Abstract
Cancer-associated thrombosis remains a significant complication in the clinical management of cancer and interactions of the hemostatic system with cancer biology continue to be elucidated. Here, we review recent progress in our understanding of tissue factor (TF) regulation and procoagulant activation, TF signaling in cancer and immune cells, and the expanding roles of the coagulation system in stem cell niches and the tumor microenvironment. The extravascular functions of coagulant and anti-coagulant pathways have significant implications not only for tumor progression, but also for the selection of appropriate target specific anticoagulants in the therapy of cancer patients.
Keywords: Tissue Factor, tumor microenvironment, coagulation, inflammation
Introduction
The clinical association of the hemostatic system and cancer progression has long been recognized from the high incidence of venous thromboembolism, including Trousseau's syndrome, in patients with advanced cancers (1). Cancer cells express tissue factor (TF) and, upon exposure to blood, activate the plasmatic coagulation cascade and platelets during metastasis (2). Consequently, metastatic disease is associated with an increased risk for thrombosis. A large body of experimental evidence has delineated a multitude of interactions between cancer cells, the blood and the vascular endothelium that contribute to the efficiency of metastatic tumor cell dissemination (3;4). However, tumor cell TF expression is not the sole determinant for cancer-associated thrombosis and cancer types display marked differences in the incidence of clinically relevant thromboembolism (5;6). Contributing factors are direct activations of platelets (7) and the contact phase (8) by which tumor cells independently promote intravascular coagulation and thrombosis. Moreover, while tumor cell TF expression is sufficient to cause signs of systemic coagulation activation, it does not predict the release of TF bearing microvesicles (MV) with measurable procoagulant activity into the circulation of tumor bearing mice (9). The cellular mechanisms of TF activation, regulation, and incorporation into procoagulant MV remain active areas of research and recent advances on the functional regulation of the TF pathway in untransformed and transformed cells will be discussed in this review.
Understanding the cellular and tumor specific mechanisms of coagulation activation is pivotal for therapy and prevention of cancer-associated thrombosis, a major complication of cancer progression as well as cancer therapy (10). However, the last two decades have provided us with a wealth of new information demonstrating that the hemostatic system influences substantially and directly aspects of tumor cell biology ranging from angiogenesis, cancer stem cell maintenance implicated in therapy resistance, to the immune cell composition of the tumor micro-environment. While cancer cell proangiogenic effects of TF had originally established the pathophysiological relevance of upstream coagulation signaling (11), recent progress has elucidated additional novel roles of the coagulant and anti-coagulant balance in physiological innate immune signaling and hematopoiesis. These new directions of research have potentially far reaching implications for cancer progression and immune evasion. As discussed below, the broader roles of the hemostatic system in the response to injury and infection may contribute to the characteristics of cancers as “wounds that do not heal”, a term originally coined to describe the similarities of tumor and regenerative angiogenesis in the context of a deposited transitional fibrin-rich extracellular matrix (12). Most important, a better understanding of the physiological and pathophysiological functions of coagulant signaling in stem cell niches and tumor microenvironments may provide additional guidance for choices of Vitamin K antagonists and target-selective oral anticoagulants in adjuvant cancer therapy.
Regulation of TF activity and procoagulant MV release
The procoagulant activity of TF is crucial for metastasis and a unique property of full-length, but not alternatively spliced (as) TF, a circulating, soluble isoform of TF with a unique C-terminus replacing the cytoplasmic and trans-membrane domains of full-length TF (13). Untransformed cells maintain TF mainly in a non-coagulant state on the cell surface and require activating signals to convert TF to a procoagulant receptor. Non-coagulant, cryptic TF in non-tumorogenic epithelial cells binds FVIIa with low affinity, but fully supports proteolytic signaling of the TF-FVIIa complex through protease activated receptor 2 (PAR2) (14). TF is maintained in a cryptic state by protein disulfide isomerase (PDI) dependent thiol-disulfide exchange reactions that modify an allosteric disulfide bond in the TF extracellular domain (14;15). Activating stimuli, including Ca2+ fluxes (15;16), can convert cryptic TF to a high affinity receptor for FVIIa and fully procoagulant state in the context of cell surface exposure of negatively charged phosphatidylserine (PS) (17). Subsequent studies have shown that TF prothrombotic activity and fibrin formation in vivo is indeed dependent on PDI-activity (18-20).
While initial studies employed rather non-physiological stimuli, including strong oxidizing substances, to activate cell surface TF, pathophysiological relevant scenarios of TF activation on untransformed hematopoietic and vascular cells have been delineated, including platelet-leukocyte interactions (20), cell injury signals activating the purinergic P2X7 receptor (18), and activation of the complement cascade (21) (Fig. 1). In the latter case, conversion of monocyte TF to a procoagulant form depends on both, redox changes of surface PDI caused by complement factor C5 activation as well as PS exposure by the subsequent membrane insertion of activated C7. Aggressive cancer cells do not regulate TF activity through PDI and thiol-disulfide exchange pathways in the same way as non-malignant cells do (22), which may contribute to the latent procoagulant state in cancer patients.
Fig. 1. Cellular Regulation of TF with activating pathways in red and inhibitory mechanisms in green.
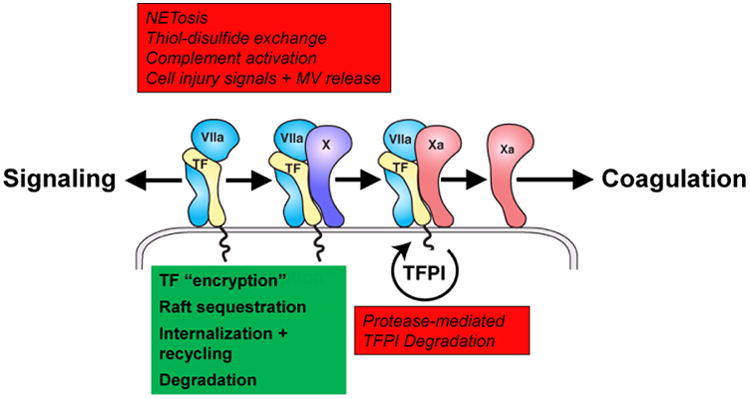
Another important aspect of TF activation on cells is the coupling of these events to molecular pathways that culminate in the generation of procoagulant MV. A hallmark of cancer cells is the spontaneous release of MV bearing TF (23). Procoagulant MV generation, rather than cancer cell procoagulant activity per se, strongly correlates with metastatic behavior of syngeneic mouse tumor cells (24). TF is released on MV that also incorporate adhesion receptors, including P-selectin glycoprotein ligand-1 (PSGL1) and β1 integrins (18), and remodeling of the actin cytoskeleton plays a crucial role in facilitating TF insertion into MV. The TF cytoplasmic domain interacts with the actin binding protein filamin A (25) which is crucial for targeting of TF to MV released from PAR2-stimulated cancer cells (26;27). A different role for cytoskeletal anchoring of TF emerged from the study of macrophages that tightly control TF activity dependent on cell adhesion (28). In primed macrophages, filamin A restricts mobility of TF located primarily in glycosphingolipid-rich raft domains and thus prevents TF recruitment onto highly procoagulant MV, unless filamin A is proteolytically cleaved by calpain.
