Abstract
Diverse molecular mechanisms that confer acquired resistance to EGFR tyrosine kinase inhibitors (TKIs) in lung cancers with sensitive EGFR mutations have been reported. However, it is not realistic to analyze for all these mechanisms at the time of resistance in clinical practice and establish adequate treatment targeting these numerous resistance mechanisms. Therefore, we believe that we should move our research focus from the exploration of “established” diverse resistance mechanisms to the elucidation of molecular mechanisms that enable cancer cells to remain alive at the early phase of the treatment. Here in this review, we summarize up-to-date molecular mechanisms that maintain residual tumor cells against EGFR TKI monotherapy in lung cancers with EGFR mutations. We classified these mechanisms into three categories. The first is a pre-existing minor subpopulation with a resistance mechanism such as a pretreatment T790M mutation that can be detected by highly sensitivity methods. The second is the reversible drug-tolerant state that is often observed in cell line models and accounts for the lack of complete response and continued survival of cells exposed to EGFR TKIs in patients. And the last is the role of the microenvironment, including survival signaling from fibroblasts or dying cancer cells and the role of poor vascularization. Primary double-strike cancer therapy, or even initial multiple-strike therapy, to cancer cells that cotarget EGFR and survival mechanism(s) simultaneously would be a promising strategy to improve the outcomes of patients with EGFR mutations.
Keywords: EGFR mutation, Acquired resistance, Molecular mechanisms, Drug-tolerant state, Microenvironment, Tumor heterogeneity
On the basis of data from six phase III trials that compared gefitinib,1,2 erlotinib,3,4 or afatinib5,6 with chemotherapy as initial treatment of patients with advanced NSCLC with sensitive EGFR mutations (exon 19 deletion or L858R mutation), EGFR tyrosine kinase inhibitor (TKI) monotherapy has become the standard frontline treatment for these patients.7–9 However, acquisition of resistance to these EGFR TKIs at a median of 9 to 13 months is inevitable, thus restricting the improvement of patients’ outcomes. Despite the fact that almost all cancer cells in these patients harbor “sensitive” EGFR mutations10,11 and most patients have tumor shrinkage, complete responses are rare and all patients progress, indicating that a large number of cancer cells survive with the inevitable acquired resistance.
To understand and ultimately overcome the molecular mechanisms underlying the acquired resistance, a number of studies analyzed tissue specimens obtained from patients in whom acquired resistance developed.12–17 Analyses of cell line models or xenograft models of development of acquired resistance against chronic exposure to these drugs have also shed light on mechanisms of acquired resistance.18–23
Resistance mechanism–based second-line treatment would be one of a number of reasonable treatment strategies to further improve patients’ outcomes. However, our experience with the HCC827 lung adenocarcinoma cell line model24 indicates that cancer cells are flexible enough to always find a way to survive. Therefore, we believe that we should move our research focus from the exploration of “established” diverse resistance mechanisms to the elucidation of molecular mechanisms that enable cancer cells to remain alive at the early phase of the treatment (mechanisms that allow survival of residual tumor cells25). Upfront polytherapy that cotargets residual tumor cells may improve treatment outcomes, as shown in highly active antiretroviral therapy, a combination of antiretroviral agents with different mechanisms of action against highly flexible human immunodeficiency virus.26 Highly active antiretroviral therapy has changed a fatal disease, acquired immunodeficiency syndrome, into a chronic disorder in developed countries. Similar strategies involving a combination of agents with different mechanisms of action to prevent the emergence of resistance have also been applied in the treatment of tuberculosis27 and hepatitis C virus.28
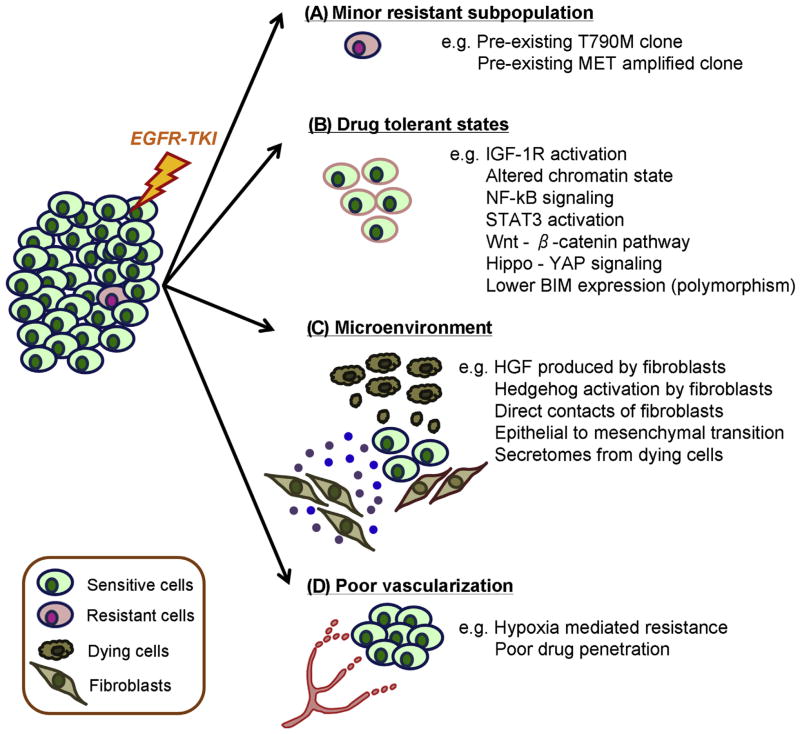
Here in this review, we summarize up-to-date molecular mechanisms that allow survival in the presence of EGFR TKI monotherapy in lung cancers with EGFR mutations. As shown in Figure 1, we classified these mechanisms into three categories, including a preexisting minor subpopulation with a resistance mechanism (Fig. 1A), a reversible drug-tolerant state (Fig. 1B), and the microenvironment (Fig. 1C and D).
