Abstract
Bardet–Biedl syndrome is a rare autosomal recessive, multisystem disease characterized by retinal dystrophy, renal malformation, obesity, intellectual disability, polydactyly, and hypogonadism. Nineteen disease-causing genes (BBS1–19) have been identified, of which mutations in BBS1 are most common in North America and Europe. A hallmark of the disease, renal malformation is heterogeneous and is a cause of morbidity and mortality through the development of CKD. We studied the prevalence and severity of CKD in 350 patients with Bardet–Biedl syndrome–related renal disease attending the United Kingdom national Bardet–Biedl syndrome clinics to further elucidate the phenotype and identify risk indicators of CKD. Overall, 31% of children and 42% of adults had CKD; 6% of children and 8% of adults had stage 4–5 CKD. In children, renal disease was often detected within the first year of life. Analysis of the most commonly mutated disease-associated genes revealed that, compared with two truncating mutations, two missense mutations associated with less severe CKD in adults. Moreover, compared with mutations in BBS10, mutations in BBS1 associated with less severe CKD or lack of CKD in adults. Finally, 51% of patients with available ultrasounds had structural renal abnormalities, and 35% of adults were hypertensive. The presence of structural abnormalities or antihypertensive medication also correlated statistically with stage 3b–5 CKD. This study describes the largest reported cohort of patients with renal disease in Bardet–Biedl syndrome and identifies risk factors to be considered in genetic counseling.
Keywords: genetic renal disease, ESRD, human genetics, risk factors
Bardet–Biedl syndrome (BBS) is a rare autosomal recessive ciliopathy characterized by rod-cone dystrophy, renal malformations, learning difficulties, obesity, postaxial polydactyly, and hypogonadism.1 Nineteen disease-causing genes have been identified (BBS1–BBS19) in the last two decades. BBS genes code for proteins that localize to the cilia or the basal body and are thought to be involved in cilia development and maintenance.2 Mutations in BBS genes lead to defective cilia. Sequencing of known disease-causing genes confirms a clinical diagnosis of BBS in around 80% of patients.2 Variable expressivity is a hallmark of BBS and both inter- and intrafamilial phenotypic variation is observed.2
Structural renal and urinary tract anomalies and renal dysfunction is a cause of considerable morbidity and reported to affect 53%–82% of patients with BBS.2–6 The primary renal phenotype is highly variable, ranging from cystic tubular disease, dysplastic renal disease, and FSGS to concentrating defects.3–7 Lower urinary tract dysfunction is observed in many patients and may have upper renal tract sequelae.8 It is thought that ciliary dysfunction leads to disturbance of the noncanonical Wnt-signaling pathway which may contribute to the development of cystic kidney disease classically associated with BBS.2 Secondary renal disease may occur as a consequence of hypertension and diabetes, both of which are frequently observed in this population.
The high frequency of renal disease in BBS is a cause of great anxiety among patients because of the devastating effect this can have on quality of life, morbidity, and mortality.4–6 This study examines renal disease in the largest reported cohort of patients with BBS and identifies indicators of disease that are directly relevant to patient management and clinical stratification.
Results
Overview of the BBS Population
Three hundred fifty patients attended the adult and pediatric national BBS clinics in Birmingham and London over a 4-year period (2010–2014). The patient population ranged in age from birth to 60 years old, with a peak frequency in the 6–10 years-of-age category, and with few older adults.
Diagnosis was on the basis of clinical phenotyping. All patients underwent genetic testing. On sequencing 13 disease-related genes, molecular confirmation of the diagnosis was achieved in 80% of all pedigrees (216 of 270) and in 77% of all patients (265 of 350). A full list of genotypes can be found in Supplemental Table 1. The sex distribution was 54% male and 46% female. Sixty-nine percent of patients were white, 28% were Southeast Asian, and the remaining 3% had a mixture of other backgrounds (black, Chinese, and Ashkenazi Jewish).
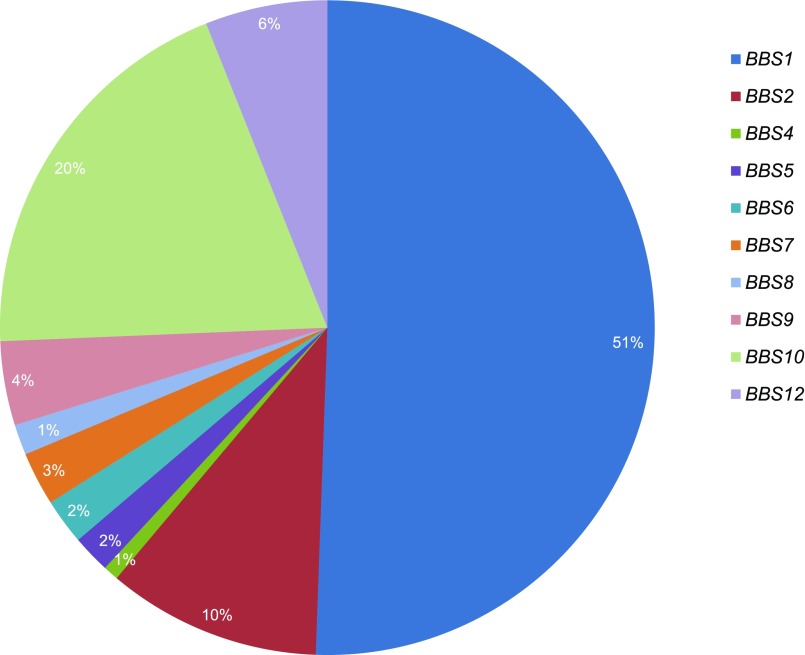
The distribution of genotypes is demonstrated in Figure 1. For the purpose of statistical analysis, mutation type was classified according to severity. One hundred twenty-five patients had two missense mutations, 82 had two truncating mutations (nonsense, frameshift, splice site, or a combination), 39 had a combination of missense and truncating mutations, and the remaining 19 patients had other mutation combinations, including exon deletions and start-codon aberrations.
