Abstract
Background
Although both men and women use e-cigarettes, most preclinical nicotine research has focused on its effects in male rodents following injection. The goals of the present study were to develop an effective e-cigarette nicotine delivery system, to compare results to those obtained after subcutaneous (s.c.) injection, and to examine sex differences in the model.
Methods
Hypothermia and locomotor suppression were assessed following aerosol exposure or s.c. injection with nicotine in female and male mice. Subsequently, plasma and brain concentrations of nicotine and cotinine were measured.
Results
Passive exposure to nicotine aerosol produced concentration-dependent and mecamylamine reversible hypothermic and locomotor suppressant effects in female and male mice, as did s.c. nicotine injection. In plasma and brain, nicotine and cotinine concentrations showed dose/concentration-dependent increases in both sexes following each route of administration. Sex differences in nicotine-induced hypothermia were dependent upon route of administration, with females showing greater hypothermia following aerosol exposure and males showing greater hypothermia following injection. In contrast, when they occurred, sex differences in nicotine and cotinine levels in brain and plasma consistently showed greater concentrations in females than males, regardless of route of administration.
Discussion
In summary, the e-cigarette exposure device described herein was used successfully to deliver pharmacologically active doses of nicotine to female and male mice. Further, plasma nicotine concentrations following exposure were similar to those after s.c. injection with nicotine and within the range observed in human smokers. Future research on vaped products can be strengthened by inclusion of translationally relevant routes of administration.
Keywords: cotinine, electronic cigarette, metabolism, nicotine, sex differences
1.0 Introduction
Since their introduction to the U.S. market, use of electronic cigarettes (e-cigarettes) has risen dramatically, particularly in youth in grades 6–12 (Bunnell et al., 2015; McMillen et al., 2015). For example, recent data from national surveys conducted by the Centers for Disease Control show that over 10% of male youth reported e-cigarette use in the last 30 days compared to less than 5% of adult males (Table 1). While significantly fewer female youth reported past 30-day use (~8%) compared to male youth, their recent use still remains twice that of adult females. Yet, examination of prevalence figures for more frequent use (i.e., some days or every day) reveals that the percentage of users of both sexes is higher for adults than for youth. Hence, the overall percentages across frequency suggest that youth are more likely to try e-cigarettes whereas adults are more consistent in their use, with similar percentages of men and women reporting regular use. This interpretation is consistent with previous literature reporting that adults use e-cigarettes primarily for smoking cessation (Dawkins et al., 2013) whereas adolescents use primarily for experimentation (Hughes et al., 2015), although longitudinal analysis suggests increasing adolescent use over time (Lippert, 2016).
Table 1.
Self-reported e-cigarette use among male and female youth and adults 1
| CDC National Youth Tobacco Survey 2014 |
CDC National Adult Tobacco Survey 2013 |
|||
|---|---|---|---|---|
| Current E- cigarette Use |
Male N (weighted %) |
Female N (weighted %) |
Male N (weighted %) |
Female N (weighted %) |
| Past 30-day use | 1,156 (10.3%) |
833 * (8.1%) |
809 (4.7%) |
854 * (3.6%) |
| Some days | 1,021 (9.2%) |
782 * (7.6%) |
691 (23.2%) |
640 (25.7%) |
| Every day | 135 (1.2%) |
51 * (0.6%) |
163 (5.5%) |
169 (5.1%) |
N = number of individuals who responded positively to the indicated question (weighted % of total number of males or females surveyed). For the youth survey, the use of e-cigarette use options were presented as “0 days”, “1 or 2 days”, “3 to 5”, “6 to 9”, “10 to 19”, “20 to 29”, and “all 30 days”. 1–29 days were recoded as “Some days”, 30 was recoded as “Everyday”.
P ≤ 0.001 (males vs females) based upon Wald test.
The use of e-cigarettes by women and men argues for inclusion of both sexes in research on biological mechanisms and consequences associated with their use. To date, however, most preclinical research on tobacco and the nascent research on e-cigarettes have focused on examination of nicotine effects in male rodents following injection. Recently, several laboratories have reported on the development of methods to expose rodents to nicotine and/or tobacco via inhalation (George et al., 2010; Ponzoni et al., 2015; Smith et al., 2015). While many of these studies concentrated primarily on examination of the effects of inhaled nicotine on the pulmonary system or on developmental or toxicological effects (McGrath-Morrow et al., 2015; Misra et al., 2014; Smith et al., 2015; Sussan et al., 2015), a few studies have investigated behavioral effects of inhaled tobacco smoke (Bruijnzeel et al., 2011; de la Pena et al., 2014; de la Pena et al., 2015; Harris et al., 2010; Yamada et al., 2010) or nicotine vapor generated by bubbling air through a nicotine solution (George et al., 2010; Gilpin et al., 2014) and one lab compared the effects of chronic exposure to cigarette smoke or e-cigarette vapor (Ponzoni et al., 2015). However, none of these studies examined sex differences and only the latter study focused on a model of e-cigarette exposure. Further, most of these studies were conducted in rats. The primary metabolic enzyme for nicotine in rats is in the CYP2B family (Nakayama et al., 1993), whereas the primary enzyme in mice is CYP2A5 (Murphy et al., 2005; Siu et al., 2006), which is more closely related (84% sequence homology) to CYP2A6 (Murphy et al., 2005), the predominant liver enzyme in humans that metabolizes nicotine to cotinine (Messina et al., 1997). Hence, mice may represent a better animal model for studies with a pharmacokinetics component (Matta et al., 2007; Siu et al., 2006).
In the present study, a commercially available tank-based e-cigarette (Brown and Cheng, 2014) was modified to permit rodent exposure to aerosolized e-liquids (i.e., solutions containing a vehicle of propylene glycol and/or vegetable glycerin with nicotine and added flavors). Hypothermia and locomotor suppression, characteristic effects of nicotine in mice (Damaj, 2001), were assessed following inhalational exposure to nicotine aerosol or after subcutaneous (s.c.) injection with nicotine in female and male mice. As a preliminary step towards verifying similar mechanisms, reversal of these effects following injection of the noncompetitive nicotine receptor antagonist mecamylamine was also assessed. Subsequently, plasma and brain concentrations of nicotine and its major metabolite cotinine (Benowitz et al., 1983; Petersen et al., 1984) were measured. Results reported here serve as proof-of-principle for a novel device capable of translationally relevant delivery of nicotine aerosol for use in mechanistic studies of behavioral and biological effects of e-cigarettes. This apparatus has also been used to deliver aerosolized stimulants to rodents (Marusich et al., 2016).
