Abstract
A Bayesian model and design are described for a phase I-II trial to jointly optimise the doses of a targeted agent and a chemotherapy agent for solid tumors. A challenge in designing the trial was that both the efficacy and toxicity outcomes were defined as four-level ordinal variables. To reflect possibly complex joint effects of the two doses on each of the two outcomes, for each marginal distribution a generalised continuation ratio model was assumed, with each agent’s dose parametrically standardised in the linear term. A copula was assumed to obtain a bivariate distribution. Elicited outcome probabilities were used to construct a prior, with variances calibrated to obtain small prior effective sample size. Elicited numerical utilities of the 16 elementary outcomes were used to compute posterior mean utilities as criteria for selecting dose pairs, with adaptive randomisation to reduce the risk of getting stuck at a suboptimal pair. A simulation study showed that parametric dose standardisation with additive dose effects provides a robust, reliable model for dose pair optimisation in this setting, and it compares favourably with designs based on alternative models that include dose-dose interaction terms. The proposed model and method are applicable generally to other clinical trial settings with similar dose and outcome structures.
Keywords: adaptive design, Bayesian design, combination trial, ordinal variables, phase I-II clinical trial, utility
1. Introduction
This paper was motivated by the problem of designing an early phase clinical trial of a three agent combination for treatment of cancer patients with advanced solid tumors. The first agent is a novel molecule (M) designed to inhibit the protein kinase complexes mTORC1 and mTORC2, and thus interfere with cancer cell proliferation and survival, among other cancer properties. M also has anti-angiogenic properties, through which it deprives the cancer of essential blood vessels that invest the tumors. The other two treatment components are the widely used chemotherapeutic agents carboplatin and paclitaxel. Paclitaxel, when given weekly, has been shown to act as an angiogenesis inhibitor as well. The property of antiangiogensis shared by M and weekly paclitaxel motivates this combination regimen, through which a more powerful antiangiogenic, and therefore anticancer effect is hypothesised. All three drugs also are expected to directly target the cancer cells through additional, different mechanisms, thereby complementing each other.
For the three-agent regimen in this trial, carboplatin is administered at a fixed dose based on the patient’s age, weight, and kidney function. The doses of the two agents that are varied are dM = 4, 5, or 6 mg of M given orally each day, and dP = 40, 60, or 80 mg/m2 of paclitaxel given intravenously twice weekly. A total of nine (M, paclitaxel) dose pairs d = (dM, dP) are studied, with the goal to find the optimal d. Our proposed method will define “optimal” d by assigning joint utilities to Toxicity and Efficacy, assuming a Bayesian model, and identifying the d having largest posterior mean utility. Toxicity is defined as a four-level ordinal variable, YT, with possible levels yT ∈ {Mild, Moderate, High, Severe}. As shown in Table 1, YT is defined in terms of the severity grades of many qualitatively different toxicities, with the level of YT determined by the highest level of any individual toxicity experienced by the patient. Reducing the many toxicities in Table 1 to the four-level ordinal outcome YT required many subjective decisions by the clinical oncologist planning the trial (the third author of this paper, RGZ). Efficacy is a four-level ordinal variable, YE, with possible values yE ∈ {PD, SD1, SD2, PR/CR}, where PD = [progressive disease] = [> 20% increase in tumor size], SD1 = [stable disease level 1] = [0 to 20% increase in tumor size], SD2 = [stable disease level 2] = [0 to 30% reduction in tumor size], and PR/CR = [partial or complete response] = [> 30% reduction in tumor size]. This is a refinement of the commonly used 3-category definition where SD1 and SD2 are combined as SD = stable disease, sometimes with the 30% replaced by 20% so that SD is a change ≤ 20% in tumor size in either direction. In the trial, both YE and YT are scored within 42 days from the start of treatment. Thus, a criterion for determining an optimal dose pair must be defined in terms of the joint effect of d on Y = (YE, YT), which has 16 possible values.
Table 1.
Definitions of overall toxicity severity levels by grades of individual toxicities. Overall toxicity is scored as the maximum individual severity level. Grades are defined using NCI criteria.
| Overall Toxicity Severity Level (YT) | ||||
|---|---|---|---|---|
|
| ||||
| Mild | Moderate | High | Severe | |
|
|
||||
| Fatigue | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Nausea | Grade 1 | Grade 2 | Grade 3 | – |
| Neuropathy | – | Grade 1 | Grade 2 | Grade ≥ 3 |
| Hyperglycemia | Grade 2 | Grade 3 | Grade 4 | – |
| Rash | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Diarrhea | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Stomatitis | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Pneumonitis | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Febrile neutropenia | – | – | Grade 3 | Grade 4 |
| Other Non-hematologic | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Hyperlipidemia | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Anemia | Grade 3 | Grade 4 | – | – |
| Thrombocytopenia | Grade 2 | Grade 3 | – | – |
| Neutropenia | Grade 3 | Grade 4 | – | – |
| AST/ALT | Grade 2 | Grade 3 | Grade 4 | – |
| Blindness | – | – | – | Grade 4 |
| Myocardioal Infarction | – | – | – | Grade 4 |
| Stroke | – | – | – | Grade 4 |
| Regimen-Related Death | – | – | – | Grade 5 |
An ordinal categorisation of solid tumor response is used commonly in oncology to compute descriptive statistics, but almost never is used for decision making by dose-finding designs. The most common practice is to define YT and YE as binary variables. In the present setting, this would be done by defining YE to indicate a “Response” event, which could be PR/CR, {PR/CR or SD2}, or {PR/CR, SD2, or SD1}. Most commonly, YT indicates a composite adverse event, “dose limiting toxicity (DLT).” These assumptions usually are made for phase I-II designs (cf. Braun 2002; Thall and Cook 2004; Bekele and Shen 2005; Zhang, Sargent, and Mandrekar 2006; Yin, Li, and Ji 2006). A further reduction is the conventional approach of ignoring efficacy and conducting a phase I trial based on the probability of DLT as a function of dose (cf. Storer, 1989; O’Quigley, Pepe, and Fisher, 1990; Babb, Rogatko, and Zacks, 1998). Curve-free dose-finding methods have been proposed by Gasparini and Eisele (2000) and Whitehead, et al. (2010) for phase I trials, and by Whitehead, et al. (2011) for phase I-II combination trials. Bekele and Shen (2005) and Zhou et al. (2006) proposed parametric model-based phase I-II methods to accommodate binary YT and continuous YE.
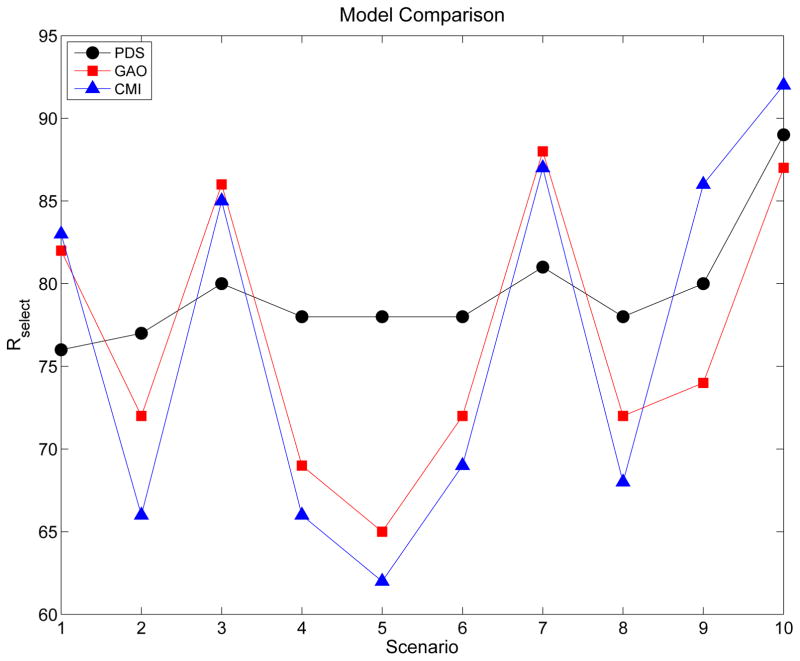
The utility-based two-agent phase I-II design of Houede et al. (2010) accounts for bivariate ordinal Y and models marginal dose-dose interactions by using a generalisation of the Aranda-Ordaz model (1981), given in the Appendix. Since this design deals with the same general problem as that addressed here, it is a natural comparator to our proposed methodology. The main differences between our methodology and that of Houede al. (2010) are that (i) we account for joint effects of two doses on each marginal outcome distribution using parametric dose standardisation, and (ii) we use adaptive randomisation to reduce the probability of getting stuck at a suboptimal dose pair. Additionally, our motivating application has an outcome of dimension (4,4) while that in the application of Houede et al. (2010) is (3,3) dimensional. Our simulations comparing the methods show that our proposal has more consistent performance across a range of dose-outcome scenarios, and in particular has better worst-case performance (Tables 5 and 6, Figure 5).
Table 5.
