Abstract
The discovery of a link between mutations in GBA1, encoding the lysosomal enzyme glucocerebrosidase, and the synucleinopathies directly resulted from the clinical recognition of patients with Gaucher disease with parkinsonism. Mutations in GBA1 are now the most common known genetic risk factor for several Lewy body disorders, and an inverse relationship exists between levels of glucocerebrosidase and oligomeric α-synuclein. While the underlying mechanisms are still debated, this complicated association is shedding light on the role of lysosomes in neurodegenerative disorders, demonstrating how insights from a rare disorder can direct research into the pathogenesis and therapy of seemingly unrelated common diseases.
Keywords: Gaucher disease, glucocerebrosidase, parkinsonism, alpha-synuclein, lysosome, autophagy
Introduction
While a link between two different medical disorders, Parkinson disease and Gaucher disease, has now been appreciated for over a decade, the basis for this association has remained elusive. The many theories proposed all have shortcomings with conflicting data, and what was once considered obvious, currently does not pass muster. There are important aspects of each disorder that have attracted attention as potential clues, but ultimately it may be time to return to the drawing board to rethink how glucocerebrosidase, the lysosomal enzyme deficient in Gaucher disease can impact Parkinson disease pathogenesis.
Parkinson disease, first described by James Parkinson two hundred years ago, is the second most common neurodegenerative disorder with an average age at diagnosis of 62 years and lifetime risk in developed nations of 3–4%. While it is now accepted that Parkinson disease is a complex multigenic disorder with both genetic and environmental contributions, major gaps remain in our understanding of disease pathogenesis and in our ability to treat patients. The recognition of the role of dopamine led to improved management of patients with Parkinson disease, but there continues to be no cure. Understanding the pathways contributing to disease etiology may better inform the design of new therapeutics. Human genetics has offered the opportunity to explore more “etiologic-based” treatments (Singleton & Hardy, 2016). Indeed, the genetics of Parkinson disease has been rigorously pursued using state-of-the-art unbiased and system-wide approaches, beginning with linkage analysis, genome wide association studies (GWAS) and more recently, whole-exome and whole genome sequencing approaches. However, the most common known genetic risk factor for Parkinson disease identified to date, mutations in GBA1, the gene encoding for the lysosomal enzyme glucocerebrosidase, was not discovered by such large system-wide endeavors. Rather it is a story that was directly born from the Medical Genetics clinic (Neudorfer et al, 1996; Tayebi et al, 2001).
Gaucher disease (GD, OMIM #606463), resulting from the inherited deficiency of glucocerebrosidase (GCase. E.C.3.2.1.45), is a rare disorder primarily affecting cells of the reticulo-endothelial lineage. Lysosomes within macrophages become engorged with the undigested substrates glucocerebroside and glucosylsphingosine, giving rise to the characteristic-appearing “Gaucher cell” (Beutler & Grabowski, 2001). GD is an extremely heterogeneous disorder with multi-organ involvement. While many patients with this disorder are so mildly affected that they can evade medical attention their entire lives, others present in infancy, childhood or adulthood. Systemic manifestations commonly include hepatosplenomegaly, which can be massive, anemia, thrombocytopenia and bony involvement including osteopenia, fractures and painful bone crises. Patients also can develop gammopathies, inflammation, and immunological and coagulation impairments. Nosebleeds, bony symptoms or painless organomegaly are commonly the presenting complaints. Patients without primary neurological manifestations have been classified as having type 1 or non-neuronopathic GD. Such patients have deficient GCase, but there is typically some residual enzymatic activity (Beutler & Grabowski, 2001). Type 2 or acute neuronopathic GD manifests in infancy or perinatally. These babies develop organomegaly and pancytopenia, as well as devastating and progressive neurodegeneration resulting in bulbar findings, opisthotonus and seizures (Weiss et al, 2015). Some are identified in the neonatal period or prenatally, when they manifest with hydrops fetalis, ichthyosis or arthrogryposis. Patients with type 2 GD make little or no GCase as a result of severe or null mutations, and the disorder is uniformly lethal in the first years of life (Beutler & Grabowski, 2001; Weiss et al, 2015). By default, patients with type 3 GD have some degree of neurological involvement, but survive infancy. Manifestations are diverse, and include slowing of the horizontal eye movements, myoclonic epilepsy and, at times, cognitive impairment (Beutler & Grabowski, 2001).
While studying the natural history of type 1 GD, it was noted that a few rare patients developed parkinsonian manifestations. Although initially these symptoms were considered to be unrelated, the observation persisted, and by the early 2000s several papers detailed series of patients sharing the two disorders. There was no consistent age, sex, ethnicity, degree of disease severity, treatment or Gaucher genotype shared by the patients who developed parkinsonism. Generally, the age of onset of parkinsonian manifestations was relatively young, and more rapid disease progression and cognitive impairment were noted (Neudorfer et al, 1996; Tayebi et al, 2001).
The next clinical observation was that first-degree family members of patients with GD also seemed to have an increased frequency of parkinsonism. When family histories were explored focusing on symptoms associated with parkinsonism, around 25% of patients had a family history of Parkinson disease. Often this was a parent, sibling or grandparent of the Gaucher proband who was an obligate GBA1 mutation carrier. This surprising finding led investigators to posit that perhaps even Gaucher heterozygotes might be at increased risk of developing PD (Goker-Alpan et al, 2004).
Two early studies indicated that this was indeed the case. Studying autopsy samples acquired from different brain banks, investigators noted that 12 of 57 subjects with a post-mortem diagnosis of PD carried GBA1 mutations (eight subjects) or the common alterations E326K or T369M (four cases), while no GBA1 alterations were identified in samples from 44 controls without parkinsonism (Lwin et al, 2004). In the same year, a cohort of 148 Ashkenazi Jewish patients with PD from Northern Israel were screened for selected GBA1 mutations with mutations identified in 40 patients (Aharon-Peretz et al, 2004). These publications were followed by replication studies in cohorts with PD around the world. Ultimately a large multicenter collaborative study was conducted which included 5691 patients with PD and 4898 controls screened in 14 centers on four continents. The odds ratio (OR) for carrying either GBA1 mutation N370S or L444P in subjects with PD was 5.43 across centers. When all exons of GBA1 were sequenced, approximately 7% of subjects with PD carried mutations in GBA1 (Sidransky et al, 2009). It is now accepted that depending on ethnicity and screening techniques used, between 2.3–9.4% of patients with PD (11–31% in Ashkenazi Jews) carry a GBA1 mutation. Two GBA1 alterations that do not appear to cause GD, E236K and T369M are similarly more common in subjects with PD, albeit with a lower OR i(Davis et al, 2016; Mallett et al, 2016).
Other reports have concluded that patients carrying more severe or null GBA1 mutations are at a higher risk for PD (Gan-Or et al, 2015). Clinical, neuropathological and imaging studies suggested that some GBA1 carriers had features characteristic of a related synucleinopathy, dementia with Lewy bodies (DLB) (Goker-Alpan et al, 2008; Goker-Alpan et al, 2012). While patients with DLB also develop parkinsonian manifestions, they tend to have a more rapidly progressive course, with pronounced cognitive impairment developing early in their cinical course. These observations, as well as pilot studies demonstrating GBA1 mutations in 3.5% to 28% of subjects with DLB, prompted a second multicenter analysis. Eleven centers from around the world joined contributing 721 cases that met clinical or pathological diagnostic criteria for DLB and 151 with Parkinson disease with dementia (PDD). The genotypes of these patients were compared to 1952 controls from the same centers, yielding an OR of 8.28 (95%CL =4.78–14.88), which was even higher than the OR for PD, further confirming the clinical impression of more prevalent cognitive impairment associated with mutations of GBA1 (Nalls et al, 2013).
A major player in PD pathogenesis is α-synuclein (α-syn), an intrinsically disordered 140 amino acid protein which is encoded by the gene SNCA. α-syn is abundant in brain, interacts with lipid membranes (Pfefferkorn et al, 2012), and has a propensity to aggregate.Its role in PD was appreciated in 1997 when Polymeropolous et.al. demonstrated segregation of PD with a mutation in α-syn in two Italian and one Greek families, and Spillantini et.al. showed that α-syn was present in Lewy bodies (Polymeropoulos et al, 1997; Spillantini et al, 1997). Dupliclations or triplications of α-syn also lead to familial PD. Unfolded momomeric α-syn can aggregate first into small oligomeric species that can be stabilized by β-sheet-like interactions, and then into higher molecular weight insoluble fibrils, seen in different synucleinopaties. The interaction of α-syn with the membranes of presynaptic and synaptic vesicles plays a role in synaptic vesicle docking, priming, clustering, fusion and recycling (Snead & Eliezer, 2014). The phosphorylation at specific serine residues of α-syn also can be important. Phosphorylation of S87 reduces its interaction with membranes, while phosphorylation of S129 may affect membrane binding of mutant, but not wildtype α-syn (Visanji et al, 2011).