2.0 Materials and Methods
2.1 Subjects
Adult male and female ICR mice (25–35 g) [Harlan/Envigo Laboratories, Frederick, MD] were singly housed in polycarbonate cages with hardwood bedding in a temperature-controlled environment (20–24°C) with a 12 h light-dark cycle (lights on at 0600). All mice had ad libitum access to food and water while in their home cages. The studies were carried out in accordance with federal and state regulatory guidelines and were IACUC-approved.
2.2 Drugs and Chemicals
Mecamylamine HCl and (-)-nicotine hydrogen tartrate salt (Sigma-Aldrich, St. Louis, MO) were dissolved in physiological saline (Patterson Veterinary, Devens, MA), and the pH was adjusted to approximately neutral (pH ~ 7), as necessary. (-)-Nicotine free base (Sigma-Aldrich) was mixed with a 50:50 propylene glycol and glycerin solution (Sigma-Aldrich). Doses of nicotine for injection are expressed as mg/kg of the base. Nicotine and mecamylamine were injected subcutaneously (s.c.) at a volume of 10 ml/kg. Concentrations for aerosol administration are expressed as mg/ml in the e-cigarette tank, and may not be representative of the actual amount of nicotine inhaled.
Chemicals and reagents for the analysis of biological samples were purchased commercially and included nicotine (Sigma-Aldrich), cotinine (Toronto Research Chemicals, Toronto, ON), nicotine-d3 (Cambridge Isotope Laboratories, Tewksbury, MA), cotinine-d3 (Santa Cruz Biotechnology, Dallas, TX), ammonium acetate (Sigma-Aldrich), and formic acid and acetonitrile (Fisher Scientific, Fair Lawn, NJ). An internal standard solution was prepared in methanol (Fisher Scientific) containing 48 µg/mL nicotine-d3 and 38 μg/mL cotinine-d3. Working solutions containing both nicotine and cotinine were prepared in methanol at concentrations of 10,000 and 100 ng/mL.
2.3 Apparatus
Aerosol was generated using a modified commercially available electronic cigarette (Figure S11). An iStick 30W variable wattage (eLeaf, Irvine, CA) supplied power (7W) to a CE5-S tank/clearomizer with bottom dual coil atomizer (1.8Ω) (Aspire, Kent, WA). Air/aerosol was pumped (1L/min) through the bottom of the tank and into an EZ-177 Sure-Seal 1L mouse induction anesthesia chamber (10 cm × 10 cm × 10 cm) [EZ-Anesthesia, Palmer, PA] via Tygon tubing (Fisher Scientific, Pittsburgh, PA) and controlled by 3-way stopcocks (Grainger, Raleigh, NC). The aerosol generation system was placed in a hood to avoid exposure of laboratory technicians to aerosol. Mouse locomotor activity was assessed in separate clear Plexiglas activity chambers (47 cm × 25.5 cm × 22 cm). Each chamber was surrounded by two arrays of 4 × 8 infrared photocell beams, interfaced with software for automated data collection (San Diego Instruments, San Diego, CA). Temperature readings were taken using a BAT-12 Microprobe Thermometer with RET-3 Rectal Probe (PhysiTemp Instruments Inc., Clifton, NJ). Analgesia was measured by a Tail Flick Analgesia Meter (IITC Inc. Life Science, Woodland Hills, CA).
2.4 In Vivo Pharmacology Procedure
In Experiment 1, pharmacological effects of nicotine were evaluated following nicotine exposure via aerosol (0, 12, 24, or 30 mg/ml) or subcutaneous (s.c.) injection (0, 0.5, 1.0, or 1.5 mg/kg). Mice (n=8/sex/group) were brought into the test room and weighed. After a minimum of 30-min acclimation, baseline temperature and tail flick latency were taken, as described previously (Wiley et al., 2015). The mice were then exposed to nicotine via aerosol (see below) or s.c. injection and placed back into their home cage. For aerosol exposure, mice were placed into the anesthesia chambers, where they were allowed to move freely. Subsequently, aerosol was generated for 10 seconds and held in the chamber for 1 minute. Mice were then placed back in their home cage for 2 minutes before being exposed to aerosol again for 1 minute. This process was repeated five times, such that each mouse was exposed to aerosol for 5 minutes over a 13- minute period. This procedure for aerosol exposure was based upon an initial pilot experiment showing that staggered nicotine exposure for 5 minutes over a period of time resulted in higher brain nicotine levels in male mice than did continuous exposure for 5 minutes. Ten minutes after the final aerosol exposure (or s.c. injection), temperature and tail flick latency were measured again and the mice were immediately placed into locomotor chambers for 10 minutes. Thirty-five minutes after the final exposure/s.c. injection, temperature was taken a third time. After a one-week washout, vehicle- or high dose nicotine-treated mice were treated with a single 1 mg/kg s.c. injection of mecamylamine 10 min before exposure to vehicle or high dose nicotine, respectively, aerosol or injection and were tested again as described above. Because antinociception was not observed following either route of administration at the time point measured, these data have been omitted from the results and discussion.
2.5 Biological Sample Preparation
Biological samples (plasma and brain) were obtained from experimentally naïve mice administered nicotine via s.c. injection or aerosol exposure, as described above. Sacrifice and collection of blood and tissue occurred 10 minutes after the final aerosol exposure or s.c. injection.
2.5.1 Plasma Samples
Plasma calibration standards and quality control samples were prepared using pooled plasma from remaining control samples. Standards, quality controls, and samples were prepared by spiking 200 µL of plasma with 10 µL of internal standard solution, and an appropriate volume of calibration solution. Calibration standards were created at 1, 2, 5, 10, 100, 500, and 1000 ng/mL for both analytes, and quality control samples were made at 5 and 500 ng/mL for both analytes. Sample extraction was achieved by diluting to a final volume of 1 mL with methanol, vortex mixing for 2 minutes followed by centrifugation at 12,000 RCF for 10 minutes. The supernatants were analyzed by high performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS).
