Abstract
Objective
To compare outcomes of women with advanced stage low-grade serous ovarian cancer and high-grade serous ovarian cancer, and identify factors associated with survival among patients with advanced stage low-grade serous ovarian cancer.
Methods
A retrospective study of patients diagnosed with grade 1 or 3, advanced-stage (stage IIIC and IV) serous ovarian cancer between 2003 and 2011 was undertaken using the National Cancer Database, a large administrative database. The effect of grade on survival was analyzed using the Kaplan-Meier method. Factors predictive of outcome were compared using the Cox proportional hazards model. Among women with low-grade serous ovarian cancer, propensity score matching was used to compare all-cause mortality among similar women who underwent chemotherapy and lymph node dissection and those who did not.
Results
A total of 16,854 (95.7%) patients with high-grade serous ovarian cancer and 755 (4.3%) patients with low-grade serous ovarian cancer were identified. Median overall survival was 40.7 months among high-grade patients and 90.8 months among women with low-grade tumors (p<0.001). Among patients with low-grade serous ovarian cancer in the propensity-score matched cohort, the median overall survival was 88.2 months among the 140 patients who received chemotherapy and 95.9 months among the 140 that did not received chemotherapy (p=0.7). Conversely, in the lymph node dissection propensity-matched cohort, median overall survival was 106.5 months among the 202 patients who underwent lymph node dissection and 58 months among the 202 who did not (p<0.001).
Conclusions
When compared to high-grade serous ovarian cancer, low-grade serous ovarian cancer is associated with improved survival. In patients with advanced-stage low-grade serous ovarian cancer, lymphadenectomy but not adjuvant chemotherapy was associated with improved survival.
Precis
In advanced serous ovarian cancer, low-grade tumors have a better prognosis than high-grade, and in low-grade tumors, lymphadenectomy, but not chemotherapy, is associated with improved survival.
Introduction
Serous epithelial ovarian tumors are the most common sub-type of ovarian cancer. Numerous studies have supported a two–tier grading system for serous ovarian carcinoma based on histology: low-grade and high-grade.(1–3) Low-grade serous ovarian carcinomas are rare, accounting for only 5–10% of all epithelial ovarian cancers.(4) Low-grade serous ovarian tumors typically demonstrate KRAS or BRAF mutations (5–8), while high-grade serous ovarian tumors are often genetically unstable and express p53 mutations.(9–11)
High-grade serous ovarian tumors usually present at advanced stages and are frequently responsive to first-line platinum-based chemotherapy.(12) However, women with high-grade serous ovarian cancer experience frequent recurrences and decreased survival as compared to patients with low-grade tumors.(13) While low-grade serous ovarian tumors tend to follow a more indolent course, chemotherapy is less effective in this population.(14–16) However, these patients often receive adjuvant chemotherapy. According to a single-institution study, 55% of patients with low-grade serous ovarian cancer received adjuvant therapy.(15) There is a lack of high-quality data to guide clinician and patient decision-making regarding treatment. A PubMed and Medline search of articles published in English from January 1, 1980 – August 31, 2016 using the term “low-grade serous ovarian cancer” confirmed that there are no randomized controlled trials comprised exclusively of women with low-grade serous ovarian cancer, and retrospective studies are limited by small numbers of patients. The objective of this study was to use a large national cancer database to compare outcomes of women with advanced stage low-grade serous ovarian cancer and high-grade serous ovarian cancer, and identify factors associated with survival among patients with advanced stage low-grade serous ovarian cancer.
Materials and Methods
This is a retrospective study using the National Cancer Database is a clinical oncology database sourced from over 1,500 hospitals, capturing approximately 70% of newly diagnosed cancer cases across the United States. The National Cancer Database was used to identify women 18 years and older diagnosed with advanced stage (IIIC and IV) serous ovarian carcinoma from January 1, 2003 until December 31, 2011. Patients with grade 1 (low-grade) and grade 3 (high- grade) tumors were included. Patients with grade 2 tumors were excluded. There is substantial variation in classification of grade 2 tumors (17), and we sought to define the high-grade and low-grade populations as accurately as possible in the absence of central pathology review. Patients were excluded if they were diagnosed with other primary cancers (n=5,201), if they had missing data on lymph node dissection or chemotherapy utilization or received chemotherapy or radiation prior to surgery (n=6,435). We also excluded patients who were treated outside of the facility where they were diagnosed with ovarian cancer (n=308) as well as patients with stage I-IIIB or unknown stage (n=9,573). Patients with unknown survival (n=1), race (n=201), or facility type (n=30) were also excluded. This study was exempt from our Institutional Review Board because all information from the National Cancer Database is deidentified.
Participant User Files were used to abstract demographic, socioeconomic and clinical variables and descriptive statistics were calculated. Adjuvant therapy (chemotherapy or radiation) was defined as any additional therapy received within the first 6 months of primary surgery. Staging was determined in accordance to the 1988 International Federation of Gynecology and Obstetrics (FIGO) staging system.(18) Registry location was divided into geographic area of residence at the time of diagnosis: central, eastern and western. Year of diagnosis was classified as 2003–2005, 2006–2008, 2009–2011. Median household income from zip code of residence was categorized into quartiles and used as a proxy for socioeconomic status. Census data and the American Community Survey also contributed to socioeconomic classifications depending on the date of diagnosis and death. The treating facility was categorized according to the Commission on Cancer Accreditation program as a Community Cancer Program, Comprehensive Community Cancer Program, Academic/Research Program, or other. Insurance status was categorized as uninsured, private insurance, Medicare, or another type of government insurance (military or Medicaid). Educational attainment for each patient’s area of residence was estimated by matching the zip code of the patient recorded at the time of diagnosis against files derived from the 2012 American Community Survey data thereby providing a measure of the proportion of adults in the patient’s zip code who did not graduate from high school. The Charlson/Deyo co-morbidity index was used to report comorbidities among the patients. The Charlson/Deyo Comorbidity Index includes comorbidities likely to impact survival among hospitalized cancer patients; a score of zero means none of the comorbidities were present (19). Subjects were assigned a score of 0, 1, or >1. Survival time was measured from the date of diagnosis until death from any cause, censoring, or last follow-up, as verified by the National Cancer Database program vital status determination. Given the cohort of patients diagnosed from 1/1/2003 through 12/31/2011 and available follow-up data through 1/1/2014, in those patients who survived we had a minimum of 2 years of follow-up data in this study.
