Abstract
Fear of diabetes and major surgery may prohibit referral of young children severely impacted by pancreatitis for total pancreatectomy and islet autotransplant (TPIAT). We evaluated outcomes in our youngest TPIAT recipients, age 3–8 years at surgery.
Medical records were reviewed for 17 children (9 female) age ≤8 years undergoing TPIAT from 2000–2014. Most (14/17) had genetic risk factors for pancreatitis. Since 2006, TPIAT recipients were followed prospectively with health questionnaires including assessments of pain and narcotic use, and scheduled HbA1c and mixed meal tolerance tests (6 mL/kg Boost HP) before surgery, and at regular intervals after. Patients are 1–11 years post TPIAT (median 2.2 years). Data are reported as median (25th, 75th percentile).
All had relief of pain, with all 17 patients off narcotics at most recent follow up. Hospitalization rates decreased from 5.0 hospitalization episodes per person-year of follow up before TPIAT, to 0.35 episodes per person-year of follow up after TPIAT. Fourteen (82%) discontinued insulin, higher than the observed insulin independence rate of 41% in 399 patients >8 years of age undergoing TPIAT over the same interval (p=0.004). Median post-TPIAT HbA1c was 5.9% (5.6, 6.3%), and within patient post-TPIAT mean HbA1c was ≤6.5% for all but 2 patients.
Very young children with severe refractory chronic pancreatitis may be good candidates for TPIAT, with high rates of pain relief and insulin independence, and excellent glycemic control in the majority.
Introduction
Pancreatitis is rare in young children, and when present, is often attributed to pre-disposing genetic mutations in trypsinogen, trypsinogen inhibitory pathways, or bicarbonate secretory pathways (1, 2). For those children who are afflicted with recurrent acute or chronic pancreatitis at a young age, the disease burden is great. Significant pain, narcotic use, and missed school in school-age children are common consequences of severe disease (1).
First-line therapies include pain management, pancreatic enzyme therapy for pancreatic suppression, and dietary changes including low fat diet or avoidance of oral intake with nutrition via nasojejunal or gastrojejunal feeds (3). Endoscopic retrograde cholangiopancreatography (ERCP) is often performed, in particular when ductal stones or strictures are present (4). Those who fail these medical and endoscopic interventions may be candidates for surgical intervention. A number of surgical techniques have been used in an attempt to ameliorate pain and restore quality of life, including partial resection, or drainage procedures such as lateral pancreaticojejunostomy (e.g. Puestow), or variants (e.g. Frey or Beger procedures) (5, 6). Patients often have transient pain relief, but due to the diffuse nature and involvement of the entire pancreas, pain eventually recurs in up to 50% of patients (7–12), and subsequent exocrine and endocrine insufficiency often develops over time (13). Total pancreatectomy with islet autotransplant (TPIAT) is the only procedure that removes the root cause of pain (14, 15) and provides long term sustained relief (5). With this procedure, the islets are isolated and infused back into the recipient’s liver to reduce the risk of post-operative diabetes.
Understandably, pediatricians and gastroenterologists may be reluctant to refer very young children for such a major TPIAT surgery with risk of diabetes mellitus. In addition, in small children, the technical aspects of surgical reconstruction of the biliary tract and islet isolation are challenging (14, 16). However, in the most severely affected children, allowing ongoing chronic pain, suffering and recurrent hospitalizations may be equally unacceptable. While we have previously reported in our cohort high rates of success for both pain and diabetes resolution in children under 12 years of age compared to adolescents, very few of these children were under 9 years of age (14). Because of an increase in the number of referrals of very young children over the past 4 years, herein we studied and reported outcomes for our youngest patients, age 3–8 years at the time of surgery.
Methods
Subjects
From all TPIAT recipients at the University of Minnesota, 17 children met inclusion criteria of age 3–8 years at time of surgery. TPIAT procedures were performed from 2000 to 2014, and all patients have at least 1 year of follow up (figure 1). Early surgical outcomes of 7 of these children have been partially reported as part of past large pediatric series (14, 17), but outcomes of this subcohort have not been previously reported. Data was collected for participants under two IRB-approved single center protocols, and informed parental consent and assent (as relevant) were obtained from all participants.
Figure 1.
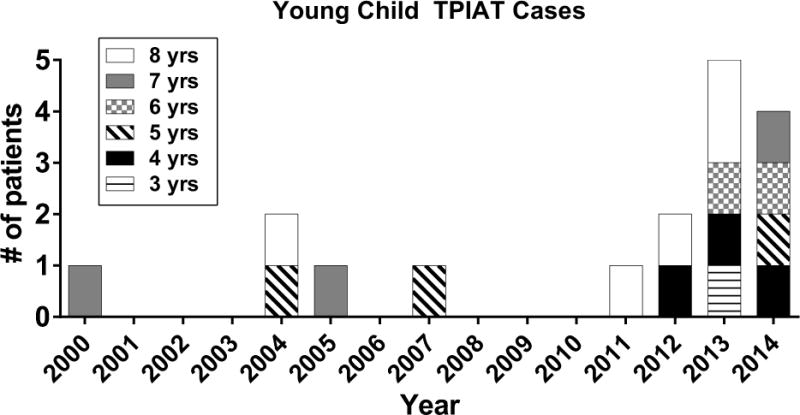
Patient number and age at TPIAT, of 3–8 years old, by year that TPIAT was performed.