Macrophage calpain is activated as the consequence of a series of events that results from triggering of the purinergic P2X7 receptor by high concentrations of ATP, a cell injury signal that may be released from tumor cells under hypoxic stress or chemotherapeutic insult. P2X7 receptor activation, dependent on endosomal reactive oxygen species (ROS) and activation of thioredoxin reductase, causes extracellular release of thioredoxin and thereby reductive changes of cell surface proteins, PS exposure and TF activation (28). While the allosteric TF disulfide can be reduced by thioredoxin to inactivate TF (29), TF procoagulant activation and release of TF on MV also requires PDI (18;28). The release of TF and other cell surface receptors on these highly procoagulant and prothrombotic MV requires trafficking of TF to the tips of filopodia that form during macrophage activation by P2X7 receptor signaling. The thiol disulfide exchange reactions following this injury response also trigger activation of the inflammasome and the effector caspase 1 that not only promotes the release of pro-inflammatory IL1β, but also -through actin destabilization- the final severing and release of highly procoagulant MV. Thus, the P2X7 receptor triggered activation of TF procoagulant activity is an apparently evolutionary conserved mechanism to couple coagulation and inflammation.
The finding that cell adhesion is required for primary macrophages to maintain TF in a non-coagulant state is in line with the known crosstalk of TF and its cytoplasmic domain with integrin and cell adhesion signaling (25;30;31), but the precise mechanism by which normal cells control cell surface TF procoagulant activity remains to be elucidated. It is tempting to speculate that the same pathways that control coagulation may in turn serve signaling functions of the TF-FVIIa complex in extravascular locations. Conversely, deregulated cell adhesion and integrin function of tumor cells may disable adhesion-dependent control of TF activity and thus favor prothrombotic MV release. However, tumor cells release also TF with little procoagulant activity into the circulation (9) and these forms of TF may primarily serve signaling roles after uptake by vascular and perivascular cells (32-34), similar to recently elucidated integrin-dependent exosome preconditioning of metastatic niches (35).
There is also expanding genetic and experimental evidence that in addition to TF expression, regulation of TF procoagulant activity is a relevant determinant for cancer progression. In addition to the demonstrated role of the anticoagulant protein C (PC) pathway in controlling intravascular tumor dissemination (13;36-39), endothelial TF pathway inhibitor (TFPI) expression limits TF-dependent experimental metastasis (40). Tumor cell expression of TFPI is regulated by hypoxia inducible factor (HIF) 1α (41) and TFPI expression by tumor cells is correlated with improved outcome in breast cancer (42). Genetic polymorphisms associated with breast cancer furthermore show that traditional prothrombotic risk factors are not the sole determinant for tumor progression and that additional hemostatic components, i.e. FX and the endothelial protein C receptor (EPCR), may be contributing to the development of cancer (43).
TF signaling in tumorcells and angiogenesis
The coagulation cascade influences many facets of cancer development beyond the metastatic process, including angiogenesis, immune evasion and tumor growth. Simon Karpatkin proposed that thrombin is a key effector protease that facilitates the transition from tumor dormancy (4), but a wealth of additional data implicate direct TF signaling as a central pathway promoting tumor progression. Oncogenic mutations in a number of key signaling pathways cause the constitutive upregulation of TF, including activating mutations in the phosphatidylinositol 3 (PI-3) kinase pathway, the epidermal growth factor receptor (EGFR), and the hepatocyte growth factor receptor (MET proto-oncogene), as well as loss of tumor suppressors (44). TF is frequently upregulated in concert with its main cell signaling receptor, PAR2, and hypoxia through induction of HIF2α, epigenetic changes in chromatin structure, and androgen receptor signaling can induce tumor cell autonomous synthesis of FVIIa (45-51). Thrombin-independent, tumor cell TF-FVIIa-PAR2 signaling induces a range of pro-angiogenic and monocyte/macrophage-stimulating growth factors and cytokines that promote tumor angiogenesis and progression in both xenograft and genetic models of spontaneous tumor development (49;52-54). In turn, the TF-induced exit from tumor dormancy and recruitment of inflammatory cells amplifies genetic alterations in tumor cells and thus directly impacts tumor progression (55).
The tumor promoting effects of TF signaling employ, in a deregulated manner, cellular pathways that are related to physiological functions of TF in adaptation and repair. TF-PAR2 signaling regulates physiological and pathological angiogenesis that involves in vascular and/or perivascular cells crosstalk with platelet-derived growth factor receptor β (PDGFRβ) signaling (56-59), chemokine regulation (60), and Wnt signaling (61;62). TF-VIIa-PAR2 signaling also synergizes with EGFR and insulin growth factor receptor (IGFR) signaling (63). While IGFR cross-activation protects tumor cells from apoptosis through activation of the PI-3 kinase pathway, this pathway may participate in PI-3 kinase-Akt signaling dependent weight gain regulation by TF-FVIIa signaling (64). Cancer cell signaling of the TF-FVIIa-FXa coagulation initiation complex induces PI-3 kinase-mTOR signaling (65), which may be related to the finding that autophagy is suppressed by TF-PAR2 signaling in hepatocellular carcinoma (66). In addition to growth factor transactivation, TF-FVIIa influences tyrosine receptor signaling by direct proteolysis of the ephrin tyrosine kinase receptors B2 and A2 (67). Since these receptors control cell-cell interaction, it is tempting to speculate that TF-FVIIa induced cleavage releases growth inhibition and enables migration in support of physiological tissue repair. If TF-FVIIa activity remains unchecked by extracellular inhibitors or cellular degradation pathways, TF-FVIIa may foster tumor invasion by the same mechanism.
TF centrally controls the cell adhesion and migration machinery through interactions with integrins. The extracellular domain of TF is a ligand for several integrin β1 heterodimers and αvβ3 (30) and ligand FVIIa binding to TF regulates association specifically with the basement matrix laminin-binding integrins α3β1 and α6β1 (49). The alternative splicing of TF preserves binding with αvβ3 and α6β1 and soluble asTF can enhance tumorigenicity of luminal breast cancer cells through autocrine integrin β1 signaling as well as through endothelial cell αvβ3 ligation promoting sprouting angiogenesis and upregulation of myeloid cell adhesion molecules (68-70). While integrin ligation pathways of asTF and transmembrane TF are overlapping, asTF, unlike full-length TF, cannot rescue early embryonic failure of the yolk sac vasculature caused by TF-deficiency in mice (71).
In addition, full-length TF plays a particularly important role in promoting tumor growth of highly aggressive triple negative breast cancer. The concept that TF-PAR2 signaling is linked to integrin association emerged from the inhibitory properties of a unique monoclonal antibody, 10H10, that has minimal effects on FVIIa binding or TF procoagulant activity, but abolishes constitutive and FVIIa-induced interaction of TF with integrins (49). This antibody potently inhibits TF-FVIIa induction of proangiogenic cytokines and consistently blocks, unlike anti-coagulant anti-TF antibodies, angiogenesis and tumor growth of aggressive breast cancer, melanoma, and glioblastoma xenograft tumors (49;72;73). Genetic studies in spontaneously developing breast cancer in mice furthermore showed that PAR2 and TF cytoplasmic domain signaling have overlapping functions in tumor progression from adenoma to invasive carcinoma (53;54), a process requiring macrophage recruitment and angiogenesis.
