Abstract
Poor sleep is thought to interfere with children’s learning and academic achievement (AA). However, existing research and theory indicate there are factors that may mitigate the academic risk associated with poor sleep. The purpose of this study was to examine the moderating role of children’s effortful control (EC) on the relation between sleep and AA in young children. One hundred and three 4.5- to 7-year-olds (M = 5.98 years, SD = 0.61) wore a wrist-based actigraph for five continuous weekday nights. Teachers and coders reported on children’s EC. EC was also assessed with a computer-based task at school. Additionally, we obtained a standardized measure of children’s AA. There was a positive main effect of sleep efficiency to AA. Several relations between sleep and AA were moderated by EC and examination of the simple slopes indicated that the negative relation between sleep and AA was only significant at low levels of EC.
Keywords: Sleep, Effortful Control, Academic Achievement, Early Elementary School
Children’s Sleep and Academic Achievement: The Moderating Role of Effortful Control
Early academic achievement (AA) plays a central role in children’s opportunities for later academic, social, and professional success (Duncan et al., 2007). Efforts to understand AA are increasingly focusing on the role of children’s sleep, in part because sleep is believed to play a critical role in learning, memory consolidation, and brain plasticity (Walker, 2009). Despite the hypothesized importance of sleep to children’s learning and AA, the empirical literature illustrates that poor sleep is not always associated with low levels of AA (Mayes et al., 2008), suggesting that there are other factors that influence the strength of the relation between sleep and AA. Consistent with this pattern, El-Sheikh and colleagues recently hypothesized that the interaction between sleep and contextual-ecological factors may predict AA (El-Sheikh, Tu, Erath, & Buckhalt, 2014). The principal aim of this study was to examine the relations between sleep (quantity, timing, and quality) and AA and to test whether children’s effortful control (EC) moderates these relations.
The Importance of Sleep
Sleep is a multidimensional construct, characterized by changes in brain wave activity, breathing, heart rate, and other physiological functions (National Sleep Foundation, 2006). It is defined as “a reversible behavioral state of perceptual disengagement from and unresponsiveness to the environment” (Carskadon, & Dement, 2011, p.16). The purposes and mechanisms of sleep are only partially understood and are the subject of substantial ongoing research (Frank, 2006). However, sleep is thought to be essential for revitalizing and restoring the physiological processes that keep the body and mind healthy and functioning properly (Horne, 1988; Oswald, 1980).
Sleep is especially important for children because it supports growth and development. Children between the ages of 6–13 require between 9–11 hours of sleep per night to minimize behavioral problems and cognitive deficits believed to impact the ability to learn in school (National Sleep Foundation, 2014). Nonetheless, a considerable proportion of school-aged children get less sleep than is recommended, with 23% of children sleeping only eight hours per night and 8% sleeping seven hours or less (National Sleep Foundation, 2014). Despite the importance of sleep to brain maturation and cognitive development (Dahl, 2005; Walker, 2009), less attention has been paid to individual differences during this time period compared to infant and adult research (c.f., Vaughn, Elmore-Staton, Shin, & El Sheikh, 2015). We attempted to partially address this gap by examining sleep in kindergarteners and first graders because these students are adapting to academic and social demands often predictive of later academic success (Duncan et al., 2007).
Parental reports have been a main source of information on children’s sleep (Sadeh, 2008), but the reliance on parent-report is problematic. Subjective parent reports overestimate children’s sleep by 30 to 60 or more minutes per night (Biggs, Lushington, Van den Heuvel, Martin, & Kennedy, 2011; Dayyat, Spruyt, Molfese, & Gozal, 2011). Additionally, parents can only report about what they are aware of or are told and will likely not know if a child spends a significant time awake or if the child’s sleep was disturbed throughout the night (Sadeh, 1994, Sadeh, Raviv, & Gruber, 2000; Werner, Molinari, Guyer, & Jenni, 2008). To overcome this bias, a growing number of scholars suggest that objective measures, including actigraphy, are more reliable measure of sleep than parental reports (see Dayyat et al., 2011 for review). Actigraphy is a non-invasive method of monitoring human rest/activity cycles worn on the wrist for several days that can provide a reliable index of sleep (Spruyt, Gozal, Dayyat, Roman, & Molfese, 2011). Thus, in this study, we utilized actigraphy as an objective measure of children’s sleep. Although researchers have examined sleep and its impact on child functioning, most have focused on sleep quantity (Astill, Van der Heijden, Van Ijzendoorn, & Van Someren, 2012; Lushington, Pamula, Martin, & Kennedy, 2013; c.f., Vaughn et al., 2015). Sleep quantity captures the actual time during which an individual is asleep (sleep duration) as well as the night-to-night variability in hours of sleep (sleep duration variability). It is also important to consider sleep timing and quality. Sleep timing refers to the initiation of the transition from wakefulness into sleep (sleep onset) with higher scores indicating later sleep start times and likely less sleep. Sleep quality, on the other hand, indexes difficulties initiating sleep and maintaining sleep. For example, the length of time that it takes to accomplish the transition from full wakefulness to sleep (sleep latency) as well as the percentage of time spent asleep compared to overall time spent in bed (sleep efficiency) are common measures of sleep quality (Respironics, 2008).
Support for the perspective that sleep quantity, timing, and quality represent separate dimensions of sleep comes from studies showing that there are often weak correlations between these dimensions of sleep (Buckhalt, El-Sheikh & Keller, 2007; Meijer, Habekothe, & van den Wittenboer, 2000). Additionally, there is evidence for compensatory mechanisms in sleep, whereby when sleep quantity or sleep quality decrease, the body adapts by improving the opposite sleep component (Sadeh, Gruber, & Raviv, 2003). For example, reduction in sleep duration may result in the consolidation of sleep with a higher sleep efficiency. Focusing on only one facet of sleep, therefore, may not provide a complete understanding of the role of sleep in important outcomes such as AA.
Sleep and Academic Achievement
Dahl’s model (Dahl, 1996) suggests that sleep deficits produce desynchronization of neural communication, which may lead to poorer performance in complex, high-challenging situations such as the classroom. Poor sleep is also believed to be linked to reduced activations in critical brain regions (e.g., frontal, parietal), thereby likely impinging on alertness and attention, impairing restorative processes, and resulting in subsequent difficulties in impulsivity and working memory that are important for AA (Buckhalt, Wolfson, & El-Sheikh, 2009; Dahl, 1996; Steenari et al., 2003). When children get insufficient sleep or poor quality sleep, their motivation and engagement for carrying out tasks may diminish, as well as their concentration, reasoning, and problem-solving skills (Buckhalt, El-Sheikh, Keller, & Kelley, 2009; Meijer et al., 2000). Additionally, insufficient sleep may promote feelings of sleepiness and irritability, influence task completion and persistence, and lead to poor task performance (Dahl, 1996; Steenari et al., 2003). An increasing number of researchers have found strong relations between sleep and memory, with sleep being a vital component for the processing of newly acquired information and for the long-term storage of memory, both of which are essential for learning (see Walker, 2009, for review). Moreover, poor, insufficient, or disorganized sleep is associated with lower cognitive functioning and academic performance in 8–16 year olds (Buckhalt, El-Sheikh, et al., 2009; Dewald, Meijer, Oort, Kerkhof, & Bögels, 2010; Epstein, Chillag, & Lavie, 1998).
