Abstract
Skeletal muscle atrophy and dysfunction are common complications in the chronic obstructive pulmonary disease (COPD). However, the underlying molecular mechanism remains elusive. Serum response factor (SRF) is a transcription factor which is critical in myocyte differentiation and growth. In this study, we established a mouse COPD model induced by cigarette smoking (CS) exposure for 24 weeks, with apparent pathophysiological changes, including increased airway resistance, enlarged alveoli, and skeletal muscle atrophy. Levels of upstream regulators of SRF, striated muscle activator of Rho signaling (STARS), and ras homolog gene family, member A (RhoA) were decreased in quadriceps muscle of COPD mice. Meanwhile, the nucleic location of SRF was diminished along with its cytoplasmic accumulation. There was a downregulation of the target muscle-specific gene, Igf1. These results suggest that the CS is one of the major causes for COPD pathogenesis, which induces the COPD-associated skeletal muscle atrophy which is closely related to decreasing SRF nucleic translocation, consequently downregulating the SRF target genes involved in muscle growth and nutrition. The STARS/RhoA signaling pathway might contribute to this course by impacting SRF subcellular distribution.
Keywords: SRF, chronic obstructive pulmonary disease, skeletal muscle atrophy, cigarette smoking
Introduction
Cigarette smoke (CS) is the primary risk factor of chronic obstructive pulmonary disease (COPD). CS exposure contributes not only to the development of irreversible lung damage but also to the extrapulmonary complications, including skeletal muscle atrophy. Clinical and animal studies indicated that CS exposure may cause muscle morphological, metabolism, bioenergetics, and functional changes.1–3 In COPD patients, skeletal muscle atrophy is an independent predictor of morbidity and mortality.4 Muscle atrophy exacerbates the decline in physical function, which causes further impaired lung function, exercise limitation, and poor health status. However, the molecular mechanisms underlying in this phenotype are not known.
Serum response factor (SRF) is a MADS-box transcription factor that regulates muscle-specific gene expression by binding to a consensus sequence of high similarity to the motif CC[A/T]6GG termed the CArG box. SRF plays a central role in the regulation of skeletal muscle development and differentiation,5–7 the essential processes of muscle growth and regeneration. Tamoxifen-inducible SRF knockout mice showed significant signs of muscle atrophy.7 Age or physical activity-related changes in the expression and subcellular location of SRF had been reported.8–11 A recent study has described that reduced activity of SRF contributes to the reduction of miR-1 expression in quadriceps of COPD patients, which as a part involved in skeletal muscle dysfunction.12
The activity of SRF can be modulated by regulating its nuclear localization.13 Ras homolog gene family, member A (RhoA), has been shown to increase the nuclear localization of SRF by increasing actin polymerization.14 Striated muscle activator of Rho signaling (STARS) – the actin-binding protein, known as an activator of SRF transcriptional activity, partly cooperates with RhoA.15–18 Recently, several reports suggested that the STARS/RhoA/SRF pathway contributes to adult skeletal muscle wasting and remodeling.8–11 STARS/RhoA/SRF signaling pathway was up- and downregulated, respectively, in adult skeletal muscle hypertrophy and atrophy. STARS protein and nuclear SRF content were increased following endurance exercise. However, STARS/RhoA/SRF axis participates in COPD-related muscle atrophy remained to be elucidated.
In the present study, we investigated the protein expression levels of STARS, RhoA, and SRF; and the subcellular localization of SRF in the quadriceps muscle from COPD model mice induced by 24 weeks CS exposure. We found that STARS/RhoA/SRF pathway signaling was suppressed in COPD mice muscle, accompanied with the decrease of the SRF-targeted genes expression, including Igf1 (insulin-like growth factor 1), myosin heavy chain 2 (Myh2), and skeletal muscle α-actin (Acta1). Thus, we suggest that CS contributes to the skeletal muscle atrophy in COPD by inhibiting STARS/RhoA/SRF signaling pathway and its target SRF-dependent muscle-specific genes.