2.5.2 Brain Samples
Whole brains were placed into a 15 mL falcon tube along with 20 2.3-mm stainless steel beads (BioSpec Products, Bartlesville, OK) and 4 µL of WFI quality water (Corning, Manassas, VA) for every mg of brain tissue. Samples were homogenized in a SPEX Sample Prep Geno-Grinder (Metuchen, NJ) at 1750 RPM for 2 minutes, then centrifuged in a Beckman Coulter Allegra X-15R Centrifuge (Pasadena, CA) for 2 minutes at 1750 RCF to remove any tissue from the lid. Since two layers were observed, the samples were lightly vortexed to assure a homogenous solution. Pooled homogenate used for calibration standards and quality control samples was created using 500 µL from each control sample. Standards, quality controls and samples were prepared by spiking 200 µL of brain homogenate with 10 µL of internal standard solution, and an appropriate volume of calibration solution. The calibration range in homogenate was the same as for plasma, described above. Sample extraction was achieved by diluting to a final volume of 1 mL with methanol, vortex mixing for 2 minutes followed by centrifugation at 12,000 RCF for 10 minutes. The supernatants were analyzed by HPLC-MS/MS.
2.6 Analysis of Biological Samples
The HPLC-MS/MS system consisted of an Agilent 1100 (Santa Clara, CA) coupled to an API-4000 with a TurboIonSpray source (Sciex, Framingham, MA). The auto sampler was maintained at 35°C for the brain tissues and 10°C for plasma. Chromatographic analysis was performed using a Phenomenex (Torrance, CA) Luna Phenyl-Hexyl column (150 × 4.60 mm i.d., 3-µm particle size) and a Phenomenex SecurityGuard AQ C18 4 × 2.0 mm column filter. Five microliters of sample were injected onto the column and elution of the analytes and internal standard was achieved at 35°C using a binary gradient and a flow rate of 0.8 mL/min. The mobile phases consisted of 5 mM ammonium acetate in water with 0.1% formic acid (mobile phase A) and acetonitrile with 0.1% formic acid (mobile phase B). The gradient was 5% mobile phase B for 2 minutes, 5 to 10% B from 2 to 5 minutes, then to 95% B in 2 minutes and held for 0.5 minutes. MS detection was performed with the electrospray ionization source operated in positive ion mode with an ion source temperature of 500 °C and an ion spray voltage of 5000V. Transitions monitored were m/z 163.2 → 84.0 for nicotine, 177.1 → 80.1 for cotinine, 166.1 → 89.1 for nicotine-d3, and 180.2 → 101.0 for cotinine-d3. The retention times were 2.4 and 3.0 min for nicotine and cotinine, respectively. Analyst software version 1.6.2 was used for data acquisition and analysis.
2.7 Statistical Analysis
Temperature readings were analyzed as change in temperature from pre-dosing baseline and locomotor activity was measured as total beam breaks during the 10-min session. Mean (±SEM) values for these in vivo measures and for plasma and brain concentrations of nicotine and cotinine were calculated separately for each sex and administered nicotine dose/concentration. For each route of administration, separate factorial ANOVAs (sex X concentration/dose) were performed. Tukey-Kramer post-hoc tests (α=0.05) were used to specify individual differences in the means for all significant ANOVAs.
3.0 Results
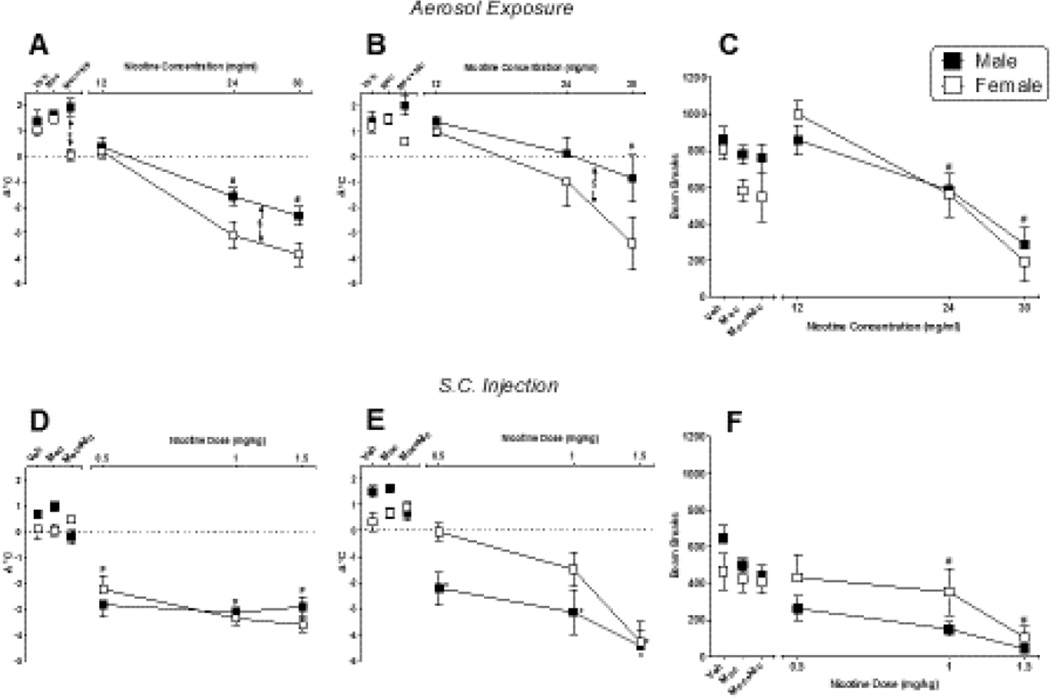
Figure 1 shows the effects of nicotine delivered via aerosol (top panels) and s.c. injection (bottom panels) on change in temperature 10 min after exposure (left panels), change in temperature 35 min after exposure (middle panels), and locomotor activity (right panels) in male and female mice. Regardless of route of administration, nicotine significantly decreased temperature at both time points and suppressed locomotor activity in both sexes. Significant decreases in temperature (at both time points) and locomotor activity were observed following aerosol delivery of nicotine [Fig. 1, panel A, main effects of concentration for 10-min temp: F(3,56)=56.93, p<0.05; Fig. 1, panel B, 35-min temp: F(3,56)=11.25, p<0.05; and Fig. 1, panel C, locomotor activity: F(3,56)=24.15, p<0.05] and following s.c. nicotine injection [Fig. 1, panel D, main effects of dose for 10-min temp: F(3,56)=48.92, p<0.05; Fig. 1, panel E, 35-min temp: F(3,56)=27.79, p<0.05; and Fig. 1, panel F, locomotor activity: F(3,56)=11.66, p<0.05]. At 10 and 35 minutes post-exposure, the magnitude of temperature decrease produced by nicotine aerosol in females exceeded that seen with males [Fig. 1, panel A, main effect of sex: F(1,56)=10.80, p<0.05 and Fig. 1, panel B, main effect of sex: F(1,56)=4.82, p<0.05, respectively]. Concentration-dependent decreases in locomotor activity were similar across both sexes (Fig. 1, panel C). Temperature decreases were also observed at 10 minutes following s.c. nicotine injection; however, sex differences were not apparent (Fig. 1, panel D). Further, temperatures were decreased to a similar magnitude across all s.c. nicotine doses, suggesting that these doses were at the maximal asymptotic end of the dose-effect curve at this time-point. By 35 minutes post-injection, females, but not males, that received lower nicotine doses had recovered baseline body temperature (Fig. 1, panel E). S.c. nicotine decreased locomotor activity in both sexes to a similar extent (Fig. 1, panel F). Nicotine-induced decreases in body temperature and locomotor activity were reversed by pre-treatment with mecamylamine (1 mg/kg, s.c.) following both routes of administration (left side of each panel). While female and male rats significantly differed in the degree of temperature change at the 10 minutes time-point following exposure to nicotine + mecamylamine [Fig. 1, panel A; F(3,56)=3.34, p<0.05], this difference may have been driven by the fact that the 24 mg/ml concentration of nicotine alone produced significant sex differences in hypothermia.
