Abstract
An improved understanding of pathogenic pathways in AKI may identify novel therapeutic approaches. Previously, we conducted unbiased liquid chromatography-tandem mass spectrometry–based protein expression profiling of the renal proteome in mice with acute folate nephropathy. Here, analysis of the dataset identified enrichment of pathways involving NFκB in the kidney cortex, and a targeted data mining approach identified components of the noncanonical NFκB pathway, including the upstream kinase mitogen-activated protein kinase kinase kinase 14 (MAP3K14), the NFκB DNA binding heterodimer RelB/NFκB2, and proteins involved in NFκB2 p100 ubiquitination and proteasomal processing to p52, as upregulated. Immunohistochemistry localized MAP3K14 expression to tubular cells in acute folate nephropathy and human AKI. In vivo, kidney expression levels of NFκB2 p100 and p52 increased rapidly after folic acid injection, as did DNA binding of RelB and NFκB2, detected in nuclei isolated from the kidneys. Compared with wild-type mice, MAP3K14 activity–deficient aly/aly (MAP3K14aly/aly) mice had less kidney dysfunction, inflammation, and apoptosis in acute folate nephropathy and less kidney dysfunction and a lower mortality rate in cisplatin-induced AKI. The exchange of bone marrow between wild-type and MAP3K14aly/aly mice did not affect the survival rate of either group after folic acid injection. In cultured tubular cells, MAP3K14 small interfering RNA targeting decreased inflammation and cell death. Additionally, cell culture and in vivo studies identified the chemokines MCP-1, RANTES, and CXCL10 as MAP3K14 targets in tubular cells. In conclusion, MAP3K14 promotes kidney injury through promotion of inflammation and cell death and is a promising novel therapeutic target.
Keywords: chemokine, renal failure, kidney tubule, renal injury, acute kidney injury
The incidence of AKI is increasing.1 However, there is currently no therapy that reliably prevents the progression to AKI or accelerates recovery of renal function.2,3 Thus, reliable biomarkers and novel therapeutic approaches are needed.4 AKI is characterized by kidney inflammation and tubular cell death, dedifferentiation, and subsequent proliferation.5–9 However, given the complexity and redundancies of the process, it is unlikely that targeting a single inflammatory molecule provides the kind of benefit that will be clinically relevant. Thus, attention has focused on upstream signaling pathways that may regulate the coordinated expression of an array of inflammatory molecules. The combination of unbiased protein expression profiling with focused data mining is a powerful tool to expand our knowledge of relevant pathways and key factors in disease. Liquid chromatography-tandem massspectrometry (LC-MS/MS) identified approximately 2000 proteins in murine renal cortex.10 However, its applications to the study of AKI has been limited and mainly concentrated in the analysis of biofluids such as urine or in the study of the metabolome rather than the proteome.11–14 To identify novel pathways and mediator networks active in AKI in a comprehensive manner, we used tissue LC-MS/MS to assess changes in the renal proteome of experimental acute folate nephropathy. Bioinformatics analysis of 41,235 peptides in cortical kidney tissue by LC-MS/MS proteomics allowed the identification of 6516 unique proteins, of which 1480 were differentially expressed in samples from experimental nephrotoxic AKI as compared with controls.15 On this previously reported raw dataset, we have now performed novel pathway analysis in search of cell death or inflammatory pathways that are activated in acute folate nephropathy. This analysis indicated enrichment of proteins from the noncanonical activation pathway for transcription factor NFκB. NFκB promotes inflammation by modulating gene transcription.16,17 Canonical NFκB activation is rapidly initiated through degradation of IκB proteins by the proteasome, thus releasing complexes that translocate to the nucleus to promote transcription of proinflammatory genes and downregulate the expression of anti-inflammatory molecules such as Klotho.18,19 By contrast, noncanonical NFκB activation is a delayed response that is engaged by a limited number of stimuli and involves activation of the mitogen-activated protein kinase kinase kinase 14 (MAP3K14)/NFκB-inducing kinase, proteasomal processing of NFκB p100 to p52, and nuclear translocation of p52/RelB complexes.16,20 The role and regulation of MAP3K14 in AKI is poorly understood.
The combined proteomic and bioinformatics approach enabled identification of evidence for MAP3K14 activation and the upregulation of several proteins of the noncanonical NFκB activation pathway in acute folate nephropathy that were confirmed by Western blot and immunohistochemistry. Functional studies identified chemokine expression and cell death and proliferation as novel MAP3K14-regulated processes in tubular cells. Furthermore, MAP3K14 was overexpressed during human AKI and genetically modified mice confirmed the key role of MAP3K14 in acute folate nephropathy and cisplatin-induced AKI.
Results
Kidney Tissue Proteomics Bioinformatics Analysis Identifies Upregulation of MAP3K14 and Noncanonical NFκB Components in Folate Nephropathy–Associated AKI
Acute folate nephropathy is characterized by increased serum creatinine (0.53±0.25 versus 0.10±0.02 mg/dl at 24 hours; P<0.05), tubular cell death, and interstitial inflammation.15 As previously described, unbiased proteomics combined with focused data analysis was conducted in 24-hour kidney cortex control and folate nephropathy samples.15 LC-MS/MS identified 41,235 peptides in the kidney cortex corresponding to 6516 unique, nonredundant proteins (Supplemental Figure 1).15 This study represents a new complimentary analysis of this previously generated dataset. KEGG pathway analysis identified the enrichment of several pathways on the basis of the upregulation of key proteins in folate nephropathy samples (Table 1). NFκB was at the crossroads of several of these pathways. NFκB activation is regulated by MAPK, requires ubiquitination and proteasomal processing or degradation, and regulates apoptosis and chemokine secretion. Canonically activated NFκB signaling has long been implicated in kidney injury.16 However, there is much less information on noncanonical NFκB activation and its components. A targeted data mining approach searched for components of the noncanonical NFκB pathway. A KEGG-generated NFκB signaling pathway map (Supplemental Figure 2) summarizes the expression of noncanonical NFκB signaling pathway components and NFκB2 (p100/p52) ubiquitination and proteasomal activation. Upregulation was observed for MAP3K14, the essential upstream kinase activating the noncanonical NFκB pathway,21,22 for proteins required for NFκB2 p100 ubiquitination and proteasomal processing to active NFκB2 p52, such as UBE2M/UBC12 (E2) and CULLIN-1 (E3), and for the two main components of noncanonical NFκB DNA-binding heterodimers, NFκB2 and RELB (Table 2).
Table 1.
Signaling pathways modulated in folate nephropathy–associated AKI samples and identified by pathway analysis using KEGG database searching
| KEGG Map | Number of Hits | Name |
|---|---|---|
| mmu04151 | 46 | PI3K-Akt signaling pathway |
| mmu04010 | 29 | MAPK signaling pathwaya |
| mmu04910 | 27 | Insulin signaling pathway |
| mmu04020 | 22 | Calcium signaling pathway |
| mmu04120 | 21 | Ubiquitin mediated proteolysisa |
| mmu04062 | 19 | Chemokine signaling pathwaya |
| mmu04310 | 17 | Wnt signaling pathway |
| mmu04630 | 16 | Jak-STAT signaling pathway |
| mmu04660 | 15 | T cell receptor signaling pathway |
| mmu03320 | 13 | PPAR signaling pathway |
| mmu04064 | 13 | NFκB signaling pathwaya |
| mmu04370 | 11 | VEGF signaling pathway |
| mmu04912 | 11 | GnRH signaling pathway |
| mmu04210 | 11 | Apoptosisa |
| mmu04722 | 10 | Neurotrophin signaling pathway |
NFκB activation is at the crossroads of these pathways.
Table 2.
Noncanonical NFκB signaling pathway and ubiquitination and proteasomal degradation proteins significantly modulated in acute folate nephropathy
| Name | Gene | Fold Change | P Value |
|---|---|---|---|
| Noncanonical NFκB pathway | |||
| Nuclear factor NFκB p100 subunit | Nfkb2 | 1.46 | 0.03 |
| Mitogen-activated protein kinase kinase kinase 14 (NIK) | Map3k14 | 1000 | 0.05 |
| Transcription factor RelB | Relb | 3.33 | 0.01 |
| Ubiquitination system | |||
| Cullin-1 | Cul1 | 1000 | 0.02 |
| NEDD8-conjugating enzyme Ubc12 | Ube2m | 3.01 | 0.01 |
Data represent focused data mining after nonbiased analysis of the significant dataset. NIK, NF-κ-B-inducing kinase.