How the TF cytoplasmic domain is contributing to proangiogenic PAR2 signaling remains to be elucidated, but the phosphorylation status of the TF cytoplasmic domain may serve as a potential biomarker to indicate active or deregulated TF-PAR2 signaling crosstalk in vivo. The human TF cytoplasmic domain is sequentially phosphorylated by protein kinase C at Ser253 and by Pro-directed kinases, including p38 MAP kinase (74), at Ser258 (75;76) and adopts a more compact structure upon phosphorylation (77). PAR2 activation induces TF phosphorylation (78), and in primary breast cancer biopsies Ser258 phosphorylation status of TF is correlated with PAR2 expression and only found in patients with relapse after initial therapy (79). While the TF cytoplasmic domain of TF is not required for embryogenesis, it regulates integrin function (30), activation of p38 MAPkinase (31;74;80), and PAR2 signaling in angiogenesis (56). Increased TF expression in macrophages of TF cytoplasmic domain deleted mice indicates that TF degradation may also be regulated by this domain (28;80). The phenocopy of PAR2-deficient and TF cytoplasmic domain-deleted mice in different disease models (53;64) suggests that TF intracellular interactions may be particularly important for binary TF-FVIIa signaling.
Coagulation signaling of immune cells
TF signaling is not restricted to the non-coagulant binary TF-FVIIa complex, but the nascent product FXa of coagulant TF-FVIIa also efficiently activates PAR2 and PAR1 (81). The remarkable switch in protease utilization from FVIIa to FXa in TF-dependent PAR2 cleavage remained mechanistically poorly understood until the demonstration that EPCR is required for signaling of the TF-FVIIa-FXa complex in a variety of cell types from mouse and man (82;83). The γ-carboxyglutamic acid-rich (Gla) domains of activated PC (aPC) and FVIIa are essentially identical and human FVIIa can interact with EPCR similar to aPC and apparently independent of TF (84-86). Binding of the FXa Gla-domain, however, is distinctly dependent on the presence of Mg2+ that also substantially alters the interaction of FX with TF-FVIIa (83). EPCR not only supports ternary TF-FVIIa-FXa signaling by presumably stabilizing the signaling conformation of this complex, but the soluble form of EPCR can also alter FX activation by TF-FVIIa, even when its interaction with TF-FVIIa is prevented through mutation of the FVIIa Gla-domain. While these data strongly supported a direct interaction of EPCR with FXa, it remains an open question whether the binary TF-FVIIa complex can engage EPCR for biologically relevant signaling responses or anticoagulation (87).
Further progress in understanding biological functions of the ternary complex came from studies of the LPS response in innate immune cells. A subset of LPS-responsive genes in dendritic cells of septic animals was no longer induced in the absence of both, EPCR and PAR2 (88). TF deficiency, TF antibody blockade, inhibition of FXa, or rendering PAR2 cleavage-insensitive by mutation in mice abolished the induction of a gene signature known to involve the recruitment of the adaptor TRIF to toll-like receptor (TLR) 4. The crucial importance of the TF ternary complex in activating this TLR4 pathway was confirmed by a unique TFPI-like inhibitor, nematode anticoagulant protein (NAP) c2, that stabilizes the TF-FVIIa-FXa ternary complex by blocking FVIIa without inhibiting FXa signaling (81;83;89). In the presence of NAPc2, the TF-induced TRIF response was not only preserved, but stabilization of the ternary complex with NAPc2 prevented the suppression of TF signaling by aPC. This signaling role of aPC is, similar to aPC anticoagulant activity, dependent on protein S as well as FV anticoagulant cofactor function that is lost in FVLeiden (90). These data suggest that EPCR ligand occupancy by either FXa or aPC determines TLR4-TRIF activation and indicate a control switch function for EPCR in the hardwiring of innate immune and coagulation signaling.
Despite its stabilizing effect on the ternary TF-FVIIa-FXa signaling complex, NAPc2 is primarily a potent anticoagulant as well as an inhibitor of TF-FVIIa-PAR2 signaling in angiogenesis (57;91). While the pronounced anti-tumor effects of NAPc2 in spontaneous colon cancer development may be due to either of these activities, one should consider whether effects on immune cell signaling are contributing factors in reducing oncogenesis (92). Effects of NAPc2 on immunity have been documented, including reduction in viral load in Ebola virus infected animals (93;94), which in part may be caused by diminished viral uptake, as demonstrated by the effect of the inhibitor on herpes simplex virus infection of endothelial cells (95). In addition, there is expanding evidence that TF and PAR signaling not only regulates TLR4 (96-98), but also a variety of anti-viral responses (99). While there is considerable cell type variability in the effects of PAR deletion and PAR agonist stimulation on these TLR signaling responses, data are sparse on the involvement of specific proteases that activate PARs and thus alter viral pathogenesis.
Similar to infectious scenarios, coagulation and immune cell derived proteases characterize the tumor microenvironment (TME). Inflammation is an essential part of tumor biology and contributes to both, tumor elimination as well as reprogramming of immune responses to promote immune evasion (100). The plasticity of macrophages renders these innate immune cells particularly susceptible to dynamic changes in the TME and tumor associated macrophages (TAM) are therefore involved in multiple aspects of tumor progression (101-103). The tumor microenvironment through incompletely defined effector pathways induces alternative macrophage activation to a repair-type phenotype supporting angiogenesis and matrix remodeling. It is tempting to speculate that alternative macrophage activation involves, in part, the intrinsic coupling of PAR signaling and innate immune signaling. Indeed, the upstream coagulation factors VIIa and X are specifically expressed by TAMs (48;104), directly positioning the proteases participating in TF signaling complexes as relevant players in the TME.
Coagulant pathways in extravascular stem cells environments
The bone marrow environment recently emerged as another example where the coagulation system has major extravascular functions beyond the regulation of hemostasis and thrombosis with potential implications for cancer stem cell biology (105). Long-term repopulating hematopoietic stem cells (HSC) are tightly regulated by their bone marrow niches to undergo proliferation upon demand, while returning to quiescence to prevent bone marrow exhaustion after stress and injury situations are resolved. The extravascular balance of pro-coagulant and anti-coagulant pathways in the bone marrow niche turned out to be crucial to regulate HSC mobilization and retention. Specifically, PAR1 signaling by thrombin or aPC bound to EPCR provide opposing signals controlling HSC fate. The most primitive HSC with long-term repopulating capacity express the highest levels of EPCR (106), but EPCR expression is lost when HSC exit the bone marrow. Thrombin in the bone marrow is crucial for the exit of HSC by PAR1-dependent release of the mobilizing chemokine CXCL12 from stromal cells, induction of ADAM17/TACE-mediated EPCR shedding from HSC, and mobility increase of HSC (105).
In contrast, EPCR expression is crucial for HSC retention specifically in bone marrow niches and loss of EPCR, function blocking antibodies, or mutations in the aPC binding site (107) cause increased numbers of circulating HSC. EPCR engagement by active aPC, but not by FVIIa, leads to endothelial cell nitric oxide synthase (eNOS) phosphorylation at the Thr495 negative regulatory site that limits NO production (105). The suppressive effect of aPC/EPCR/PAR1 signaling can be reversed by thrombin-PAR1 signaling leading to activating eNOS Ser1177 phosphorylation and NO production. This regulatory switch of PAR1 signaling controls motility and adhesion of HSC. In the EPCR-induced quiescent state, HSC have reduced levels of active CDC42, a motility promoting small GTPase, adopt polarization, and increase affinity of the integrin α4β1 that plays a pivotal role for HSC retention through VCAM1 and fibronectin interaction in the bone marrow niche. HSC aPC/EPCR/PAR1 signaling supports engraftment in bone marrow transplantation, and, conversely, loss of this pathway increases the susceptibility to bone marrow failure under myelo-ablative chemotherapy.