Figure 1.
Pharmacological effects of nicotine administered via whole body aerosol exposure (top panels) or s.c. injection (bottom panels) in male (filled squares) and female (unfilled squares) mice. Changes in rectal temperature 10 and 35 min after the final aerosol exposure (panels A and B, respectively) or s.c. injection (panels D and E, respectively) are shown, as are the effects of aerosolized and s.c. nicotine on locomotor activity (panels C and F, respectively). Results of tests with vehicle/saline, mecamylamine alone (1 mg/kg, s.c.), and mecamylamine (1 mg/kg, s.c.) plus nicotine (30 mg/mL aerosol exposure or 1.5 mg/kg s.c. injection) are shown at the left side of each panel. Each value represents the mean (± SEM) of 8 mice. $ indicates significant main effect for sex. # indicates significant main effect of dose/concentration (compared to vehicle). * indicates significant interaction and difference compared to vehicle for specific sex. P<0.05 for all post hoc comparisons.
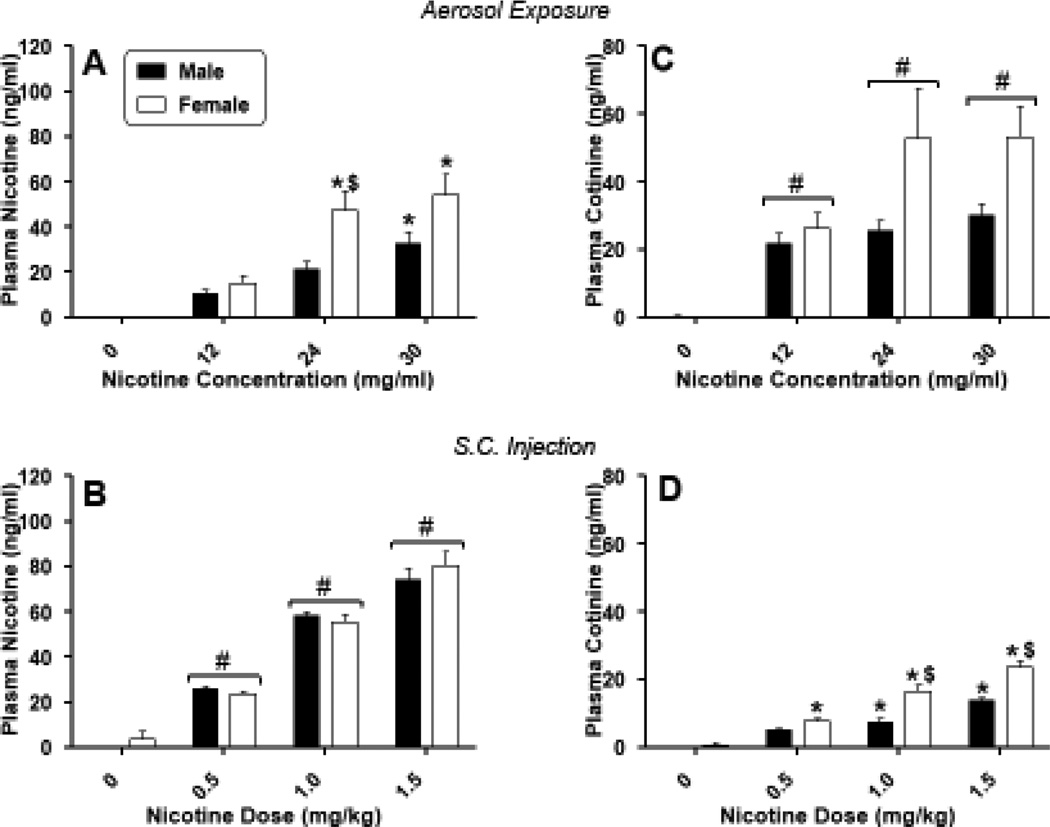
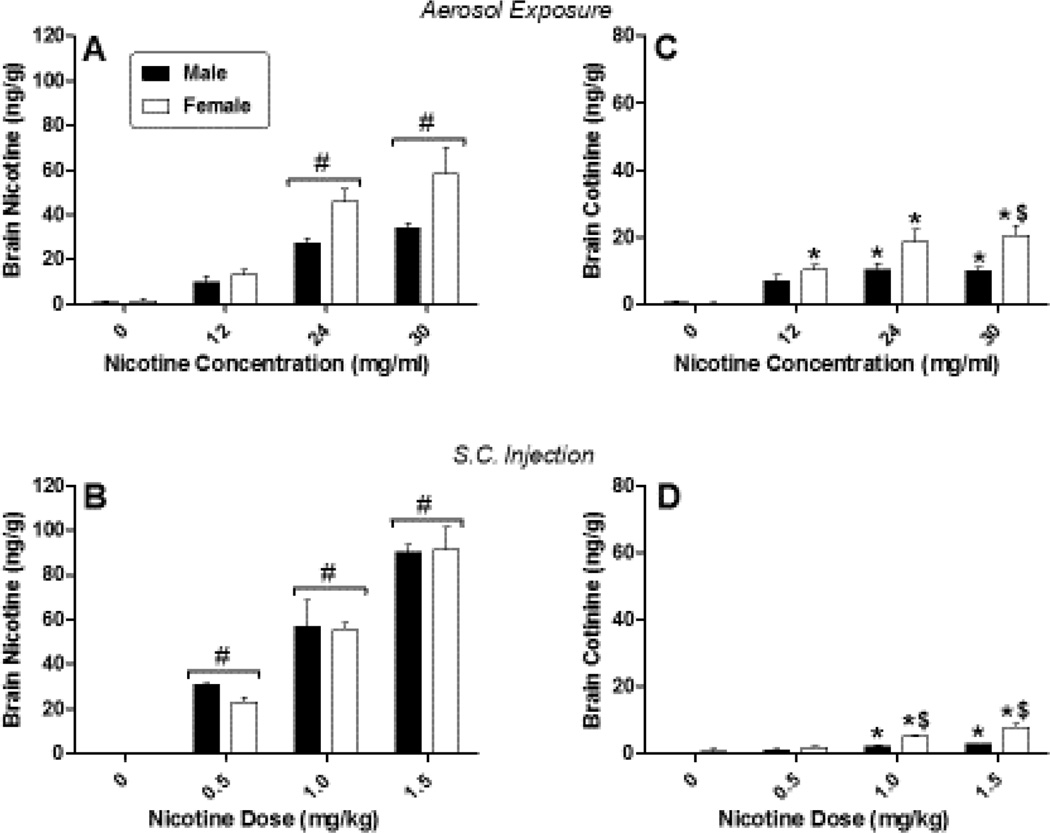
Concomitant with the observed pharmacological effects of nicotine, concentrations of nicotine and cotinine in the plasma (Figure 2) and brain (Figure 3) showed dose/concentration-dependent increases in mice of both sexes following each route of administration. Significant concentrations of nicotine were absorbed into the plasma [Figure 2, panels A and B; F(3,40)=30.21, p<0.05 and F(3,40)=186.79, p<0.05 for dose/concentration main effects for aerosol and injection, respectively] and subsequently metabolized to cotinine [Figure 2, panels C and D; F(3,40)=16.34, p<0.05 and F(3,40)=89.90, p<0.05 for dose/concentration main effects for aerosol and injection, respectively]. Similarly, nicotine and cotinine were distributed to the brain, with significantly increased concentrations of both compounds at higher doses, regardless of route of administration [Figure 3; F(3,40)=36.60, p<0.05 and F(3,40)=90.80, p<0.05 for dose/concentration main effects for brain nicotine level after aerosol exposure or injection, respectively and F(3,40)=23.58, p<0.05 and F(3,40)=47.85, p<0.05 for dose/concentration main effects for brain cotinine levels after aerosol exposure or injection, respectively]. Direct comparisons of plasma and brain levels across route of administration were precluded because of differences in concentrations/doses administered and in the amount of time required to deliver aerosol (~ 13 minutes) vs. injection (< 1 minute), as well as differences in experimental time points: i.e., mice received initial exposure to aerosol 25 min, whereas injections were administered 10 min, prior to biological sample collection. For example, consistent with the longer exposure time before sample collection, the ratios of cotinine: nicotine in the plasma and in the brain were reliably higher for inhalation than for s.c. injection across all concentrations (Table 2), suggesting that the longer time since