Validation of Noncanonical NFκB Pathway Activation in Acute Folate Nephropathy
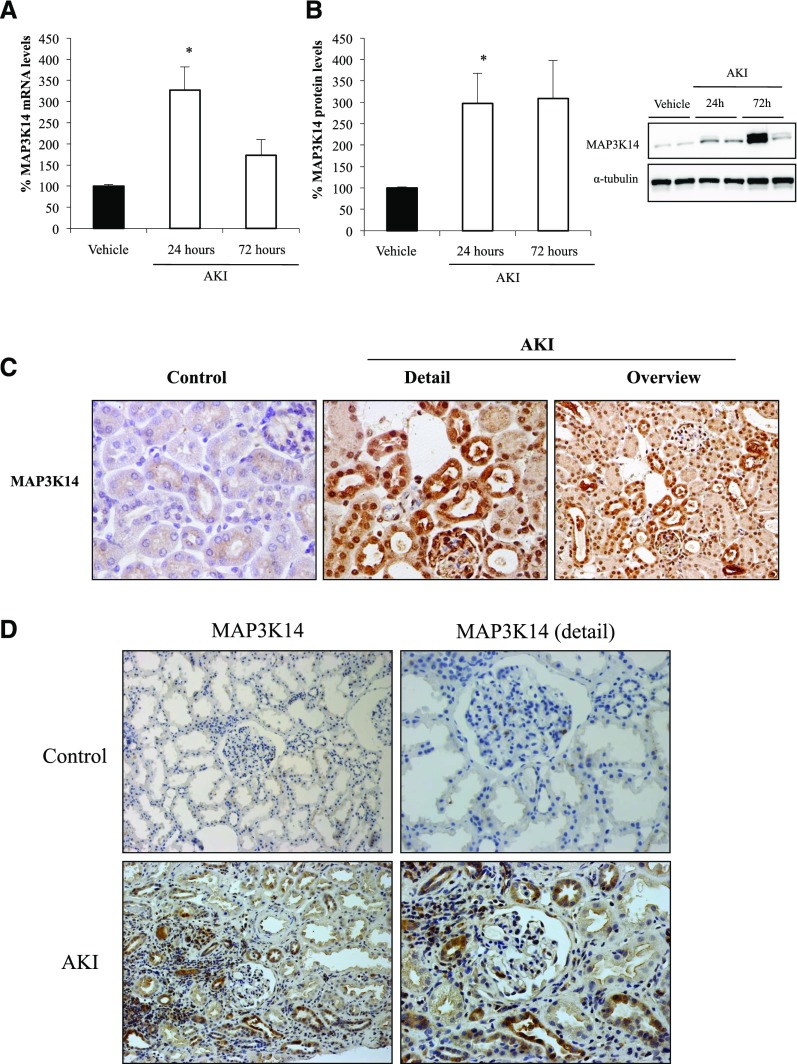
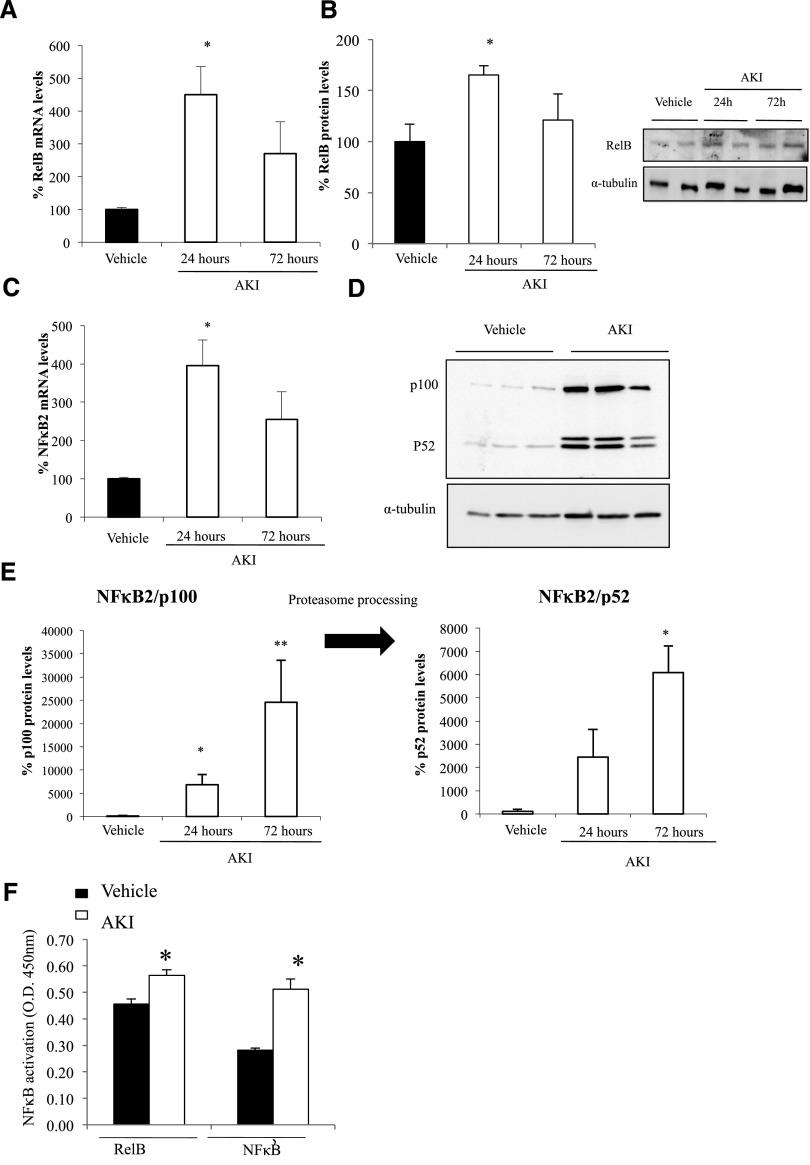
The proteomics findings of increased MAP3K14, RELB, and NFκB2 p100/p52 were validated by Western blot and immunohistochemistry and the mechanisms by which the system is upregulated were explored by assessing mRNA expression. Kidney Map3k14, RelB, and NFκB2 mRNA expression was increased in folate nephropathy, suggesting transcriptional upregulation (Figures 1A and 2, A and C). Western blot confirmed increased kidney MAP3K14, RELB, NFκB2 p100, and NFκB2 p52 in folate nephropathy (Figures 1B and 2, B, D, and E). Immunohistochemistry localized the increased expression of these proteins to tubular cells (Figure 1C). In addition, NFκB2 p52 and RELB DNA-binding activity was increased in nuclear extracts from folate nephropathy kidneys (Figure 2F). Thus, evidence for increased activation of MAP3K14 includes processing of NFκB2 p100 to NFκB2 p52 and nuclear translocation and increased DNA binding activity of the RelB/NFκB2 p52 transcription factor. The expression of the ubiquitination pathway CULLIN-1 protein was also confirmed to be increased in folate nephropathy (Supplemental Figure 3).
Figure 1.
Increased kidney mRNA and protein expression of MAP3K14 in acute folate nephropathy and human AKI. Kidney mRNA levels were assessed by quantitative RT-PCR and protein levels by Western blot. (A) MAP3K14 mRNA, *p<0.01 versus vehicle. (B) MAP3K14 protein, *p<0.005 versus vehicle. (C) MAP3K14 immunohistochemistry. Increased MAP3K14 expression was localized to tubular cells in nephropathy samples from WT mice at 24 hours. Original magnification ×40; n=6 animals per group. (D) MAP3K14 expression in human kidney tissue. Immunohistochemistry was performed in human control and AKI tissue. Increased tubular cell immunostaining for MAP3K14 was observed in AKI. Original magnification ×20; detail ×100.
Figure 2.
Increased kidney RelB and NFκB2 expression and evidence for noncanonical NFκB activation in acute folate nephropathy. Kidney mRNA levels (A and C) were assessed by quantitative RT-PCR and protein levels by Western blot (B and D). (A) RelB mRNA, *p<0.01 versus vehicle. (B) RelB protein, *p<0.03 versus vehicle. (C) NFκB2 mRNA, *p<0.01 versus vehicle. (D) NFκB2 p100 and p52 proteins, representative Western blot. (E) NFκB2 p100 and p52 protein quantification, *p<0.03 and **p<0.05 versus vehicle. NFκB2 p100 is processed to NFκB p52 by the proteasome. (F) Increased nuclear DNA-binding activity of NFκB2 p52 and RelB in folate nephropathy. A DNA-binding ELISA was used to quantify DNA-binding activity of NFκB2 p52 and RelB in nuclei obtained from kidneys 24 hours after induction of folate nephropathy or vehicle administration. *p<0.01 versus vehicle; n=6 animals per group.
Given the poor understanding of its role in kidney injury and its upstream situation in the pathway, we further explored the role of MAP3K14 in folate nephropathy–associated AKI. In this regard, extensive MAP3K14 immunostaining was also observed in kidney tubules in human AKI (Figure 1D).
MAP3K14 Deficient Mice Were Protected from Folate Nephropathy and Cisplatin-Induced AKI
To explore the role of MAP3K14 in folate nephropathy, we used MAP3K14 activity–deficient alymphoplasia (MAP3K14aly/aly) mice, which carry a point mutation causing an amino acid substitution in the carboxy-terminal interaction domain of MAP3K14.23,24 MAP3K14+/aly heterozygote mice and MAP3K14+/+ mice were used as controls.
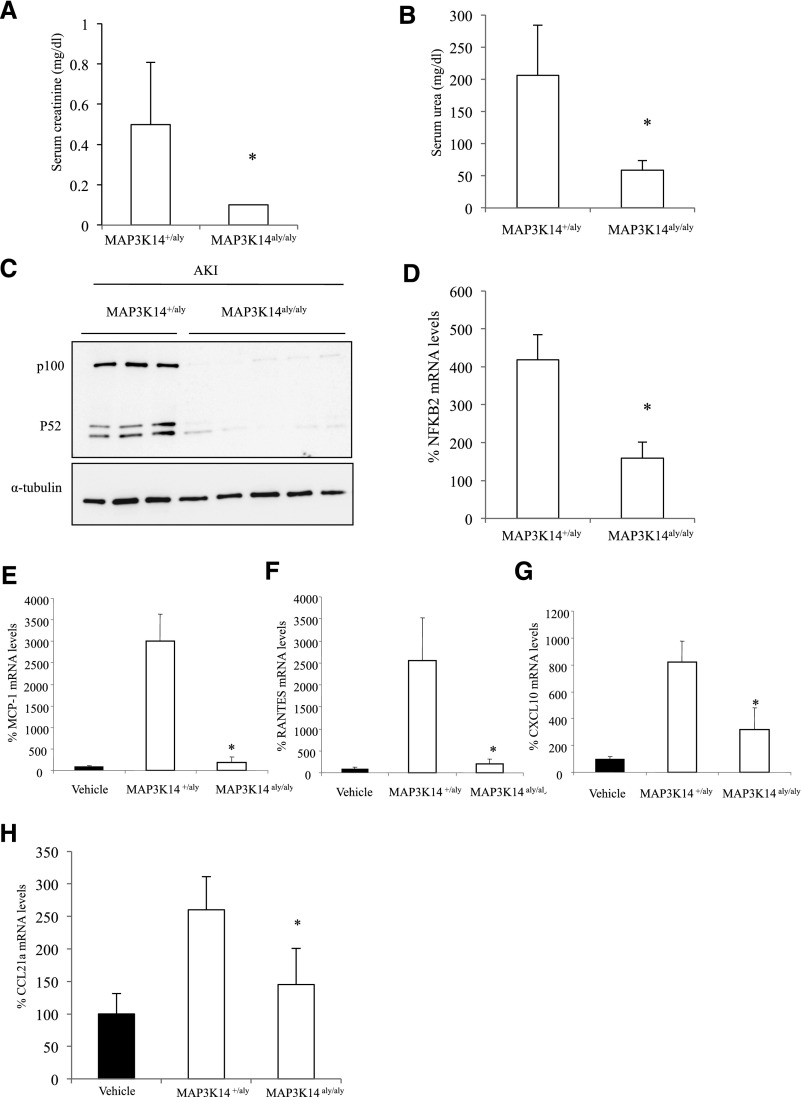
MAP3K14+/+ or MAP3K14+/aly heterozygote mice developed folate nephropathy characterized by increased serum creatinine and urea levels (Figure 3, A and B, Supplemental Figure 4) and increased kidney NFκB2 activation (Figure 3, C and D, Supplemental Figure 4), expression of chemokines (Figure 3, E–G, Supplemental Figure 4), and interstitial macrophages.