The coagulant and anticoagulant pathways thus play important regulatory roles not only within the vasculature, but also in perivascular and extravascular environments. These physiological processes may be relevant e.g. for the retention and survival of leukemic cells in bone marrow niches. EPCR not only marks HSC, but also neuronal, epithelial and pluripotent mammary gland progenitor populations (108-112), and broader roles for EPCR in cancer and normal stem cell regulation can be expected. EPCR is best studied in breast cancer where it marks cancer stem cells (113) and is functionally important for their population of tumor niches at very low numbers (48). Function blocking antibodies to EPCR also block growth of heterogeneous tumor consisting of cancer stem and more differentiated cells. Intriguingly, the studied breast cancer stem cell population also expresses integrin α4β1 and EPCR ligation increased proliferation specifically on matrices for integrin α4β1. Although EPCR suppresses tumor progression of procoagulant TF-PAR1 dependent mesothelioma (114), EPCR supports lung cancer aggressiveness (115), suggesting that EPCR may influence stem cell phenotypes in several cancer types. It will be of interest for future studies to better understand how cancer cells utilize the coagulant and anticoagulant pathways in the TME and metastatic niches for survival and the escape from cytotoxic cancer therapy.
Targeting the coagulant pathways in cancer
The emerging functions of the pro- and anti-coagulant pathways in cell signaling and regulation of extracellular microenvironments give new perspectives on challenges and opportunities in treating cancer patients with anticoagulants. While the focus in the therapy of non-malignant thromboembolic disease is on restoring the intravascular pro- and anticoagulant balance, removal of flow restrictions and therapeutic interventions in disease-causing risk factors, cancer-associated thrombosis is frequently chronic and even aggravated by therapeutic intervention. The distinct contributions of extravascular coagulation activation in the TME may limit efficacy of plasma protease inhibitor-dependent anticoagulants, like heparin, and make cancer-associated thrombosis more approachable with oral small molecular target selective anticoagulants. Although certain prostate cancer cells may utilize contact pathway activation for cancer-associated thrombosis (8), redundant coagulation activation by stromal cell TF (72) likely limits the utility of selective targeting the contact pathway as primary therapy for cancer-associated thrombosis.
In the pioneering studies by Leo Zacharski (116), anticoagulation by blocking activity of Gla-domain-containing proteins with Vitamin K antagonists produced a remarkable survival benefit in patients with small cell lung cancer. The expanding knowledge of both tumor promoting and pathogenic mechanisms of coagulation activation in cancer and molecular definition of cancer subtypes may eventually lead to improved matching of patients for therapy with the expanding repertoire of target selective anticoagulants. The elucidation of cancer cell TF signaling mechanisms indicates that targeting the upstream extrinsic coagulation pathway may have beneficial effects on both cancer-associated thrombosis as well as tumor-promoting activities of the hemostatic system. TFPI-like inhibitors, like NAPc2 and Ixolaris have anticancer activities (117;118) that are expected to be recapitulated by direct inhibitors of FVIIa. While it is unclear whether targeting FVIIa directly carries an increased bleeding risk compared to direct inhibitors of FXa or thrombin, the latter are less likely to attenuate tumor cells TF signaling and tumor promoting activities of the extrinsic pathway. The concept that TF signaling can be selectively inhibited by antibody or other modulators of TF function (49) has not been evaluated clinically, but may be particularly attractive as adjuvant therapy, even in patients with increased risk for bleeding.
Highlights.
Progress in the understanding of TF activation and signaling provides new perspectives for coagulation contributions to cancer-associated thrombosis and tumor progression
Extravascular coagulation signaling plays novel roles in stem cell niches and the tumor microenvironment with implications for anticoagulant therapy in cancer
Acknowledgments
The work described in the review was supported by NIH grants HL-31950 and HL-60742, the Humboldt Foundation, and BMBF grant 01EO1503.
Footnotes
Conflict of interest statement: W.R. consults for Iconic Therapeutics.
References
- 1.Falanga A, Schieppati F, Russo D. Cancer Tissue Procoagulant Mechanisms and the Hypercoagulable State of Patients with Cancer. Semin Thromb Hemost. 2015 Oct;41(7):756–64. doi: 10.1055/s-0035-1564040. [DOI] [PubMed] [Google Scholar]
- 2.Mueller BM, Reisfeld RA, Edgington TS, Ruf W. Expression of tissue factor by melanoma cells promotes efficient hematogenous metastasis. Proc Natl Acad Sci USA. 1992;89(24):11832–6. doi: 10.1073/pnas.89.24.11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruf W, Mueller BM. Thrombin generation and the pathogenesis of cancer. Semin Thromb Hemost. 2006 Apr;32(Suppl 1):61–8. doi: 10.1055/s-2006-939555. [DOI] [PubMed] [Google Scholar]
- 4.Nierodzik ML, Karpatkin S. Thrombin induces tumor growth, metastasis, and angiogenesis: Evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell. 2006 Nov;10(5):355–62. doi: 10.1016/j.ccr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Hisada Y, Geddings JE, Ay C, Mackman N. Venous thrombosis and cancer: from mouse models to clinical trials. J Thromb Haemost. 2015 Aug;13(8):1372–82. doi: 10.1111/jth.13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falanga A, Russo L, Milesi V. The coagulopathy of cancer. Curr Opin Hematol. 2014 Sep;21(5):423–9. doi: 10.1097/MOH.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 7.Shao B, Wahrenbrock MG, Yao L, David T, Coughlin SR, Xia L, et al. Carcinoma mucins trigger reciprocal activation of platelets and neutrophils in a murine model of Trousseau syndrome. Blood. 2011 Aug 22;118(15):4015–23. doi: 10.1182/blood-2011-07-368514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nickel KF, Ronquist G, Langer F, Labberton L, Fuchs TA, Bokemeyer C, et al. The polyphosphate-factor XII pathway drives coagulation in prostate cancer-associated thrombosis. Blood. 2015 Sep 10;126(11):1379–89. doi: 10.1182/blood-2015-01-622811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang JG, Geddings JE, Aleman MM, Cardenas JC, Chantrathammachart P, Williams JC, et al. Tumor-derived tissue factor activates coagulation and enhances thrombosis in a mouse xenograft model of human pancreatic cancer. Blood. 2012 Jun 7;119(23):5543–52. doi: 10.1182/blood-2012-01-402156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013 Sep 5;122(10):1712–23. doi: 10.1182/blood-2013-04-460121. [DOI] [PubMed] [Google Scholar]