exposure initiation in the aerosol exposed mice may have allowed greater metabolism of nicotine to cotinine. Sex differences in nicotine concentrations in plasma and brain occurred only in plasma at the 24 mg/ml aerosolized nicotine concentration, with females exhibiting significantly greater concentration of nicotine than males [Figure 2, panel A; sex X dose interaction: F(3,40)=2.94, p<0.05]. For s.c. injections, females also showed higher levels of cotinine in plasma [Figure 2, panel D; sex X dose interaction: F(3,40)=7.41, p<0.05]. Sex differences in brain cotinine concentrations occurred at higher concentrations/doses for both routes of administration [Figure 3, panels C and D; sex X dose interactions: F(3,40)=2.98, p<0.05 and F(3,40)=10.72, p<0.05 for aerosol and s.c. injection, respectively]. Again, concentrations were significantly higher in females than in males.
Figure 2.
Concentrations of nicotine (left panels) and cotinine (right panels) in the plasma of male (filled squares) and female (unfilled squares) mice after exposure to nicotine aerosol (top panels) or s.c. nicotine injection (bottom panels). Each value represents the mean (± SEM) of 8 mice. # indicates main effect of nicotine concentration/dose (compared to vehicle). * indicates significant interaction and difference compared to vehicle for specific sex. $ indicates significant interaction and sex difference at specified dose. P<0.05 for all post hoc comparisons.
Figure 3.
Concentrations of nicotine (left panels) and cotinine (right panels) in the brains of male (filled squares) and female (unfilled squares) mice after exposure to nicotine aerosol (top panels) or s.c. nicotine injection (bottom panels). Each value represents the mean (± SEM) of 8 mice. # indicates main effect of nicotine concentration/dose (compared to vehicle). * indicates significant interaction and difference compared to vehicle for specific sex. $ indicates significant interaction and sex difference at specified dose. P<0.05 for all post hoc comparisons.
Table 2.
Cotinine: nicotine ratio in plasma and brain following aerosol exposure and s.c. injection with nicotine*
| Route of Administration |
Concentration/Dose | Plasma | Brain | ||
|---|---|---|---|---|---|
| Males | Females | Males | Females | ||
| Aerosol Exposure |
12 mg/ml | 2.16 (0.31) |
1.81 (0.27) |
0.73 (0.14) |
0.86 (0.18) |
| 24 mg/ml | 1.27 (0.20) |
1.07 (0.13) |
0.39 (0.09) |
0.44 (0.08) |
|
| 30 mg/ml | 0.96 (0.13) |
1.0 (0.07) |
0.30 (0.04) |
0.37 (0.04) |
|
| S.C. Injection | 0.5 mg/kg | 0.20 (0.01) |
0.34 (0.06) |
0.04 (0) |
0.09 (0.01) |
| 1 mg/kg | 0.13 (0.02) |
0.31 (0.05) |
0.03 (0) |
0.10 (0.01) |
|
| 1.5 mg/kg | 0.20 (0.02) |
0.30 (0.02) |
0.03 (0) |
0.10 (0.02) |
|
Each value represents the mean (± SEM) of data from 6 mice. Plasma and brain concentrations were measured in the same mice. Values > 1 indicate greater cotinine than nicotine concentrations whereas values < 1 indicate greater concentrations of nicotine.