Figure 3.
MAP3K14 deficient mice were protected from folate nephropathy–associated AKI. (A) Serum creatinine, *p<0.02 versus heterozygous mice. (B) Serum urea, *p<0.001 versus heterozygous mice. (C) NFκB2 p100 and p52 proteins (representative Western blot). (D) NFκB2 mRNA, *p<0.01 versus heterozygous mice with folate nephropathy. (E) Decreased whole kidney MCP-1, (F) RANTES, and (G) CXCL10 mRNA expression in MAP3K14 deficient mice with folate nephropathy compared with heterozygous mice. *p<0.02 versus heterozygous mice with folate nephropathy. (H) CCL21a mRNA expression. Mean±SD of six mice per group at the 72-hour time-point; *p<0.03 versus heterozygous mice with folate nephropathy.
MAP3K14 deficient mice were protected from folate nephropathy. Serum creatinine and urea (Figure 3, A and B, Supplemental Figure 4) and kidney expression of NFκB2 p100/52 protein and mRNA (Figure 3, C and D, Supplemental Figure 4), MCP-1 (Figure 3E, Supplemental Figure 4), RANTES (Figure 3F, Supplemental Figure 4), CXCL10 (Figure 3G, Supplemental Figure 4), and Ccl21a mRNA expression (Figure 3H, Supplemental Figure 4) were lower than in control MAP3K14+/+ mice or MAP3K14+/aly heterozygote mice with folate nephropathy.
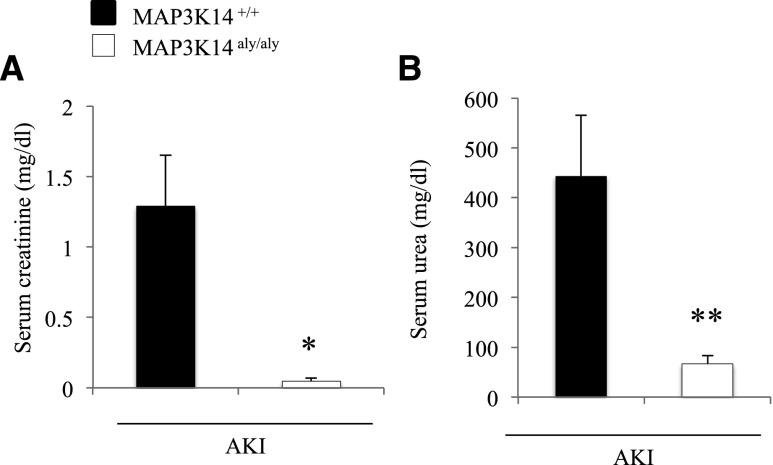
Finally, we tested a different model, cisplatin-induced AKI. An initial study at 72 hours resulted in 5/5 (100%) mortality in wild-type (WT) mice, whereas mortality in MAP3K14 deficient mice was 0/5 (0%). Next we studied the outcome at 48 hours. Mortality was 1/7 (14%) in WT mice and 0/5 (0%) in MAP3K14 deficient mice. Furthermore, renal function was preserved in MAP3K14 deficient mice as compared with WT mice (Figure 4), suggesting that mortality was due to severe AKI.
Figure 4.
MAP3K14 deficient mice were protected from experimental cisplatin-induced AKI. (A) Serum creatinine. (B) Serum urea. *p<0.02, **p<0.04 versus MAP3K14+/+ cisplatin-induced AKI mice. Mean±SD of 5–6 mice per group at the 48-hour time-point.
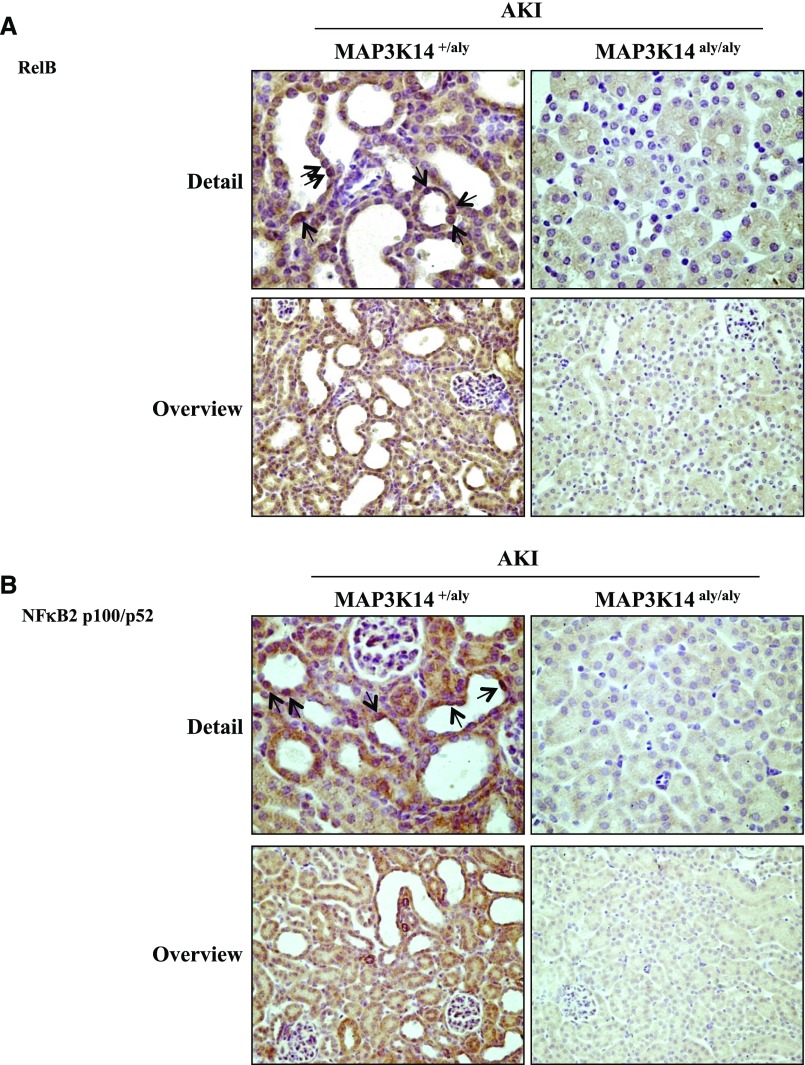
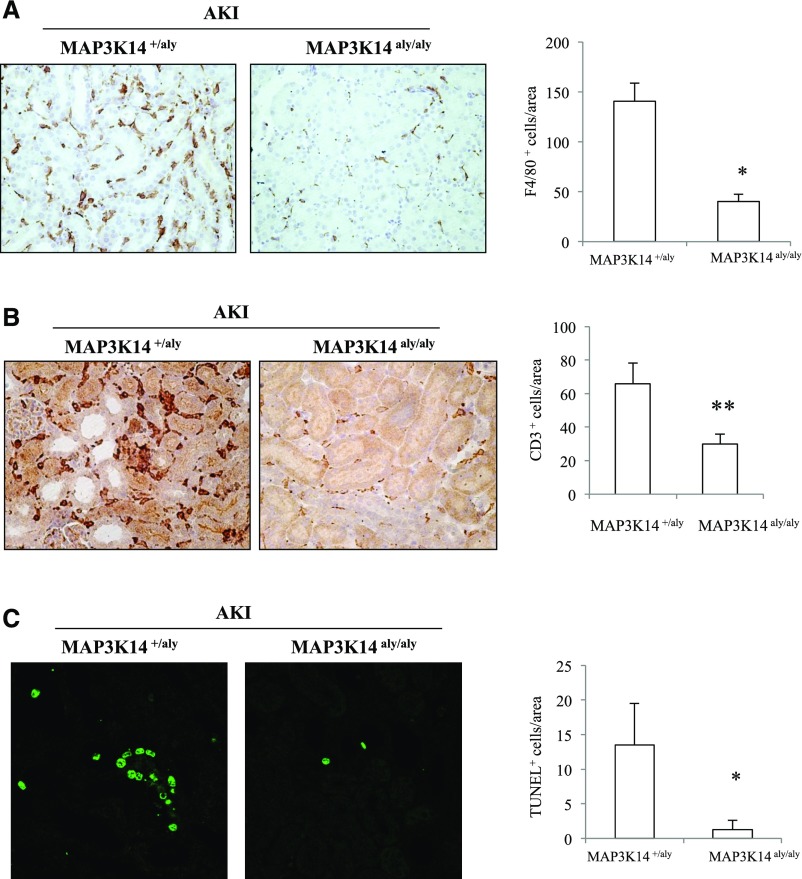
Immunohistochemistry confirmed the lack of RELB (Figure 5A) and NFκB2 P52 expression in MAP3K14 deficient mice with folate nephropathy (Figure 5B), and disclosed decreased F4/80 macrophages and CD3 T lymphocytes (Figure 6, A and B) and deoxynucleotidyl-transferase–mediated dUTP nick-end labeling (TUNEL) positive tubular cells representing dying cells (Figure 6C) in MAP3K14 deficient mice compared to heterozygous controls with folate nephropathy.
Figure 5.