- 11.Ruf W, Disse J, Carneiro-Lobo TC, Yokota N, Schaffner F. Tissue factor and cell signalling in cancer progression and thrombosis. J Thromb Haemost. 2011 Jul;9(Suppl 1):306–15. doi: 10.1111/j.1538-7836.2011.04318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dvorak HF. Tumors: wounds that do not heal-redux. Cancer Immunol Res. 2015 Jan;3(1):1–11. doi: 10.1158/2326-6066.CIR-14-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yokota N, Zarpellon A, Chakrabarty S, Bogdanov VY, Gruber A, Castellino FJ, et al. Contributions of thrombin targets to tissue factor-dependent metastasis in hyperthrombotic mice. J Thromb Haemost. 2014 Jan;12(1):71–81. doi: 10.1111/jth.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahamed J, Versteeg HH, Kerver M, Chen VM, Mueller BM, Hogg PJ, et al. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proc Natl Acad Sci USA. 2006;103(38):13932–7. doi: 10.1073/pnas.0606411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen VM, Ahamed J, Versteeg HH, Berndt MC, Ruf W, Hogg PJ. Evidence for activation of tissue factor by an allosteric disulfide bond. Biochemistry. 2006;45(39):12020–8. doi: 10.1021/bi061271a. [DOI] [PubMed] [Google Scholar]
- 16.Bach RR, Moldow CF. Mechanism of tissue factor activation on HL-60 cells. Blood. 1997;89:3270–6. [PubMed] [Google Scholar]
- 17.Langer F, Ruf W. Synergies of phosphatidylserine and protein disulfide isomerase in tissue factor activation. Thromb Haemost. 2014 Apr 1;111(4):590–7. doi: 10.1160/TH13-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furlan-Freguia C, Marchese P, Gruber A, Ruggeri ZM, Ruf W. P2X7 receptor signaling contributes to tissue factor-dependent thrombosis in mice. J Clin Invest. 2011 Jul 1;121(7):2932–44. doi: 10.1172/JCI46129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho J, Furie BC, Coughlin SR, Furie B. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J Clin Invest. 2008 Mar;118(3):1123–31. doi: 10.1172/JCI34134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinhardt C, von Bruhl ML, Manukyan D, Grahl L, Lorenz M, Altmann B, et al. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J Clin Invest. 2008 Mar 3;118(3):1110–22. doi: 10.1172/JCI32376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langer F, Spath B, Fischer C, Stolz M, Ayuk FA, Kroger N, et al. Rapid activation of monocyte tissue factor by antithymocyte globulin is dependent on complement and protein disulfide isomerase. Blood. 2013 Mar 21;121(12):2324–35. doi: 10.1182/blood-2012-10-460493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang HP, Hogg PJ. Critical importance of the cell system when studying tissue factor de-encryption. Blood. 2008 Aug 1;112(3):912–3. doi: 10.1182/blood-2008-05-158766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dvorak HF, Quay SC, Orenstein NS, Dvorak AM, Hahn P, Bitzer AM, et al. Tumor shedding and coagulation. Science. 1981;212:923–4. doi: 10.1126/science.7195067. [DOI] [PubMed] [Google Scholar]
- 24.Grimstad IA, Prydz H. Thromboplastin release, but not content, correlates with spontaneous metastasis of cancer cells. Int J Cancer. 1988;41:427–31. doi: 10.1002/ijc.2910410319. [DOI] [PubMed] [Google Scholar]
- 25.Ott I, Fischer EG, Miyagi Y, Mueller BM, Ruf W. A role for tissue factor in cell adhesion and migration mediated by interaction with actin binding protein 280. J Cell Biol. 1998;140(5):1241–53. doi: 10.1083/jcb.140.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koizume S, Ito S, Yoshioka Y, Kanayama T, Nakamura Y, Yoshihara M, et al. High-level secretion of tissue factor-rich extracellular vesicles from ovarian cancer cells mediated by filamin-A and protease-activated receptors. Thromb Haemost. 2015 Oct 8;115(2) doi: 10.1160/TH15-03-0213. [DOI] [PubMed] [Google Scholar]
- 27.Collier ME, Maraveyas A, Ettelaie C. Filamin-A is required for the incorporation of tissue factor into cell-derived microvesicles. Thromb Haemost. 2014 Apr 1;111(4):647–55. doi: 10.1160/TH13-09-0769. [DOI] [PubMed] [Google Scholar]
- 28.Rothmeier AS, Marchese P, Petrich BG, Furlan-Freguia C, Ginsberg MH, Ruggeri ZM, et al. Caspase-1-mediated pathway promotes generation of thromboinflammatory microparticles. J Clin Invest. 2015 Apr 1;125(4):1471–84. doi: 10.1172/JCI79329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang P, Wu Y, Li X, Ma X, Zhong L. Thioredoxin and thioredoxin reductase control tissue factor activity by thiol redox-dependent mechanism. J Biol Chem. 2013 Feb 1;288(5):3346–58. doi: 10.1074/jbc.M112.418046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dorfleutner A, Hintermann E, Tarui T, Takada Y, Ruf W. Crosstalk of integrin α3β1 and tissue factor in cell migration. Mol Biol Cell. 2004;15(10):4416–25. doi: 10.1091/mbc.E03-09-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ott I, Weigand B, Michl R, Seitz I, Sabbari-Erfani N, Neumann FJ, et al. Tissue factor cytoplasmic domain stimulates migration by activation of the GTPase Rac1 and the mitogen-activated protein kinase p38. Circulation. 2005 Jan 25;111(3):349–55. doi: 10.1161/01.CIR.0000153333.52294.42. [DOI] [PubMed] [Google Scholar]
- 32.Svensson KJ, Kucharzewska P, Christianson HC, Skold S, Lofstedt T, Johansson MC, et al. Hypoxia triggers a pro-angiogenic pathway involving cancer cell microvesicles and PAR-2 mediated HB-EGF signaling in endothelial cells. Proc Natl Acad Sci USA. 2011;108:13147–52. doi: 10.1073/pnas.1104261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collier ME, Ettelaie C. Induction of endothelial cell proliferation by recombinant and microparticle-tissue factor involves beta1-integrin and extracellular signal regulated kinase activation. Arterioscler Thromb Vasc Biol. 2010 Sep;30(9):1810–7. doi: 10.1161/ATVBAHA.110.211854. [DOI] [PubMed] [Google Scholar]
- 34.Arderiu G, Pena E, Badimon L. Angiogenic microvascular endothelial cells release microparticles rich in tissue factor that promotes postischemic collateral vessel formation. Arterioscler Thromb Vasc Biol. 2015 Feb;35(2):348–57. doi: 10.1161/ATVBAHA.114.303927. [DOI] [PubMed] [Google Scholar]
- 35.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic MM, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015 Nov 19;527(7578):329–35. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bezuhly M, Cullen R, Esmon CT, Morris SF, West KA, Johnston B, et al. Role of activated protein C and its receptor in inhibition of tumor metastasis. Blood. 2009 Apr 2;113(14):3371–4. doi: 10.1182/blood-2008-05-159434. [DOI] [PubMed] [Google Scholar]
- 37.Van Sluis GL, Niers TM, Esmon CT, Tigchelaar W, Richel DJ, Buller HR, et al. Endogenous activated protein C limits cancer cell extravasation through sphingosine-1-phosphate receptor 1-mediated vascular endothelial barrier enhancement. Blood. 2009 Aug 27;114(9):1968–73. doi: 10.1182/blood-2009-04-217679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruggemann LW, Versteeg HH, Niers TM, Reitsma PH, Spek CA. Experimental melanoma metastasis in lungs of mice with congenital coagulation disorders. J Cell Mol Med. 2008 Dec;12(6B):2622–7. doi: 10.1111/j.1582-4934.2008.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horowitz NA, Blevins EA, Miller WM, Perry AR, Talmage KE, Mullins ES, et al. Thrombomodulin is a determinant of metastasis through a mechanism linked to the thrombin binding domain but not the lectin-like domain. Blood. 2011 Sep 8;118(10):2889–95. doi: 10.1182/blood-2011-03-341222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, Xiao J, Wen D, Wu X, Mao Z, Zhang J, et al. Endothelial cell-anchored tissue factor pathway inhibitor regulates tumor metastasis to the lung in mice. Mol Carcinog. 2015 May 6; doi: 10.1002/mc.22329. [DOI] [PubMed] [Google Scholar]
