Abstract
Importance
Intracerebral hemorrhage (ICH) survivors appear to develop cognitive impairment at high rates, both early after ICH and long-term.
Objective
to identify and compare risk factors for early and delayed dementia after ICH.
Design
longitudinal study enrolling ICH survivors from January 2006 to December 2013.
Setting
tertiary care academic institution.
Participants
1141 individuals with primary ICH, age ≥ 18 years and without pre-ICH dementia were considered for study enrollment. Of these, 24 refused consent and 379 were otherwise ineligible. A total of 738 ICH survivors were therefore included in Early Post-ICH Dementia (EPID) analyses. After accounting for incident dementia and mortality at 6 months, 435 participants were included in analyses of Delayed Post-ICH Dementia (DPID).
Exposure(s)
clinical history and demographic information, medications exposure during follow-up. All subjects underwent CT scanning at time of ICH to determine hematoma location and size, as well as severity of CT-defined White Matter Disease (CT-WMD).
Main Outcome and Measure(s)
Cognitive performance was captured using the Modified Telephone Interview for Cognitive Status test. Outcomes included: 1) EPID, diagnosed within 6 months after ICH; 2) DPID, diagnosed beyond 6 months after ICH.
Results
Among 738 ICH survivors, 140 (19%) developed dementia within 6 months. A total of 435 ICH without dementia at 6 months were followed longitudinally (median follow-up 47.4 months, Inter-Quartile Range [IQR] 43.4 - 52.1), with estimated yearly dementia incidence of 5.8% (95%CI 5.1-7.0%). Larger hematoma size (Hazard Ratio [HR] 1.47 per 10cc increase, 95% Confidence Interval [95%CI] 1.09-1.97) and lobar ICH location (HR 2.04, 95% CI 1.06 - 3.91) were specifically associated with EPID, but not with DPID (heterogeneity p<0.05 for both). Educational level (HR 0.60, 95%CI 0.40-0.89), incident mood symptoms (HR 1.29, 95%CI 1.02-1.63), and CT-WMD (HR 1.70, 95%CI 1.07-2.71) were specifically associated with DPID, but not EPID (heterogeneity p<0.05 for all).
Conclusions and Relevance
Early incident dementia after ICH is strongly associated with hematoma size and location. Delayed incident dementia is frequent among ICH survivors, and not prominently associated with acute ICH characteristics. These findings suggest the existence of heterogeneous biological mechanisms accounting for early vs. delayed cognitive decline among ICH survivors.
INTRODUCTION
Intracerebral Hemorrhage (ICH) accounts for 15% of all strokes and ~ 50% of stroke-related associated mortality and disability worldwide.1,2 ICH survivors are at high risk for several negative outcomes including rebleeding, ischemic stroke, and progressive cognitive impairment. These findings likely reflect the detrimental impact of underlying Cerebral Small Vessel Disease (CSVD), which is presumed to be the etiological factor responsible for both primary ICH and the associated conditions listed above.3 Indeed, ICH survivors demonstrate higher prevalence of genetic and neuroimaging markers of underlying CSVD than the general elderly population.4-6
While progressive cognitive decline (including incident dementia) is frequent after ICH, we possess very limited understanding of its associated risk factors. Prior studies reported very high rates of dementia after ICH, but were unable to fully describe its predictors.7-10 In particular, they did not investigate whether early and delayed dementias after ICH demonstrate comparable or distinct risk factor profiles.
We hypothesized that the extent of central nervous system injury associated with acute hematoma formation would be strongly associated with Early Post-ICH Dementia (EPID), but confer limited risk for Delayed Post-ICH Dementia (DPID). We sought to test this hypothesis in a ongoing longitudinal study enrolling primary ICH survivors, by means of two separate analyses focused on: 1) risk of developing dementia within 6 months after acute ICH (EPID); 2) risk of developing dementia 6 months after acute ICH and thereafter (DPID). Risk factors associated with either outcome were tested to clarify whether they conferred risk for cognitive decline only in the early or delayed timeframe.
METHODS
Overall Study Design
In order to investigate whether risk factors for post-ICH dementia differ based on temporal relationship to acute bleeding, we designed a two-stage study format (eFigure 1), including separate analyses for EPID risk (onset within 6 months after ICH), and DPID risk (onset beyond 6 months after ICH). We restricted analyses to individuals surviving beyond 3 months after ICH, in order to limit the impact on cognitive status of unrelated medical and neurological comorbidities leading to early case fatality. We therefore selected the next available follow-up time-point in our study (6 months), to separate EPID from DPID. Participants were first included in the early dementia analyses. Within this group, patients that survived and remained dementia free at 6 months were then included in the delayed dementia risk analyses. Visual inspection of dementia incidence rates supported our study design format by identifying a substantial decrease in incident dementia cases beyond 6 months from index ICH (eFigure 2).