Figure 1.
Molecular mechanisms that support residual tumor cells in the early phase of EGFR tyrosine kinase inhibitor (TKI) monotherapy. Preexistence of a minor resistance subpopulation that will survive frontline EGFR TKI monotherapy (A), reversible drug-tolerant state mainly observed in cell line models (B), survival signaling from microenvironment (fibroblasts or dying cancer cells) (C), and role of poor vascularization (D) are shown. MET, MET proto-oncogene, receptor tyrosine kinase gene; IGF-1R, insulin-like growth factor 1 receptor; NF-κB, nuclear factor kappa light-chain enhancer of activated B cells; STAT3, signal transducer and activator of transcription 3; YAP, yes-associated protein; BIM, BCL2 like 11; HGF, human growth factor.
Preexisting Minor Resistant Subpopulation
The evidence of a preexistent minor subpopulation with T790M mutation12 has been reported since 2006,29 with high-sensitivity methods used to detect this resistance mutation.30–34 Patients with the scant T790M mutation should be strictly distinguished from rare patients with double mutations (an activating EGFR mutation together with the abundant T790M mutation that is detectable in routine clinical molecular testing35), and some of them carry T790M mutation as germline mutations.36–38 A recent ultrasensitive detection study in which droplet digital polymerase chain reaction was used to identify T790M mutation observed that 298 of 373 NSCLCs with activating EGFR mutation (79.9%) carried pretreatment T790M mutation. It is of note that the allele frequency of the EGFR T790M mutation was between 0.001% and 0.1% in most of the cases (95%),39 and cases with abundant T790M allele (≥10%) are very rare (0.5%). It is unclear why cancer cells “prepare” this resistance mutation before EGFR TKI therapy. However, a recent study suggested that hypermethylation of the CpG dinucleotide in EGFR codon 790 easily leads to the C-to-T transition mutation, causing T790M mutation.40 Therefore, it is possible that cancer cells may harbor several minor subpopulations with different a EGFR secondary mutation, including a TKI-resistant T790M mutation. Several studies have suggested that the pre-existing minor T790M mutation is related to decreased efficacy of gefitinib or erlotinib monotherapy,30,34 whereas others have observed the opposite results.32
Preexistence of another resistance mechanism, MET proto-oncogene, receptor tyrosine kinase gene (MET) amplification, is also reported in clinical specimens by high-throughput fluorescence in situ hybridization analyses.41 There are currently no data, as far as we know, regarding the preexistence of other resistance mechanisms, such as erb-b2 receptor tyrosine kinase 2 gene (ERBB2) amplification, AXL receptor tyrosine kinase activation, or SCLC transformation (although the presence of mixed small cell/adenocarcinoma at diagnosis has been recognized for many years).
In lung cancer cell lines with activating EGFR mutations, evidence supports the preexistence of minor resistant subpopulations, such as T790M mutation in PC9 cells42 and MET gene amplification in HCC827 cells.41 H1975 cells, with L858R mutation and T790M mutation, are the cell line model for aforementioned double mutations, but not for ones with a preexistent minor T790M subpopulation.
It is natural to consider that these preexistent minor subpopulation cells with a resistance mechanism are selected through the EGFR TKI therapy (see Fig. 1A) because most of the sensitive cells are killed and emerge as acquired resistance. This hypothesis is also supported by the fact that each lung cancer cell line with EGFR mutation has its “favorite” resistance mechanism: T790M mutation in PC9 cells, MET gene amplification in HCC827 and H4011 cells, and epithelial-mesenchymal transition (EMT) phenotype in HCC4006 and H1975 cells.16,21,43–47
Upfront polytherapy, targeting both EGFR and minor subpopulation cells with a resistance mechanism, may be a promising treatment strategy to overcome or delay the emergence of resistance. At the laboratory bench, studies using upfront polytherapies conferred different resistance mechanisms in PC9 cells,48,49 HCC827 cells,14 and HCC4006 cells50,51 as compared with their favorite ones. In addition, in our experience, a longer exposure period was needed to establish cells resistant to a combination therapy of erlotinib plus a MET proto-oncogene, receptor tyrosine kinase (MET) TKI than to erlotinib alone in HCC827 cells.14 In a clinical trial, osimertinib, a third-generation EGFR TKI that is able to inhibit the T790M mutation, has shown longer progression-free survival (PFS) in the frontline setting (19.3 months)52 as compared with data from first- or second-generation EGFR TKIs (9.6–13.7 months1–6). This may due to the fact that most patients with EGFR-mutated lung cancer (79.9%) carry a tumor subpopulation with T790M mutation.39 It is interesting to theorize whether a difference in PFS could be found between patients with a minor T790M subpopulation and those without T790M at frontline osimertinib treatment. Regarding MET gene amplification targeting, clinical trials with tivantinib, a small-molecule MET inhibitor, combined with erlotinib did not show a PFS benefit over erlotinib alone in a small EGFR-mutant subgroup with prior cytotoxic chemotherapy treatment.53,54 Currently, most of the trials with a combination of EGFR TKI and MET TKIs (crizotinib, tivantinib, cabozantinib, volitinib, MSC2156119J, or INC280)55 are focusing on EGFR-mutated patients after treatment failure with prior EGFR TKI (the phase II group 2 part of NCT02335944 uses patients without prior EGFR TKI therapy). Frontline combination of EGFR TKI and MET TKI in patients with EGFR-mutated lung cancer with a preexistent minor subpopulation with MET-amplified cells is an interesting strategy for another primary double-strike therapy candidate.