In contrast, the pattern of findings is limited in young children (Vaughn et al., 2015) and, at times, contradictory. Bernier et al. (2013) followed children from one to four years of age and found that better parent-reported sleep at age one was associated with better abstract reasoning skills, concept formation, and problem-solving skills at age four. Parent-reported sleep problems in preschool have also been negatively associated with performance on tasks assessing working memory (Karpinski, Scullin, & Montgomery-Downs, 2008; Nelson, Nelson, Kidwell, James, & Espy, 2015). Ravid and colleagues found that shorter sleep duration and reduced sleep quality (objectively measured) were associated with kindergarten retention (Ravid, Afek, Suraiya, Shahar, & Pillar, 2009a). Additionally, children who performed poorly on first grade achievement tests were more likely to have sleep disturbances such as increased sleep latency and lower sleep efficiency compared to their peers (Ravid, Afek, Suraiya, Shahar, & Pillar, 2009b). Greater sleep latency and lower sleep efficiency have also been associated with greater memory errors and poorer grades in math and English in 6-to 13-year olds (Gruber et al., 2014; Steenari et al., 2003). In addition, in a study with 2.5–6 year-olds, Touchette et al. (2007) found significant negative associations between sleep duration and neurodevelopmental tests.
However, several researchers have found non-significant or inconsistent relations between sleep and AA (Buckhalt, El-Sheikh, & Keller, 2007; Cooper, Kohler, & Blunden, 2012; Mayes et al., 2008). Additionally, the average effect sizes from a meta-analysis involving relations between 5–12 year-olds sleep efficiency and multiple cognitive/memory variables and school performance, as well as the average effect of sleep duration on children’s memory and intelligence were nonsignificant (Astill et al., 2012). One reason for these discrepancies may be whether or not sleep was assessed for a short period (i.e., overnight) or over several nights (Astill et al., 2012). Moreover, mixed findings may exist because the relation between sleep and AA is moderated by other contextual factors or developmental processes (Buckhalt et al., 2007; El-Sheikh et al., 2014; Mayes et al., 2008).
Effortful Control and Academic Achievement
Temperament theorists such as Rothbart identify EC as a protective factor for later adjustment (Rothbart, Ellis, Rosario Rueda, & Posner, 2003) and, therefore, EC may mitigate the effects of poor sleep on AA. EC has been defined as “the efficiency of executive attention— including the ability to inhibit a dominant response and/or to activate a subdominant response, to plan, and to detect errors” (Rothbart & Bates, 2006, p. 126). EC reflects an individual’s ability to willfully modulate thoughts, emotions, and behavior and is the temperamental basis for self-regulation (Rothbart & Bates, 2006). EC is thought to foster children’s engagement in learning activities and to facilitate classroom processes (Ladd, Birch, & Buhs, 1999), perhaps making it easier for children high in EC to master reading and math concepts (Duncan et al., 2007).
Executive functioning, a closely related construct, reflects higher-level cognitive processes important for engaging in deliberate, goal-directed thought and action (inhibitory control), cognitive flexibility (attention shifting), and working memory processes (see Bridgett, Oddi, Laake, Murdock, & Bachmann, 2013). Central to both EC and executive functioning constructs are the abilities to focus attention, shift attention to new tasks, and inhibit impulsive behaviors which are especially important in the classroom, where paying attention and following instructions are necessary for limiting class disruptions and for efficient learning. Children’s EC and executive functioning positively predict various components of AA, including emergent literacy and math skills and school readiness (Blair & Razza, 2007; Brock, Rimm-Kaufman, Nathanson, & Grimm, 2009; Ponitz, McClelland, Matthews, & Morrison, 2009; Valiente et al., 2010; Valiente et al., 2008), even after accounting for initial cognitive and/or language ability (Blair & Razza, 2007; Duncan et al., 2007; McClelland et al., 2007).
The Moderating Role of EC
Although the importance of examining relations among sleep, self-regulation, and AA in children has been recognized (Dahl, 1996; 2005; Sadeh et al., 2003), EC has not, to our knowledge, been investigated as a potential moderator of the relations between children’s sleep and their AA. In the social competence and adjustment literature, however, there is preliminary evidence for interaction effects between sleep, albeit measured through subjective parent-reports, and self-regulation. Five-year olds’ sleep deficits were most strongly related to problem behaviors at low levels of self-control in infancy (Goodnight, Bates, Staples, Pettit, & Dodge, 2007). Moreover, El-Sheikh (2007) suggested that regulatory functioning may be a moderator that either decreases or aggravates negative child outcomes in the context of objectively measured sleep disruptions. In a study of 8- and 9- year-olds, actigraph-measured sleep disruptions were related to increased adjustment problems when children exhibited lower regulation (measured by vagal tone withdrawal; El-Sheikh, Erath, & Keller, 2007). Although the previous studies focused on maladjustment, recently Elmore-Staton and colleagues (2014) argued that there is substantial variability in the relation between sleep and AA and that assessment of moderators, including children’s regulation, is critical for elucidating this association. Indeed, findings suggest that links between AA and sleep may be weaker when children have other resources (e.g., income) in conjunction with regulatory capacities to compensate for poor sleep quality (Elmore-Staton, Hinnant, Buckhalt, & El-Sheikh, 2014). Accordingly, we expected the negative relation between poor sleep and AA to be most pronounced for children low in EC. In contrast, we did not expect a negative relation between poor sleep and AA at high levels of EC.
The Present Study
In the current study, we examined the degree to which objectively measured sleep in young children, as well as individual differences in EC (assessed with multiple methods), were related to AA, and whether children’s EC moderated the relations between sleep and AA. We utilized actigraphy to assess children’s sleep quantity, timing, and quality. Poor sleep was expected to relate negatively to AA, but only at low levels of EC. Due to evidence pointing towards gender differences in AA (Matthews, Ponitz, & Morrison, 2009) and because children in our sample ranged from 4 ½ to 7 years of age, we included sex and age as covariates. Moreover, SES and ethnicity (Hispanic or non-Hispanic) were entered as additional covariates as educational attainment differences have been noted (Davis-Kean, 2005; Stevenson, Chen, & Uttal, 2008). Despite the fact that the majority of children did not use medications that may influence sleep (e.g., allergy medication, cough medicine, etc.) during the week of actigraphy, use of medication was also controlled for in all analyses.
Method
Participants
Participants included 103 kindergarteners and first graders (50.5% girls) in 33 elementary classrooms. They were recruited through phone calls and mailings from a larger study of emotions, relationships in school, and AA. According to parents’ reports, 49.5% of children were Hispanic, 44.7% were non-Hispanic and 5.8% did not report; of the non-Hispanic children, 82.6% were White, 4.3% were Black, 2.2% were Asian, 8.7% were American Indian, and 2.2% reported they were of mixed race. Children were between the ages of 4.64 and 7.51 years (M = 5.98 years, SD = 0.61) and were predominantly from two-parent homes (86.7%) with an average income between $60,000-$69,999 (ranging from less than $9,999 to more than $100,000). Five percent of mothers did not obtain a high school diploma, 14% had only a high school diploma or equivalent, 33% had some college education, 47% were college graduates or higher, and 1% did not report on their level of education.
Procedure
Data were obtained from multiple sources and methods. During the fall, teachers and coders reported on children’s EC. Each child was observed by 2 or 3 different coders on three days each week in school for approximately 9–12 weeks. EC was also assessed with a computer-based task at school. In a series of home visits that took place in early spring, actigraphs were delivered to participants’ homes and caregivers were instructed to have their child wear the actigraph for five consecutive weekday nights. Later in the spring semester, AA was measured with subtests from the Woodcock Johnson III (WJ-III) tests of achievement at school. Research assistants who administered the continuous performance task (CPT) and the WJ-III were trained for approximately five weeks and monitored by expert staff. We compensated parents and teachers monetarily, and children received two small toys for their participation.