Clinical Research
These initial genetic studies stimulated both clinical and basic science investigations probing the etiology and impact of this association. On the clinical side, studies prospectively evaluating patients with GBA1 mutations with and without overt manifestations of parkinsonism are currently in progress in several centers, aiming to better characterize the parkinsonian phenotype, identify potential biomarkers and recognize early signs of Parkinson disease in this at-risk population. The evaluations include olfactory testing, neurologic assessments, neurocognitive testing, surveys for non-motor, sleep and/or psychiatric manifestations and imaging with transcranial ultrasonography, MRI scans, DAT scans, MRI spectroscopy, 18F-fluoro-L-DOPA and 15O-H2O PET scans (Beavan et al, 2015; Chetrit et al, 2013; Goker-Alpan et al, 2012). The clinical course of subjects with either two or one mutant GBA1 alleles have been studied. A report of the clinical course of 19 patients with both GD and parkinsonism found that these patients with two mutant GBA1 alleles had an earlier age at disease onset and more cognitive changes than encountered in sporadic PD. However, there was not a uniformly aggressive clinical course or distinguishing features in these patients, and in fact, some had a late onset or relatively slow disease progression(Lopez et al, 2016). Imaging studies in GBA1-associated PD have revealed that the pattern of dopamine loss is similar to sporadic PD, with the greatest loss seen in the caudal striatum. Resting cerebral blood flow studies demonstrated that subjects with GD and PD had less activity in specific brain regions affected in neurodegenerative disorders with cognitive impairment (Goker-Alpan et al, 2012).
Among GBA1 carriers with PD there is also a spectrum of associated PD manifestations. Most studies do indicate an earlier age at PD onset among mutation carriers, and a more rapid progression of both motor features and cognitive decline (Mata et al, 2016). Unilateral tremor and bradykinesia are the most common presenting features, although gait abnormalities and postural instability are also relatively frequent. Levodopa responsiveness is largely positive. Rapid-eye-movement (REM) sleep disorder, impaired olfaction, depression and anxiety have been reported. It has been suggested that the extent of cognitive impairment is related to mutation severity (Cilia et al, 2016; Liu et al, 2016).
Biological Relationships
On the basic science side, despite a considerable number of investigations, the basis of this relationship remains confusing. There is a reciprocal relationship between levels of GCase and α-syn present in patients with synucleinopathies (Mazzulli et al, 2011). Evaluating brain bank samples, it was found that even subjects with idiopathic PD have decreased levels of GCase on Western blots (Gegg et al, 2012; Murphy et al, 2014; Rocha et al, 2015). When mutant GBA1 was over-expressed in neuronal cells, α-syn levels increased. This was found to be related to GCase levels rather than activity (Cullen et al, 2011). Knocking-down wildtype GCase in neurons with shRNA led to increased levels of oligomeric α-syn (Mazzulli et al, 2011).Two groups have reported that inhibition GCase activity with the highly selective inhibitor Conduritol B Epoxide (CBE) in both mice and neuroblastoma cells led to elevated levels of α-syn (Cleeter et al, 2013; Manning-Bog et al, 2009), although Dermentzaki and co-workers (Dermentzaki et al, 2013) did not observe accumulation of α-syn in CBE treated differentiated SH-SY5Y neuroblastoma cells. This discrepancy could result from different lengths of exposure to 50 µM CBE (ranging from 48 hours to 30 days), differences in assay conditions or the use of different buffers and α-syn antibodies in the three studies.
Using a different approach, it was shown in rodent models of PD that direct delivery of GCase to the brain resulted in a reduction of α-syn levels and an improvement of symptoms associated with parkinsonism (Rockenstein et al, 2016; Sardi et al, 2013). It was also found that infants with type 2 GD disease who often have extremely low residual GCase activity exhibit increased neuronal α-syn levels (Aflaki et al, 2016; Mazzulli et al, 2011), although no α-syn pathology is noted at autopsy (Berger-Sieczkowski et al, 2016).
However despite this evidence, the basis for and significance of this reciprocal relationship remains unclear. Both gain- and loss-of-function theories have been postulated, but there are serious deficiencies with each (Sardi et al, 2015; Siebert et al, 2014), (Schapira, 2015) (Blanz and Saftig 2016). The gain-of-function hypothesis is supported by the observation that many of the mutations identified result in a misfolded protein that could enhance a-syn aggregation and lead to lysosomal dysfunction, over-burdening of the ubiquitin-proteasome pathway or impairments of autophagy due to defective fusion between autophagosomes and lysosomes. However, the observation that some GBA1 mutations (i.e. c.84dupG) found in patients with PD are null mutations conflicts with this hypothesis, especially since null alleles are associated with an even higher risk for PD (Gan-Or et al, 2015).
Alternatively, parkinsonism could arise from the loss of GCase activity, where glucosylceramide accumulation could change lipid homeostasis, resulting in altered α-syn processing. Arguing against this theory is the finding that most patients with GD do not develop PD, despite having significantly less GCase than heterozygotes. Based upon data from a large Gaucher registry (Rosenbloom et al, 2011), among patients with GD, the probability of developing PD before age 70 is only 5–7%, about five times higher than in the general population. But over 90% of older patients still do not develop PD and thus, the enzymatic deficiency itself is not predictive of PD.
There is experimental evidence for a direct physical interaction between α-syn and GCase (Yap et al, 2011), indicating that the C-terminus of α-syn and GCase interact selectively under acidic conditions (pH 5.5) at residues 118–137. It was also found that α-syn efficiently inhibits GCase activity, and that saposin C (Sap C), a protein vital for GCase activity in vivo, protects GCase against α-syn inhibition (Yap et al, 2013). Using nuclear magnetic resonance spectroscopy, site-specific fluorescence, and Förster energy transfer probes, Sap C was observed to displace α-syn from GCase in solution in the presence of lipid, suggesting a role for Sap C in Gaucher-related PD (Yap et al, 2015). Sap C associates with GCase in solution in a 1:1 complex, and the region of Sap C contacting GCase is distinct from the region known to enhance GCase activity (Gruschus et al, 2015). Neutron reflectometry revealed that GCase binds to and partially inserts into the membrane bilayer, with its active site most likely lying just above the membrane-water interface (Yap et al, 2015). Using deuterated α-syn versus protiated GCase, changes in the membrane-bound structure of α-syn in the complex were demonstrated, indicating that the interaction with α-syn could displace GCase from the membrane, impeding substrate access and perturbing the active site. GCase would then move membrane-bound α-syn away from the bilayer, preventing its lysosomal degradation.
Insights from New Models
It has been challenging to study the mechanisms underlying GBA1-associated parkinsonism in part because most of the different cellular and animal models available each have inherent deficiencies. Attempts to use immortalized neuronal lines such as human neuroblastoma lines or SH-SY5Y cells by gene silencing, introducing mutations with nucleases, or pharmacological inhibition of GCase have been attempted, albeit with mixed success. Studying α-syn in cellular models can also be challenging as α-syn may not accumulate analogously during the shortened timeframe of cell culture, and may not be in the same forms found in human brain (Xin et al, 2015).
The challenge in creating appropriate disease models is not unique to GD. A better understanding of mechanisms underlying disease progression in both rare and common diseases can enhance the development of treatments specifically targeting aspects related to disease etiology. Many different types of animal models using rats, mice, dogs, flies and zebrafish have been developed. However, in animal models the observed phenotype can, at times, be species-specific and therefore not adequately mimic the human phenotype. The discovery of induced pluripotent stem cells (iPSCs) has opened new avenues for many aspects of clinical research, regenerative medicine and drug discovery because pluripotency technology can be used to better mimic conditions occurring in patients. Moreover, new technologies such as gene editing with CRISPR/Cas9 or TALENs can introduce or correct different mutations in order to study the role of these specific changes in humans. While such technologies are very labor-intensive, time-consuming and expensive, they can uncover mechanisms underlying disease in ways never previously possible. Recently, teams around the world have successfully established different iPSC lines from patients with PD harboring different GBA1 mutations.
In the first of these studies, iPSCs were generated from patients with PD harboring heterozygous GBA1 mutations (L444P, RecNcil and N370S). (Schondorf et al, 2014). This group differentiated iPSCs into dopaminergic neurons and observed reduced GCase activity and increased α-syn levels. They performed multiplexed quantitative proteomics on an enriched neuronal fraction, which indicated an elevation of NECAB2 in iPSC-neurons carrying GBA1 mutations. NECAB2 belongs to the family of neuronal calcium-binding proteins highly expressed in the dorsal substantia nigra (SN) (Ganat et al, 2012). Their research demonstrates the calcium buffering of NECAB2 and supports the hypothesis that the increase in NECAB2 seen in neurons carrying GBA1 mutations represents a compensatory mechanism. Additionally, iPSC-derived dopaminergic neurons from PD subjects carrying GBA1 mutations exhibit ER stress, reflected in elevated levels of binding immunoglobulin protein (BiP), protein disulfide-isomerase (PDI), and calnexin (Fernandes et al, 2016), which correlate with increased calcium release from the ER to the cytosol. This is in agreement with previous studies where altered calcium release from the ER in the cytosol was observed in GD rodent cell models and GD patient-derived tissues with GlcCer and GlcSph storage. These studies suggest that elevated levels of GlcCer and GlcSph might facilitate neuronal cell death through increased calcium release in the cytosol via a ryanodine receptor-dependent and an inositol (1,4,5)-trisphosphate receptor (InsP3R) and sarcoplasmic /endoplasmic reticulum Ca2+-ATPase (SERCA) mechanism, respectively (Korkotian & Segal, 1999; Pelled et al, 2005). While initially no difference in α-syn levels in iPSC-derived dopaminergic neurons was observed, α-syn secretion increased over time in iPSC-DA neurons from patients with PD carrying GBA1 mutations (Fernandes et al, 2016).
A study by Woodard et.al. described iPSC-derived dopaminergic neurons from monozygotic twins carrying an N370S GBA1 allele, where only one twin developed PD. The neurons from both twins had approximately 50% glucocerebrosidase activity, 3-fold elevated α-syn protein levels, and reduced dopamine levels, but neurons from the twin with PD had less dopamine, increased monoamine oxidase B (MAO-B) expression, and impaired intrinsic network activity (Woodard et al, 2014). This suggests that non-genetic factors may also contribute to PD pathogenesis in at-risk individuals.