Reversible Drug-Tolerant State
In vitro observations demonstrated that the vast majority of EGFR-mutated lung cancer cells are killed within a few days upon exposure to a clinically relevant concentration of EGFR TKIs, whereas a small fraction of viable, largely quiescent cells (~0.3%) can still be detected 9 days later (see Fig. 1B).56 In clinical practice, we can say that when a patient starts with 1011 cancer cells,57 there are still 3 × 108 cells or more remaining even if an EGFR TKI eliminates cancer cells as effectively as in cell culture dishes. This drug-tolerant status is reversible, as cells propagated in drug-free media rapidly reacquire EGF TKI sensitivity and have also been shown to arise in cells established from a single-cell clone. Therefore, the mechanism(s) of cell survival is different from genetic changes such as acquisition of T790M mutation or MET gene amplification. However, recent studies have demonstrated that “irreversible” resistance mechanisms such as T790M mutation will occur in these drug-tolerant persisting cells after continuous exposure to EGFR TKIs.42,58
Survival signaling in drug-tolerant persisting cells is well studied for PC9 cells; it involves the insulin-like growth factor 1 receptor (IGF-1R) pathway or an altered chromatin state that requires the histone demethylase retinol binding protein 2/lysine demethylase 5A.42,56 In PC9 cells, exposure to a combination therapy with an IGF-1R inhibitor, AEW541, and an EGFR TKI eliminated these persisting drug-tolerant cells.42,56 However, it is unclear whether IGF-1R signaling or an altered chromatin state plays an important role in all EGFR-mutated lung cancer cell survival. Currently, there are no clinical data regarding the combination of IGF-1R inhibitor with EGFR TKI in patients with EGFR-mutated lung cancer, but discouraging results for this combination in a cohort of unselected patients with lung cancer have been reported.59,60
Another candidate pathway involved in drug-tolerant status against EGFR TKIs is nuclear factor kappa light-chain enhancer of activated B cells (NF-κB) signaling. A pooled RNA interference screen identified that down-regulation of several components of the NF-κB pathway specifically enhanced cell death induced by EGFR TKI in lung cancer cells with EGFR mutation.61 The role of NF-κB activation in resistance to apoptosis with EGFR TKI treatment was confirmed by later research using patient-derived tumor xenograft models62 and in three-dimensional cultured cell models.63 The former study also identified signal transducer and activator of transcription 3 as the important downstream molecule in the NF-κB pathway that confers resistance to apoptosis,62 confirming the importance of feedback activation of signal transducer and activator of transcription 3 in primary insensitivity to EGFR TKIs.64,65 Recently, inhibition of casein kinase 1 alpha has been reported to prevent acquired resistance to EGFR TKI in vitro and in vivo through suppression of the NF-κB signaling pathway.66
Another RNA interference screen found that the canonical Wnt/beta-catenin pathway (in particular, the poly–adenosine diphosphate–ribosylating enzymes tankyrase 1 and 2 that positively regulate this signaling) contributes to the survival of cancer cells during EGFR inhibition.67 In addition, tankyrase has recently been reported to allow cancer cells to survive against EGFR TKI through the Hippo/yes-associated protein pathway.68 A tankyrase inhibitor, AZ1366, showed synergistic efficacy with gefitinib in H3255, H1650, HCC4006, and HCC4011 cells but not in HCC827 and PC9 cells.69
BCL2 like 11 (BIM) is another molecule that may be related to decreased apoptosis in response to EGFR TKIs. EGFR TKIs cause a rapid increase of BIM protein expression with proapoptotic BCL2 homology domain 3 (BH3) in lung cancer cells with EGFR mutation, and RNA interference–mediated knockdown of BIM eliminates EGFR TKI–inducible apoptosis, whereas addition of the BH3 mimetics (ABT-737) enhances EGFR TKI–mediated apoptosis.70,71 Later studies found that pretreatment RNA levels of BIM predicted the capacity of EGFR TKIs to induce apoptosis,72 and a common BCL2 like 11 gene (BIM) deletion polymorphism mediates intrinsic resistance to EGFR TKIs through the expression of BIM isoforms lacking BH3.73 In addition, EGFR-mutant lung cancer cell lines that harbor the BIM deletion polymorphism are much less sensitive to EGFR TKI–inducible apoptosis, and the histone deacetylase inhibitor vorinostat could circumvent low EGFR TKI sensitivity in these cell lines.74 However, the impact of BIM deletion polymorphism as a predictive biomarker for EGFR TKI is still controversial in clinical settings.75–77
Signals from the Microenvironment
It has also been shown that cancer cells receive survival signaling from the microenvironment that may modify drug efficacy (see Fig. 1C).78 In 2008, Yano, et al. showed that addition of hepatocyte growth factor (HGF), but not epidermal growth factor, transforming growth factor-α, or insulin-like growth factor 1, in the conditioned media conferred resistance to EGFR TKIs in lung cancer cells with EGFR mutations.79 HGF is often produced by fibroblasts, and lung cancer cells became resistant to EGFR TKIs when cocultured in vitro with HGF-producing fibroblasts or coinjected into immune-deficient mice.80 Survival signaling may also be mediated by fibroblasts through hedgehog signaling.81 Other growth factors or chemokines, such as fibroblast growth factor 244,82 or interleukin-8,83,84 are also reported to cause established acquired resistance to EGFR TKIs in vitro through overexpression by cancer cells themselves (autocrine). However, the role of these