Socioeconomic Status (SES)
In the fall, parents provided an estimate of their combined family income (1= $0-$9,999 to 11= $100,000 or over) as well as maternal education (1 = less than HS diploma to 4 = college graduate or higher). Income and education were significantly positively correlated, r(91) = .73, p < .001; therefore, a measure of SES was created by standardizing income and education and averaging the z scores.
Effortful Control
During the fall, teachers and coders rated (1 = extremely false to 7 = extremely true) children’s EC in school using the Attentional Focusing (11 for teachers and 5 for coders; e.g., “Is easily distracted when listening to a story”; αs = .92 and .92), Inhibitory Control (13 items for teachers and 4 for coders; e.g., “Can easily stop an activity when she/he is told "no"”; .90 and .90), and Attention Shifting (12 items for teachers; e.g., “Needs to complete one activity before being asked to start on another one”; α = .93) subscales from the Children’s Behavioral Questionnaires (CBQ, Rothbart, Ahadi, Hershey, & Fisher, 2001). Coders did not report on attention shifting. Some CBQ questions were slightly modified to increase comprehension and because some of the original items did not fit the school context. Correlations among the subscales ranged from rs(99)= .62 to .79 for teachers and r(102)=.87 for coders, all ps < .001. The scales were averaged within reporter (αs =. 92 and .93 for teachers and coders, respectively).
In addition, trained research assistants administered a computer-based CPT to assess EC (adapted from NICHD, 2003) in school. Children were seated in front of a computer and were asked to press the space bar as soon as the target stimulus appeared on the screen. Two hundred twenty pictures of different familiar objects (e.g., butterfly, flower) were randomly presented on the screen, including 44 presentations of the target stimulus and 176 presentations of non-target stimuli. Stimuli appeared on the screen for either 0.2 or 0.5 seconds (first graders had a shorter viewing window to avoid ceiling effects) with 1.5 second intervals between stimuli. Children were asked to press the space bar upon seeing the target stimulus in order to get the trial correct. The proportion of false alarms (e.g., saw no target stimulus and pressed) was subtracted from the proportion of correct hits (e.g., saw target stimulus and pressed); high scores reflect higher EC. Children needed to complete 75% of trials to be included in analyses; all children had a sufficient number of trials.
Sleep Measures
In early spring, children wore wrist-based actigraphs (Actiwatch 2; Philips Respironics Inc) on their non-dominant hand for five consecutive school days. During a home visit, caregivers and children were instructed to remove the actigraph only if the child was showering or engaging in activities where the actigraph could be damaged (i.e., swimming). Additionally, caregivers were instructed to give their child a sticker for each day that he or she successfully wore the actigraph. The actigraph continuously measured motion using a piezoelectric accelerometer, allowing the detection of sleep/wake states (Weiss, Johnson, Berger, & Redline, 2010). The threshold was set to 40 counts per epoch, with a range of 20–80 (Meltzer, Walsh, Traylor, & Westin, 2012b). For school-age children, lower thresholds have been found to underestimate while higher thresholds overestimate sleep duration and sleep efficiency (Meltzer et al., 2012b). Sleep variables were computed utilizing 1-minute epochs and based on significant movement after at least 10 minutes of inactivity.
Researchers scored sleep data using the Phillips Actiware V5.7 program, which includes a validated algorithm to measure sleep in children (Hyde et al., 2007; Meltzer et al., 2012b). Actigraphy data were validated with parental reports of sleep behavior. Parents were asked to keep a daily sleep diary recording their child’s sleep-wake times. Sleep diaries were available in either English or Spanish; 13 caregivers completed their diaries in Spanish (Spanish translations were back translated as a quality check). Procedures for setting sleep-wake times followed previously established protocols (Acebo & Carskadon, 2002). Sleep start and end times were set by the researcher based on the daily diaries. Sleep onset and sleep end time were then calculated by the software (Respironics, 2008).
For this report, two actigraph measures of sleep quantity were used: sleep duration (time spent asleep in minutes) and sleep duration variability (day-to-day variability of time spent asleep). We also used sleep onset (average sleep start time) as an index of sleep timing. In addition, we used two indices of sleep quality: sleep latency (time required for sleep to start after initiating the intent to sleep) and sleep efficiency (percent of minutes scored as sleep compared to overall time spent in bed). Children’s average weekday sleep quality and quantity were calculated from a minimum of three weekday nights of data collection (Acebo et al., 1999). On average, 4.88 nights (SD = .35) of actigraphy data were available per child. Eighty-nine percent of children had data for the entire five nights, 10% had data for four nights, and less than 1% had only three nights of data. Reasons for missing data included forgetting to wear the actigraph and mechanical problems.
Academic Achievement
During school days in late spring, an experimenter administered the Applied Problems and Passage Comprehension subtests from the WJ-III to each child (Woodcock, McGrew, & Mather, 2001). The WJ-III is appropriate for assessing academic abilities in individuals ranging from 2 to 90 years of age. Applied problems has a mean reliability range of .88 – .94 and passage comprehension has a mean range reliability of .94 – .96 in children 4 to 7 years of age (Woodcock, McGrew, & Mather, 2001). W scores from these subtests were used in the analyses. W scores, which are similar to standardized scores, were computed from raw scores using the WJ-III software. The W score allows for a comparison with a normative population based on a score provided by the WJ-III. The correlation between the two subscales was r(99) = .59, p < .001; thus, they were averaged and used as a measure of AA.
Results
Prior to addressing the primary research question, a series of preliminary analyses were conducted. Intra-class correlations (ICCs) considering multiple children sampled from the same classroom ranged from .03 −.16 for sleep variables and ranged from .03 – .53 for EC and AA. Consequently, a multilevel statistical approach—specified using the type=complex command in Mplus 6, with classroom as the cluster variable—was used in all analyses to account for the nested data structure (Muthén, & Muthén, 1998–2010). Full information maximum likelihood estimation with robust standard errors (MLR) was used. We applied confirmatory factor analysis (CFA) to create factor scores for children’s EC. We then examined correlations among the key variables and whether the interactions between EC and sleep predicted AA.
Preliminary Analyses
In order to reduce the number of analyses, we computed a CFA for EC using teacher-reported EC, coder-reported EC, and CPT performance as indices of children’s EC. The model was saturated and therefore fit could not be evaluated. EC variables were positively correlated, rs ranged from r(99) =.26 to .47, ps < .01. Consistent with the significant zero-order correlations among the three indices, the model-estimated standardized loadings were significant, all ps < .01, in the expected direction, and ranged from .39 to .71. A factor score was estimated using Mplus and used in all subsequent analyses.
The correlations shown in Table 1 were estimated in Mplus to account for clustering and missing data. Consistent with expectations, the control variables were related to a number of the focal variables. Sleep variables were correlated with one another in the expected directions. EC did not significantly correlate with AA. There were no significant correlations between the measures of sleep and AA.
Table 1.
