched-chain amino acid arterial concentration with enteral menhaden oil administration. Furthermore, these are the most sensitive markers of essential amino acid concentration for maintaining euaminoacidemia during clamps as they exhibit the most extensive decline in arterial concentration during the hyperinsulinemic–euaminoacidemic clamp procedure (Wray-Cahen et al., 1997). Furthermore, the central role of leucine in protein synthesis regulation of the insulin resistant elderly musculature (Rieu et al., 2006) supports the speculation that leucine would be a more sensitive and reliable marker of this regulation. The number of animals used in this study was small, further evidences collated with previous studies conducted on the topic (Bergeron et al., 2007; Gingras et al., 2007) will help to further understand the involvement of muscle membrane fatty acid composition on muscle insulin action.
In conclusion, this study shows that the enteral administration of incremental amounts of menhaden oil rich in LCn-3PUFA induces a linear increase in the insulin sensitivity of protein metabolism with a maximal response achieved with 4% menhaden oil. Augmentation in responsiveness of protein metabolism to insulin may also be a feature of the anabolic response. Insulin sensitivity of glucose metabolism was differentially regulated with a quadratic increase in insulin sensitivity with the menhaden oil feeding; a maximal glucose utilization rate was achieved with only 2% menhaden oil. During a stable insulin stimulation obtained through a fed-steady state, many essential amino acid concentrations in arterial plasma were reduced, and this effect is consistent with a more anabolic environment with a greater amino acid use for metabolic purposes, although WB ILR of phenylalanine remained unaltered. BCAA, such as leucine, may be a reliable sensitive marker to assess amino acid metabolism sensitivity to insulin when membrane fatty acid composition is altered, as these are profoundly altered by insulin and menhaden oil feeding. These current findings complement previous studies highlighting LCn-3PUFA as fatty acids that can indirectly exert anabolic regulation of protein metabolism through improvement of insulin sensitivity once these are incorporated in muscle membranes. This study specifically shows that this regulation is dose-dependent. The role of membrane fatty acids on insulin action and on protein metabolism needs further investigations to establish whether functional nutrition could strategically be used to improve performance and further improve the knowledge of development, growth and potentially old age.
Acknowledgements
This work is funded in part by grants from the ‘Conseil de recherches en pêche et en agroalimentaire du Québec (COR-PAQ)’ and the ‘Fédération des producteurs de bovins du Québec’. A special thanks is extended to Omega Protein Inc., Reedville, VA, USA, who kindly provided menhaden oil required in this study. We gratefully thank Mrs Richard Prince and O’Neil Fecteau for their animal care and assistance. Appreciations are extended to Ms Line Berthiaume who performed the analyses of the fatty acid profile of muscle lipid fractions. Ms Thérèse Carbonneau – Ferme Simmental Thérèse et Claude Carbonneau Inc. and Mr Simon Marcotte – Élevage bovins St-Gilbert are gratefully acknowledged for their collaboration in providing crossbred steers.