Standard univariate analyses were performed and the distribution of demographic, clinical, and treatment characteristics were compared using chi-square tests. A student t test was used to assess the significance of differences in the mean values of continuous variables. The association of grade with overall survival was assessed using the Kaplan-Meier method and the log-rank test. Cox proportional hazards models were used to calculate adjusted group Hazard Ratios, and their 95% confidence intervals (CI) were utilized to assess the importance of histology as an independent predictor of survival after adjusting for the following prognostic factors: age, race, socioeconomic status, facility type, stage, surgery, lymph node dissection, co-morbidity index, period of diagnosis, education, insurance, hospital volume, treatment, and registry location.
Patients with low-grade serous ovarian cancer were then analyzed separately to assess the impact of chemotherapy and lymph node dissection on overall survival. Propensity score matching was undertaken to create a cohort in which subjects who did and did not receive chemotherapy were balanced on covariates that could affect the decision to administer chemotherapy and thereby con found the association between use of chemotherapy and survival.(20) We included factors that the clinician would have known at the time of treatment decision-making which were available in the National Cancer Database (e.g. age, race, stage, lymph node status, etc). We fit logistic regression models to estimate the probability of receiving chemotherapy; independent variables included age, period of diagnosis, race–ethnicity, treating facility type, insurance status, income, geographic region, rural–urban status, education level, tumor size, stage and lymph node dissection. We then matched each woman who received chemotherapy with a woman who did not who had the same propensity to undergo chemotherapy using a caliper of 0.2.(21) We plotted survival functions for women who underwent chemotherapy and those who did not in the propensity score matched cohort using the Kaplan Meier method, and compared these using the log rank test. We then calculated the relative hazard of overall mortality associated with receipt of chemotherapy using a univariate Cox proportional hazard model. We employed a similar approach to assess the impact of lymph node dissection on overall survival. Propensity score for receipt of lymphadenectomy was calculated in a logistic regression model using all aforementioned independent variable, with lymph node dissection used as the dependent variable. Since information regarding chemotherapy utilization was unknown by the surgeon when choosing to perform or omit lymphadenectomy, this variable was not used to calculate propensity score. To estimate the association of lymphadenectomy and overall survival independent of chemotherapy use, we used a multivariable Cox proportional hazard model with chemotherapy as a covariate.
All statistical tests were 2 sided and differences were considered statistically significant at p < 0.05 and when hazard ratio confidence intervals did not include or cross 1.00. R version 3.0.3 was used for all statistical analyses.
Results
