Abstract
Objective
Vasomotor symptoms (VMS, i.e. hot flashes or night sweats) are reported by many, but not all, women. The extent to which VMS are genetically determined is unknown. We evaluated the relationship of genetic variation and VMS.
Methods
In this observational study, we accessed data from three genome-wide association studies (GWAS)(SHARe, WHIMS+, GARNET studies, total n= 17,695) of European American (EA), African American (AA), and Hispanic American (HA) postmenopausal women aged 50-79 years at baseline in the Women’s Health Initiative Study. We examined genetic variation in relation to VMS (yes/no) in each study and using trans-ethnic inverse variance fixed-effects meta-analysis.11,078,977 single-nucleotide polymorphisms (SNPs) met the quality criteria.
Results
After adjustment for covariates and population structure 3 SNPs (on chromosomes 3 and 11) were associated with VMS at the genome-wide threshold of 5 × 10-8 in the AA SHARE GWAS but were not associated in the other cohorts. However, in the meta-analysis, 14 SNPs, all located on chromosome 4 in the tachykinin receptor 3 (TACR3) locus, had p-values of <5×10-8. These SNPs’ effect sizes were similar across studies/participants’ ancestry (OR~1.5).
Conclusions
Genetic variation in TACR3 may contribute to the risk of VMS. To our knowledge, this is the first GWAS to examine SNPs associated with VMS. These results support the biological hypothesis of a role for TACR3 in VMS, which was previously hypothesized from animal and human studies. Further study of these variants may lead to new insights into the biological pathways involved in VMS, which are poorly understood.
Keywords: menopause, vasomotor symptoms, hot flashes, genome-wide association study
Introduction
Menopausal vasomotor symptoms (VMS), defined as hot flashes or night sweats, are reported by the majority of U.S. women regardless of ethnic ancesty. More than 70% of late perimenopausal women, and more than 60% of postmenopausal women, experience VMS 1-3. Moreover, VMS may last many years, lasting more than 7 years for more than half of women, and ten percent of women report having VMS 12 years after the final menstrual period 4, 5. Ethnicity (African American vs. White), greater body mass index (BMI), lower education level, current smoking, anxiety, and depressive symptoms are associated with an increased risk for frequent VMS1.
The extent to which VMS are genetically determined is unclear. Genome-wide association studies (GWAS), which are studies of common genetic variation across the entire human genome, identify genetic associations with observable traits 6. In GWAS, each person’s complete chromosomal deoxyribonucleic acid is scanned by machines that quickly survey each person’s genome for strategically selected markers of genetic variation called single-nucleotide polymorphisms (SNPs) 7. GWAS can provide valuable clues about genomic function and pathophysiologic mechanisms6.
GWAS regarding VMS have never been performed among U.S. women. Candidate gene studies suggest that genetic variants may be associated with VMS, including single-nucleotide polymorphisms (SNPs) in estrogen receptor genes and the estrogen metabolism pathway (cytochrome P450 [CYP] CYP1A1, CYP1A2, CYP1B1, and CYP19A1, catechol-O-methyltransferase [COMT], 17-beta-hydroxysteroid dehydrogenase [17HSD], and sulfotransferase A1 [SULT1A1] genes)8-16. Other candidate gene studies have suggested associations of drug metabolism-related SNPs17, 18 and serotonin transporter SNPs 19 with VMS. Another candidate gene study suggested associations between genes that control angiogenesis (endothelial nitric oxide synthase [eNOS] and hypoxia inducible factor-1 alpha [HIF1α]) and VMS20. Because the pathophysiology of VMS is not well understood, and because GWAS does not require a priori hypotheses regarding associations, GWAS is an invaluable tool to help fill knowledge gaps regarding the pathophysiology of VMS.
The goal of this study was to evaluate the relationship of genetic variation (assessed by GWAS) and VMSin the Women’s Health Initiative Study, which collected GWAS data and information regarding VMS in several independent cohorts of postmenopausal women21. We hypothesized that we would find multiple genetic variants to be associated with VMS. Elucidation of these variants may lead to new insights into the biological pathways involved in VMS, which are poorly understood. By using a meta-analytic approach to combine GWAS that span multiple ethnic groups (a trans-ethnic meta-analysis)22, we found that genetic variation in the tachykinin receptor 3 gene (TACR3), which encodes the receptor for neurokinin B (NKB), was associated with VMS. NKB mRNA-expressing neurons are located predominantly in the infundibular nucleus and the anterior hypothalamic area 23. In humans, NKB neurons co-localize with the gonadotropin-releasing hormone tract in the infundibular stalk, and the NKB pathway is implicated in pubertal development and hypogonadatropic hypogonadism 23-25.
Methods
The Women’s Health Initiative Study and Women’s Health Initiative Genetic Studies
The Women’s Health Initiative Observational Study (WHI-OS) and Clinical Trials (WHI-CT) were carried out at 40 U.S. clinical centers between 1993 and 1998 21, 26. Participants were postmenopausal women aged 50-79 years at baseline, free from serious cardiac, pulmonary, renal, and hepatic conditions, with at least three years’ life expectancy. The WHI-CTs performed randomized controlled trial evaluation of 3 distinct interventions: a low-fat eating pattern, menopausal hormone therapy, and calcium plus vitamin D supplementation 21. The WHI-OS was designed to provide information about disease risk factors, including cancer, cardiovascular disease, and fractures 21. The combined studies enrolled 161,808 participants (93,676 in the WHI-OS and 68,132 in the WHI-CTs). The WHI Hormone Therapy trials did not exclude women on the basis of having severe VMS, but they excluded women who did not wish to forgo the use of personal menopausal hormone therapy 21.
There were several independent GWAS studies conducted within the WHI study: 1) the SNP Health Association Resource cohort (SHARe) (n = 12,157 African American [SHARe-AA] and Hispanic women [SHARe-HA]), 2) the Genome-wide Association Studies of Treatment Response in Randomized Clinical Trials (GARNET) (n = 4,416 women of non-Hispanic, European ancestry), and 3) the WHI Memory Study cohort (WHIMS+) (n = 5,840 women of non-Hispanic, European ancestry) 27. We used all available GWAS data from all 3 studies, the characteristics of which are summarized in Table 1.
Table 1.
Sample sizes and racial/ethnic composition of the WHI GWAS studiesa
| Study Name | Study population (synopsis) |
GWAS method | Link to further information on dbgapb |
|---|---|---|---|
|
| |||
| SHARe AA & H GWAS SNP Health Association Resource | African American (AA) and Hispanic (H) participants in the WHI CT or OS | Affymetrix 6.0 | http://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000386.v6.p3 |
| Racial/ethnic distribution: 70% AA, 30% H | |||
|
| |||
| GARNET HT GWAS: Genomics and Randomized Trials Network | Cases and controls from the WHI HT trials | Illumina HumanOmni1-Quad v1-0 B | http://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000315.v6.p3 |
| Cases: all of the WHI participants with confirmed cases of CHD, stroke, VTE, or incident diabetes, or more than one of the case conditions in the WHI HT trials. | |||
| Controls: free of those conditions by the end of the WHI HT trials. | |||
| Racial/ethnic distribution: white (87%), black (5%), Hispanic (3%), Asian/Pacific Islander (1.8%), American Indian (0.7%), unknown (1.9%) | |||
|
| |||
| WHIMS + EA GWAS | WHI HT Trial participants of non- Hispanic European ancestry from 3 subgroups: | Illumina HumanOmniExpress Exome-8v1_B | http://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000675.v2.p3 |
| WHIMS = WHIMemory Study cohort | 1) WHI Memory Study (WHIMS) participants who were not in GARNET | ||
| 2) Women at least 65 years old at enrollment who were neither in WHIMS nor GARNET | |||
| 3) Women younger than age 65 at enrollment who were neither in WHIMS nor GARNET | |||
| Racial/ethnic distribution: non-Hispanic European ancestry | |||
All participants were postmenopausal women. Further information regarding the Genome Wide Association Studies (GWAS) within the Women’s Health Initiative (WHI) Clinical Trials and Observational Studies is available at http://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000746.v1.p3 and at https://www.whi.org/researchers/data/SitePages/GWAS%20Data.aspx. QC denotes quality control, CHD denotes coronary heart disease (myocardial infarction or coronary death), VTE denotes venous thromboembolism, and HT denotes menopausal hormone therapy.
dbGAP denotes the Database of Genotype and Phenotype created by the National Center for Biotechnology Information (NCBI).