Materials and methods
Animals
Adult male Balb/c mice (6–8 weeks old, 18–20 g) were purchased from Guangdong Medical Laboratory Animal Center, housed under controlled conditions in standard laboratory cages (PAB-S200, Biolab) under a 12-hour light–dark cycle, and given food and water ad libitum. All experimental procedures were approved by the Animal Ethics Committee of the First Affiliated Hospital, Guangzhou Medical College. The present study was performed in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, revised 1996) and was approved by Animal Care and Use Committee of the Guangzhou Medical University.
CS exposure
The mice were divided into two groups. For CS exposure, the mice (n=15) were placed in a 27 L chamber and exposed to the smoke produced by nine commercially filtered cigarettes (1.3 mg of nicotine, 13 mg of tar, and 15 mg of carbon monoxide per cigarette), for 4 hours a day and 5 days a week. The inhalable particle concentration (PM10) was 34.6±4.3 mg/m3, and the concentration for carbon monoxide in the exposure chamber was 500–800 ppm, as monitored by a real-time ambient particle monitor (P-5L2C). Body weights of the mice were measured once a week throughout the study. The control animals (n=15) were exposed to room air for 24 weeks.
Lung function measurement
Twenty-four hours after the last CS exposure, the mice were anesthetized, tracheostomized, and mechanically ventilated using a Harvard ventilator. Airway resistance (RL) and dynamic lung compliance (cdyn) were obtained according to the Buxco resistance/compliance application manual. RL and cdyn were calibrated to body weight. Tissue samples were collected after lung function measurement for further analyses.
Histological and lung morphometric analysis
The left lung was perfused, through the main bronchus, with a fixative solution (4% paraformaldehyde) at a pressure of 25 cmH2O,19,20 and then immediately immersed and maintained in the fixative solution for 24 hours. Quadriceps muscle was also fixed in 4% paraformaldehyde for 24 hours. The tissue was embedded in paraffin and 5-µm-thick sections were stained with hematoxylin and eosin (H&E). The lung mean linear intercept (MLI), mean alveolus number (MAN), and muscle fiber cell cross-sectional area were measured by Image Pro Plus 6.0.
Immunoblotting
Total protein of quadriceps muscle was harvested and lysed in buffer containing complete protease inhibitor cocktail (Sigma) as described previously.3 Muscle tissue cytosolic and nuclear proteins were separated by using a NE-PER kit (Pierce Biotechnology, Rockford, IL, USA) according to the manufacturer’s instructions. The protein concentration was determined by BCA Protein Assay (Pierce Biotechnology) and equal amount of total protein were loaded to sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel for Western blotting analysis described previously.21 The following primary antibodies were used in the analysis: anti-STARS (1:1,000; Abcam), anti-RhoA (1:2,000; Abcam), anti-SRF (1:1,000; Abcam), anti-histone H3 (1:1,000; Abcam), anti-glyceraldehyde 3-phosphate dehydrogenase (1:30,000; CST), and anti-β-tubulin (1:30,000; Sigma).
Immunofluorescence
Paraffin sections of quadriceps muscle tissue were used to detect the subcellular localization of SRF by immunofluorescence staining as described previously,8 with the antibody against SRF and corresponding conjugated secondary antibody (Sigma). The nuclei were indicated by 4′,6-diamidino-2-phenylindole staining. Images were captured under a Leica DMI 4000 B fluorescence microscope.
Real-time quantitative polymerase chain reaction (PCR)
Total RNA was extracted from quadriceps muscle tissue by using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. cDNA was then prepared from 1,000 ng total RNA using a PrimeScript reverse transcription (RT) reagent kit (Takara). The RNA expression levels were quantified by real-time PCR using iScript one-step rt-PCR kit with the following primers:
SRF, forward: 5′-cctaccaggtgtcggaatct-3′, reverse: 5′-tctggattgtggaggtggta-3′; Igf1, forward: 5′-tgctgtccctctatgcttcc-3′, reverse: 5′-gaaggaatagccacgctcag-3′; Myh2, forward: 5′-acacgagagacgagtgaagga-3′, reverse : 5′-tgcggaacttggatagatttg-3′; Acta1, forward: 5′-tgctgtccctctatgcttcc-3′, reverse: 5′-gaaggaatagccacgctcag-3′.