A total of 17,609 patients with grade 1 and 3 advanced stage serous ovarian cancer were identified. Of these, 755 (4.3%) were diagnosed with low-grade serous ovarian cancer while 16,854 (95.7%) had high-grade serous ovarian cancer. Table 1 illustrates the demographic differences between these two groups. High-grade serous ovarian cancer patients were older at diagnosis compared to women with low-grade serous ovarian cancer (62.3 years versus 53.6 years; p<0.001). Patients with high-grade serous ovarian cancer had higher rates of stage IV disease (26.1% versus 17.2%; p<0.001) and lower rate of lymph node dissection (55.6% versus 67.4%; p<0.001). The majority of women were white in both groups (90.1% versus 92.1%; p=0.1). Comorbidities scores were similar among high-grade serous ovarian cancer patients and low-grade serous ovarian cancer patients, with the majority of women presenting with a score of 0 (83.3% versus 83.4%, p= 0.3). Women with high-grade serous ovarian cancer had higher rates of chemotherapy delivery (83.5% versus 80.6%; p=0.01).
Table 1.
Demographic and clinical characteristics of the study population.
| Characteristic | Low-Grade Tumor | High-Grade Tumor | P | |
|---|---|---|---|---|
| N(%) | 755 (4.29) | 16,854 (95.71) | ||
| Mean Age [Years (standard deviation)] | 53.6 (±15.34) | 62.3 (±11.66) | <0.001 | |
| Race [N (%)] | 0.1 | |||
| White | 695 (92.1) | 15,186 (90.1) | ||
| African-American | 43 (5.7) | 1,108 (6.6) | ||
| Other | 17 (2.3) | 560 (3.3) | ||
| Year of diagnosis [N (%)] | 0.03 | |||
| 2003–2005 | 268 (35.5) | 5,349 (31.7) | ||
| 2006–2008 | 264 (35.0) | 5,864 (34.8) | ||
| 2009–2011 | 223 (29.5) | 5,641 (33.5) | ||
| Region [N (%)] | <0.001 | |||
| Northeast | 128 (17.0) | 3,023 (17.9) | ||
| Midwest | 201 (26.6) | 4,397 (26.1) | ||
| South | 333 (44.1) | 6,507 (38.6) | ||
| West | 93 (12.3) | 2,927 (17.4) | ||
| Charlson/Deyo Score [N (%)] | 0.3 | |||
| 0 | 630 (83.4) | 14,032 (83.3) | ||
| 1 | 109 (14.4) | 2,320 (13.8) | ||
| 2 | 16 (2.1) | 502 (3.0) | ||
| Stage [N (%)] | <0.001 | |||
| IIIC | 625 (82.8) | 12,454 (73.9) | ||
| IV | 130 (17.2) | 4,400 (26.1) | ||
| Lymph Node Dissection [N (%)] | 509 (67.4) | 9,379 (55.6) | <0.001 | |
| Adjuvant Treatment [N (%)] | 0.01 | |||
| None | 146 (19.3) | 2,776 (16.5) | ||
| Chemotherapy | 603 (80.6) | 14,026 (83.5) | ||
| Radiation | 1 (0.1) | 5 (0.0) | ||
| Chemo-radiation | 5 (0.7) | 47 (0.3) | ||
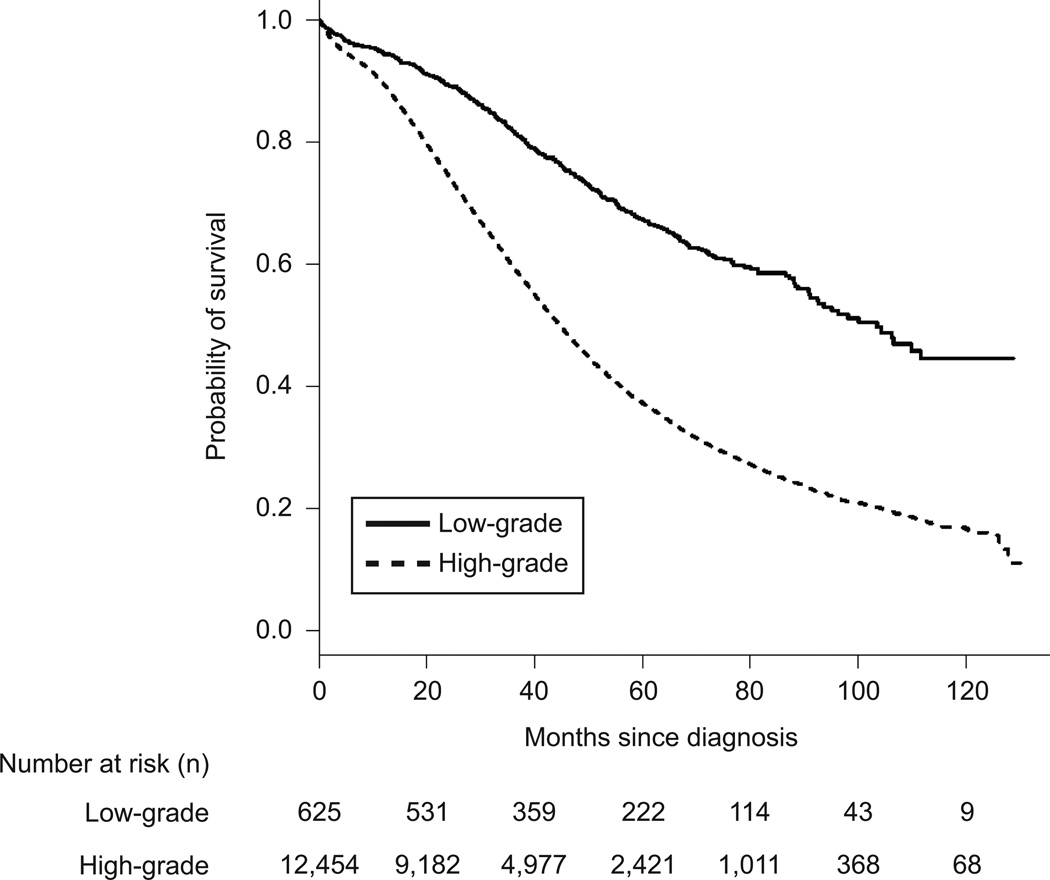
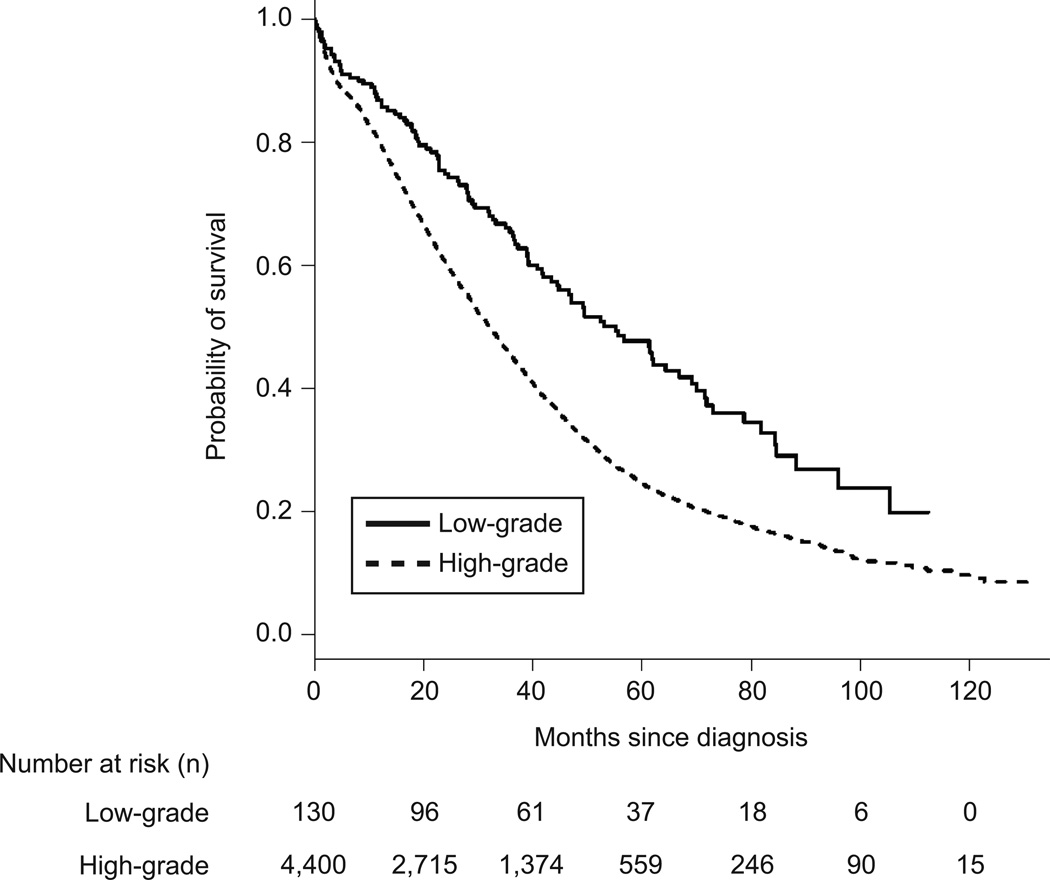
The median overall survival for women with high-grade serous ovarian cancer was 40.7 months (95%CI=40.08–41.5) and 90.8 months (95%CI=78.7–106.3) in women with low-grade serous ovarian cancer (p<0.001) When patients with stage IIIC and stage IV disease were evaluated separately, the median overall survival among women with stage IIIC high-grade serous ovarian cancer was 43.8 months (95%CI 42.9–44.8) compared to 98.1 months (95%CI=88.02.3–111.6) in low-grade serous ovarian cancer (p<0.001) (Figure 1). Among patients with stage IV disease, the median overall survival in high-grade serous ovarian cancer was 32.5 months (95%CI=31.2–33.8) and 55.2 months (95%CI=40.8–84.5) in low-grade serous ovarian cancer patients (p<0.001) (Figure 2).