Patient Recruitment and Baseline Data Collection
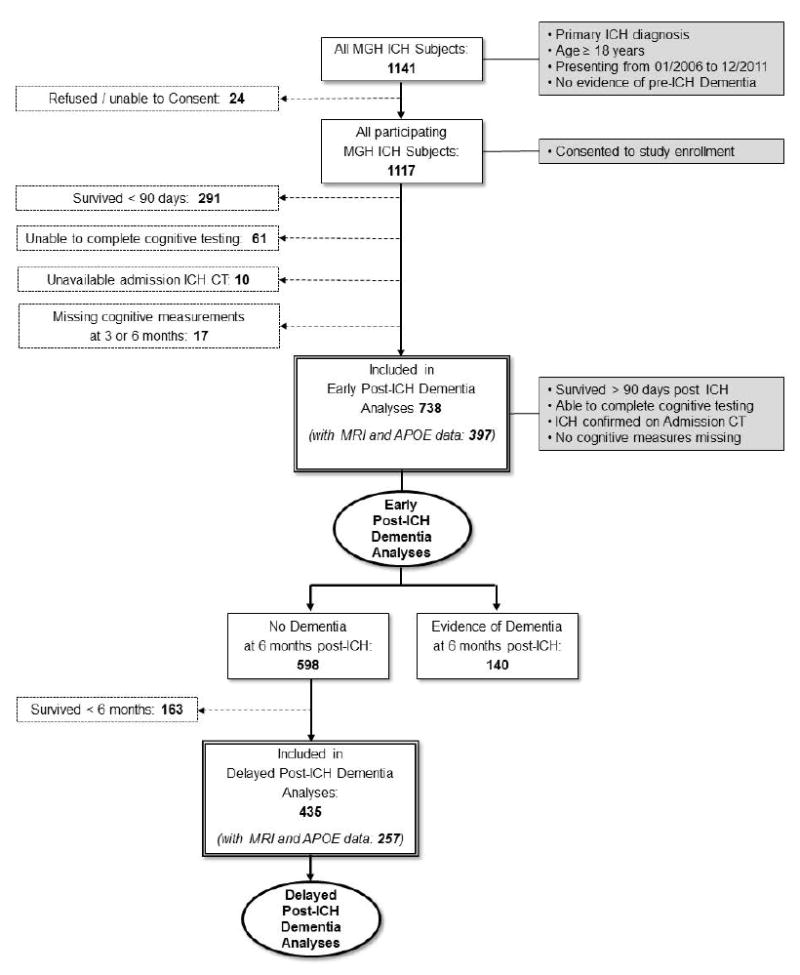
Participating individuals were enrolled in an ongoing single-center longitudinal cohort study of ICH as previously described.4,11,12 Particiapnts were selected among consecutive patients age ≥ 18 years, admitted to Massachusetts General Hospital (MGH) from January 2006 to December 2013 with primary ICH, i.e. not related to trauma, conversion of an ischemic infarct, rupture of a vascular malformation / aneurysm, or a brain tumor (Figure 1).
Figure 1. Study Design and Inclusion / Exclusion Criteria.
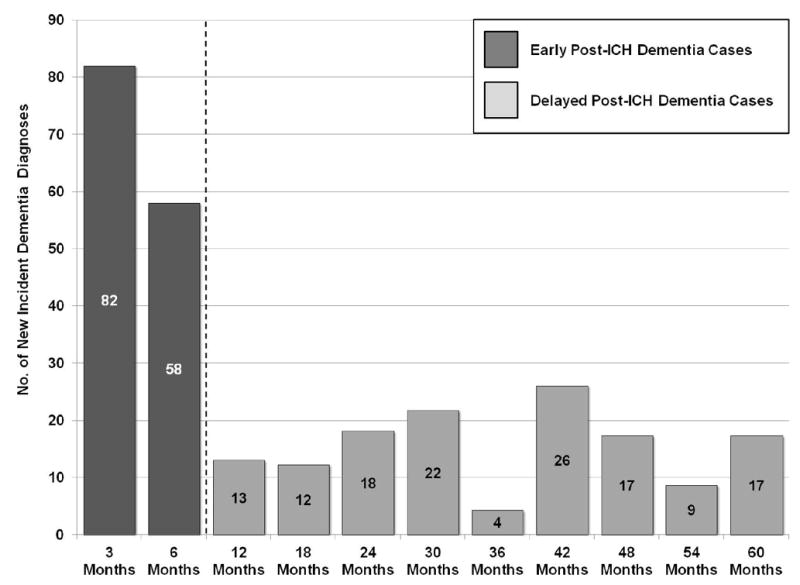
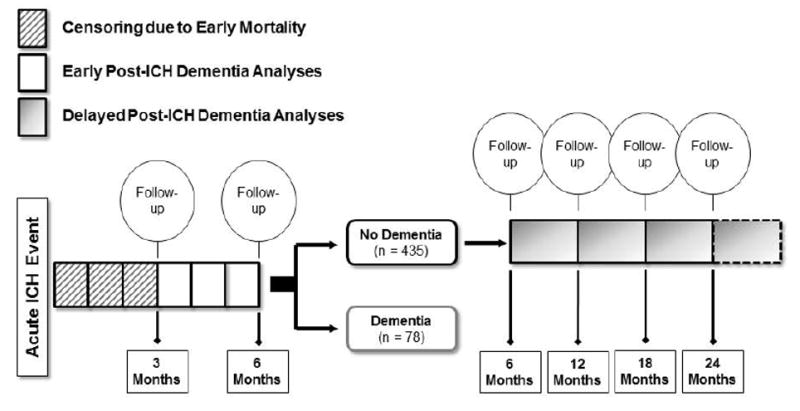
Figure presents study design, inclusion / exclusion criteria and patient groups retained for analysis. Solid single-line boxes represent subjects meeting criteria for inclusion in the present study at each step. Eligibility criteria for inclusion in the study are listed in grey-background boxes. Dashed lines connect to dashed-bordered boxes listing criteria for exclusion and number of subjects excluded. Double-line bordered boxes indicate study group subsets selected for analyses mentioned in the Results section. Oval shapes with bolded margins identify planned analyses.