One patient in this series was lost to follow up at 1 year after TPIAT. Long-term continuous follow up is available for the remaining 16 cases. One patient is deceased at 7 years post-TPIAT from respiratory complications related to infectious complications of spinal surgery. This patient was insulin independent and off narcotics at the time of death, which was unrelated to TPIAT.
Pre-operative assessment
All patients are reviewed by a multi-disciplinary chronic pancreatitis workgroup for pancreatitis diagnosis and appropriateness for TPIAT surgery, as previously described (14, 15, 17, 18). As part of the evaluation process, all children are assessed for risk factors for pancreatitis, including genetic testing for mutations in the protease serine 1 (PRSS1), serine protease inhibitor Kazal-type 1 (SPINK1), and cystic fibrosis transmembrane regulator (CFTR) genes, and, recently, chymotrypsin C (CTRC) gene. Magnetic resonance cholangiopancreateogram (MRCP) is standardly obtained in the evaluation process for imaging features of chronic pancreatitis such as atrophy, ductal dilatation and side branches. In the more the recent era, liver volume, bile duct diameter, and portal vein diameter were also measured by MRCP.
Surgical Procedure
The surgical technique for TPIAT for small children is similar to previously described (14, 19). In contrast to total pancreatectomy alone, for islet autotransplantation, the blood supply to the pancreas must be preserved until the dissection is completed for resection, thus minimizing the warm ischemia time, and maximizing islet cell preservation. In addition, important surgical steps in the pediatric patient include special attention to avoid any inadvertent injury or spasm of the small vessels to the liver (specially the abberant right and left hepatic arteries), pylorus preservation, and use of a Roux-en-Y loop for duodenojejunostomy and choledochojeujunostomy to minimize postoperative gastrointestinal complications such as bile reflux gastritis. All patients also receive splenectomy and cholecystectomy if not already done. Due to the small bile duct diameter in the children, biliary anastamosis was performed over an internal biliary stent. A laparoscopic-assisted approach was performed in 3 patients. A gastro-jejunal tube was placed at the time of surgery for enteral feeding post-operatively as post-operative gastroparesis is universal in these patients. Islet isolation was performed by enzymatic digestion with collagenase followed by mechanical disruption using the semi-automated method of Ricodi, as previously described (20, 21). Islet purification was performed only if necessary for high tissue volume, in the modern era defined by total tissue mass >0.25 mL/kg. Islets were infused into the liver under gravity via cannulation of a tributary of the portal vein. In 2 cases, a portion of the islets was placed in the peritoneal cavity due to elevation of portal pressures (>25 cm saline) during islet infusion. To minimize risk of portal vein thrombosis, dextran 40 at 0.5 cc/Kg was started 1 hour prior to islet infusion and continued for 48 hours post operatively after which aspirin 2–3 mg/Kg was initiated and continued for 1 year. Heparin was administered in all cases as a bolus of 70 units/kg with the islet product, followed by a heparin drip (10 units/Kg) for 1 week post-operatively (22).
Insulin was administered post-operatively in all patients, initially as a continuous intravenous insulin infusion until nutritional and clinical status was stable (23), and then transitioned to subcutaneous insulin therapy, targeting glucoses of 80–125 mg/dL for the first 4–6 weeks post-transplant, and thereafter 80–120 mg/dL fasting and 80–150 mg/dL at 2 hours post-prandial. Insulin was weaned as tolerated at ≥3 months post-TPIAT. Patients were considered insulin independent only when glucose targets were met and hemoglobin A1c (HbA1c) was maintained at ≤6.5% in the absence of insulin therapy. Insulin dependent patients were considered to have partial islet graft function if stimulated C-peptide was ≥0.6 ng/mL, indicating endogenous islet function despite the need for supplemental exogenous insulin therapy.
Data collection
Medical records and islet lab records were reviewed for surgical details, islet mass infused, post-operative complications, insulin use, glycemic control, pain medication use, and growth parameters. As part of routine post-TPIAT care, TPIAT recipients since 2007 undergo assessment of islet function and glycemic control before surgery, and at 3 months, 6 months, 1 year and then yearly after surgery. This includes mixed meal tolerance testing (6 mL/kg of Boost HP) with fasting baseline and stimulated glucose and C-peptide, and hemoglobin A1c levels. Insulin dose is assessed based on patient report. Patients and families (parents) since 2007 have completed health questionnaires including health related quality of life assessments, and assessment of pain medication and insulin use at 3 months, 6 months, and yearly after surgery.
Statistical analysis
Data are reported as median values (25th, 75th percentile). The number of patients achieving insulin independence and nacrotic independence in the young child cohort vs all other patients over the same interval was compared using chi-square. All data analysis was performed with PC SAS (version 9.3).