References
- Abel S, Gelderblom WCA, Smuts CM, Kruger M. Thresholds and kinetics of fatty acid replacement in different cellular compartments in rat liver as a function of dietary n-6/n-3 fatty acid content. Prostaglandins, Leukotrienes and Essential Fatty Acids. 1997;56:29–39. doi: 10.1016/s0952-3278(97)90522-6. [DOI] [PubMed] [Google Scholar]
- Adams HR. Veterinary pharmacology and therapeutics. Iowa State University Press; Ames, IA, USA: 2001. [Google Scholar]
- Association of Official Analytical Chemists . Official Methods of Analysis. AOAC; Arlington, VA, USA: 1990. [Google Scholar]
- Bergeron K, Julien P, Davis TA, Myre A, Thivierge MC. Long-chain n-3 fatty acids enhance neonatal insulin-regulated protein metabolism in piglets by differentially altering muscle lipid composition. Journal of Lipid Research. 2007;48:2396–2410. doi: 10.1194/jlr.M700166-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black AL, Anand RS, Bruss ML, Brown CA, Nakagiri JA. Partitioning of amino acids in lactating cows: Oxidation to carbon dioxide. The Journal of Nutrition. 1990;120:700–710. doi: 10.1093/jn/120.7.700. [DOI] [PubMed] [Google Scholar]
- Canadian Council on Animal Care . Guide to care and used of experimental animals. 2nd Vol. 1. Bradda Printing Services; Ottawa, ON, Canada: 1993. [Google Scholar]
- Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RA, Mackay K. The estimation of peroxides in fats and oils by the ferric thiocyanate method. Journal of American Oil Chemists Society. 1949;26:321–325. [Google Scholar]
- Chouinard PY, Corneau L, Barbano DM, Metzger LE, Bauman DE. Conjugated linoleic acids alter milk fatty acid composition and inhibit milk fat secretion in dairy cows. The Journal of Nutrition. 1999;129:1579–1584. doi: 10.1093/jn/129.8.1579. [DOI] [PubMed] [Google Scholar]
- Combaret L, Dardevet D, Rieu I, Pouch M-N, Bechet D, Taillandier D, Grizard J, Attaix D. A leucine-supplemented diet restores the defective postprandial inhibition of proteasome-dependent proteolysis in aged rat skeletal muscle. The Journal of Physiology. 2005;569:489–499. doi: 10.1113/jphysiol.2005.098004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counil E, Julien P, Lamarche B, Chateau-Degat M-L, Ferland A, Dewailly E. Association between trans-fatty acids in erythrocytes and proatherogenic lipid profiles among Canadian Inuit of Nunavik: possible influences of sex and age. The British Journal of Nutrition. 2009;102:766–776. doi: 10.1017/S0007114509297182. [DOI] [PubMed] [Google Scholar]
- Daveloose D, Linard A, Asfi T, Viret J, Christon R. Simultaneous changes in lipid composition, fluidity and enzyme activity in piglet intestinal brush border membrane as affected by dietary polyunsaturated fatty acid deficiency. Biochimica Biophysica Acta. 1993;1166:229–237. doi: 10.1016/0005-2760(93)90102-f. [DOI] [PubMed] [Google Scholar]
- Davis TA, Burrin DG, Fiorotto ML, Reeds PJ, Jahoor F. Roles of insulin and amino acids in the regulation of protein synthesis in the neonate. The Journal of Nutrition. 1998;128:347S–350S. doi: 10.1093/jn/128.2.347S. [DOI] [PubMed] [Google Scholar]
- Davis TA, Suryawan A, Bush JA, O’Connor PMJ, Thivierge MC. Interaction of amino acids and hormones in the regulation of protein metabolism in growing animals. Canadian Journal of Animal Science. 2003;83:353–364. [Google Scholar]
- Dudouet C. La croissance et le développement. In: Dudouet C, editor. La production des bovins allaitants. Groupe France Agricole; France: 1999. p. 39. [Google Scholar]
- Eisemann JH, Huntington GB, Catherman DR. Insulin sensitivity and responsiveness of portal-drained viscera, liver, hindquarters, and whole body of beef steers weighing 275 or 490 kilograms. Journal of Animal Science. 1997;75:2084–2091. doi: 10.2527/1997.7582084x. [DOI] [PubMed] [Google Scholar]