Abbreviations: ICH = Intracerebral Hemorrhage
All pre-enrollment data were collected via review of existing medical records and billing information, combined with a structured standardized in-person interview. As the primary goal of this study was to investigate incident cognitive decline after ICH, all participants diagnosed with dementia prior to index were excluded. Pre-ICH dementia was identified by administering to reliable informants the 16-item (short) version of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE). As per reported normative data any subject with an average score of > 3.3 was diagnosed with pre-ICH dementia.13 In light of known ethnic and racial variations in outcome after ICH,14 participants were asked at enrollment to self-identify race and ethnicity, choosing from the options recommended for use in research studies by the Office for Management and Budget and the National Institutes of Health. All subjects required and index ICH admission CT scan, obtained within 24 hours of onset of symptoms.
The study protocol was approved by the Massachusetts General Hospital Institutional Review Board. Written informed consent was obtained from all study participants or their surrogates.
Genetic and Neuroimaging Data Acquisition and Interpretation
APOE genotype was determined for subjects consenting to blood draw ccording to previously published methods, to determine number of ε2/ε3/ε4 copies.4,11 ICH location was assigned based on consensus review of index ICH CT scans by study staff as previously described. 4,11 CT-defined white matter disease (CT-WMD) was quantified using a previously validated 4-point scale, separately grading severity of anterior and posterior WMD as none / mild (grade 0), moderate (grade 1), or severe (grade 2).4,15,16 MRI with axial gradient-echo images was performed in a subset of patients within 90 days of onset of symptoms, according to previously described methods.4 On available MRI scans we quantified: 1) MRI-defined White Matter Hyperintensity (MRI-WMH) volume17; 2) Cerebral Micro-Bleeds (CMBs) burden and location (lobar vs. non-lobar).4 All imaging analyses were performed and results recorded by study investigators without knowledge of participating subjects’ clinical and/or genetic information.
Longitudinal Follow-up
ICH survivors and/or their caregivers were contacted and interviewed by dedicated study staff at 3 and 6 months after index ICH, and every 6 months thereafter, per established protocols.4,11,12. Investigators inquired about and collected medical records pertaining to ICH recurrence, death, functional status, medication regimens according to previously published methods.12 Cognitive testing was performed at all follow-up times using the Modified Telephone Interview for Cognitive Status (TICS-m) test.18-22 The TICS-m is validated telephone-based global cognitive assessment tool, that measures overall cognitive performance with scores ranging from 0 (worst performance) to 39 (best performance).19 We supplemented telephone-based collection of follow-up data with semi-automated review of longitudinal Electronic Medical Records (EMR) as previously described.4,11,12 Patients who were missing ≥ 1 cognitive measurement(s) were excluded based on pre-specified criteria (Figure 1). Subjects’ data were censored in case of incident dementia diagnosis, death or loss to follow-up.
Statistical Methods
Variable Definition and Handling
Age at index ICH was analyzed as a continuous variable. Race/ethnicity was analyzed as a categorical variable, with European-American subjects as reference group due to their numerical preponderance. Educational level was dichotomized using a cut-off of ≥ 10 years of education.4 APOE genotype was analyzed using two categorical variables indicating presence of any ε2 or ε4 alleles.4 CT-defined volumes for index ICH (and intraventricular component, if any) were analyzed as continuous variables. CT-WMD was analyzed as an ordinal variable indicating increasing burden.4 CMBs counts were analyzed using previously employed cut-off of 0, 1, 2-4, or ≥ 5 microbleeds, with separate variables created for lobar and non-lobar CMBs.4 MRI-WMH volumes were log-transformed and analyzed as a continuous variable. 17
We defined incident dementia for outcome analyses based on: 1) relevant ICD-9 codes being entered in EMR records and/or 2) subjects assigned TICS-m scores < 20 (based on published normative data).21,23 We estimated sensitivity and specificity of dementia diagnosis via ICD-9 / TICS-m in comparison to clinical evaluation by the attending neurologist (when documented in medical records). We performed secondary analyses investigating rate of cognitive decline, which was quantified by calculating individual-specific slopes for TICS-m scores over time and analyzed as a continuous variable.
Statistical Models
We used a time-to-event analysis framework to identify risk factors associated with post-ICH early vs. delayed dementia risk. Separate analyses were conducted risk of EPID and DPID. Risk factors associated with incident dementia (defined as per above), were first assessed in univariable analyses using log-rank tests. Candidate variables for multivariable modeling included all those with p < 0.20 for association with incident dementia in univariable analyses. Multivariable analyses employed Cox regression models. Medication exposures were treated as time-varying variables in all Cox regression analyses. After variable selection, a minimal model was generated by backward elimination of non-significant variables (p > 0.05). Of note, variables associate with either EPID or DPID risk were included in both multivariable analyses for comparison purposes (even if failing to achieve p < 0.05 for association in the parallel analysis). We created multivariable linear regression models to analyze predictors of TICS-m slope as outcome variable. Variable selection procedures for linear regression models were identical to those for Cox regression analyses. Heterogeneity of effects for association of risk factors with early vs. delayed dementia after ICH were evaluated for statically significance using the metareg function, part of the meta package for the R statistical program.
We addressed multiple testing burden by adopting the False Discovery Rate (FDR) method as developed by Benjamini and Hochberg.24 All p-values reported are adjusted for multiple testing with the FDR methods. All significance tests were 2-tailed, and significance threshold was set at p < 0.05 (after FDR adjustment). All analyses were performed with R software v 3.2.0 (The R Foundation for Statistical Computing).