Comparison of design performance using three alternative generalised continuation ratio models for (4,4) dimensional bivariate ordinal outcomes. Scenarios 11 and 12 have no acceptable dose, so Rselect values are less relevant and thus have a gray background.
| Scenario
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Parametric Dose Standardisation (PDS) | ||||||||||||
| Rselect | 76 | 77 | 80 | 78 | 78 | 78 | 81 | 78 | 80 | 89 | 93 | 94 |
| Rtreat | 65 | 63 | 61 | 72 | 70 | 67 | 70 | 70 | 65 | 85 | 79 | 67 |
| % None | 2 | 2 | 1 | 0 | 1 | 7 | 1 | 1 | 0 | 0 | 95 | 96 |
| % Best | 32 | 28 | 32 | 46 | 39 | 34 | 39 | 33 | 48 | 44 | – | – |
| # Pats | 59.4 | 59.4 | 59.6 | 59.9 | 59.8 | 57.9 | 59.7 | 59.8 | 59.9 | 59.9 | 26.5 | 27.7 |
| # Eff | 36.9 | 27.1 | 29.3 | 29.4 | 30.2 | 24.1 | 29.8 | 30.0 | 31.9 | 31.9 | 13.2 | 5.0 |
| # Tox | 28.6 | 25.2 | 23.6 | 19.2 | 22.9 | 20.8 | 22.1 | 22.1 | 19.8 | 22.6 | 18.1 | 7.7 |
| Generalised Aranda-Ordaz Model (GAO) | ||||||||||||
| Rselect | 82 | 72 | 86 | 69 | 65 | 72 | 88 | 72 | 74 | 87 | 93 | 95 |
| Rtreat | 71 | 65 | 63 | 68 | 65 | 63 | 75 | 68 | 57 | 82 | 79 | 66 |
| % None | 2 | 3 | 1 | 0 | 1 | 6 | 1 | 1 | 1 | 0 | 94 | 94 |
| % Best | 49 | 25 | 64 | 31 | 8 | 19 | 67 | 22 | 47 | 54 | – | – |
| # Pats | 59.5 | 59.3 | 59.7 | 60.0 | 59.8 | 58.4 | 59.7 | 59.7 | 59.8 | 59.9 | 27.1 | 32.2 |
| # Eff | 36.2 | 25.8 | 28.1 | 27.9 | 28.3 | 23.3 | 29.2 | 28.7 | 30.9 | 30.5 | 13.5 | 5.7 |
| # Tox | 25.7 | 22.9 | 21.2 | 17.6 | 22.1 | 20.6 | 19.7 | 20.8 | 19.0 | 20.9 | 18.5 | 8.9 |
| Conventional Multiplicative Interaction (CMI) | ||||||||||||
| Rselect | 83 | 66 | 85 | 66 | 62 | 69 | 87 | 68 | 86 | 92 | 95 | 97 |
| Rtreat | 69 | 60 | 61 | 67 | 62 | 64 | 73 | 68 | 65 | 85 | 80 | 68 |
| % None | 1 | 3 | 1 | 0 | 1 | 6 | 1 | 1 | 1 | 0 | 91 | 95 |
| % Best | 56 | 14 | 62 | 20 | 2 | 9 | 65 | 11 | 68 | 50 | – | – |
| # Pats | 59.7 | 59.3 | 59.7 | 59.9 | 59.7 | 58.3 | 59.7 | 59.7 | 59.8 | 60.0 | 29.3 | 29.0 |
| # Eff | 36.8 | 26.0 | 28.8 | 29.4 | 28.9 | 24.0 | 29.9 | 29.9 | 31.9 | 32.2 | 14.6 | 5.3 |
| # Tox | 26.9 | 24.8 | 23.1 | 19.6 | 24.2 | 21.6 | 22.0 | 23.0 | 19.7 | 22.9 | 19.9 | 8.1 |
Table 6.
Comparison of summary statistics for two-agent dose-finding designs, with (4,4), (3,3), or (2,2) dimensional bivariate outcomes.
| Scenario
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| 4 Eff, 4 Tox Levels PDS |
Rselect | 76 | 77 | 80 | 78 | 78 | 78 | 81 | 78 | 80 | 89 | 93 | 94 |
| Rtreat | 65 | 63 | 61 | 72 | 70 | 67 | 70 | 70 | 65 | 85 | 79 | 67 | |
| % None | 2 | 2 | 1 | 0 | 1 | 7 | 1 | 1 | 0 | 0 | 95 | 96 | |
| % Best | 32 | 28 | 32 | 46 | 39 | 34 | 39 | 33 | 48 | 44 | – | – | |
|
| |||||||||||||
| 3 Eff, 3 Tox Levels PDS |
Rselect | 77 | 75 | 83 | 75 | 76 | 81 | 84 | 77 | 66 | 88 | 91 | 92 |
| Rtreat | 66 | 63 | 63 | 70 | 69 | 67 | 71 | 69 | 59 | 85 | 79 | 67 | |
| % None | 2 | 2 | 1 | 0 | 1 | 6 | 1 | 1 | 1 | 0 | 94 | 95 | |
| % Best | 35 | 28 | 38 | 36 | 33 | 40 | 47 | 35 | 25 | 46 | – | – | |
|
| |||||||||||||
| 3 Eff, 3 Tox Levels GAO |
Rselect | 84 | 71 | 87 | 67 | 63 | 72 | 89 | 73 | 60 | 87 | 93 | 97 |
| Rtreat | 72 | 64 | 64 | 67 | 64 | 63 | 76 | 68 | 51 | 81 | 77 | 67 | |
| % None | 2 | 3 | 1 | 0 | 1 | 4 | 1 | 1 | 0 | 1 | 94 | 93 | |
| % Best | 53 | 25 | 67 | 24 | 5 | 19 | 70 | 26 | 25 | 61 | – | – | |
|
| |||||||||||||
| 2 Eff, 2 Tox Levels PDS |
Rselect | 77 | 79 | 72 | 67 | 80 | 77 | 75 | 78 | 61 | 84 | 89 | 89 |
| Rtreat | 67 | 67 | 60 | 69 | 72 | 67 | 67 | 70 | 58 | 83 | 79 | 66 | |
| % None | 3 | 2 | 1 | 0 | 1 | 7 | 1 | 1 | 1 | 0 | 95 | 96 | |
| % Best | 34 | 30 | 19 | 13 | 43 | 30 | 23 | 32 | 15 | 46 | – | – | |
|
| |||||||||||||
| 2 Eff, 2 Tox Levels Yuan-Yin (2011) Design |
Rselect | 81 | 77 | 67 | 65 | 75 | 72 | 71 | 73 | 57 | 86 | 91 | 51 |
| Rtreat | 74 | 66 | 57 | 59 | 63 | 67 | 53 | 58 | 62 | 81 | 96 | 64 | |
| % None | 20 | 10 | 8 | 2 | 12 | 14 | 7 | 11 | 9 | 2 | 100 | 8 | |
| % Best | 31 | 29 | 20 | 8 | 26 | 22 | 23 | 21 | 18 | 48 | – | – | |
|
| |||||||||||||
| 2 Eff, 2 Tox Levels Wages-Conaway (2014) Design |
Rselect | 74 | 77 | 70 | 69 | 76 | 65 | 77 | 74 | 51 | 86 | 100 | 77 |
| Rtreat | 73 | 69 | 60 | 66 | 68 | 58 | 64 | 62 | 47 | 79 | 97 | 61 | |
| % None | 0 | 2 | 1 | 0 | 2 | 5 | 0 | 2 | 0 | 0 | 71 | 63 | |
| % Best | 12 | 34 | 21 | 11 | 29 | 13 | 33 | 23 | 9 | 62 | – | – | |
|
| |||||||||||||
| No Eff, 2 Tox Levels PO-CRM, Target 0.35 |
Rselect | 79 | 73 | 55 | 64 | 71 | 64 | 60 | 61 | 61 | 90 | 100 | 81 |
| Rtreat | 78 | 68 | 49 | 62 | 63 | 57 | 56 | 55 | 55 | 84 | 99 | 71 | |
| % None | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| % Best | 27 | 28 | 21 | 3 | 20 | 17 | 13 | 15 | 19 | 65 | – | – | |
Figure 5.
Rselect values for designs based on three different bivariate ordinal outcome models, PDS, GAO, and CMI, that account for dose-dose interactions differently. All three designs determine an optimal dose pair based on (4,4) dimensional bivariate ordinal outcomes under generalised continuation ratio models for the marginals.
Medically, the trial considered here is similar to the trial motivating the phase I-II design of Riviere, Yuan, Dubois, and Zohar (2015), in that both trials aim to find an optimal dose pair of a targeted agent and a chemotherapy agent. Key differences are that Riviere et al. address settings where toxicity is a binary variable and efficacy is a time-to-event variable and, assuming a proportional hazards model, the dose-efficacy curve may increase initially but then reach a plateau. The problem of optimising the doses of a two-agent combination based on bivariate binary (YE, YT) outcomes has been addressed in the phase I-II designs proposed by Yuan and Yin (2011) and Wages and Conaway (2014). Our computer simulations, reported in Section 4 and Table 6, show that defining efficacy and toxicity as ordinal variables with three or more levels is more informative than collapsing categories and defining two binary indicators, for example by dichotomising YE in one of the ways noted above and defining binary YT = I(High or Severe Toxicity).
Formulating a probability model and decision rules that use a (4,4) dimensional bivariate ordinal outcome to choose dose pairs in a sequentially adaptive phase I-II trial is challenging. In this trial, a maximum of 60 patients will be accrued, treated in 20 cohorts of size 3, starting at d = (4, 60). Denote an elementary outcome by y = (yE, yT), with the efficacy outcomes ordered from worst to best by yE = 0, 1, ···, LE, and the toxicity outcomes ordered from least to most severe by yT = 0, 1, ···, LT. Even if the trial’s 60 patients were distributed evenly among the 16 possible y pairs at completion, there would only be about four patients per outcome. This sample size allocation is an unrealistic ideal, however, because the elementary outcomes are not equally likely for any d, and moreover dose pairs are assigned in a sequentially adaptive manner. Unavoidably, in practice, the final distribution of patients among the 144 possible (d, y) combinations will be very unbalanced. Consequently, a dose-outcome model π(y, d, θ) = Pr(Y = y | d, θ), parameterised by θ, must borrow strength across many possible (d, y) values. We will take the common practical approach of modeling the marginal probabilities πk(yk, d, θk) = P(Yk = yk | d, θk) for k = E and T, and using a bivariate copula (Nelsen, 2006) to induce association between YE and YT and obtain π(y, d, θ).