Figure 1.
The BBS1 genotype predominates in the UK population of patients with Bardet-Biedl syndrome followed by mutations in BBS10 and BBS2. Distribution of genotypes.
Age of Onset of Renal Disease
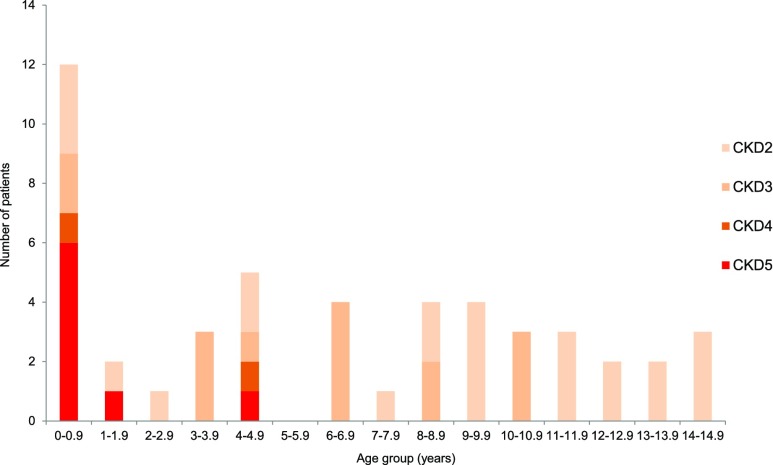
One hundred fifty-six pediatric patients were seen in the pediatric clinics. Of these, we were able to retrospectively obtain the earliest recorded age of onset of stage9 2–5 CKD (CKD2–5) in 49 pediatric patients attending the BBS clinic (Figure 2). All pediatric patients with CKD4–5 were diagnosed before the age of 5 years. The majority of patients with any stage of CKD presented before the age of 10 years. Because the peak referral age to the clinic is late childhood, a later-recorded onset of CKD is likely to reflect significant ascertainment bias as patients may have asymptomatic renal disease and no previous renal sonography.
Figure 2.
CKD4-5 was predominantly diagnosed within the first year of life in pediatric patients with BBS. Age at which renal disease (CKD2–5) was first noted in pediatric patients with BBS (n=49).
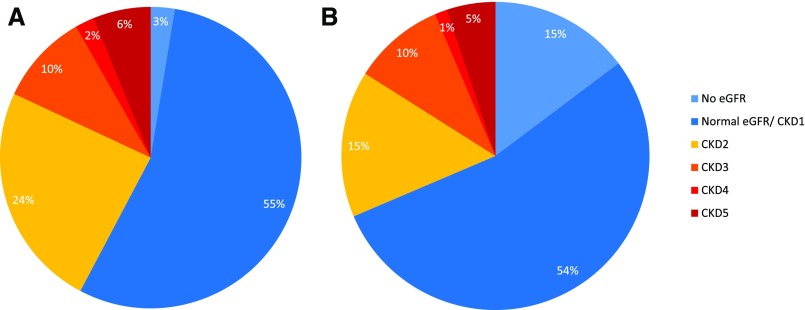
Observations from the national BBS clinics suggest that patients either develop CKD4–5 in childhood or maintain normal or near-normal renal function into adulthood. Figure 3 demonstrates that the frequency of CKD4–5 remains similar in adults and children (8% and 6% respectively).
Figure 3.
The majority of patients with BBS have normal eGFR. Distribution of CKD stages in (A) adults (n=194) and (B) children (n=156).
Prevalence of CKD
eGFR results were available for 189 adults and 133 pediatric patients. Seventy percent of adult patients had at least two eGFR readings. The prevalence of each stage of CKD in adults and children is demonstrated in Figure 3, where CKD2–5 is present in 42% and 31%, respectively. In the adult population (n=194), 107 patients were considered free of renal disease or had CKD1 and five did not have an eGFR or a renal ultrasound scan. Forty-three adult patients had a normal renal ultrasound scan and 47 had a structural abnormality. In the pediatric population (n=156), 84 patients were considered free of renal disease or had CKD1, and 23 patients did not have an eGFR. Eighty-seven pediatric patients had a renal ultrasound scan, 43 of which revealed a structural abnormality. Nine patients had neither a documented eGFR nor an ultrasound scan. All patients were requested to provide a sample for urinalysis. Ninety percent of children and 96% of adults were able to comply. Urinalysis was normal in all those considered free of renal disease. Table 1 demonstrates the number of patients seen in the adult and pediatric clinics and the prevalence of CKD indicators. Table 2 demonstrates the prevalence of CKD2–5 in the adult and pediatric populations presenting to the BBS clinics.