- Else PL, Hulbert AJ. Membranes as metabolic pacemakers. Clinical Experimental Pharmacology & Physiology. 2003;30:559–564. doi: 10.1046/j.1440-1681.2003.03883.x. [DOI] [PubMed] [Google Scholar]
- Fink RI, Kolterman OG, Griffin J, Olefsky JM. Mechanisms of insulin resistance in aging. The Journal of Clinical Investigation. 1983;71:1235–1523. doi: 10.1172/JCI110908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox DG, Sniffen CJ, O’Connor JD, Russell JB, Soest PJV. A net carbohydrate and protein system for evaluating cattle diets: III. Cattle requirements and diet adequacy. Journal of Animal Science. 1992;70:3578–3596. doi: 10.2527/1992.70113578x. [DOI] [PubMed] [Google Scholar]
- Gelfand RA, Glickman MG, Castellino P, Louard RJ, DeFronzo RA. Measurement of L-[1-14C]leucine kinetics in splanchnic and leg tissues in humans. Diabetes. 1988;37:1365–1372. doi: 10.2337/diab.37.10.1365. [DOI] [PubMed] [Google Scholar]
- Gingras AA, White PJ, Chouinard PY, Julien P, Davis TA, Dombroski L, Couture Y, Dubreuil P, Myre A, Bergeron K, Marette A, Thivierge MC. Long-chain omega-3 fatty acids regulate whole-body protein metabolism by promoting muscle insulin signaling to the Akt-mTOR-S6K1 pathway and insulin sensitivity. The Journal of Physiology. 2007;579:269–284. doi: 10.1113/jphysiol.2006.121079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PM, Skene PA, Buchan V, Milne E, Calder AG, Anderson SE, Connell A, Lobley GE. Effect of food intake on hind-limb and whole-body protein metabolism in young growing sheep: chronic studies based on arteriovenous techniques. The British Journal of Nutrition. 1992;68:389–407. doi: 10.1079/bjn19920097. [DOI] [PubMed] [Google Scholar]
- Innis SM, Clandinin MT. Dynamic modulation of mitochondrial inner-membrane lipids in rat heart by dietary fat. The Biochemical Journal. 1981;193:155–167. doi: 10.1042/bj1930155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobgen WS, Fried SK, FU WJ, Meininger CJ, Wu G. Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrate. Journal of Nutritional Biochemistry. 2006;17:571–588. doi: 10.1016/j.jnutbio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Jones PJH, Toy BR, Cha MC. Differential fatty acid accretion in hearth, liver, and adipose tissues of rats fed beef tallow, fish oil, olive oil and safflower oil at three levels of energy intake. The Journal of Nutrition. 1995;125:1175–1182. doi: 10.1093/jn/125.5.1175. [DOI] [PubMed] [Google Scholar]
- Li P, Kim SW, Li X, Datta S, Pound WG, Wu G. Dietary supplementation with cholesterol and docosahexaenoic acid affects concentrations of amino acids in tissues of young pigs. Amino Acids. 2008a doi: 10.1007/s00726-008-0196-5. doi:10.1007/s00726-008-0196-5, Published Online by Springer Wien 30 October 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Sung WK, Li X, Datta S, Pond WG, Wu G. Dietary supplementation with cholesterol and docosahexaenoic acid increases the activity of the arginine-nitric oxide pathway in tissues of young pigs. Nitric Oxide. 2008b;19:259–265. doi: 10.1016/j.niox.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Baracos VE, Quinney HA, Clandinin MT. Dietary omega-3 and polyunsaturated fatty acids modify fatty acyl composition and insulin binding in skeletal-muscle sarcolemma. The Biochemical Journal. 1994;299:831–837. doi: 10.1042/bj2990831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald JIS, Sprecher H. Phospholipid fatty acid remodeling in mammalian cells. Biochemica Biophysica Acta. 1991;1084:105–121. doi: 10.1016/0005-2760(91)90209-z. [DOI] [PubMed] [Google Scholar]
- National Research Council . Nutrient Requirements of Beef Cattle. National Academy of Science; Washington, DC: 2000. Last updated. [Google Scholar]
- Olefsky JM. Fat talks, liver and muscle listen. Cell. 2008;134:914–916. doi: 10.1016/j.cell.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Palmquist DL. Omega-3 fatty acids in metabolism, health, and nutrition and for modified animal product foods. The Professional Animal Scientist. 2009;25:207–249. [Google Scholar]