Results
Patient characteristics
Patients underwent TPIAT at a median age of 6.8 years (IQR 5.1 – 8.3), and have been followed for a median duration of 2.24 years (IQR 1.5 – 4.3) after TPIAT. Pancreatitis was attributed to genetic mutations in 14/17, including PRSS1 mutation in 11 patients (see Table 1); one child considered idiopathic, however, was not tested for genetic mutations (earliest case). The indication for TPIAT in all children was painful recurrent or chronic pancreatitis, necessitating daily or intermittent narcotic therapy, and repeated hospitalizations, with a median of 14 hospitalizations/patient (IQR 4–20). All 17 children had previously documented recurrent acute pancreatitis, and all children with imaging available for review had imaging changes of chronic pancreatitis (11/11 with MRCP, 1/1 with endoscopic ultrasound; see example in supplemental Figure 1), and histopathology showed chronic pancreatitis in 12/12 cases (pathology not done or inadequate specimen obtained in 5). All children were non-diabetic prior to surgery. All received opioid analgesics intermittently, and in 7 cases daily opioids were required for pain control.
Table 1.
Characteristics of the 17 patients undergoing total pancreatectomy with islet autotransplantation at age 3–8 years.
| Median/n | (IQR) | |
|---|---|---|
|
| ||
| Age at TPIAT (years) | 6.8 | (5.1 – 8.3) |
| Gender, M/F | 8/9 | |
| Duration diagnosed pancreatitis (years) | 2.65 | (1.77 – 3.94) |
| Age at diagnosis of pancreatitis (years) | 3.39 | (2.50 – 4.9) |
| Etiology of disease (n) | ||
| PRSS1 | 9 | |
| PRSS1 + CFTR | 2 | |
| SPINK1 | 1 | |
| SPINK1 + CTRC | 1 | |
| Cystic Fibrosis | 1 | |
| Pancreas Divisum | 1 | |
| Idiopathic | 2 | |
| Prior ERCP/stent (n) | 11 | |
| Prior pancreatic surgery (n) | 0 | |
| Islet mass transplanted | ||
| Total islet equivalents (IEQ) | 140,049 | (108,000 – 189,400) |
| IEQ/kg body weight | 6.366 | (5,287 – 10,647) |
TPIAT= total pancreatectomy with islet autotransplant; PRSS1 = protease, serine, 1 ; SPINK1= serine protease inhibitor Kazal-type 1; CFTR= Cystic fibrosis transmembrane conductance regulator; CTRC= chymotrypsin C; ERCP = endoscopic retrograde cholangiopancreatography ; IEQ= islet equivalents
Median patient weight before surgery was 25.6 kg (IQR 21.1– 32.0, smallest 15.2 kg). On MRCP imaging, liver volume was 592 mL (IQR 551– 700). All children had liver volumes >400cc. In two children, with liver volumes <400cc, the surgery was deferred until they attained a liver volume >400cc. All children had failed medical and or endoscopic management and were not candidates for less invasive surgical drainage procedures (due to non-dilated pancreatic duct) or partial resection procedures (due to diffuse involvement of the gland).
Surgical procedure and post-operative complications
Median duration in the operating room was 8.5 hours (IQR 8.0 –10.5). Patients received a median islet mass of 140,049 IEQ (IQR 108,000 – 189,400), equivalent to 6,366 IEQ/kg (IQR 5,287 – 10,647 IEQ/kg). Median tissue volume transplanted was 5.0 cc (IQR 4.0– 8.5 cc). Patients were hospitalized for a median of 15 days (IQR 13 – 18) and in the intensive care unit (ICU) for 7 days (IQR 5– 9), with duration of ICU stay primarily dictated by duration of intravenous insulin drip therapy. Seven patients required packed red blood cell (PRBC) infusion intraoperatively, and an additional 4 received PRBC transfusion post-operatively. There were 15 complications in 7 patients: gastrojejunal tube-related complications (n=4), wound complications (n=3), bowel obstruction (n=2), intra-abdominal hemorrhage (n=2), lower gastrointestinal bleeding (n=1), clinical peritonitis (n=1), biliary leak (n=1), intra-abdominal abscess (n=1). Four patients required re-operation for bowel obstruction (n=2), intraabdominal abscess/wound dehiscence (n=1), and bile leak (n=1). All were successfully managed with no long-term sequelae observed.
Pain relief after TPIAT
The primary indication for TPIAT procedure was pain relief. All 17 patients had relief of pain, and successfully discontinued narcotic therapy after surgery. All 17 were off all opioid analgesics at 1 year after TPIAT surgery, vs 51% (n=174/340) patients age 9 and older with data available for the same interval (p<0.0001). Patients discontinued both scheduled and intermittent oral narcotics by a median of 68 days (IQR 45 – 96) post-TPIAT, and by 6 months post-operatively in all patients. One patient had additionally required hydromorphone by intrathecal pump for 2 years before surgery which was weaned off successfully after TPIAT.