Table 1.
CKD assessment in the adult and pediatric populations
| CKD Marker | n/Total (% of Total) | |
|---|---|---|
| Pediatric Patients | Adult Patients | |
| Total no. of patients | 156 (100%) | 194 (100%) |
| eGFR | ||
| Total no. of patients who had eGFR | 133/156 (86%) | 189/194 (97%) |
| Normal or CKD1 | 84/133 (63%) | 107/189 (57%) |
| CKD2–5 (<90 ml/min per 1.73 m2) | 49/133 (37%) | 82/189 (43%) |
| CKD5 (<15 ml/min per 1.73 m2) | 8/133 (5%) | 12/189 (8%) |
| Renal ultrasound scan | 87/156 (55%) | 90/194 (46%) |
| Normal | 44/87 (51%) | 43/90 (48%) |
| Abnormal | 43/87 (49%) | 47/90 (52%) |
| No ultrasound scan and no eGFR | 9/156 (6%) | 5/194 (3%) |
| Urinalysis | 140/156 (90%) | 186/194 (96%) |
Table 2.
Prevalence of CKD in adults and children, according to age group
| Age Group, yr | Normal/CKD1/No eGFR | CKD2 | CKD3 | CKD4 | CKD5 | Total |
|---|---|---|---|---|---|---|
| Pediatric patients, n | ||||||
| 0–5 | 22 | 2 | 7 | 0 | 5 | 36 |
| 6–10 | 42 | 7 | 5 | 2 | 1 | 57 |
| 11–15 | 29 | 6 | 2 | 0 | 1 | 38 |
| 16–18 | 14 | 9 | 1 | 0 | 1 | 25 |
| Total (% of total) | 107 (69%) | 24 (15%) | 15 (10%) | 2 (1%) | 8 (5%) | 156 |
| Adult patients, n | ||||||
| 16–20 | 27 | 0 | 3 | 0 | 2 | 32 |
| 21–25 | 29 | 5 | 1 | 1 | 3 | 39 |
| 26–30 | 17 | 5 | 1 | 1 | 1 | 25 |
| 31–35 | 14 | 4 | 1 | 1 | 3 | 23 |
| 36–40 | 6 | 7 | 5 | 0 | 1 | 19 |
| 41–45 | 5 | 8 | 2 | 1 | 1 | 17 |
| 46–50 | 6 | 5 | 2 | 0 | 1 | 14 |
| 51–55 | 6 | 8 | 3 | 0 | 0 | 17 |
| 56–60+ | 2 | 5 | 1 | 0 | 0 | 8 |
| Total (% of total) | 112 (58%) | 47 (24%) | 19 (10%) | 4 (2%) | 12 (6%) | 194 |
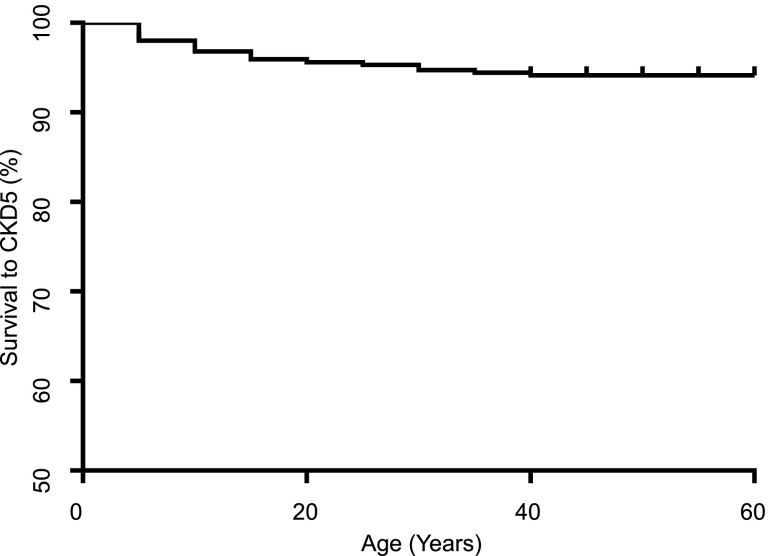
The age at which patients reached CKD5 is known for 20 patients in this cohort. Of these patients, 70% (n=14) had reached CKD5 by the age of 20 years. The age of onset of CKD5 was delineated retrospectively and Kaplan–Meier survival to CKD5 is demonstrated in Figure 4.
Figure 4.
End stage renal disease is not a common feature in the UK BBS population. Kaplan–Meier survival curve: age at which patients were first diagnosed with CKD5 (n=350).