Our goal in modeling the marginals is to obtain a dose-finding design with desirable properties. Each marginal model must account for four outcome level main effects, two dose effects on each outcome level, and possibly complex dose-dose interactions. The most difficult dose-outcome scenarios are those where the optimal pair d is located in a middle portion of the two-dimensional domain, rather than at one of its four corners. To address these issues in a practical way, we assume a generalisation of the continuation ratio (CR) model (Fienberg, 1980; Cox, 1988) for each marginal. Our main departure from conventional approaches to constructing a dose-finding model is that we standardise each agent’s dose parametrically in the linear term of each marginal. This gives a robust model that accounts for a wide variety of possible effects of d on πE(yE, d, θE) and πT(yT, d, θT).
Once the 16 possible elementary outcomes y = (yE, yT) were established, their numerical utilities U(y) were elicited from RGZ to quantify their relative desirability. These elicited utilities subsequently were reviewed by members of the Department of Investigational Cancer Therapeutics at M.D. Anderson Cancer Center, and a consensus was obtained without changing any of the numerical values. In practice, utility elicitation may be carried out more formally using the so-called “Delphi method” (Dalkey, 1969; Brook et al., 1986) or, for example, the methods described by Hunink, et al. (2014) or Swinburn et al. (2010). Our elicited utilities are given in Table 2. A general admissibility criterion for any utility function U(yE, yT) in this setting is that it should increase as either yE or yT becomes more desirable on its ordinal scale. That is, one should not use a utility function that does not make sense. These utilities are used during the trial as a basis for computing the posterior mean utility of each dose pair, which is the design’s optimality criterion. Adaptive randomisation (AR) among nearly optimal dose pairs is used to avoid getting stuck at a suboptimal pair (cf. Azriel, Mandel, and Rinott, 2011; Thall and Nguyen, 2012). Our simulations, given below in Section 4, show that parametrically standardising the two doses and including them additively in the model’s linear terms provides a robust basis for dose-finding for a wide variety of πk(y|d) probability surfaces. In particular, our design’s performance compares favourably to what is obtained assuming a more conventional model with multiplicative dose-dose interaction terms.
Table 2.
Elicited numerical utilities of the 16 joint (Efficacy, Toxicity) outcomes.
| Toxicity |
Disease Status (Efficacy)
|
|||
|---|---|---|---|---|
| PD | SD1 | SD2 | PR/CR | |
| Mild | 25 | 55 | 80 | 100 |
| Moderate | 20 | 35 | 70 | 90 |
| High | 10 | 25 | 50 | 70 |
| Severe | 0 | 10 | 25 | 40 |
The dose-outcome model is given in Section 2. Decision criteria and algorithms for trial conduct are presented in Section 3. The methodology is applied to the motivating trial in Section 4, including a simulation study. We close with a discussion in Section 5.
2. Dose-Response Models
2.1 Parametric Dose Standardisation
In phase I-II trials, a key issue is modeling the effects of intermediate doses on both πE(y, d, θE) and πT(y, d, θT). First, consider a single agent trial with lowest dose d1, highest dose dM, and mean dose d̄. For a given intermediate dose dj between d1 and dM, and each k = E, T, the actual value of πk(dj, θ) may be, approximately, close to πk(d1, θ), midway between πk(d1, θ) and πk(dM, θ), or close to πk(dM, θ). If πE and πT both are defined using the same standardised dose, say x = d − d̄ or d/d̄, a problem arises from the facts that the shapes of the two curves πE(x, θ) and πT(x, θ) may be very different, and the desirabilities of an intermediate dj in terms of πE(xj, θ) and πT(xj, θ) also may be very different. For example, dj may have desirably low πT(dj, θ) close to πT(d1, θ), and low, intermediate, or high πE(dj, θ). An important case is one where πT(dj, θ) is close to πT(d1, θ) and πE(dj, θ) is close to πE(dM, θ), so dj is optimal for any reasonable criterion. If the model does not accurately reflect the different shapes of πT(d, θ) and πE(d, θ) as functions of d, the utility-based method may not select dj with sufficiently high probability.
Next, consider a phase I-II combination trial. For each agent, a = 1, 2, denote the dose vector by da = (da,1, ···, da,Ma) with mean d̄a = (da,1 + ··· + da,Ma)/Ma. The modeling problem here is to characterise the joint effects of (d1,j, d2,r) on both YE and YT. An intermediate dose pair is any d = (d1,j, d2,r) not located at one of the four corners of the rectangular dose pair domain, i.e. 1 < j < M1 and 1 < r < M2. Standardising each dose as xa,j = da,j − d̄a or da,j/d̄a, suffers from the same limitations described above for an individual agent. Consequently, the problems described above for a single agent are more complex in that they now are elaborated in terms of the two probability surfaces πE(d1,j, d2,r) and πT(d1,j, d2,r).
These problems motivate the use of two parametrically standardised versions of each dose, one with parameters corresponding to πE and the other with parameters corresponding to πT. For each outcome k = E, T and agent a, we define parametric dose standardisation (PDS) for da,j to be
| (1) |
where all entries of the dose standardisation parameter vector λ = (λE,1, λE,2, λT,1, λT,2) are positive-valued. This construction gives two parametrically standardised versions of each dose of each agent, one for each outcome, mapping each d1,j for agent 1 to ( ), j = 1, ···, M1 and each d2,r for agent 2 to ( ), r = 1, ···, M2. The formula (1) is a two-agent version of that used by Thall et al. (2013) in the context of a design for optimising the dose and schedule of one agent.
For each agent a, the lowest and highest standardised doses in (1) are and . Thus, the parametrically standardised doses at the lower and upper limits of the dose domain are usual standardised doses, and do not depend on either λ or the outcome k. These serve as anchors for the intermediate doses, 1 < j < Ma, where the PDS involves λ and k, and is a parametric, outcome-specific modification of the commonly used form da,j/d̄a, which corresponds to λk,a ≡ 1. Exponentiating the proportion (da,j − da,1)/(da,Ma − da,1) by the model parameter λk,a in (1) shifts each intermediate dose, da,j/d̄a, either up toward da,Ma/d̄a or down toward da,1/d̄a. Since λ is updated along with the other model parameters in the posterior, the formulation (1) provides a data-driven refinement of dose effects on each outcome that is not obtained if one uses the usual standardised values da,j/d̄a or da,j − d̄a.
2.2 Generalised Continuation Ratio Models
Reviews of generalised continuation ratio (GCR) models, and of copulas used to obtain bivariate distributions having given marginals, are given in the Appendix. Given the PDS form (1), one may stabilise numerical computations by using either or in the model’s linear component. For a given dose pair (d1,j, d2,r), when no meaning is lost we will suppress the dose indices j = 1, ···, M1 and r = 1, ···, M2, and use the generic notation d = (d1,j, d2,r) and . Denote the conditional probabilities
| (2) |
To construct the GCR model with PDS, we define the linear components
| (3) |
To enhance robustness, we use the parametric link function of Aranda-Ordaz (AO) (1981), which defines a probability p in terms of a real-valued linear term η and parameter ϕ > 0 as
| (4) |
The AO link gives a very flexible model for p as a function of η, with ϕ = 1 corresponding to the logit link and the complementary log-log link obtained as the limiting case when ϕ → 0. For the GCR model with PDS, we define the marginal of [Yk | d] by the equation
That is, we assume an AO link with PDS in the linear terms. We define , and to ensure that . We require βk,y,1, βk,y,2 > 0 for each y ≥ 1 to ensure that increases with each dose. Writing αk = {αk,y, y = 1, 2, 3} and βk = {βk,y,a, y = 1, 2, 3, a = 1, 2}, the marginal parameter vector is θk = (αk, βk, λk,1, λk,2, ϕk). The key components of the marginal model are that the linear components (3) include the doses of the two agents additively using PDS (1), it has a GCR form (2), and it uses an AO link (4). In the sequel, for brevity we will abuse the notation slightly by identifying this model and the corresponding dose finding method using the acronym ‘PDS.’
Since each intermediate standardised dose varies between the positive values da,1/d̄a and da,Ma/d̄a, we may consider 1 to be the middle numerical dose value. Mapping each to either or has the same effect as centering the covariates at their means to reduce collinearity in conventional regression. Similarly, we define so that it varies around 0 rather than 1 to improve numerical stability. If, instead, one were to transform to maximise numerical stability at either the minimum or maximum of the dose domain, this would have the effect of destabilising computations at the other end. Consequently, it is very desirable to transform to stabilise computations in the middle portion of the dose domain, and for values of near 1/2. For , this implies that
with is obtained if and ϕk = 1, corresponding to a logit link in (2). In this case, , and if then αk,y = 0. Thus, numerical stability is greatest in this dose pair neighborhood, equivalently for . Alternatively, one could use .
Figure 1 illustrates possible shapes of the probability surface γE(1, d, θE) = Pr(YE ≥ 1|d, θE) as a function of the pair d = (dM, dP) = (Dose of Targeted Agent, Dose of Paclitaxel), for each of four different numerical dose standardisation parameter pairs (λE,1, λE,2). The surface in the upper left for λE,1 = λE,2 = 1 corresponds to the additive model with linear term
Figure 1.