- 41.Cui XY, Tinholt M, Stavik B, Dahm AE, Kanse S, Jin Y, et al. Effect of hypoxia on tissue factor pathway inhibitor expression in breast cancer. J Thromb Haemost. 2015 Nov 24; doi: 10.1111/jth.13206. [DOI] [PubMed] [Google Scholar]
- 42.Tinholt M, Vollan HK, Sahlberg KK, Jernstrom S, Kaveh F, Lingjaerde OC, et al. Tumor expression, plasma levels and genetic polymorphisms of the coagulation inhibitor TFPI are associated with clinicopathological parameters and survival in breast cancer, in contrast to the coagulation initiator TF. Breast Cancer Res. 2015;17:44. doi: 10.1186/s13058-015-0548-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tinholt M, Viken MK, Dahm AE, Vollan HK, Sahlberg KK, Garred O, et al. Increased coagulation activity and genetic polymorphisms in the F5, F10 and EPCR genes are associated with breast cancer: a case-control study. BMC Cancer. 2014;14:845. doi: 10.1186/1471-2407-14-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Magnus N, D'Asti E, Meehan B, Garnier D, Rak J. Oncogenes and the coagulation system--forces that modulate dormant and aggressive states in cancer. Thromb Res. 2014 May;133(2):S1–S9. doi: 10.1016/S0049-3848(14)50001-1. [DOI] [PubMed] [Google Scholar]
- 45.Koizume S, Jin MS, Miyagi E, Hirahara F, Nakamura Y, Piao JH, et al. Activation of cancer cell migration and invasion by ectopic synthesis of coagulation factor VII. Cancer Res. 2006 Oct 16;66(19):9453–60. doi: 10.1158/0008-5472.CAN-06-1803. [DOI] [PubMed] [Google Scholar]
- 46.Koizume S, Yokota N, Miyagi E, Hirahara F, Nakamura Y, Sakuma Y, et al. Hepatocyte nuclear factor-4-independent synthesis of coagulation factor VII in breast cancer cells and its inhibition by targeting selective histone acetyltransferases. Mol Cancer Res. 2009;7(12):1928–36. doi: 10.1158/1541-7786.MCR-09-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koizume S, Ito S, Miyagi E, Hirahara F, Nakamura Y, Sakuma Y, et al. HIF2alpha-Sp1 interaction mediates a deacetylation-dependent FVII-gene activation under hypoxic conditions in ovarian cancer cells. Nucleic Acids Res. 2012 Jul 1;40(12):5389–401. doi: 10.1093/nar/gks201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaffner F, Yokota N, Carneiro-Lobo TC, Kitano M, Schaffer M, Anderson GM, et al. Endothelial Protein C Receptor Function in Murine and Human Breast Cancer Development. PLoS ONE. 2013 Apr 9;8(4):e61071. doi: 10.1371/journal.pone.0061071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Versteeg HH, Schaffner F, Kerver M, Petersen HH, Ahamed J, Felding-Habermann B, et al. Inhibition of tissue factor signaling suppresses tumor growth. Blood. 2008;111(1):190–9. doi: 10.1182/blood-2007-07-101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Magnus N, Garnier D, Rak J. Oncogenic epidermal growth factor receptor up-regulates multiple elements of the tissue factor signaling pathway in human glioma cells. Blood. 2010 Aug 5;116(5):815–8. doi: 10.1182/blood-2009-10-250639. [DOI] [PubMed] [Google Scholar]
- 51.Naderi A. Coagulation factor VII is regulated by androgen receptor in breast cancer. Exp Cell Res. 2015 Feb 1;331(1):239–50. doi: 10.1016/j.yexcr.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Albrektsen T, Sorensen BB, Hjortoe GM, Fleckner J, Rao LVM, Petersen LC. Transcriptional program induced by factor VIIa-tissue factor, PAR1 and PAR2 in MDA-MB-231 cells. J Thromb Haemost. 2007;5:1588–97. doi: 10.1111/j.1538-7836.2007.02603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schaffner F, Versteeg HH, Schillert A, Yokota N, Petersen LC, Mueller BM, et al. Cooperation of tissue factor cytoplasmic domain and PAR2 signaling in breast cancer development. Blood. 2010 Dec 23;116(26):6106–13. doi: 10.1182/blood-2010-06-289314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Versteeg HH, Schaffner F, Kerver M, Ellies LG, Andrade-Gordon P, Mueller BM, et al. Protease activated receptor (PAR)2, but not PAR1 signaling promotes the development of mammary adenocarcinoma in PyMT mice. Cancer Res. 2008;68(17):7219–27. doi: 10.1158/0008-5472.CAN-08-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Magnus N, Garnier D, Meehan B, McGraw S, Lee TH, Caron M, et al. Tissue factor expression provokes escape from tumor dormancy and leads to genomic alterations. Proc Natl Acad Sci U S A. 2014 Mar 4;111(9):3544–9. doi: 10.1073/pnas.1314118111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Belting M, Dorrell MI, Sandgren S, Aguilar E, Ahamed J, Dorfleutner A, et al. Regulation of angiogenesis by tissue factor cytoplasmic domain signaling. Nature Med. 2004;10(5):502–9. doi: 10.1038/nm1037. [DOI] [PubMed] [Google Scholar]
- 57.Uusitalo-Jarvinen H, Kurokawa T, Mueller BM, Andrade-Gordon P, Friedlander M, Ruf W. Role of Protease Activated Receptor 1 and 2 Signaling in Hypoxia-Induced Angiogenesis. Arterioscler Thromb Vasc Biol. 2007 Mar 15;27(6):1456–62. doi: 10.1161/ATVBAHA.107.142539. [DOI] [PubMed] [Google Scholar]
- 58.Siegbahn A, Johnell M, Rorsman C, Ezban M, Heldin CH, Rönnstrand L. Binding of factor VIIa to tissue factor on human fibroblasts leads to activation of phospholipase C and enhanced PDGF-BB-stimulated chemotaxis. Blood. 2000;96:3452–8. [PubMed] [Google Scholar]
- 59.Siegbahn A, Johnell M, Nordin A, Aberg M, Velling T. TF/FVIIa transactivate PDGFRbeta to regulate PDGF-BB-induced chemotaxis in different cell types: involvement of Src and PLC. Arterioscler Thromb Vasc Biol. 2008 Jan;28(1):135–41. doi: 10.1161/ATVBAHA.107.155754. [DOI] [PubMed] [Google Scholar]
- 60.Arderiu G, Pena E, Aledo R, Juan-Babot O, Badimon L. Tissue Factor Regulates Microvessel Formation and Stabilization by Induction of Chemokine (C-C motif) Ligand 2 Expression. Arterioscler Thromb Vasc Biol. 2011 Nov;31(11):2607–15. doi: 10.1161/ATVBAHA.111.233536. [DOI] [PubMed] [Google Scholar]
- 61.Pena E, Arderiu G, Badimon L. Tissue factor induces human coronary artery smooth muscle cell motility through Wnt-signalling. J Thromb Haemost. 2013 Oct;11(10):1880–91. doi: 10.1111/jth.12327. [DOI] [PubMed] [Google Scholar]
- 62.Versteeg HH, Ruf W. New helpers in TF-dependent migration. J Thromb Haemost. 2013 Oct;11(10):1877–9. doi: 10.1111/jth.12378. [DOI] [PubMed] [Google Scholar]
- 63.Aberg M, Eriksson O, Siegbahn A. Tissue Factor Noncoagulant Signaling: Mechanisms and Implications for Cell Migration and Apoptosis. Semin Thromb Hemost. 2015 Oct;41(7):691–9. doi: 10.1055/s-0035-1564046. [DOI] [PubMed] [Google Scholar]