Genotyping was performed using baseline blood samples collected at the time of enrollment. Of the 22,413 total women for whom genotyping data were available in all 3 GWAS studies combined, data from 21,753 passed quality control criteria, including minimum genotyping efficiencies and no near relatives; see Supplementary Information for details. Of these women, information regarding baseline self-reported VMS was available for 20,482 women. Of these 20,482 women, information regarding primary analysis covariates was missing for 2,787 women. Therefore, our total analytic sample consisted of 17,695 participants from 3 ethnic groups(GARNET non-Hispanic European n = 3,282, SHARe African American n = 6,732, SHARe Hispanic n = 2,778, WHIMS+non-Hispanic European n = 4,903) (Figure 1).
Figure 1.
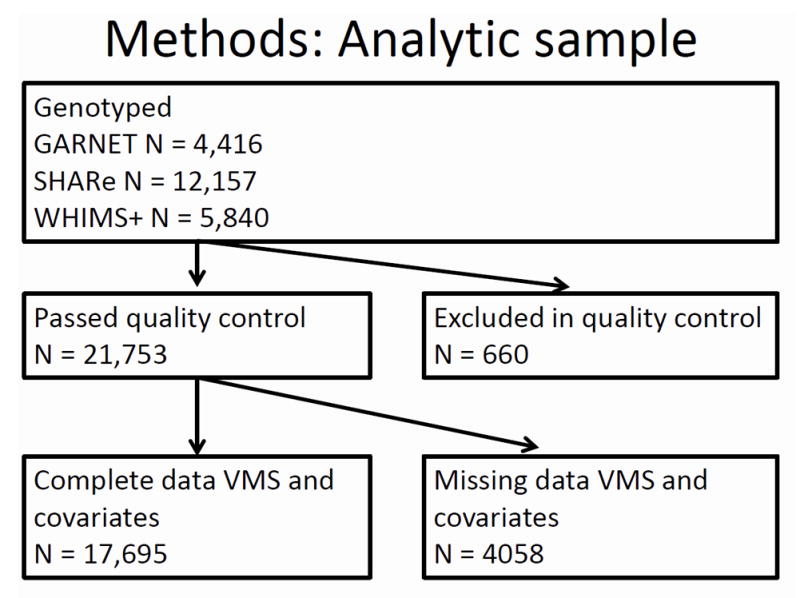
Flow diagram of analytic cohort
Participants were asked to complete baseline self-assessment questionnaires. Weight and height were measured at baseline visit with standardized protocols. BMI was calculated as body weight in kilograms (kg) divided by the square of height in meters.
Each institution obtained human subjects committee approval. Each participant provided written informed consent.
Outcome: Vasomotor symptoms (VMS)
On baseline questionnaires, participants were asked whether they had ever experienced VMS (hot flashes and/or night sweats). Hot flashes and night sweats were assessed as individual symptoms on the symptom checklist3, 28.
Primary exposure: Genotyping
For SHARe, genome-wide SNP genotyping was performed using the Affymetrix Genome-wide Human SNP Array 6.0 (Affymetrix®, Inc., Santa Clara, CA) 29-31. An ND-8000 spectrophotometer was used to quantitate genomic DNA. DNA quality was assessed using gel electrophoresis. Affymetrix ® Genotypic Console software was used to process data for genotype calling by the Birdseed calling algorithm version 2.0 (Affymetrix ®, Inc., Santa Clara, CA) 29. For GARNET, genotyping was performed with the Illumina Human Omni Quad 1.0M chip (Illumina ®, Inc., San Diego, CA)31. WHIMS+ used the Illumina Omni Express Array.
All three sub-studies were imputed to the same 1000 Genomes reference panel. Prior to imputation, we excluded data from samples with low genotyping efficiency (sample call rates <95%) for SHARe, <98% for GARNET, and <97% for WHIMS+), SNP assays with locus call rates <90% for SHARe and <98% for GARNET and WHIMS+, SNPs that deviated from Hardy-Weinberg equilibrium (HWE, p-value threshold <1 X 10-6 for SHARe and <1 X 10-4 for GARNET and WHIMS+). SNP assays with a minor allele frequency <0.01 were removed from SHARe and WHIMS+. Imputation was carried out with MINIMAC-OMP (version 1, Ann Arbor, MI32, 33) for WHIMS+ and GARNET, and MACH for SHARe (version 1.0, Ann Arbor, MI, 34, 35). All SNPs in the final analytic dataset were imputed, including those that had been directly genotyped. Any SNP whose post-imputation minor allele frequency was less than 0.001 when averaged across the studies, or that had an imputation quality R-squared measure of <0.01, was removed. Data from genetically related individuals were excluded. Allelic R2, the squared correlation between the imputed allele dosage with the highest posterior probability and true allele dosage, is a measure of imputation accuracy 36. A SNP was required to meet the minimum thresholds in all 3 component studies to be included in the meta-analysis. 19,335,715 SNPs met inclusion criteria in at least one of the studies; 11,078,977 SNPs were available for analysis across all of the studies. A detailed description of the imputation and harmonization effort of the WHI GWAS data is available 37.
Other covariates
Baseline self-report questionnaires were used to gather information regarding age, race/ethnicity, education, income, cigarette smoking, alcohol intake, physical activity38-40, years since menopause, and oophorectomy. Clinic staff examined the labels of drug containers to collect information regarding medication use.
Statistical Analysis
In each separate study, and subsequently in meta-analysis of the all studies, we analyzed associations between genetic variants and VMS using additive models. In the primary analyses, ever experiencing VMS was categorized as a binary (yes vs. no) variable. Principal components were calculated with R using a subset of 5,665 SNPs and all non-duplicate samples across WHIGWAS studies. We used the first 10 principal components as covariates in each of these analyses to adjust for ethnic differences between participants that remained after separating the samples based on self-reported ethnicity 41.
We included the following covariates: bilateral oophorectomy, age at baseline, smoking (never/past/current), alcohol intake (drinks/d, continuous), physical activity (total METs/week), population structure (principal components 1 through 10), BMI (continuous), education (0-8 years, some high school, high school diploma, some school after high school, college degree or higher), income (total annual household income (categorical, <$20,000, $20,000-$49,999, >$50,000), and menopausal estrogen therapy use (ever use, yes/no).
In a secondary analyses, we repeated the analyses in the subset of women who reporting never having used menopausal hormone therapy (HT), because HT could mask VMS.
For the meta-analysis, we used R version 3.2.2 42. The effect size for each SNP from each of the sample data sets was estimated using inverse variance fixed-effects meta-analysis using rmeta package for R version 2.16 (Vienna, Austria)42, 43.