A recent study uniquely focused on iPSC-derived neuronal lines from subjects with GD with and without parkinsonism, including a pair of siblings with GD disease discordant for PD. Preliminary assays indicated that these neurons do store and release dopamine. Dopaminergic neurons from patients with GD appropriately exhibit deficient enzymatic activity, reduced levels of GCase in lysosomes as well as storage of the glycolipid substrates glucosylceramide (GlcCer) and glucosylsphingosine (GlcSph). Aged neurons from the subjects with PD recapitulate the patient phenotype by displaying increased α-syn levels. This work also demonstrated that iPSC-dopaminergic neurons from patients with type 2 GD, the most severe acute neuronopathic form, also show increased α-syn accumulation (Aflaki et al, 2016).
Understanding the Reciprocal Relationship Between Glucocerebrosidase and α-syn
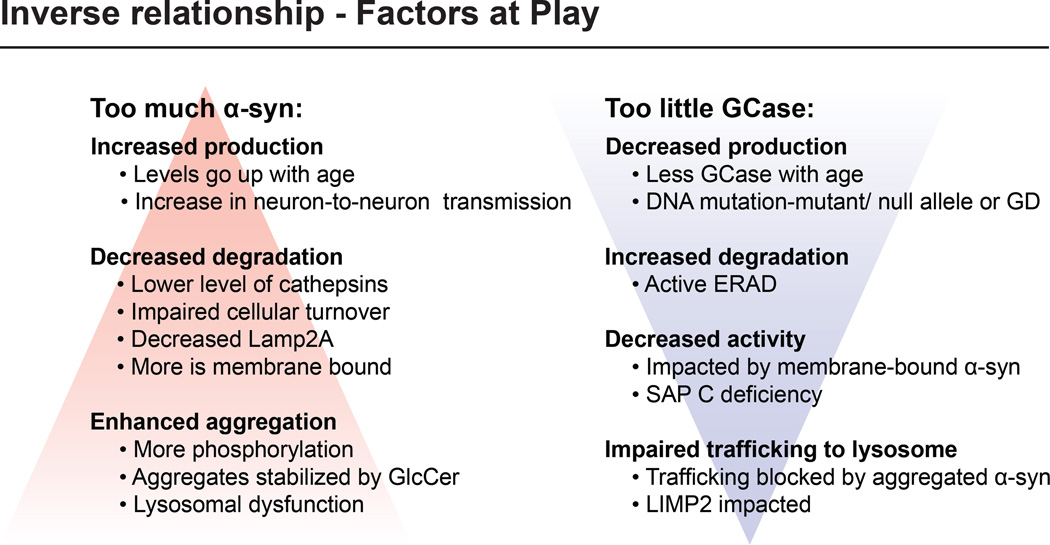
There are multiple factors that could potentially sway the reciprocal relationship between GCase and α-syn and thus impact the pathogenesis of PD or provide targets for therapeutic intervention (Fig 1). The normal balance between the two proteins could be altered by either an increase in the amount of α-syn present or by decreased GCase levels.
Figure 1. The inverse relationship between α-synuclein and glucocerebrosidase.
Multiple factors can impact the inverse relationship including those leading to too much α-syn or two little GCase.
Elevations in α-syn can result from increased production, decreased degradation or enhanced aggregation. It is known that levels of α-syn increase with age (Chu & Kordower, 2007) (Bobela et al, 2015). Also, α-syn aggregates may be spread or increased by neuron-to-neuron transmission (Lopes da Fonseca et al, 2015). Factors that might contribute to decreased degradation include lower levels of cathepsins, proteins responsible for α-syn breakdown, impaired intracellular turnover of α-syn, and an increase in membrane-bound α-syn (McGlinchey & Lee, 2015). Enhanced aggregation can result from increased phosphorylation, stabilization of aggregates by lipids like glucosylceramide and other causes of lysosomal dysfunction (Roberts & Brown, 2015; Wong & Krainc, 2016).
On the other hand, factors that can lead to decreased GCase levels include misfolded or absent protein secondary to gene mutations or enhanced enzyme degradation via ERAD (Blanz & Saftig, 2016; Horowitz et al, 2016). It has also been reported that levels of GCase go down with age (Rocha, Smith et al. 2015). GCase may be inactivated by membrane bound α-syn present in the lysosomal membrane (Yap et al, 2015). Also, trafficking of GCase to the lysosome could be blocked by aggregated intracellular α-syn as part of a bidirectional vicious cycle (Fig 2-pathway #1) (Mazzulli et al, 2011).
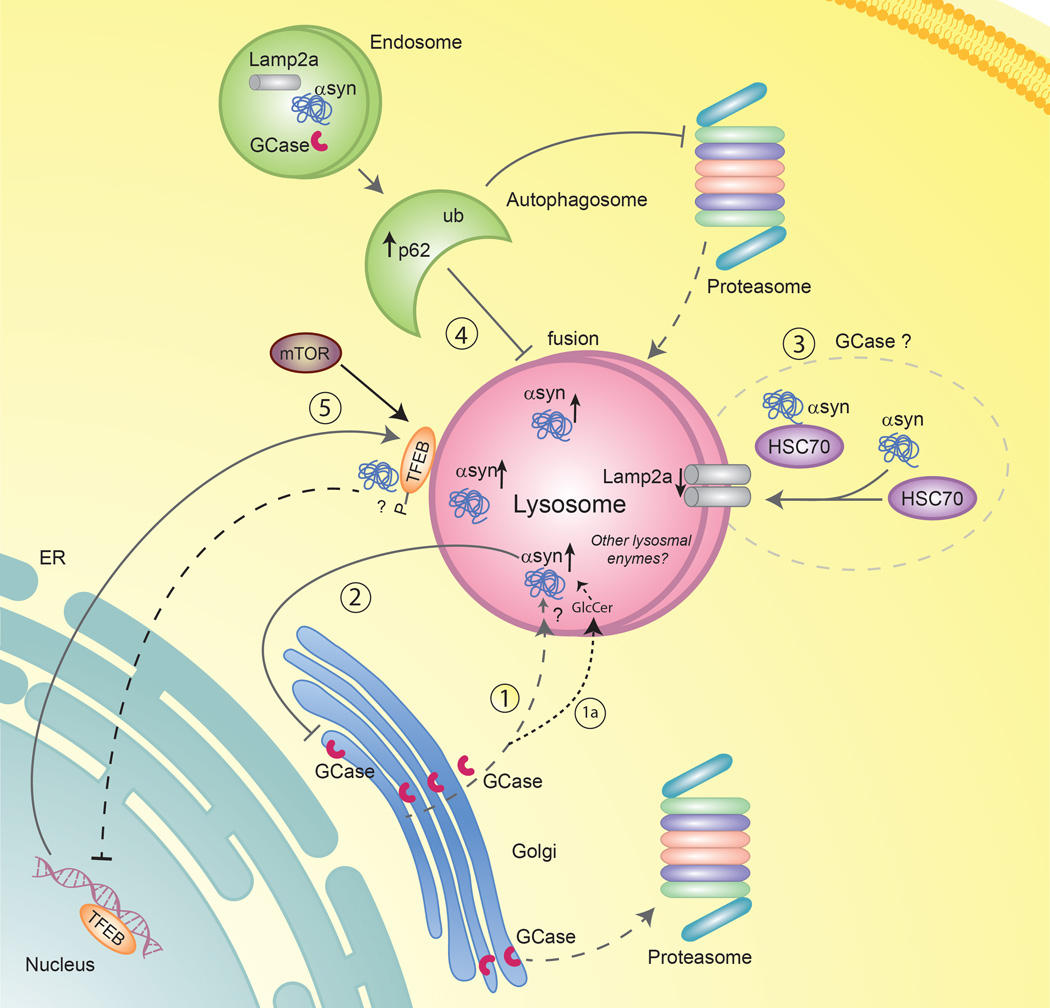
Figure 2. Pathways that might contribute to the association between GD and PD.
Wild-type GCase is produced in the ER, glycosylated in the Golgi and is translocated to the lysosome where it degrades its substrate, GlcCer. (1) Mutant GCase may undergo proteosomal breakdown, is not translocated to lysosomes. (1a) GlcCer accumulates in the lysosome, which subsequently may lead to α-syn aggregation; however, not all individuals carrying a mutation develop PD. (2) In PD, the oligomeric form of α-syn may suppress ER-Golgi trafficking of GCase which can result in reduced GCase activity. (3) α-syn is a substrate for a selective form of autophagy (chaperone mediated autophagy, CMA) and interacts with the cytosolic chaperone (HSC70), enabling its translocation to the lysosome with the help of Lamp2a, a receptor for CMA on the lysosomal membrane. It is not known whether GCase is a substrate for CMA or if CMA is affected in patients with PD and GD. (4) Macroautophagy could be affected. Accumulation of ubiquitinated proteins and p62 in the autophagosome inhibits fusion between the autophagosome and the lysosome, which augments the accumulation of autophagosomes in the cells. These eventually inhibit macroautophagy and impair lysosomal function. (5) Transcription factor EB (TFEB), a key regulator of lysosome biogenesis may play a role. Under normal conditions, mTOR interacts with TFEB on the surface of the lysosome, which leads to TFEB phosphorylation and cytosolic sequestration of this transcription factor. When autophagy is impaired or mTOR is inhibited, TFEB is no longer phosphorylated, which results in the dissociation of TFEB from the lysosome and its translocation to the nucleus. α-syn accumulation or lysosomal dysfunction could inhibit translocation of TFEB to the nucleus, leading to the accumulation of phosphorylated TFEB on the lysosome.