4.0 Discussion
The results of the present study offer proof-of-principle evidence that characteristic concentration-dependent nicotine-induced pharmacological effects can be elicited following exposure to nicotine aerosol delivered via an e-cigarette device in female and male mice. Maximal effects in female mice were similar to those observed after s.c. injection with nicotine, a more traditional route of administration for exposure of rodents to nicotine. In male mice, similarity of the maximal effects was time- and task-dependent, with similar between-route maximal hypothermic effect at 10 minutes, but less pronounced locomotor activity suppression and hypothermia at 35 minutes following aerosol exposure. In both sexes, nicotine concentrations in the plasma after aerosol administration were within the range (10–50 ng/ml) of those observed in human cigarette smokers (Matta et al., 2007) and similar to or higher than those reported after human e-cigarette use in an experimental setting (Lopez et al., 2016; Ramoa et al., 2016; Velez de Mendizabal et al., 2015). Brain nicotine levels following exposure to aerosol concentrations of 24 and 30 mg/ml nicotine reached those observed with pharmacologically active s.c. nicotine doses of 0.5–1 mg/kg. Since nicotine discrimination studies in mice typically employ approximate parenteral doses of 0.3–1 mg/kg (Caine et al., 2014; Cunningham and McMahon, 2013; Varvel et al., 1999), these results suggest that the e-cigarette apparatus described here exposed mice to the interoceptive cues of nicotine, which may play a role in maintaining nicotine addiction. Previous studies have also demonstrated that repeated exposure to nicotine aerosol/vapor can induce dependence in rats (George et al., 2010; Gilpin et al., 2014) and mice (Ponzoni et al., 2015). In all groups, the noncompetitive nicotinic receptor antagonist mecamylamine attenuated the acute hypothermic and locomotor suppressant effects of nicotine.
Despite the similarity in mecamylamine’s effects, sex differences in nicotine's effects on temperature were observed. Interestingly, however, the direction of the differences was dependent upon route of administration, with females showing greater sensitivity to nicotine's hypothermic effects via aerosol exposure and males showing greater sensitivity following s.c. injection. These differences in the in vivo pharmacological effects were not associated with sex differences in brain nicotine concentrations, although plasma nicotine levels were elevated at 24 mg/ml nicotine aerosol in female (vs. male) mice. Since aerosol nicotine concentration was not adjusted for bodyweight, this difference may have resulted from exposure to different nicotine doses. However, sex differences were not observed in nicotine's suppressant effects on locomotion for either route of administration. Although concentration-dependent sex differences in the metabolism of nicotine to cotinine have been reported in mice (Siu et al., 2006), “response specificity” in nicotine’s effects across sex has been noted previously in ICR mice (Damaj, 2001), suggesting that sex differences in nicotine-induced pharmacological effects in mice are not likely to be mediated solely by pharmacokinetic factors. In humans, nicotine is more reinforcing in men than women (Perkins et al., 2009), whereas cigarette smoking is associated with greater conditioned reinforcement and sensory effects in women than men (Perkins et al., 1999; Perkins et al., 2001); and while both men and women show cigarette smoking-related cue reactivity, they have different physiological responses to these cues (Pogun and Yararbas, 2009). Results from preliminary studies have suggested that sensory cues (e.g., flavors) associated with e-cigarettes may also differ between women and men (Dawkins et al., 2013). In the absence of aerosol exposure techniques such as the one described herein, investigation of a potential modulatory role for flavors in animal models of nicotine dependence may be difficult.
Direct comparison of results in mice to topography and use profiles in humans is complicated somewhat by between-species differences in respiratory physiology. Unlike adult humans, rodents are obligate nose breathers. Further, their respiratory rate is much higher than the rate for humans (~ 163 vs. 12–20 breaths/min, respectively). In this study, mouse exposure to nicotine aerosol was, by necessity, passive and duration was controlled by the experimenter. In contrast, human exposure is active. In the natural environment, puff duration and other puff parameters (e.g., number of puffs, inter-puff interval, flow rate, volume) are controlled by the user (Robinson et al., 2015; Robinson et al., 2016), although manipulation of these parameters through controlled vaping bouts has occurred in laboratory settings (Spindle et al., 2016). In humans, average puff duration for e-cigarettes (“cigalikes” and tank-based systems) appeared dependent upon nicotine concentration (Lopez et al., 2016; Ramoa et al., 2016; Spindle et al., 2016) and showed considerable variability, ranging from 1.8 to 6.1 s (Cunningham et al., 2016; Ramoa et al., 2016; Robinson et al., 2015; Spindle et al., 2016). In the present study, duration of aerosol generation was 10 seconds, with subsequent 1-minute hold in the chamber. Exposure occurred for a total of 5 minutes over the course of a 13-minute period. By comparison, Spindle et al. (2016) reported that average puff durations of 4.5 seconds occurred in experienced e-cigarette users during a controlled vaping bout of 10 puffs with 30 seconds inter-puff intervals. Hence, human users received 45 seconds active exposure to nicotine aerosol over a ~ 5-minute period versus 50 seconds active aerosol generation and a total 5-minute exposure period over a 13-minute time span for the mice. Under conditions of ad libitum access, humans averaged puff durations of 5.3 seconds and puff numbers of 62.55 for a total exposure duration of ~ 332 seconds (or 5 minutes) over a 90-minute session. While caveats apply with regard to the impact of species differences, total exposure periods in the mice were similar to those reported in the Spindle et al. (2016) study in humans.
In summary, the e-cigarette exposure device described herein was used successfully to deliver pharmacologically active doses of nicotine to female and male mice. Further, plasma nicotine concentrations following exposure were similar to those observed after s.c. injection with a low dose of nicotine as well as within the range observed in human smokers. Consequently, this mouse model of exposure to actual e-cigarette emissions has high translational relevance, although issues related to control of exposure dose still need to be solved through additional modification of the system. In May 2016, the U.S. Food and Drug Administration (FDA) extended their regulatory purview to e-cigarettes in a process known as “deeming.” Deeming placed emphasis on data-driven conclusions regarding the potential harms of e-cigarettes and emphasized the need for additional research, including preclinical investigation of underlying mechanisms. The present results suggest that route of administration should be an integral consideration of this research and may play a crucial role in examination of the contribution of flavors to nicotine dependence.
Supplementary Material
Highlights.
E-cigarette use is prevalent in youth and in adult men and women.
This study compared effects of nicotine aerosol and s.c. nicotine in mouse model.
Female mice were more sensitive to the hypothermic effects of aerosolized nicotine.