molecules produced from microenvironment noncancerous cells in the survival of residual tumor cells is unclear to date. Direct interaction of podoplanin-positive cancer-associated fibroblasts with cancer cells is also reported to cause resistance to EGFR TKIs in vitro, and EGFR-mutated lung cancer patients with podoplanin-positive cancer-associated fibroblasts demonstrated reduced PFS when treated with EGFR TKIs.85 Fibroblast-mediated stimulation,81 or many other factors including cigarette smoke extract,86 may also cause a reduced EGFR TKI sensitivity at the early phase of the treatment through the induction of EMT.18 Transforming growth factor-β receptor inhibition, together with EGFR TKI, is reported to inhibit EMT-mediated acquired resistance to EGFR TKI in vitro.50 In addition, histone deacetylase inhibitors have been shown to overcome EGFR TKI resistance linked to epigenetic changes and EMT state in vitro87–89; however, the clinical data are limited so far.90,91
A recent study demonstrated that in addition to survival signaling from fibroblasts, secretomes produced by dying cells in response to EGFR TKI therapy may help residual cancer cells survive against the drug.92 It is unclear whether this resistance conferred via the microenvironment can lead to development of bona fide resistance mechanism(s) in cancer cells. However, in one in vitro study, researchers observed that HGF exposure facilitated HCC827 cells’ acquisition of MET gene–amplified resistance.41
Nonfunctional blood vessels in tumor tissues are also one of hallmarks of the tumor microenvironment (see Fig. 1D). This poor vascularization causes a hypoxic environment, and tumor hypoxia is associated with aggressive tumor phenotypes, treatment resistance, and poor clinical prognosis.93 Several in vitro studies observed that EGFR-mutated lung cancer cells showed insensitivity to EGFR TKIs under hypoxic cell culture conditions compared with under normoxia conditions.94,95 Poor vascularization is also considered to provide poor drug delivery to cancer cells, causing a lower EGFR TKI concentration, which is related to earlier development of resistance than with higher drug concentrations.96,97 In clinical settings, the addition of bevacizumab (an antiangiogenic monoclonal antibody that targets vascular endothelial growth factor and may modify aberrant vessels around a tumor) to the EGFR TKI erlotinib has shown dramatic improvement of PFS (16.0 months versus 9.7 months).98
Conclusions and Future Perspectives
Here, we have summarized possible scenarios that lung cancer cells with EGFR mutation utilize to survive the early phase of treatment with EGFR TKIs. As shown in clinical trials with frontline osimertinib52 or the combination of erlotinib plus bevacizumab,98 primary double-strike therapies for cancer cells that cotarget EGFR and survival mechanism(s) simultaneously would be a promising strategy to improve the outcomes of patients with EGFR mutations. In addition, abundant data from the bench, as summarized here, may provide additional ideas for primary double-strike therapies for cancer cells. Outside the scope of this review, there are additional ideas for improved treatments for patients with lung cancer with EGFR mutations, such as combination of EGFR TKI with MEK inhibitors,49,99 combination of EGFR TKI with immune checkpoint inhibitors, or combination of EGFR TKI with cytotoxic agents100, as summarized in a recent clinical review.55 Although a dozen years have passed since discovery of the EGFR mutation,101–103 this research area still holds substantial interest in the research community.
Acknowledgments
This study was supported by an International Association for the Study of Lung Cancer Young Investigator Award (to Dr. Suda). We declare that we had no writing assistance.
Footnotes
The remaining authors declare no conflict of interest.
Disclosure: Dr. Bunn has received consulting income from Genentech, Eli Lilly, Merck, Merck Serono, Pfizer, and Clovis Oncology. Dr. Mitsudomi has received honoraria from AstraZeneca, Chugai, Boehringer-Ingelheim, Pfizer, and Roche; compensation from AstraZeneca, Chugai, Boehringer-Ingelheim, Pfizer, Roche, and Clovis Oncology for participating in advisory boards; and research funding (through Kinki University Faculty of Medicine) from AstraZeneca and Chugai. Dr. Hirsch has received compensation from Genentech/Roche, Pfizer, Bristol-Myers Squibb, Lilly, Merck, AstraZeneca, Boehringer-Ingelheim, and Ventana/Roche for participating in advisory boards, and he has received research funding (through the University of Colorado) from Genetech/Roche, BMS, Lilly, Bayer, Amgen, and Ventana/Roche.
References
- 1.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 2.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 3.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 4.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 5.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 6.Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–222. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 7.Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8:823–859. doi: 10.1097/JTO.0b013e318290868f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leighl NB, Rekhtman N, Biermann WA, et al. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology guideline. J Clin Oncol. 2014;32:3673–3679. doi: 10.1200/JCO.2014.57.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerr KM, Bubendorf L, Edelman MJ, et al. Second ESMO consensus conference on lung cancer: pathology and molecular biomarkers for non-small-cell lung cancer. Ann Oncol. 2014;25:1681–1690. doi: 10.1093/annonc/mdu145. [DOI] [PubMed] [Google Scholar]