Figure 1.
Mortality for women diagnosed with stage IIIC low-grade and high-grade serous ovarian carcinoma.
Figure 2.
Mortality for women diagnosed with stage IV low-grade and high-grade serous ovarian carcinoma.
Over the entire study period, and after adjusting for age, race, socioeconomic status, facility type, stage, surgery, lymph node dissection, chemotherapy, co-morbidity index, period of diagnosis, education, hospital volume, and registry location, high-grade serous ovarian cancer was associated with worse overall survival (Hazard Ratio 2.13; 95%CI=1.89–2.40) (Table 2).
Table 2.
Effect of various factors on mortality in patients with advanced stage (IIIC and IV) papillary serous ovarian cancer.
| Age (Years) | Adjusted Hazard Ratio (95% Confidence Interval) |
|
|---|---|---|
| <60 | Ref. | |
| 60–69 | 1.12 (1.07–1.18) | |
| 70–79 | 1.40 (1.31–1.50) | |
| ≥ 80 | 2.14 (1.97–2.33) | |
| Time Period | ||
| 2003–2005 | Ref. | |
| 2006–2008 | 0.90 (0.86–0.94) | |
| 2009–2011 | 0.90 (0.85–0.95) | |
| Race | ||
| White | Ref. | |
| African-American | 1.12 (1.03–1.21) | |
| Other | 0.89 (0.79–1.00) | |
| Charlson/Deyo Score | ||
| 0 | Ref. | |
| 1 | 1.18 (1.12–1.25) | |
| 2 | 1.42 (1.28–1.58) | |
| Histology Type | ||
| Low-Grade | Ref. | |
| High-Grade | 2.13 (1.89–2.40) | |
| Lymph Node Dissection | ||
| No | Ref. | |
| Yes | 0.73 (0.70–0.76) | |
| Stage | ||
| IIIC | Ref. | |
| IV | 1.35 (1.29–1.41) | |
| Adjuvant Treatment | ||
| None | Ref. | |
| Chemotherapy | 0.57 (0.54–0.60) | |
| Radiotherapy | 0.52 (0.17–1.62) | |
| Chemo-radiotherapy | 0.74 (0.54–1.03) | |
Also included in the model: insurance, income level, education, registry location, facility type, hospital volume
Patients with low-grade serous ovarian cancer were analyzed separately. In a multivariable model, patients with income in the 4th quartile and other government insurance were more likely to receive chemotherapy. Women located in the west geographic location and classified as “other” race were less likely to receive chemotherapy. With regards to lymph node dissection, women age 80 and older, with stage IV disease and tumor size <3cm were less likely to undergo dissection. Women with private insurance were more likely to undergo lymph node dissection than women with other types of insurance (Table 3).
Table 3.
Adjusted associations between patients’ socio-demographic and disease characteristics and use of chemotherapy and lymph node dissection in women with low-grade serous ovarian cancer
| Age (Years) | Chemotherapy [Adjusted HR (95% Confidence Interval)] |
Lymph Node dissection [Adjusted HR (95% Confidence Interval)] |
|
|---|---|---|---|
| < 60 | Ref. | Ref. | |
| 60–69 | 1.17 (0.66–2.09) | 0.68 (0.43–1.08) | |
| 70–79 | 0.67 (0.30–1.49) | 0.54 (0.27–1.05) | |
| ≥ 80 | 0.47 (0.17–1.27) | 0.40 (0.16–0.99) | |
| Time Period | |||
| 2003–2005 | Ref. | Ref. | |
| 2006–2008 | 1.18 (0.75–1.85) | 1.63 (1.10–2.40) | |
| 2009–2011 | 1.67 (1.00–2.77) | 1.18 (0.79–1.78) | |
| Race | |||
| White | Ref. | Ref. | |
| Black | 0.52 (0.24–1.12) | 1.08 (0.53–2.24) | |
| Other | 0.29 (0.09–0.87) | 1.27 (0.39–4.14) | |
| Registry Location | |||
| Northeast | Ref. | Ref. | |
| Midwest | 1.37 (0.69–2.71) | 1.36 (0.81–2.26) | |
| South | 0.67 (0.37–1.23) | 1.26 (0.78–2.03) | |
| West | 0.33 (0.16–0.68) | 1.45 (0.77–2.72) | |
| Facility Type | |||
| Academic | Ref. | Ref. | |
| Community | 0.72 (0.29–1.81) | 1.22 (0.53–2.81) | |
| Comprehensive | 0.91 (0.60–1.37) | 0.84 (0.59–1.18) | |
| Insurance | |||
| None | Ref. | Ref. | |
| Private | 1.57 (0.73–3.39) | 2.13 (1.11–4.11) | |
| Medicare | 1.24 (0.47–3.25) | 1.57 (0.70–3.52) | |
| Other Government | 3.21 (1.10–9.35) | 1.34 (0.59–3.04) | |
| Unknown | 1.27 (0.31–5.15) | 1.02 (0.32–3.28) | |
| Income | |||
| 1st Quartile | Ref. | Ref. | |
| 2nd Quartile | 1.70 (0.90–3.21) | 1.21 (0.70–2.08) | |
| 3rd Quartile | 1.39 (0.76–2.56) | 1.28 (0.74–2.20) | |
| 4th Quartile | 2.06 (1.06–3.98) | 1.38 (0.79–2.42) | |
| Unknown | 1.09 (0.24–4.87) | 2.22 (0.54–9.10) | |
| Charlson/Deyo Score | |||
| 0 | Ref. | Ref. | |
| 1 | 0.98 (0.56–1.71) | 1.28 (0.80–2.06) | |
| 2 | 0.51 (0.16–1.67) | 0.95 (0.32–2.83) | |
| Tumor Size | |||
| <1cm | Ref. | Ref. | |
| <2cm | 0.32 (0.06–1.86) | 0.22 (0.04–1.28) | |
| <3cm | 0.77 (0.12–5.01) | 0.10 (0.02–0.62) | |
| <4cm | 4.83 (0.36–64.11) | 0.33 (0.05–2.15) | |
| <5cm | 0.26 (0.04–1.52) | 0.30 (0.05–1.82) | |
| >5cm | 0.71 (0.14–3.44) | 0.25 (0.05–1.26) | |
| Unknown | 0.63 (0.13–3.14) | 0.19 (0.04–0.97) | |
| Stage | |||
| IIIC | Ref. | Ref. | |
| IV | 0.64 (0.39–1.03) | 0.35 (0.23–0.53) | |
Also included in the model urban vs. rural setting and education level not shown.