RESULTS
Study Subjects, Follow-up Information and Dementia Incidence after ICH
A total of 1141 patients age ≥ 18 years presented to our center and were diagnosed with primary ICH without pre-existing dementia during the pre-specified enrollment time (Figure 1). Of these, 738 met all eligibility criteria and participated in analyses of early dementia risk after ICH. Of note, 291 of the 379 total excluded patients (77%) were ineligible due to early case fatality (Figure 1). Eligible patients’ characteristics are presented in Table 1. Overall, a total of 279/738 (39%) ICH survivors in our study developed dementia. We compared dementia diagnostic accuracy against clinical examination by the attending neurologist in 522/738 (71%) cases with documented cognitive evaluations. Sensitivity and specificity for a TICS-m / ICD-9 based dementia diagnosis were 90% and 94% respectively (compared to in-person evaluation). We observed a total of 55 recurrent ICH events (44 recurrent lobar ICH cases, 11 deep ICH cases), for an overall recurrence rate of 5.2% / year. We repeated all multivariable analyses after removal of recurrent ICH cases with no significant difference in results (eTable 1).
Table 1.
Cohort Characteristics
| Variables | All ICH Survivors | ICH Survivors with no Dementia at 6 months | ||
|---|---|---|---|---|
|
| ||||
| No. 738 | % 100 | No. 435 | % 100 | |
|
| ||||
| Demographics | ||||
| Age at Enrollment (Mean, SD) | 74.3 (12.1) | - | 71.2 (11.3) | - |
| Sex (Male) | 384 | 52 | 226 | 52 |
| Race / Ethnicity | ||||
| - European American | 605 | 82 | 331 | 76 |
| - African American | 74 | 10 | 65 | 15 |
| - Asian American | 21 | 3 | 15 | 3 |
| - Hispanic | 23 | 3 | 13 | 3 |
| - Other | 15 | 2 | 11 | 3 |
| Education (≥ 10 years) | 465 | 63 | 222 | 51 |
|
| ||||
| Past Medical History (at Time of Enrollment) | ||||
| Hypertension | 546 | 74 | 305 | 70 |
| Ischemic Heart Disease | 133 | 18 | 65 | 15 |
| Atrial Fibrillation | 118 | 16 | 39 | 9 |
| Diabetes | 125 | 17 | 65 | 15 |
| Mood Disorder | 111 | 15 | 78 | 18 |
| Anxiety Disorder | 52 | 7 | 52 | 12 |
| Prior lobar ICH | 15 | 2 | 13 | 3 |
| Prior non-lobar ICH | 7 | 1 | 1 | 0.2 |
| Prior Ischemic Stroke / TIA | 31 | 4 | 20 | 5 |
|
| ||||
| CT Imaging Data | ||||
| ICH Location | ||||
| - Lobar | 289 | 39 | 187 | 43 |
| - Deep | 359 | 49 | 204 | 47 |
| - Cerebellar | 69 | 9 | 26 | 6 |
| - Multiple Locations | 21 | 3 | 18 | 4 |
| ICH Volume (Median, IQR) | 19.4 (4.5 - 24.1) | - | 17.8 (3.9 - 25.0) | - |
| Intraventricular Extension | 214 | 29 | 117 | 27 |
| CT-WMD Presence | 494 | 67 | 318 | 73 |
|
| ||||
| Post-ICH Medication Use | ||||
| Antiplatelet agents | 103 | 14 | 65 | 15 |
| Warfarin | 74 | 10 | 39 | 9 |
| Statins | 258 | 35 | 157 | 36 |
| Antihypertensive Agents | 435 | 59 | 291 | 67 |
| SSRI | 207 | 28 | 117 | 27 |
Abbreviations: ICH = Intra-Cerebral Hemorrhage, IQR = Inter-Quartile Range, SD = Standard Deviation, SSRI = Selective Serotonin Reuptake Inhibitor Antidepressants, CT-WMD = CT-defined White Matter Disease, TIA = Transient Ischemic Attack.
A total of 140 participants (19%) developed incident dementia within 6 months. Among subjects included in these analyses 397 (54%) had available APOE and MRI data for additional analyses (Table 2). Among the 598 participants who did not develop dementia within 6 months of ICH, 435 were alive at 6 months and therefore eligible delayed dementia incidence analyses. Of these, 257 (59%) had available APOE and MRI data for additional analyses (Table 2). Median longitudinal follow-up duration was 47.4 months (IQR 43.4 - 52.1). Average censoring due to loss to follow-up (other than death or incident dementia) was 1.2% per year. We estimated a yearly dementia incidence rate of 5.8% / year (95%CI 5.1-7.0%), corresponding to 139/435 (32%) diagnoses during follow-up. Cumulative delayed incident dementia rates after ICH are graphically detailed in Figure 2. Based on findings reported above, EPID (140 of 279 dementia cases) and DPID (139 of 279 dementia cases) accounted for approximately 50% of diagnoses each.
Table 2.