Male mice were more sensitive to the hypothermic effects of s.c. nicotine.
Feasibility of aerosol nicotine delivery via e-cigarette device is demonstrated.
Acknowledgments
Role of Funding Sources. Research and preparation of this manuscript were supported by RTI International Internal Research and Development Funds and by National Institute on Drug Abuse grants DA-003672 and DA-016644. NIH/NIDA did not have any other role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The opinions, findings, and conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect those of NIH or NIDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Contributors
All authors have read and approved the final version of the manuscript.
Participated in research design: Lefever, Thomas, Silinski, Wiley, Marusich, Lee
Conducted experiments: Lefever, Kovach
Invented new device: Lefever
Performed data analysis: Wiley, Lee
Wrote or contributed to the writing of the manuscript: Lefever, Wiley, Marusich, Thomas, Silinski, Lee
Declaration of interests. The authors report no conflicts of interest.
References
- Benowitz NL, Kuyt F, Jacob P, III, Jones RT, Osman AL. Cotinine disposition and effects. Clin. Pharmacol. Ther. 1983;34:604–611. doi: 10.1038/clpt.1983.222. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Cheng JM. Electronic cigarettes: Product characterisation and design considerations. Tob. Control. 2014;23(Suppl. 2):ii4–ii10. doi: 10.1136/tobaccocontrol-2013-051476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Rodrick G, Singh RP, Derendorf H, Bauzo RM. Repeated pre-exposure to tobacco smoke potentiates subsequent locomotor responses to nicotine and tobacco smoke but not amphetamine in adult rats. Pharmacol. Biochem. Behav. 2011;100:109–118. doi: 10.1016/j.pbb.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Bunnell RE, Agaku IT, Arrazola RA, Apelberg BJ, Caraballo RS, Corey CG, Coleman BN, Dube SR, King BA. Intentions to smoke cigarettes among never-smoking US middle and high school electronic cigarette users: National Youth Tobacco Survey, 2011–2013. Nicotine Tob. Res. 2015;17:228–235. doi: 10.1093/ntr/ntu166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Collins GT, Thomsen M, Wright C, Lanier RK, Mello NK. Nicotine-like behavioral effects of the minor tobacco alkaloids nornicotine, anabasine, and anatabine in male rodents. Exp. Clin. Psychopharmacol. 2014;22:9–22. doi: 10.1037/a0035749. [DOI] [PubMed] [Google Scholar]
- Cunningham A, Slayford S, Vas C, Gee J, Costigan S, Prasad K. Development, validation and application of a device to measure e-cigarette users' puffing topography. Sci. Rep. 2016;6:35071. doi: 10.1038/srep35071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CS, McMahon LR. Multiple nicotine training doses in mice as a basis for differentiating the effects of smoking cessation aids. Psychopharmacology (Berl.) 2013;228:321–333. doi: 10.1007/s00213-013-3037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI. Influence of gender and sex hormones on nicotine acute pharmacological effects in mice. J. Pharmacol. Exp. Ther. 2001;296:132–140. [PubMed] [Google Scholar]
- Dawkins L, Turner J, Roberts A, Soar K. 'Vaping' profiles and preferences: An online survey of electronic cigarette users. Addiction. 2013;108:1115–1125. doi: 10.1111/add.12150. [DOI] [PubMed] [Google Scholar]
- de la Pena JB, Ahsan HM, Botanas CJ, Sohn A, Yu GY, Cheong JH. Adolescent nicotine or cigarette smoke exposure changes subsequent response to nicotine conditioned place preference and self-administration. Behav. Brain Res. 2014;272:156–164. doi: 10.1016/j.bbr.2014.06.044. [DOI] [PubMed] [Google Scholar]
- de la Pena JB, Ahsan HM, Tampus R, Botanas CJ, dela Pena IJ, Kim HJ, Sohn A, dela Pena I, Shin CY, Ryu JH, Cheong JH. Cigarette smoke exposure during adolescence enhances sensitivity to the rewarding effects of nicotine in adulthood, even after a long period of abstinence. Neuropharmacology. 2015;99:9–14. doi: 10.1016/j.neuropharm.2015.06.014. [DOI] [PubMed] [Google Scholar]
- George O, Grieder TE, Cole M, Koob GF. Exposure to chronic intermittent nicotine vapor induces nicotine dependence. Pharmacol. Biochem. Behav. 2010;96:104–107. doi: 10.1016/j.pbb.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Whitaker AM, Baynes B, Abdel AY, Weil MT, George O. Nicotine vapor inhalation escalates nicotine self-administration. Addict. Biol. 2014;19:587–592. doi: 10.1111/adb.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Mattson C, Lesage MG, Keyler DE, Pentel PR. Comparison of the behavioral effects of cigarette smoke and pure nicotine in rats. Pharmacol. Biochem. Behav. 2010;96:217–227. doi: 10.1016/j.pbb.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K, Bellis MA, Hardcastle KA, McHale P, Bennett A, Ireland R, Pike K. Associations between e-cigarette access and smoking and drinking behaviours in teenagers. BMC Pub. Health. 2015;15:244. doi: 10.1186/s12889-015-1618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippert AM. Temporal changes in the correlates of U.S. adolescent electronic cigarette use and utilization in tobacco cessation, 2011 to 2013. Health Educ. Behav. 2016 doi: 10.1177/1090198116650150. pii: 1090198116650150. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Lopez AA, Hiler MM, Soule EK, Ramoa CP, Karaoghlanian NV, Lipato T, Breland AB, Shihadeh AL, Eissenberg T. Effects of electronic cigarette liquid nicotine concentration on plasma nicotine and puff topography in tobacco vigarette smokers: A preliminary report. Nicotine Tob. Res. 2016;18:720–723. doi: 10.1093/ntr/ntv182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Lefever TW, Blough BE, Thomas BF, Wiley JL. Pharmacological effects of methamphetamine and alpha-PVP vapor and injection. Neurotoxicology. 2016;55:83–91. doi: 10.1016/j.neuro.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl.) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- McGrath-Morrow SA, Hayashi M, Aherrera A, Lopez A, Malinina A, Collaco JM, Neptune E, Klein JD, Winickoff JP, Breysse P, Lazarus P, Chen G. The effects of electronic cigarette emissions on systemic cotinine levels, weight and postnatal lung growth in neonatal mice. PLoS One. 2015;10:e0118344. doi: 10.1371/journal.pone.0118344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen RC, Gottlieb MA, Shaefer RM, Winickoff JP, Klein JD. Trends in electronic cigarette use among U.S. adults: Use is increasing in both smokers and nonsmokers. Nicotine Tob. Res. 2015;17:1195–1202. doi: 10.1093/ntr/ntu213. [DOI] [PubMed] [Google Scholar]