Propensity score matching between patients with low-grade serous ovarian cancer yielded 140 patients who received chemotherapy and 140 patients who did not. (Table 4). After propensity score matching, all characteristics were balanced in the two groups. The median follow up time was 72.9 months (66.4–78.1) and there were 56 deaths among patients who received chemotherapy and 52 among those who did not. The median overall survival in the chemotherapy administration propensity-matched cohort was 88.2 months (95%CI=68.6-not reached) for the women who underwent chemotherapy and 95.9 months (95%CI=73.7-not reached) in those who did not (p=.0.7). In a Cox regression model that included the propensity-matched cohorts, chemotherapy delivery was not associated with risk of death (HR 0.96, 95%CI=0.66–1.4).
Table 4.
Demographic and clinical characteristics of women with low-grade serous ovarian cancer after propensity score matching for utilization of chemotherapy.
| Chemotherapy | No Chemotherapy |
P | ||
|---|---|---|---|---|
| N | 140 | 140 | ||
| Mean Age [Years (SD)] | 57.68 (15.71) | 55.99 (15.90) | 0.9 | |
| Race [N (%)] | 0.6 | |||
| White | 115 (84.6) | 120 (88.22) | ||
| African-America | 16 (11.8) | 11 (8.1) | ||
| Other | 5 (3.7) | 5 (3.7) | ||
| Period of Diagnosis [N (%)] |
0.8 | |||
| 2003–2005 | 57 (41.9) | 52 (38.2) | ||
| 2006–2008 | 49 (36.0) | 50 (36.8) | ||
| 2009–2011 | 30 (22.1) | 34 (25.0) | ||
| Facility Type [N (%)] | 0.7 | |||
| Academic | 55 (40.4) | 59 (43.4) | ||
| Community | 10 (7.4) | 7 (5.1) | ||
| Comprehensive | 71 (52.2) | 70 (51.5) | ||
| Urban vs. Rural [N (%)] | 0.8 | |||
| Urban | 120 (88.2) | 123 (90.4) | ||
| Rural | 2 (1.5) | 2 (1.5) | ||
| Unknown | 14 (10.3) | 11 (8.1) | ||
| Registry Location [N (%)] | 0.9 | |||
| Northeast | 23 (16.9) | 20 (14.7) | ||
| Midwest | 25 (18.4) | 24 (17.6) | ||
| South | 68 (50.0) | 68 (50.0) | ||
| West | 20 (14.7) | 24 (17.6) | ||
| Income [N (%)] | 1.0 | |||
| 1st Quartile | 26 (19.1) | 25 (18.4) | ||
| 2nd Quartile | 32 (23.5) | 33 (24.3) | ||
| 3rd Quartile | 42 (30.9) | 41 (30.1) | ||
| 4th Quartile | 30 (22.1) | 30 (22.1) | ||
| Unknown | 6 (4.4) | 7 (5.1) | ||
| Insurance [N (%)] | 0.7 | |||
| None | 10 (7.4) | 9 (6.6) | ||
| Private | 66 (48.5) | 75 (55.1) | ||
| Medicare | 41 (30.1) | 40 (29.4) | ||
| Other Government | 14 (10.3) | 8 (5.9) | ||
| Unknown | 5 (3.7) | 4 (2.9) | ||
| Charlson/Deyo Score [N (%)] |
0.9 | |||
| 0 | 107 (78.7) | 110 (80.9) | ||
| 1 | 23 (16.9) | 21 (15.4) | ||
| 2 | 6 (4.4) | 5 (3.67 | ||
| Education Level [N (%)] | 0.9 | |||
| <7% | 23 (16.9) | 22 (16.2) | ||
| 7–13% | 39 (28.7) | 44 (32.4) | ||
| 13–21% | 42 (30.9) | 37 (27.2) | ||
| >21% | 26 (19.1) | 26 (19.1) | ||
| Unknown | 6 (4.4) | 7 (5.1) | ||
| Stage [N (%)] | 0.5 | |||
| IIIC | 110 (80.9) | 104 (76.5) | ||
| IV | 26 (19.1) | 32 (23.5) | ||
| Tumor size [N (%)] | 1.0 | |||
| <1cm | 3 (2.2) | 2 (1.5) | ||
| <2cm | 9 (6.6) | 7 (5.1) | ||
| <3cm | 5 (3.7) | 5 (3.7) | ||
| <4cm | 1 (0.7) | 1 (0.7) | ||
| <5cm | 9 (6.6) | 8 (5.9) | ||
| >5cm | 58 (42.6) | 65 (47.8) | ||
| Unknown | 51 (37.5) | 48 (35.3) | ||
SD: Standard Deviation.
Propensity score matching for lymph node dissection yielded 202 patients who underwent lymphadenectomy and 202 patients who did not. All variables were also well balanced between the groups (Table 5). The median follow-up was 72.7 months (66.7–75.2) and there were 65 deaths among patients who underwent lymphadenectomy and 102 among those who did not. The median overall survival in the lymph node dissection propensity-matched cohort was 106.5 months (95%CI=103.5-not reached) for the women who underwent lymph node dissection and 58 months (95%CI=46.7–87.2) in those who did not (p<0.001). In a Cox regression model that included the lymph node dissection propensity-matched cohorts, lymph node dissection was associated with improved survival after adjustment for chemotherapy (Hazard Ratio 0.54, 95%CI=0.39–0.74).
Table 5.