- 64.Badeanlou L, Furlan-Freguia C, Yang G, Ruf W, Samad F. Tissue factor-PAR2 signaling promotes diet-induced obesity and adipose inflammation. Nat Med. 2011 Oct 23;17(11):1490–7. doi: 10.1038/nm.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jiang X, Zhu S, Panetti TS, Bromberg ME. Formation of tissue factor-factor VIIa-factor Xa complex induces activation of the mTOR pathway which regulates migration of human breast cancer cells. Thromb Haemost. 2008 Jul;100(1):127–33. doi: 10.1160/TH07-12-0722. [DOI] [PubMed] [Google Scholar]
- 66.Chen KD, Wang CC, Tsai MC, Wu CH, Yang HJ, Chen LY, et al. Interconnections between autophagy and the coagulation cascade in hepatocellular carcinoma. Cell Death Dis. 2014;5:e1244. doi: 10.1038/cddis.2014.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eriksson O, Ramstrom M, Hornaeus K, Bergquist J, Mokhtari D, Siegbahn A. The Eph tyrosine kinase receptors EphB2 and EphA2 are novel proteolytic substrates of tissue factor/coagulation factor VIIa. J Biol Chem. 2014 Nov 21;289(47):32379–91. doi: 10.1074/jbc.M114.599332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kocaturk B, van den Berg YW, Tieken C, Mieog JS, de Kruijf EM, Engels CC, et al. Alternatively spliced Tissue Factor promotes breast cancer growth in a B1 integrin-dependent manner. PNAS. 2013 Jul 9;110(28):11517–22. doi: 10.1073/pnas.1307100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Srinivasan R, Ozhegov E, van den Berg YW, Aronow BJ, Franco RS, Palascak MB, et al. Splice Variants of Tissue Factor Promote Monocyte-Endothelial Interactions by Triggering the Expression of Cell Adhesion Molecules via Integrin-Mediated Signaling. J Thromb Haemost. 2011 Oct;9(10):2087–96. doi: 10.1111/j.1538-7836.2011.04454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van den Berg YW, van den Hengel LG, Myers HR, Ayachi O, Jordanova E, Ruf W, et al. Alternatively spliced tissue factor induces angiogenesis through integrin ligation. Proc Natl Acad Sci U S A. 2009 Nov 17;106(46):19497–502. doi: 10.1073/pnas.0905325106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sluka SH, Akhmedov A, Vogel J, Unruh D, Bogdanov VY, Camici GG, et al. Alternatively spliced tissue factor is not sufficient for embryonic development. PLoS ONE. 2014 May 30;9(5):e97793. doi: 10.1371/journal.pone.0097793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Magnus N, Meehan B, Garnier D, Hashemi M, Montermini L, Lee TH, et al. The contribution of tumor and host tissue factor expression to oncogene-driven gliomagenesis. Biochem Biophys Res Commun. 2014 Nov 14;454(2):262–8. doi: 10.1016/j.bbrc.2014.10.041. [DOI] [PubMed] [Google Scholar]
- 73.Gessler F, Voss V, Dutzmann S, Seifert V, Gerlach R, Kogel D. Inhibition of tissue factor/protease-activated receptor-2 signaling limits proliferation, migration and invasion of malignant glioma cells. Neuroscience. 2010 Feb 17;165(4):1312–22. doi: 10.1016/j.neuroscience.2009.11.049. [DOI] [PubMed] [Google Scholar]
- 74.Ettelaie C, Elkeeb AM, Maraveyas A, Collier ME. p38alpha phosphorylates serine 258 within the cytoplasmic domain of tissue factor and prevents its incorporation into cell-derived microparticles. Biochim Biophys Acta. 2013 Mar;1833(3):613–21. doi: 10.1016/j.bbamcr.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 75.Zioncheck TF, Roy S, Vehar GA. The cytoplasmic domain of tissue factor is phosphorylated by a protein kinase C-dependent mechanism. J Biol Chem. 1992;267:3561–4. [PubMed] [Google Scholar]
- 76.Dorfleutner A, Ruf W. Regulation of tissue factor cytoplasmic domain phosphorylation by palmitoylation. Blood. 2003;102(12):3998–4005. doi: 10.1182/blood-2003-04-1149. [DOI] [PubMed] [Google Scholar]
- 77.Sen M, Herzik M, Craft JW, Jr, Creath AL, Agrawal S, Ruf W, et al. Spectroscopic characterization of successive phosphorylation of the tissue factor cytoplasmic region. Open Spectrosc J. 2009;3:58–65. doi: 10.2174/1874383800903010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ahamed J, Ruf W. Protease-activated receptor 2-dependent phosphorylation of the tissue factor cytoplasmic domain. J Biol Chem. 2004;279(22):23038–44. doi: 10.1074/jbc.M401376200. [DOI] [PubMed] [Google Scholar]
- 79.Ryden L, Grabau D, Schaffner F, Jonsson PE, Ruf W, Belting M. Evidence for tissue factor phosphorylation and its correlation with protease activated receptor expression and the prognosis of primary breast cancer. Int J Cancer. 2010;126(10):2330–40. doi: 10.1002/ijc.24921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ahamed J, Niessen F, Kurokawa T, Lee YK, Bhattacharjee G, Morrissey JH, et al. Regulation of macrophage procoagulant responses by the tissue factor cytoplasmic domain in endotoxemia. Blood. 2007 Mar 1;109(12):5251–9. doi: 10.1182/blood-2006-10-051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Riewald M, Ruf W. Mechanistic coupling of protease signaling and initiation of coagulation by tissue factor. Proc Natl Acad Sci USA. 2001;98(14):7742–7. doi: 10.1073/pnas.141126698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Disse J, Ruf W. Endothelial protein C receptor is required for tissue factor ternary complex signaling in the mouse. J Thromb Haemost. 2011 Dec;9(12):2516–8. doi: 10.1111/j.1538-7836.2011.04521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Disse J, Petersen HH, Larsen KS, Persson E, Esmon N, Esmon CT, et al. The Endothelial Protein C Receptor Supports Tissue Factor Ternary Coagulation Initiation Complex Signaling through Protease-activated Receptors. J Biol Chem. 2011 Feb 18;286(7):5756–67. doi: 10.1074/jbc.M110.201228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ghosh S, Pendurthi UR, Steinoe A, Esmon CT, Rao LV. Endothelial cell protein C receptor acts as a cellular receptor for factor VIIa on endothelium. J Biol Chem. 2007 Apr 20;282(16):11849–57. doi: 10.1074/jbc.M609283200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oganesyan V, Oganesyan N, Terzyan S, Qu D, Dauter Z, Esmon NL, et al. The crystal structure of the endothelial protein C receptor and a bound phospholipid. J Biol Chem. 2002 Jul 12;277(28):24851–4. doi: 10.1074/jbc.C200163200. [DOI] [PubMed] [Google Scholar]
- 86.Sen P, Gopalakrishnan R, Kothari H, Keshava S, Clark CA, Esmon CT, et al. Factor VIIa bound to endothelial cell protein C receptor activates protease activated receptor-1 and mediates cell signaling and barrier protection. Blood. 2011 Mar 17;117(11):3199–208. doi: 10.1182/blood-2010-09-310706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lopez-Sagaseta J, Montes R, Puy C, Diez N, Fukudome K, Hermida J. Binding of factor VIIa to the endothelial cell protein C receptor reduces its coagulant activity. J Thromb Haemost. 2007 Sep;5(9):1817–24. doi: 10.1111/j.1538-7836.2007.02648.x. [DOI] [PubMed] [Google Scholar]
- 88.Liang HP, Kerschen EJ, Hernandez I, Basu S, Zogg M, Botros F, et al. EPCR-dependent PAR2 activation by the blood coagulation initiation complex regulates LPS-triggered interferon responses in mice. Blood. 2015 Apr 30;125(18):2845–54. doi: 10.1182/blood-2014-11-610717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bergum PW, Cruikshank A, Maki S, Kelly CR, Ruf W, Vlasuk G. Role of zymogen and activated factor X as scaffolds for the inhibition of the blood coagulation factor VIIa-tissue factor complex by recombinant nematode anticoagulant protein c2. J Biol Chem. 2001;276(13):10063–71. doi: 10.1074/jbc.M009116200. [DOI] [PubMed] [Google Scholar]