Illustration of the probability surface γE(1, d, θE) as a function of dose pair d, for four different values of the dose standardisation parameters (λE,1, λE,2). The upper left plot is the surface corresponding to λE,1 = λE,2 =1, as a basis for comparison.
which may be used as a basis for visual comparison. Other probability surfaces as functions of d may be drawn similarly, such as γE(y, d, θE), γT(y, d, θT), πE(y, d, θE), or πT(y, d, θT), for integer y ≥ 1. Figure 1 shows that parametrically standardising the doses in this way give a very flexible model for the probabilities that are the basis for the dose-finding design.
Index patients by i = 1, ···, n for interim sample size n ≤ N, and denote the dose pair given to the ith patient by d[i]. The likelihood is the product
and the posterior is
where p(θ | θ̃) denotes the prior with fixed hyperparameters θ̃. Collecting terms, for k = E, T, y = 1, 2, 3, and a = 1, 2, the model parameters are λ = {λk,a} for parametric dose standardisation, the intercepts α = {αk,y}, the dose effects β = {βk,y,a}, the AO link parameters ϕ = {ϕE, ϕT} and the copula’s association parameter ρ. Thus θ = (λ, α, β, ϕ, ρ).
2.3 Establishing Priors
Normal priors were assumed for the real-valued parameters {αk,y}, the positive-valued dose main effect coefficients {βk,y,a} were assumed to follow normal priors truncated below at 0, the copula association parameter was assumed to be uniform on [−1, +1], and each λk,a and the AO link parameter ϕ were assumed to follow lognormal priors. Prior means were estimated from the elicited probabilities given in Table 3 using the pseudo sampling method described in Thall, et al. (2011, Section 4.2) and Thall and Nguyen (2012, Section 4.3). Prior variances were calibrated to make the effective sample size (ESS), as defined by Morita, Thall, and Mueller (2008, 2010), of the prior of each marginal probability πk(y, d, θk) suitably small, and to give a design with good operating characteristics over a diverse set of scenarios. The ESS of each prior was approximated by equating the prior mean and variance of πk(y, d, θk) to the mean μ̃ = a/(a+b) and variance σ̃2 = μ̃(1 − μ̃)/(a+ b + 1) of a Beta(a, b). Thus, a + b was used to approximate the ESS of the prior of πk(y, d, θk). The overall mean of these ESS values was .09 for the selected prior standard deviation of 20. Detailed descriptions of the prior parameters are given in Supplementary Table S1.
Table 3.
Elicited prior mean marginal outcome probabilities, for each dose pair.
| (dM, dP) |
Efficacy
|
Toxicity
|
||||||
|---|---|---|---|---|---|---|---|---|
| PD | SD1 | SD2 | PR/CR | Mild | Mod | High | Severe | |
| (4, 40) | .70 | .10 | .10 | .10 | .70 | .20 | .05 | .05 |
| (5, 40) | .50 | .10 | .20 | .20 | .60 | .20 | .10 | .10 |
| (6, 40) | .30 | .20 | .20 | .30 | .50 | .20 | .15 | .15 |
| (4, 60) | .50 | .10 | .20 | .20 | .60 | .20 | .10 | .10 |
| (5, 60) | .30 | .20 | .20 | .30 | .50 | .20 | .15 | .15 |
| (6, 60) | .20 | .20 | .20 | .40 | .30 | .20 | .30 | .20 |
| (4, 80) | .30 | .20 | .20 | .30 | .50 | .20 | .15 | .15 |
| (5, 80) | .20 | .20 | .20 | .40 | .30 | .20 | .30 | .20 |
| (6, 80) | .10 | .20 | .20 | .50 | .20 | .20 | .30 | .30 |
3. Posterior Decision Criteria and Trial Design
3.1 Utility Based Decision Criteria
Given the Bayesian dose outcome model and elicited numerical utilities U(y) in Table 2, the trial is conducted using the following decision criteria. Given θ, the mean utility of dose pair d is
where the sum is over all y pairs in the support of Y. Since θ is not known, we compute each dose pair’s posterior mean utility,
| (5) |
given the data on n patients available when an interim decision must be made. This integral is approximated by generating a posterior sample θ(1), ···, θ(M) using Markov chain Monte Carlo (MCMC) (Robert and Cassella, 1999) and computing the sample mean of Ū(d, θ(1)), ···, Ū(d, θ(M)).
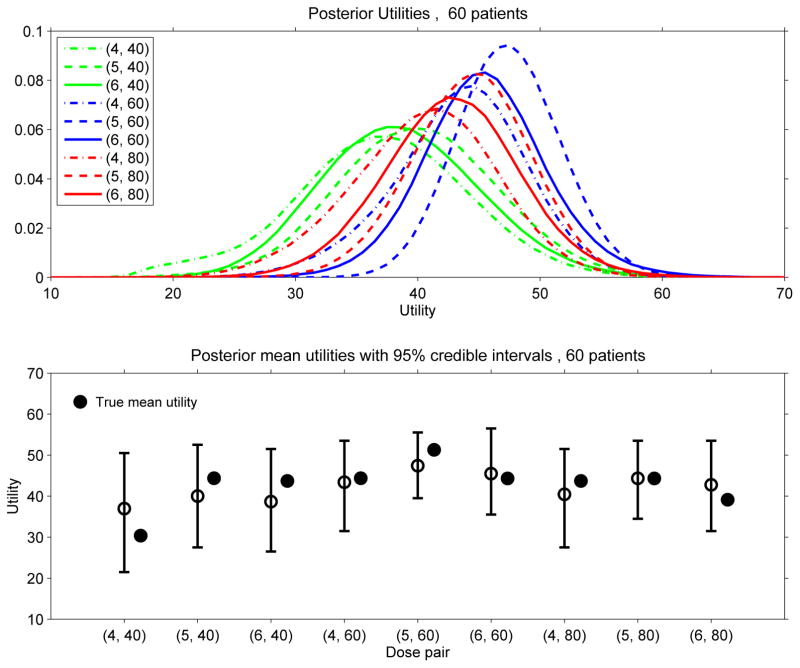
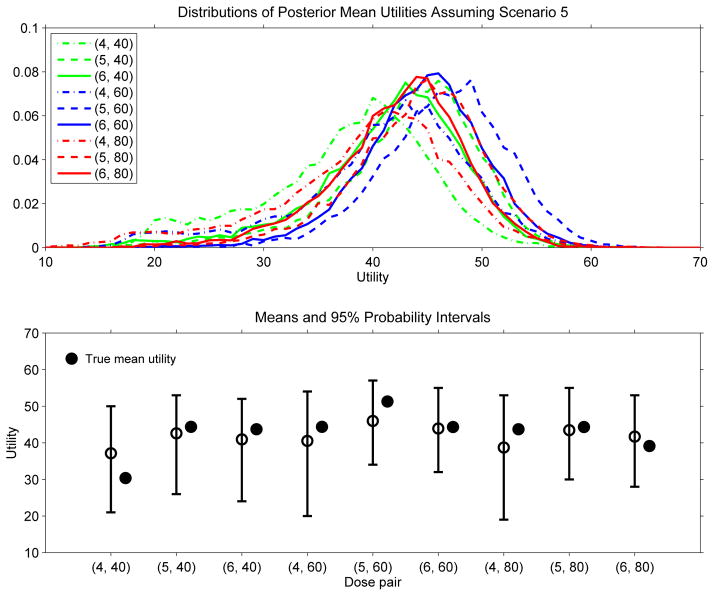
The posterior mean utilities given by (5) are the basis for the design’s sequential decision rules to select dose pairs during the trial. It is very important to bear in mind that each posterior mean utility is a statistic that can be quite variable. This is illustrated by Figure 2, which plots the distributions of u(d | data60) and corresponding 95% probability intervals for each of the nine dose pairs, based on one 60-patient data set from a trial simulated under Scenario 5. To illustrate how such final utility distributions may vary across trials, Figure 3 provides similar plots based on a sample of 10,000 trials, each of size n = 60, with the data generated under Scenario 5. From a Bayesian perspective, the randomness of each distribution in Figure 2 is due to posterior uncertainty about θ, whereas the randomness of each distribution in Figure 3 is due to the random variation in the data. It is also is important to bear in mind that, for the smaller sample sizes that are the basis for interim decisions during the trial, the variability of u(d | datan) for each d is greater than that shown by Figure 2 for the final data of n = 60 patients. In general, the substantial variability of each u(d | datan) also would be the case for any statistic used as a decision criterion in this or similar small scale trial settings using any other adaptive design. These considerations motivate, in part, our use of adaptive randomisation between nearly optimal dose pairs in the trial design. The general point is that, in early phase trials, decision making must be done under great uncertainty.
Figure 2.
Posterior distributions of the mean utilities u(θ | data60) for each of the nine dose pairs, based on a selected 60-patient data set obtained from one trial simulated under Scenario 5.
Figure 3.
Distributions of the final posterior mean utility u(θ | data60) and 95% probability intervals for each of the nine dose pairs for the proposed PDS model-based method, based on a sample of 10,000 trials, each of size n = 60, with the data for each trial generated under Scenario 5.