Demographic and clinical characteristics of women with LGCS after propensity score matching for utilization of lymph node dissection.
| Lymph Node Dissection | No Lymph Node Dissection |
P | ||
|---|---|---|---|---|
| N | 202 | 202 | ||
| Mean Age (Years, SD) | 54.08 (15.14) | 55.54 (14.83) | 0.3 | |
| Race [N (%)] | 0.6 | |||
| White | 185 (91.6) | 187 (92.6) | ||
| African-America | 10 (5.0) | 11 (5.4) | ||
| Other | 7 (3.5) | 4 (2.0) | ||
| Period of Diagnosis [N (%)] | 0.3 | |||
| 2003–2005 | 87 (43.1) | 81 (40.1) | ||
| 2006–2008 | 54 (26.7) | 68 (33.7) | ||
| 2009–2011 | 61 (30.2) | 53 (26.2) | ||
| Facility Type [N (%)] | 0.8 | |||
| Academic | 91 (45.0) | 96 (47.5) | ||
| Community | 10 (5.0) | 9 (4.5) | ||
| Comprehensive | 101 (50.0) | 97 (48.0) | ||
| Urban vs. Rural [N (%)] | 0.8 | |||
| Urban | 188 (93.1) | 188 (93.1) | ||
| Rural | 2 (1.0) | 3 (1.5) | ||
| Unknown | 12 (5.9) | 11 (5.4) | ||
| Registry Location [N (%)] | 0.9 | |||
| Northeast | 40 (19.8) | 37 (18.3) | ||
| Midwest | 46 (22.8) | 50 (24.8) | ||
| South | 93 (46.0) | 89 (44.1) | ||
| West | 23 (11.4) | 26 (12.9) | ||
| Income [N (%)] | 0.9 | |||
| 1st Quartile | 26 (12.9) | 25 (12.4) | ||
| 2nd Quartile | 53 (26.2) | 56 (27.7) | ||
| 3rd Quartile | 58 (28.7) | 59 (29.2) | ||
| 4th Quartile | 59 (29.2) | 55 (27.2) | ||
| Unknown | 6 (3.0) | 7 (3.5) | ||
| Insurance [N (%)] | 0.9 | |||
| None | 17 (8.4) | 15 (7.4%) | ||
| Private | 111 (55.0) | 111 (55.0%) | ||
| Medicare | 50 (24.8) | 54 (26.7%) | ||
| Other Government | 16 (7.9) | 17 (8.4%) | ||
| Unknown | 8 (4.0) | 5 (2.5%) | ||
| Charlson/Deyo Score [N (%)] |
0.7 | |||
| 0 | 167 (82.7) | 168 (83.2) | ||
| 1 | 31 (15.3) | 28 (13.9) | ||
| 2 | 4 (2.0) | 6 (3.0) | ||
| Education Level [N (%)] | 0.7 | |||
| <7% | 48 (23.8) | 46 (22.8) | ||
| 7–13% | 75 (37.1) | 65 (32.2) | ||
| 13–21% | 48 (23.8) | 53 (26.2) | ||
| >21% | 25 (12.4) | 31 (15.3) | ||
| Unknown | 6 (3.0) | 7 (3.5) | ||
| Stage [N (%)] | 171 (84.7) | 165 (81.7) | ||
| IIIC | 31 (15.3) | 37 (18.3) | ||
| IV | ||||
| Tumor size [N (%)] | 0.7 | |||
| <1cm | 4 (2.0) | 2 (1.0) | ||
| <2cm | 9 (4.5) | 9 (4.5) | ||
| <3cm | 10 (5.0) | 6 (3.0) | ||
| <4cm | 5 (2.5) | 4 (2.0) | ||
| <5cm | 12 (5.9) | 8 (4.0) | ||
| > 5cm | 95 (47.0) | 98 (48.5) | ||
SD: Standard Deviation.
Discussion
Low-grade serous ovarian cancer is a rare histological subtype of ovarian cancer lacking specific data to inform prognosis and appropriate treatment strategies. In this investigation we used a large national cancer database to compare the presenting characteristics and outcomes of women with advanced stage low-grade serous ovarian cancer and high-grade serous ovarian cancer, as well as identify factors associated with survival among patients with low-grade serous ovarian cancer. The current study suggests that there are several distinct clinical characteristics between women with low-grade serous ovarian cancer and high-grade serous ovarian cancer, such as a younger age at diagnosis and a significantly longer median overall survival in patients with low-grade serous ovarian cancer. When patients with low-grade serous ovarian cancer were separately analyzed, we found that chemotherapy was not associated with improved survival; conversely, lymph node dissection was associated with improved survival in this population.
Our finding of a more favorable outcome and younger age at presentation in women with low-grade serous ovarian cancer compared to high-grade serous ovarian cancer parallels the results of prior studies. In a retrospective study using the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) database, Plaxe (13) reported that in women with metastatic disease median overall survival was 85 months in low-grade serous patients and 36 months among the high-grade serous group. Other single institution studies demonstrate similar results.(15,22) These findings support that low-grade serous ovarian tumors represent a distinct entity, and their presentation should rank high in the differential diagnoses list of younger women presenting with metastatic epithelial ovarian cancer.
Currently, many patients with stage II–IV low-grade serous ovarian cancer undergo cytoreductive surgery followed by platinum-based chemotherapy.(23) However, prior studies have shown that low-grade serous ovarian cancers are less sensitive to conventional chemotherapy compared to high-grade serous ovarian tumors. Schmeler et al. (16) identified 25 women who received neoadjuvant chemotherapy, however, only one patient (4%) demonstrated a complete response to treatment. A separate analysis of 39 women diagnosed with low-grade serous ovarian cancer who underwent a suboptimal debulking showed an objective response in only 10 patients (23.1%), compared to 90.1% in the control cohort of high-grade serous ovarian cancer (p<0.001).(24) In a recent study of patients with low-grade primary peritoneal cancer, of the 48 patients that received chemotherapy 66.7% of patients were noted to have persistent or progressive disease.(25) Furthermore, Gershenson et al.(14) found a response rate of less than 4% among women with recurrent low-grade serous ovarian cancer. Our results also suggest that chemotherapy offers limited benefit in the adjuvant treatment of women with low-grade serous ovarian cancer. In the current analysis, we found that chemotherapy administration was not associated with improved survival in the propensity score-matched cohort of women with low-grade serous ovarian cancer. While this is compelling and argues against chemotherapy as adjuvant treatment for low-grade serous ovarian cancer patients, there may be clinically relevant factors that may be associated with a worse prognosis (such as residual disease) that are not available in the National Cancer Database data and which drove the decision to give these patients chemotherapy. Additionally, it is important to note that only a small number of patients with low-grade serous ovarian cancer (N=140) who did not received chemotherapy were included in the propensity score analysis, and this might limit our findings.
