Abstract
TGF-β has been implicated as a major pathogenic factor in diabetic nephropathy. This randomized, double-blind, phase 2 study assessed whether modulating TGF-β1 activity with a TGF-β1–specific, humanized, neutralizing monoclonal antibody (TGF-β1 mAb) is safe and more effective than placebo in slowing renal function loss in patients with diabetic nephropathy on chronic stable renin-angiotensin system inhibitor treatment. We randomized 416 patients aged ≥25 years with type 1 or type 2 diabetes, a serum creatinine (SCr) level of 1.3–3.3 mg/dl for women and 1.5–3.5 mg/dl for men (or eGFR of 20–60 ml/min per 1.73 m2), and a 24-hour urine protein-to-creatinine ratio ≥800 mg/g to TGF-β1 mAb (2-, 10-, or 50-mg monthly subcutaneous dosing for 12 months) or placebo. We assessed a change in SCr from baseline to 12 months as the primary efficacy variable. Although the Data Monitoring Committee did not identify safety issues, we terminated the trial 4 months early for futility on the basis of their recommendation. The placebo group had a mean±SD change in SCr from baseline to end of treatment of 0.33±0.67 mg/dl. Least squares mean percentage change in SCr from baseline to end of treatment did not differ between placebo (14%; 95% confidence interval [95% CI], 9.7% to 18.2%) and TGF-β1 mAb treatments (20% [95% CI, 15.3% to 24.3%], 19% [95% CI, 14.2% to 23.0%], and 19% [95% CI, 14.0% to 23.3%] for 2-, 10-, and 50-mg doses, respectively). Thus, TGF-β1 mAb added to renin-angiotensin system inhibitors did not slow progression of diabetic nephropathy.
Keywords: Diabetic Kidney Disease, Transforming growth factor beta, proteinuria, renal fibrosis
Diabetic nephropathy (DN) is a common cause of ESRD in much of the world, including regions where the availability of RRT is limited.1,2 The global prevalence of diabetes is growing rapidly, especially in the developing world, and the prevalence of DN and ESRD continues to rise.2–5 Furthermore and regardless of the underlying cause of ESRD, patients treated with RRT experience substantial morbidity and mortality and consume disproportionate heath care expenditure. Therefore, major efforts must be made in attempting to prevent ESRD and the need for RRT.
The prevention of DN progression and the development of ESRD has been a particularly challenging task. The only well established therapeutic means to slow the progression of DN has been through inhibition of the renin-angiotensin system (RAS). Currently, angiotensin-converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARBs) are the recommended standard of care in DN because of their established effect in slowing the progressive decline in renal function.6–8 Their routine use contributed to reducing the rate of ESRD in patients with diabetes, although the decrease is less than that observed with other proven interventions that reduce the risk of other major diabetes-related complications, such as stroke and myocardial infarction.9 Because the residual risk for ESRD remains high in patients with DN treated with RAS inhibitors, new therapies are needed that provide additional renal protection when combined with ACEi or ARB treatment.3,4
TGF-β has been implicated as an important pathogenic factor in various types of CKD, including DN.10 The TGF-β superfamily consists of >30 members of evolutionarily conserved proteins that contribute to diverse developmental and regulatory processes.11,12 The first member, TGF-β1, was discovered >20 years ago and is one of three distinct TGF-β isoforms encoded by separate genes (TGF-β1, -β2, and -β3). The mature forms of these proteins are 60%–80% conserved and activate SMAD signaling through the same receptor-signaling complexes.13,14 These pathways activate and regulate multiple gene responses that can influence a number of cellular processes and disease states, including DN.10,12,15
Renal fibrosis, one of the cardinal histologic features of progressive DN, has been associated with increased renal expression of TGF-β; inhibition of TGF-β has been shown to attenuate fibrosis in animal models of diabetes.16,17 Therefore, targeting TGF-β seemed a logical course to pursue. Among the three TGF-β isoforms, experimental data suggest that the β1 isoform may be the most important cytokine causing progressive renal injury and loss of function.18,19 Consequently, we developed a humanized monoclonal antibody (mAb) with full neutralizing action that was selective against active TGF-β1, and would potentially avoid the risk of serious toxicity that might be associated with chronic inhibition of all three TGF-β isoforms.