MAP3K14 deficient mice are protected from tubular noncanonical NFκB pathway activation in acute folate nephropathy. (A) RelB and (B) p100/52 immunohistochemistry. Nuclear p52 is observed in renal tubules from heterozygous mice with folate nephropathy (arrows), whereas no staining was observed in MAP3K14 deficient mice with folate nephropathy. Immunohistochemistry does not discriminate between NFκB2 p100 and NFκB2 p52. However, Western blot shown in Figure 4C shows the presence of the active NFκB2 p52 protein. Images representative of six animals per group at the 72-hour time-point; original magnification ×40; detail ×400; n=6 animals per group.
Figure 6.
MAP3K14 deficient mice were protected from acute folate nephropathy–induced inflammation and cell death. (A) F4/80 macrophage and (B) CD3 immunohistochemistry. Macrophage infiltration is milder in MAP3K14 deficient mice with folate nephropathy than in heterozygous mice with folate nephropathy. * P<0.001; ** P<0.02; original magnification ×20. (C) TUNEL for fragmented DNA characteristic of apoptosis was frequently positive in tubular cells in heterozygous mice with folate nephropathy. The rate of apoptosis was lower in MAP3K14 deficient mice with folate nephropathy. * P<0.03; original magnification ×20; mean±SD of six mice per group at the 72-hour time-point.
We next induced folate nephropathy in bone marrow chimeras to test whether MAP3K14 deficiency in kidney cells or in bone marrow–derived cells was responsible for nephroprotection. Mice on a MAP3K14aly/aly background were protected from folate nephropathy–induced death when compared with MAP3K14 +/+ mice and this was independent of the bone marrow genotype (Supplemental Figure 5).
MAP3K14 Function in Tubular Cells
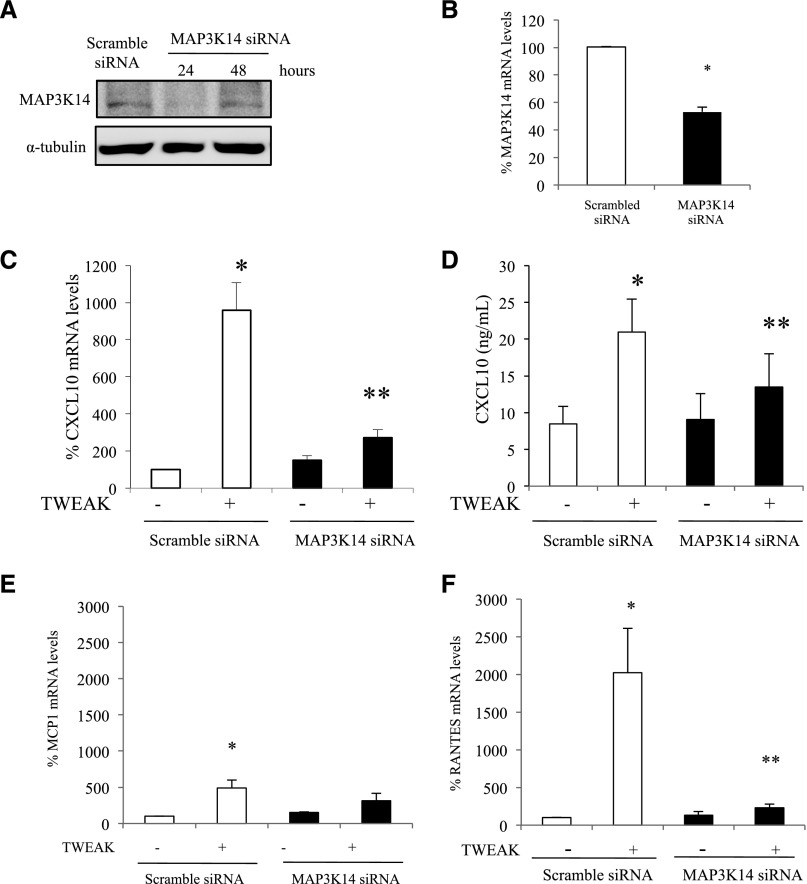
After the findings of MAP3K14 upregulation and of evidence for MAP3K14 activation (NFκB2 P100 processing to P52) in tubular cells in vivo, and a beneficial effect of MAP3K14 deficiency in vivo, the function of MAP3K14 was explored in cultured murine tubular epithelial cells by small interfering RNA (siRNA) targeting (Figure 7, A and B). Because KEGG pathway analysis had identified chemokine signaling and apoptosis as enriched pathways and MAP3K14 deficiency indeed resulted in lower inflammation and tubular cell death in vivo, we explored the potential regulation of chemokine secretion and cell death by MAP3K14 in tubular cells. For this we took advantage of TWEAK, the only cytokine characterized to date to activate the noncanonical NFκB pathway in tubular cells.25 In order to assess for further functions of MAP3K14 we explored canonical NFκB targets, including CXCL10, whose expression was recently related to MAP3K14 polymorphisms in human lymphoblastoid cells, but that had not previously been linked to MAP3K14 by functional studies.26 MAP3K14 silencing by specific siRNAs prevented TWEAK-induced upregulation of Cxcl10 mRNA (Figure 7C) and protein (Figure 7D) as well as of canonical NFκB targets such as Mcp1 and Rantes27 (Figure 7, E and F, Supplemental Figure 6). Differences in MCP-1 expression, which peaks earlier than RANTES, were more evident at earlier time points (Supplemental Figure 6). In this regard some genes are targeted by both canonical and noncanonical NFκB, whereas others such as CCL21 are specifically targeted by noncanonical NFκB activation in tubular and extrarenal cells.25,28,29
Figure 7.
Functional characterization of MAP3K14 actions on cultured proximal tubular cells: chemokine expression. (A) MAP3K14 siRNA silencing in cultured murine proximal tubular cells suppressed MAP3K14 protein expression. Representative Western blot. (B) MAP3K14 siRNA silencing in cultured murine proximal tubular cells suppressed MAP3K14 mRNA expression. (C) MAP3K14 siRNA silencing prevents CXCL10 mRNA upregulation induced by a 24-hour stimulation by the noncanonical NFκB activator TWEAK (100 ng/ml). qRT-PCR. *p<0.01 versus control; **p<0.01 versus TWEAK alone. (D) MAP3K14 siRNA silencing prevents the increase in culture supernatants of the CXCL10 chemokine induced by exposure for 24 hours to 100 ng/ml TWEAK (ELISA). *p<0.001 versus control; **p<0.01 versus TWEAK alone. (E) MAP3K14 siRNA silencing prevents MCP1 mRNA upregulation induced by the noncanonical NFκB activator TWEAK. qRT-PCR. *p<0.001 versus scrambled; **p<0.001 versus TWEAK alone. (F) MAP3K14 siRNA silencing prevents RANTES mRNA upregulation induced by TWEAK. qRT-PCR. *p<0.002 versus scrambled; **p<0.003 versus TWEAK alone. Cells were treated with scramble or MAP3K14 siRNA before addition of 100 ng/ml TWEAK for 24 hours; mean±SD of three independent experiments.
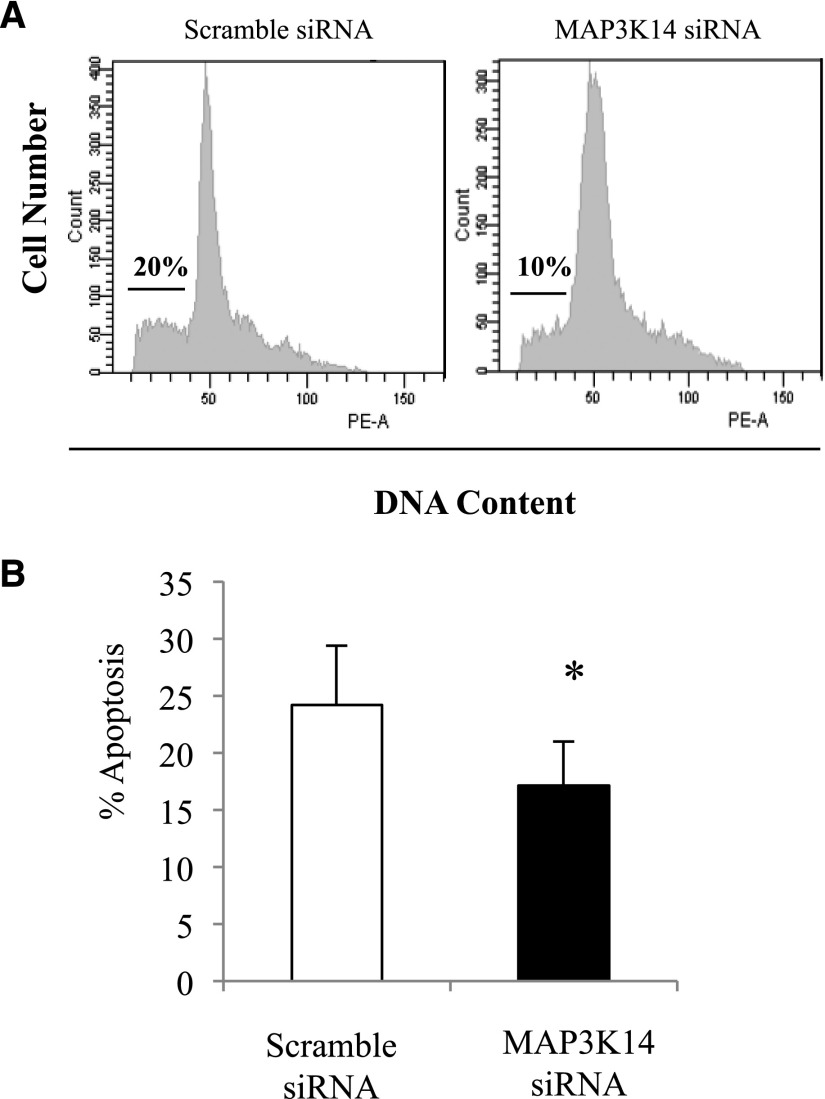
Deprivation of the survival factors from serum is a classic inducer of apoptosis.30 MAP3K14 targeting decreased apoptosis in tubular cells cultured in the absence of survival factors (Figure 8).
Figure 8.