Emerging data suggests that other agents may be active in low-grade serous ovarian cancer. In a single-institution retrospective study of stage II –IV low-grade serous ovarian cancer patients who underwent cytoreductive surgery and platinum-based chemotherapy, 114 patients underwent surveillance and 66 patients received hormonal maintenance with letrazole, anastrazole or leuprolide. Patients on hormonal maintenance had a significantly improved median progression free survival (52.0 months versus 29.9 months) and decreased risk of recurrence (Hazard Ratio=0.21; 95% CI .10–0.43) when compared to women on surveillance alone (26). A small number of patients in our cohort received hormonal treatment, and we were not able to perform a separate analysis of these patients. Another single-institution series reported median progression-free survival of 48 months for 12 low-grade serous ovarian cancer patients receiving bevacizumab. (27) While further trials are needed to identify what, if any, chemotherapy or hormonal regimen is beneficial, clinicians should consider discussing with patients the lack of evidence for adjuvant chemotherapy. setting.
The role of lymphadenectomy in advanced ovarian cancer remains controversial. (28–29) Interestingly, we found that among the lymph node dissection propensity-matched cohort, lack of lymph node dissection was associated with increased risk of death. Based on results of our literature search, this association between lymphadenectomy and survival for low-grade serous ovarian cancer patients has not been previously reported. However, it is possible that lymph node dissection upstaged some women with no evidence of macroscopic disease, who may have had a survival advantage based on their initial low tumor volume rather than the lymph node dissection. Unfortunately, the information regarding the specifics of lymphadenectomy was not available in our data. Prior studies have shown that women with retroperitoneal lymph node metastasis as the only site of extra-ovarian involvement have a better prognosis compared to patients with macroscopic peritoneal metastases.(30) The patients in this study were staged according to the FIGO criteria prior to the most recent updated criteria in 2014. Therefore there may be patients classified as IIIC due to isolated lymph node metastases in our study, which by the 2014 FIGO staging system would be stage IIIA1. It is possible that the benefit of lymphadenectomy for some of these patients was diagnostic and not therapeutic, however, we attempted to reduce potential bias by including initial tumor size in the propensity score analysis and restricting the analysis to women with stage IIIC and IV disease. Additionally, healthier patients have a baseline better prognosis and these women are also more likely to have a lymph node dissection. In order to reduce this selection bias, we included comorbidity index score in the propensity score analysis.
Although our analysis benefits from a large study population and its ability to adjust for patients’ demographic characteristics, socioeconomic status, comorbidities and adjuvant treatment, there are several limitations that must be considered when interpreting the findings. The retrospective nature of this study creates inherent bias due to non-randomized allocation to treatment groups, as well as other limitations including reliance on database information from a wide variety of institutions, absence of central pathology review, as well as lack of information regarding specific surgical and treatment factors and missing data points for some patients. Clinically important factors such as gross residual disease and chemotherapy regimens are not available in this database for this cohort of patients. These factors likely have a significant impact on the clinical course of both high-grade and low-grade serous ovarian cancer and deserve further investigation.. However, given the rarity of low-grade serous ovarian cancer, large-scale prospective trials are very challenging to conduct and large database analyses can provide valuable insights in an otherwise difficult to study disease.
Acknowledgments
Supported by R25CA092203 from the National Cancer Institute at the National Institutes of Health and The Deborah Kelly Center for Outcomes Research, Massachusetts General Hospital.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
Presented as a poster at the 2016 Society of Gynecologic Oncology Annual Meeting, San Diego, California. March 19–22, 2016
References
- 1.Malpica A, Deavers MT, Lu K, Bodurka DC, Atkinson EN, Gershenson DM, et al. Grading ovarian cancer serous carcinoma using a two-tier system. Am J Surg Pathol. 2000;19:7–15. doi: 10.1097/00000478-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Malpica A, Deavers MT, Tornos C, Kurman RJ, Soslow R, Seidman JD, et al. Interobserver and intraobserver variability of a to-tier system for grading ovarian serous carcinoma. Am J Surg Pathol. 2007;31:1168–1174. doi: 10.1097/PAS.0b013e31803199b0. [DOI] [PubMed] [Google Scholar]
- 3.Gilks CB. Subclassification of ovarian surface epithelial tumors based on correlation of histologic and molecular pathologic data. Int J Gynecol Pathol. 2004;23:200–205. doi: 10.1097/01.pgp.0000130446.84670.93. [DOI] [PubMed] [Google Scholar]
- 4.Kurman RJ, Shih IM. The dualistic model of ovarian carcinogenesis: Revisited, revised and expanded. Am J Pathology. 2016;186:733–747. doi: 10.1016/j.ajpath.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer G, Stohr R, Cope L, Dehari R, Hartmann A, Cao DF, et al. Patterns of p53 mutations separate ovarian serous borderline tumors and low- and high-grade carcinomas and provide support for a new model of ovarian carcinogenesis: a mutational analysis with immunohistochemical correlation. Am J Surg Pathol. 2005;29:218–224. doi: 10.1097/01.pas.0000146025.91953.8d. [DOI] [PubMed] [Google Scholar]