GCase is one of the few lysosomal enzymes which does not utilize the mannose-6-phosphate receptor to enter the lysosome, but rather it is transported to lysosome bound to the protein lysosomal integral membrane protein type-2 (LIMP-2)(Gonzalez et al, 2014). A deficiency in LIMP-2 results in the reduction of lysosomal GCase, and hence levels of LIMP-2 could also impact the reciprocal relationship between GCase and α-syn. In a mouse model, LIMP-2 induced deficiency of GCase led to increased α-syn aggregation and PD-like pathology (Blanz & Saftig, 2016; Rothaug et al, 2014). Furthermore, LIMP-2 overexpression in cell lines, including those over-expressing α-syn, led to reduced α-syn levels (Rothaug et al, 2014). There is also genetic evidence that LIMP-2 may be linked to PD (Michelakakis et al, 2012), although this it is not yet clear whether the reported SNPs indeed impact LIMP-2 expression (Maniwang et al, 2013).
The Role of the Autophagosomal/Lysosomal Pathway
Elucidating the causes of accumulation and aggregation of α-syn and the degradative pathways involved are essential for understanding disease pathogenesis and in designing treatments. Recent evidence that mutations in genes coding for other lysosomal proteins also may increase the risk of parkinsonism supports the premise that lysosomal dysfunction may be an important factor (Dagan et al, 2015; Shachar et al, 2011). Thus, degradation pathways involved in clearing accumulated α-syn, notably the autophagosome-lysosome systems, merit close attention. Different forms of autophagy may play a role in this process.
In macroautophagy, autophagosomes engulf cytosolic cargo before they are delivered to the lysosomes for degradation. Autophagosomes are increased in DA neurons in the substantia nigra (SN) of patients with PD, and lysosomal markers such as Cathespin D are decreased, suggesting impaired autophagic flux in patients with PD (Chu et al, 2009). Moreover, the presence of aggregated α-syn containing K48-linked polyubiquitin, and the ubiquitin-binding protein p62 in DA neurons, also indicates that autophagosomal/lysosomal and proteosomal pathways are defective, resulting in the failure to appropriately reduce misfolded proteins (Fig 2-pathway #4).
In addition to macroautophagy, defects in chaperone mediated autophagy (CMA), a specific form of autophagy, have been implicated in PD (Fig 2-pathway #3). In CMA, lysosomal targeting of proteins containing a KFERQ-like motif occurs via the cytosolic chaperone HSC70. The chaperone/substrate complex interacts with Lamp2a, which acts as a receptor on the lysosome. The substrate then passes through the lysosomal membrane and is rapidly degraded. It has been suggested that wild-type α-syn is a substrate for CMA, providing a possible link between CMA and PD (Cuervo et al, 2004). Additionally, there is evidence that Lamp2a and HSC70 levels are reduced in patients with PD, which could be attributed to reduced CMA activity (Alvarez-Erviti et al, 2010). However, there is currently no data indicating that GBA1 is a substrate for chaperone mediated autophagy or that CMA is defective in neuronopathic forms of GD.
A third pathway which may play a role in the link between PD and GD involves Transcription Factor EB (TFEB), a master regulator of the autophagy-lysosomal pathway (ALP) (Fig 2-pathway #5). In normal, nutrient-rich conditions, there is an abundance of amino acids available in lysosomes due to protein hydrolysis, which leads to activation and translocation of mammalian target of rapamycin (mTOR) to the lysosomal surface, where mTOR-dependent phosphorylation of TFEB facilitates its cytoplasmic sequestration. (Settembre & Ballabio, 2011; Settembre et al, 2013). Under starvation conditions, mTOR is inactivated and dissociates from the lysosomal surface. TFEB undergoes nuclear translocation and activates transcription of the coordinated lysosomal expression and regulation (CLEAR) network genes (Settembre et al, 2012). The association between lysosomal storage disorders and neurodegenerative diseases like PD highlights the importance of lysosomal degradation pathways in these disorders. Studying postmortem PD brain samples, it was shown that nuclear TFEB is reduced in the midbrain. There also appears to be an interaction of α-syn with TFEB (Decressac et al, 2013), which could lead to sequestering of the transcription factor in the cytosol, preventing its activation. Therefore, enhancing TFEB could be a potential therapeutic strategy for PD. One report showed down-regulated TFEB levels in type 2 GD iPSC-neurons (Awad et al, 2015), but data in dopaminergic neurons from patients with GBA1-associated PD is not currently available. Since TFEB has been reported to be a direct regulator of GCase expression, further studies probing the role of this pathway might enhance our understanding of the association between GD and PD (Sardiello et al, 2009).
While current evidence suggests that the autophagosome-lysosome system is impaired in PD, it will be essential to confirm this in relevant models of neuronopathic GD to determine if and how these pathways impact the association between PD and GD. These theories must be reconciled with the fact that carrying one GBA1 mutation, even a mild one, increases the risk of PD, although many of these patients have well above 50% of control GCase activity. It is theoretically possible that even a mild reduction in GCase activity leads to sub-optimal lysosomal function and an impaired ability to dispose of misfolded proteins, although most patients with GD that have much lower GCase levels and activity do not develop PD. Moreover, elevations of glucosylceramide, an important component of membranes, can impact internalization of α-syn into the lysosome. It is very important to emphasize that regardless of how mutations in GBA1 contribute to the development of parkinsonism, the vast majority of patients and carriers with GD never develop parkinsonism. Thus, it is quite likely that multiple factors, many of which are impacted by the aging process, work in concert with deficient GCase to cause parkinsonism.
There is also preliminary evidence linking other lysosomal enzymes to parkinsonism. In both control iPSC-dopaminergic neurons overexpressing α-syn, and neurons derived from patients with PD, it was observed that in acidic compartments, the activity of other lysosomal enzymes like β-galactosidase and hexosaminidase were reduced. (Mazzulli et al, 2016a). Genetic studies of patients with PD indicate that mutations in sphingomyelin phosphodiesterase 1 which cause Niemann-Pick disease may be more frequent than in controls, although larger studies are still required.(Dagan et al, 2016; Mao et al, 2017). A recent study in the Twitcher mouse model of Krabbe disease demonstrated α-syn aggregation in the brain and possible dysfunction in synaptic function, which was believed to be associated with the altered lipid profile (Marshall & Bongarzone, 2016).
Therapeutics for Gaucher Disease May Also Impact Parkinson Disease
The reciprocal relationship between GCase and α-syn has spurred a growing interest in the development of novel treatments for synucleinopathies based on the hypothesis that therapeutic enhancement of GCase activity might diminish aggregation and accumulation of α-syn in the brains of patients. This idea was supported by a promising proof-of-concept study in which a mouse model over-expressing A53T α-syn showed diminished α-syn accumulation when wild type GBA1 was introduced in the central nervous system by viral infection (Sardi et al, 2013).
The two FDA-approved therapies for patients with GD type 1 currently on the market are enzyme replacement therapy (ERT) and substrate reduction therapy (SRT). Patients on ERT receive life-long intravenous infusions of recombinant GCase enzyme on a regular basis. While ERT greatly improves hematologic and visceral symptoms, there is no alleviation of neurological manifestations in patients with type 2 and type 3 GD since ERT does not cross the blood-brain-barrier (BBB) (Jung et al, 2016). Thus, the current FDA-approved ERTs for GD are not suitable for treatment of synucleinopathies, and long-term ERT administration to patients with GD type 1 does not prevent the development of PD. Current research suggests that tagging of therapeutic proteins with BBB-crossing peptides or loading them into exosomes could enhance delivery to the brain. It remains to be tested if these strategies could indeed render ERT suitable for treatment of neuropathic forms of GD or synucleinopathies (Gramlich et al, 2016; Hall et al, 2016).
The second FDA-approved treatment for GD is SRT, where patients are treated with selective molecules that inhibit glucosylceramide synthase, thereby reducing the formation of the GCase substrate glucosylceramide (Van Rossum & Holsopple, 2016). While current SRT drugs on the market, which include Miglustat and Eliglustat, do not show efficacy in the brain, in neuropathic GD mouse models a novel SRT compound named Genz-682452 appeared to reduce storage and alleviate neurological symptoms (Marshall et al, 2016). Thus, the effect of Genz-682452 on α-syn levels should be evaluated in models of synucleinopathies.
There currently remains an urgent need for an effective treatment for neuronopathic forms of GD. A decade long collaboration between NHGRI and the National Chemical Genomics Center (NCGC) yielded significant progress in the development of small chemical chaperone therapy for GD (Jung et al, 2016). Various mutations in GBA1 lead to protein misfolding in the endoplasmic reticulum (ER), followed by premature proteasome-mediated degradation, reduced translocation of GCase from the ER to lysosomes and increased substrate accumulation (Maor et al, 2013). High throughput screening (HTS) was employed to identify small chemical chaperones that specifically bind to various mutant forms of GCase and increase enzyme stability, lysosomal translocation, lysosomal enzyme activity and substrate turnover. Small chemical chaperones for GCase can successfully cross the BBB and might modulate GCase activity and protein levels in the brain (Patnaik et al, 2012), and subsequently increase substrate turnover due to residual enzyme activity. Not only would such a therapy be beneficial for the treatment of neuronopathic forms of GD, but it might also hold promise for treatment of the synucleinopathies.