3.2 Dose Acceptability Criteria and Adaptive Randomisation
To ensure that the trial is ethically acceptable, rather than simply choosing d from the nine pairs to maximise u(d | datan), we impose additional constraints to ensure that any dose pair used to treat patients is both acceptably safe and acceptably efficacious. This follows the approach used by Thall and Cook (2004) and many others. We use the following two posterior acceptability criteria. For each k = E or T, denote π̄k(y, d, θk) = Pr(Yk ≥ y | d, θk). Indexing the toxicity levels by y = 0, 1, 2, 3 for mild, moderate, high, severe, π̄T (2, d, θ) is the probability of high or severe toxicity with d. A dose pair d is considered unacceptably toxic if
| (6) |
That is, d is not acceptable if, based on the current data, it is likely that d has a probability of high or severe toxicity that is above .45. For the efficacy rule, we similarly index the outcomes {PD, SD1, SD2, PR/CR} by 0, 1, 2, 3, so that π̄E(2, d, θ) is the probability of SD2 or better with dose pair d. A dose pair d is considered unacceptably inefficacious if
| (7) |
This says that d is not acceptable if, given the current data, it is likely that achieving SD2 or better occurs at a rate below 40%. A dose pair d is considered acceptable if it has both acceptable toxicity and acceptable efficacy, and we denote the set of acceptable dose pairs based on datan by 𝒜n. As data are acquired during the trial and the posterior becomes more reliable, 𝒜n may change, so that a given d not in 𝒜n may be in 𝒜n+k, or conversely. The events used to define (6) and (7) and the corresponding numerical probabilities .45 and .40 are specific to the solid tumor trial. These particular values were determined by RGZ in collaboration with oncologist colleagues involved in planning the trial. In other trials, different toxicity and efficacy events and probability cut-offs should be chosen as appropriate.
Given the acceptability criteria, it may seem that one simply may choose the d ∈ 𝒜n that maximises u(d | datan). This may lead to a design with undesirable properties, in some cases, due to the well known “optimisation-versus-exploration” dilemma in sequential decision making (cf. Gittins, 1979; Sutton and Barto, 1998). The problem is that, given some optimality criterion, a “greedy” sequential decision rule that always takes the empirically optimal action based on the current data carries a risk of getting stuck at a truly suboptimal action. The problem that greedy sequential algorithms are “sticky” in this sense only recently has been discussed in the context of dose-finding trials, by Azriel, Mandel, and Rinott (2011), Thall and Nguyen (2012), Oron and Hoff (2013), Braun, Kang, and Taylor (2013), and Thall, et al. (2014).
We address the problem of stickiness by applying adaptive randomisation (AR) among d having u(d | datan) close to the maximum, similarly to Thall and Nguyen (2012). Denote the acceptable dose pair maximising u(d | datan) by . While nominally this dose pair is “optimal,” it is only empirically optimal based on the most recent data, and it may not be the truly optimal pair that would maximise Ū(d, θ) if θ were known. In practice the truly optimal dose pair cannot be known, but in a simulation study all assumed πtrue(y | d) are specified, so the dopt under this assumed state of nature is known, and design performance can be evaluated accordingly. While this distinction may seem obvious, the difference between an empirically optimal action and the truly optimal action is at the heart of the optimisation-versus-exploration dilemma. A general form for AR probabilities for dose pair d* based on the posterior mean utilities of the acceptable dose pairs is
We studied several modified versions of AR, called AR(m), which is limited to randomising among only the best m dose pairs based on their current posterior mean utilities, for m = 1 (a greedy design with no AR), 2, 3, 4 and 9. The results are summarised in Supplementary Table S5. Additionally, we studied the required difference between the sub-sample sizes of the empirically best and other acceptable dose pairs, to ensure that an adequate number of patients have been treated at before applying any AR rule. Based on this preliminary study, for the actual trial design, we used AR(2), with AR applied only if at least three or more patients have been treated at the current than at any other acceptable d. Denote the empirically second best acceptable dose pair by , that is, is the second largest posterior mean utility. For our implementation of AR(2), the next cohort of patients are treated with dose pair with probability
and treated with dose pair with probability 1 − rn.
3.3 Trial Conduct
Using the above decision criteria, the trial is conducted as follows. Recall that the maximum sample size is N = 60, and the cohort size is c = 3.
The first cohort is treated at d = (dM, dP) = (4, 60).
For each cohort after the first, the posterior decision criteria (5), (6), and (7) are computed based on the most current data.
When escalating, an untried dose of either agent may not be skipped.
If no d is acceptable, the trial is terminated with no d selected.
If exactly one d is acceptable, the next cohort is treated at that dose pair.
-
For cohort size c, if two or more d’s are acceptable and the number of patients treated at minus the largest number of patients treated at any other acceptable dose is
≥ c, then apply AR(2) to choose randomly between and .
< c, then treat the next cohort at .
4. Simulations
4.1 General Design Performance Evaluation
The trial design was simulated under each of 12 dose-outcome scenarios, given in Supplementary Table S2, assuming an accrual rate of 1.5 patients per month. Each scenario is specified in terms of fixed true four-level marginal efficacy and toxicity probabilities, which are not based on the design’s model or any other model. Association was induced by assuming a Gaussian copula with true association parameter 0.10. Additional simulations were conducted using alternative models, or different cohort size or maximum sample size. For each case studied, the trial was replicated 3000 times, and all posterior quantities were computed using MCMC with Gibbs sampling.
We use the following summary statistics, given by Thall and Nguyen (2012), to quantify overall design performance. For given d and assumed true outcome probabilities {πtrue(y|d)}, we define the true mean utility of d to be
Thus, Ūtrue(d) is analogous to, but different from, the mean utility Ū(d, θ) based on the unknown parameter θ, and the posterior mean utility u(d | datan), which is a statistic. Let and denote the largest and smallest possible true mean utilities among all dose pairs. To quantify the method’s reliability for selecting a dose pair with high true utility, which benefits future patients, denoting the final selected dose pair by dselect, we use the statistic
To quantify benefit to the patients enrolled in the trial, we use the statistic
where d[i] is the dose pair given to the ith patient, and N is the final sample size. For both statistics, a larger value in the domain [0, 100] corresponds to better performance. We also report the selection percentage of the best acceptable d, denoted by % Best.
Simulation results for six selected scenarios are summarised in Table 4. The results for all 12 scenarios are given in Supplementary Table S3 and Supplementary Figure S1. In terms of true utilities and selection percentages of the nine dose pairs, Table 4 shows that the design does a reliable job of selecting acceptable dose pairs having true mean utility at or near the maximum, while also reliably avoiding unacceptable dose pairs. Figure 4 illustrates how the utility function U(y) maps the eight assumed true outcome probability pairs ( ) for yE = 0, 1, 2, 3 and yT = 0, 1, 2, 3 to Ūtrue(d) for each d, in Scenario 5. For each outcome, the assumed probabilities for y = 0, 1, 2, 3 are represented by successively darker shades of red for k = T and green for k = E. Figure 4 shows, for the PDS model based design, how the dose pair selection probabilities follow the magnitudes of the true mean utilities. A key point is that, if one wishes to compare dose pairs, inevitably a one-dimensional criterion is needed. The utility function provides this in a way that makes sense medically, provided that one accepts the particular numerical utilities given in Table 2.
Table 4.
Simulation results for the PDS-GCR-PO model based design. For each dose pair d = (dM, dP), Sel = selection percentage and Npat = number of patients treated. Utilities of unacceptable doses have a gray background. The highest utility among acceptable doses is given in boldface.
| Scenario 1 |
dP
|
Scenario 2 |
dP
|
|||||
|---|---|---|---|---|---|---|---|---|
| dM | 40 | 60 | 80 | dM | 40 | 60 | 80 | |
|
|
|
|||||||
|
Ūtrue(d) Sel, Npat |
4 | 56.0 | 51.8 | 48.3 | 4 | 43.2 | 44.4 | 37.9 |
| 32, 9.4 | 25, 14.8 | 9, 8.4 | 8, 3.5 | 17, 12.2 | 3, 5.3 | |||
| 5 | 51.8 | 47.2 | 44.7 | 5 | 49.7 | 46.8 | 38.9 | |
| 15, 7.4 | 7, 6.8 | 2, 3.2 | 28, 8.7 | 24, 10.5 | 2, 5.2 | |||
| 6 | 48.3 | 44.7 | 39.4 | 6 | 45.5 | 39.7 | 33.6 | |
| 8, 6.0 | 2, 2.6 | 0, 0.9 | 10, 6.7 | 5, 5.6 | 0, 1.7 | |||
| Percent none selected = 2 | Percent none selected = 2 | |||||||
|
| ||||||||
| Scenario 3 |
dP
|
Scenario 5 |
dP
|
|||||
| dM | 40 | 60 | 80 | dM | 40 | 60 | 80 | |
|
|
|
|||||||
|
Ūtrue(d) Sel, Npat |
4 | 39.4 | 40.1 | 36.7 | 4 | 30.4 | 44.4 | 43.7 |
| 1, 1.1 | 3, 8.6 | 1, 3.2 | 1, 1.2 | 15, 11.7 | 9, 6.3 | |||
| 5 | 48.9 | 47.6 | 42.7 | 5 | 44.4 | 51.3 | 44.3 | |
| 12, 6.1 | 17, 9.1 | 3, 5.7 | 10, 4.4 | 39, 12.7 | 9, 8.5 | |||
| 6 | 52.6 | 49.8 | 44.6 | 6 | 43.7 | 44.3 | 39.1 | |
| 32, 10.0 | 27, 11.2 | 3, 4.7 | 6, 4.0 | 10, 7.8 | 1, 3.4 | |||
| Percent none selected = 1 | Percent none selected = 1 | |||||||
|
| ||||||||
| Scenario 8 |
dP
|
Scenario 9 |
dP
|
|||||
| dM | 40 | 60 | 80 | dM | 40 | 60 | 80 | |
|
|
|
|||||||
|
Ūtrue(d) Sel, Npat |
4 | 33.8 | 45.4 | 48.2 | 4 | 43.8 | 50.8 | 52.6 |
| 1, 0.8 | 9, 10.0 | 15, 7.6 | 1, 0.5 | 2, 8.3 | 4, 5.3 | |||
| 5 | 37.2 | 48.953.2 | 5 | 50.8 | 52.6 | 58.4 | ||
| 2, 2.6 | 21, 9.7 | 33, 12.6 | 1, 1.6 | 4, 5.9 | 17, 11.5 | |||
| 6 | 41.3 | 45.9 | 45.6 | 6 | 52.6 | 58.4 | 64.0 | |
| 3, 2.5 | 9, 6.2 | 6, 7.9 | 4, 3.4 | 18, 8.6 | 48, 14.7 | |||
| Percent none selected = 1 | Percent none selected = 0 | |||||||
Figure 4.