- 90.Liang HP, Kerschen EJ, Basu S, Hernandez I, Zogg M, Jia S, et al. Coagulation factor V mediates inhibition of tissue factor signaling by activated protein C in mice. Blood. 2015 Nov 19;126(21):2415–23. doi: 10.1182/blood-2015-05-644401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hembrough TA, Swartz GM, Papathanassiu A, Vlasuk GP, Rote WE, Green SJ, et al. Tissue factor/factor VIIa inhibitors block angiogenesis and tumor growth through a nonhemostatic mechanism. Cancer Res. 2003 Jun 1;63(11):2997–3000. [PubMed] [Google Scholar]
- 92.Zhao J, Aguilar G, Palencia S, Newton E, Abo A. rNAPc2 inhibits colorectal cancer in mice through tissue factor. Clin Cancer Res. 2009 Jan 1;15(1):208–16. doi: 10.1158/1078-0432.CCR-08-0407. [DOI] [PubMed] [Google Scholar]
- 93.Geisbert TW, Hensley LE, Jahrling PB, Larsen T, Geisbert JB, Paragas J, et al. Treatment of Ebola virus infection with a recombinant inhibitor of factor VIIa/tissue factor: a study in rhesus monkeys. Lancet. 2003 Dec 13;362(9400):1953–8. doi: 10.1016/S0140-6736(03)15012-X. [DOI] [PubMed] [Google Scholar]
- 94.Ruf W. Emerging roles of tissue factor in viral hemorrhagic fever. Trends Immunol. 2004;25(9):461–4. doi: 10.1016/j.it.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 95.Sutherland MR, Ruf W, Pryzdial ELG. Tissue factor and glycoprotein C on herpes simplex virus type 1 are protease activated receptor 2 cofactors that enhance infection. Blood. 2012 Apr 12;119:3638–45. doi: 10.1182/blood-2011-08-376814. [DOI] [PubMed] [Google Scholar]
- 96.Moretti S, Bellocchio S, Bonifazi P, Bozza S, Zelante T, Bistoni F, et al. The contribution of PARs to inflammation and immunity to fungi. Mucosal Immunol. 2008 Mar;1(2):156–68. doi: 10.1038/mi.2007.13. [DOI] [PubMed] [Google Scholar]
- 97.Nhu QM, Shirey K, Teijaro JR, Farber DL, Netzel-Arnett S, Antalis TM, et al. Novel signaling interactions between proteinase-activated receptor 2 and Toll-like receptors in vitro and in vivo. Mucosal Immunol. 2010 Jan;3(1):29–39. doi: 10.1038/mi.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rallabhandi P, Nhu QM, Toshchakov VY, Piao W, Medvedev AE, Hollenberg MD, et al. Analysis of proteinase-activated receptor 2 and TLR4 signal transduction: a novel paradigm for receptor cooperativity. J Biol Chem. 2008 Sep 5;283(36):24314–25. doi: 10.1074/jbc.M804800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Antoniak S, Mackman N. Multiple roles of the coagulation protease cascade during virus infection. Blood. 2014 Apr 24;123(17):2605–13. doi: 10.1182/blood-2013-09-526277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012 Feb;22(1):33–40. doi: 10.1016/j.semcancer.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 101.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010 Mar 19;140(6):883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Semin Immunopathol. 2013 Sep;35(5):585–600. doi: 10.1007/s00281-013-0367-7. [DOI] [PubMed] [Google Scholar]
- 103.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014 Jul 17;41(1):49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation) Blood. 2006 Mar 1;107(5):2112–22. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- 105.Gur-Cohen S, Itkin T, Chakrabarty S, Graf C, Kollet O, Ludin A, et al. PAR1 signaling regulates the retention and recruitment of EPCR-expressing bone marrow hematopoietic stem cells. Nature Med. 2015 Nov;21(11):1307–17. doi: 10.1038/nm.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wilson NK, Kent DG, Buettner F, Shehata M, Macaulay IC, Calero-Nieto FJ, et al. Combined Single-Cell Functional and Gene Expression Analysis Resolves Heterogeneity within Stem Cell Populations. Cell Stem Cell. 2015 Jun 4;16(6):712–24. doi: 10.1016/j.stem.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pepler L, Yu P, Dwivedi DJ, Trigatti BL, Liaw PC. Characterization of mice harboring a variant of EPCR with impaired ability to bind protein C: novel role of EPCR in hematopoiesis. Blood. 2015 Jul 30;126(5):673–82. doi: 10.1182/blood-2014-02-558940. [DOI] [PubMed] [Google Scholar]
- 108.Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA, Lemischka IR. A stem cell molecular signature. Science. 2002 Oct 18;298(5593):601–4. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- 109.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004 Sep 3;118(5):635–48. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 110.Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. “Stemness”: transcriptional profiling of embryonic and adult stem cells. Science. 2002 Oct 18;298(5593):597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- 111.Balazs AB, Fabian AJ, Esmon CT, Mulligan RC. Endothelial protein C receptor (CD201) explicitly identifies hematopoietic stem cells in murine bone marrow. Blood. 2005 Nov 22;107(6):2317–21. doi: 10.1182/blood-2005-06-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang D, Cai C, Dong X, Yu QC, Zhang XO, Yang L, et al. Identification of multipotent mammary stem cells by protein C receptor expression. Nature. 2015 Jan 1;517(7532):81–4. doi: 10.1038/nature13851. [DOI] [PubMed] [Google Scholar]
- 113.Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007 Mar;11(3):259–73. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 114.Keshava S, Sahoo S, Tucker TA, Idell S, Rao LV, Pendurthi UR. Endothelial cell protein C receptor opposes mesothelioma growth driven by tissue factor. Cancer Res. 2013 Jul 1;73(13):3963–73. doi: 10.1158/0008-5472.CAN-12-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Anton I, Molina E, Luis-Ravelo D, Zandueta C, Valencia K, Ormazabal C, et al. Receptor of Activated Protein C Promotes Metastasis and Correlates with Clinical Outcome in Lung Adenocarcinoma. Am J Respir Crit Care Med. 2012 Jul 1;186(1):96–105. doi: 10.1164/rccm.201110-1826OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zacharski LR, Henderson WG, Rickles FR, Forman WB, Cornell CJ, Jr, Forcier RJ, et al. Effect of warfarin anticoagulation on survival in carcinoma of the lung, colon, head and neck, and prostate. Cancer. 1984;53:2046–52. doi: 10.1002/1097-0142(19840515)53:10<2046::aid-cncr2820531007>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 117.Carneiro-Lobo TC, Konig S, Machado DE, Nasciutti LE, Forni MF, Francischetti IM, et al. Ixolaris, a tissue factor inhibitor, blocks primary tumor growth and angiogenesis in a glioblastoma model. J Thromb Haemost. 2009 Nov;7(11):1855–64. doi: 10.1111/j.1538-7836.2009.03553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carneiro-Lobo TC, Schaffner F, Disse J, Ostergaard H, Francischetti IM, Monteiro RQ, et al. The tick-derived inhibitor Ixolaris prevents tissue factor signaling on tumor cells. Journal of Thrombosis and Haemostasis. 2012 doi: 10.1111/j.1538-7836.2012.04864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