As part of our quality control, we plotted the quantile-quantile plot (q-q plot) of the test statistics as compared with their expected values under the null to determine whether our adjustments for study and ethnic differences were effective44, 45. As we expected a small number of significant results (10-20), we removed the 14 most significant results before producing the q-q plot.
Results
At baseline, the mean age of participants was 63.8 years, mean BMI was 29.7 kg/m2, 9.6% were current smokers, and 39.3% of participants reported current use of menopausal hormone therapy (Table 2). Thirty-eight percent of participants were African American, 16%were Hispanic, and 46% were non-Hispanic European ancestry.
Table 2.
Baseline characteristics of participants included in the analytic sample
| SHARe AA | SHARe HA | GARNET EA | WHIMS+ EA | Total | P-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | ||
| Any vasomotor symptoms (e.g. hot flashes, night sweats) | <.001 | ||||||||||
| No | 1527 | 22.7 | 902 | 32.5 | 1144 | 34.9 | 1846 | 37.7 | 5419 | 30.6 | |
| Yes | 5205 | 77.3 | 1876 | 67.5 | 2138 | 65.1 | 3057 | 62.3 | 12276 | 69.4 | |
|
| |||||||||||
| Smoking status | <.001 | ||||||||||
| Never | 3217 | 47.8 | 1741 | 62.7 | 1625 | 49.5 | 2520 | 51.4 | 9103 | 51.4 | |
| Past | 2731 | 40.6 | 848 | 30.5 | 1276 | 38.9 | 2038 | 41.6 | 6893 | 39.0 | |
| Current | 784 | 11.6 | 189 | 6.8 | 381 | 11.6 | 345 | 7.0 | 1699 | 9.6 | |
|
| |||||||||||
| Alcohol intake daily | <.001 | ||||||||||
| nondrinker | 4172 | 62.0 | 1435 | 51.7 | 1558 | 47.5 | 2009 | 41.0 | 9174 | 51.8 | |
| <= 1 drink | 2257 | 33.5 | 1190 | 42.8 | 1323 | 40.3 | 2207 | 45.0 | 6977 | 39.4 | |
| > 1 drink | 303 | 4.5 | 153 | 5.5 | 401 | 12.2 | 687 | 14.0 | 1544 | 8.7 | |
|
| |||||||||||
| Education | <.001 | ||||||||||
| 0-8 years | 123 | 1.8 | 282 | 10.2 | 34 | 1.0 | 38 | 0.8 | 477 | 2.7 | |
| Some high school | 468 | 7.0 | 219 | 7.9 | 150 | 4.6 | 174 | 3.5 | 1011 | 5.7 | |
| High school diploma/GED | 870 | 12.9 | 485 | 17.5 | 758 | 23.1 | 1051 | 21.4 | 3164 | 17.9 | |
| School after high school | 2671 | 39.7 | 1102 | 39.7 | 1375 | 41.9 | 2004 | 40.9 | 7152 | 40.4 | |
| College degree or higher | 2600 | 38.6 | 690 | 24.8 | 965 | 29.4 | 1636 | 33.4 | 5891 | 33.3 | |
|
| |||||||||||
| Income, annual household | <.001 | ||||||||||
| <$20,000 | 1810 | 26.9 | 912 | 32.8 | 824 | 25.1 | 1006 | 20.5 | 4552 | 25.7 | |
| $20,000-$49,999 | 2909 | 43.2 | 1141 | 41.1 | 1649 | 50.2 | 2585 | 52.7 | 8284 | 46.8 | |
| >$50,000 | 2013 | 29.9 | 725 | 26.1 | 809 | 24.6 | 1312 | 26.8 | 4859 | 27.5 | |
|
| |||||||||||
| Bilateral oophorectomy | <.001 | ||||||||||
| No | 5133 | 76.2 | 2280 | 82.1 | 2583 | 78.7 | 4271 | 87.1 | 14267 | 80.6 | |
| Yes | 1599 | 23.8 | 498 | 17.9 | 699 | 21.3 | 632 | 12.9 | 3428 | 19.4 | |
|
| |||||||||||
| Menopausal hormone therapy use ever | |||||||||||
| No | 3952 | 58.7 | 1344 | 48.4 | 2090 | 63.7 | 3348 | 68.3 | 10734 | 60.7 | |
| Yes | 2780 | 41.3 | 1434 | 51.6 | 1192 | 36.3 | 1555 | 31.7 | 6961 | 39.3 | <.001 |
|
| |||||||||||
| Age (Mean, SD) | 61.3 | 6.9 | 60.2 | 6.6 | 65.7 | 6.9 | 68.2 | 5.8 | 17695 | 63.8, 7.3 | <.001 |
|
| |||||||||||
| Body mass index, kg/m2 Mean, SD) | 31.0 | 6.6 | 28.8 | 5.8 | 29.7 | 6.2 | 28.4 | 5.7 | 17695 | 29.7, 6.3 | <.001 |
|
| |||||||||||
| Physical Activity (Mean, SD)a | 9.7 | 12.7 | 10.8 | 13.5 | 10.2 | 12.7 | 11.7 | 13.2 | 17695 | 10.5, 13.0 | <.001 |
Total energy expended from recreational physical activity (MET-hours/week)
At baseline, 69% of participants reported ever experiencing VMS. Sixty-three percent of participants of non-Hispanic European ancestry experienced VMS, compared with 77% of African American participants (p-value <0.001 vs. participants of non-Hispanic European ancestry) and 68% of Hispanic participants (p-value <0.001 vs. participants of non-Hispanic European ancestry).
After removal of the 14 most significant results, quantile-quantile plot of the test statistics as compared with the expected distribution suggests that the likelihood of substantial hidden population substructure or differential genotype calling between cases and controls was unlikely (λ = 0.9987071)(Supplemental Fig. 1).
The Manhattan signal plot is a genome-wide plot of –log10 of the P-value for the SNP-phenotype association versus chromosomal position 46. That is, the association statistics are shown as –log10 of the P value; a P value of 0.01 would be plotted as “2” on the y-axis. The Manhattan signal plots show the statistical significance of SNP-VMS associations vs. chromosomal position for all genotyped SNPs for each of the 4 separate GWAS (Fig. 2a GARNET, Fig. 2b SHARe-AA, Fig. 2c SHARe-HA, Fig. 2d WHIMS+). In the individual studies, three SNPs reached the p-value threshold of 5×10-8, all of them in the SHARe-AA Study: rs75699757 and rs11518608 on chromosome 11, and rs148680409 on chromosome 3 (Fig. 2b). The odds ratios[ORs] for VMS were statistically significantly higher for rs75699757 (OR 1.98) and for rs11518608 (OR 1.07) in SHARe, indicating that the minor allele was positively associated with risk of VMS; in contrast, the minor alleles of these two SNPs were inversely associated with VMS in the SHARe-HA and WHIMS+ studies (Table 3). The opposite directions of the ORs across the individual studies accounts for the lack of statistically significant association of these two SNPs with VMS in the meta-analysis. Although rs148680409 passed the initial quality control and was associated with a 7.47-fold higher odds of VMS in the SHARe-AA study, this SNP did not pass the quality control criteria for the meta-analysis. SNPs on chromosome 4 were not associated with VMS in the individual studies, even in SHARe, which had the largest sample size.
Figure 2.