Genetic and MRI data for participating individuals
| Variables | All ICH Survivors | ICH Survivors with no Dementia at 6 months | ||
|---|---|---|---|---|
|
| ||||
| No. 397 | % 100 | No. 257 | % 100 | |
|
| ||||
| MRI Imaging Data | ||||
| Cerebral MBs (Presence) | ||||
| - Any location | 270 | 68 | 180 | 70 |
| - Strictly Lobar | 123 | 31 | 86 | 33 |
| - Strictly Deep | 111 | 28 | 70 | 27 |
| - Mixed | 36 | 9 | 24 | 9 |
| CMBs Count (Any Location) | ||||
| - 0 | 127 | 32 | 77 | 30 |
| - 1 or 2 | 92 | 23 | 62 | 24 |
| - 2 to 4 | 79 | 20 | 51 | 20 |
| - ≥ 5 | 99 | 25 | 67 | 26 |
| MRI-WMH Volume | 17.4 | 15.8 | ||
| (Median, IQR in cc) | (7.2 - 35.1) | - | (7.3 - 28.9) | - |
|
| ||||
| APOE Genotype | ||||
| APOE ε2 (Minor Allele Frequency) | 0.11 | - | 0.08 | - |
| APOE ε4 (Minor Allele Frequency) | 0.17 | - | 0.20 | - |
Abbreviations: APOE = Apolipoprotein E Gene, CMBs = Cerebral Microbleeds, ICH = Intra-Cerebral Hemorrhage, IQR = Inter-Quartile Range, MRI-WMH = MRI-defined White Matter Hyperintensity.
Figure 2. Incident Delayed Cognitive Decline among ICH Survivors.
Cumulative incidence of Delayed Post-ICH Dementia (DPID), expressed as percentages of total study population. Rates computed among all study participants that were free of dementia at 6 months. Number of patients alive and being followed at each time point is listed at the bottom. Legend located in left lower corner.
Abbreviations: ICH = Intracerebral Hemorrhage
Risk Factors for Early Incident Dementia after ICH
We initially performed univariable analyses to identify associations between characteristics listed in Table 1 and EPID risk, and selected for further multivariable modeling (univariable p < 0.20) age at index ICH, African-American ethnicity, ICH location, and ICH volume. Multivariable analyses results are presented in Table 3 (Model 1), with additional risk factors associated with DPID risk included for comparison purposes. Significant associations with EPID risk included age at index ICH, ICH location, and ICH volume. We repeated univariable and multivariable analyses including APOE and MRI data listed in Table 2 in the smaller groups of ICH survivors with these data points available (n = 397). In multivariable analyses (Table 3, Model 2) an additional significant association with EPID risk was uncovered for the APOE ε2 variant.
Table 3.
Multivariable Analyses of Risk Factors for Early vs. Delayed Dementia Incidence after ICH
| Model 1 (Includes: Demographics, Medical History and CT Imaging Data)* | Early Post-ICH Dementia Risk (n = 619) | Delayed Post-ICH Dementia Risk (n = 435) | Heterogeneity p-value | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | ||
| Age | 1.02 | 1.00 – 1.04 | 0.034 | 1.01 | 1.00 - 1.01 | 0.049 | 0.78 |
| Education (≥ 10 years) | 0.89 | 0.61 – 1.30 | 0.55 | 0.60 | 0.40 - 0.89 | 0.012 | <0.001 |
| Race: African American | 1.22 | 0.96 – 1.55 | 0.11 | 1.48 | 1.09 - 2.02 | 0.014 | 0.55 |
| Mood Disorder | 0.66 | 0.04 – 11.11 | 0.77 | 1.29 | 1.02 - 1.63 | 0.036 | 0.012 |
| ICH Volume (per 10 cc increase) | 1.47 | 1.09 – 1.97 | 0.011 | 1.10 | 0.70 – 1.73 | 0.68 | < 0.001 |
| ICH Location: Lobar | 2.04 | 1.06 – 3.91 | 0.033 | 1.33 | 0.25 – 7.03 | 0.74 | 0.024 |
| CT-WMD Severity | 1.34 | 0.23 – 7.76 | 0.74 | 1.70 | 1.07 - 2.71 | 0.027 | 0.044 |
| Model 2 (Includes: Model 1 data, APOE Genotype and MRI Imaging Data)† | Early Post-ICH Dementia Risk (n = 397) | Delayed Post-ICH Dementia Risk (n = 257) | Heterogeneity p-value | ||||
| HR | 95% CI | p | HR | 95% CI | p | ||
| Age | 1.02 | 1.00 – 1.04 | 0.011 | 1.01 | 1.00 - 1.01 | 0.032 | 0.94 |
| Education (≥ 10 years) | 1.12 | 0.82 – 1.54 | 0.48 | 0.57 | 0.36 – 0.90 | 0.016 | <0.001 |
| Race: African American | 1.42 | 0.64 – 3.17 | 0.39 | 1.39 | 1.07 – 1.81 | 0.015 | 0.42 |
| Mood Disorder | 0.59 | 0.13 – 2.77 | 0.51 | 1.56 | 1.11 – 2.19 | 0.011 | 0.006 |
| ICH Volume (per 10 cc increase) | 1.52 | 1.12 – 2.07 | 0.008 | 0.89 | 0.58 – 1.37 | 0.60 | <0.001 |
| ICH Location: Lobar | 1.66 | 1.01 – 2.73 | 0.047 | 1.19 | 0.46 – 3.09 | 0.72 | 0.19 |
| CT-WMD Severity | 0.89 | 0.54 – 1.36 | 0.52 | 1.70 | 1.07 - 2.71 | 0.027 | 0.011 |
| APOE ε2 (1+ copies) | 1.69 | 1.14 – 2.50 | 0.009 | 1.29 | 0.79 – 2.10 | 0.31 | 0.031 |
| APOE ε4 (1+ copies) | 1.06 | 0.68 – 1.64 | 0.80 | 2.12 | 1.45 - 3.09 | < 0.001 | 0.002 |
| CMBs (Lobar) | 0.82 | 0.26 – 2.62 | 0.74 | 1.78 | 1.07 - 2.98 | 0.029 | 0.042 |
Table presents detailed results of multivariable analyses only, please refer to Methods section for information on univariable testing for association. Results shown only for variables significantly associated with early and/or delayed dementia risk, and thus selected for heterogeneity of effects testing. Heterogeneity p-values < 0.05 indicate statistically significant differences in effects sizes for a certain variable when comparing its association with early vs. delayed post-ICH dementia risk.