Statistical analysis
All values are expressed as mean ± SEM and were analyzed with the two-tailed Student’s t-test. GraphPad Prism 5 software (GraphPad Software, Inc., San Diego, CA, USA) was used for statistical calculations. Values of P<0.05 were considered statistically significant.
Results
CS exposed mice develop the hallmarks of COPD and skeletal muscle atrophy
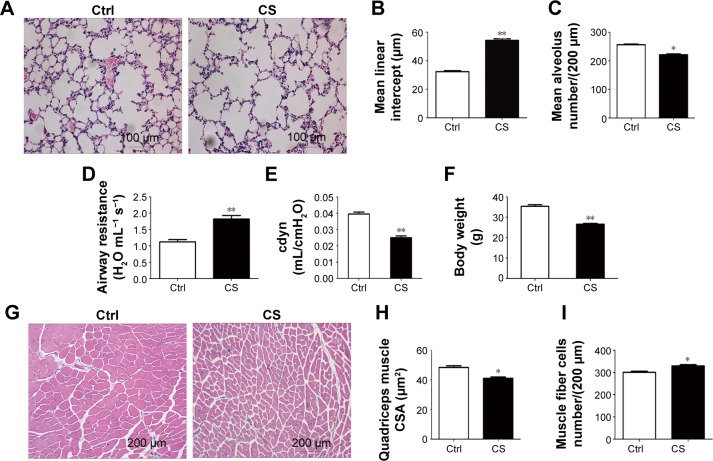
After 24 weeks CS exposure, the mice showed typical pathological features of chronic bronchitis and emphysema with significant enlargement of alveolar spaces distributed throughout the parenchyma (Figure 1A), while normal lung structure in the control group, quantitative morphological analysis showed that MLI was much higher in CS exposed mice than in normal mice (54.25±1.140 vs 32.37±0.6557 µm2; P<0.001; Figure 1B) and MAN was significantly decreased in CS mice than that in the normal group (221.6±2.783 vs 256.6±2.592 per LP [low-power field, 200 µm]; P<0.05; Figure 1C). Lung functional analysis showed that RL was significantly increased in CS mice (1.819±0.0278 vs 1.123±0.0184 H2O mL−1s−1; P<0.001; Figure 1G), whereas cdyn was much lower in CS mice than in normal mice (cdyn: 0.02502±0.00109 vs 0.03956±0.001209 mL/cmH2O; P<0.001; Figure 1H), showed that CS-induced mouse COPD model was successfully achieved.
Figure 1.
CS-exposed mice develop the hallmarks of COPD and skeletal muscle atrophy.
Notes: (A) Lung sections of the CS-group and control mice stained with H&E. (B) The MLI of the CS-group was significantly higher than that of the control group. (C) The MAN of the CS-group was significantly lower than that of the control group. (D) The airway resistance of the CS-group was significantly higher than that of the control group. (E) The dynamic lung compliance of the CS-group was significantly lower than that of the control group. (F) After 24 weeks of CS exposure, the body weight of the CS-group mice was significantly lower than that of the control group mice. (G) Quadriceps muscle sections stained with H&E. (H) Muscle fiber cross-sectional area in the CS-group was significantly lower than that in the control group. (I) The number of muscle fiber cells per LP of the CS-group was significantly higher than that of the control group. The values are shown as mean ± SEM (n= five per group). *P<0.05, **P<0.001 compared with the control group.
Abbreviations: cdyn, dynamic lung compliance; Ctrl, control; CS, cigarette smoke; CSA, cross-sectional area; H&E, hematoxylin and eosin; MLI, mean linear intercept; MAN, mean alveolus number; LP, low-power field; SEM, standard error of mean.