Genotype and mutation-type analyses in adults with BBS revealed statistically significant correlations with the presence of severe renal disease defined as CKD3b–5 (eGFR<45 ml/min per 1.73 m2). Univariable logistical regression analysis indicated that mutations in BBS2, BBS10, and BBS12 were more likely to be associated with severe renal disease than mutations in BBS1 (P values of 0.02, <0.001, and 0.03, respectively) (Table 3). Univariable logistical regression analysis of mutation type revealed that truncating mutations and truncating/missense mutations were statistically associated with severe renal disease in comparison to two missense mutations (P values of <0.001 and 0.01, respectively) (Table 3).
Table 3.
Univariable logistical regression analysis of risk factors for severe renal disease (eGFR<45 ml/min per 1.73 m2) in adults with known common genotypes
| Risk Factor | Odds Ratio | Confidence Interval | P Value | |
|---|---|---|---|---|
| 2.5% | 97.5% | |||
| Genetic factors | ||||
| Genotype (n=154) | ||||
| BBS1 mutation (n=90) | (Reference) | |||
| BBS2 mutation (n=22) | 4.4 | 1.28 | 15.19 | 0.02a |
| BBS9 mutation (n=6) | 2.4 | 0.12 | 17.74 | 0.46 |
| BBS10 mutation (n=26) | 7.4 | 2.49 | 23.32 | <0.01a |
| BBS12 mutation (n=10) | 5.9 | 1.08 | 28.39 | 0.03a |
| Mutation type (n=149) | ||||
| Missense/missense (n=76) | (Reference) | |||
| Truncating/truncating (n=40) | 11.4 | 3.9 | 41.8 | <0.01a |
| Missense/truncating (n=33) | 6.3 | 1.5 | 28.6 | 0.01a |
| Diabetes (n=137) | 0.62 | 0.14 | 0.99 | 0.47 |
| Hypertension (n=137) | 5.43 | 2.21 | 14.29 | <0.01a |
| Body mass index (n=93) | 1.04 | 0.96 | 1.10 | 0.32 |
| Age (n=154) | 1.02 | 0.99 | 1.96 | 0.15 |
Statistically significant result.
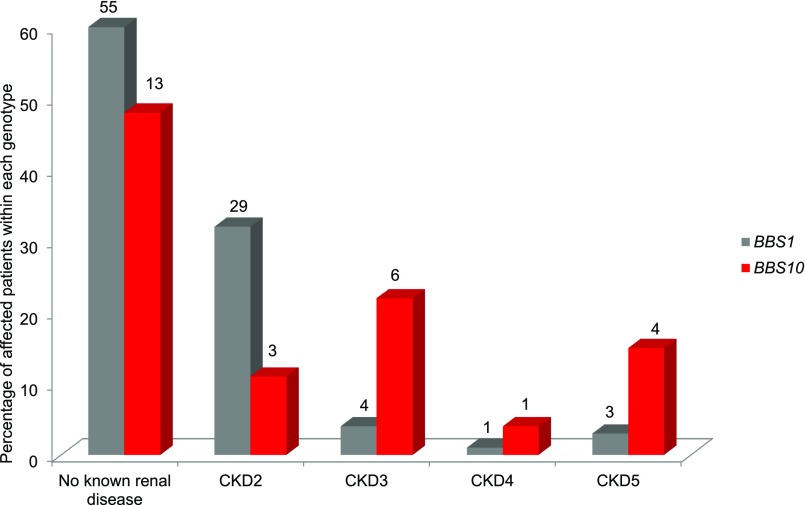
Proportional frequencies of stage 2, 3, 4, and 5 CKD in adults were compared for the commonest genotypes, BBS1 and BBS10, as outlined in Figure 5. Patients with BBS1 mutations are more likely to be disease-free or have early stage CKD. Mutations in BBS10 were more frequently represented with increasing stage of CKD. Of note, patients attending our clinics with mutations in BBS1 are statistically significantly older than those with mutations in BBS10 (P=0.001).
Figure 5.
Mutations in BBS10 area associated with more severe renal disease as compared to mutations in BBS1. Percentage distribution of CKD stages in adults. Mutations in BBS1 versus BBS10; absolute numbers are indicated above each column.
Previous research indicates that the recurring missense mutation M390R in BBS1 may be hypomorphic.10,11 We assessed this by comparing adult patients who were homozygous for M390R to patients with other mutation types in BBS1 (Supplemental Figure 1). This did not reveal an obvious hypomorphic effect of homozygous BBS1 M390R mutations. It may reflect the relatively small group of patients, or that the majority of patients with mutations in BBS1 who are not homozygous for M390R are heterozygous for this mutation. Because BBS is an autosomal recessive disease, it is possible that the hypomorphic effect of M390R predominates even in those who are heterozygous. Of note, only one patient homozygous for BBS1 M390R had progressed beyond CKD3. The patient had renal transplant aged 23 and was subsequently referred to the Bardet-Biedl syndrome service.