The majority of small chemical chaperones described in the literature are GCase inhibitors that stabilize the enzyme by interacting with the active site (Jung et al, 2016). However, once the enzyme-inhibitor complex reaches the lysosome, the substrate has to out-compete the inhibitor for optimal substrate turnover, which makes balancing drug dosing challenging. For example, Isofagomine, an imminosugar-based inhibitor molecule that showed much promise in cell and mouse models of GD, failed to demonstrate efficacy in a phase 2 clinical trial (Khanna et al, 2010; Sun et al, 2012). One potential candidate chaperone, the expectorant ambroxol, was identified as a mixed inhibitor of GCase in a high throughput screen (HTS) of an FDA-approved drug library (Maegawa et al, 2009). The potency of ambroxol for GD has been demonstrated in various in vitro and in vivo models such as cells, mice, and flies (Luan et al, 2013; Maor et al, 2016). Pilot studies conducted in 12 patients with GD type 1 and five with neuronopathic GD demonstrated no further deterioration of clinical symptoms in the patients with GD type 1 and improvement of myoclonus and pupillary light reflex in patients with GD type 3 (Narita et al, 2016; Zimran et al, 2013). In an in vitro study where α-syn was reduced in an ambroxol-treated α-syn over-expressing neuroblastoma cell line and preliminary reports of the efficacy of ambroxol in neuronopathic GD, support further study of this molecule (McNeill et al, 2014).
In contrast, non-inhibitory chaperones stabilize enzyme by binding to a site other than the active site, thereby rendering it available for substrate turnover even after the enzyme becomes lysosome-resident (Jung et al, 2016). Non-inhibitory chaperones for GCase were identified using a unique HTS strategy utilizing a spleen homogenate from a patient with type 1GD as the source of mutant GCase enzyme, and increases in enzyme activity as the outcome parameter. The screen identified about 30 non-inhibitory chaperones, amongst them NCGC758 and NCGC607 (Goldin et al, 2012; Patnaik et al, 2012). Initial microscopy-based translocation assays in GD fibroblasts showed a significant increase in GCase in lysosomal compartments of cells treated with NCGC758 compared to non-treated cells (Patnaik et al., 2012). Further cell-based evaluation was restricted as the GD fibroblast cell model lacks lysosomal substrate storage. The development of relevant GD macrophage and neuronal cell models proved to be invaluable for the evaluation of the non-inhibitory chaperones identified in the spleen homogenate-based HTS. Treatment of primary human macrophages derived from monocytes and macrophages derived from induced pluripotent stem cells (iPSCs) with the non-inhibitory chaperone NCGC758 resulted in increased GCase enzyme activity and translocation to lysosomes, improved chemotaxis, and reduced substrate storage (Aflaki et al, 2014). However, the binding site on the enzyme must be intact, which makes this therapy unsuitable for patients where GCase expression is absent or where mutations affect binding sites.
Small chemical chaperones can cross the BBB, as demonstrated for the non-inhibitory chaperone NCGC758 (Patnaik et al, 2012), and have the potential to modulate α-syn levels in the brain by increasing GCase protein levels and enzyme activity. This property renders them attractive therapeutic candidates for the treatment of synucleinopathies. Two very recent studies on iPSC-derived human midbrain dopaminergic neurons from patients with PD, GD and PD, and GD types 1 and 2 showed that treatment with the non-inhibitory chaperones NCGC758 and NCGC607 reduced substrate accumulation, increased lysosomal activity, enhanced GCase translocation to lysosomes, and reversed α-syn accumulation and downstream toxicity (Aflaki et al, 2016; Mazzulli et al, 2016b). Although such non-inhibitory compounds have not yet been evaluated in animal models, these studies showcase the relevance of appropriate cell-based models in evaluating modulators of GCase as a novel therapy for the synucleinopathies.
Identifying the Missing Pieces
Despite a decade of work, and several hundred related publications, the field remains stymied by the enigmatic role that GCase plays in Parkinson pathogenesis. The observation that patients with null GBA1 alleles still are at increased risk for parkinsonism negates gain-of-function theories, while the fact that the risk is also increased in heterozygous carriers of extremely mild mutations indicates that deficient enzymatic activity alone cannot be held responsible. It is intriguing to speculate that GCase could have another totally unrelated “moonlighting” function which, when absent or deficient, contributes to parkinsonism. Most likely, multiple factors play into the equation, not the least of which is the aging process itself, and its impact on gene regulation, autophagy and lysosomal function.
While the genetics of parkinsonism is certainly complex, the glucocerebrosidase story may provide an anchor to help in the identification of other contributing genetic, epigenetic and environmental risk factors. By utilizing whole exome or genome sequencing, targeted microarrays, methylation chips, and other “omic” strategies, the genetic signatures of large numbers of patients with PD with and without GBA1 mutations, or subjects with GBA1 mutations with or without PD can be compared. Such studies, both ambitious and quintessentially collaborative, may ultimately reveal “the magic in the web of it” (Othello) and enable us to discover and untangle the many contributing strands.
In brief.
Aflaki et al provide new perspectives on potential mechanisms and pathways underlying the enigmatic association between the lysosomal enzyme glucocerebrosidase and Parkinson disease. They posit that glucocerebrosidase may provide a novel drug target for patients with parkinsonism.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aflaki E, Borger DK, Moaven N, Stubblefield BK, Rogers SA, Patnaik S, Schoenen FJ, Westbroek W, Zheng W, Sullivan P, Fujiwara H, Sidhu R, Khaliq ZM, Lopez GJ, Goldstein DS, Ory DS, Marugan J, Sidransky E. A New Glucocerebrosidase Chaperone Reduces alpha-Synuclein and Glycolipid Levels in iPSC-Derived Dopaminergic Neurons from Patients with Gaucher Disease and Parkinsonism. J Neurosci. 2016;36(28):7441–7452. doi: 10.1523/JNEUROSCI.0636-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aflaki E, Stubblefield BK, Maniwang E, Lopez G, Moaven N, Goldin E, Marugan J, Patnaik S, Dutra A, Southall N, Zheng W, Tayebi N, Sidransky E. Macrophage models of Gaucher disease for evaluating disease pathogenesis and candidate drugs. Sci Transl Med. 2014;6(240):240ra73. doi: 10.1126/scitranslmed.3008659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R. Mutations in the glucocerebrosidase gene and Parkinson’s disease in Ashkenazi Jews. N Engl J Med. 2004;351(19):1972–1977. doi: 10.1056/NEJMoa033277. [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Rodriguez-Oroz MC, Cooper JM, Caballero C, Ferrer I, Obeso JA, Schapira AH. Chaperone-mediated autophagy markers in Parkinson disease brains. Arch Neurol. 2010;67(12):1464–1472. doi: 10.1001/archneurol.2010.198. [DOI] [PubMed] [Google Scholar]
- Awad O, Sarkar C, Panicker LM, Miller D, Zeng X, Sgambato JA, Lipinski MM, Feldman RA. Altered TFEB-mediated lysosomal biogenesis in Gaucher disease iPSC-derived neuronal cells. Hum Mol Genet. 2015;24(20):5775–5788. doi: 10.1093/hmg/ddv297. [DOI] [PubMed] [Google Scholar]
- Beavan M, McNeill A, Proukakis C, Hughes DA, Mehta A, Schapira AH. Evolution of prodromal clinical markers of Parkinson disease in a GBA mutation-positive cohort. JAMA Neurol. 2015;72(2):201–208. doi: 10.1001/jamaneurol.2014.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger-Sieczkowski E, Lutz MI, Auff E, Kovacs GG. Gaucher cells are not associated with alpha-synuclein neuropathology in infants. Clin Neuropathol. 2016;35(3):122–128. doi: 10.5414/NP300901. [DOI] [PubMed] [Google Scholar]
- Beutler E, Grabowski GA. Gaucher disease, in. In: Scriver CBA, Beaudet AL, Sly WS, Valle D, editors. The metabolic & molecular bases of inherited disease. New York: McGraw-Hill; 2001. pp. 3635–3668. [Google Scholar]
- Blanz J, Saftig P. Parkinson’s disease: acid-glucocerebrosidase activity and alpha-synuclein clearance. J Neurochem. 2016;139(Suppl 1):198–215. doi: 10.1111/jnc.13517. [DOI] [PubMed] [Google Scholar]
- Bobela W, Aebischer P, Schneider BL. Alphalpha-Synuclein as a Mediator in the Interplay between Aging and Parkinson’s Disease. Biomolecules. 2015;5(4):2675–2700. doi: 10.3390/biom5042675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetrit EB, Alcalay RN, Steiner-Birmanns B, Altarescu G, Phillips M, Elstein D, Zimran A. Phenotype in patients with Gaucher disease and Parkinson disease. Blood Cells Mol Dis. 2013;50(3):218–221. doi: 10.1016/j.bcmd.2012.11.011. [DOI] [PubMed] [Google Scholar]