- Messina ES, Tyndale RF, Sellers EM. A major role for CYP2A6 in nicotine C-oxidation by human liver microsomes. J. Pharmacol. Exp. Ther. 1997;282:1608–1614. [PubMed] [Google Scholar]
- Misra M, Leverette RD, Cooper BT, Bennett MB, Brown SE. Comparative in vitro toxicity profile of electronic and tobacco cigarettes, smokeless tobacco and nicotine replacement therapy products: E-liquids, extracts and collected aerosols. Int. J. Environ. Res. Public Health. 2014;11:11325–11347. doi: 10.3390/ijerph111111325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SE, Raulinaitis V, Brown KM. Nicotine 5'-oxidation and methyl oxidation by P450 2A enzymes. Drug Metab. Dispos. 2005;33:1166–1173. doi: 10.1124/dmd.105.004549. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Okuda H, Nakashima T, Imaoka S, Funae Y. Nicotine metabolism by rat hepatic cytochrome P450s. Biochem. Pharmacol. 1993;45:2554–2556. doi: 10.1016/0006-2952(93)90238-r. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Coddington SB, Karelitz JL, Jetton C, Scott JA, Wilson AS, Lerman C. Variability in initial nicotine sensitivity due to sex, history of other drug use, and parental smoking. Drug Alcohol Depend. 2009;99:47–57. doi: 10.1016/j.drugalcdep.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: Review of human and animal evidence. Nicotine Tob. Res. 1999;1:301–315. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Vender J, Grobe J, Meeker J, Hutchison S. Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nicotine Tob. Res. 2001;3:141–150. doi: 10.1080/14622200110043059. [DOI] [PubMed] [Google Scholar]
- Petersen DR, Norris KJ, Thompson JA. A comparative study of the disposition of nicotine and its metabolites in three inbred strains of mice. Drug Metab. Dispos. 1984;12:725–731. [PubMed] [Google Scholar]
- Pogun S, Yararbas G. Sex differences in nicotine action. Handb. Exp. Pharmacol. 2009;192:261–291. doi: 10.1007/978-3-540-69248-5_10. [DOI] [PubMed] [Google Scholar]
- Ponzoni L, Moretti M, Sala M, Fasoli F, Mucchietto V, Lucini V, Cannazza G, Gallesi G, Castellana CN, Clementi F, Zoli M, Gotti C, Braida D. Different physiological and behavioural effects of e-cigarette vapour and cigarette smoke in mice. Eur. Neuropsychopharmacol. 2015;25:1775–1786. doi: 10.1016/j.euroneuro.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Ramoa CP, Hiler MM, Spindle TR, Lopez AA, Karaoghlanian N, Lipato T, Breland AB, Shihadeh A, Eissenberg T. Electronic cigarette nicotine delivery can exceed that of combustible cigarettes: A preliminary report. Tob. Control. 2016;25:e6–e9. doi: 10.1136/tobaccocontrol-2015-052447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RJ, Hensel EC, Morabito PN, Roundtree KA. Electronic cigarette topography in the natural environment. PLoS One. 2015;10:e0129296. doi: 10.1371/journal.pone.0129296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RJ, Hensel EC, Roundtree KA, Difrancesco AG, Nonnemaker JM, Lee YO. Week long topography study of young adults using electronic cigarettes in their natural environment. PLoS One. 2016;11:e0164038. doi: 10.1371/journal.pone.0164038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu EC, Wildenauer DB, Tyndale RF. Nicotine self-administration in mice is associated with rates of nicotine inactivation by CYP2A5. Psychopharmacology (Berl.) 2006;184:401–408. doi: 10.1007/s00213-006-0306-6. [DOI] [PubMed] [Google Scholar]
- Smith D, Aherrera A, Lopez A, Neptune E, Winickoff JP, Klein JD, Chen G, Lazarus P, Collaco JM, McGrath-Morrow SA. Adult behavior in male mice exposed to e-Cigarette nicotine vapors during late prenatal and early postnatal life. PLoS One. 2015;10:e0137953. doi: 10.1371/journal.pone.0137953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindle TR, Hiler MM, Breland AB, Karaoghlanian NV, Shihadeh AL, Eissenberg T. The influence of a mouthpiece-based topography measurement device on electronic cigarette user's plasma nicotine concentration, heart rate, and subjective effects under directed and ad libitum use conditions. Nicotine Tob Res. 2016 doi: 10.1093/ntr/ntw174. pii: ntw174. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussan TE, Gajghate S, Thimmulappa RK, Ma J, Kim JH, Sudini K, Consolini N, Cormier SA, Lomnicki S, Hasan F, Pekosz A, Biswal S. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One. 2015;10:e0116861. doi: 10.1371/journal.pone.0116861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvel SA, James JR, Bowen S, Rosecrans JA, Karan LD. Discriminative stimulus (DS) properties of nicotine in the C57BL/6 mouse. Pharmacol. Biochem. Behav. 1999;63:27–32. doi: 10.1016/s0091-3057(98)00262-7. [DOI] [PubMed] [Google Scholar]
- Velez de Mendizabal N, Jones DR, Jahn A, Bies RR, Brown JW. Nicotine and cotinine exposure from electronic cigarettes: a population approach. Clin. Pharmacokinet. 2015;54:615–626. doi: 10.1007/s40262-014-0221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Lefever TW, Antonazzo KR, Wallgren MT, Cortes RA, Patel PR, Grabenauer M, Moore KN, Thomas BF. AB-CHMINACA, AB-PINACA, and FUBIMINA: Affinity and potency of novel synthetic cannabinoids in producing delta-9-tetrahydrocannabinol-like effects in mice. J. Pharmacol. Exp. Ther. 2015;354:328–339. doi: 10.1124/jpet.115.225326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Bishnoi M, Keijzers KF, van Tuijl IA, Small E, Shah HP, Bauzo RM, Kobeissy FH, Sabarinath SN, Derendorf H, Bruijnzeel AW. Preadolescent tobacco smoke exposure leads to acute nicotine dependence but does not affect the rewarding effects of nicotine or nicotine withdrawal in adulthood in rats. Pharmacol. Biochem. Behav. 2010;95:401–409. doi: 10.1016/j.pbb.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.