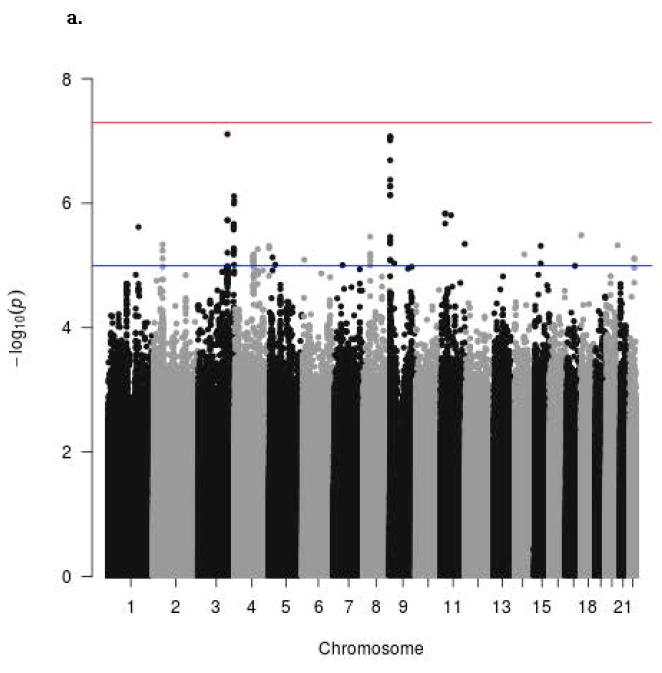
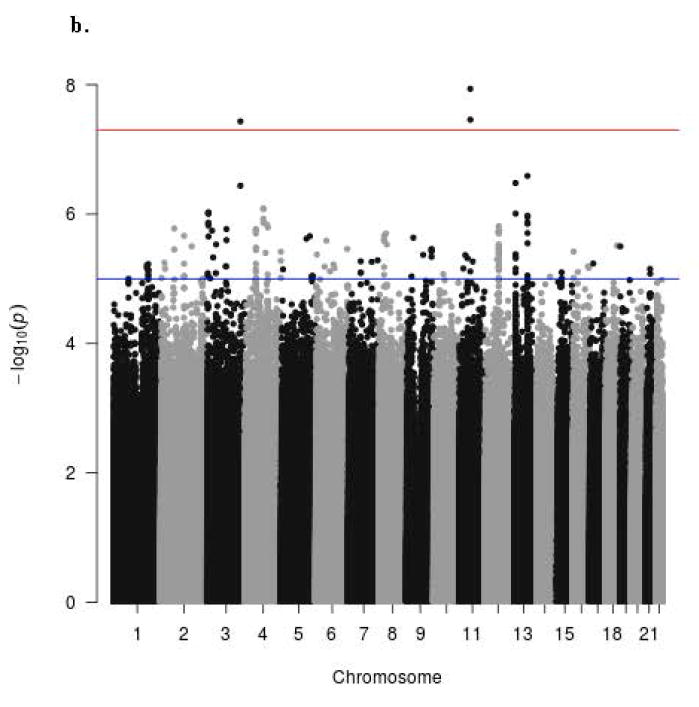
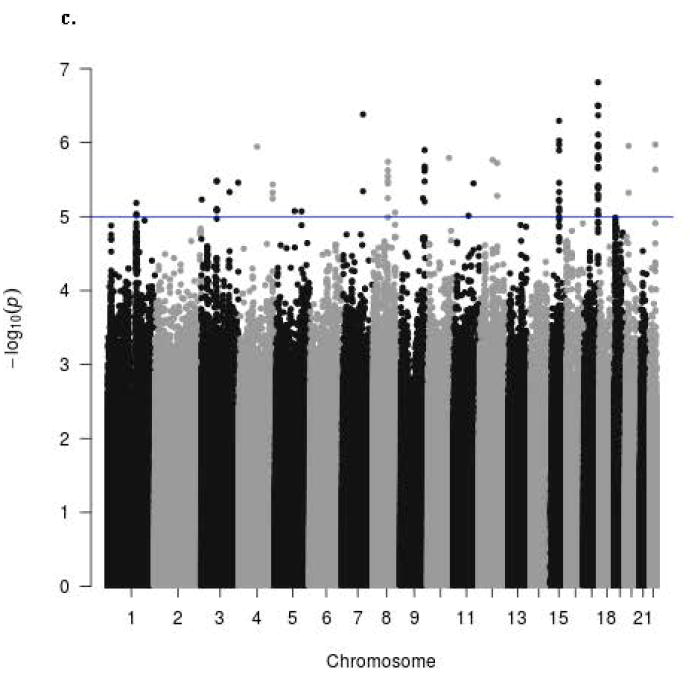
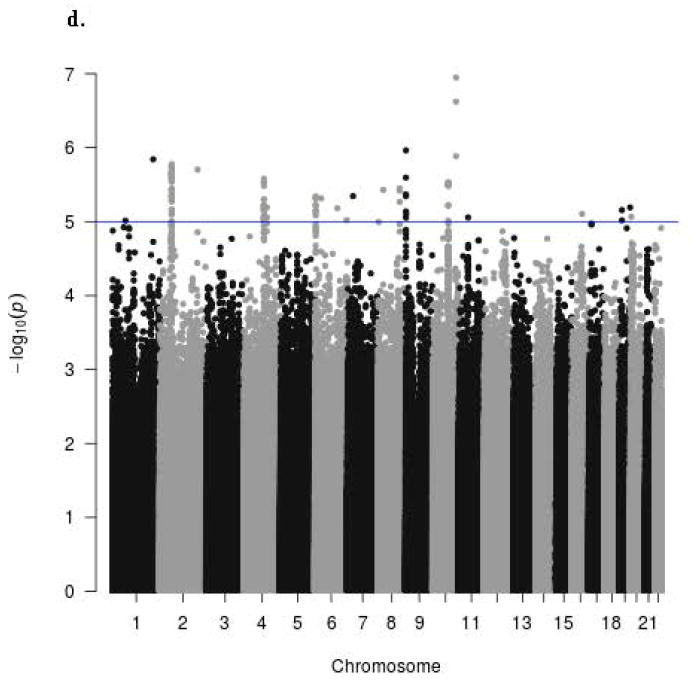
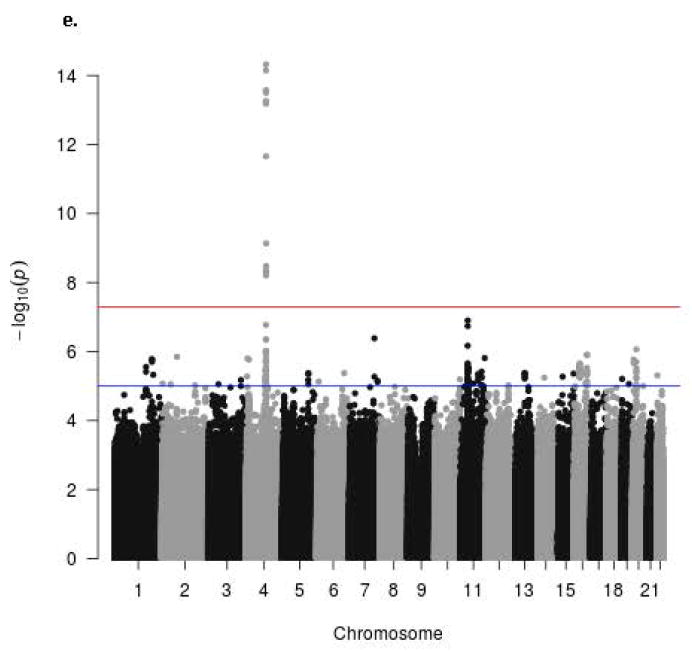
Manhattan signal plot of meta-analysis of associations of all genotyped single-nucleotide polymorphisms (SNPs) with vasomotor symptoms. Blue line denotes p-value below 5 X 10-5; red line denotes p-value below 5 X 10-8.
a. Manhattan plot for participants of GARNET Study
b. Manhattan plot for participants of SHARE-AA Study
c. Manhattan plot for participants of SHARE-HA Study
d. Manhattan plot for participants of WHIMS+ Study
e. Manhattan plot of meta-analysis showing statistical significance of all genotyped single-nucleotide polymorphisms (SNPs)
Table 3.