Hospitalizations after TPIAT
Complete pre- and post-TPIAT hospitalization history is known for 15/17 cases (unknown for two of the early patients who are not available for contact: case 1 died related to respiratory complications from cerebral palsy and spinal rod placement, and case 4 was lost to follow up). In these 15 patients, there were a total of 187 hospital admissions from the time of diagnosis of pancreatitis until TPIAT was performed, equating to 5.0 hospitalizations per person-year of follow up. After TPIAT, these same patients have had 20 hospitalizations, equating to 0.35 hospitalizations per person-year of follow up (p<0.001 vs preTPIAT). Eight of the 20 readmissions occurred in the first 8 weeks after TPIAT (usually related to feeding tube dysfunction, constipation, or bowel obstruction concern), and only 12 occurred more than 2 months after the TPIAT, equating to 0.22 hospitalizations per patient-year follow up. Of note, at least 4 of these 12 late admissions were unrelated to TPIAT or abdominal symptoms (bradycardia, cystic fibrosis flare, metabolic disease, and rhabdomyolysis); and 1 admit was for overnight fever observation due to post-splenectomy state.
Insulin therapy and glycemic control after TPIAT
Fourteen patients (82%) had any period of insulin independence, and 11 (64%) were insulin independent at last follow up (Figure 2). By comparison, over the same time interval, only 41% (166/399) of patients >9 years of age undergoing TPIAT with at least 1 year of follow up data available had any period of insulin independence (p=0.004).
Figure 2.
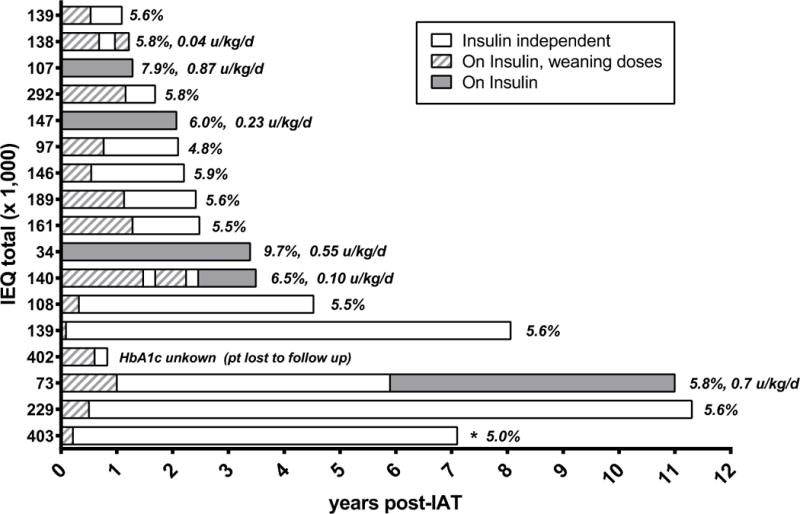
Insulin use after TPIAT, most recent hemoglobin A1c level, and insulin dose in insulin dependent patients (ordered by historical sequence of TPIAT surgery, earliest cases lowest ascending to most recent).
HbA1c results were available for 16/17 patients, with 1–8 post-TPIAT HbA1c values per patient and a median of 4 HbA1c measures (IQR 3 – 6) per patient. To account for changes in HbA1c over time, and in order to represent each patient equivalently in our cohort, we calculated the within patient average HbA1c value post-TPIAT for each child in our cohort for analysis, and report the latest HbA1c value in Figure 2. The median post-transplant TPIAT value was 5.9% (IQR 5.6– 6.3). All but 2 patients had an average post-TPIAT HbA1c ≤6.5%. In the two patients with highest HbA1c values, both above the American Diabetes Association goal of 7.5% at most recent measurement, one had the lowest islet mass transplant (34,000 IEQ total), and the other had a parent with type 1 diabetes and developed insulin antibodies post-transplant. All insulin dependent patients had partial islet graft function (C-peptide positive). Glycemic measures over the first year post-TPIAT are displayed in Table 2. One insulin independent patient has reported episodes of recurrent hypoglycemia (no severe hypoglycemic events to date) starting several years after TPIAT.
Table 2.
Glycemic parameters and islet function over the first year post-TPIAT.
| Pre-TPIAT (n=13) |
3 months (n=12) |
6 months (n=11) |
1 year (n=13) |
|
|---|---|---|---|---|
|
| ||||
| HbA1c (%) | 5.4 (5.2, 5.6) | 6.1 (5.9, 6.3) | 5.9 (5.8, 6.3) | 6.0 (5.6, 6.1) |
| Fasting Glucose (mg/dL) | 84 (82, 90) | 101 (84, 107) | 94 (90, 104) | 88 (83, 92) |
| Fasting C-peptide (ng/mL) | 0.75 (0.5, 0.95) | 0.6 (0.4, 0.8) | 0.7 (0.6, 0.9) | 0.9 (0.2, 1.1) |
| Max Stimulated Glucose (mg/dL) | 108 (89, 129) | 123 (112, 136) | 145 (130, 186) | 113 (102, 146) |
| Max Stimulated C-peptide (ng/mL) | 2.9 (2.3, 6.0) | 1.5 (0.9, 2.6) | 2.15 (1.3, 2.8) | 2.3 (0.9, 3.2) |
Metabolic assessment protocols were implemented in 2007. N completing each time point is indicated.