Patients’ characteristics screened via univariable comparisons for inclusion in multivariable analysis are listed in Table 1.
Patients’ characteristics screened via univariable comparisons for inclusion in multivariable analysis were those entered in Model 1 (see above), and genetic and MRI data listed in Table 2. These analyses were restricted to participants with available genotype and MRI data (Table 2).
Abbreviations: 95% CI = 95% Confidence Interval, APOE = Apolipoprotein E Gene, CMBs = Cerebral Microbleeds, CT-WMD = CT-defined White Matter disease, HR = Hazard Ratio, ICH = Intracerebral Hemorrhage, IVH = Intraventricular Hemorrhage.
Risk Factors for Delayed Incident Dementia after ICH
Univariable analyses of DPID risk identified significant associations with age at index ICH, educational level, African-American ethnicity, diagnosis of mood disorder, and increasing CT-WMD severity (all p < 0.05). All these associations were confirmed in multivariable analysis, as detailed in Table 3, Model 1 (as above, additional risk factors associated with EPID risk are included for comparison purposes only). We separately included APOE and MRI data in univariable and multivariable analyses (n = 257), as presented in Table 3 (Model 2); in these analyses we uncovered additional significant associations with DPID risk for: 1) increasing burden of lobar CMBs; 2) the APOE ε4 variant.
Comparison of Risk Factors for Early and Delayed Dementia after ICH
We formally compared risk factors profiles for EPID vs. DPID, as presented in detailed in Table 3 (Heterogeneity; right-most column). Among all identified risk factors, the following showed statistically significant (p < 0.05) preferential association with early incident dementia: ICH volume, lobar ICH location, and the APOE ε2 variant. Conversely, educational level, diagnosis of mood disorder, increasing CT-WMD severity, increasing lobar CMBs burden and the APOE ε4 variant were specifically associated with delayed dementia risk, but not with EPID diagnosis. Of all identified risk factors, only advancing age at index ICH represented a shared risk factor for both early and delayed incident dementia after ICH. We performed secondary comparative analyses of cognitive decline rates before vs. after 6 months post-ICH (eTable2). ICH volume and lobar ICH location were confirmed as speficially associated with cognitive decline rate before 6 months post-ICH, while educational level, diagnosis of mood disorder, and increasing CT-WMD severity were specifically associated with cognitive decline rate after 6 months post-ICH (all heterogeneity p < 0.05).
DISCUSSION
We conducted the first comprehensive longitudinal study of cognitive impairment after primary ICH, leveraging a large cohort of consecutive cases with in-depth characterization and extended follow-up to demonstrate a high incidence of dementia after primary intraparenchymal hemorrhage. Of great interest, we showed those early and delayed dementias were closely matched in incidence. We also demonstrated a significant discrepancy in risk factor profile between EPID and DPID risks; the former was primarily associated with acute hematoma parameters (location and size), whereas the latter was not. These findings support the hypothesis that different mechanisms underlie cognitive impairment after ICH, depending on temporal dynamics.
Post-ICH dementia rate derived from our analyses are substantial, particularly as all dementia diagnoses were incident during follow-up. Removal of the small number of subjects experiencing recurrent ICH did not lower cognitive decline rates substantially. As mentioned above, far from being a limited occurrence, incident dementia among ICH survivors free of dementia at 6 months accounts for half of all cases. These findings are of immediate clinical relevance to healthcare providers and ICH survivors. Assuming replication of our findings in future studies, adequate communication of cognitive decline risk (especially beyond the immediate post-ICH period) will represent a critical issue for clinicians, their ICH patients and family / caregivers.
Previous studies of cognitive impairment after ICH or hemorrhagic stroke have been limited in their ability to disentangle the impact on cognitive outcomes of the acute bleeding event vs. potential underlying disease processes.7-10 As a result, identified predictors of cognitive performance (other than age), were primarily hematoma size and location. We have indeed confirmed that these variables are strongly associated with EPID risk, likely reflecting cortical areas or networks directly damaged by acute bleeding. By virtue of our study design (and due to the study’s large sample size and long follow-up) we were able to demonstrate that DPID risk has little to no association with the acute bleeding event.