Genome-wide association study (GWAS) results by study sample and combined in meta-analysis: Vasomotor symptoms ever vs. nevera
| GARNET Study | SHARe - AA Study | SHARe - HA Study | WHIMS+ Study | Meta-analysis p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Refsnp IDb | CHR:POSc | OR (SE) | p-value | OR (SE) | p-value | OR (SE) | p-value | OR (SE) | p-value | |
| rs74827081 | 4:104556732 | 1.65 (0.18) | 7.88e-6 | 1.83(0. 34) | 1.25e-3 | 1.64 (0.24) | 7.46e-4 | 1.54 (0.15) | 6.41e-6 | 4.77e-15 |
| rs74589515 | 4:104584258 | 1.60 (0.18) | 2.71e-5 | 1.80 (0.32) | 1.11e-3 | 1.61 (0.23) | 9.62e-4 | 1.57 (0.15) | 2.92e-6 | 7.11e-15 |
| rs79246187 | 4:104580809 | 1.61 (0.18) | 2.1e-5 | 1.51 (0.23) | 7.46e-3 | 1.60 (0.23) | 1.02e-3 | 1.57 (0.15) | 2.63e-6 | 2.64e-14 |
| rs112390256 | 4:104575473 | 1.64 (0.18) | 9.46e-6 | 1.51 (0.24) | 8.92e-3 | 1.61 (0.23) | 9.87e-4 | 1.54 (0.15) | 4.97e-6 | 2.81e-14 |
| rs75544266 | 4:104584997 | 1.60 (0.18) | 2.84e-5 | 1.51 (0.23) | 6.55e-3 | 1.60 (0.23) | 9.42e-4 | 1.57 (0.15) | 2.89e-6 | 3.15e-14 |
| rs78154848 | 4:104562840 | 1.65 (0.18) | 8.16e-6 | 1.46 (0.24) | 1.94e-2 | 1.62 (0.24) | 8.61e-4 | 1.54 (0.15) | 6.05e-6 | 5.65e-14 |
| rs76643670 | 4:104562842 | 1.65 (0.18) | 8.16e-6 | 1.46 (0.24) | 1.94e-2 | 1.62 (0.24) | 8.59e-4 | 1.54 (0.15) | 6.05e-6 | 5.65e-14 |
| rs78289784 | 4:104580155 | 1.61 (0.18) | 2.12e-5 | 1.46 (0.23) | 1.51e-2 | 1.60 (0.23) | 9.92e-4 | 1.56 (0.15) | 3.28e-6 | 6.55e-14 |
| rs77322567 | 4:104569676 | 1.64 (0.18) | 8.62e-6 | 1.23 (0.13) | 5.12e-2 | 1.55 (0.22) | 2.24e-3 | 1.53 (0.15) | 6.52e-6 | 2.17e-12 |
| rs78141901 | 4:104593977 | 1.42 (0.16) | 2.67e-3 | 1.80 (0.37) | 3.88e-3 | 1.35 (0.23) | 7.62e-2 | 1.55 (0.16) | 1.31e-5 | 7.27e-10 |
| rs78844131 | 4:104600029 | 1.41 (0.16) | 2.85e-3 | 1.49 (0.26) | 2.08e-2 | 1.30 (0.22) | 1.17e-1 | 1.56 (0.16) | 1.14e-5 | 3.34e-09 |
| rs79852843 | 4:104628587 | 1.44 (0.17) | 1.68e-3 | 1.49 (0.29) | 4.45e-2 | 1.22 (0.20) | 2.22e-1 | 1.58 (0.16) | 5.08e-6 | 4.38e-09 |
| rs80328778 | 4:104612447 | 1.42 (0.16) | 2.15e-3 | 1.46 (0.27) | 3.67e-2 | 1.24 (0.21) | 1.95e-1 | 1.57 (0.16) | 7.06e-6 | 4.97e-09 |
| rs112623956 | 4:104623714 | 1.42 (0.16) | 2.33e-3 | 1.49 (0.27) | 2.83e-2 | 1.20 (0.20) | 2.67e-1 | 1.57 (0.16) | 6.19e-6 | 6.19e-09 |
| rs75699757 | 11:56818268 | 1.08 (0.08) | 3.01e-01 | 1.98 (0.24) | 1.16e-08 | 0.84 (0.10) | 1.16e-01 | 0.86 (0.05) | 1.51e-02 | 7.07e-01 |
| rs11518608 | 11:56767184 | 1.07 (0.08) | 3.67e-01 | 1.91 (0.22) | 3.47e-08 | 0.87 (0.10) | 2.13e-01 | 0.91 (0.06) | 1.09e-01 | 3.80e-01 |
| rs148680409 | 3:173330544 | Missing | Missing | 7.47 (2.73) | 3.69e-08 | 2.90 (3.33) | 3.54e-01 | Missing | Missing | Missing |
Table displays the results that were statistically significant (p-value threshold <5x10-8) in the meta-analysis of data from all 3 GWAS studies and/or in the individual studies. Odds ratio (OR) is expressed as allelic OR (standard error). Reference group is “never had vasomotor symptoms”. Adjusted for bilateral oophorectomy, age, smoking, alcohol intake, physical activity, population structure, body mass index, education, income, and menopausal estrogen therapy use. GARNET Study denotes the Genome-wide Association Studies of Treatment Response in Randomized Clinical Trials cohort of women of non-Hispanic European ancestry. SHARe-AA Study denotes the SNP Health Association Resource cohort African American women. SHARe-HA Study denotes the SNP Health Association Resource cohort Hispanic American women. WHIMS+ Study denotes the Women’s Health Initiative Memory Study cohort of women of non-Hispanic European ancestry.
Refsnp identification numbers (IDs) were obtained from the SNP locations for Homo sapiens (dbSNP Build 144) Bioconductor package.
CHR:POS denotes chromosome assignment and position of SNP according to Build 37.
In the Manhattan signal plot for the meta-analysis (Figure 2e), only the locus on chromosome 4 exceeds genome-wide significance. After adjustment for bilateral oophorectomy, age, smoking, alcohol intake, physical activity, population structure, BMI, education, income, and menopausal hormone therapy use, 14 SNPs were associated with increased odds of VMS at a p-value threshold <5×10-8 (Table 3). The regional Manhattan plot provides a detailed view of the SNPs on chromosome 4 that were significantly associated with VMS (Supplemental Fig. 2). These 14 SNPs are located within the same region of chromosome 4, the gene for tachykinin receptor 3. Per allele, the SNPs were associated with 1.20 to 1.83-fold higher odds of VMS.
The magnitudes of associations were consistent in each individual study. Although the GARNET substudy was enriched with women who had cardiovascular disease and/or diabetes, the magnitudes of associations among the GARNET substudy participants were similar to those of the other substudies.