- Pan DA, Lillioja S, Milner MR, Kriketos AD, Baur LA, Bogardus C, Storlien LH. Skeletal muscle membrane lipid composition is related to adiposity and insulin action. The Journal of Clinical Investigation. 1995;96:2802–2808. doi: 10.1172/JCI118350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires JAA, Pescara JB, Brickner AE, Silvia del Rio N, Cunha AP, Grummer RR. Effects of abomasal infusion of linseed oil on responses to glucose and insulin in Holstein cows. Journal of Dairy Science. 2007;91:1378–1390. doi: 10.3168/jds.2007-0714. [DOI] [PubMed] [Google Scholar]
- Rieu I, Balage M, Sornet C, Giraudet C, Pujos E, Grizard J, Mosoni L, Dardevet D. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. Journal of Physiology. 2006;575:305–315. doi: 10.1113/jphysiol.2006.110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc. Statistical Analysis System. SAS Institute Inc.; Cary, NC, USA: 2000. [Google Scholar]
- Simopoulos AP. Omega-3 fatty acids in health and disease and in growth and development. American The Journal of Clinical Nutrition. 1991;54:438–463. doi: 10.1093/ajcn/54.3.438. [DOI] [PubMed] [Google Scholar]
- Storlien LH, Jenkins AB, Chishlom DJ, Pascoe WS, Khouri S, Kraegen EW. Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglycerides and omega-3 fatty acids in muscle phospholipids. Diabetes. 1991;40:280–289. doi: 10.2337/diab.40.2.280. [DOI] [PubMed] [Google Scholar]
- Storlien LH, Kraegen EW, Chishlom DJ, Ford GL, Bruce DG, Pascoe WS. Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science. 1987;237:885–888. doi: 10.1126/science.3303333. [DOI] [PubMed] [Google Scholar]
- Stubbs CD, Smith AD. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochemica et Biophysica Acta. 1984;779:89–137. doi: 10.1016/0304-4157(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Taouis M, Dagou C, Ster C, Durand G, Pinault M, Delarue J. N-3 polyunsaturated fatty acids prevent the defect of insulin receptor signaling in muscle. American Journal of Physiology Endocrinology and Metabolism. 2002;282:E664–E671. doi: 10.1152/ajpendo.00320.2001. [DOI] [PubMed] [Google Scholar]
- Thivierge MC, Bernier JF, Dubreuil P, Lapierre H. The effect of jugular or abomasal infusion of amino acids on milk yield in lactating cows fed a protein deficient diet. Reproduction, Nutrition and Development. 2002;42:1–13. doi: 10.1051/rnd:2002001. [DOI] [PubMed] [Google Scholar]
- Thivierge MC, Bush JA, Suryawan A, Nguyen HV, Orellana RA, Burrin DG, Jahoor F, Davis TA. Whole body and hindlimb protein breakdown are differentially altered by feeding in neonatal piglets. The Journal of Nutrition. 2005;135:1430–1435. doi: 10.1093/jn/135.6.1430. [DOI] [PubMed] [Google Scholar]
- Voelker DR. Organelle biogenesis and intracellular lipid transport in eukaryotes. Microbiology Review. 1991;55:543–560. doi: 10.1128/mr.55.4.543-560.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi E, Ferrando A, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulates net muscle protein synthesis in the elderly. The Journal of Clinical Investigation. 1998;101:2000–2007. doi: 10.1172/JCI939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray-Cahen D, Beckett PR, Nguyen HV, Davis TA. Insulin-stimulated amino acid utilization during glucose and amino acids clamps decreases with development. American Journal of Physiology Endocrinology and Metabolism. 1997;273:E305–E314. doi: 10.1152/ajpendo.1997.273.2.E305. [DOI] [PubMed] [Google Scholar]
- Yang X, Yang C, Farberman A, Rideout TC, de Lange CFM, France J, Fan MZ. The mammalian target of rapamycin-signaling pathway in regulating metabolism and growth. Journal of Animal Science. 2008;86:E36–E50. doi: 10.2527/jas.2007-0567. [DOI] [PubMed] [Google Scholar]