- 10.Yatabe Y, Matsuo K, Mitsudomi T. Heterogeneous distribution of EGFR mutations is extremely rare in lung adenocarcinoma. J Clin Oncol. 2011;29:2972–2977. doi: 10.1200/JCO.2010.33.3906. [DOI] [PubMed] [Google Scholar]
- 11.Suda K, Murakami I, Yu H, et al. Heterogeneity of EGFR aberrations and correlation with histological morphologies: analyses of therapy naive isogenic lung cancer lesions with EGFR mutation. J Thorac Oncol. 2016;11:1711–1717. doi: 10.1016/j.jtho.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 13.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suda K, Murakami I, Katayama T, et al. Reciprocal and complementary role of MET amplification and EGFR T790M mutation in acquired resistance to kinase inhibitors in lung cancer. Clin Cancer Res. 2010;16:5489–5498. doi: 10.1158/1078-0432.CCR-10-1371. [DOI] [PubMed] [Google Scholar]
- 15.Takezawa K, Pirazzoli V, Arcila ME, et al. HER2 amplification: a potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site EGFRT790M mutation. Cancer Discov. 2012;2:922–933. doi: 10.1158/2159-8290.CD-12-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19:2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 19.Engelman JA, Mukohara T, Zejnullahu K, et al. Allelic dilution obscures detection of a biologically significant resistance mutation in EGFR-amplified lung cancer. J Clin Invest. 2006;116:2695–2706. doi: 10.1172/JCI28656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guix M, Faber AC, Wang SE, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J Clin Invest. 2008;118:2609–2619. doi: 10.1172/JCI34588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suda K, Tomizawa K, Fujii M, et al. Epithelial to mesenchymal transition in an epidermal growth factor receptor-mutant lung cancer cell line with acquired resistance to erlotinib. J Thorac Oncol. 2011;6:1152–1161. doi: 10.1097/JTO.0b013e318216ee52. [DOI] [PubMed] [Google Scholar]
- 22.Pirazzoli V, Nebhan C, Song X, et al. Acquired resistance of EGFR-mutant lung adenocarcinomas to afatinib plus cetuximab is associated with activation of mTORC1. Cell Rep. 2014;7:999–1008. doi: 10.1016/j.celrep.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Z, Lee JC, Lin L, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet. 2012;44:852–860. doi: 10.1038/ng.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suda K, Tomizawa K, Osada H, et al. Conversion from the “oncogene addiction” to “drug addiction” by intensive inhibition of the EGFR and MET in lung cancer with activating EGFR mutation. Lung Cancer. 2012;76:292–299. doi: 10.1016/j.lungcan.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Bivona TG, Doebele RC. A framework for understanding and targeting residual disease in oncogene-driven solid cancers. Nat Med. 2016;22:472–478. doi: 10.1038/nm.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chmielecki J, Pao W. Highly active antitumor therapy (HAATT) for epidermal growth factor receptor-mutant lung cancer. Clin Cancer Res. 2010;16:5371–5373. doi: 10.1158/1078-0432.CCR-10-2405. [DOI] [PubMed] [Google Scholar]
- 27.Saltini C. Chemotherapy and diagnosis of tuberculosis. Respiratory Med. 2006;100:2085–2097. doi: 10.1016/j.rmed.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Dhingra A, Kapoor S, Alqahtani SA. Recent advances in the treatment of hepatitis C. Discovery Med. 2014;18:203–208. [PubMed] [Google Scholar]
- 29.Inukai M, Toyooka S, Ito S, et al. Presence of epidermal growth factor receptor gene T790M mutation as a minor clone in non-small cell lung cancer. Cancer Res. 2006;66:7854–7858. doi: 10.1158/0008-5472.CAN-06-1951. [DOI] [PubMed] [Google Scholar]
- 30.Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359:366–377. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosell R, Molina MA, Costa C, et al. Pretreatment EGFR T790M mutation and BRCA1 mRNA expression in erlotinib-treated advanced non-small-cell lung cancer patients with EGFR mutations. Clin Cancer Res. 2011;17:1160–1168. doi: 10.1158/1078-0432.CCR-10-2158. [DOI] [PubMed] [Google Scholar]
- 32.Fujita Y, Suda K, Kimura H, et al. Highly sensitive detection of EGFR T790M mutation using colony hybridization predicts favorable prognosis of patients with lung cancer harboring activating EGFR mutation. J Thorac Oncol. 2012;7:1640–1644. doi: 10.1097/JTO.0b013e3182653d7f. [DOI] [PubMed] [Google Scholar]
- 33.Su KY, Chen HY, Li KC, et al. Pretreatment epidermal growth factor receptor (EGFR) T790M mutation predicts shorter EGFR tyrosine kinase inhibitor response duration in patients with non-small-cell lung cancer. J Clin Oncol. 2012;30:433–440. doi: 10.1200/JCO.2011.38.3224. [DOI] [PubMed] [Google Scholar]
- 34.Costa C, Molina MA, Drozdowskyj A, et al. The impact of EGFR T790M mutations and BIM mRNA expression on outcome in patients with EGFR-mutant NSCLC treated with erlotinib or chemotherapy in the randomized phase III EURTAC trial. Clin Cancer Res. 2014;20:2001–2010. doi: 10.1158/1078-0432.CCR-13-2233. [DOI] [PubMed] [Google Scholar]
- 35.Yu HA, Arcila ME, Hellmann MD, Kris MG, Ladanyi M, Riely GJ. Poor response to erlotinib in patients with tumors containing baseline EGFR T790M mutations found by routine clinical molecular testing. Ann Oncol. 2014;25:423–428. doi: 10.1093/annonc/mdt573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bell DW, Gore I, Okimoto RA, et al. Inherited susceptibility to lung cancer may be associated with the T790M drug resistance mutation in EGFR. Nat Genet. 2005;37:1315–1316. doi: 10.1038/ng1671. [DOI] [PubMed] [Google Scholar]