- Chu Y, Dodiya H, Aebischer P, Olanow CW, Kordower JH. Alterations in lysosomal and proteasomal markers in Parkinson’s disease: relationship to alpha-synuclein inclusions. Neurobiol Dis. 2009;35(3):385–398. doi: 10.1016/j.nbd.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Chu Y, Kordower JH. Age-associated increases of alpha-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: Is this the target for Parkinson’s disease? Neurobiol Dis. 2007;25(1):134–149. doi: 10.1016/j.nbd.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Cilia R, Tunesi S, Marotta G, Cereda E, Siri C, Tesei S, Zecchinelli AL, Canesi M, Mariani CB, Meucci N, Sacilotto G, Zini M, Barichella M, Magnani C, Duga S, Asselta R, Solda G, Seresini A, Seia M, Pezzoli G, Goldwurm S. Survival and dementia in GBA-associated Parkinson’s disease: The mutation matters. Ann Neurol. 2016;80(5):662–673. doi: 10.1002/ana.24777. [DOI] [PubMed] [Google Scholar]
- Cleeter MW, Chau KY, Gluck C, Mehta A, Hughes DA, Duchen M, Wood NW, Hardy J, Mark Cooper J, Schapira AH. Glucocerebrosidase inhibition causes mitochondrial dysfunction and free radical damage. Neurochemistry international. 2013;62(1):1–7. doi: 10.1016/j.neuint.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305(5688):1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- Cullen V, Sardi SP, Ng J, Xu YH, Sun Y, Tomlinson JJ, Kolodziej P, Kahn I, Saftig P, Woulfe J, Rochet JC, Glicksman MA, Cheng SH, Grabowski GA, Shihabuddin LS, Schlossmacher MG. Acid beta-glucosidase mutants linked to Gaucher disease, Parkinson disease, and Lewy body dementia alter alpha-synuclein processing. Annals of neurology. 2011;69(6):940–953. doi: 10.1002/ana.22400. [DOI] [PubMed] [Google Scholar]
- Dagan E, Schlesinger I, Ayoub M, Mory A, Nassar M, Kurolap A, Peretz-Aharon J, Gershoni-Baruch R. The contribution of Niemann-Pick SMPD1 mutations to Parkinson disease in Ashkenazi Jews. Parkinsonism Relat Disord. 2015;21(9):1067–1071. doi: 10.1016/j.parkreldis.2015.06.016. [DOI] [PubMed] [Google Scholar]
- Dagan E, Schlesinger I, Kurolap A, Ayoub M, Nassar M, Peretz-Aharon J, Gershoni-Baruch R. LRRK2, GBA and SMPD1 Founder Mutations and Parkinson’s Disease in Ashkenazi Jews. Dement Geriatr Cogn Disord. 2016;42(1–2):1–6. doi: 10.1159/000447450. [DOI] [PubMed] [Google Scholar]
- Davis MY, Johnson CO, Leverenz JB, Weintraub D, Trojanowski JQ, Chen-Plotkin A, Van Deerlin VM, Quinn JF, Chung KA, Peterson-Hiller AL, Rosenthal LS, Dawson TM, Albert MS, Goldman JG, Stebbins GT, Bernard B, Wszolek ZK, Ross OA, Dickson DW, Eidelberg D, Mattis PJ, Niethammer M, Yearout D, Hu SC, Cholerton BA, Smith M, Mata IF, Montine TJ, Edwards KL, Zabetian CP. Association of GBA Mutations and the E326K Polymorphism With Motor and Cognitive Progression in Parkinson Disease. JAMA Neurol. 2016;73(10):1217–1224. doi: 10.1001/jamaneurol.2016.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decressac M, Mattsson B, Weikop P, Lundblad M, Jakobsson J, Bjorklund A. TFEB-mediated autophagy rescues midbrain dopamine neurons from alpha-synuclein toxicity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(19):E1817–E1826. doi: 10.1073/pnas.1305623110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermentzaki G, Dimitriou E, Xilouri M, Michelakakis H, Stefanis L. Loss of beta-glucocerebrosidase activity does not affect alpha-synuclein levels or lysosomal function in neuronal cells. PLoS One. 2013;8(4):e60674. doi: 10.1371/journal.pone.0060674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes HJ, Hartfield EM, Christian HC, Emmanoulidou E, Zheng Y, Booth H, Bogetofte H, Lang C, Ryan BJ, Sardi SP, Badger J, Vowles J, Evetts S, Tofaris GK, Vekrellis K, Talbot K, Hu MT, James W, Cowley SA, Wade-Martins R. ER Stress and Autophagic Perturbations Lead to Elevated Extracellular alpha-Synuclein in GBA-N370S Parkinson’s iPSC-Derived Dopamine Neurons. Stem Cell Reports. 2016;6(3):342–356. doi: 10.1016/j.stemcr.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan-Or Z, Amshalom I, Kilarski LL, Bar-Shira A, Gana-Weisz M, Mirelman A, Marder K, Bressman S, Giladi N, Orr-Urtreger A. Differential effects of severe vs mild GBA mutations on Parkinson disease. Neurology. 2015;84(9):880–887. doi: 10.1212/WNL.0000000000001315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganat YM, Calder EL, Kriks S, Nelander J, Tu EY, Jia F, Battista D, Harrison N, Parmar M, Tomishima MJ, Rutishauser U, Studer L. Identification of embryonic stem cell-derived midbrain dopaminergic neurons for engraftment. J Clin Invest. 2012;122(8):2928–2939. doi: 10.1172/JCI58767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegg ME, Burke D, Heales SJ, Cooper JM, Hardy J, Wood NW, Schapira AH. Glucocerebrosidase deficiency in substantia nigra of parkinson disease brains. Annals of neurology. 2012;72(3):455–463. doi: 10.1002/ana.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goker-Alpan O, Lopez G, Vithayathil J, Davis J, Hallett M, Sidransky E. The spectrum of parkinsonian manifestations associated with glucocerebrosidase mutations. Arch Neurol. 2008;65(10):1353–1357. doi: 10.1001/archneur.65.10.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goker-Alpan O, Masdeu JC, Kohn PD, Ianni A, Lopez G, Groden C, Chapman MC, Cropp B, Eisenberg DP, Maniwang ED, Davis J, Wiggs E, Sidransky E, Berman KF. The neurobiology of glucocerebrosidase-associated parkinsonism: a positron emission tomography study of dopamine synthesis and regional cerebral blood flow. Brain. 2012;135(Pt 8):2440–2448. doi: 10.1093/brain/aws174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goker-Alpan O, Schiffmann R, LaMarca ME, Nussbaum RL, McInerney-Leo A, Sidransky E. Parkinsonism among Gaucher disease carriers. Journal of medical genetics. 2004;41(12):937–940. doi: 10.1136/jmg.2004.024455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin E, Zheng W, Motabar O, Southall N, Choi JH, Marugan J, Austin CP, Sidransky E. High Throughput Screening for Small Molecule Therapy for Gaucher Disease Using Patient Tissue as the Source of Mutant Glucocerebrosidase. PLoS ONE. 2012;7(1):e29861. doi: 10.1371/journal.pone.0029861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A, Valeiras M, Sidransky E, Tayebi N. Lysosomal integral membrane protein-2: a new player in lysosome-related pathology. Mol Genet Metab. 2014;111(2):84–91. doi: 10.1016/j.ymgme.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramlich PA, Westbroek W, Feldman RA, Awad O, Mello N, Remington MP, Sun Y, Zhang W, Sidransky E, Betenbaugh MJ, Fishman PS. A peptide-linked recombinant glucocerebrosidase for targeted neuronal delivery: Design, production, and assessment. J Biotechnol. 2016;221:1–12. doi: 10.1016/j.jbiotec.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruschus JM, Jiang Z, Yap TL, Hill SA, Grishaev A, Piszczek G, Sidransky E, Lee JC. Dissociation of glucocerebrosidase dimer in solution by its co-factor, saposin C. Biochem Biophys Res Commun. 2015;457(4):561–566. doi: 10.1016/j.bbrc.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Prabhakar S, Balaj L, Lai CP, Cerione RA, Breakefield XO. Delivery of Therapeutic Proteins via Extracellular Vesicles: Review and Potential Treatments for Parkinson’s Disease, Glioma, and Schwannoma. Cell Mol Neurobiol. 2016;36(3):417–427. doi: 10.1007/s10571-015-0309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz M, Elstein D, Zimran A, Goker-Alpan O. New Directions in Gaucher Disease. Hum Mutat. 2016;37(11):1121–1136. doi: 10.1002/humu.23056. [DOI] [PubMed] [Google Scholar]