Growth after TPIAT
Before TPIAT, patients had a height Z-score of −0.5 (IQR −1.06– 0.43) standard deviation scores (SDS) for age; weight Z-score of 0.43 (IQR −0.58 – 1.26) SDS for age, and BMI Z-score of 0.99 (−0.08 – 1.06) SDS for age. Based on available data in this cohort, normal growth was observed over 2 years post-TPIAT, with gains in Z-score from baseline of: +0.17 (IQR −0.02 – 0.39) for height, +0.19 (IQR −0.21 – 0.43) for weight, and +0.09 (IQR −0.44 – 0.46) for BMI at 1 year (n=11); and at 2 years gains from baseline of: +0.32 (IQR 0.09 – 0.44) for height, +0.24 (IQR−0.22 – 0.41) for weight, and +0.08 (IQR −0.47 – 0.20) for BMI (n=9).
Discussion
Early-onset painful recurrent acute and chronic pancreatitis result in intractable pain and recurrent hospitalizations in afflicted children, with few medical treatment options (1). Surgical therapy may be considered in carefully selected children who have failed appropriate medical and endoscopic treatments. Total pancreatectomy with islet autotransplantation is the preferred surgical approach for most patients with small-duct genetic pancreatitis and diffuse gland involvement (18, 24–26). In our experience with very young children (age 3–8 years) undergoing TPIAT, long-term outcomes are favorable, with successful relief of pain and insulin independence in the majority. All patients in our cohort had cessation of need for opioid analgesics, and three-fourths obtained insulin independence.
Surgical intervention in this age group was implemented only after careful consideration and failure of conservative treatment and/or endoscopic stenting. Part of the concern of performing major surgery at such a young age is the risk for specific surgical complications. Gastro-biliary reconstruction confers a risk for biliary stricture (early or late) or biliary leak (19, 27); based on experience in the pediatric liver transplant population, small children may be at particular risk for biliary complications (28). Reassuringly, only one patient in our series experienced biliary leak after choledochojejunostomy. Another concern in small children is the risk of portal vein thrombosis; historically, there is a ~3% risk overall of partial or complete portal thrombosis after TPIAT amongst all patients (22). Risk of portal thrombosis has previously been shown to be increased by high islet graft tissue volume and smaller liver volume (22, 29). To minimize or prevent this risk in small children, a low tissue volume islet product is preferred, ≤0.25 mL/kg body weight—a threshold that we have previously demonstrated is associated with low risk of portal hypertension and thrombosis (22, 29, 30). However, islet isolation from pediatric pancreata is challenged by greater embedding of the islets in surrounding exocrine tissue in pancreata from younger patients (31–33). To optimize the likelihood that the portal vasculature will accept the islet graft without worrisome portal hypertension or thrombosis, in our current protocols, we evaluate liver volume by MRCP and target a liver volume of > 400cc before TPIAT is considered, based on experience in islet transplantation (29) as well as living donor liver transplantation (34). As consistent with the standard of care in islet allo- and autotransplantation (30, 35), heparin is administered with the islet infusion, and more recently, based on promising data in liver and islet transplantation (36–38), dextran sulfate has been included in our own institutional protocol along with heparin infusion. While we do not have a control group without anticoagulation for comparison, reassuringly we did not see any portal vein thrombosis with our current institutional protocols, consistent with an overall lower risk of portal thrombosis seen in children in earlier reports (22).
These young children were twice as likely to achieve insulin independence after transplant as compared to the older children and adults in our database, with more than 80% of children in this cohort coming off insulin for variable durations post-TPIAT. Although children in this age group received over 5,000 IEQ/kg transplanted on average, a yield where we historically see about two-thirds of patients reach insulin independence, the total islet mass (IEQ) was lower than average and yet insulin independence appears to persist despite growth and development. All children in our series had islet graft function after surgery, and all but two maintained a HbA1c level, on average, below 6.5%. This is in stark contrast to children in this age group with type 1 diabetes mellitus; by comparison, children <8 years of age in the U.S. Type 1 Diabetes Exchange registry have an overall mean HbA1c >8% and less than 25% of these patients have an HbA1c below the target of 7.5% (39). In the only two patients in our series with highly elevated HbA1c values (HbA1c >7% for both), one of these patients had the lowest islet mass transplanted in our series (only 34,000 IEQ) and the other had a parental history of type 1 diabetes and had de novo appearance of insulin antibodies post-TPIAT.