Indeed, we identified several CSVD-related markers and risk factors (APOE ε4, WMD and CMBs) as associated with DPID risk. The known association between CSVD and ICH may represent the underlying unifying etiological factor explaining the aforementioned results, but we are unable to further address this question in our analyses. Of note, we identified opposite associations patterns for the ε2 and ε4 variants of the APOE gene. We previously showed that the APOE ε2 variant is associated with larger hematoma volume and/or hematoma expansion, and thus with worse functional outcome at 3 months.25,26 It seems likely that, having adjusted only for initial hematoma volume in our analyses, the known association with hematoma expansion risk accounts for its relationship with EPID incidence. In contrast, the APOE ε4 variant is known to increase amyloid burden in both the brain parenchyma and vasculature, and is more likely to exert a long-term effect on cognitive outcome after ICH.27,28
Our study has limitations. Firstly, the vast majority of our cognitive outcome information was obtained using the TICS-m tool for phone-based cognitive evaluations, rather than in-person interviews. The TICS-m, however, has been validated multiple times as reliable, efficient tool for global cognitive assessment.18-22 TICS-m scores have been shown in multiple prior studies to correlate closely with in-person testing scores (e.g. Mini-Mental State Examination [MMSE] or Montreal Cognitive Assessment [MoCA]), and to diagnose dementia with high sensitivity and specificity.19-22 Only a small percentage of ICH survivors were unable to complete one or more TICS-m questionnaires, and were therefore excluded or censored. We also observed excellent concordance between TICS-m dementia diagnoses and in-person clinical evaluations. Secondly, although we detected an association between self-reported African-American racial background and cognitive decline after ICH, it is possible that other minority groups may be also at higher risk of post-ICH cognitive impairment. However, their inadequate representation in this cohort likely limited power to uncover such associations. Third, because of our follow-up frequency we had limited ability to capture precise timing of dementia onset, particularly for EPID. Finally, we cannot exclude the possibility that Alzheimer’s disease pathology plays a role in the observed findings. Indeed, all CSVD-related markers associated with DPID risk in our analyses have previously been observed in Late-Onset Alzheimer’s Disease (LOAD) patients.29-31 Additional studies will be required to better clarify the contribution of LOAD to cognitive decline after ICH.
In summary, we identified a substantial risk of incident cognitive decline among ICH survivors in the first large, comprehensive longitudinal study on the topic. In the short-term, within 6 months of ICH, we confirmed that hematoma location and volume are strongly associated with incident dementia risk. However, we diagnosed at least half of dementia cases beyond the 6 months mark, and demonstrated the existence of a significantly different risk factor profile. This latter group of patients will benefit from additional studies investigating the pathophysiology of long-term cognitive decline after ICH.
Supplementary Material
Acknowledgments
The authors’ work on this study was supported by funding from the National Institute of Health (R25 NS065743, R01 NS063925, R01 NS059727, K23 NS086873, P50 NS051343 and R01 AG26484). The funding entities had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
All statistical analyses performed by: Alessandro Biffi, MD (Massachusetts General Hospital). Dr. Alessandro Biffi had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis
Footnotes
Disclosures
Dr. Alessandro Biffi is supported by R25NS065743.
Ms. Destiny Bailey reports no disclosures
Dr. Christopher D. Anderson is supported by K23NS086873
Ms. Alison M. Ayres reports no disclosures.
Dr. Edip M Gurol is supported by K23NS083711
Dr. Steven M Greenberg is supported by R01AG26484
Dr. Jonathan Rosand is supported by U01NS069208, R01NS073344 and R01NS059727
Dr. Anand Viswanathan is supported by R01AG047975, R01AG026484, P50AG005134, K23 AG028726
Statement of contribution
Study design: AB, JR, AV; Data acquisition: AB, DB, CDA, AA, EMG, SMG, JR, AV; Statistical analysis: AB; Study management: AB, CDA, AA, EMG, SMG, JR, AV; Manuscript preparation: AB, JR, AV; Manuscript review: AB, DB, CDA, AA, EMG, SMG, JR, AV
References
- 1.Badjatia N, Rosand J. Intracerebral hemorrhage. Neurologist. 2005;11(6):311–324. doi: 10.1097/01.nrl.0000178757.68551.26. [DOI] [PubMed] [Google Scholar]
- 2.Poon MT, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. Journal of neurology, neurosurgery, and psychiatry. 2014;85(6):660–667. doi: 10.1136/jnnp-2013-306476. [DOI] [PubMed] [Google Scholar]
- 3.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet neurology. 2010;9(7):689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 4.Biffi A, Halpin A, Towfighi A, et al. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology. 2010;75(8):693–698. doi: 10.1212/WNL.0b013e3181eee40f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koennecke HC. Cerebral microbleeds on MRI: Prevalence, associations, and potential clinical implications. Neurology. 2006;66(2):165–171. doi: 10.1212/01.wnl.0000194266.55694.1e. [DOI] [PubMed] [Google Scholar]
- 6.Zhu YC, Chabriat H, Godin O, et al. Distribution of white matter hyperintensity in cerebral hemorrhage and healthy aging. Journal of neurology. 2012;259(3):530–536. doi: 10.1007/s00415-011-6218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia PY, Roussel M, Bugnicourt JM, et al. Cognitive impairment and dementia after intracerebral hemorrhage: a cross-sectional study of a hospital-based series. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2013;22(1):80–86. doi: 10.1016/j.jstrokecerebrovasdis.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Tveiten A, Ljostad U, Mygland A, Naess H. Functioning of long-term survivors of first-ever intracerebral hemorrhage. Acta neurologica Scandinavica. 2014;129(4):269–275. doi: 10.1111/ane.12185. [DOI] [PubMed] [Google Scholar]