Illustration of true marginal outcome probabilities { , y = 0, 1, 2, 3}, the resulting true mean utility Ūtrue(d), and simulation results %Sel = percent selection and %Pat = percent of patients treated in the trial for each dose pair, using the proposed PDS model based method, under Scenario 5. for y = 0, 1, 2, 3 are represented by successively darker shades of red for k = T and green for k = E.
4.2 Comparison to Models with Qualitatively Different Dose-Dose Effects
The generalised Aranda-Ordaz (GAO) model used by the two-agent phase I–II design of Houede et al. (2010) to account for dose-dose interactions is given in the Appendix. As noted earlier, because this design addresses the same problem of choosing optimal d based on ordinal (YE, YT), it is a natural comparator to the PDS model based design proposed here. Another comparator may be obtained from the more conventional model formulation in which all λk,a = 1 in the PDS linear components and a multiplicative dose-dose interaction term is inclued in the linear term, using the usual standardised doses xa,j = log(da,j/d̄a). The linear components then would take the commonly assumed form
The βk,12’s are real-valued and assumed to have normal priors. The element βk,12x1,jx2,r of this linear term is widely considered to be an “interaction” between two covariates in their joint effect on the outcome in a regression model. Here, the interaction is the joint effect of d1 and d2 on the marginal probability distribution of Yk. We will refer to this as the conventional multiplicative interaction (CMI) model.
Table 5 summarises how well the design performs assuming each of these three alternative models, for (4,4) dimensional bivariate ordinal outcomes. All three designs reliably stop the trial early if no d pairs are acceptable, in Scenarios 11 and 12. For Scenarios 1 – 10, Figure 5 shows the comparative Rselect results graphically. In the five scenarios {2, 4, 5, 6, 8} where dopt is a middle dose pair, not located at one of the four corners of the 3 × 3 matrix of d pairs, the PDS model gives much larger Rselect values than the other two designs. The differences Rselect(PDS) - Rselect(GAO) vary from 5 to 13 (7% to 20%), while Rselect(PDS) - Rselect(CMI) vary from 9 to 16 (13% to 26%). In the four scenarios {1, 3, 7, 9} where dopt is located at one of the four corners of the matrix of d pairs, the GAO and the CMI model give Rselect values that are larger than those of the PDS model by the smaller differences 5 to 7 (6% to 9%). The Rtreat and % Best d selected values also follow these general patterns. Scenario 10 corresponds to the prior, and has three acceptable dose pairs all having the same maximum true utility. An important property of the PDS method is that it gives much more stable behavior across Scenarios 1 – 10, with Rselect values in the range [76, 89], compared to ranges [65, 88] for the GAO method and [62, 92] for the CMI model. Similarly, the % Best d selected values have range [28, 48] for the PDS method versus ranges [8, 67] for the GAO model and [2, 68] for the CMI model. It thus appears that using parametrically standardised doses gives much more stable behavior across a range of scenarios, and provides insurance against very poor performance in some scenarios. The PDS model gives substantially larger Rselect values in the harder cases where dopt is a middle dose pair, with the price being smaller Rselect values in the easier cases where dopt is located at a corner of the rectangular dose pair domain.
4.3 Comparison to Designs that Reduce the Ordinal Outcomes
We next compare our proposed method, based on the (4,4) dimensional ordinal outcome Y = (YE, YT), to alternative designs that reduce this outcome by combining categories. The first two comparators are versions of the PDS and GAO designs based on (3,3) ordinal outcomes obtained by combining SD2 and CR/PR for YE and combining High and Severe for YT. We obtained a (2,2) outcome by also combining the YE events PD and SD1 so that YE became the binary indicator of [CR/PR or SD2], and combining the YT events Mild and Moderate so that YT became the binary indicator of [High or Severe]. For each of these (3,3) and (2,2) cases, in each scenario the outcome probabilities were obtained from those in Supplementary Table S2 by summing the corresponding elementary event probabilities. For the (2,2) case, in addition to the reduced version of the PDS design, we also included as comparators the phase I–II designs of Yuan and Yin (YY, 2011) and Wages and Conaway (WC, 2014), both of which rely on bivariate binary Y. A final comparator is the partial orders continual reassessment method (PO-CRM) of Wages, Conaway, and O’Quigley (2011), which uses only a binary version of YT to choose optimal d.
The YY design uses a copula to model the probability of toxicity as a function of d in phase I, and chooses a set of admissible d for subsequent efficacy evaluation in parallel treatment arms in phase II. The design applies AR based on the probability of a binary efficacy outcome in phase II, assuming a hierarchical binomial-beta-gamma model. At the end of phase II, the YY design selects the dose pair with acceptable toxicity that has highest posterior mean efficacy. Since the YY design allows one to vary the cohort size c and sub-sample sizes nI and nII in phases I and II, for comparison to the PDS model based design, we first simulated versions of the YY design with (c, nI, nII) = (3, 30, 30), (1, 30, 30), and (1, 20, 40), given in Supplementary Table S8. Since the YY design with (c, nI, nII) = (1, 30, 30) has slightly better overall performance than the other two, this version is used for comparison to the PDS design.
The WC design is based on partial orderings of d. Like the YY design, the WC design also chooses the dose pair d with acceptable toxicity that maximises the probability of efficacy. We simulated both the YY and WC designs using the same toxicity probability acceptability upper limit, .45, and efficacy probability lower limit, .40, as those used by the PDS design. Since the total number of possible partial orderings in the rectangle of d pairs is impractically large, a subset must be chosen. For comparison to the PDS model based design, we first simulated versions of the WC design with either six partial orderings, starting the trial at d = (1,2) as in our design, or 26 partial orderings, starting the trial at either d = (1,2) or (1,1), summarised in Supplementary Table S9. Since the version with 26 partial orderings, starting the trial at d = (1,2) had slightly better overall performance than the other two, it is included in Table 6.
An important point is that both the YY and WC designs choose d that has acceptably low toxicity and maximum efficacy, whereas the PDS design chooses d that has acceptably low toxicity, acceptably high efficacy, and maximum posterior mean utility. That is, the criteria are qualitatively different. The three designs have the same “best” d is scenarios 5, 6, 8, 9, and 10, and different “best” d in scenarios 1, 2, 3, 4, and 7. To compare the methods, we used the same utility-based criteria, namely Rselect, Rtreat, and true mean utility Ūtrue(d) to define % Best d selected.
The results are given in Table 6. Comparing the PDS model based design with (4,4) versus (3,3) dimensional outcomes shows that the Rselect values differ by at most ±3 for Scenarios 1 – 8 and 10, but in Scenario 9 using a (3,3) outcome greatly reduces Rselect, from 80 to 66. A similar pattern is seen for Rtreat and % Best d selected. Comparison of the PDS model, with either (4,4) or (3,3) outcomes, to the GAO model with (3,3) outcomes shows that the latter has much larger variability between scenarios in terms of Rselect, Rtreat, and % Best. Thus, as in Table 5, it appears that the PDS model provides a much more stable design, and in particular protects against very poor performance in some cases, as seen in Scenarios 4, 5, and 9 with the GAO model.
Simulation results for four designs in Table 6 are illustrated graphically for Rselect in Figure 6, which shows that, in general, dichotomising the ordinal outcomes substantively decreases Rselect values in some scenarios, regardless of the design used. Figure 6 also illustrates that the PDS model based design using the full (4,4) dimensional ordinal outcome is robust, in the sense that the Rselect values stay consistently high across all scenarios. In the special case of Scenario 10, which corresponds to the prior, three of the nine d pairs are optimal, hence selecting an optimal d pair is much easier for all designs. Supplementary Table S10 shows that the PO-CRM has greatly inferior performance compared to the PDS based design. This may be attributed to the general fact that using binary toxicity alone for dose-finding may ignore useful efficacy information.
Figure 6.
Rselect values of competing phase I-II designs to choose an optimal dose pair, given (4,4) dimensional ordinal (efficacy, toxicity) outcomes. ‘PDS 4 Levels’ denotes the proposed PDS model-based design. The other three designs all reduce both outcomes to binary variables, including the PDS design with dichotomised outcomes, YY = Yuan and Yin (2011) design, and WC = Wages and Conaway (2014) design.