The linkage disequilibrium (LD) plot (Figure 3) shows the strength of the associations, the LDpattern (r2 estimates), and the position of the associated SNPs relative to the most strongly associated SNP (rs74827981, represented by a purple circle) and the genes in the region(LocusZoom,47). The SNPs that we found to be associated with vasomotor symptoms are clustered tightly within the region of the TACR3 gene, and are not in LD with other SNPs found in other genes within the 3Mb region.
Figure 3.
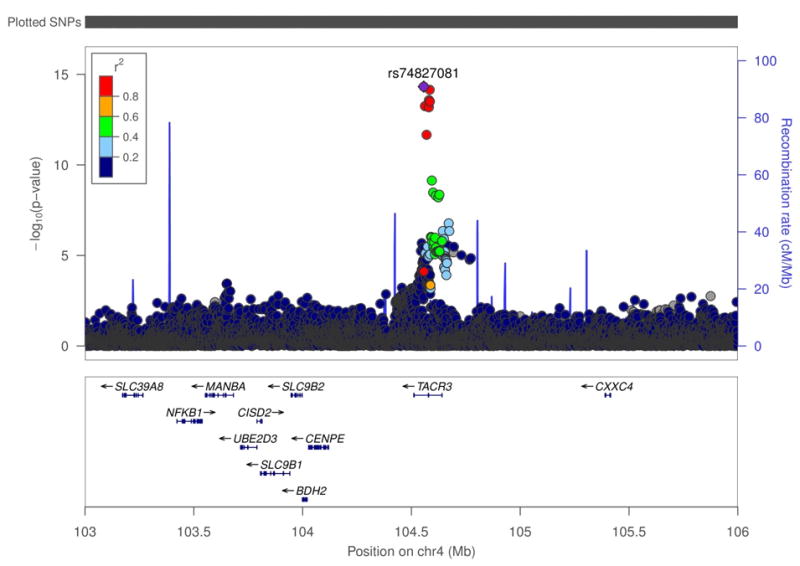
Linkage disequilibrium plot showing the position of the SNPs and their r2 values relative to the mostly strongly associated SNP (rs74827981) and genes in the region
In secondary analyses designated a priori, we repeated the analyses among participants who had never used HT. Among the HT never-users, the strongest signals and corresponding odds ratios were similar to those of the main analysis (Supplemental Table 3).
To facilitate comparison of our results with previous and future studies, we provide the rs numbers and allele frequencies corresponding to the UCSC genome browser48 chromosomal position of each of the 14 SNPs that met the p-value threshold <5×10-8 (Supplemental Table 1). Supplemental Table 2 displays the observed allele frequencies for the 14 SNPs that were associated with higher odds of VMS.
Discussion
To our knowledge, this is the first published GWAS study to focus on genetic variation in relation to VMS. In this trans-ethnicmeta-analysis of several studies, we identified several SNPs that were associated with experiencing hot flashes. The SNPs that were significantly associated with hot flashes at P < 5 X 10-8 were all located on chromosome 4 in the intronic regions of the tachykinin receptor 3 gene (TACR3). TACR3 encodes the NK3R receptor (NK3R). Neurokinin B (NKB), a member of the tachykinin family of peptides, preferentially binds to NK3R 23. The meta-analysis combined studies of women with different ethnicities. Because regions of high linkage disequilibrium (haplotype blocks) will vary by ethnicity, this trans-ethnic approach to meta-analysis can increase statistical power and narrow the region that should be considered for fine-mapping 22. The effect sizes (ORs) were very similar across racial/ethnic groups, suggesting a relatively ancient origin of the mutation. Statistical power was greatest in European Americans, which can be at least partially accounted for by the differences in minor allele frequencies among the racial/ethnic groups.
There are no previous GWAS studies of hot flashes or hot flushes posted in the GWAS Catalog (https://www.ebi.ac.uk/gwas/), although age at menarche has been associated with upstream gene variants of TACR3 in a prior GWAS 49. None of the SNPs that were associated with VMS in the current study produced any search results in the GWAS catalog, highlighting that our research question is novel.
The associations that we observed between genetic variation in NKB pathway genes and hot flashes are biologically plausible. The strongest evidence comes from a recent randomized double-blind placebo-controlled cross-over study of healthy women demonstrating that the infusion of NKB induces hot flashes 50. Other lines of evidence also implicate a role for NKB in VMS. First, colocalization studies support physiologic interaction between the NKB pathway and gonadotropin pathways. For example, NKB and estrogen receptor alpha messenger RNA are co-expressed in the human infundibular nucleus23 and the descending gonadotropin-releasing hormone tract that courses through the infundibular stalk to the neurohypophysis is exposed to dense NKB fiber plexuses 51. In rats, monkeys, and sheep, NKB fibers project from the arcuate nucleus (the area corresponding to the human infundibular nucleus) to the median eminence, a site with dense gonadotropin-releasing hormone terminals 23. Second, menopause and/or oophorectomy are associated with changes in NKB gene expression in human and animal studies. For example, NKB gene expression in the human infundibular nucleus increases after menopause and, in monkeys and rats, ovariectomy induces similar increases in NKB gene expression that are reversed by estradiol therapy 23, 24. Compared with premenopausal women, postmenopausal women have hypertrophy of neurons expressing NKB 23. Third, in humans, inactivating mutations of the NKB gene (TAC3) and TACR3 induce hypogonadotropic hypogonadism. For example, in humans, TAC3 and TACR3 inactivating mutations are associated with absent pubertal development as well as low circulating serum luteinizing hormone and gonadal steroid levels 23, 25. In rhesus monkeys, intervention to slow the frequency of gonadotropin-releasing hormone pulses can reproduce the pattern of low serum luteinizing hormone seen in humans with TAC3 and TACR3 mutations, suggesting that women with deficits in NKB/NK3R signaling have changes in the pattern of gonadotropin-releasing hormone pulses23. Finally, the infusion of a potent selective NK3R agonist (senktide) into the median preoptic nucleus of the rat markedly reduces core body temperature, suggesting that hypertrophy of NKB neurons characterizing postmenopausal women could be involved in the biology underlying menopausal hot flashes23, 52.
On the National Institutes of Health Common Fund’s Genotype-Tissue Expression (GTEx) website (https://commonfund.nih.gov/GTEx/index), the highest gene expression (reads per kilobase of transcript per million mapped reads) for TACR3 is located in the hypothalamus, followed by the amygdala http://www.gtexportal.org/home/gene/TACR3). Searches for the TACR3 SNPs associated with VMS in the current study produced no results in GTEx.
The results of the current study do not confirm the results of the previous candidate gene studies, and previous candidate gene studies of genetic variation on chromosome 4 found no associations between SNPs in candidate genes (sulfotransferase family 1E, estrogen-preferring, member 1 [SULT1E1], UDP glucuronosyltransferase 2 family, polypeptide B7 [UGT2B7], UDP glucuronosyltransferase 2 family, polypeptide B15 [UGT2B15], kinase insert domain receptor [VEGFR2]10, 13, 20. However, according to the National Center for Biotechnology Information gene dbSNP webpage, the locations of these genes (SULT1E1 69841212..69860765; UGT2B7 69051249..69112987; UGT2B15 ; 68646597..68670776 VEGFR2 55078259..55125595) are not located in close proximity to the tachykinin receptor 3 gene (103589468..103719816).