Functional characterization of MAP3K14 actions on cultured proximal tubular cells: cell death. (A) MAP3K14 siRNA silencing decreases spontaneous apoptosis of serum-deprived tubular cells. Representative flow cytometry diagrams of cell DNA content. Hypodiploid cells consistent with apoptosis are indicated by a horizontal bar. (B) Quantification of hypodiploid apoptotic cells. *p<0.05 versus control; **p<0.03 versus TWEAK/TNFα/INFγ alone; mean±SD of three independent experiments.
Discussion
For the first time we have uncovered evidence that MAP3K14 is a therapeutic target in kidney injury. A nonbiased proteomics characterization of acute folate nephropathy kidneys disclosed enrichment for components of the noncanonical NFκB activation, chemokine, and apoptosis pathways. Further studies evidenced activation of the apical kinase of the noncanonical NFκB pathway, and showed that in kidney tubular cells MAP3K14 regulates the expression of chemokines not previously associated with noncanonical NFκB, such as CXCL10, and cell survival. In vivo MAP3K14 targeting protected from folate nephropathy and cisplatin-induced AKI, improving renal function and decreasing inflammation and tubular cell death.
NFκB is a family of structurally homologous proteins, including NFκB1, NFκB2, RELA, RELB, and C-REL, which form homo- or hetero-dimers that bind to κB enhancers in DNA to promote or inhibit gene transcription.16 Two main pathways for NFκB activation are known. Canonical NFκB activation is usually a rapid, protein synthesis–independent and transient response to a wide range of stimuli that involves proteasomal degradation of cytosolic IκB inhibitory proteins leading to the release of RELA/P50 and other dimers that then migrate to the nucleus. NFκB-driven IκBα resynthesis contributes to a fast turn-off of the response. By contrast, noncanonical NFκB activation requires MAP3K14 activation and NFκB2 P100 processing to P52 by the proteasome, resulting in a delayed nuclear translocation of RELB/P52 heterodimers and in prolonged activation of NFκB target genes.31–33 Increased transcription of NFκB2 and RELB may contribute to persistence of the response.34 In contrast to the canonical pathway, only a limited number of stimuli are known to activate the noncanonical NFκB pathway. These include advanced glycosylation end products33 and TNF receptor superfamily members such as lymphotoxin-β receptor, B-cell activating factor, CD40, receptor activator for NFκB, CD27, and the TWEAK receptor Fn14.20,25,35 None of these receptors or their ligands was identified in the proteomic analysis of kidney cortex. However, some of them had been previously shown to contribute to AKI. A literature search revealed a role in AKI for CD27 and TWEAK/Fn14.36–38 CD27 was localized to sloughed cells in tubular lumens postischemia and CD27-deficient mice were protected from AKI and tubular cell apoptosis.36,37 In contrast to this single report, multiple studies from several institutions have provided evidence for a role of TWEAK/FN14 in kidney injury.39 Furthermore, TWEAK targeting decreased the expression of the noncanonical NFκB target CCL21 in tubular cells.25 Thus for cell culture studies we chose TWEAK as a pathophysiologically relevant activator of the noncanonical NFκB pathway.
There is evidence for a role of canonical activation of NFκB in kidney injury.16 However, no therapeutic approach specifically targeting NFκB systemically is undergoing clinical trials, suggesting a fundamental lack of understanding of the system. In this regard, the role of MAP3K14 and noncanonical NFκB activation in AKI has not been well characterized. There is very little and scattered information on activation of this pathway in kidney disease. MAP3K14 was phosphorylated in tubular cells during kidney ischemia-reperfusion40 and levels were increased in experimental diabetic nephropathy and human delayed graft function.40,41 TWEAK-dependent nuclear translocation of RELB and p52 was observed in tubular cells in nephrotoxic AKI.25 In this report, kidney tissue proteomics identified upregulation of several proteins in the noncanonical NFκB pathway, upregulation of these proteins was confirmed and localized to tubular cells, the contribution of transcriptional regulation was identified, and the role of MAP3K14 in tubular cell injury was characterized. In this regard, MAP3K14 activity–deficient mice were protected from folate nephropathy and cisplatin-induced AKI. Thus, MAP3K14 represents a key regulated step promoting AKI that may potentially be subject to therapeutic manipulation, although at present there are no satisfactory MAP3K14 inhibitors.42
MAP3K14 is the essential upstream serine/threonine kinase of the noncanonical NFκB pathway that binds to TRAF2 and participates in NFκB signaling in response to the TNF superfamily and IL-1 receptors.22 MAP3K14 protein concentrations are low in quiescent cells as a result of rapid degradation. Cytokines and oxidative stress may increase MAP3K14 protein stability, leading to MAP3K14 activation.20 In addition to this universal mechanism of MAP3K14 regulation, we found increased steady-state Map3k14 mRNA levels to be an additional regulatory mechanism of MAP3K14 expression in tubular epithelium that also takes place in vivo during AKI. MAP3K14 induces IκB kinase-α–mediated phosphorylation of NFκB2 p100, a prerequisite for p100 ubiquitination and subsequent proteasomal processing to active NFκB2 p52.32
Ubiquitination is required for targeting of specific proteins to the proteasome. F-box proteins provide specificity for substrate recognition in the S-phase kinase associated protein 1-cullin 1-F-box protein (SCF) family of the Cullin-RING ligases E3 ubiquitin ligase superfamily.43 The F-box protein β-transducin repeat containing (β-TrCP; FBW1A) provides substrate specificity for MAP3K14-phosphorylated p100, allowing ubiquitination by SCFβ-TrCP.44–47 Efficient NFκB2 p100 ubiquitination requires UBA3 (ube1c) and UBE2M (UBC12), UBE2D3 (UBCH5c), and intact CULLIN-1 in SCFβ-TrCP.45 Interestingly, the UBE2M E2 ubiquitin conjugating enzyme and CULLIN-1 of the SCFβ-TrCP E3 ubiquitin ligase were found to be upregulated in folate nephropathy.
Evidence for MAP3K14 activation in vivo in folate nephropathy included increased MAP3K14 levels, NFκB p100 processing to NFκB p52, increased nuclear localization and DNA binding activity of P52/RELB, and decreased kidney inflammation and cell death and preserved renal function in MAP3K14 activity–deficient mice. Protection from folate nephropathy may depend on systemic MAP3K14 deficiency (e.g., leukocyte MAP3K14 deficiency) and/or kidney MAP3K14 deficiency. Bone marrow transplantation experiments results suggest that MAP3K14 deficiency in marrow-derived cells is not required for nephroprotection, and are consistent with, but do not confirm, the hypothesis that renal cell MAP3K14 targeting is important for nephroprotection. Alternatively, MAP3K14 targeting in additional, nonrenal, nonmarrow-derived cells may play a role. The fact that nonrenal cells also express MAP3K14 may result in undesired side effects when targeting MAP3K14 with small molecules. MAP3K14 and noncanonical NFκB gene targets have been characterized in the immune system, but there is little information on kidney cells.16 We now provide evidence of a role of MAP3K14 in the regulation of the inflammatory and cell death/proliferation responses in cultured tubular cells that together with the upregulation of MAP3K14 in tubular cells in folate nephropathy suggest at least a partial contribution of MAP3K14 targeting in tubular cells to the therapeutic responses.
The P52/RELB heterodimers characteristic of MAP3K14-initiated noncanonical NFκB activation share a number of gene targets with RELA-containing, classically activated NFκB complexes.16,29 Because canonical NFκB activation is an early transient response peaking at around 1–3 hours, whereas noncanonical NFκB activation is delayed and peaks at around 24 hours, noncanonical NFκB activation may contribute to sustained NFκB-dependent gene expression.16,28,48 In this regard, the CC genotype of the MAP3K14 SNP rs7222094 was recently associated with increased mortality and renal dysfunction in septic shock patients.26 CXCL10 was the gene with the greatest difference in expression between major and minor MAP3K14 genotypes. The rs7222094 genotype strongly associated with decreased CXCL10 levels in lymphoblastoid cell lines and in septic shock patients.26 Urinary CXCL10 is increased in AKI patients49,50 and, as shown here, in folate nephropathy kidney tissue. We now provide for the first time direct functional evidence that persistent CXCL10 expression in response to TWEAK is regulated by MAP3K14. CXCL10 (IP-10) had long been associated with kidney injury in animals and humans.51,52 MCP-1 and RANTES, which are targets of canonical NFκB activation,53 were also found to be MAP3K14-dependent in tubular cells. CCL21a was previously shown to be MAP3K14-dependent in this cell system.27 Thus, a wide spectrum of chemokines, commonly considered as both canonical NFκB targets and noncanonical NFκB targets, is regulated by MAP3K14 in cultured tubular cells and during folate nephropathy.
KEGG pathway analysis also disclosed apoptosis pathways as overrepresented in the folate nephropathy–associated AKI proteome. MAP3K14 had been identified as a cell death regulator in cancer cells. Indeed, Map3k14 siRNA targeting reduced serum deprivation–induced death in tubular cells. These results were consistent with decreased tubular cell apoptosis in vivo in MAP3K14 activity–deficient mice during folate nephropathy. These results are also consistent with observations targeting another component of the noncanonical NFκB pathway, RELB. Thus, RelB targeting by siRNA protected mice against lethal kidney ischemia,54 and in cultured proximal tubular cells knockdown of RELB abrogated the excess apoptosis induced by TNF in combination with cisplatin.55
In conclusion, preclinical functional studies in cell culture and in vivo identified MAP3K14 as a promising therapeutic target in kidney injury. In this regard MAP3K14 was upregulated during human kidney injury, suggesting that experimental findings may be applicable to the clinical settings. This information sets the stage for the exploration of the potential of MAP3K14 as a therapeutic target in humans.