We do not know the mechanisms that underlie this high rate of diabetes success in young children. Autopsy studies do suggest that children under the age of 13 years, and particularly under the age of 6 years, retain capacity for beta cell expansion and growth, so there is theoretical potential that the transplanted beta cell mass is able to adapt to the patients’ needs after TPIAT in this age group in a way that does not occur in older individuals (40); furthermore, young children have relatively low insulin requirements and therefore may have a better metabolic milieu for islet engraftment. Also these patients did not have prior resection or drainage procedures that impair islet yield (19, 41), and relative islet mass (IEQ/kg body weight) was generally high. However, it is important to note that much of the data are short-term, and continued follow up will be needed to determine longevity of function of the islet graft in children undergoing TPIAT at a young age. Whether these islets can sustain insulin independence into young adulthood remains unknown. With administration of pancreatic enzymes for pancreatic exocrine insufficiency and management of diabetes mellitus, children exhibited normal growth after TPIAT, and a mild gain in height and weight Z-scores.
Most importantly, TPIAT is performed primarily for relief of debilitating pain. All of our patients reported pain relief at most recent follow up, and all discontinued any opioid analgesics after surgery. These young children may have certain favorable characteristics that increase likelihood of complete and sustained pain relief: shorter duration of disease, lack of daily opioid therapy, and fewer ERCP procedures have been associated with better response to surgical drainage procedures and TPIAT in adults (42). All patients are eating normally, and were able to resume a normal childhood. Rate of hospitalization after TPIAT was reduced by more than 15 fold, from 5.0 admissions per patient-year to 0.35 admissions per patient year, and even lower long-term, as many of the post-TPIAT admissions occur in the first 8 weeks after surgery due to early complications of surgery or enteral feeding tube dysfunction which subsequently were treated and resolved. Of note, this cohort was selected for TPIAT based on severe disease course, failing medical and endoscopic therapy, and thus the improvements seen in this age group may not be generalizable to all young children with hereditary pancreatitis; medical and endoscopic therapy remains the first line of treatment.
In conclusion, TPIAT can be successfully and safely performed in small children with excellent pain relief and good islet graft function. Although the decision to proceed to TPIAT must be carefully considered in the management of young children with chronic pancreatitis, taking into consideration the risks of major surgery, we found that the age and size of the child does not negatively affect outcomes. Younger age should not be a contraindication to referral.
Supplementary Material
Supplemental Figure: An example of chronic pancreatitis by MRCP in an 8 year old child with PRSS1-mediated hereditary pancreatitis. This 3D MRCP maximum intensity projection shows that the pancreatic duct was markedly dilated and irregular with multiple sidebranches, compatible with chronic pancreatitis.
What is known
Total pancreatectomy with islet autotransplantation (TPIAT) has emerged as a promising therapy to relieve pain in children with severe chronic pancreatitis.
Children under age 12 years have higher rates of pain relief and insulin independence compared to adolescents undergoing TPIAT
What is new
Only recently has TPIAT been used for very young children. For the first time, we report outcomes in these very young children.
In children ages 3–8 years with severe refractory pancreatitis, TPIAT provided pain relief and >80% chance of insulin independence, with low risk of long-term complications.
Acknowledgments
Funding: 5K23DK084315 (Bellin).
No honorarium, grant, or other form of payment was given to anyone to produce the manuscript.
Abbreviations
- CFTR
cystic fibrosis transmembrane regulator
- CTRC
chymotrypsin C
- ERCP
endoscopic retrograde cholangiography
- HbA1c
hemoglobin A1c level
- MRCP
magnetic resonance cholangiopancreatography
- PRSS1
protease serine 1
- SPINK1
serine protease inhibitor Kazal-type 1
- TPIAT
total pancreatectomy with islet autotransplantation
Footnotes
Conflicts of interest: None
Author Contributions:
Study design: MDB, MLF, GJB, TBD, TLP, MLF, DERS, SJS, SC
Study funding: MDB
Data collection: MDB, GF, KM, MB, SC, SJS, DERS, JJW, MM, MC
Data analysis: MDB
Authored manuscript first draft: MDB
Manuscript revisions/critical review: GF, KM, MB, JJW, MM, MC, MLF, GJB, TBD, TLP, DERS, SJS, SC
Approved final version of manuscript: all authors
References