- 37.Oxnard GR, Miller VA, Robson ME, et al. Screening for germline EGFR T790M mutations through lung cancer genotyping. J Thorac Oncol. 2012;7:1049–1052. doi: 10.1097/JTO.0b013e318250ed9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu HA, Arcila ME, Harlan Fleischut M, et al. Germline EGFR T790M mutation found in multiple members of a familial cohort. J Thorac Oncol. 2014;9:554–558. doi: 10.1097/JTO.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watanabe M, Kawaguchi T, Isa S, et al. Ultra-sensitive detection of the pretreatment EGFR T790M mutation in non-small cell lung cancer patients with an EGFR-activating mutation using droplet digital PCR. Clin Cancer Res. 2015;21:3552–3560. doi: 10.1158/1078-0432.CCR-14-2151. [DOI] [PubMed] [Google Scholar]
- 40.Fujii A, Harada T, Iwama E, et al. Hypermethylation of the CpG dinucleotide in epidermal growth factor receptor codon 790: implications for a mutational hotspot leading to the T790M mutation in non-small-cell lung cancer. Cancer Genet. 2015;208:271–278. doi: 10.1016/j.cancergen.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Turke AB, Zejnullahu K, Wu YL, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17:77–88. doi: 10.1016/j.ccr.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hata AN, Niederst MJ, Archibald HL, et al. Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat Med. 2016;22:262–269. doi: 10.1038/nm.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suda K, Mizuuchi H, Maehara Y, Mitsudomi T. Acquired resistance mechanisms to tyrosine kinase inhibitors in lung cancer with activating epidermal growth factor receptor mutation-diversity, ductility, and destiny. Cancer Metastasis Rev. 2012;31:807–814. doi: 10.1007/s10555-012-9391-7. [DOI] [PubMed] [Google Scholar]
- 44.Ware KE, Hinz TK, Kleczko E, et al. A mechanism of resistance to gefitinib mediated by cellular reprogramming and the acquisition of an FGF2-FGFR1 autocrine growth loop. Oncogenesis. 2013;2:e39. doi: 10.1038/oncsis.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shien K, Toyooka S, Yamamoto H, et al. Acquired resistance to EGFR inhibitors is associated with a manifestation of stem cell-like properties in cancer cells. Cancer Res. 2013;73:3051–3061. doi: 10.1158/0008-5472.CAN-12-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hashida S, Yamamoto H, Shien K, et al. Acquisition of cancer stem cell-like properties in non-small cell lung cancer with acquired resistance to afatinib. Cancer Sci. 2015;106:1377–1384. doi: 10.1111/cas.12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshida T, Song L, Bai Y, et al. ZEB1 mediates acquired resistance to the epidermal growth factor receptor-tyrosine kinase inhibitors in non-small cell lung cancer. PloS One. 2016;11:e0147344. doi: 10.1371/journal.pone.0147344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cortot AB, Repellin CE, Shimamura T, et al. Resistance to irreversible EGF receptor tyrosine kinase inhibitors through a multistep mechanism involving the IGF1R pathway. Cancer Res. 2013;73:834–843. doi: 10.1158/0008-5472.CAN-12-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eberlein CA, Stetson D, Markovets AA, et al. Acquired resistance to the mutant-selective EGFR inhibitor AZD9291 is associated with increased dependence on RAS signaling in preclinical models. Cancer Res. 2015;75:2489–2500. doi: 10.1158/0008-5472.CAN-14-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soucheray M, Capelletti M, Pulido I, et al. Intratumoral heterogeneity in EGFR mutant NSCLC results in divergent resistance mechanisms in response to EGFR tyrosine kinase inhibition. Cancer Res. 2015;75:4372–4383. doi: 10.1158/0008-5472.CAN-15-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suda K, Rivard CJ, Mitsudomi T, Hirsch FR. Heterogeneity in tumors and resistance to EGFR TKI therapy-Letter. Cancer Res. 2016;76:3109–3110. doi: 10.1158/0008-5472.CAN-15-2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramalingam S, Yang JC, Lee CK, et al. LBA1_PR: osimertinib as first-line treatment for EGFR mutation-positive advanced NSCLC: updated efficacy and safety results from two phase I expansion cohorts. J Thorac Oncol. 2016;11(suppl 4):S152. [Google Scholar]
- 53.Sequist LV, von Pawel J, Garmey EG, et al. Randomized phase II study of erlotinib plus tivantinib versus erlotinib plus placebo in previously treated non-small-cell lung cancer. J Clin Oncol. 2011;29:3307–3315. doi: 10.1200/JCO.2010.34.0570. [DOI] [PubMed] [Google Scholar]
- 54.Scagliotti G, von Pawel J, Novello S, et al. Phase III multinational, randomized, double-blind, placebo-controlled study of tivantinib (ARQ 197) plus erlotinib versus erlotinib alone in previously treated patients with locally advanced or metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2015;33:2667–2674. doi: 10.1200/JCO.2014.60.7317. [DOI] [PubMed] [Google Scholar]
- 55.Zhou C, Yao LD. Strategies to improve outcomes of patients with EGRF-mutant non-small cell lung cancer: review of the literature. J Thorac Oncol. 2016;11:174–186. doi: 10.1016/j.jtho.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 56.Sharma SV, Lee DY, Li B, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell sub-populations. Cell. 2010;141:69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Del Monte U. Does the cell number 10(9) still really fit one gram of tumor tissue? Cell Cycle. 2009;8:505–506. doi: 10.4161/cc.8.3.7608. [DOI] [PubMed] [Google Scholar]