Concise Methods
Animal Model
Studies were conducted in accord with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Acute folate nephropathy causes reversible increase in serum creatinine and urea, tubular cell death, compensatory tubular cell proliferation, activation of an inflammatory response, and eventual progression to mild fibrosis.27,56–58 Indeed, folic acid nephropathy has been reported in humans.59 C57/BL6 female mice (12- to 14-week-old) from the Instituto Investigacion Sanitaria-Fundacion Jimenez Diaz animal facilities received a single intraperitoneal (ip) injection of folic acid (Sigma-Aldrich, St. Louis, MO), 250 mg/kg in 0.3 mol/L sodium bicarbonate or vehicle, and were euthanized 24 hours or 72 hours after injection (n=6 per group). The kidneys were perfused in situ with cold saline before removal. One half-kidney from each mouse was fixed in buffered formalin, embedded in paraffin, and used for immunohistochemistry, and the other half was snap-frozen in liquid nitrogen for RNA and protein studies. The cortex from one kidney obtained 24 hours after folic acid or vehicle injection was carefully separated and snap-frozen for proteomics analysis.
To assess the role of MAP3K14 in folate nephropathy, MAP3K14aly/aly mice deficient in MAP3K14 and MAP3K14+/aly heterozygote or MAP3K14 +/+ littermate control mice received a single ip injection of folic acid (Sigma-Aldrich), 250 mg/kg in 0.3 mol/L sodium bicarbonate or vehicle, and were euthanized 72 hours after injection (n=5 per group). MAP3K14aly/aly mice deficient in MAP3K14 were from the Centro Biologia Molecular (Madrid, Spain) animal facilities.23
A different model of AKI was induced by the ip injection of a single dose of 25 mg/kg cisplatin (Sigma-Aldrich) dissolved in 0.9% saline solution. The cisplatin dose was based on literature analysis and results of preliminary experiments, showing renal function impairment at day three after cisplatin injection. MAP3K14aly/aly mice (n=10) and MAP3K14+/+ littermate control mice (n=12) were used in these experiments and sacrificed at 48 and 72 hours.
Generation of Bone Marrow Chimera
Recipient MAP3K14+/+ littermate control mice and MAP3K14aly/aly mice at age 6 weeks were γ-irradiated with two doses of 5 Gy for ablation of endogenous bone marrow cells. For bone marrow transplantation, bone marrow cells were isolated (donor) by flushing the femurs and tibias using a 25 g needle with DMEM (Invitrogen, Carlsbad, CA). After resuspension, bone marrow cells were centrifuged (300 × g, 5 minutes, 4 °C). After resuspension with ice-cold DMEM, bone marrow cells were filtered through a 35 μm filter. Irradiated recipient MAP3K14+/+ littermate control and MAP3K14aly/aly mice were injected intravenously with 4 × 106 donor bone marrow cells (in 100 μl per recipient) within 4 hours after the last irradiation dose. Eight weeks after bone marrow transplantation, bone marrow chimeric mice (four groups of five mice: recipient MAP3K14+/+ with donor MAP3K14+/+, recipient MAP3K14+/+ with donor MAP3K14aly/aly, recipient MAP3K14aly/aly with donor MAP3K14+/+, and recipient MAP3K14aly/aly with donor MAP3K14aly/aly) were subjected to folic acid nephropathy and euthanized at 72 hours.
Sample Preparation and Mass Spectrometry Analysis
Tissue samples were weighed out and extracted using the filter aided sample preparation method,60 as described previously.15 Briefly, tissue samples were homogenized in SDS-lysis buffer (1:10 sample to buffer ratio) (0.1 M Tris-HCl pH 7.6 supplemented with 4% SDS and 0.1 M DTT) using an Ultra-Turrax T 25 (IKA, Staufen, Germany), incubated at 95°C for 3 minutes, and clarified by centrifugation at 16,000 × g for 5 minutes at room temperature. An aliquot of the supernatant was taken and placed in a Micron YM-30 filter device (EMD Millipore, Watford, UK). Eight M urea buffer was added to the protein extract, centrifuged at 14,000 × g for 15 minutes, and then repeated. The protein extract was then mixed gently for 1 minute with 0.05 M iodoacetamide buffer and incubated for a further 20 minutes before centrifugation. Eight M urea buffer was again added and centrifuged (twice). Ammonium bicarbonate buffer (50 mM NH4HCO3, pH 8) was added and centrifuged (twice) before incubating overnight with trypsin. The trypsin homogenate was centrifuged and washed with the ammonium bicarbonate buffer before acidification with 10% formic acid. Sample volumes were adjusted to match final concentration of protein before analysis by LC-MS/MS.
Tissue extracts were separated on a Dionex Ultimate 3000 RSLS nano flow system (Dionex, Camberly, UK). A 5 µl sample was loaded in 0.1% formic acid and acetonitrile (98:2) onto a Dionex 100 µm × 2 cm, 5 µm C18 nano trap column at a flow rate of 5 µl/min. Elution was performed on an Acclaim PepMap C18 nano column 75 μm × 50 cm, 2 μm, 100 Å with a linear gradient of solvent A, 0.1% formic acid and acetonitrile (98:2), against solvent B, 0.1% formic acid and acetonitrile (20:80), starting at 1% B for 5 minutes rising to 30% at 400 minutes then to 50% B at 480 minutes. The sample was ionized in positive ion mode using a Proxeon nano spray ESI source (Thermo Fisher Scientific, Vernon Hills, IL) and analyzed in an Orbitrap Velos FTMS (Thermo Finnigan, Bremen, Germany). The MS was operated in a data-dependent mode (top 40) to switch between MS and MS/MS acquisition and parent ions were fragmented by collision-induced dissociation. Data files were searched against the IPI mouse nonredundant database using SEQUEST with enzyme specified as trypsin. A fixed modification of carbamidomethylation was set and oxidation of methionine and proline as variable modifications were selected. Mass error windows of 20 ppm and 0.8 Da were allowed for MS and MS/MS, respectively. In SEQUEST, only peptides that showed mass deviation of less than 10 ppm were passed, and the peptide data were extracted using high peptide confidence and top one peptide rank filters. Statistical P value analysis was performed using the Mann–Whitney U test.
Bioinformatics Analysis
Protein identification and a significant dataset of 1480 entries with P values <0.05 and fold changes of >2 have been previously described.15 This dataset was used for metabolic and signaling pathway analysis using the KEGG web-resource (www.genome.jp/kegg-bin/) or with PathVisio (www.pathvisio.org). Focused data mining was then amplified to all molecules with a P value ≤0.05.
Cells and Reagents
MCT cells are a cultured line of proximal tubular epithelial cells harvested originally from the renal cortex of SJL mice and have been extensively characterized.61 MCT cells were cultured in RPMI 1640 (Gibco, Carlsbad, CA), 10% decomplemented FBS, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin, in 5% CO2 at 37°C.61 Recombinant human soluble TWEAK (EMD Millipore, Billerica, MA) was used at 100 ng/ml.
Western Blot Analysis
Tissue and cell samples were homogenized in lysis buffer62 then separated by 10% or 12% SDS-PAGE under reducing conditions and transferred to PVDF membranes (EMD Millipore, Bedford, MA), blocked with 5% skimmed milk in PBS/0.5% v/v Tween 20 for 1 hour, and washed with PBS/Tween. Primary antibodies were rabbit polyclonal anti-p100/52 (1:500; Cell Signaling Technology, Danvers, MA), anti-RelB (1:500; Santa Cruz Biotechnology, Santa Cruz, CA), anti-MAP3K14 (1:1000; Cell Signaling Technology), anti-Cyclin D1 (1:1000; Cell Signaling Technology) and anti–cullin-1 (1:500; Santa Cruz Biotechnology). Antibodies were diluted in 5% milk PBS/Tween. Blots were washed with PBS/Tween and subsequently incubated with appropriate horseradish peroxidase–conjugated secondary antibody (1:2000; GE Healthcare, Waukesha, WI). After washing, the blots were developed with the chemiluminescence method. Blots were then reprobed with monoclonal anti-mouse α-tubulin antibody (1:2000; Sigma-Aldrich) and levels of expression were corrected for minor differences in loading.
Quantitative RT-PCR
One µg RNA isolated by Trizol (Invitrogen) was reverse transcribed with High Capacity cDNA Archive Kit and real-time PCR was performed on an ABI Prism 7500 PCR system (Applied Biosystems, Foster City, CA) using the DeltaDelta Ct method.63 Expression levels are given as ratios to GAPDH. Predeveloped primer and probe assays were from Applied Biosystems, Foster City, CA.
Immunohistochemistry
Immunohistochemistry was carried out as previously described on paraffin-embedded 5 µm thick tissue sections.62 Primary antibodies were rabbit polyclonal anti-RelB (1:50; Santa Cruz Biotechnology), anti-NFκB2 p100/p52 (1:20; Santa Cruz Biotechnology), anti-MAP3K14 (1:100; Cell Signaling Technology, Danvers, MA), rat polyclonal anti-F4/80 antigen (1:50; Serotec, Oxford, UK), rabbit monoclonal anti-CD3 (1:100; Dako, Denmark), and anti–Cullin-1 (1:80; Santa Cruz Biotechnology). Sections were counterstained with Carazzi hematoxylin. Negative controls included incubation with a nonspecific immunoglobulin of the same isotype as the primary antibody.
Apoptosis was assayed by TUNEL (In Situ Cell Death Detection Kit; Roche, Basel, Switzerland) according to the manufacturer’s instructions.63
For human kidney immunohistochemistry, control kidney tissue from nephrectomy specimens (n=4) and AKI tissue (n=7) diagnosed as “acute tubular necrosis” was studied. Mean age was 36.4±18.6 years, four patients were females, and serum creatinine ranged from 1.7 to 10.0 mg/dl (5.7±3.5 mg/dl). Immunohistochemistry was performed as described above by using anti-human MAP3K14 from Abcam.
Transfection with Small Interfering RNA
Cells were grown in six-well plates (Costar, Cambridge, MA) and transfected with a mixture of 20 nmol/ml MAP3K14 siRNA (Santa Cruz Biotechnology), Opti-MEM I Reduced Serum Medium, and Lipofectamine 2000 (Invitrogen).64 After 18 hours, cells were washed and cultured for 6 hours in complete medium, and serum-depleted for 24 hours before addition of stimulus. This time point was selected from a time-course of decreasing MAP3K14 protein expression in response to siRNA. A negative control scrambled siRNA provided by the manufacturer did not reduce MAP3K14 protein.