Descriptive Statistics and Correlations among the Key Study Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Sex | - | |||||||||||
| 2. Age | −.06 | - | ||||||||||
| 3. Hispanic | −.26* | .05 | - | |||||||||
| 4. Socioeconomic Status(SES) | .19* | −.16 | −.41** | - | ||||||||
| 5. Medication | .31* | −.08 | −.10 | .22* | - | |||||||
| 6. Sleep duration | −.05 | −.17+ | −.01 | .25* | −.02 | - | ||||||
| 7. Sleep duration variability | .21* | .12 | .002 | −.17 | .19* | −.32* | - | |||||
| 8. Sleep onset | .02 | .09 | .18+ | −.19* | −.02 | −.67** | .17+ | - | ||||
| 9. Sleep latency | .07 | .27* | −.18+ | −.002 | .04 | −.32* | .37** | .28* | - | |||
| 10. Sleep efficiency | −.18* | −.10 | .18+ | −.10 | −.11 | .26* | −.13+ | −.08 | −.43* | - | ||
| 11. Effortful Control | −.24** | −.11 | −.07 | .08 | −.14* | −.11* | −.14 | .08 | .10 | .02 | - | |
| 12. Academic Achievement | .13 | .40** | −.23* | .35** | .07 | .06 | −.09 | −.04 | .16 | .06 | .14 | - |
| Mean | .50 | 5.98 | .53 | −.05 | .12 | 561.82 | 35.65 | 21.02 | 25.89 | 81.01 | .00 | 453.80 |
| SD | .50 | .61 | .50 | 2.00 | .26 | 30.50 | 20.61 | .59 | 14.30 | 4.23 | .44 | 18.06 |
Note. N = 103. Sex was coded as Female = 0 and Male = 1; Hispanic was coded as Non-Hispanic = 0 and Hispanic = 1; Sleep variables are in minutes except Efficiency, which is a percentage.
p <.10,
p < .05,
p < .01
Prediction of AA
To test our hypotheses, we computed five regression analyses (five measures of sleep X one moderator). In each regression, we controlled for sex, age, Hispanic (ethnicity), SES, and medication use. Because Mplus does not provide an F test for regression analyses, regression models were compared against a baseline model in which all paths from the predictors to AA were fixed to zero. As is the case for an F test, a significant χ2 for the baseline model indicates that the combined contribution of predictors is statistically significant (Muthén, & Muthén, 1998–2010). The effect of EC was positive and significant in each model, whereas only sleep efficiency was positively related to AA (see Table 2).
Table 2.
Sleep by Effortful Control Interaction Effects in Predicting Academic Achievement
| Academic Achievement | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sleep predictors: |
Sleep Duration | Duration Variability | Sleep Onset | Sleep Latency | Sleep Efficiency | ||||||||||
| B(SE) | t | CI 95% | B(SE) | t | CI 95% | B(SE) | t | CI 95% | B(SE) | t | CI 95% | B(SE) | t | CI 95% | |
| Sex | 5.08 (2.85) |
1.78+ | −.51– 10.66 |
5.08 (3.23) |
1.57 | −1.25– 11.42 |
4.43 (2.90) |
1.53 | −1.26– 10.12 |
2.58 (2.71) |
.95 | 2.73– 7.89 |
4.82 (2.99) |
1.61 | −1.04– 10.70 |
| Age | 14.73 (2.93) |
5.02** | 8.97– 20.48 |
14.36 (3.13) |
4.58** | 8.22– 19.51 |
14.34 (2.92) |
4.91** | 8.62– 20.06 |
15.60 (2.98) |
5.23** | 9.76– 21.44 |
15.50 (2.75) |
5.63** | 10.10– 20.89 |
| Hispanic | −3.14 (3.94) |
−.80 | −10.87– 4.59 |
−3.36 (3.69) |
−.91 | −10.59– 2.71 |
−3.29 (4.02) |
−.82 | −11.17– 4.60 |
−3.69 (4.13) |
−.89 | −11.78– 4.40 |
−3.94 (4.02) |
−.98 | −11.81– 3.93 |
| Socioeconomic status |
6.59 (1.87) |
3.53** | 2.93– 10.25 |
6.09 (1.68) |
3.62** | 2.79– 8.85 |
7.16 (1.88) |
3.81** | 3.48– 10.84 |
6.95 (1.93) |
3.60** | 3.16– 10.73 |
6.58 (1.98) |
3.33* | 2.71– 10.46 |
| Medication | .28 (5.75) |
−.05 | −10.98– 11.54 |
2.13 (4.70) |
.45 | −7.08– 9.87 |
−.30 (6.08) |
−.05 | −12.21– 11.61 |
1.45 (5.27) |
.28 | −8.87– 11.78 |
2.23 (5.73) |
.39 | −9.01– 13.47 |
| Effortful Control |
8.65 (3.59) |
2.41* | 1.60– 15.69 |
7.25 (3.99) |
1.82+ | .56– 13.81 |
7.83 (3.35) |
2.33* | 1.25– 14.40 |
6.30 (3.52) |
1.79+ | −.60– 13.20 |
7.39 (3.66) |
2.02* | .22– 14.56 |
| Sleep predictor | .06 (.05) |
1.09 | −.05−.16 | −.05 (.05) |
−1.19 | −.14– .04 |
−1.56 (2.79) |
−.56 | −7.02– 3.90 |
−.06 (.09) |
−.67 | −.25– .12 |
.87 (.38) |
2.33* | .14– 1.61 |
| Sleep * Effortful Control |
−.15 (.09) |
−1.60 | −.33−.3 | .27 (.11) |
2.48* | .06−.48 | 15.95 (6.65) |
2.40* | 2.92– 28.98 |
.68 (.20) |
3.47** | .30– 1.06 |
−.82 (1.02) |
−.80 | −2.81– 1.18 |
| χ2 (8) = 48.57** R2 = .40** | χ2 (8) = 55.57** R2 = .60** | χ2 (8) = 48.06** R2 = .43** | χ2(8) = 50.88** R2 = .44** | χ2(8) = 41.71** R2 = .41** | |||||||||||
Note. N = 103. Sleep predictor for each model is denoted on the top of the c. Coefficients are unstandardized. Sex was coded as Female = 0 and Male = 1; Hispanic was coded as Non-Hispanic = 0 and Hispanic = 1.