- 58.Ramirez M, Rajaram S, Steininger RJ, et al. Diverse drug-resistance mechanisms can emerge from drug-tolerant cancer persister cells. Nature Commun. 2016;7:10690. doi: 10.1038/ncomms10690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramalingam SS, Spigel DR, Chen D, et al. Randomized phase II study of erlotinib in combination with placebo or R1507, a monoclonal antibody to insulin-like growth factor-1 receptor, for advanced-stage non-small-cell lung cancer. J Clin Oncol. 2011;29:4574–4580. doi: 10.1200/JCO.2011.36.6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goldberg SB, Supko JG, Neal JW, et al. A phase I study of erlotinib and hydroxychloroquine in advanced non-small-cell lung cancer. J Thorac Oncol. 2012;7:1602–1608. doi: 10.1097/JTO.0b013e318262de4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bivona TG, Hieronymus H, Parker J, et al. FAS and NF-kappaB signalling modulate dependence of lung cancers on mutant EGFR. Nature. 2011;471:523–526. doi: 10.1038/nature09870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blakely CM, Pazarentzos E, Olivas V, et al. NF-kappaB-activating complex engaged in response to EGFR oncogene inhibition drives tumor cell survival and residual disease in lung cancer. Cell Rep. 2015;11:98–110. doi: 10.1016/j.celrep.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sakuma Y, Yamazaki Y, Nakamura Y, et al. NF-kappaB signaling is activated and confers resistance to apoptosis in three-dimensionally cultured EGFR-mutant lung adenocarcinoma cells. Biochem Biophys Res Commun. 2012;423:667–671. doi: 10.1016/j.bbrc.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 64.Lee HJ, Zhuang G, Cao Y, Du P, Kim HJ, Settleman J. Drug resistance via feedback activation of Stat3 in oncogene-addicted cancer cells. Cancer Cell. 2014;26:207–221. doi: 10.1016/j.ccr.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 65.Kim SM, Kwon OJ, Hong YK, et al. Activation of IL-6R/ JAK1/STAT3 signaling induces de novo resistance to irreversible EGFR inhibitors in non-small cell lung cancer with T790M resistance mutation. Mol Cancer Ther. 2012;11:2254–2264. doi: 10.1158/1535-7163.MCT-12-0311. [DOI] [PubMed] [Google Scholar]
- 66.Lantermann AB, Chen D, McCutcheon K, et al. Inhibition of casein kinase 1 alpha prevents acquired drug resistance to erlotinib in EGFR-mutant non-small cell lung cancer. Cancer Res. 2015;75:4937–4948. doi: 10.1158/0008-5472.CAN-15-1113. [DOI] [PubMed] [Google Scholar]
- 67.Casas-Selves M, Kim J, Zhang Z, et al. Tankyrase and the canonical Wnt pathway protect lung cancer cells from EGFR inhibition. Cancer Res. 2012;72:4154–4164. doi: 10.1158/0008-5472.CAN-11-2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang H, Lu B, Castillo J, et al. Tankyrase inhibitor sensitizes lung cancer cells to endothelial growth factor receptor (EGFR) inhibition via stabilizing angiomotins and inhibiting YAP signaling. J Biol Chem. 2016;291:15256–15266. doi: 10.1074/jbc.M116.722967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scarborough HA, Helfrich BA, Casas-Selves M, et al. AZ1366: An inhibitor of tankyrase and the canonical Wnt pathway that limits the persistence of non-small cell lung cancer cells following EGFR inhibition [e-pub ahead of print] [accessed October 6, 2016];Clin Cancer Res. doi: 10.1158/1078-0432.CCR-16-1179. http://dx.doi.org/10.1158/1078-0432.CCR-16-1179. [DOI] [PMC free article] [PubMed]
- 70.Gong Y, Somwar R, Politi K, et al. Induction of BIM is essential for apoptosis triggered by EGFR kinase inhibitors in mutant EGFR-dependent lung adenocarcinomas. PLoS Med. 2007;4:e294. doi: 10.1371/journal.pmed.0040294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cragg MS, Kuroda J, Puthalakath H, Huang DC, Strasser A. Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires BIM and can be enhanced by BH3 mimetics. PLoS Med. 2007;4:1681–1689. doi: 10.1371/journal.pmed.0040316. discussion 1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Faber AC, Corcoran RB, Ebi H, et al. BIM expression in treatment-naive cancers predicts responsiveness to kinase inhibitors. Cancer Discov. 2011;1:352–365. doi: 10.1158/2159-8290.CD-11-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ng KP, Hillmer AM, Chuah CT, et al. A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nat Med. 2012;18:521–528. doi: 10.1038/nm.2713. [DOI] [PubMed] [Google Scholar]
- 74.Nakagawa T, Takeuchi S, Yamada T, et al. EGFR-TKI resistance due to BIM polymorphism can be circumvented in combination with HDAC inhibition. Cancer Res. 2013;73:2428–2434. doi: 10.1158/0008-5472.CAN-12-3479. [DOI] [PubMed] [Google Scholar]
- 75.Lee JK, Shin JY, Kim S, et al. Primary resistance to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) in patients with non-small-cell lung cancer harboring TKI-sensitive EGFR mutations: an exploratory study. Ann Oncol. 2013;24:2080–2087. doi: 10.1093/annonc/mdt127. [DOI] [PubMed] [Google Scholar]
- 76.Isobe K, Hata Y, Tochigi N, et al. Clinical significance of BIM deletion polymorphism in non-small-cell lung cancer with epidermal growth factor receptor mutation. J Thorac Oncol. 2014;9:483–487. doi: 10.1097/JTO.0000000000000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu SG, Liu YN, Yu CJ, Yang PC, Shih JY. Association of BIM deletion polymorphism with intrinsic resistance to EGFR tyrosine kinase inhibitors in patients with lung adenocarcinoma. JAMA Oncol. 2016;2:826–828. doi: 10.1001/jamaoncol.2016.0016. [DOI] [PubMed] [Google Scholar]
- 78.McMillin DW, Negri JM, Mitsiades CS. The role of tumour-stromal interactions in modifying drug response: challenges and opportunities. Nat Rev Drug Disc. 2013;12:217–2128. doi: 10.1038/nrd3870. [DOI] [PubMed] [Google Scholar]
- 79.Yano S, Wang W, Li Q, et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res. 2008;68:9479–9487. doi: 10.1158/0008-5472.CAN-08-1643. [DOI] [PubMed] [Google Scholar]
- 80.Wang W, Li Q, Yamada T, et al. Crosstalk to stromal fibroblasts induces resistance of lung cancer to epidermal growth factor receptor tyrosine kinase inhibitors. Clin Cancer Res. 2009;15:6630–6638. doi: 10.1158/1078-0432.CCR-09-1001. [DOI] [PubMed] [Google Scholar]