Cell Death and Apoptosis
Cells were cultured to subconfluence in six-well plates and transfected with MAP3K14 siRNA as previously described.65 Apoptosis was assessed by flow cytometry of DNA content. For assessment of the cell cycle and apoptosis, adherent cells were pooled with spontaneously detached cells, and stained in 100 µg/ml propidium iodide, 0.05% NP-40, 10 µg/mL RNAse A in PBS at 4°C for >1 hour. This assay permeabilizes the cells. Permeabilization allows entry of propidium iodide into all cells, dead or alive. Apoptotic cells are characterized by a lower DNA content (hypodiploid cells) because of nuclear fragmentation. Thus, this assay is not based on the known ability of propidium iodide to enter dead cells. The percentage of apoptotic cells with decreased DNA content (Ao) was counted.30
ELISA
Cells were transfected with MAP3K14 siRNA and stimulated with 100 ng/ml TWEAK. Murine CxCL10 in the supernatants was determined by ELISA (BD Pharmingen, San Diego, CA).
NFκB DNA-Binding Activity
RelB and NFκB2 p52 subunits in nuclear extracts from kidney tissue were assessed by their binding to an oligonucleotide containing the NFκB consensus site using TransAM NFκB Family Kit (Active Motif, Carlsbad, CA).
Statistics
Statistical analysis was performed using SPSS 11.0 statistical software (IBM, White Plains, NY). Results are expressed as mean±SD. Significance at the P<0.05 level was assessed by t test for two groups of data and ANOVA for three or more groups.
Disclosures
H.M. is the cofounder and co-owner of Mosaiques Diagnostics (Hannover, Germany).
Supplementary Material
Acknowledgments
Thanks to Beatriz Barrocal, Dr. Daniel Carpio, and M. Eugenia Burgos for their technical help.
Grant support: Fondo Europeo de Desarrollo Regional funds and Fondo de Investigacion en Salud Instituto de Salud Carlos III PI13/00047, PI15/00298, CP14/00133, CP12/03262, Red Tematica de Investigacion Cooperativa en Salud, Red de Investigacion Renal RD12/0021, European Uremic Toxin Work Group, Sociedad Española de Nefrologia, Comunidad de Madrid (CIFRA S2010/BMD-2378), Fondecyt 1160465. Salary support: FIS Miguel Servet to M.D.S.-N., Programa Intensificación Actividad Investigadora (ISCIII/Agencia Laín-Entralgo/CM) to A.O. The research presented in this manuscript was supported in part by the FP7 programs “Improvement of tools and portability of MS-based clinical proteomics as applied to chronic kidney disease” (Protoclin, PEOPLE-2009-IAPP, GA 251368), “Clinical and system –omics for the identification of the Molecular Determinants of established Chronic Kidney Disease” (iMODE-CKD, PEOPLE-ITN-GA-2013-608332) and “Systems biology towards novel chronic kidney disease diagnosis and treatment” (SysKID HEALTH–F2–2009–241544).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015080898/-/DCSupplemental.
References
- 1.Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, Liu KD, Mehta RL, Pannu N, Van Biesen W, Vanholder R: Acute kidney injury: an increasing global concern. Lancet 382: 170–179, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Bellomo R, Kellum JA, Ronco C: Acute kidney injury. Lancet 380: 756–766, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Fliser D, Laville M, Covic A, Fouque D, Vanholder R, Juillard L, Van Biesen W; Ad-hoc working group of ERBP : A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transplant 27: 4263–4272, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanmassenhove J, Vanholder R, Nagler E, Van Biesen W: Urinary and serum biomarkers for the diagnosis of acute kidney injury: an in-depth review of the literature. Nephrol Dial Transplant 28: 254–273, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Akcay A, Nguyen Q, Edelstein CL: Mediators of inflammation in acute kidney injury. Mediators Inflamm 2009: 137072, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sánchez-González PD, López-Hernández FJ, López-Novoa JM, Morales AI: An integrative view of the pathophysiological events leading to cisplatin nephrotoxicity. Crit Rev Toxicol 41: 803–821, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Wen X, Murugan R, Peng Z, Kellum JA: Pathophysiology of acute kidney injury: a new perspective. Contrib Nephrol 165: 39–45, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Hotta K, Sho M, Yamato I, Shimada K, Harada H, Akahori T, Nakamura S, Konishi N, Yagita H, Nonomura K, Nakajima Y: Direct targeting of fibroblast growth factor-inducible 14 protein protects against renal ischemia reperfusion injury. Kidney Int 79: 179–188, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Xu C, Chang A, Hack BK, Eadon MT, Alper SL, Cunningham PN: TNF-mediated damage to glomerular endothelium is an important determinant of acute kidney injury in sepsis. Kidney Int 85: 72–81, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Y, Denner L, Haidacher SJ, LeJeune WS, Tilton RG: Comprehensive analysis of the mouse renal cortex using two-dimensional HPLC - tandem mass spectrometry. Proteome Sci 6: 15, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Yan S, Ji C, Dai W, Hu W, Zhang W, Mei C: Metabolomic changes and protective effect of (L)-carnitine in rat kidney ischemia/reperfusion injury. Kidney Blood Press Res 35: 373–381, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Zgoda-Pols JR, Chowdhury S, Wirth M, Milburn MV, Alexander DC, Alton KB: Metabolomics analysis reveals elevation of 3-indoxyl sulfate in plasma and brain during chemically-induced acute kidney injury in mice: investigation of nicotinic acid receptor agonists. Toxicol Appl Pharmacol 255: 48–56, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Prasain JK, Arabshahi A, Taub PR, Sweeney S, Moore R, Sharer JD, Barnes S: Simultaneous quantification of F2-isoprostanes and prostaglandins in human urine by liquid chromatography tandem-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 913-914: 161–168, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beger RD, Holland RD, Sun J, Schnackenberg LK, Moore PC, Dent CL, Devarajan P, Portilla D: Metabonomics of acute kidney injury in children after cardiac surgery. Pediatr Nephrol 23: 977–984, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Husi H, Sanchez-Niño MD, Delles C, Mullen W, Vlahou A, Ortiz A, Mischak H: A combinatorial approach of Proteomics and Systems Biology in unravelling the mechanisms of acute kidney injury (AKI): involvement of NMDA receptor GRIN1 in murine AKI. BMC Syst Biol 7: 110, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanz AB, Sanchez-Niño MD, Ramos AM, Moreno JA, Santamaria B, Ruiz-Ortega M, Egido J, Ortiz A: NF-kappaB in renal inflammation. J Am Soc Nephrol 21: 1254–1262, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Hoesel B, Schmid JA: The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer 12: 86, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreno JA, Izquierdo MC, Sanchez-Niño MD, Suárez-Alvarez B, Lopez-Larrea C, Jakubowski A, Blanco J, Ramirez R, Selgas R, Ruiz-Ortega M, Egido J, Ortiz A, Sanz AB: The inflammatory cytokines TWEAK and TNFα reduce renal klotho expression through NFκB. J Am Soc Nephrol 22: 1315–1325, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiggins JE, Patel SR, Shedden KA, Goyal M, Wharram BL, Martini S, Kretzler M, Wiggins RC: NFkappaB promotes inflammation, coagulation, and fibrosis in the aging glomerulus. J Am Soc Nephrol 21: 587–597, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun SC: The noncanonical NF-κB pathway. Immunol Rev 246: 125–140, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Razani B, Reichardt AD, Cheng G: Non-canonical NF-κB signaling activation and regulation: principles and perspectives. Immunol Rev 244: 44–54, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Thu YM, Richmond A: NF-κB inducing kinase: a key regulator in the immune system and in cancer. Cytokine Growth Factor Rev 21: 213–226, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sánchez-Valdepeñas C, Martín AG, Ramakrishnan P, Wallach D, Fresno M: NF-kappaB-inducing kinase is involved in the activation of the CD28 responsive element through phosphorylation of c-Rel and regulation of its transactivating activity. J Immunol 176: 4666–4674, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Shinkura R, Kitada K, Matsuda F, Tashiro K, Ikuta K, Suzuki M, Kogishi K, Serikawa T, Honjo T: Alymphoplasia is caused by a point mutation in the mouse gene encoding Nf-kappa b-inducing kinase. Nat Genet 22: 74–77, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Sanz AB, Sanchez-Niño MD, Izquierdo MC, Jakubowski A, Justo P, Blanco-Colio LM, Ruiz-Ortega M, Selgas R, Egido J, Ortiz A: TWEAK activates the non-canonical NFkappaB pathway in murine renal tubular cells: modulation of CCL21. PLoS One 5: e8955, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thair SA, Walley KR, Nakada TA, McConechy MK, Boyd JH, Wellman H, Russell JA: A single nucleotide polymorphism in NF-κB inducing kinase is associated with mortality in septic shock. J Immunol 186: 2321–2328, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Sanz AB, Justo P, Sanchez-Niño MD, Blanco-Colio LM, Winkles JA, Kreztler M, Jakubowski A, Blanco J, Egido J, Ruiz-Ortega M, Ortiz A: The cytokine TWEAK modulates renal tubulointerstitial inflammation. J Am Soc Nephrol 19: 695–703, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonizzi G, Bebien M, Otero DC, Johnson-Vroom KE, Cao Y, Vu D, Jegga AG, Aronow BJ, Ghosh G, Rickert RC, Karin M: Activation of IKKalpha target genes depends on recognition of specific kappaB binding sites by RelB:p52 dimers. EMBO J 23: 4202–4210, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffmann A, Baltimore D: Circuitry of nuclear factor kappaB signaling. Immunol Rev 210: 171–186, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Niño MD, Sanz