4.4 Additional Sensitivity Analyses
Supplementary Table S6 shows that the PDS based design’s behavior is insensitive to cohort size c = 1, 2, or 3. Supplementary Table S4 summarises the PDS model-based design’s sensitivity to maximum sample sizes N = 30 to 300. The design’s operating characteristic improve greatly as N increases. For example, in Scenario 1, for N = 30, 60, 300, the corresponding Rselect values are 67, 76, 95 and Rtreat values are 61, 76, 78. The same pattern is seen for all other scenarios with acceptable dose pairs. In the two Scenarios 11 and 12, where there is no acceptable d, the simulated probability that no pair is selected is 1 for N ≥ 120. These results provide an empirical validation of the method’s consistency, in terms of both optimal dose pair selection and stopping the trial early for futility or safety in cases where this should be done. These numerical results also show that the maximum sample size 60 cannot reliably achieve Rselect values of 80 or larger across the scenarios studied, in this particular setting, and that N ≥ 90 is needed to achieve Rselect ≥ 80, and N roughly 200 to 240 is needed if Rselect ≥ 90 is desired.
Supplementary Table S12 summarises the behavior of the PDS based design when the trial is conducted using each of three different numerical utilities. One is the elicited utility in Table 2, and two are hypothetical, given in Supplementary Table S11, constructed to place greater value on either lower toxicity or greater efficacy. The simulations show that, across the 12 scenarios, the three resulting designs behave differently, but with no general pattern favoring one utility over the others. The utility favoring higher efficacy gives a design that escalates more aggressively and thus has greater observed toxicity and efficacy. Analogously, the utility favoring lower toxicity results in less toxicity but also less efficacy. A general conclusion is that the design behaves in a way that reflects the numerical values of U(y), which is the intention.
Table 7 gives a patient-by-patient illustration of how the design may behave as the trial plays out, and what the interim estimates look like during the trial, for patients 1 – 12, 15, 30, 45, and 60. Since the maximum posterior mean utility after the first cohort is u(4, 80 | data3) = 35.5, the pair d = (4,80) is used to treat cohort 2. Although u(6, 60 | data6) = 34.0 is largest for n = 6, the constraint that an untried dose may not be skipped when escalating results in d = (5,60) being used to treat cohort 3. The trial continues similarly, applying the AR method as described in step 6 of the design in Section 3.3. For each d, the posterior variability of u(d | datan) decreases with sample size n, but not monotonically. At the end of the trial, d = (6,60) is optimal with u(6, 60 | data60) = 57.0, but d = (6,40) also is a good choice since it is nearly optimal with u(6, 40 | data60) = 56.1.
Table 7.
Case-by-case example of a 60 patient trial. The largest current posterior mean utility is given in boldface.
| Patient | Dose Pair
|
Outcomes
|
Posterior mean utility u(d | datan) and its (std dev) for each (d1, d2)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d1 | d2 | YE | YT | (4,40) | (4,60) | (4,80) | (5,40) | (5,60) | (5,80) | (6,40) | (6,60) | (6,80) | |
| (Prior) | – | – | – | – | 34.8 (22.3) | 37.3 (25.5) | 38.0 (26.4) | 36.3 (24.2) | 39.0 (27.3) | 39.7 (28.1) | 36.8 (24.8) | 39.5 (27.9) | 40.2 (28.7) |
| 1 | 4 | 60 | 1 | 0 | 49.2 (11.8) | 54.2 (6.0) | 54.8 (10.9) | 51.0 (14.8) | 55.9 (13.2) | 56.5 (16.1) | 51.6 (15.7) | 56.4 (14.9) | 56.9 (17.6) |
| 2 | 4 | 60 | 1 | 0 | 49.8 (11.1) | 54.7 (3.5) | 55.2 (9.9) | 51.5 (14.2) | 56.3 (12.3) | 56.7 (15.5) | 52.0 (15.2) | 56.7 (14.2) | 57.1 (17.0) |
| 3 | 4 | 60 | 0 | 2 | 33.6 (9.9) | 35.5 (8.5) | 35.5 (12.3) | 33.8 (14.5) | 34.9 (14.4) | 34.9 (16.6) | 33.3 (15.5) | 34.0 (15.3) | 34.1 (17.3) |
| 4 | 4 | 80 | 1 | 1 | 35.2 (9.4) | 37.2 (7.2) | 37.4 (8.1) | 37.9 (12.5) | 39.6 (11.6) | 39.7 (12.5) | 38.7 (13.2) | 40.2 (12.5) | 40.3 (13.4) |
| 5 | 4 | 80 | 0 | 1 | 31.7 (8.3) | 33.7 (6.4) | 31.8 (6.8) | 35.3 (13.2) | 37.4 (12.6) | 35.5 (13.0) | 36.3 (14.1) | 38.3 (13.5) | 36.5 (14.0) |
| 6 | 4 | 80 | 0 | 1 | 30.4 (6.9) | 31.4 (5.7) | 29.6 (5.9) | 32.5 (11.4) | 33.6 (11.4) | 32.3 (11.7) | 32.9 (12.3) | 34.0 (12.3) | 33.0 (12.7) |
| 7 | 5 | 60 | 1 | 1 | 30.4 (7.4) | 31.8 (5.5) | 30.0 (5.5) | 34.2 (10.4) | 35.5 (7.6) | 34.8 (8.2) | 34.6 (11.0) | 36.0 (8.7) | 35.4 (9.3) |
| 8 | 5 | 60 | 1 | 0 | 30.4 (7.4) | 32.9 (5.3) | 31.7 (5.5) | 37.8 (10.6) | 40.0 (6.4) | 38.7 (7.4) | 37.6 (11.2) | 39.4 (8.0) | 38.3 (9.0) |
| 9 | 5 | 60 | 0 | 2 | 31.5 (7.2) | 31.6 (4.9) | 30.7 (5.3) | 32.3 (7.9) | 31.9 (5.5) | 31.1 (6.1) | 34.5 (10.6) | 33.8 (9.5) | 33.0 (10.1) |
| 10 | 6 | 40 | 2 | 2 | 30.9 (6.4) | 31.9 (5.2) | 30.4 (5.5) | 31.3 (7.5) | 32.3 (6.6) | 31.1 (7.1) | 48.4 (16.7) | 50.2 (17.8) | 49.6 (18.4) |
| 11 | 6 | 40 | 2 | 2 | 31.7 (6.6) | 32.2 (5.3) | 29.5 (5.6) | 30.9 (7.9) | 31.4 (6.8) | 29.0 (7.1) | 52.7 (12.6) | 54.7 (14.1) | 53.3 (15.4) |
| 12 | 6 | 40 | 3 | 2 | 31.3 (6.5) | 31.8 (5.6) | 29.6 (5.9) | 30.8 (7.7) | 31.4 (7.0) | 29.4 (7.4) | 57.0 (13.9) | 62.9 (16.8) | 62.7 (17.8) |
| 15 | 6 | 60 | 1 | 2 | 32.0 (6.9) | 32.6 (6.2) | 30.4 (6.4) | 32.1 (9.5) | 32.8 (9.1) | 31.2 (9.3) | 56.0 (10.6) | 56.9 (10.3) | 57.3 (12.0) |
| 30 | 6 | 80 | 2 | 1 | 35.8 (7.4) | 36.2 (6.3) | 36.1 (6.7) | 38.9 (10.4) | 39.1 (9.6) | 39.1 (10.0) | 53.7 (5.7) | 53.6 (5.9) | 53.9 (6.2) |
| 45 | 6 | 40 | 1 | 0 | 33.6 (6.2) | 33.6 (6.0) | 28.2 (6.1) | 34.6 (9.6) | 34.3 (9.4) | 28.5 (9.6) | 55.5 (4.7) | 55.4 (4.7) | 48.7 (6.1) |
| 60 | 6 | 60 | 3 | 1 | 36.7 (6.9) | 37.9 (6.4) | 31.5 (7.7) | 37.2 (10.3) | 38.2 (9.9) | 32.1 (10.5) | 56.1 (4.0) | 57.0 (3.8) | 50.4 (5.5) |
|
| |||||||||||||
| Total number of patients assigned | 0 | 3 | 3 | 0 | 3 | 0 | 21 | 24 | 6 | ||||
5. Discussion
Because the generalised CR model given by (2) links the conditional probability to the linear term , it has the computational advantage that there are no order constraints on the intercept parameters αk,1, ···, αk,Lk. An alternative model may be defined by
This generalises the proportional odds model (McCullagh, 1980) by replacing the logit link with the AO link. Because this model links the unconditional probability rather than the conditional probability to the linear term, it requires the order constraints αk,1 > ··· > αk,Lk for the probabilities to be well defined. Using this model for dose-finding, the need to impose these constraints on each parameter vector αk = (αk,1, ···, αk,Lk), k = E, T makes the MCMC computations to obtain posteriors much more difficult, especially for small amounts of data. This is one important motivation for our use of the generalised CR model.
Various special cases or alternative formulations of the PDS model can be obtained by changing one or more of its components. A natural question is whether adding a multiplicative dose-dose interaction term to the model with parametric dose standardisation would improve the design’s behavior. This model would have linear components
It may be considered a hybrid of the PDS and CMI model, in that it includes both parametric dose standardisation and a conventional multiplicative interaction term. Supplementary Table S7 shows that, compared to the PDS model, the hybrid model gives a design with Rselect values 1 to 6 smaller in eight scenarios, 1 to 3 larger in two scenarios, and slightly larger incorrect early stopping probabilities. Thus, on average, this more complex hybrid model produces a design with slightly worse performance than the PDS model.
A computer program named “U2OET” for implementing this methodology is available from the website https://biostatistics.mdanderson.org/SoftwareDownload.
Supplementary Material
Acknowledgments
This research was supported by NIH NCI grant RO1-CA-83932. We thank Nolan Wages and Mark Conaway for providing computer programs to simulate their designs. We also are grateful to two referees and an associate editor for their constructive comments and suggestions.