- 1.Schwarzenberg SJ, Bellin M, Husain SZ, et al. Pediatric chronic pancreatitis is associated with genetic risk factors and substantial disease burden. J Pediatr. 2015;166(4):890–6 e1. doi: 10.1016/j.jpeds.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitcomb DC. Genetic risk factors for pancreatic disorders. Gastroenterology. 2013;144(6):1292–302. doi: 10.1053/j.gastro.2013.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morinville VD, Husain SZ, Bai H, et al. Definitions of pediatric pancreatitis and survey of present clinical practices. J Pediatr Gastroenterol Nutr. 2012;55(3):261–5. doi: 10.1097/MPG.0b013e31824f1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giefer MJ, Kozarek RA. Technical outcomes and complications of pediatric ERCP. Surgical endoscopy. 2015 doi: 10.1007/s00464-015-4105-1. [DOI] [PubMed] [Google Scholar]
- 5.Clifton MS, Pelayo JC, Cortes RA, et al. Surgical treatment of childhood recurrent pancreatitis. Journal of pediatric surgery. 2007;42(7):1203–7. doi: 10.1016/j.jpedsurg.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Iqbal CW, Moir CR, Ishitani MB. Management of chronic pancreatitis in the pediatric patient: endoscopic retrograde cholangiopancreatography vs operative therapy. J Pediatr Surg. 2009;44(1):139–43. doi: 10.1016/j.jpedsurg.2008.10.023. discussion 43. [DOI] [PubMed] [Google Scholar]
- 7.Gachago C, Draganov PV. Pain management in chronic pancreatitis. World journal of gastroenterology : WJG. 2008;14(20):3137–48. doi: 10.3748/wjg.14.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradley EL., 3rd Long-term results of pancreatojejunostomy in patients with chronic pancreatitis. Am J Surg. 1987;153(2):207–13. doi: 10.1016/0002-9610(87)90816-6. [DOI] [PubMed] [Google Scholar]
- 9.Holmberg JT, Isaksson G, Ihse I. Long term results of pancreaticojejunostomy in chronic pancreatitis. Surg Gynecol Obstet. 1985;160(4):339–46. [PubMed] [Google Scholar]
- 10.Markowitz JS, Rattner DW, Warshaw AL. Failure of symptomatic relief after pancreaticojejunal decompression for chronic pancreatitis. Strategies for salvage. Archives of surgery (Chicago, Ill: 1960) 1994;129(4):374–9. doi: 10.1001/archsurg.1994.01420280044006. discussion 9–80. [DOI] [PubMed] [Google Scholar]
- 11.O’Neil SJ, Aranha GV. Lateral pancreaticojejunostomy for chronic pancreatitis. World journal of surgery. 2003;27(11):1196–202. doi: 10.1007/s00268-003-7238-7. [DOI] [PubMed] [Google Scholar]
- 12.Cahen DL, Gouma DJ, Nio Y, et al. Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis. The New England journal of medicine. 2007;356(7):676–84. doi: 10.1056/NEJMoa060610. [DOI] [PubMed] [Google Scholar]
- 13.Sasikala M, Talukdar R, Pavan kumar P, et al. beta-Cell dysfunction in chronic pancreatitis. Dig Dis Sci. 2012;57(7):1764–72. doi: 10.1007/s10620-012-2086-7. [DOI] [PubMed] [Google Scholar]
- 14.Chinnakotla S, Bellin MD, Schwarzenberg SJ, et al. Total pancreatectomy and islet autotransplantation in children for chronic pancreatitis: indication, surgical techniques, postoperative management, and long-term outcomes. Ann Surg. 2014;260(1):56–64. doi: 10.1097/SLA.0000000000000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellin MD, Sutherland DE. Pediatric islet autotransplantation: indication, technique, and outcome. Current diabetes reports. 2010;10(5):326–31. doi: 10.1007/s11892-010-0140-4. [DOI] [PubMed] [Google Scholar]
- 16.Bottino R, Bertera S, Grupillo M, et al. Isolation of human islets for autologous islet transplantation in children and adolescents with chronic pancreatitis. Journal of transplantation. 2012;2012:642787. doi: 10.1155/2012/642787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellin MD, Freeman ML, Schwarzenberg SJ, et al. Quality of life improves for pediatric patients after total pancreatectomy and islet autotransplant for chronic pancreatitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2011;9(9):793–9. doi: 10.1016/j.cgh.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chinnakotla S, Radosevich DM, Dunn TB, et al. Long-term outcomes of total pancreatectomy and islet auto transplantation for hereditary/genetic pancreatitis. J Am Coll Surg. 2014;218(4):530–43. doi: 10.1016/j.jamcollsurg.2013.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutherland DE, Radosevich DM, Bellin MD, et al. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg. 2012;214(4):409–24. doi: 10.1016/j.jamcollsurg.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balamurugan AN, Loganathan G, Bellin MD, et al. A new enzyme mixture to increase the yield and transplant rate of autologous and allogeneic human islet products. Transplantation. 2012;93(7):693–702. doi: 10.1097/TP.0b013e318247281b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ricordi C, Lacy PE, Scharp DW. Automated islet isolation from human pancreas. Diabetes. 1989;38(Suppl 1):140–2. doi: 10.2337/diab.38.1.s140. (Journal Article): [DOI] [PubMed] [Google Scholar]