p < .10,
p < .05,
p < .01
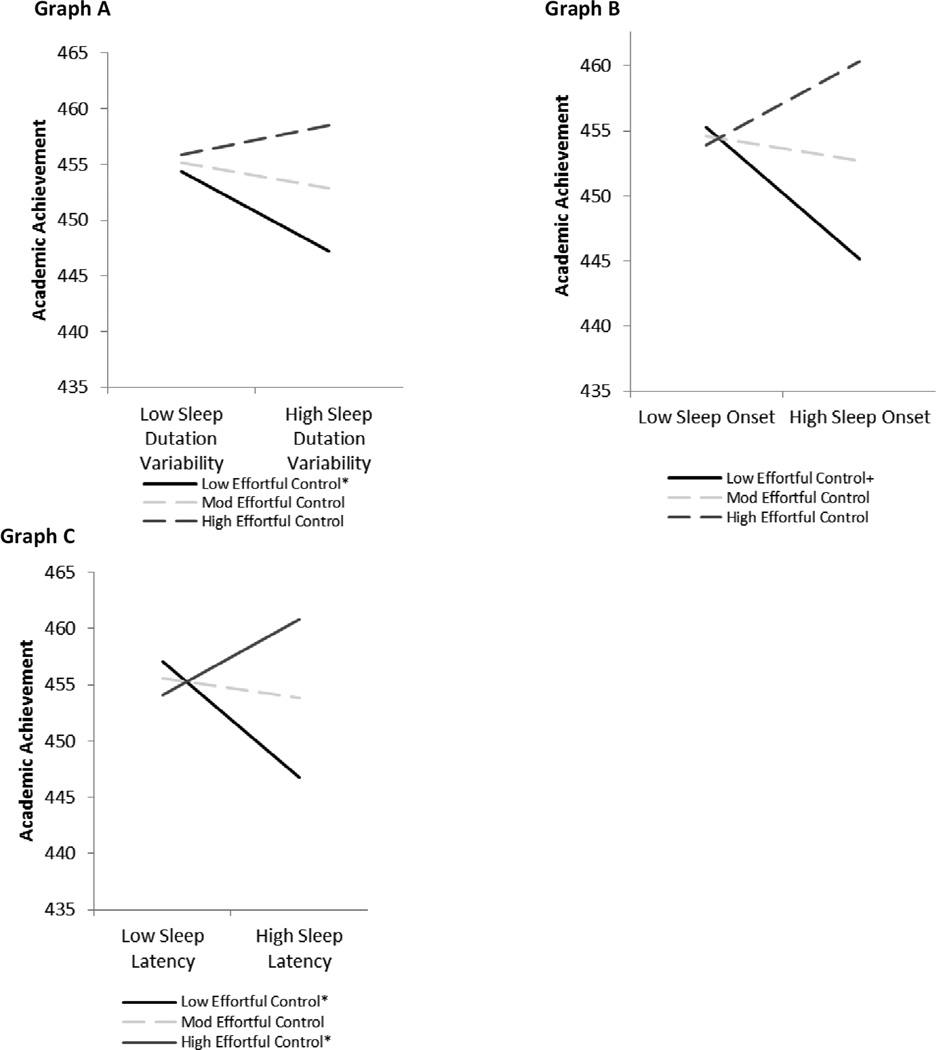
Table 2 summarizes the interactions between EC and sleep. EC significantly interacted with duration variability, sleep onset, and sleep latency. Significant interactions were examined in Mplus and plotted in accordance with the procedures outlined by Aiken and West (1991). As shown in Figure 1, Graph A, sleep duration variability was negatively related to AA at low levels of EC, b = −.17, z = −4.72, p < .001, but was unrelated to AA at moderate or high levels of EC. In addition, the negative relation between sleep onset and AA approached significance at low levels of EC, b = −8.58, z = −1.88, p = .061, but was unrelated to AA at moderate or high levels of EC (Figure 1, Graph B). Sleep latency was also negatively related to AA at low EC, b = −.36, z = −2.90, p < .01. At high EC, the positive relation between sleep latency and AA approached significance, b = .24, z = 1.82, p = .068 (Figure 1, Graph C). The pattern of findings remained the same when controlling for outliers.
Figure 1.
Children’s effortful control moderates the relation between sleep variables and academic achievement.
Note: N = 103. Significant simple slopes are represented by an * and slopes approaching significance are denoted by a +. Levels of effortful control were tested at low (1 SD below the mean), moderate (mean), and high (1 SD above the mean).
Discussion
The goal of this study was to examine the relations between sleep and AA in a sample of kindergarten and first graders by testing the moderating role of children’s EC. In support for our hypotheses, there were significant negative relations between poor sleep and AA, but only for children low in EC. These findings represent an important extension of the existing body of sleep literature because, to our knowledge, no study has examined the moderating effect of EC in associations between sleep and AA. These findings provide insight into why nonsignificant relations between sleep and early AA might be found and under what conditions poor sleep is particularly detrimental to children’s AA. Previous researchers have reported interactions between sleep and children’s regulatory abilities, but such interactions have only been examined in the context of problem behaviors (El-Sheikh et al., 2007). In an extension of this line of research, our findings illustrate that the relation between poor sleep quantity (sleep duration variability) or quality (sleep latency) and AA were negative at low levels of EC. A similar, albeit weaker, negative relation between sleep timing (sleep onset) and AA emerged at low levels of EC. Children with poor sleep (later sleep onset, longer sleep latencies, or greater night-to-night variability in sleep duration) are hypothesized to exhibit low alertness and impaired concentration, making it difficult to learn efficiently. Poor sleep may also make children susceptible to a wide range of problems, including lack of motivation to complete school work, and reduced engagement in class activities as well as compromised cognitive and behavioral functioning (Buckhalt, Wolfson, et al., 2009; Dahl, 1996, 2005; Meijer et al., 2000). However, our findings indicate that the expected negative relations may be observed only when children are low in EC.
Children with lower EC may not be able to attend to information, or regulate their behavior in the classroom, in order to compensate for the effects that poor sleep has on AA. However, when children are high in EC (i.e., they can focus, inhibit dominant responses, and detect errors), they may be able to overcome and even compensate for the learning and achievement challenges believed to be associated with poor sleep (Rothbart & Bates, 1998). In support of this idea, at high levels of EC, there was evidence for a positive relation between sleep latency and AA that approached significance. Children with greater EC may spend the time they are awake assessing the day’s performance and identifying their errors, resulting in longer sleep latencies, but benefiting their AA. In future work, it would be interesting to examine whether EC helps children use the time they are awake more constructively. For example, it is possible that children high in EC spend some of their awake time planning ways to overcome academic demands.
A significant interaction was found for sleep duration variability but not sleep duration. Having high levels of variability might be more detrimental to daytime functioning (Acebo & Carskadon, 2002). Specifically, sleeping poorly one night may prompt an individual to ‘catch up’ by sleeping longer the subsequent night (Bagley & El-Sheikh, 2013). The act of ‘recovery’ sleep can then result in even worse sleep during the following night, as overly long sleep tends to undermine the ability to fall asleep (Lemola, Ledermann, & Friedman, 2013). Such a pattern is consistent with homeostatic sleep regulation, in which deficient sleep leads to compensatory increases in sleep duration, while excessive sleep results in the opposite effect (Mezick et al., 2009). Our findings are also consistent with prior research with both preschoolers and adolescents that suggests that low sleep duration variability is more important for an individual’s functioning and school adjustment than average sleep time (Bates, Richard, Douglas, Beyers, & Stockton, 2002; Fuligni & Hardway, 2006; Lemola et al., 2013).
Additionally, an interaction was found for a component of sleep quality that happens right at bedtime (i.e., sleep latency) but not for overall sleep efficiency. There was, however, a positive main effect for sleep efficiency. Higher sleep efficiency is believed to strengthen neural connections enabling better memory consolidation and information processing (Walker, 2008; 2009) and has been associated with better grades in math and in English (Gruber et al., 2014). However, research on ADHD and anxiety indicates that the strongest associations between sleep and maladaptive outcomes—for which EC would be most beneficial—are those that happen as children are trying to fall asleep (Owens, 2009). Hence, EC may be especially important in the relation between sleep initiation difficulties and early AA, in contrast to sleep efficiency that takes into account sleep quality throughout the night.
Although not the focus of this study, EC was negatively correlated with sleep duration. Similarly, Vaughn and colleagues (2015) found a positive relation between EC and variability in sleep onset (less variability in sleep is believed to be associated with better adaptation). Why greater EC is correlated with poorer sleep, and why it might occur are topics for future work.
EC has often been shown to be positively related to AA over and above a number of control variables. EC has been positively related to mathematics, vocabulary, literacy skills, and grade point average (Blair & Razza, 2007; McClelland et al., 2007; Valiente et al., 2008), perhaps because children high in EC are better able to focus, complete assignments, and avoid distractions in the classroom than children with lower EC (Duncan et al., 2007; Raver, 2002).