- 81.Choe C, Shin YS, Kim C, et al. Crosstalk with cancer-associated fibroblasts induces resistance of non-small cell lung cancer cells to epidermal growth factor receptor tyrosine kinase inhibition. Onco Targets Ther. 2015;8:3665–3678. doi: 10.2147/OTT.S89659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Terai H, Soejima K, Yasuda H, et al. Activation of the FGF2-FGFR1 autocrine pathway: a novel mechanism of acquired resistance to gefitinib in NSCLC. Mol Cancer Res. 2013;11:759–767. doi: 10.1158/1541-7786.MCR-12-0652. [DOI] [PubMed] [Google Scholar]
- 83.Liu YN, Chang TH, Tsai MF, et al. IL-8 confers resistance to EGFR inhibitors by inducing stem cell properties in lung cancer. Oncotarget. 2015;6:10415–10431. doi: 10.18632/oncotarget.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fernando RI, Hamilton DH, Dominguez C, David JM, McCampbell KK, Palena C. IL-8 signaling is involved in resistance of lung carcinoma cells to erlotinib [e-pub ahead of print] [accessed August 10, 2016];Oncotarget. 2016 doi: 10.18632/oncotarget.9662. http://dx.doi.org/10.18632/oncotarget.9662. [DOI] [PMC free article] [PubMed]
- 85.Yoshida T, Ishii G, Goto K, et al. Podoplanin-positive cancer-associated fibroblasts in the tumor microenvironment induce primary resistance to EGFR-TKIs in lung adenocarcinoma with EGFR mutation. Clin Cancer Res. 2015;21:642–651. doi: 10.1158/1078-0432.CCR-14-0846. [DOI] [PubMed] [Google Scholar]
- 86.Li D, Zhang L, Zhou J, Chen H. Cigarette smoke extract exposure induces EGFR-TKI resistance in EGFR-mutated NSCLC via mediating Src activation and EMT. Lung Cancer. 2016;93:35–42. doi: 10.1016/j.lungcan.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 87.Edwards A, Li J, Atadja P, Bhalla K, Haura EB. Effect of the histone deacetylase inhibitor LBH589 against epidermal growth factor receptor-dependent human lung cancer cells. Mol Cancer Ther. 2007;6:2515–2524. doi: 10.1158/1535-7163.MCT-06-0761. [DOI] [PubMed] [Google Scholar]
- 88.Zhang W, Peyton M, Xie Y, et al. Histone deacetylase inhibitor romidepsin enhances anti-tumor effect of erlotinib in non-small cell lung cancer (NSCLC) cell lines. J Thorac Oncol. 2009;4:161–166. doi: 10.1097/JTO.0b013e318194fae7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Greve G, Schiffmann I, Pfeifer D, Pantic M, Schuler J, Lubbert M. The pan-HDAC inhibitor panobinostat acts as a sensitizer for erlotinib activity in EGFR-mutated and -wildtype non-small cell lung cancer cells. BMC Cancer. 2015;15:947. doi: 10.1186/s12885-015-1967-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Witta SE, Jotte RM, Konduri K, et al. Randomized phase II trial of erlotinib with and without entinostat in patients with advanced non-small-cell lung cancer who progressed on prior chemotherapy. J Clin Oncol. 2012;30:2248–2255. doi: 10.1200/JCO.2011.38.9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gray JE, Haura E, Chiappori A, et al. A phase I, pharmacokinetic, and pharmacodynamic study of panobinostat, an HDAC inhibitor, combined with erlotinib in patients with advanced aerodigestive tract tumors. Clin Cancer Res. 2014;20:1644–1655. doi: 10.1158/1078-0432.CCR-13-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Obenauf AC, Zou Y, Ji AL, et al. Therapy-induced tumour secretomes promote resistance and tumour progression. Nature. 2015;520:368–372. doi: 10.1038/nature14336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wigerup C, Pahlman S, Bexell D. Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacol Ther. 2016;164:152–169. doi: 10.1016/j.pharmthera.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 94.Minakata K, Takahashi F, Nara T, et al. Hypoxia induces gefitinib resistance in non-small-cell lung cancer with both mutant and wild-type epidermal growth factor receptors. Cancer Sci. 2012;103:1946–1954. doi: 10.1111/j.1349-7006.2012.02408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Murakami A, Takahashi F, Nurwidya F, et al. Hypoxia increases gefitinib-resistant lung cancer stem cells through the activation of insulin-like growth factor 1 receptor. PloS One. 2014;9:e86459. doi: 10.1371/journal.pone.0086459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hayakawa H, Ichihara E, Ohashi K, et al. Loer gefitinib dose led to earlier resistance acquisition before emergence of T790M mutation in epidermal growth factor receptor-mutated lung cancer model. Cancer Sci. 2013;104:1440–1446. doi: 10.1111/cas.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Furugaki K, Iwai T, Moriya Y, Harada N, Fujimoto-Ouchi K. Loss of an EGFR-amplified chromosome 7 as a novel mechanism of acquired resistance to EGFR-TKIs in EGFR-mutated NSCLC cells. Lung Cancer. 2014;83:44–50. doi: 10.1016/j.lungcan.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 98.Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014;15:1236–1244. doi: 10.1016/S1470-2045(14)70381-X. [DOI] [PubMed] [Google Scholar]
- 99.Tricker EM, Xu C, Uddin S, et al. Combined EGFR/MEK inhibition prevents the emergence of resistance in EGFR-mutant lung cancer. Cancer Discov. 2015;5:960–971. doi: 10.1158/2159-8290.CD-15-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cheng Y, Murakami H, Yang PC, et al. Randomized phase II trial of gefitinib with and without pemetrexed as first-line therapy in patients with advanced nonsquamous non-small-cell lung cancer with activating epidermal growth factor receptor mutations. J Clin Oncol. 2016;34:3258–3266. doi: 10.1200/JCO.2016.66.9218. [DOI] [PubMed] [Google Scholar]
- 101.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 102.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 103.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]