To evaluate the skeletal muscle atrophy in COPD model mice, we monitored the body weight of mice from both groups during the CS exposure. We found although the body weights in both groups increased stably during 24 weeks exposure to room air or CS, a slower increase on the body weight was observed in CS mice, which led to a significantly lower body weight in CS group as compared in the control group (26.6067±0.3794 g in CS mice vs 35.3533±0.8051 g in normal mice; P<0.001; Figure 1I). Then we compared the quadriceps muscle morphologies in CS mice with those in normal mice. A random loose arrangement in muscle fibers was found in the CS mice (Figure 1D), accompanied with reduced muscle fiber cell cross-sectional area (41.05±0.098 µm2 in CS mice vs 48.43±1.171 µm2 in normal mice; P<0.05; Figure 1E) and increased number of cells per LP (329.4±6.440 for CS mice vs 300.8±4.826 for normal mice; P<0.05; Figure 1F).
Reduced nuclear expression of SRF in quadriceps muscle from COPD-like mice
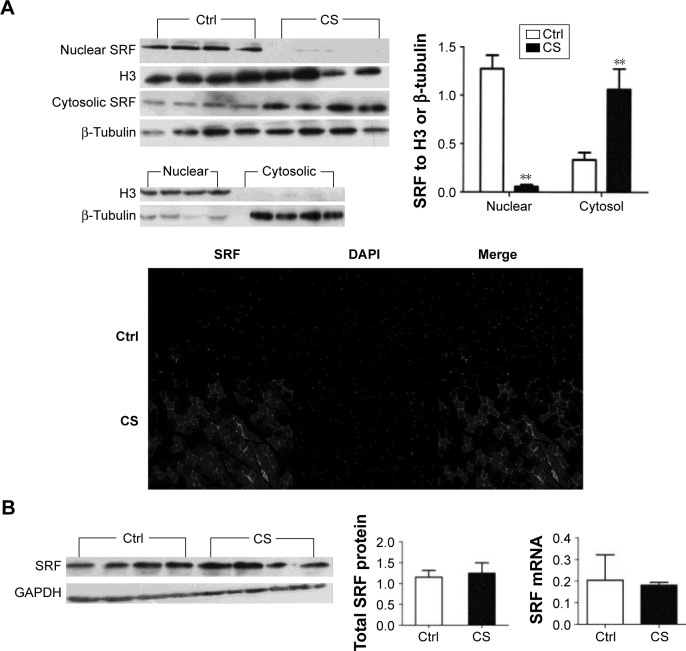
We analyzed the SRF protein and mRNA levels in normal and CS mice. The total protein and mRNA levels of SRF were not changed after CS exposure. However, when we separated the nucleic and cytoplasmic fraction of quadriceps muscle cells and analyzed the SRF levels in both fractions, around 95% reduction of nucleic SRF (P<0.001) and more than two time increase of cytoplasmic SRF (P<0.001) were found in CS mice (Figure 2). The reduced nucleic distribution and increased cytoplasmic accumulation of SRF in CS mice were observed in immunostaining of quadriceps muscle sections (Figure 2A). These results indicated that CS exposure arrests the nucleic translocation of SRF in quadriceps muscle.
Figure 2.
SRF expression and subcellular redistribution.
Notes: (A) The cytosolic and nuclear fractions were harvested and subjected to Western blot analysis to examine the subcellular localization of SRF. Beta-tubulin and histone H3 were included as an internal control for the cytosol and nuclear fraction, respectively. The protein level of SRF in the cytosolic fraction was greater in the quadriceps muscle of CS-group mice than in that of control mice; conversely, in the nuclear fraction it was lower in the CS-group compared with controls. Accordingly, immunofluorescence indicated fewer SRF-positive nuclei in the muscle fibers in CS-group mice and more SRF in the cytosol compared with the control group. (B) The amount of SRF protein and mRNA in the whole quadriceps tissue was not significantly different between the two groups. The values are shown as mean ± SEM (n= four per group). **P<0.001 compared with the control group.