We assessed for the presence of micro- and macroalbuminuria by analyzing urinary albumin-to-creatinine ratios as a proxy for glomerular injury. Urinary albumin-to-creatinine ratios were available for 139 adult and pediatric patients. Seven (5%) had proteinuria (defined as urinary albumin-to-creatinine ratio >30 mg/mmol); three of whom were diabetic. Thirty-two patients (28%) had microalbuminuria (defined as urinary albumin-to-creatinine ratio >3.5 mg/mmol12), and two of these patients were diabetic. This could be matched to an eGFR in 119 patients. There was a statistically significant correlation between severe renal disease and urinary albumin-to-creatinine ratios (P=0.01). The sample size was inadequate for correlation with genotype and mutation type.
Although under-reported, 6% of the adult BBS population reported urological complications requiring specialist management. Urological abnormalities included neuropathic bladder, vesico-ureteric reflux, urinary incontinence, and bladder outflow obstructions.
Presence of Structural Abnormalities
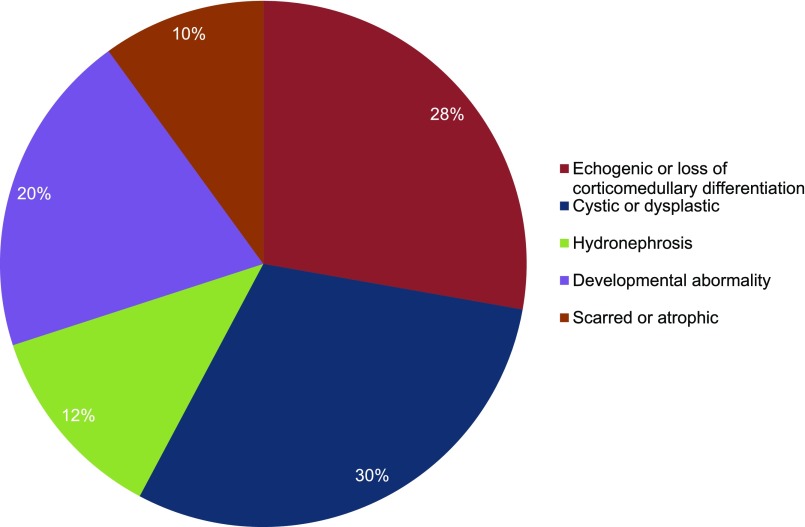
One hundred seventy-seven ultrasound reports from the entire cohort were available for our assessment. Of these, 87 (49%) were unremarkable and 90 (51%) revealed structural defects. Abnormalities were categorized as atrophic/scarring, echogenic or loss of corticomedullary differentiation, cystic or dysplastic, other developmental abnormality, or hydronephrosis, as seen in Figure 6. No consistent pattern was evident in the type of renal structural aberrations observed; cystic disease in patients ranged from unilateral single cysts to multiple bilateral cystic disease. Developmental abnormalities included horseshoe, ectopic, duplex, and absent kidneys. Where several abnormalities were present, the predominant structural defect is reported. On assessing genotype correlations (BBS1 versus BBS10), no association with the presence of structural abnormality was identified (P=0.19).
Figure 6.
Structural renal abnormalities are highly variable in BBS. Prevalence of structural abnormalities detected on sonography.
Correlating the presence of all causes of structural abnormality in adults with CKD staging revealed a strong correlation with CKD3b–5 (P=0.04). All patients with a reported renal ultrasound scan and severe renal disease had a detectable structural abnormality (n=7).
Ultrasound reports from 39 pediatric patients with known renal structural abnormalities who had both antenatal and postnatal sonography failed to identify the anatomic aberration prenatally in 14 patients (36%).
Five pediatric patients had abnormal antenatal renal ultrasound reports and normal postnatal sonography. In all patients, nonspecific echogenicity was reported antenatally and no specific structural abnormalities were detected.
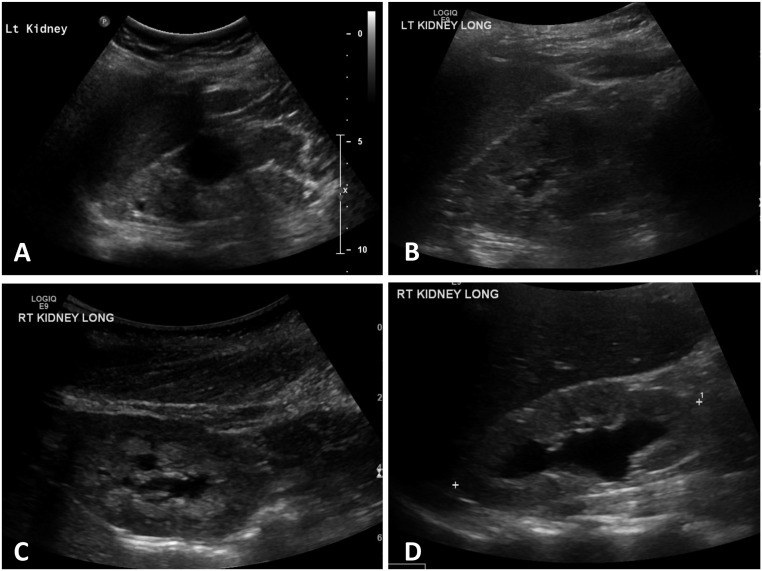
In this cohort, 30 patients presented with sonographic evidence of cystic kidney disease which is classically associated with BBS. Twenty-four of these patients had molecular confirmation of BBS. Patients with mutations in BBS1 and BBS10 accounted for the majority of genotypes represented (42% and 21%, respectively). Figure 7 demonstrates some of the structural renal aberrations commonly detected on ultrasonography.