- 22.Wilhelm JJ, Bellin MD, Dunn TB, et al. Proposed thresholds for pancreatic tissue volume for safe intraportal islet autotransplantation after total pancreatectomy. Am J Transplant. 2013;13(12):3183–91. doi: 10.1111/ajt.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forlenza GP, Chinnakotla S, Schwarzenberg SJ, et al. Near-Euglycemia Can Be Achieved Safely in Pediatric Total Pancreatectomy Islet Autotransplant Recipients Using an Adapted Intravenous Insulin Infusion Protocol. Diabetes Technol Ther. 2014 doi: 10.1089/dia.2014.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson GC, Sutton JM, Salehi M, et al. Surgical outcomes after total pancreatectomy and islet cell autotransplantation in pediatric patients. Surgery. 2013;154(4):777–83. doi: 10.1016/j.surg.2013.07.003. discussion 83–4. [DOI] [PubMed] [Google Scholar]
- 25.Ziff OJ, Lawrence A, Al-Adra DP, et al. Pediatric Islet Autotransplantation after Total Pancreatectomy for Hereditary Pancreatitis with Defined Genetic Variants. CellR4. 2014;2(5):e1197. [Google Scholar]
- 26.Sutton JM, Schmulewitz N, Sussman JJ, et al. Total pancreatectomy and islet cell autotransplantation as a means of treating patients with genetically linked pancreatitis. Surgery. 2010;148(4):676–85. doi: 10.1016/j.surg.2010.07.043. discussion 85–6. [DOI] [PubMed] [Google Scholar]
- 27.Jimenez RE, Fernandez-del Castillo C, Rattner DW, et al. Outcome of pancreaticoduodenectomy with pylorus preservation or with antrectomy in the treatment of chronic pancreatitis. Ann Surg. 2000;231(3):293–300. doi: 10.1097/00000658-200003000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feier FH, da Fonseca EA, Seda-Neto J, et al. Biliary complications after pediatric liver transplantation: Risk factors, diagnosis and management. World journal of hepatology. 2015;7(18):2162–70. doi: 10.4254/wjh.v7.i18.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawahara T, Kin T, Shapiro AM. A comparison of islet autotransplantation with allotransplantation and factors elevating acute portal pressure in clinical islet transplantation. Journal of hepato-biliary-pancreatic sciences. 2012;19(3):281–8. doi: 10.1007/s00534-011-0441-2. [DOI] [PubMed] [Google Scholar]
- 30.Kawahara T, Kin T, Kashkoush S, et al. Portal vein thrombosis is a potentially preventable complication in clinical islet transplantation. Am J Transplant. 2011;11(12):2700–7. doi: 10.1111/j.1600-6143.2011.03717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ricordi C, Alejandro R, Zeng Y, et al. Human islet isolation and purification from pediatric-age donors. Transplant Proc. 1991;23(1 Pt 1):783–4. [PMC free article] [PubMed] [Google Scholar]
- 32.Balamurugan AN, Chang Y, Bertera S, et al. Suitability of human juvenile pancreatic islets for clinical use. Diabetologia. 2006;49(8):1845–54. doi: 10.1007/s00125-006-0318-0. [DOI] [PubMed] [Google Scholar]
- 33.Meier RP, Sert I, Morel P, et al. Islet of Langerhans isolation from pediatric and juvenile donor pancreases. Transpl Int. 2014;27(9):949–55. doi: 10.1111/tri.12367. [DOI] [PubMed] [Google Scholar]
- 34.D’Onofrio M, De Robertis R, Demozzi E, et al. Liver volumetry: Is imaging reliable? Personal experience and review of the literature. World journal of radiology. 2014;6(4):62–71. doi: 10.4329/wjr.v6.i4.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casey JJ, Lakey JR, Ryan EA, et al. Portal venous pressure changes after sequential clinical islet transplantation. Transplantation. 2002;74(7):913–5. doi: 10.1097/00007890-200210150-00002. [DOI] [PubMed] [Google Scholar]
- 36.Mazzaferro V, Esquivel CO, Makowka L, et al. Hepatic artery thrombosis after pediatric liver transplantation–a medical or surgical event? Transplantation. 1989;47(6):971–7. doi: 10.1097/00007890-198906000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Johansson H, Goto M, Dufrane D, et al. Low molecular weight dextran sulfate: a strong candidate drug to block IBMIR in clinical islet transplantation. Am J Transplant. 2006;6(2):305–12. doi: 10.1111/j.1600-6143.2005.01186.x. [DOI] [PubMed] [Google Scholar]
- 38.Spirig R, Gajanayake T, Korsgren O, et al. Low molecular weight dextran sulfate as complement inhibitor and cytoprotectant in solid organ and islet transplantation. Molecular immunology. 2008;45(16):4084–94. doi: 10.1016/j.molimm.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 39.Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes care. 2015;38(6):971–8. doi: 10.2337/dc15-0078. [DOI] [PubMed] [Google Scholar]
- 40.Meier JJ, Butler AE, Saisho Y, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 2008;57(6):1584–94. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morgan KA, Theruvath T, Owczarski S, et al. Total pancreatectomy with islet autotransplantation for chronic pancreatitis: do patients with prior pancreatic surgery have different outcomes? Am Surg. 2012;78(8):893–6. [PubMed] [Google Scholar]
- 42.Ahmed Ali U, Nieuwenhuijs VB, van Eijck CH, et al. Clinical outcome in relation to timing of surgery in chronic pancreatitis: a nomogram to predict pain relief. Arch Surg. 2012;147(10):925–32. doi: 10.1001/archsurg.2012.1094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure: An example of chronic pancreatitis by MRCP in an 8 year old child with PRSS1-mediated hereditary pancreatitis. This 3D MRCP maximum intensity projection shows that the pancreatic duct was markedly dilated and irregular with multiple sidebranches, compatible with chronic pancreatitis.