Abbreviations: CS, cigarette smoke; Ctrl, control; DAPI, 4′,6-diamidino-2-phenylindole; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; mRNA, messenger RNA; SRF, serum response factor; SEM, standard error of mean.
Expression of Igf1, Myh2, and Acta1 in quadriceps muscle from CS-exposed, COPD-like mice
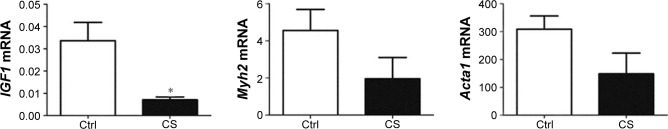
We examined the mRNA levels of several target genes of SRF: Igf1, Myh2, and Acta1 in the quadriceps muscle from normal and CS mice. Igf1 was decreased by 97% in CS mice. The mRNA levels of Myh2 and Acta1 showed trends to decline in CS mice, though no significant difference was found compared with normal (Figure 3).
Figure 3.
Reduced mRNA levels of Igf1, Acta1, and Myh2 mRNA in CS-exposed mice.
Notes: The mRNA levels of Igf1, Acta1, and Myh2 were quantified in the quadriceps muscle of control and CS-group mice by real-time reverse transcription polymerase chain reaction. The levels of IGF1 mRNA were decreased by 97% in CS-group mice compared with controls. The mRNA levels of Acta1 and Myh2 were reduced in the CS-group compared with the control group, but the difference was not significant. The values are shown as mean ± SEM (n= four per group). *P<0.05 compared with the control group.
Abbreviations: CS, cigarette smoke; Ctrl, control; mRNA, messenger RNA; SEM, standard error of mean.
Skeletal muscle STARS and RhoA levels
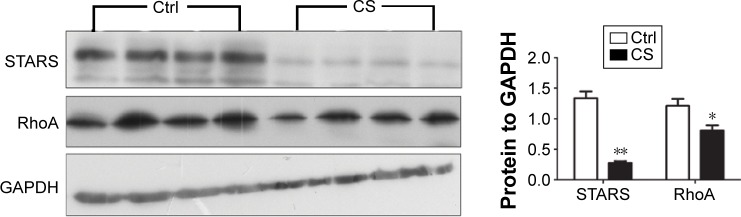
As we observed skeletal muscle atrophy and reduced nucleic SRF in CS mice, which hinted a possible reduction on SRF transcriptional activity, we then analyzed the protein levels of several genes involved in regulating SRF activity, including STARS and RhoA. The results showed that both of the two proteins were decreased in quadriceps muscle from CS mice (Figure 4).
Figure 4.
The expression of STARS and RhoA protein in CS-exposed and control mice.
Notes: Western blot analysis showed that the protein levels of STARS and RhoA in the quadriceps muscle of CS-group mice were markedly lower than those in the control group. Densitometric analysis showed the amount of STARS and RhoA in the quadriceps muscle of CS-group mice to be 79% and 50% lower than that in control mice, respectively. The protein levels of STARS and RhoA were normalized to those of GAPDH protein. The values are shown as mean ± SEM (n=4 per group). *P<0.05, **P<0.001 compared with the control group.
Abbreviations: CS, cigarette smoke; Ctrl, control; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; RhoA, ras homolog gene family, member A; STARS, striated muscle activator of Rho signaling; SEM, standard error of mean.
Discussion
CS not only leads to pulmonary impairment but also results in extrapulmonary manifestations,22 including peripheral skeletal muscle wasting.23 Long-term exposure to CS in mice is a widely accepted animal model of COPD with systemic manifestations.24 Exposure to smoke for 24 weeks is long enough to incur muscular changes.25 In the present study, we found that exposure to CS for 24 weeks caused the development of the hallmarks of COPD in Balb/c mice, accompanied by skeletal muscle atrophy, which is commonly observed in COPD patients. Our results showed that chronic exposure of Balb/c mice to CS led to a marked decrease in quadriceps muscle cross-sectional area and body weight.