Figure 7.
Renal ultrasonography demonstrates common structural abnormalities associated with Bardet-Biedl syndrome. (A) Demonstrates the typical cystic dysplastic appearance associated with BBS: a small subcortical cyst, one large cyst, and loss of corticomedullary differentiation. (B) Subcapsular cysts and increased echogenicity. (C) Nephrocalcinosis. (D) Renal pelvic dilation. LT, left; RT, right.
Hypertension and Diabetes
Thirty-five percent of adult patients (n=67) in this cohort were hypertensive. There was a statistically significant correlation between CKD3b–5 and the presence of antihypertensive medication (P<0.01) (Table 3). There was a significant association between the presence of hypertension and albuminuria (P<0.001). The most commonly prescribed antihypertensive medications were angiotensin-converting enzyme inhibitors (52%), followed by diuretics (21%), calcium channel blockers (15%), beta blockers (8%), and angiotensin-receptor blockers (3%).
Fifteen percent of adult patients (n=28) were on hypoglycemic medication. There was no statistically significant association with CKD3b–5 (P=0.47).
Discussion
To our knowledge, this is the largest reported study characterizing the renal phenotype in BBS. The age distribution of our patient population is most likely a reflection of a number of factors affecting patient referral. Children are often referred to the service after the onset of visual decline, which typically occurs toward the end of the first decade of life, accounting for the high frequency of children aged 6–10 years old. Many children presenting in the first year of life are siblings of patients known to the service. The clinical service has been in operation since 2010 and patients >60 years old may not have a known diagnosis of BBS, as familiarity with the syndrome has only increased in the last two decades. The variation of genotypes presented in this study reflects the United Kingdom BBS population and is similar to that observed by others in Europe and North America.13,14 Patients with mutations in BBS1 generally present later to the BBS clinics than patients with mutations in BBS10. This may relate to a milder phenotype and later onset of retinal degeneration.
Our study suggests that the onset of primary renal disease in children predominantly occurs in infancy. For many adult patients it was difficult to accurately determine the age of onset of renal disease because patients with CKD are managed locally with an annual specialist BBS review. A striking observation is the relatively modest difference in prevalence of CKD4–5 between adults (8%) and children (6%) (Figure 3). This supports our hypothesis that patients with BBS primarily either develop CKD4–5 in childhood or remain entirely or relatively free of severe renal disease. The small proportion of adult-onset severe renal disease may relate to comorbidities associated with BBS, such as urological complications, hypertension, obesity, and diabetes. These are potentially modifiable risk factors which should be managed appropriately. The number of patients >30 years is limited in this study and it is therefore not possible to draw conclusions about the risk of developing renal disease in older patients with BBS.
The prevalence of CKD in this cohort is lower than anticipated on the basis of previous estimates.3 Forty-two percent of adults have CKD2–5 and only 8% of adult patients develop CKD3b–5. There appears to be both genotype and mutation-type correlations with CKD, with increased risk of developing CKD3b–5 for those patients who have truncating mutations and mutations in BBS10. This is in keeping with a previous study indicating similar findings for cardiovascular risk factors in this group.10 However, it is difficult to ascertain if genotype and mutation type represent independent risk factors, or if the statistical significance of mutation types reflects the high prevalence of missense mutations in BBS1 and truncating mutations in other genotypes. Only one of the patients in this study with homozygous BBS1 M390R mutations developed CKD5. BBS1 M390R is the most common mutation observed in the BBS population in Europe and Northern America, and patients homozygous for M390R make up a significant proportion of the patient population (45% of the United Kingdom population are homozygous or heterozygous for this mutation [B. Hoskins, P.L. Beales, unpublished data]). This significant subgroup of patients could be counseled regarding their lower risk of progressing to CKD4–5.
Guidelines for the management of BBS recommend that every patient should have a baseline renal ultrasound examination to assess for the presence of any structural abnormalities.15 Although some patients who have abnormal renal ultrasound scans do not go on to develop CKD, there is a statistically significant correlation between structural abnormalities and CKD. This study validates the requirement for a baseline postnatal renal ultrasound scan after a diagnosis of BBS. The presence of a structural abnormality should alert clinicians that close monitoring is required to identify any deterioration in renal function. Anecdotal reports of structural renal abnormalities detected antentally but absent on postnatal sonography could not be confirmed in this study.
One report16 suggests that mutations in BBS10 are associated with antenatal severe cystic kidney disease, which is incompatible with life. In this study, the prevalence of BBS10 mutations among patients presenting with postnatal sonographic evidence of cystic kidney disease (21%; n=5) was consistent with that of the overall study population (20%). The authors are not aware of a higher rate of pregnancy losses in families with BBS10 mutations.