AB, Lorz C, Gnirke A, Rastaldi MP, Nair V, Egido J, Ruiz-Ortega M, Kretzler M, Ortiz A: BASP1 promotes apoptosis in diabetic nephropathy. J Am Soc Nephrol 21: 610–621, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Senftleben U, Cao Y, Xiao G, Greten FR, Krähn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, Karin M: Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science 293: 1495–1499, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Xiao G, Harhaj EW, Sun SC: NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol Cell 7: 401–409, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Zhao Y, Banerjee S, LeJeune WS, Choudhary S, Tilton RG: NF-κB-inducing kinase increases renal tubule epithelial inflammation associated with diabetes. Exp Diabetes Res 2011: 192564, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mordmüller B, Krappmann D, Esen M, Wegener E, Scheidereit C: Lymphotoxin and lipopolysaccharide induce NF-kappaB-p52 generation by a co-translational mechanism. EMBO Rep 4: 82–87, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saitoh T, Nakayama M, Nakano H, Yagita H, Yamamoto N, Yamaoka S: TWEAK induces NF-kappaB2 p100 processing and long lasting NF-kappaB activation. J Biol Chem 278: 36005–36012, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Padanilam BJ, Lewington AJ, Hammerman MR: Expression of CD27 and ischemia/reperfusion-induced expression of its ligand Siva in rat kidneys. Kidney Int 54: 1967–1975, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Singaravelu K, Padanilam BJ: p53 target Siva regulates apoptosis in ischemic kidneys. Am J Physiol Renal Physiol 300: F1130–F1141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanz AB, Izquierdo MC, Sanchez-Niño MD, Ucero AC, Egido J, Ruiz-Ortega M, Ramos AM, Putterman C, Ortiz A: TWEAK and the progression of renal disease: clinical translation. Nephrol Dial Transplant 29[Suppl 1]: i54–i62, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanz AB, Sanchez-Niño MD, Ortiz A: TWEAK, a multifunctional cytokine in kidney injury. Kidney Int 80: 708–718, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Loverre A, Ditonno P, Crovace A, Gesualdo L, Ranieri E, Pontrelli P, Stallone G, Infante B, Schena A, Di Paolo S, Capobianco C, Ursi M, Palazzo S, Battaglia M, Selvaggi FP, Schena FP, Grandaliano G: Ischemia-reperfusion induces glomerular and tubular activation of proinflammatory and antiapoptotic pathways: differential modulation by rapamycin. J Am Soc Nephrol 15: 2675–2686, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Starkey JM, Haidacher SJ, LeJeune WS, Zhang X, Tieu BC, Choudhary S, Brasier AR, Denner LA, Tilton RG: Diabetes-induced activation of canonical and noncanonical nuclear factor-kappaB pathways in renal cortex. Diabetes 55: 1252–1259, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Li K, McGee LR, Fisher B, Sudom A, Liu J, Rubenstein SM, Anwer MK, Cushing TD, Shin Y, Ayres M, Lee F, Eksterowicz J, Faulder P, Waszkowycz B, Plotnikova O, Farrelly E, Xiao SH, Chen G, Wang Z: Inhibiting NF-κB-inducing kinase (NIK): discovery, structure-based design, synthesis, structure-activity relationship, and co-crystal structures. Bioorg Med Chem Lett 23: 1238–1244, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Lee EK, Diehl JA: SCFs in the new millennium. Oncogene 33: 2011–2018, 2014 [DOI] [PubMed] [Google Scholar]
- 44.Fong A, Sun SC: Genetic evidence for the essential role of beta-transducin repeat-containing protein in the inducible processing of NF-kappa B2/p100. J Biol Chem 277: 22111–22114, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Amir RE, Haecker H, Karin M, Ciechanover A: Mechanism of processing of the NF-kappa B2 p100 precursor: identification of the specific polyubiquitin chain-anchoring lysine residue and analysis of the role of NEDD8-modification on the SCF(beta-TrCP) ubiquitin ligase. Oncogene 23: 2540–2547, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Arabi A, Ullah K, Branca RM, Johansson J, Bandarra D, Haneklaus M, Fu J, Ariës I, Nilsson P, Den Boer ML, Pokrovskaja K, Grandér D, Xiao G, Rocha S, Lehtiö J, Sangfelt O: Proteomic screen reveals Fbw7 as a modulator of the NF-κB pathway. Nat Commun 3: 976, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Busino L, Millman SE, Pagano M: SCF-mediated degradation of p100 (NF-κB2): mechanisms and relevance in multiple myeloma. Sci Signal 5: pt14, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fusco AJ, Huang DB, Miller D, Wang VY, Vu D, Ghosh G: NF-kappaB p52:RelB heterodimer recognizes two classes of kappaB sites with two distinct modes. EMBO Rep 10: 152–159, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaidya VS, Waikar SS, Ferguson MA, Collings FB, Sunderland K, Gioules C, Bradwin G, Matsouaka R, Betensky RA, Curhan GC, Bonventre JV: Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci 1: 200–208, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ho J, Lucy M, Krokhin O, Hayglass K, Pascoe E, Darroch G, Rush D, Nickerson P, Rigatto C, Reslerova M: Mass spectrometry-based proteomic analysis of urine in acute kidney injury following cardiopulmonary bypass: a nested case-control study. Am J Kidney Dis 53: 584–595, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Gómez-Chiarri M, Ortiz A, González-Cuadrado S, Serón D, Emancipator SN, Hamilton TA, Barat A, Plaza JJ, González E, Egido J: Interferon-inducible protein-10 is highly expressed in rats with experimental nephrosis. Am J Pathol 148: 301–311, 1996 [PMC free article] [PubMed] [Google Scholar]
- 52.Suthanthiran M, Schwartz JE, Ding R, Abecassis M, Dadhania D, Samstein B, Knechtle SJ, Friedewald J, Becker YT, Sharma VK, Williams NM, Chang CS, Hoang C, Muthukumar T, August P, Keslar KS, Fairchild RL, Hricik DE, Heeger PS, Han L, Liu J, Riggs M, Ikle DN, Bridges ND, Shaked A; Clinical Trials in Organ Transplantation 04 (CTOT-04) Study Investigators : Urinary-cell mRNA profile and acute cellular rejection in kidney allografts. N Engl J Med 369: 20–31, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoffmann A, Levchenko A, Scott ML, Baltimore D: The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science 298: 1241–1245, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Feng B, Chen G, Zheng X, Sun H, Zhang X, Zhang ZX, Xiang Y, Ichim TE, Garcia B, Luke P, Jevnikar AM, Min WP: Small interfering RNA targeting RelB protects against renal ischemia-reperfusion injury. Transplantation 87: 1283–1289, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Benedetti G, Fokkelman M, Yan K, Fredriksson L, Herpers B, Meerman J, van de Water B, de Graauw M: The nuclear factor κB family member RelB facilitates apoptosis of renal epithelial cells caused by cisplatin/tumor necrosis factor α synergy by suppressing an epithelial to mesenchymal transition-like phenotypic switch. Mol Pharmacol 84: 128–138, 2013 [DOI] [PubMed] [Google Scholar]
- 56.Fang TC, Alison MR, Cook HT, Jeffery R, Wright NA, Poulsom R: Proliferation of bone marrow-derived cells contributes to regeneration after folic acid-induced acute tubular injury. J Am Soc Nephrol 16: 1723–1732, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Doi K, Okamoto K, Negishi K, Suzuki Y, Nakao A, Fujita T, Toda A, Yokomizo T, Kita Y, Kihara Y, Ishii S, Shimizu T, Noiri E: Attenuation of folic acid-induced renal inflammatory injury in platelet-activating factor receptor-deficient mice. Am J Pathol 168: 1413–1424, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ortega A, Rámila D, Ardura JA, Esteban V, Ruiz-Ortega M, Barat A, Gazapo R, Bosch RJ, Esbrit P: Role of parathyroid hormone-related protein in tubulointerstitial apoptosis and fibrosis after folic acid-induced nephrotoxicity. J Am Soc Nephrol 17: 1594–1603, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Metz-Kurschel U, Kurschel E, Wagner K, Aulbert E, Graben N, Philipp T: Folate nephropathy occurring during cytotoxic chemotherapy with high-dose folinic acid and 5-fluorouracil. Ren Fail 12: 93–97, 1990 [DOI] [PubMed] [Google Scholar]
- 60.Wiśniewski JR, Zougman A, Mann M: Combination of FASP and StageTip-based fractionation allows in-depth analysis of the hippocampal membrane proteome. J Proteome Res 8: 5674–5678, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Haverty TP, Kelly CJ, Hines WH, Amenta PS, Watanabe M, Harper RA, Kefalides NA, Neilson EG: Characterization of a renal tubular epithelial cell line which secretes the autologous target antigen of autoimmune experimental interstitial nephritis. J Cell Biol 107: 1359–1368, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanchez-Niño MD, Poveda J, Sanz AB, Mezzano S, Carrasco S, Fernandez-Fernandez B, Burkly LC, Nair V, Kretzler M, Hodgin JB, Ruiz-Ortega M, Selgas R, Egido J, Ortiz A: Fn14 in podocytes and proteinuric kidney disease. Biochim Biophys Acta 1832: 2232–2243, 2013 [DOI] [PubMed] [Google Scholar]
- 63.Sanchez-Niño MD, Bozic M, Córdoba-Lanús E, Valcheva P, Gracia O, Ibarz M, Fernandez E, Navarro-Gonzalez JF, Ortiz A, Valdivielso JM: Beyond proteinuria: VDR activation reduces renal inflammation in experimental diabetic nephropathy. Am J Physiol Renal Physiol 302: F647–F657, 2012 [DOI] [PubMed] [Google Scholar]
- 64.Sanchez-Niño MD, Sanz AB, Sanchez-Lopez E, Ruiz-Ortega M, Benito-Martin A, Saleem MA, Mathieson PW, Mezzano S, Egido J, Ortiz A: HSP27/HSPB1 as an adaptive podocyte antiapoptotic protein activated by high glucose and angiotensin II. Lab Invest 92: 32–45, 2012 [DOI] [PubMed] [Google Scholar]
- 65.Sanz AB, Sanchez-Niño MD, Izquierdo MC, Jakubowski A, Justo P, Blanco-Colio LM, Ruiz-Ortega M, Egido J, Ortiz A: Tweak induces proliferation in renal tubular epithelium: a role in uninephrectomy induced renal hyperplasia. J Cell Mol Med 13[9B]: 3329–3342, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.