SRF is an important transcription factor that regulates the expression of skeletal muscle differentiation- and actin cytoskeleton-associated genes.26,27 STARS is an actin-binding protein specifically expressed in the striated muscle. By stabilizing actin polymerization, STARS stimulates SRF-dependent transcription through RhoA signaling, and therefore plays an important role in regulating muscle development and differentiation.15 Further study on RhoA/Rho kinase pathway and SRF signaling showed that RhoA/Rho modulated SRF transcriptional activity by regulating SRF subcellular localization through dynamic actin polymerization.14
Here we found CS exposure impaired the STARS/RhoA signaling in the skeletal muscle of COPD model mice. Furthermore, the SRF nucleic location was diminished by reducing its nucleic translocation, which might lead to weaken the SRF-associated signaling. A recent report also indicated that the activity of SRF was reduced in the quadriceps muscle of COPD patients.12 Thus, it is possible that CS exposure inhibited SRF signaling by limiting its nucleic distribution which might caused by STARS/RhoA signaling impairment.
The SRF signaling attenuation was proved by the reduced expressions of its target genes in the quadriceps muscle. The genes Igf1, Myh2, and Acta1 are involved in muscle structure, function, and growth. A resistance training for skeletal muscle atrophy enhanced the expression of these three genes.11 Igf1 is known to be a key regulator of muscle mass28 by stimulating muscle growth and repair.29 IGF1 is markedly reduced in the skeletal muscle of COPD patients.30 We also found that Igf1 was significantly reduced in the quadriceps muscle of CS-exposed mice. Myh2 and Acta1 are SRF-targeted genes that are related to skeletal muscle phenotype and structure. In our COPD model mice, although the Myh2 and Acta1 expressions were not statistically significant, lower than those in normal mice, there was a clear reduction trend for both genes in CS mice. Thus, we supposed CS in airway impacts the STARS/RhoA signaling in quadriceps muscle, which resulted an accumulation of SRF in quadriceps muscle cytoplasm, consequently weaken the SRF transcriptional activity and its downstream gene expression involved in maintaining the muscle growth and differentiation.
Conclusion
The conclusions drawn from the present study are limited by only descriptive data regarding the role of SRF in CS-induced atrophy and did not examine further mechanism. It should be demonstrated mechanistically through in vitro and in vivo studies. Furthermore, human samples will be analyzed in further development.
In summary, the present study showed that chronic exposure to CS decreased the levels of STARS and RhoA, reduced nucleic distribution of SRF, and downregulated the expression of its target genes in the quadriceps muscle, which contributed to the pathogenesis of skeletal muscle atrophy during COPD.
Acknowledgments
The authors would like to thank Dr Yuting Liang for his hard work at establishing animal model and lung function measurement. We would like to thank Dr Bo Jiang, Dr Yongfeng Luo, Dr Yuting Liang, and the members of State Key Laboratory of Respiratory Diseases for their excellent technological assistance.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Barreiro E, Peinado VI, Galdiz JB, et al. Cigarette smoke-induced oxidative stress: A role in chronic obstructive pulmonary disease skeletal muscle dysfunction. Am J Respir Crit Care Med. 2010;182(4):477–488. doi: 10.1164/rccm.200908-1220OC. [DOI] [PubMed] [Google Scholar]