Strengths and Limitations
A strength of this study was the use of actigraphy to assess sleep quantity, timing, and quality in young children. Additionally, to our knowledge, it is the only study to empirically examine EC as a potential moderator of the relations between sleep and AA. Although the findings advance the literature in important ways, several limitations should be noted. To date, there is not consistency in the literature regarding recording parameters and actigraphy scoring methods (Meltzer, Montgomery-Downs, Insana, & Walsh, 2012a). Further research is needed to examine the validity of algorithms and wake sensitivity thresholds for younger populations (Meltzer et al., 2012a), as adjusting scoring and sensing algorithms may alter results and conclusions. Additionally, the study was cross-sectional and does not address the direction of causal relations. Future sleep research could also include measures of family relationships and family functioning in order to examine the role of bedtime routines, parenting, and/or familial stress in children’s sleep (e.g., Buckhalt et al., 2009; El-Sheikh, 2014).
Conclusion
In summary, we found evidence that EC moderated the relation between multiple sleep dimensions and AA, such that sleep was related to AA especially for children low in EC. These findings have implications regarding the role of sleep and EC in children’s AA. Children with low EC need plenty of sleep in order to be successful in school. Parents should encourage their children to sleep 10–11 hours each night and have consistent bedtimes (National Sleep Foundation, 2014). Given that greater EC may work as a buffer for children with sleep difficulties, fostering EC may be especially important for children experiencing the poorest quality and quantity of sleep. These findings highlight the need to consider children’s EC in future efforts to understand the complex relation between sleep and AA.
Acknowledgments
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under award numbers R01HD068522 and 3R01HD068522-02S1, awarded to Carlos Valiente and Nancy Eisenberg. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank the participating families, schools, staff, and research assistants who took part in this study.
References
- Acebo C, Sadeh A, Seifer R, Tzischinsky O, Wolfson AR, Hafer A, Carskadon MA. Estimating sleep patterns with activity monitoring in children and adolescence: How many nights are necessary for reliable measures? Sleep. 1999;22:95–103. doi: 10.1093/sleep/22.1.95. [DOI] [PubMed] [Google Scholar]
- Acebo C, Carskadon MA. Influence of irregular sleep/wake patterns on waking behavior. In: Carskadon MA, editor. Adolescent Sleep Patterns: Biological, Social, and Psychological Influences. Cambridge, UK: Cambridge University Press; 2002. pp. 220–235. [Google Scholar]
- Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Newbury Park: Sage; 1991. [Google Scholar]
- Asarnow L, Harvey A, McGlinchey E. The effects of bedtime and sleep duration on academic and emotional outcomes in a nationally representative sample of adolescents. Adolescent Health. 2013;54:350–356. doi: 10.1016/j.jadohealth.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astill RG, Van der Heijden KB, Van Ijzendoorn MH, Van Someren EJ. Sleep, cognition, and behavioral problems in school-age children: A century of research metaanalyzed. Psychological Bulletin. 2012;138:1109–1138. doi: 10.1037/a0028204. [DOI] [PubMed] [Google Scholar]
- Bagley E, El-Sheikh M. Children’s sleep and internalizing and externalizing symptoms. In: Wolfson AR, Montgomery-Downs HE, editors. Oxford Handbook of Infant, Child and Adolescent Sleep and Behaviors. New York, NY: Oxford University Press; 2013. [Google Scholar]
- Bates JE, Viken RJ, Alexander DB, Beyers J, Stockton L. Sleep and adjustment in preschool children: Sleep diary reports by mothers relate to behavior reports by teachers. Child Development. 2002;73:62–75. doi: 10.1111/1467-8624.00392. [DOI] [PubMed] [Google Scholar]
- Beebe DW. Cognitive, behavioral, and functional consequences of inadequate sleep in children and adolescents. Pediatric Clinics of North America. 2011;58:649–665. doi: 10.1016/j.pcl.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier A, Beauchamp MH, Bouvette-Turcot AA, Carlson SM, Carrier J. Sleep and cognition in preschool years: Specific links to executive functioning. Child Development. 2013;84:1542–1553. doi: 10.1111/cdev.12063. [DOI] [PubMed] [Google Scholar]
- Biggs SN, Lushington K, Van den Heuvel CJ, Martin AJ, Kennedy JD. Inconsistent sleep schedules and daytime behavioral difficulties in school-aged children. Sleep Medicine. 2011;12:780–786. doi: 10.1016/j.sleep.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Blair C, Razza RP. Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child Development. 2007;78:647–663. doi: 10.1111/j.1467-8624.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- Bridgett DJ, Oddi KB, Laake LM, Murdock KW, Bachmann MN. Integrating and differentiating aspects of self-regulation: Effortful control, executive functioning, and links to negative affectivity. Emotion. 2013;13:47–63. doi: 10.1037/a0029536. [DOI] [PubMed] [Google Scholar]
- Brock LL, Rimm-Kaufman SE, Nathanson L, Grimm KJ. The contributions of ‘hot’ and ‘cool’ executive function to children’s academic achievement, learning-related behaviors, and engagement in kindergarten. Early Childhood Research Quarterly. 2009;24:337–349. [Google Scholar]
- Buckhalt JA, El-Sheikh M, Keller P. Children’s sleep and cognitive functioning: Race and socioeconomic status as moderators of effects. Child Development. 2007;78:213–231. doi: 10.1111/j.1467-8624.2007.00993.x. [DOI] [PubMed] [Google Scholar]
- Buckhalt JA, El-Sheikh M, Keller P, Kelley R. Concurrent and longitudinal relations between children’s sleep and cognitive functioning. Child Development. 2009;80:875–892. doi: 10.1111/j.1467-8624.2009.01303.x. [DOI] [PubMed] [Google Scholar]
- Buckhalt JA, Wolfson A, El-Sheikh M. Children’s sleep and school psychology practice. School Psychology Quarterly. 2009;24:60–69. [Google Scholar]
- Carskadon MA, Dement WC. Monitoring and staging human sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 5th. St. Louis, MO: Elsevier Saunders; 2011. [Google Scholar]
- Cooper P, Kohler M, Blunden S. Sleep and academic performance in indigenous australian children from a remote community: An exploratory study. Journal of Pediatrics and Child Health. 2012;48:122–127. doi: 10.1111/j.1440-1754.2011.02059.x. [DOI] [PubMed] [Google Scholar]
- Dahl RE. The impact of inadequate sleep on children’s daytime cognitive function. Seminars in Pediatric Neurology. 1996;3:44–50. doi: 10.1016/s1071-9091(96)80028-3. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Sleep, learning, and the developing brain: Early-to-bed as a healthy and wise choice for school aged children: Comment on Fallone et al. Sleep. 2005;28:1498–1499. [PubMed] [Google Scholar]
- Davis-Kean PE. The influence of parent education and family income on child achievement: The indirect role of parental expectations and the home environment. Journal of Family Psychology. 2005;19:294–304. doi: 10.1037/0893-3200.19.2.294. [DOI] [PubMed] [Google Scholar]