Appendix: Review of Generalised Continuation Ratio Models and Copulas
Recall that γk(y, d, θk) = P(Yk ≥ y | Yk ≥ y − 1, d, θk), for y = 0, ···, Lk. For given link function and linear term η(y, d, θk), a GCR model defines this conditional probability as
The marginal probabilities of a GCR model are given by
Since
the GCR model may be specified equivalently in the more commonly used form
In general, the joint pmf of Y =(YE, YT) given by a copula (Nelsen, 2006) can be defined in terms of the marginal cdfs
by applying the formula
where Cρ(ua, vb) denotes the copula and u1 = FE(yE|d, θ), v1 = FT(yT|d, θ), u2 = FE(yE − 1|d, θ) and v2 = FT(yT − 1|d, θ). To obtain a bivariate distribution under the the PDS model, we assume a Farlie-Gumbel-Morgenstern (FGM) copula
The GCR model given by Houede et al. (2010) accounts for the joint effects of the two doses on each ordinal outcome in a qualitatively different way. First, a conventional linear term for each agent a = 1, 2, level y of outcome Yk for k = E, T, and dose d(a) is defined as
A generalised Aranda-Ordaz (GAO) link is then defined as
where κ > 0 is a dose-dose interaction parameter. Houede et al. (2010) obtain bivariate distributions by assuming a Gaussian copula,
where Φρ denotes a bivariate normal cdf with correlation ρ and Φ denotes a N(0,1) cdf.
References
- 1.Aranda-Ordaz FJ. On two families of transformations to additivity for binary response data. Biometrika. 1981;68:357–363. [Google Scholar]
- 2.Azriel D, Mandel M, Rinott Y. The treatment versus experimentation dilemma in dose-finding studies. J Statist Planning and Inference. 2011;141:2759–2758. [Google Scholar]
- 3.Babb J, Rogatko A, Zacks S. Cancer phase I clinical trials: Efficient dose escalation with overdose control. Statist Med. 1998;17:1103–1120. doi: 10.1002/(sici)1097-0258(19980530)17:10<1103::aid-sim793>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Bekele BN, Shen Y. A Bayesian approach to jointly modeling toxicity and biomarker expression in a phase I/II dose-finding trial. Biometrics. 2005;61:344–354. doi: 10.1111/j.1541-0420.2005.00314.x. [DOI] [PubMed] [Google Scholar]
- 5.Braun TM. The bivariate continual reassessment method: extending the CRM to phase I trials of two competing outcomes. Controlled Clin Trials. 2002;23:240–256. doi: 10.1016/s0197-2456(01)00205-7. [DOI] [PubMed] [Google Scholar]
- 6.Braun TM, Kang S, Taylor JMG. A phase I/II trial design when response is unobserved in subjects with dose-limiting toxicity. Statist Meth Medical Res. 2013;22:1–15. doi: 10.1177/0962280212464541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox C. Multinomial regression models based on continuation ratios. Statist Med. 1988;7:435–441. doi: 10.1002/sim.4780070309. [DOI] [PubMed] [Google Scholar]
- 8.Brook RH, Chassin MR, Fink A, Solomon DH, Kosecoff J, Park RE. A method for the detailed assessment of the appropriateness of medical technologies. International J Technology Assessment and Health Care. 1986;2:53–63. doi: 10.1017/s0266462300002774. [DOI] [PubMed] [Google Scholar]
- 9.Dalkey NC. An experimental study of group opinion. Futures. 1969;1:408–426. [Google Scholar]
- 10.Fienberg SE. The Analysis of Cross-Classified Categorical Data. 2. Cambridge: M.I.T. Press; 1980. [Google Scholar]
- 11.Gasparini M, Eisele J. A curve-free method for phase I clinical trials. Biometrics. 2000;56:609–615. doi: 10.1111/j.0006-341x.2000.00609.x. [DOI] [PubMed] [Google Scholar]
- 12.Gittins JC. Bandit processes and dynamic allocation indices. J R Statist Soc B. 1979;41:148–177. [Google Scholar]
- 13.Houede N, Thall PF, Nguyen H, Paoletti X, Kramar A. Utility-based optimization of combination therapy using ordinal toxicity and efficacy in phase I/II trials. Biometrics. 2010;66:532–540. doi: 10.1111/j.1541-0420.2009.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunink MGM, Weinstein MC, Wittenberg E, Pliskin JS, Drummond MF, Glasziou PP, Wong JB. Decision Making in Health and Medicine: Integrating Evidence and Values. Cambridge: Cambridge University Press; 2014. [Google Scholar]
- 15.McCullagh P. Regression models for ordinal data (with discussion) J R Stat Soc B. 1980;42:109–142. [Google Scholar]
- 16.Morita S, Thall PF, Müller P. Determining the effective sample size of a parametric prior. Biometrics. 2008;64:595–602. doi: 10.1111/j.1541-0420.2007.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morita S, Thall PF, Müller P. Evaluating the impact of prior assumptions in Bayesian biostatistics. Stat Biosciences. 2010;2:1–17. doi: 10.1007/s12561-010-9018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelsen RB. An Introduction to Copulas. 2. New York: Springer-Verlag; 2006. [Google Scholar]
- 19.O’Quigley J, Pepe M, Fisher L. Continual reassessment method: A practical design for phase I clinical trials in cancer. Biometrics. 1990;46:33–48. [PubMed] [Google Scholar]
- 20.Oron AP, Hoff PD. Small-sample behavior of novel phase I designs. Clin Trials. 2013;10:63–80. doi: 10.1177/1740774512469311. [DOI] [PubMed] [Google Scholar]
- 21.Riviere MK, Yuan Y, Dubois F, Zohar S. A Bayesian dose finding design for clinical trials combining a cytotoxic agent with a molecularly targeted agent. J R Statist Soc C. 2015;64:215–229. [Google Scholar]
- 22.Robert CP, Cassella G. Monte Carlo Statistical Methods. New York: Springer; 1999. [Google Scholar]
- 23.Storer B. Design and analysis of phase I clinical trials. Biometrics. 1989;45:925–937. [PubMed] [Google Scholar]
- 24.Sutton RS, Barto AG. Reinforcement Learning: An Introduction. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- 25.Swinburn P, Lloyd A, Nathan P, Choueiri TK, Cella D, Neary MP. Elicitation of health state utilities in metastatic renal cell carcinoma. Current Med Res and Opinion. 2010;26:1091–1096. doi: 10.1185/03007991003712258. [DOI] [PubMed] [Google Scholar]
- 26.Thall PF, Cook JD. Dose-finding based on efficacy-toxicity trade-offs. Biometrics. 2004;60:684–693. doi: 10.1111/j.0006-341X.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- 27.Thall PF, Nguyen HQ. Adaptive randomization to improve utility-based dose-finding with bivariate ordinal outcomes. J Biopharmaceutical Statist. 2012;22:785–801. doi: 10.1080/10543406.2012.676586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thall PF, Nguyen HQ, Braun TM, Qazilbash MH. Using joint utilities of the times to response and toxicity to adaptively optimize schedule-dose regimes. Biometrics. 2013;69:673–682. doi: 10.1111/biom.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thall PF, Szabo A, Nguyen HQ, Amlie-Lefond CM, Zaidat OO. Optimizing the concentration and bolus of a drug delivered by continuous infusion. Biometrics. 2011;67:1638–1646. doi: 10.1111/j.1541-0420.2011.01580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thall PF, Nguyen HQ, Zohar S, Maton P. Optimizing sedative dose in preterm infants undergoing treatment for respiratory distress syndrome. J Amer Statist Ass. 2014;109:931–943. doi: 10.1080/01621459.2014.904789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wages NA, Conaway MR, O’Quigley J. Dose-finding design for multi-drug combinations. Clin Trials. 2011;8:380–389. doi: 10.1177/1740774511408748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wages NA, Conaway MR. Phase I/II adaptive design for drug combination oncology trials. Statist Med. 2014;33:1990–2003. doi: 10.1002/sim.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitehead J, Thygesen H, Whitehead A. A Bayesian dose-finding procedure for phase I clinical trials based only on the assumption of monotonicity. Statistics in Medicine. 2010;29:1808–1824. doi: 10.1002/sim.3963. [DOI] [PubMed] [Google Scholar]
- 34.Whitehead J, Thygesen H, Whitehead A. Bayesian procedures for phase I/II clinical trials investigating the safety and efficacy of drug combinations. Statistics in Medicine. 2011;30:1952–1970. doi: 10.1002/sim.4267. [DOI] [PubMed] [Google Scholar]
- 35.Yin G, Li Y, Ji Y. Bayesian dose-finding in phase I/II clinical trials using toxicity and efficacy odds ratios. Biometrics. 2006;62:777–787. doi: 10.1111/j.1541-0420.2006.00534.x. [DOI] [PubMed] [Google Scholar]
- 36.Yuan Y, Yin G. Bayesian phase I/II adaptively randomized oncology trials with combined drugs. Ann Applied Statist. 2011;5:924–942. doi: 10.1214/10-AOAS433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang W, Sargent DJ, Mandrekar S. An adaptive dose-finding design incorporating both efficacy and toxicity. Statist Med. 2006;25:2365–2383. doi: 10.1002/sim.2325. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Y, Whitehead J, Bonvini E, Stevens J. Bayesian decision procedures for binary and continuous bivariate dose-escalation studies. Pharmaceutical Statistics. 2006;5:125–133. doi: 10.1002/pst.222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.