- 2.Barreiro E, del Puerto-Nevado L, Puig-Vilanova E, et al. Cigarette smoke-induced oxidative stress in skeletal muscles of mice. Respir Physiol Neurobiol. 2012;182(1):9–17. doi: 10.1016/j.resp.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Liu Q, Xu WG, Luo Y, et al. Cigarette smoke-induced skeletal muscle atrophy is associated with up-regulation of USP-19 via p38 and ERK MAPKs. J Cell Biochem. 2011;112(9):2307–2316. doi: 10.1002/jcb.23151. [DOI] [PubMed] [Google Scholar]
- 4.Agusti AGN, Noguera A, Sauleda J, Sala E, Pons J, Busquets X. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:347–360. doi: 10.1183/09031936.03.00405703. [DOI] [PubMed] [Google Scholar]
- 5.Gauthier-Rouviere C, Vandromme M, Tuil D, et al. Expression and activity of serum response factor is required for expression of the muscle-determining factor MyoD in both dividing and differentiating mouse C2C12 myoblasts. Mol Biol Cell. 1996;7(5):719–729. doi: 10.1091/mbc.7.5.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soulez M, Rouviere CG, Chafey P, et al. Growth and differentiation of C2 myogenic cells are dependent on serum response factor. Mol Cell Biol. 1996;16(11):6065–6074. doi: 10.1128/mcb.16.11.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lahoute C, Sotiropoulos A, Favier M, et al. Premature aging in skeletal muscle lacking serum response factor. PLoS One. 2008;3(12):e3910. doi: 10.1371/journal.pone.0003910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakuma K, Akiho M, Nakashima H, Akima H, Yasuhara M. Age-related reductions in expression of serum response factor and myocardin-related transcription factor A in mouse skeletal muscles. Biochim Biophys Acta. 2008;1782(7–8):453–461. doi: 10.1016/j.bbadis.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Sakuma K, Watanabe K, Hotta N, et al. The adaptive responses in several mediators linked with hypertrophy and atrophy of skeletal muscle after lower limb unloading in humans. Acta Physiol (Oxf) 2009;197(2):151–159. doi: 10.1111/j.1748-1716.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 10.Wallace MA, Hock MB, Hazen BC, Kralli A, Snow RJ, Russell AP. Striated muscle activator of Rho signalling (STARS) is a PGC-1alpha/oestrogen-related receptor-alpha target gene and is upregulated in human skeletal muscle after endurance exercise. J Physiol. 2011;589(Pt 8):2027–2039. doi: 10.1113/jphysiol.2011.205468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamon S, Wallace MA, Leger B, Russell AP. Regulation of STARS and its downstream targets suggest a novel pathway involved in human skeletal muscle hypertrophy and atrophy. J Physiol. 2009;587(Pt 8):1795–1803. doi: 10.1113/jphysiol.2009.168674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis A, Riddoch-Contreras J, Natanek SA, et al. Downregulation of the serum response factor/miR-1 axis in the quadriceps of patients with COPD. Thorax. 2012;67(1):26–34. doi: 10.1136/thoraxjnl-2011-200309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camoretti-Mercado B, Liu HW, Halayko AJ, et al. Physiological control of smooth muscle-specific gene expression through regulated nuclear translocation of serum response factor. J Biol Chem. 2000;275(39):30387–30393. doi: 10.1074/jbc.M000840200. [DOI] [PubMed] [Google Scholar]
- 14.Liu HW, Halayko AJ, Fernandes DJ, et al. The RhoA/Rho kinase pathway regulates nuclear localization of serum response factor. Am J Respir Cell Mol Biol. 2003;29(1):39–47. doi: 10.1165/rcmb.2002-0206OC. [DOI] [PubMed] [Google Scholar]
- 15.Arai A, Spencer JA, Olson EN. STARS, a striated muscle activator of Rho signaling and serum response factor-dependent transcription. J Biol Chem. 2002;277(27):24453–24459. doi: 10.1074/jbc.M202216200. [DOI] [PubMed] [Google Scholar]
- 16.Troidl K, Ruding I, Cai WJ, et al. Actin-binding rho activating protein (Abra) is essential for fluid shear stress-induced arteriogenesis. Arterioscler Thromb Vasc Biol. 2009;29(12):2093–2101. doi: 10.1161/ATVBAHA.109.195305. [DOI] [PubMed] [Google Scholar]