- Jung O, Patnaik S, Marugan J, Sidransky E, Westbroek W. Progress and potential of non-inhibitory small molecule chaperones for the treatment of Gaucher disease and its implications for Parkinson disease. Expert Rev Proteomics. 2016;13(5):471–479. doi: 10.1080/14789450.2016.1174583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R, Benjamin ER, Pellegrino L, Schilling A, Rigat BA, Soska R, Nafar H, Ranes BE, Feng J, Lun Y, Powe AC, Palling DJ, Wustman BA, Schiffmann R, Mahuran DJ, Lockhart DJ, Valenzano KJ. The pharmacological chaperone isofagomine increases the activity of the Gaucher disease L444P mutant form of beta-glucosidase. FEBS J. 2010;277(7):1618–1638. doi: 10.1111/j.1742-4658.2010.07588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkotian E, Segal M. Release of calcium from stores alters the morphology of dendritic spines in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 1999;96(21):12068–12072. doi: 10.1073/pnas.96.21.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Boot B, Locascio JJ, Jansen IE, Winder-Rhodes S, Eberly S, Elbaz A, Brice A, Ravina B, van Hilten JJ, Cormier-Dequaire F, Corvol JC, Barker RA, Heutink P, Marinus J, Williams-Gray CH, Scherzer CR for theInternational Genetics of Parkinson Disease Progression, C. Neuropathic Gaucher’s mutations accelerate cognitive decline in Parkinson’s. Ann Neurol. 2016 doi: 10.1002/ana.24781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes da Fonseca T, Villar-Pique A, Outeiro TF. The Interplay between Alpha-Synuclein Clearance and Spreading. Biomolecules. 2015;5(2):435–471. doi: 10.3390/biom5020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez G, Kim J, Wiggs E, Cintron D, Groden C, Tayebi N, Mistry PK, Pastores GM, Zimran A, Goker-Alpan O, Sidransky E. Clinical course and prognosis in patients with Gaucher disease and parkinsonism. Neurol Genet. 2016;2(2):e57. doi: 10.1212/NXG.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan Z, Li L, Higaki K, Nanba E, Suzuki Y, Ohno K. The chaperone activity and toxicity of ambroxol on Gaucher cells and normal mice. Brain Dev. 2013;35(4):317–322. doi: 10.1016/j.braindev.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Lwin A, Orvisky E, Goker-Alpan O, LaMarca ME, Sidransky E. Glucocerebrosidase mutations in subjects with parkinsonism. Molecular genetics and metabolism. 2004;81(1):70–73. doi: 10.1016/j.ymgme.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Maegawa GH, Tropak MB, Buttner JD, Rigat BA, Fuller M, Pandit D, Tang L, Kornhaber GJ, Hamuro Y, Clarke JT, Mahuran DJ. Identification and characterization of ambroxol as an enzyme enhancement agent for Gaucher disease. J Biol Chem. 2009;284(35):23502–23516. doi: 10.1074/jbc.M109.012393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallett V, Ross JP, Alcalay RN, Ambalavanan A, Sidransky E, Dion PA, Rouleau GA, Gan-Or Z. GBA p.T369M substitution in Parkinson disease: Polymorphism or association? A meta-analysis. Neurol Genet. 2016;2(5):e104. doi: 10.1212/NXG.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniwang E, Tayebi N, Sidransky E. Is Parkinson disease associated with lysosomal integral membrane protein type-2?: challenges in interpreting association data. Mol Genet Metab. 2013;108(4):269–271. doi: 10.1016/j.ymgme.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning-Bog AB, Schule B, Langston JW. Alpha-synuclein-glucocerebrosidase interactions in pharmacological Gaucher models: a biological link between Gaucher disease and parkinsonism. Neurotoxicology. 2009;30(6):1127–1132. doi: 10.1016/j.neuro.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Mao CY, Yang J, Wang H, Zhang SY, Yang ZH, Luo HY, Li F, Shi M, Liu YT, Zhuang ZP, Du P, Wang YH, Shi CH, Xu YM. SMPD1 variants in Chinese Han patients with sporadic Parkinson’s disease. Parkinsonism Relat Disord. 2017;34:59–61. doi: 10.1016/j.parkreldis.2016.10.014. [DOI] [PubMed] [Google Scholar]
- Maor G, Cabasso O, Krivoruk O, Rodriguez J, Steller H, Segal D, Horowitz M. The contribution of mutant GBA to the development of Parkinson disease in Drosophila. Hum Mol Genet. 2016 doi: 10.1093/hmg/ddw129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maor G, Rencus-Lazar S, Filocamo M, Steller H, Segal D, Horowitz M. Unfolded protein response in Gaucher disease: from human to Drosophila. Orphanet J Rare Dis. 2013;8:140. doi: 10.1186/1750-1172-8-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J, Sun Y, Bangari DS, Budman E, Park H, Nietupski JB, Allaire A, Cromwell MA, Wang B, Grabowski GA, Leonard JP, Cheng SH. CNS-accessible Inhibitor of Glucosylceramide Synthase for Substrate Reduction Therapy of Neuronopathic Gaucher Disease. Mol Ther. 2016;24(6):1019–1029. doi: 10.1038/mt.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall MS, Bongarzone ER. Beyond Krabbe’s disease: The potential contribution of galactosylceramidase deficiency to neuronal vulnerability in late-onset synucleinopathies. J Neurosci Res. 2016;94(11):1328–1332. doi: 10.1002/jnr.23751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata IF, Leverenz JB, Weintraub D, Trojanowski JQ, Chen-Plotkin A, Van Deerlin VM, Ritz B, Rausch R, Factor SA, Wood-Siverio C, Quinn JF, Chung KA, Peterson-Hiller AL, Goldman JG, Stebbins GT, Bernard B, Espay AJ, Revilla FJ, Devoto J, Rosenthal LS, Dawson TM, Albert MS, Tsuang D, Huston H, Yearout D, Hu SC, Cholerton BA, Montine TJ, Edwards KL, Zabetian CP. GBA Variants are associated with a distinct pattern of cognitive deficits in Parkinson’s disease. Mov Disord. 2016;31(1):95–102. doi: 10.1002/mds.26359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, Caldwell GA, Sidransky E, Grabowski GA, Krainc D. Gaucher disease glucocerebrosidase and alpha-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146(1):37–52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzulli JR, Zunke F, Isacson O, Studer L, Krainc D. alpha-Synuclein-induced lysosomal dysfunction occurs through disruptions in protein trafficking in human midbrain synucleinopathy models. Proc Natl Acad Sci U S A. 2016a;113(7):1931–1936. doi: 10.1073/pnas.1520335113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzulli JR, Zunke F, Tsunemi T, Toker NJ, Jeon S, Burbulla LF, Patnaik S, Sidransky E, Marugan JJ, Sue CM, Krainc D. Activation of beta-Glucocerebrosidase Reduces Pathological alpha-Synuclein and Restores Lysosomal Function in Parkinson’s Patient Midbrain Neurons. J Neurosci. 2016b;36(29):7693–7706. doi: 10.1523/JNEUROSCI.0628-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlinchey RP, Lee JC. Cysteine cathepsins are essential in lysosomal degradation of alpha-synuclein. Proc Natl Acad Sci U S A. 2015;112(30):9322–9327. doi: 10.1073/pnas.1500937112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill A, Magalhaes J, Shen C, Chau KY, Hughes D, Mehta A, Foltynie T, Cooper JM, Abramov AY, Gegg M, Schapira AH. Ambroxol improves lysosomal biochemistry in glucocerebrosidase mutation-linked Parkinson disease cells. Brain. 2014;137(Pt 5):1481–1495. doi: 10.1093/brain/awu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelakakis H, Xiromerisiou G, Dardiotis E, Bozi M, Vassilatis D, Kountra PM, Patramani G, Moraitou M, Papadimitriou D, Stamboulis E, Stefanis L, Zintzaras E, Hadjigeorgiou GM. Evidence of an association between the scavenger receptor class B member 2 gene and Parkinson’s disease. Mov Disord. 2012;27(3):400–405. doi: 10.1002/mds.24886. [DOI] [PubMed] [Google Scholar]
- Murphy KE, Gysbers AM, Abbott SK, Tayebi N, Kim WS, Sidransky E, Cooper A, Garner B, Halliday GM. Reduced glucocerebrosidase is associated with increased alpha-synuclein in sporadic Parkinson’s disease. Brain. 2014;137(Pt 3):834–848. doi: 10.1093/brain/awt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalls MA, Duran R, Lopez G, Kurzawa-Akanbi M, McKeith IG, Chinnery PF, Morris CM, Theuns J, Crosiers D, Cras P, Engelborghs S, De Deyn PP, Van Broeckhoven C, Mann DM, Snowden J, Pickering-Brown S, Halliwell N, Davidson Y, Gibbons L, Harris J, Sheerin UM, Bras J, Hardy J, Clark L, Marder K, Honig LS, Berg D, Maetzler W, Brockmann K, Gasser T, Novellino F, Quattrone A, Annesi G, De Marco EV, Rogaeva E, Masellis M, Black SE, Bilbao JM, Foroud T, Ghetti B, Nichols WC, Pankratz N, Halliday G, Lesage S, Klebe S, Durr A, Duyckaerts C, Brice A, Giasson BI, Trojanowski JQ, Hurtig HI, Tayebi N, Landazabal C, Knight MA, Keller M, Singleton AB, Wolfsberg TG, Sidransky E. A multicenter study of glucocerebrosidase mutations in dementia with Lewy bodies. JAMA neurology. 2013;70(6):727–735. doi: 10.1001/jamaneurol.2013.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita A, Shirai K, Itamura S, Matsuda A, Ishihara A, Matsushita K, Fukuda C, Kubota N, Takayama R, Shigematsu H, Hayashi A, Kumada T, Yuge K, Watanabe Y, Kosugi S, Nishida H, Kimura Y, Endo Y, Higaki K, Nanba E, Nishimura Y, Tamasaki A, Togawa M, Saito Y, Maegaki Y, Ohno K, Suzuki Y. Ambroxol chaperone therapy for neuronopathic Gaucher disease: A pilot study. Ann Clin Transl Neurol. 2016;3(3):200–215. doi: 10.1002/acn3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neudorfer O, Giladi N, Elstein D, Abrahamov A, Turezkite T, Aghai E, Reches A, Bembi B, Zimran A. Occurrence of Parkinson’s syndrome in type I Gaucher disease. QJM. 1996;89(9):691–694. doi: 10.1093/qjmed/89.9.691. [DOI] [PubMed] [Google Scholar]