- 9.Koivunen RJ, Harno H, Tatlisumak T, Putaala J. Depression, anxiety, and cognitive functioning after intracerebral hemorrhage. Acta neurologica Scandinavica. 2015;132(3):179–184. doi: 10.1111/ane.12367. [DOI] [PubMed] [Google Scholar]
- 10.Benedictus MR, Hochart A, Rossi C, et al. Prognostic Factors for Cognitive Decline After Intracerebral Hemorrhage. Stroke; a journal of cerebral circulation. 2015 doi: 10.1161/STROKEAHA.115.010200. [DOI] [PubMed] [Google Scholar]
- 11.Biffi A, Shulman JM, Jagiella JM, et al. Genetic variation at CR1 increases risk of cerebral amyloid angiopathy. Neurology. 2012;78(5):334–341. doi: 10.1212/WNL.0b013e3182452b40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biffi A, Anderson C, Battey TW, et al. Association between Blood Pressure Control and Risk of Recurrent Intracerebral Hemorrhage. JAMA. 2015;9(314):904–912. doi: 10.1001/jama.2015.10082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrison JK, Fearon P, Noel-Storr AH, McShane R, Stott DJ, Quinn TJ. Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) for the diagnosis of dementia within a secondary care setting. Cochrane Database Syst Rev. 2015;3 doi: 10.1002/14651858.CD010772.pub2. CD010772. [DOI] [PubMed] [Google Scholar]
- 14.Woo D, Rosand J, Kidwell C, et al. The Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study protocol. Stroke; a journal of cerebral circulation. 2013;44(10):e120–125. doi: 10.1161/STROKEAHA.113.002332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Swieten JC, Hijdra A, Koudstaal PJ, van Gijn J. Grading white matter lesions on CT and MRI: a simple scale. Journal of neurology, neurosurgery, and psychiatry. 1990;53(12):1080–1083. doi: 10.1136/jnnp.53.12.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith EE, Gurol ME, Eng JA, et al. White matter lesions, cognition, and recurrent hemorrhage in lobar intracerebral hemorrhage. Neurology. 2004;63(9):1606–1612. doi: 10.1212/01.wnl.0000142966.22886.20. [DOI] [PubMed] [Google Scholar]
- 17.Rost NS, Rahman RM, Biffi A, et al. White matter hyperintensity volume is increased in small vessel stroke subtypes. Neurology. 2010;75(19):1670–1677. doi: 10.1212/WNL.0b013e3181fc279a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brandt J, Spencer M, Folstein MF. The telephone interview for cognitive status. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1988;1(2):111–118. [Google Scholar]
- 19.Knopman DS, Roberts RO, Geda YE, et al. Validation of the telephone interview for cognitive status-modified in subjects with normal cognition, mild cognitive impairment, or dementia. Neuroepidemiology. 2010;34(1):34–42. doi: 10.1159/000255464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seo EH, Lee DY, Kim SG, et al. Validity of the telephone interview for cognitive status (TICS) and modified TICS (TICSm) for mild cognitive imparment (MCI) and dementia screening. Archives of gerontology and geriatrics. 2011;52(1):e26–30. doi: 10.1016/j.archger.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Barber M, Stott DJ. Validity of the Telephone Interview for Cognitive Status (TICS) in post-stroke subjects. International journal of geriatric psychiatry. 2004;19(1):75–79. doi: 10.1002/gps.1041. [DOI] [PubMed] [Google Scholar]
- 22.de Jager CA, Budge MM, Clarke R. Utility of TICS-M for the assessment of cognitive function in older adults. International journal of geriatric psychiatry. 2003;18(4):318–324. doi: 10.1002/gps.830. [DOI] [PubMed] [Google Scholar]
- 23.Pendlebury ST, Welch SJ, Cuthbertson FC, Mariz J, Mehta Z, Rothwell PM. Telephone assessment of cognition after transient ischemic attack and stroke: modified telephone interview of cognitive status and telephone Montreal Cognitive Assessment versus face-to-face Montreal Cognitive Assessment and neuropsychological battery. Stroke; a journal of cerebral circulation. 2013;44(1):227–229. doi: 10.1161/STROKEAHA.112.673384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keselman HJ, Cribbie R, Holland B. Controlling the rate of Type I error over a large set of statistical tests. Br J Math Stat Psychol. 2002;55(Pt 1):27–39. doi: 10.1348/000711002159680. [DOI] [PubMed] [Google Scholar]
- 25.Biffi A, Anderson CD, Jagiella JM, et al. APOE genotype and extent of bleeding and outcome in lobar intracerebral haemorrhage: a genetic association study. Lancet neurology. 2011;10(8):702–709. doi: 10.1016/S1474-4422(11)70148-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brouwers HB, Biffi A, Ayres AM, et al. Apolipoprotein E genotype predicts hematoma expansion in lobar intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2012;43(6):1490–1495. doi: 10.1161/STROKEAHA.111.643262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Yu JT, Wang HF, et al. APOE genotype and neuroimaging markers of Alzheimer’s disease: systematic review and meta-analysis. Journal of neurology, neurosurgery, and psychiatry. 2015;86(2):127–134. doi: 10.1136/jnnp-2014-307719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holtzman DM. In vivo effects of ApoE and clusterin on amyloid-beta metabolism and neuropathology. J Mol Neurosci. 2004;23(3):247–254. doi: 10.1385/JMN:23:3:247. [DOI] [PubMed] [Google Scholar]
- 29.Radanovic M, Pereira FR, Stella F, et al. White matter abnormalities associated with Alzheimer’s disease and mild cognitive impairment: a critical review of MRI studies. Expert review of neurotherapeutics. 2013;13(5):483–493. doi: 10.1586/ern.13.45. [DOI] [PubMed] [Google Scholar]
- 30.Yates PA, Desmond PM, Phal PM, et al. Incidence of cerebral microbleeds in preclinical Alzheimer disease. Neurology. 2014 doi: 10.1212/WNL.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hommet C, Mondon K, Constans T, et al. Review of cerebral microangiopathy and Alzheimer’s disease: relation between white matter hyperintensities and microbleeds. Dementia and geriatric cognitive disorders. 2011;32(6):367–378. doi: 10.1159/000335568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