This study has potential limitations. Although it would be interesting to investigate gene-gene or gene-environment interactions, we do not have sufficient power with the current sample size to carry forward these analyses. The variants identified are non-coding and have not been implicated as regulatory. However, as is the case with all GWAS studies, genetic variation in intronic regions could indicate that SNPs exert effects on splicing or regulation of transcription or methylation sites of other genes, or they could affect translation, induce post-translational modifications, lead to changes in mRNA stability, or affect ligand binding 53. Alternatively, the identified SNPs may not be causal but may instead be tightly linked with the underlying true causal variants.
It is likely that there are still rare variants of moderate effect as well as common variants of small effect that we have not detected. By combining studies across ethnic groups using meta-analysis, we down-weight the importance of ethnic-specific association. Additionally, each of the cohorts were genotyped using SNP platforms that include ~1 million SNPs, which means that many SNPs and single-nucleotide variations are not directly genotyped, i.e. we could fail to discover a rare variant (or even a common variant) that is not included in the SNP array. However, there are two related points that ameliorate this concern. First, linkage disequilibrium (LD) leads to high correlation between the variants at a SNP that was genotyped and the variants of loci that were not genotyped. Second, based on this principle of LD, our densely genotyped marker sets, and well-characterized reference panels, we imputed 10-fold more SNPs than were directly genotyped.
Although VMS were self-reported in this study, self-reporting of VMS reflects clinical practice. Also, because individuals are unaware of their genotypes, self-report should not be differentially biased between TACR3 variant carriers and those individuals homozygous for the major allele. The numbers of women with severe VMS at baseline were too low to allow us to reliably examine genetic variation in relation to the severity of VMS. It is possible that women with severe VMS did not consent for inclusion in the study. Strengths of this study include the large sample size, ethnic diversity of participants, and detailed information regarding potential confounders. The GWAS approach provides an unbiased source of information regarding previously unknown functional pathways.
We are encouraged by the GWAS results and our bioinformatics analyses, which lend support to the hypothesis of the NKB pathway as a target for the treatment of VMS symptoms, but confirmation of these results is necessary before clinical implications can be made. Necessary steps in testing this hypothesis include replication and fine-mapping of TACR3, perhaps by targeted sequencing followed by in-silico annotation, characterization of variant function using epigenetic and expression data, and ultimately functional studies using cell lines, human tissue, and isogenetic or mouse models 54.
Conclusion
In conclusion, several SNPs located in the area coding for tachykinin receptor 3 were associated with increased odds of VMS at a p-value < 5 × 10-8. Per allele, each of these SNPs was associated with 1.4- to 1.6-fold higher odds of VMS. The odds ratios were consistent in several cohorts from diverse ethnic groups. To our knowledge, the present study is the first GWAS of VMS. Ultimately, elucidation of the mechanisms responsible for VMS could yield novel therapies for hot flashes. Our results should inform the study of biological pathways responsible for VMS. Inter-relations between genetic markers and other known environmental risk factors for VMS require study.
Supplementary Material
Acknowledgments
We thank the women who generously participated in the WHI and the WHI investigators and staff for their dedicated efforts.
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller Clinical Coordinating Center: Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
Investigators and Academic Centers: (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumake
For a list of all the investigators who have contributed to WHI science, please visit: https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf
Role of the sponsor
Representatives of the National Heart, Lung, and Blood Institute participated in Women’s Health Initiative committees that had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparations, review, or approval of the manuscript.
Funding
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C
JSS is partially funded by NIH grant GM053275 and NSF grant DMS 1264153.
Footnotes
Author contributions
Mr. Hohensee had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of data analysis.
Study concept and design: CJC, JSS
Acquisition of data: JEM, JWW
Analysis and interpretation of data: all authors
Drafting of the manuscript: CJC
Critical revision of the manuscript for important intellectual content: all authors
Statistical analysis: CH
Obtained funding: JEM, JWW
Financial Disclosures
The following authors have no relevant conflicts of interest to disclose:
CJC, SH, JSS, MZV, CH, RN, JWW, JEM
ESL’s Institution has received funding from Amgen Inc, Astrazeneca, Bristol-Meyers-Squibb, and Merck for research projects unrelated to the current manuscript.
References
- 1.Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women’s health across the nation. Am J Public Health. 2006;96(7):1226–35. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crandall CJ, Tseng CH, Crawford SL, et al. Association of menopausal vasomotor symptoms with increased bone turnover during the menopausal transition. J Bone Miner Res. 2011;26(4):840–9. doi: 10.1002/jbmr.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szmuilowicz ED, Manson JE, Rossouw JE, et al. Vasomotor symptoms and cardiovascular events in postmenopausal women. Menopause. 2011;18(6):603–10. doi: 10.1097/gme.0b013e3182014849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Politi MC, Schleinitz MD, Col NF. Revisiting the duration of vasomotor symptoms of menopause: a meta-analysis. J Gen Intern Med. 2008;23(9):1507–13. doi: 10.1007/s11606-008-0655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avis NE, Crawford SL, Greendale G, et al. Duration of Menopausal Vasomotor Symptoms Over the Menopause Transition. JAMA Intern Med. 2015 doi: 10.1001/jamainternmed.2014.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearson TA, Manolio TA. How to interpret a genome-wide association study. JAMA. 2008;299(11):1335–44. doi: 10.1001/jama.299.11.1335. [DOI] [PubMed] [Google Scholar]
- 7.National Human Genome Research Institute. Genome-wide association studies fact sheet. Available at: https://www.genome.gov/20019523/genomewide-association-studies-fact-sheet/
- 8.Crandall CJ, Crawford SL, Gold EB. Vasomotor symptom prevalence is associated with polymorphisms in sex steroid-metabolizing enzymes and receptors. Am J Med. 2006;119(9 Suppl 1):S52–60. doi: 10.1016/j.amjmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Butts SF, Freeman EW, Sammel MD, Queen K, Lin H, Rebbeck TR. Joint effects of smoking and gene variants involved in sex steroid metabolism on hot flashes in late reproductive-age women. J Clin Endocrinol Metab. 2012;97(6):E1032–42. doi: 10.1210/jc.2011-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rebbeck TR, Su HI, Sammel MD, et al. Effect of hormone metabolism genotypes on steroid hormone levels and menopausal symptoms in a prospective population-based cohort of women experiencing the menopausal transition. Menopause. 2010;17(5):1026–34. doi: 10.1097/gme.0b013e3181db61a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schilling C, Gallicchio L, Miller SR, Langenberg P, Zacur H, Flaws JA. Genetic polymorphisms, hormone levels, and hot flashes in midlife women. Maturitas. 2007;57(2):120–31. doi: 10.1016/j.maturitas.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Visvanathan K, Gallicchio L, Schilling C, et al. Cytochrome gene polymorphisms, serum estrogens, and hot flushes in midlife women. Obstet Gynecol. 2005;106(6):1372–81. doi: 10.1097/01.AOG.0000187308.67021.98. [DOI] [PubMed] [Google Scholar]