- Dayyat E, Spruyt K, Molfese DL, Gozal D. Sleep estimates in children: Parental versus actigraphic assessments. National Science of Sleep. 2011;3:115–123. doi: 10.2147/NSS.S25676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald JF, Meijer AM, Oort FJ, Kerkhof GA, Bögels SM. The influence of sleep quality, sleep duration, and sleepiness on school performance in children and adolescents: A meta-analytic review. Sleep Medicine Reviews. 2010;14:179–189. doi: 10.1016/j.smrv.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Duckworth AL, Quinn PD, Tsukayama E. What no child left behind leaves behind: The roles of IQ and self-control in predicting standardized achievement test scores and report card grades. Journal of Educational Psychology. 2012;104:439–451. doi: 10.1037/a0026280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GJ, Dowsett CJ, Claessens A, Magnuson K, Huston AC, Klebanov P, Japel C. School readiness and later achievement. Developmental Psychology. 2007;43:1428–1446. doi: 10.1037/0012-1649.43.6.1428. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Qing Z, Spinrad TL, Valiente C, Fabes RA, Liew J. Relations among positive parenting, children’s effortful control, and externalizing problems: A three-wave longitudinal study. Child Development. 2005;76:1055–1071. doi: 10.1111/j.1467-8624.2005.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore-Staton L, Hinnant JB, Buckhalt JA, El-Sheikh M. Sleep and cognitive performance: The role of income and respiratory sinus arrhythmia reactivity. Developmental Psychobiology. 2014;56:1528–1540. doi: 10.1002/dev.21247. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Erath SA, Keller PS. Children’s sleep and adjustment: The moderating role of vagal regulation. Journal of Sleep Research. 2007;16:396–405. doi: 10.1111/j.1365-2869.2007.00618.x. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Tu KM, Erath SA, Buckhalt JA. Family stress and adolescents’ cognitive functioning: Sleep as a protective factor. Journal of Family Psychology. 2014;28:887–896. doi: 10.1037/fam0000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Chillag N, Lavie P. Starting times of school: Effects on daytime functioning of fifth grade children in Israel. Sleep. 1998;21:250–256. doi: 10.1093/sleep/21.3.250. [DOI] [PubMed] [Google Scholar]
- Fallone G, Owens JA, Deane J. Sleepiness in children and adolescents: Clinical implications. Sleep Medicine Review. 2002;6:287–306. doi: 10.1053/smrv.2001.0192. [DOI] [PubMed] [Google Scholar]
- Fuligni AJ, Hardway C. Daily variation in adolescents’ sleep, activities, and psychological Well-Being. Journal of Research on Adolescence. 2006;16:353–378. [Google Scholar]
- Frank MG. The mystery of sleep function: current perspectives and future directions. Reviews in the Neurosciences. 2006;17:375–392. doi: 10.1515/revneuro.2006.17.4.375. [DOI] [PubMed] [Google Scholar]
- Goodnight JA, Bates JE, Staples AD, Pettit GS, Dodge KA. Temperamental resistance to control increases the association between sleep problems and externalizing behavior development. Journal of Family Psychology. 2007;21:39–48. doi: 10.1037/0893-3200.21.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber R, Somerville G, Enros P, Paquin S, Kestler M, Gillies-Poitras E. Sleep efficiency (but not sleep duration) of healthy school-age children is associated with grades in math and languages. Sleep Medicine. 2014;15:1517–1525. doi: 10.1016/j.sleep.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Horne JA. Sleep and body restitution. Experientia. 1908;36:11–13. doi: 10.1007/BF02003942. [DOI] [PubMed] [Google Scholar]
- Hyde M, O’Driscoll DM, Binette S, Galand C, Tan SK, Verginis N, Horne RSC. Validation of actigraphy for determining sleep and wake in children with sleep disordered breathing. Journal of Sleep Research. 2007;16:213–216. doi: 10.1111/j.1365-2869.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- Karpinski AC, Scullin MH, Montgomery-Downs HE. Risk for sleep-disordered breathing and executive function in preschoolers. Sleep medicine. 2008;9:418–424. doi: 10.1016/j.sleep.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Murray KT, Harlan E. Effortful control in early childhood: Continuity and change, antecedents, and implications for social development. Developmental Psychology. 2000;36:220–232. [PubMed] [Google Scholar]
- Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Medicine. 2001;2:389–396. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- Ladd GW, Birch SH, Buhs ES. Children’s social and scholastic lives in kindergarten: Related spheres of influence? Child Development. 1999;70:1373–1400. doi: 10.1111/1467-8624.00101. [DOI] [PubMed] [Google Scholar]
- Lemola S, Ledermann T, Friedman EM. Variability of sleep duration is related to subjective sleep quality and subjective well-being: An actigraphy study. PLoS One. 2013;8:1–9. doi: 10.1371/journal.pone.0071292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhou G, Wang Y, Ai Y, Pinto-Martin J, Liu X. Sleep problems, fatigue, and cognitive performance in Chinese kindergarten children. The Journal of Pediatrics. 2012;161:520–525. doi: 10.1016/j.jpeds.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lushington K, Pamula Y, Martin J, Kennedy J. The relationship between sleep and daytime cognitive/behavioral functioning: Infancy and preschool years. In: Wolfson AR, Montgomery-Downs HE, editors. Oxford Handbook of Infant, Child and Adolescent Sleep and Behaviors. New York, NY: Oxford University Press; 2013. [Google Scholar]
- Matthews JS, Ponitz CC, Morrison FJ. Early gender differences in self-regulation and academic achievement. Journal of Educational Psychology. 2009;101:689–704. [Google Scholar]
- Mayes SD, Calhoun SL, Bixler EO, et al. Nonsignificance of sleep relative to IQ and neuropsychological scores in predicting academic achievement. Journal of Developmental and Behavior Pediatric. 2008;29:206–212. doi: 10.1097/DBP.0b013e31816d924f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland MM, Cameron CE, Duncan R, Bowles RP, Acock AC, Miao A, Pratt ME. Predictors of early growth in academic achievement: the head-toes-knees-shoulders task. Frontiers in Psychology. 2014;5:599. doi: 10.3389/fpsyg.2014.00599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland MM, Cameron CE, Connor CM, Farris CL, Jewkes AM, Morrison FJ. Links between behavioral regulation and preschoolers’ literacy, vocabulary, and math skills. Developmental Psychology. 2007;43:947–959. doi: 10.1037/0012-1649.43.4.947. [DOI] [PubMed] [Google Scholar]
- Meijer AM, Habekothe HT, van den Wittenboer GLH. Time in bed, quality of sleep and school functioning of children. Journal of Sleep Research. 2000;9:145–153. doi: 10.1046/j.1365-2869.2000.00198.x. [DOI] [PubMed] [Google Scholar]
- Mezick EJ, Matthews KA, Hall M, Kamarck TW, Buysse DJ, Owens JF, Reis SE. Intra-individual variability in sleep duration and fragmentation: associations with stress. Psychoneuroendocrinology. 2009;34:1346–1354. doi: 10.1016/j.psyneuen.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M, Slane J, Mindell JA, Burt SA, Klump KL. Sleep problems and temperament in adolescents. Child: Care, Health and Development. 2011;37:559–562. doi: 10.1111/j.1365-2214.2010.01157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AS, Silk JS, Steinberg L, Sessa FM, Avenevoli S, Essex MJ. Temperamental vulnerability and negative parenting as interacting predictors of child adjustment. Journal of Marriage and Family. 2002;64:461–471. [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 6th. Los Angeles, CA: Muthén, & Muthén; 1998–2010. [Google Scholar]
- National Sleep Foundation. Sleep in America Poll: Sleep in the Modern Family. Washington (DC): National Sleep Foundation; 2014. Available from http://www.sleepfoundation.org/sleep-polls-data/sleep-in-americapoll/2014-sleep-in-the-modern-family. [Google Scholar]