It has previously been suggested that proteinuria is consistently absent in BBS-associated renal disease.17 This study demonstrates the coexistence of proteinuria and CKD and the correlation between CKD3b–5 and albuminuria, and is likely the result of renal mass reduction caused by structural abnormalities.
We have previously reported on the high prevalence of antihypertensive medication in this group.10 It is not clear if the statistical correlation between CKD and antihypertensive medication is related to antihypertensive agents prescribed as a part of CKD management to decrease the rate of progression or if hypertension represents an independent statistical risk factor in this cohort.
In summary, this study maps the prevalence of renal disease in BBS and characterizes the highly variable renal phenotype. We have identified risk indicators as well as potentially protective factors for renal disease. Adults who harbor missense mutations in BBS1 and have normal renal ultrasound scans in adulthood are less likely to develop CKD3b–5. Patients with truncating mutations in BBS10, hypertension, and abnormal renal ultrasound scans are at significantly increased risk of CKD3b–5 compared with the general BBS population. Primary renal disease as a consequence of BBS appears to present in early childhood.
The evidence presented here could have a direct clinical implication for patients with BBS. The presence or absence of risk factors should be considered when counseling patients and may be used to stratify the clinical service. Previous recommendations advise that patients should be reviewed by a nephrologist annually unless CKD is present, in which case closer monitoring is required. On the basis of this study, the authors suggest that adults with the lowest risk of renal disease could receive community nephrology follow-up and low-risk children could be seen less frequently in specialist clinics. All patients with ESRD require frequent specialist follow-up and those with identifiable risk factors or early stable renal disease (CKD1–3) warrant annual specialist follow-up. A multinational study could facilitate the development of a statistical renal risk calculator for this unique population.
Concise Methods
Patients
The following renal parameters were ascertained retrospectively for all 350 patients attending the national BBS clinics: known history of renal disease; stage of CKD (if present); any abnormalities noted on renal ultrasound scanning; eGFR; renal function tests; and relevant concomitant factors, including presence of hypertension, diabetes, and obesity. All patients gave informed consent or assent. Patients were seen in the pediatric clinics if they were 16 years of age or under, or 18 years of age or under and in full-time education. All other patients were seen in the adult clinics. All blood and urinary tests were completed after a 6-hour starvation period. The Modification of Diet in Renal Disease formula was applied to estimate GFR in all adults, in keeping with its common use for patients with obesity and diabetes in the general population.18 eGFR for the pediatric population was calculated according the Schwarz–Haycock formula (height [cm] × 31/creatinine [µmol/L]). Adults were categorized as hypertensive if they were on antihypertensive medication, or if they fulfilled the criteria for antihypertensive treatment in patients with diabetic according to the Kidney Disease Improving Global Outcomes study guidelines.19 Referrals were made primarily from the British national patient support group, clinical geneticists, and ophthalmologists in the United Kingdom.
Mutation Analysis
Mutation analysis was undertaken through the United Kingdom national BBS gene panel, which encompasses 11 BBS genes including BBS1–BB10 and BBS12, as well as two BBS-associated genes, MKS1 and ALMS1.
Statistical Analyses
Genotype-phenotype analysis was targeted to patients with mutations in the two most commonly affected genes, BBS1 and BBS10, as well as the less common genotypes BBS2 and BBS12, where adequate sample sizes were available. Correlation with mutation type was also assessed. Patients with two known missense mutations were compared with two known truncating (nonsense or frameshift) mutations and a combination of missense/truncating mutations. The nonparametric Mann–Whitney U test was performed to assess differences in median age for genotypes BBS1 and BBS10. For the purpose of genotype and mutation type analysis children were not included because renal failure appears to occur and progress primarily in childhood, hence CKD stage was not considered to be stable until adulthood. Multivariable regression analysis was applied to evaluate genotype-phenotype analysis and assess the effect of confounders on chronic renal disease. The relative burden of each risk factor was described in odds ratios. Statistical analyses were conducted in R (R Foundation for Statistical Computing, Vienna, Austria). A 5% confidence level was considered statistically significant. All tests were two-tailed.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors wish to thank patients and colleagues and in particular the Laurence-Moon-Bardet–Biedl Syndrome patient group for their ongoing support and E. Bagkeris (University College London) for statistical support and advice.
The national Bardet–Biedl Syndrome clinic is funded by the National Health Service (NHS) Highly Specialised Services. The National Institute for Health Research Wellcome Clinical Research Facility at Birmingham Children’s Hospital was used to host some of the data collection via the European Wolfram, Alstrom and Bardet-Biedl syndrome Registry Project.
E.F. is funded by the Medical Research Council. E.F., D.B., and P.L.B. are supported by the National Institute for Health Research Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London. D.B. receives support from the European Union, FP7 (grant agreement 2012-305608), and the European Consortium for High-Throughput Research in Rare Kidney Diseases. P.L.B. was supported by a Wellcome Trust Senior Fellowship and is a NIHR Senior Investigator
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015091029/-/DCSupplemental.
