Abstract
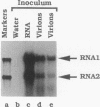
Newly synthesized virions of flock house virus (FHV), an insect nodavirus, were detected in plant cells inoculated with FHV RNA. FHV was found in whole plants of barley (Hordeum vulgare), cowpea (Vigna sinensis), chenopodium (Chenopodium hybridum), tobacco (Nicotiana tabacum), and Nicotiana benthamiana and in protoplasts derived from barley leaves. Virions produced in plants contained newly synthesized RNA as well as newly synthesized capsid protein. These results show that the intracellular environment in these plants is suitable for synthesis of a virus normally indigenous only to insects. Such synthesis involves, minimally, translation of viral RNA, RNA replication, and virion assembly. Inoculation of barley protoplasts with FHV virions resulted in synthesis of small amounts of progeny virions, suggesting that FHV virions are capable of releasing their RNA in plant cells. In N. benthamiana, virions resulting from inoculation with RNA were detected not only in inoculated leaves but also in other leaves of inoculated plants, suggesting that virions could move in this plant species. Such movement probably occurs by a passive transport through the vascular system rather than by an active transport involving mechanisms that have evolved for plant viruses.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atabekov J. G., Morozov S. Y. Translation of plant virus messenger RNAs. Adv Virus Res. 1979;25:1–91. doi: 10.1016/s0065-3527(08)60568-0. [DOI] [PubMed] [Google Scholar]
- Dasgupta R., Ghosh A., Dasmahapatra B., Guarino L. A., Kaesberg P. Primary and secondary structure of black beetle virus RNA2, the genomic messenger for BBV coat protein precursor. Nucleic Acids Res. 1984 Sep 25;12(18):7215–7223. doi: 10.1093/nar/12.18.7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasmahapatra B., Dasgupta R., Ghosh A., Kaesberg P. Structure of the black beetle virus genome and its functional implications. J Mol Biol. 1985 Mar 20;182(2):183–189. doi: 10.1016/0022-2836(85)90337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasmahapatra B., Dasgupta R., Saunders K., Selling B., Gallagher T., Kaesberg P. Infectious RNA derived by transcription from cloned cDNA copies of the genomic RNA of an insect virus. Proc Natl Acad Sci U S A. 1986 Jan;83(1):63–66. doi: 10.1073/pnas.83.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deom C. M., Oliver M. J., Beachy R. N. The 30-kilodalton gene product of tobacco mosaic virus potentiates virus movement. Science. 1987 Jul 24;237(4813):389–394. doi: 10.1126/science.237.4813.389. [DOI] [PubMed] [Google Scholar]
- Friesen P. D., Rueckert R. R. Black beetle virus: messenger for protein B is a subgenomic viral RNA. J Virol. 1982 Jun;42(3):986–995. doi: 10.1128/jvi.42.3.986-995.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen P., Scotti P., Longworth J., Rueckert R. Black beetle virus: propagation in Drosophila line 1 cells and an infection-resistant subline carrying endogenous black beetle virus-related particles. J Virol. 1980 Sep;35(3):741–747. doi: 10.1128/jvi.35.3.741-747.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher T. M., Friesen P. D., Rueckert R. R. Autonomous replication and expression of RNA 1 from black beetle virus. J Virol. 1983 May;46(2):481–489. doi: 10.1128/jvi.46.2.481-489.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiruki C. Properties of single- and double-stranded ribonucleic acid from barley plants infected with bromegrass mosaic virus. J Virol. 1969 May;3(5):498–505. doi: 10.1128/jvi.3.5.498-505.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H. T., Black L. M. Multiplication of potato yellow dwarf virus on vector cell monolayers. Virology. 1974 May;59(1):331–334. doi: 10.1016/0042-6822(74)90232-3. [DOI] [PubMed] [Google Scholar]
- Kimura I., Black L. M. Growth of wound tumor virus in vector cell monolayers. Virology. 1972 Jun;48(3):852–854. doi: 10.1016/0042-6822(72)90168-7. [DOI] [PubMed] [Google Scholar]
- Klebe R. J., Harriss J. V. A technically simple "non-lethal" vital staining procedure for viral plaque and cell transformation assays. Brief report. Arch Virol. 1984;81(3-4):359–362. doi: 10.1007/BF01310007. [DOI] [PubMed] [Google Scholar]
- Newman J. F., Matthews T., Omilianowski D. R., Salerno T., Kaesberg P., Rueckert R. In vitro translation of the Two RNAs of Nodamura virus, a novel mammalian virus with a divided genome. J Virol. 1978 Jan;25(1):78–85. doi: 10.1128/jvi.25.1.78-85.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider I. Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morphol. 1972 Apr;27(2):353–365. [PubMed] [Google Scholar]
- Scotti P. D., Dearing S., Mossop D. W. Flock House virus: a nodavirus isolated from Costelytra zealandica (White) (Coleoptera: Scarabaeidae). Arch Virol. 1983;75(3):181–189. doi: 10.1007/BF01315272. [DOI] [PubMed] [Google Scholar]
- Selling B. H., Rueckert R. R. Plaque assay for black beetle virus. J Virol. 1984 Jul;51(1):251–253. doi: 10.1128/jvi.51.1.251-253.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]