- National Sleep Foundation. The sleep-wake cycle: Its physiology and impact on health. Washington, DC: National Sleep Foundation; 2006. [Google Scholar]
- Nelson TD, Nelson JM, Kidwell KM, James TD, Espy KA. Preschool sleep problems and differential associations with specific aspects of executive control in early elementary school. Developmental Neuropsychology. 2015;40:167–180. doi: 10.1080/87565641.2015.1020946. [DOI] [PubMed] [Google Scholar]
- Oswald I. Sleep as restorative process: Human clues. Progress in Brain Research. 1980;53:279–288. doi: 10.1016/s0079-6123(08)60069-2. [DOI] [PubMed] [Google Scholar]
- Owens JA. A clinical overview of sleep and attention-deficit/hyperactivity disorder in children and adolescents. Journal of the Canadian Academy of Child and Adolescent Psychiatry. 2009;18:92–102. [PMC free article] [PubMed] [Google Scholar]
- Palermo T. A brief history of child and adolescent sleep research: Key contributions in psychology. In: Wolfson AR, Montgomery-Downs HE, editors. Oxford Handbook of Infant, Child and Adolescent Sleep and Behaviors. New York, NY: Oxford University Press; 2013. [Google Scholar]
- Ponitz CC, McClelland MM, Jewkes AM, Connor CM, Farris CL, Morrison FJ. Touch your toes! Developing a direct measure of behavioral regulation in early childhood. Early Childhood Research Quarterly. 2008;23:141–158. [Google Scholar]
- Ponitz CC, McClelland MM, Matthews JS, Morrison FJ. A structured observation of behavioral regulation and its contributions to kindergarten outcomes. Developmental Psychology. 2009;45:605–619. doi: 10.1037/a0015365. [DOI] [PubMed] [Google Scholar]
- Popham W. Modern educational measurement. 3rd. Boston, MA: Allyn & Bacon; 2000. [Google Scholar]
- Raver CC. Emotions matter: Making the case for the role of young children’s emotional development for early school readiness. Social Policy Report, Society for Research in Child Development. 2002;16:3–18. [Google Scholar]
- Ravid S, Afek I, Suraiya S, Shahar E, Pillar G. Kindergarten children’s failure to qualify for first grade could result from sleep disturbances. Journal of Child Neurology. 2009a;24:816–822. doi: 10.1177/0883073808330766. [DOI] [PubMed] [Google Scholar]
- Ravid S, Afek I, Suraiya S, Shahar E, Pillar G. Sleep disturbances are associated with reduced school achievements in first-grade pupils. Developmental Neuropsychology. 2009b;34:574–587. doi: 10.1080/87565640903133533. [DOI] [PubMed] [Google Scholar]
- Respironics. Actware Clinicians Guide. Murrysville, PA: Respironics, Inc; 2008. [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey K, Fisher P. Investigations of temperament at three to seven years: The children’s behavior questionnaire. Child Development. 2001;72:1394–1408. doi: 10.1111/1467-8624.00355. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ellis LK, Rosario Rueda M, Posner MI. Developing mechanisms of temperamental effortful control. Journal of Personality. 2003;71:1113–1144. doi: 10.1111/1467-6494.7106009. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Bates JE. Temperament. In: Damon W, Eisenberg N, editors. Handbook of Child Psychology. Vol. 3. Social, Emotional, Personality Development. New York, NY: Wiley; 1998. pp. 105–176. [Google Scholar]
- Rothbart MK, Bates JE. Temperament. In: Damon W, Eisenberg N, editors. Handbook of Child Psychology. Vol. 3. Social, Emotional, Personality Development. 6th. Hoboken, NJ: John Wiley, & Sons; 2006. pp. 99–166. [Google Scholar]
- Sadeh A. Assessment of intervention for infant night waking: Parental reports and activity-based home monitoring. Journal of Consulting and Clinical Psychology. 1994;62:63–68. doi: 10.1037//0022-006x.62.1.63. [DOI] [PubMed] [Google Scholar]
- Sadeh A. Sleep. In: Haith MM, Benson JB, editors. Encyclopedia of Infant and Early Childhood Development. San Diego, CA: Academic Press; 2008. [Google Scholar]
- Sadeh A, Gruber R, Raviv A. The effects of sleep restriction and extension on school-age children: What a difference an hour makes. Child Development. 2003;7:444–455. doi: 10.1111/1467-8624.7402008. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Raviv A, Gruber R. Sleep patterns and sleep disruptions in School-Age Children. Developmental Psychology. 2000;36:291–301. doi: 10.1037//0012-1649.36.3.291. [DOI] [PubMed] [Google Scholar]
- Simonds J, Kieras JE, Rueda MR, Rothbart MK. Effortful control, executive attention, and emotional regulation in 7-10-year-old children. Cognitive Development. 2007;22:474–488. [Google Scholar]
- Spruyt K, Gozal D, Dayyat E, Roman A, Molfese DL. Sleep assessments in healthy school-aged children using actigraphy: Concordance with polysomnography. Journal of Sleep Research. 2011;20:223–232. doi: 10.1111/j.1365-2869.2010.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenari M, Steenari M, Vuontela V, Paavonen J. Working memory and sleep in 6-to 13-year old children. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:85–92. doi: 10.1097/00004583-200301000-00014. [DOI] [PubMed] [Google Scholar]
- Stevenson HW, Chen C, Uttal DH. Beliefs and achievement: A study of Black, White, and Hispanic children. Child Development. 1990;61:508–523. doi: 10.1111/j.1467-8624.1990.tb02796.x. [DOI] [PubMed] [Google Scholar]
- Touchette É, Petit D, Séguin JR, Boivin M, Tremblay RE, Montplaisir JY. Associations between sleep duration patterns and behavioral/cognitive functioning at school entry. Sleep. 2007;30:1213–1219. doi: 10.1093/sleep/30.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiente C, Lemery-Chalfant K, Swanson J. Prediction of kindergartners’ academic achievement from their effortful control and emotionality: Evidence for direct and moderated relations. Journal of Educational Psychology. 2010;102:550–560. [Google Scholar]
- Valiente C, Lemery-Chalfant K, Swanson J, Reiser M. Prediction of children’s academic competence from their effortful control, relationships, and classroom participation. Journal of Educational Psychology. 2008;100:67–77. doi: 10.1037/0022-0663.100.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn BE, Elmore-Staton L, Shin N, El-Sheikh M. Sleep as a support for social competence, peer relations, and cognitive functioning in preschool children. Behavioral Sleep Medicine. 2015;13:1–15. doi: 10.1080/15402002.2013.845778. [DOI] [PubMed] [Google Scholar]
- Walker MP. The role of sleep in cognition and emotion. Annals of the New York Academy of Sciences. 2009;1156:168–197. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- Walker MP. Cognitive consequences of sleep and sleep loss. Sleep Medicine. 2008;9:S29–S34. doi: 10.1016/S1389-9457(08)70014-5. [DOI] [PubMed] [Google Scholar]
- Weiss AR, Johnson NL, Berger NA, Redline S. Validity of activity-based devices to estimate sleep. Journal of Clinical Sleep Medicine. 2010;6:336–342. [PMC free article] [PubMed] [Google Scholar]
- Werner H, Molinari L, Guyer C, Jenni OG. Agreement rates between actigraphy, diary, and questionnaire for children’s sleep patterns. Archives of Pediatric Adolescence Medicine. 2008;162:350–358. doi: 10.1001/archpedi.162.4.350. [DOI] [PubMed] [Google Scholar]
- Willingham WW, Pollack JM, Lewis C. Grades and test scores: Accounting for observed differences. Journal of Educational Measurement. 2002;39:1–37. [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock Johnson III Tests of Achievement. Rolling Meadows, IL: Riverside; 2007. [Google Scholar]