- 6.Singer G, Oldt R, 3rd, Cohen Y, Wang BG, Sidranksy D, Kurman RJ, et al. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst. 2003;95:484–486. doi: 10.1093/jnci/95.6.484. [DOI] [PubMed] [Google Scholar]
- 7.Singer G, Kurman RJ, Chang HW, Cho SK, Shih IeM. Diverse tumorigenic pathways in ovarian serous carcinoma. Am J Pathol. 2002;160:1223–1228. doi: 10.1016/s0002-9440(10)62549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sieben NL, Macropoulos P, Roemen GM, Kolkman-Uljee SM, Jan Fleuren G, Houmadi R, et al. In ovarian neoplasms, BRAF, but not KRAS, mutations are restricted to low grade serous tumours. J Pathol. 2004;202:336–340. doi: 10.1002/path.1521. [DOI] [PubMed] [Google Scholar]
- 9.Kohler MF, Marks JR, Wiseman RW, Jacobs IJ, Davidoff AM, Clarke-Pearson DL, et al. Spectrum of mutation and frequency of allelic deletion of the p53 gene in ovarian cancer. J Natl Cancer Inst. 1993;85:1513–1519. doi: 10.1093/jnci/85.18.1513. [DOI] [PubMed] [Google Scholar]
- 10.Milner J, Medcalf EA, Cook AC. Tumor suppressor p53: analysis of wild-type and mutant p53 complexes. Mol Cell Biol. 1991;11:12–19. doi: 10.1128/mcb.11.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salani R, Kurman RJ, Giuntoli R, 2nd, Gardner G, Bristow R, Wang TL, et al. Assessment of TP53 mutation using purified tissue samples of ovarian serous carcinomas reveals a much higher mutation rate than previously reported and does not correlate with drug resistance. Int J Gynecol Cancer. 2008;18:487–491. doi: 10.1111/j.1525-1438.2007.01039.x. [DOI] [PubMed] [Google Scholar]
- 12.Kurman RJ. Origin and molecular pathogenesis of ovarian high-grade serous carcinoma. Annal Oncol. 2013;24:16–21. doi: 10.1093/annonc/mdt463. [DOI] [PubMed] [Google Scholar]
- 13.Plaxe SC. Epidemiology of low-grade serous ovarian cancer. Am J Obstet Gynecol. 2008;198:459e1–459e9. doi: 10.1016/j.ajog.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 14.Greshenson DM, Sun CC, Bodurka D, Coleman RL, Lu KH, Sood AK, et al. Recurrent low-grade serous ovarian carcinoma is relatively chemoresistant. Gynecol Oncol. 2009;114:48–52. doi: 10.1016/j.ygyno.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Gershenson DM, Sun CC, Lu KH, Coleman RL, Sood AK, Malpica A, et al. Clinical behavior of stage II–IV low-grade serous carcinoma of the ovary. Obstet Gynecol. 2006;108:361–368. doi: 10.1097/01.AOG.0000227787.24587.d1. [DOI] [PubMed] [Google Scholar]
- 16.Schmeler KM, Sun CC, Bodurka DC, Deavers MT, Malpica A, Coleman RL, et al. Neoadjuvant chemotherapy for low-grade serous carcinoma of the ovary or peritoneum. Gynecol Oncol. 2008;108:510–514. doi: 10.1016/j.ygyno.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu Y, Kamoi S, Amada S, Hasumi K, Akiyama F, Silverberg SG. Toward the development of a universal grading system for ovarian epithelial carcinoma. I. Prognostic significance of histopathologic features – problems involved in the architectural grading system. Gynecol Oncol. 1998;70:2–12. doi: 10.1006/gyno.1998.5051. [DOI] [PubMed] [Google Scholar]
- 18.Prat J FIGO Committee on Gynecologic Oncology. Staging classification for cancer of the ovary, fallopian tube and peritoneum. Int J Gynaecol Obstet. 2014;124:1–5. doi: 10.1016/j.ijgo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for casual effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 21.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okoye E, Euscher ED, Malpica A. Ovarian Low-grade Serous Carcinoma: A Clinicopathologic Study of 33 Cases With Primary Surgery Performed at a Single Institution. Am J Surg Pathol. 2016;40:627–635. doi: 10.1097/PAS.0000000000000615. [DOI] [PubMed] [Google Scholar]
- 23.Romero I, Sun CC, Wong KK, Bast RC, Jr, Gershenson DM. Low-grade serous carcinoma: new concepts and emerging therapies. Gynecol Oncol. 2013;130:660–666. doi: 10.1016/j.ygyno.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 24.Grabowski JP, Harter P, Heitz F, Pujade-Lauraine E, Reuss A, Kristensen G, et al. Operability and chemotherapy responsiveness in advanced low-grade serous ovarian cancer. An analysis of the AGO Study Group metadatabase. Gynecol Oncol. 2016;140:457–462. doi: 10.1016/j.ygyno.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 25.Schmeler KM, Sun CC, Malpica A, Deavers MT, Bodurka DC, Gershenson DM. Low-grade serous primary peritoneal carcinoma. Gynecol Oncol. 2011;121:482–486. doi: 10.1016/j.ygyno.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Gershenson DM, Bodurka DC, Coleman RL, et al. Hormonal maintenance therapy for women with low grade serous carcinoma of the ovary or peritoneum. J Clin Oncol. 2016;34(suppl) doi: 10.1200/JCO.2016.71.0632. (abstract 5502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rose PG, Mahdi H, Jernigan A, Yang B. Activity of bevacizumab in patients with low-grade serous ovarian carcinoma. Int J Gynecol Oncol. 2016;26:1048–1052. doi: 10.1097/IGC.0000000000000742. [DOI] [PubMed] [Google Scholar]
- 28.Panici PB, Maggioni A, Hacker N, Landoni F, Ackerman S, Campagnutta E, et al. Systematic aortic and pelvic lymphadenectomy versus resection of bulky nodes only in optimally debulked advanced ovarian cancer: a randomized clinical trial. J Natl Cancer Inst. 2005;97:560–566. doi: 10.1093/jnci/dji102. [DOI] [PubMed] [Google Scholar]
- 29.du Bois A, Reuss A, Harter P, Pujade-Lauraine E, Ray-Coquard I, Pfisterer J, et al. Potential role of lymphadenectomy in advanced ovarian cancer: a combined exploratory analysis of three prospectively randomized phase III multicenter trials. J Clin Oncol. 2010;28:1733–1739. doi: 10.1200/JCO.2009.25.3617. [DOI] [PubMed] [Google Scholar]
- 30.Onda T, Yoshikawa H, Yasugi T, Mishima M, Nakagawa S, Yamada M, et al. Patients with ovarian carcinoma upstaged to stage III after systematic lymphadenctomy have similar survival to Stage I/ II patients and superior survival to other Stage III patients. Cancer. 1998;83:1555–1560. [PubMed] [Google Scholar]