- Patnaik S, Zheng W, Choi JH, Motabar O, Southall N, Westbroek W, Lea WA, Velayati A, Goldin E, Sidransky E, Leister W, Marugan JJ. Discovery, structure-activity relationship, and biological evaluation of noninhibitory small molecule chaperones of glucocerebrosidase. Journal of medicinal chemistry. 2012;55(12):5734–5748. doi: 10.1021/jm300063b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelled D, Trajkovic-Bodennec S, Lloyd-Evans E, Sidransky E, Schiffmann R, Futerman AH. Enhanced calcium release in the acute neuronopathic form of Gaucher disease. Neurobiol Dis. 2005;18(1):83–88. doi: 10.1016/j.nbd.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn CM, Jiang Z, Lee JC. Biophysics of alpha-synuclein membrane interactions. Biochim Biophys Acta. 2012;1818(2):162–171. doi: 10.1016/j.bbamem.2011.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Roberts HL, Brown DR. Seeking a mechanism for the toxicity of oligomeric alpha-synuclein. Biomolecules. 2015;5(2):282–305. doi: 10.3390/biom5020282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha EM, Smith GA, Park E, Cao H, Brown E, Hallett P, Isacson O. Progressive decline of glucocerebrosidase in aging and Parkinson’s disease. Ann Clin Transl Neurol. 2015;2(4):433–438. doi: 10.1002/acn3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenstein E, Clarke J, Viel C, Panarello N, Treleaven CM, Kim C, Spencer B, Adame A, Park H, Dodge JC, Cheng SH, Shihabuddin LS, Masliah E, Sardi SP. Glucocerebrosidase modulates cognitive and motor activities in murine models of Parkinson’s disease. Hum Mol Genet. 2016 doi: 10.1093/hmg/ddw124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom B, Balwani M, Bronstein JM, Kolodny E, Sathe S, Gwosdow AR, Taylor JS, Cole JA, Zimran A, Weinreb NJ. The incidence of Parkinsonism in patients with type 1 Gaucher disease: data from the ICGG Gaucher Registry. Blood Cells Mol Dis. 2011;46(1):95–102. doi: 10.1016/j.bcmd.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothaug M, Zunke F, Mazzulli JR, Schweizer M, Altmeppen H, Lullmann-Rauch R, Kallemeijn WW, Gaspar P, Aerts JM, Glatzel M, Saftig P, Krainc D, Schwake M, Blanz J. LIMP-2 expression is critical for beta-glucocerebrosidase activity and alpha-synuclein clearance. Proc Natl Acad Sci U S A. 2014;111(43):15573–15578. doi: 10.1073/pnas.1405700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardi SP, Cheng SH, Shihabuddin LS. Gaucher-related synucleinopathies: the examination of sporadic neurodegeneration from a rare (disease) angle. Prog Neurobiol. 2015;125:47–62. doi: 10.1016/j.pneurobio.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Sardi SP, Clarke J, Viel C, Chan M, Tamsett TJ, Treleaven CM, Bu J, Sweet L, Passini MA, Dodge JC, Yu WH, Sidman RL, Cheng SH, Shihabuddin LS. Augmenting CNS glucocerebrosidase activity as a therapeutic strategy for parkinsonism and other Gaucher-related synucleinopathies. Proc Natl Acad Sci U S A. 2013;110(9):3537–3542. doi: 10.1073/pnas.1220464110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, Di Malta C, Donaudy F, Embrione V, Polishchuk RS, Banfi S, Parenti G, Cattaneo E, Ballabio A. A gene network regulating lysosomal biogenesis and function. Science. 2009;325(5939):473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- Schapira AH. Glucocerebrosidase and Parkinson disease: Recent advances. Mol Cell Neurosci. 2015;66(Pt A):37–42. doi: 10.1016/j.mcn.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schondorf DC, Aureli M, McAllister FE, Hindley CJ, Mayer F, Schmid B, Sardi SP, Valsecchi M, Hoffmann S, Schwarz LK, Hedrich U, Berg D, Shihabuddin LS, Hu J, Pruszak J, Gygi SP, Sonnino S, Gasser T, Deleidi M. iPSC-derived neurons from GBA1-associated Parkinson’s disease patients show autophagic defects and impaired calcium homeostasis. Nat Commun. 2014;5:4028. doi: 10.1038/ncomms5028. [DOI] [PubMed] [Google Scholar]
- Settembre C, Ballabio A. TFEB regulates autophagy: an integrated coordination of cellular degradation and recycling processes. Autophagy. 2011;7(11):1379–1381. doi: 10.4161/auto.7.11.17166. [DOI] [PubMed] [Google Scholar]
- Settembre C, De Cegli R, Mansueto G, Saha PK, Vetrini F, Visvikis O, Huynh T, Carissimo A, Palmer D, Klisch TJ, Wollenberg AC, Di Bernardo D, Chan L, Irazoqui JE, Ballabio A. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nature cell biology. 2013;15(6):647–658. doi: 10.1038/ncb2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Zoncu R, Medina DL, Vetrini F, Erdin S, Erdin S, Huynh T, Ferron M, Karsenty G, Vellard MC, Facchinetti V, Sabatini DM, Ballabio A. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 2012;31(5):1095–1108. doi: 10.1038/emboj.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shachar T, Lo Bianco C, Recchia A, Wiessner C, Raas-Rothschild A, Futerman AH. Lysosomal storage disorders and Parkinson’s disease: Gaucher disease and beyond. Mov Disord. 2011;26(9):1593–1604. doi: 10.1002/mds.23774. [DOI] [PubMed] [Google Scholar]
- Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Bar-Shira A, Berg D, Bras J, Brice A, Chen CM, Clark LN, Condroyer C, De Marco EV, Durr A, Eblan MJ, Fahn S, Farrer MJ, Fung HC, Gan-Or Z, Gasser T, Gershoni-Baruch R, Giladi N, Griffith A, Gurevich T, Januario C, Kropp P, Lang AE, Lee-Chen GJ, Lesage S, Marder K, Mata IF, Mirelman A, Mitsui J, Mizuta I, Nicoletti G, Oliveira C, Ottman R, Orr-Urtreger A, Pereira LV, Quattrone A, Rogaeva E, Rolfs A, Rosenbaum H, Rozenberg R, Samii A, Samaddar T, Schulte C, Sharma M, Singleton A, Spitz M, Tan EK, Tayebi N, Toda T, Troiano AR, Tsuji S, Wittstock M, Wolfsberg TG, Wu YR, Zabetian CP, Zhao Y, Ziegler SG. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. The New England journal of medicine. 2009;361(17):1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert M, Sidransky E, Westbroek W. Glucocerebrosidase is shaking up the synucleinopathies. Brain. 2014;137(Pt 5):1304–1322. doi: 10.1093/brain/awu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton A, Hardy J. The Evolution of Genetics: Alzheimer’s and Parkinson’s Diseases. Neuron. 2016;90(6):1154–1163. doi: 10.1016/j.neuron.2016.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snead D, Eliezer D. Alpha-synuclein function and dysfunction on cellular membranes. Exp Neurobiol. 2014;23(4):292–313. doi: 10.5607/en.2014.23.4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Sun Y, Liou B, Xu YH, Quinn B, Zhang W, Hamler R, Setchell KD, Grabowski GA. Ex vivo and in vivo effects of isofagomine on acid beta-glucosidase variants and substrate levels in Gaucher disease. J Biol Chem. 2012;287(6):4275–4287. doi: 10.1074/jbc.M111.280016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayebi N, Callahan M, Madike V, Stubblefield BK, Orvisky E, Krasnewich D, Fillano JJ, Sidransky E. Gaucher disease and parkinsonism: a phenotypic and genotypic characterization. Mol Genet Metab. 2001;73(4):313–321. doi: 10.1006/mgme.2001.3201. [DOI] [PubMed] [Google Scholar]
- Van Rossum A, Holsopple M. Enzyme Replacement or Substrate Reduction? A Review of Gaucher Disease Treatment Options. Hosp Pharm. 2016;51(7):553–563. doi: 10.1310/hpj5107-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visanji NP, Wislet-Gendebien S, Oschipok LW, Zhang G, Aubert I, Fraser PE, Tandon A. Effect of Ser-129 phosphorylation on interaction of alpha-synuclein with synaptic and cellular membranes. J Biol Chem. 2011;286(41):35863–35873. doi: 10.1074/jbc.M111.253450. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Weiss K, Gonzalez AN, Lopez G, Pedoeim L, Groden C, Sidransky E. The clinical management of Type 2 Gaucher disease. Mol Genet Metab. 2015;114(2):110–122. doi: 10.1016/j.ymgme.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong YC, Krainc D. Lysosomal trafficking defects link Parkinson’s disease with Gaucher’s disease. Mov Disord. 2016;31(11):1610–1618. doi: 10.1002/mds.26802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard CM, Campos BA, Kuo SH, Nirenberg MJ, Nestor MW, Zimmer M, Mosharov EV, Sulzer D, Zhou H, Paull D, Clark L, Schadt EE, Sardi SP, Rubin L, Eggan K, Brock M, Lipnick S, Rao M, Chang S, Li A, Noggle SA. iPSC-derived dopamine neurons reveal differences between monozygotic twins discordant for Parkinson’s disease. Cell Rep. 2014;9(4):1173–1182. doi: 10.1016/j.celrep.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin W, Emadi S, Williams S, Liu Q, Schulz P, He P, Alam NB, Wu J, Sierks MR. Toxic Oligomeric Alpha-Synuclein Variants Present in Human Parkinson’s Disease Brains Are Differentially Generated in Mammalian Cell Models. Biomolecules. 2015;5(3):1634–1651. doi: 10.3390/biom5031634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap TL, Gruschus JM, Velayati A, Sidransky E, Lee JC. Saposin C protects glucocerebrosidase against alpha-synuclein inhibition. Biochemistry. 2013;52(41):7161–7163. doi: 10.1021/bi401191v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap TL, Gruschus JM, Velayati A, Westbroek W, Goldin E, Moaven N, Sidransky E, Lee JC. Alpha-synuclein interacts with Glucocerebrosidase providing a molecular link between Parkinson and Gaucher diseases. J Biol Chem. 2011;286(32):28080–28088. doi: 10.1074/jbc.M111.237859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap TL, Jiang Z, Heinrich F, Gruschus JM, Pfefferkorn CM, Barros M, Curtis JE, Sidransky E, Lee JC. Structural features of membrane-bound glucocerebrosidase and alpha-synuclein probed by neutron reflectometry and fluorescence spectroscopy. J Biol Chem. 2015;290(2):744–754. doi: 10.1074/jbc.M114.610584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimran A, Altarescu G, Elstein D. Pilot study using ambroxol as a pharmacological chaperone in type 1 Gaucher disease. Blood Cells Mol Dis. 2013;50(2):134–137. doi: 10.1016/j.bcmd.2012.09.006. [DOI] [PubMed] [Google Scholar]