References
- 1.Beales PL, Elcioglu N, Woolf AS, Parker D, Flinter FA: New criteria for improved diagnosis of Bardet-Biedl syndrome: results of a population survey. J Med Genet 36: 437–446, 1999 [PMC free article] [PubMed] [Google Scholar]
- 2.Forsythe E, Beales PL: Bardet-Biedl syndrome. Eur J Hum Genet 21: 8–13, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imhoff O, Marion V, Stoetzel C, Durand M, Holder M, Sigaudy S, Sarda P, Hamel CP, Brandt C, Dollfus H, Moulin B: Bardet-Biedl syndrome: a study of the renal and cardiovascular phenotypes in a French cohort. Clin J Am Soc Nephrol 6: 22–29, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tieder M, Levy M, Gubler MC, Gagnadoux MF, Broyer M: Renal abnormalities in the Bardet-Biedl syndrome. Int J Pediatr Nephrol 3: 199–203, 1982 [PubMed] [Google Scholar]
- 5.O’Dea D, Parfrey PS, Harnett JD, Hefferton D, Cramer BC, Green J: The importance of renal impairment in the natural history of Bardet-Biedl syndrome. Am J Kidney Dis 27: 776–783, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Gourdol O, David L, Colon S, Bouvier R, Ayral A, Aguercif M, François R: Renal involvement in the Laurence-Moon-Bardet-Biedl syndrome. Apropos of 3 cases. Pediatrie 39: 175–181, 1984 [PubMed] [Google Scholar]
- 7.Marion V, Schlicht D, Mockel A, Caillard S, Imhoff O, Stoetzel C, van Dijk P, Brandt C, Moulin B, Dollfus H: Bardet-Biedl syndrome highlights the major role of the primary cilium in efficient water reabsorption. Kidney Int 79: 1013–1025, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Harnett JD, Green JS, Cramer BC, Johnson G, Chafe L, McManamon P, Farid NR, Pryse-Phillips W, Parfrey PS: The spectrum of renal disease in Laurence-Moon-Biedl syndrome. N Engl J Med 319: 615–618, 1988 [DOI] [PubMed] [Google Scholar]
- 9.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G; National Kidney Foundation : National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 139: 137–147, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Forsythe E, Sparks K, Hoskins BE, Bagkeris E, McGowan BM, Carroll PV, Huda MS, Mujahid S, Peters C, Barrett T, Mohammed S, Beales PL: Genetic predictors of cardiovascular morbidity in Bardet-Biedl syndrome. Clin Genet 87: 343–349, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castro-Sánchez S, Álvarez-Satta M, Cortón M, Guillén E, Ayuso C, Valverde D: Exploring genotype-phenotype relationships in Bardet-Biedl syndrome families. J Med Genet 52: 503–513, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Amin R, Widmer B, Prevost AT, Schwarze P, Cooper J, Edge J, Marcovecchio L, Neil A, Dalton RN, Dunger DB: Risk of microalbuminuria and progression to macroalbuminuria in a cohort with childhood onset type 1 diabetes: prospective observational study. BMJ 336: 697–701, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deveault C, Billingsley G, Duncan JL, Bin J, Theal R, Vincent A, Fieggen KJ, Gerth C, Noordeh N, Traboulsi EI, Fishman GA, Chitayat D, Knueppel T, Millán JM, Munier FL, Kennedy D, Jacobson SG, Innes AM, Mitchell GA, Boycott K, Héon E: BBS genotype-phenotype assessment of a multiethnic patient cohort calls for a revision of the disease definition. Hum Mutat 32: 610–619, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Billingsley G, Deveault C, Héon E: BBS mutational analysis: a strategic approach. Ophthalmic Genet 32: 181–187, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Forsythe E, Beales PL: Bardet-Biedl Syndrome. GeneReviews at GeneTests: Medical Genetics Information Resource 1993–2016, 2015. Available at: http://www.genetests.org. Accessed August 11, 2015 [Google Scholar]
- 16.Putoux A, Mougou-Zerelli S, Thomas S, Elkhartoufi N, Audollent S, Le Merrer M, Lachmeijer A, Sigaudy S, Buenerd A, Fernandez C, Delezoide AL, Gubler MC, Salomon R, Saad A, Cordier MP, Vekemans M, Bouvier R, Attie-Bitach T: BBS10 mutations are common in ‘Meckel’-type cystic kidneys. J Med Genet 47: 848–852, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Putoux A, Attie-Bitach T, Martinovic J, Gubler MC: Phenotypic variability of Bardet-Biedl syndrome: focusing on the kidney. Pediatr Nephrol 27: 7–15, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Goderis G, Van Pottelbergh G, Truyers C, Van Casteren V, De Clercq E, Van Den Broeke C, Buntinx F: Long-term evolution of renal function in patients with type 2 diabetes mellitus: a registry-based retrospective cohort study. BMJ Open 3: e004029, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wheeler DC, Becker GJ: Summary of KDIGO guideline. What do we really know about management of blood pressure in patients with chronic kidney disease? Kidney Int 83: 377–383, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.