- 17.Mahadeva H, Brooks G, Lodwick D, Chong NW, Samani NJ. ms1, a novel stress-responsive, muscle-specific gene that is up-regulated in the early stages of pressure overload-induced left ventricular hypertrophy. FEBS Lett. 2002;521(1–3):100–104. doi: 10.1016/s0014-5793(02)02833-8. [DOI] [PubMed] [Google Scholar]
- 18.Kuwahara K, Barrientos T, Pipes GC, Li S, Olson EN. Muscle-specific signaling mechanism that links actin dynamics to serum response factor. Mol Cell Biol. 2005;25(8):3173–3181. doi: 10.1128/MCB.25.8.3173-3181.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nesi RT, de Souza PS, Dos Santos GP, et al. Physical exercise is effective in preventing cigarette smoke-induced pulmonary oxidative response in mice. Int J Chron Obstruct Pulmon Dis. 2016;11:603–610. doi: 10.2147/COPD.S93958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanzetti M, da Costa CA, Nesi RT, et al. Oxidative stress and nitrosative stress are involved in different stages of proteolytic pulmonary emphysema. Free Radic Biol Med. 2012;53(11):1993–2001. doi: 10.1016/j.freeradbiomed.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Sakuma K, Nakao R, Yamasa Y, Yasuhara M. Normal distribution of presenilin-1 and nicastrin in skeletal muscle and the differential responses of these proteins after denervation. Biochim Biophys Acta. 2006;1760(6):980–987. doi: 10.1016/j.bbagen.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EFM. Systemic effects of smoking. Chest. 2007;131(5):1557–1566. doi: 10.1378/chest.06-2179. [DOI] [PubMed] [Google Scholar]
- 23.ATS/ERS Skeletal muscle dysfunction in chronic obstructive pulmonary disease. A statement of the American Thoracic Society and European Respiratory Society. Am J Respir Crit Care Med. 1999;159(4 Pt 2):S1–S40. doi: 10.1164/ajrccm.159.supplement_1.99titlepage. [DOI] [PubMed] [Google Scholar]
- 24.Gosker HR, Langen RC, Bracke KR, et al. Extrapulmonary manifestations of chronic obstructive pulmonary disease in a mouse model of chronic cigarette smoke exposure. Am J Respir Cell Mol Biol. 2009;40(6):710–716. doi: 10.1165/rcmb.2008-0312OC. [DOI] [PubMed] [Google Scholar]
- 25.Rinaldi M, Maes K, De Vleeschauwer S, et al. Long-term nose-only cigarette smoke exposure induces emphysema and mild skeletal muscle dysfunction in mice. Dis Model Mech. 2012;5(3):333–341. doi: 10.1242/dmm.008508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sotiropoulos A, Gineitis D, Copeland J, Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell. 1999;98(2):159–169. doi: 10.1016/s0092-8674(00)81011-9. [DOI] [PubMed] [Google Scholar]
- 27.Li L, Liu Z, Mercer B, Overbeek P, Olson EN. Evidence for serum response factor-mediated regulatory networks governing SM22alpha transcription in smooth, skeletal, and cardiac muscle cells. Dev Biol. 1997;187(2):311–321. doi: 10.1006/dbio.1997.8621. [DOI] [PubMed] [Google Scholar]
- 28.Frost RA, Mazella J, Tseng L. Insulin-like growth factor binding protein-1 inhibits the mitogenic effect of insulin-like growth factors and progestins in human endometrial stromal cells. Biol Reprod. 1993;49(1):104–111. doi: 10.1095/biolreprod49.1.104. [DOI] [PubMed] [Google Scholar]
- 29.Butler AA, Le Roith D. Control of growth by the somatropic axis: growth hormone and the insulin-like growth factors have related and independent roles. Annu Rev Physiol. 2001;63:141–164. doi: 10.1146/annurev.physiol.63.1.141. [DOI] [PubMed] [Google Scholar]
- 30.Crul T, Spruit MA, Gayan-Ramirez G, et al. Markers of inflammation and disuse in vastus lateralis of chronic obstructive pulmonary disease patients. Eur J Clin Invest. 2007;37(11):897–904. doi: 10.1111/j.1365-2362.2007.01867.x. [DOI] [PubMed] [Google Scholar]