- 13.Dezentje VO, Gelderblom H, Van Schaik RH, et al. CYP2D6 genotype in relation to hot flashes as tamoxifen side effect in a Dutch cohort of the tamoxifen exemestane adjuvant multinational (TEAM) trial. Breast Cancer Res Treat. 2014;143(1):171–9. doi: 10.1007/s10549-013-2777-6. [DOI] [PubMed] [Google Scholar]
- 14.Jin Y, Hayes DF, Li L, et al. Estrogen receptor genotypes influence hot flash prevalence and composite score before and after tamoxifen therapy. J Clin Oncol. 2008;26(36):5849–54. doi: 10.1200/JCO.2008.16.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malacara JM, Perez-Luque EL, Martinez-Garza S, Sanchez-Marin FJ. The relationship of estrogen receptor-alpha polymorphism with symptoms and other characteristics in post-menopausal women. Maturitas. 2004;49(2):163–9. doi: 10.1016/j.maturitas.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Fontein DB, Houtsma D, Nortier JW, et al. Germline variants in the CYP19A1 gene are related to specific adverse events in aromatase inhibitor users: a substudy of Dutch patients in the TEAM trial. Breast Cancer Res Treat. 2014;144(3):599–606. doi: 10.1007/s10549-014-2873-2. [DOI] [PubMed] [Google Scholar]
- 17.Goetz MP, Rae JM, Suman VJ, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol. 2005;23(36):9312–8. doi: 10.1200/JCO.2005.03.3266. [DOI] [PubMed] [Google Scholar]
- 18.Henry NL, Rae JM, Li L, et al. Association between CYP2D6 genotype and tamoxifen-induced hot flashes in a prospective cohort. Breast Cancer Res Treat. 2009;117(3):571–5. doi: 10.1007/s10549-009-0309-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montasser ME, Ziv-Gal A, Brown JP, Flaws JA, Merchenthaler I. A potentially functional variant in the serotonin transporter gene is associated with premenopausal and perimenopausal hot flashes. Menopause. 2015;22(1):108–13. doi: 10.1097/GME.0000000000000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider BP, Radovich M, Flockhart DA, et al. Exploratory study evaluating the association of polymorphisms of angiogenesis genes with hot flashes. Breast Cancer Res Treat. 2009;116(3):543–9. doi: 10.1007/s10549-008-0178-z. [DOI] [PubMed] [Google Scholar]
- 21.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 22.Asimit JL, Hatzikotoulas K, McCarthy M, Morris AP, Zeggini E. Trans-ethnic study design approaches for fine-mapping. Eur J Hum Genet. 2016 doi: 10.1038/ejhg.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA. Neurokinin B and the hypothalamic regulation of reproduction. Brain Res. 2010;1364:116–28. doi: 10.1016/j.brainres.2010.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rance NE, Bruce TR. Neurokinin B gene expression is increased in the arcuate nucleus of ovariectomized rats. Neuroendocrinology. 1994;60(4):337–45. doi: 10.1159/000126768. [DOI] [PubMed] [Google Scholar]
- 25.Topaloglu AK, Reimann F, Guclu M, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;41(3):354–8. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13(9 Suppl):S107–21. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 27.Women’s Health Initiative Clinical Coordinating Center. GWAS data: Key WHI Genetic and Biomarker Studies. Available at: https://www.whi.org/researchers/data/Pages/GWAS-Data.aspx.
- 28.Crandall CJ, Aragaki A, Cauley JA, et al. Associations of menopausal vasomotor symptoms with fracture incidence. J Clin Endocrinol Metab. 2015;100(2):524–34. doi: 10.1210/jc.2014-3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Velez Edwards DR, Naj AC, Monda K, et al. Gene-environment interactions and obesity traits among postmenopausal African-American and Hispanic women in the Women’s Health Initiative SHARe Study. Hum Genet. 2013;132(3):323–36. doi: 10.1007/s00439-012-1246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen CT, Fernandez-Rhodes L, Brzyski RG, et al. Replication of loci influencing ages at menarche and menopause in Hispanic women: the Women’s Health Initiative SHARe Study. Hum Mol Genet. 2012;21(6):1419–32. doi: 10.1093/hmg/ddr570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan KH, Huang YT, Meng Q, et al. Shared molecular pathways and gene networks for cardiovascular disease and type 2 diabetes mellitus in women across diverse ethnicities. Circ Cardiovasc Genet. 2014;7(6):911–9. doi: 10.1161/CIRCGENETICS.114.000676. [DOI] [PubMed] [Google Scholar]
- 32.Fuchsberger C, Abecasis GR, Hinds DA. minimac2: faster genotype imputation. Bioinformatics. 2015;31(5):782–4. doi: 10.1093/bioinformatics/btu704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howie B, Fuchsberger C, Stephens M, Marchini J, Abecasis GR. Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat Genet. 2012;44(8):955–9. doi: 10.1038/ng.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol. 2010;34(8):816–34. doi: 10.1002/gepi.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Willer C, Sanna S, Abecasis G. Genotype imputation. Annu Rev Genomics Hum Genet. 2009;10:387–406. doi: 10.1146/annurev.genom.9.081307.164242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Browning BL, Browning SR. A unified approach to genotype imputation and haplotype-phase inference for large data sets of trios and unrelated individuals. Am J Hum Genet. 2009;84(2):210–23. doi: 10.1016/j.ajhg.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.WHI Harmonized and Imputed GWAS Data [Google Scholar]
- 38.Manson JE, Greenland P, LaCroix AZ, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347(10):716–25. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 39.Meyer AM, Evenson KR, Morimoto L, Siscovick D, White E. Test-retest reliability of the Women’s Health Initiative physical activity questionnaire. Med Sci Sports Exerc. 2009;41(3):530–8. doi: 10.1249/MSS.0b013e31818ace55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seguin R, Buchner DM, Liu J, et al. Sedentary behavior and mortality in older women: the Women’s Health Initiative. Am J Prev Med. 2014;46(2):122–35. doi: 10.1016/j.amepre.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904–9. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 42.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 43.Lumley T. rmeta: Meta-analysis Functions for simple fixed and random effects meta-analysis for two-sample comparisons and cumulative meta-analyses. Draws standard summary plots, funnel plots, and computes summaries and tests for association and heterogeneity. 2.16. 2012 [Google Scholar]
- 44.Turner S. qqman: Q-Q and Manhattan plots for GWAS data. R package version 0.1.2. https://cran.r-project.org/web/packages/qqman/index.html2014.
- 45.Zhao JH. gap: Genetic Analysis Package. R package version 1.1-16. http://cran.r-project.org/package=gap2015.
- 46.Laurie CC, Doheny KF, Mirel DB, et al. Quality control and quality assurance in genotypic data for genome-wide association studies. Genet Epidemiol. 2010;34(6):591–602. doi: 10.1002/gepi.20516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pruim RJ, Welch RP, Sanna S, et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26(18):2336–7. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cruz GBGoUS. UCSC Genome Browser on Human Feb 2009. The Regents of the University of California; [Google Scholar]
- 49.Perry JR, Day F, Elks CE, et al. Parent-of-origin-specific allelic associations among 106 genomic loci for age at menarche. Nature. 2014;514(7520):92–7. doi: 10.1038/nature13545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jayasena CN, Comninos AN, Stefanopoulou E, et al. Neurokinin B administration induces hot flushes in women. Sci Rep. 2015;5(8466) doi: 10.1038/srep08466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borsay BA, Skrapits K, Herczeg L, et al. Hypophysiotropic gonadotropin-releasing hormone projections are exposed to dense plexuses of kisspeptin, neurokinin B and substance p immunoreactive fibers in the human: a study on tissues from postmenopausal women. Neuroendocrinology. 2014;100(2-3):141–52. doi: 10.1159/000368362. [DOI] [PubMed] [Google Scholar]
- 52.Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34(3):211–27. doi: 10.1016/j.yfrne.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang F, Lupski JR. Non-coding genetic variants in human disease. Hum Mol Genet. 2015;24(R1):R102–10. doi: 10.1093/hmg/ddv259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Edwards SL, Beesley J, French JD, Dunning AM. Beyond GWASs: illuminating the dark road from association to function. Am J Hum Genet. 2013;93(5):779–97. doi: 10.1016/j.ajhg.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