We hypothesized that reducing TGF-β1 activity with an mAb specific for the TGF-β1 isoform would provide a safe and effective treatment for patients with DN that would complement the beneficial effect of RAS inhibition. To test this hypothesis, we conducted a placebo-controlled, dose-ranging, phase 2, clinical trial investigating a TGF-β1–specific, humanized, neutralizing mAb (TGF-β1 mAb) in patients with moderate to advanced DN. The specific aim of the study was to determine whether 12 months of treatment with the mAb, when added to standard of care, would effectively and safely attenuate the rate of decline in renal function associated with DN.
Results
Patient Characteristics and Disposition
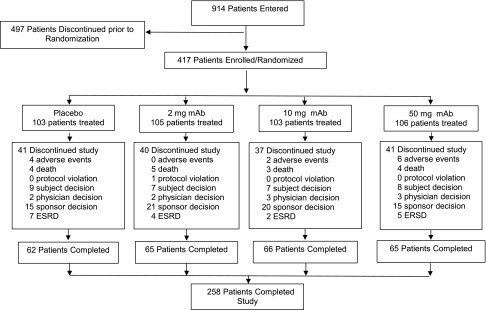
As indicated in Figure 1, 417 of 914 screened patients were included in the study: 103 patients were randomized to placebo and 105, 103, and 106 patients to TGF-β1 mAb 2-, 10-, and 50-mg monthly subcutaneous dosing, respectively. One patient assigned TGF-β1 mAb 50 mg discontinued the study before receiving study medication.
Figure 1.
Disposition of study participants by treatment group. One patient assigned TGF-β1 mAb 50 mg discontinued the study before receiving study medication.
Baseline demographic features, as well as clinical and biochemical characteristics, for the 416 treated patients were similar across the four study groups (Table 1). Patients self-reporting black race constituted 9.4% of the study population. Although some numerical differences were observed across treatment arms, these differences were not statistically significant. Eighty-nine percent of patients had type 2 diabetes. On average, study participants had long-standing diabetes. Serum creatinine (SCr) averaged 2.14±0.61 mg/dl, eGFR averaged 35.5±11.9 ml/min per 1.73 m2, and urine protein-to-creatinine ratio (UPCR) averaged 3.26±2.51 g/g. As shown in Figure 1, 258 of 417 randomized patients completed the study, whereas 159 patients discontinued before receiving the intended 12 months of treatment. The major reason for early discontinuation was sponsor decision: 67 of 71 early discontinuation patients stopped after the decision by the sponsor to terminate the study for lack of efficacy. The study was stopped 4 months before its scheduled completion after an unplanned futility analysis recommended by the independent Data Monitoring Committee (DMC) overseeing the study. Mean changes in SCr monitored by the DMC as part of their periodic review were not trending in a manner that indicated renal benefit. Although no specific safety issues were identified, because the futility analysis indicated a low probability that the primary end point would be met upon completion of the trial, the DMC concluded that the risk-to-benefit assessment no longer supported continuing the study. When the trial was stopped, the median duration of dosing was 315 days and 75.7% of patients received their final dose 9 months or longer after initiating treatment. As shown in Supplemental Figure 1, duration of dosing was comparable among the four treatment groups.
Table 1.
Baseline characteristics and demographics of randomized patients
| Variable | Placebo (n=103) | TGF-β1 mAb | Total (n=416) | P-Valuea | ||
|---|---|---|---|---|---|---|
| 2 mg (n=105) | 10 mg (n=103) | 50 mg (n=105) | ||||
| Sex, n (%) | 0.25 | |||||
| Men | 76 (73.8) | 84 (80.0) | 74 (71.8) | 86 (81.9) | 320 (76.9) | |
| Women | 27 (26.2) | 21 (20.0) | 29 (28.2) | 19 (18.1) | 96 (23.1) | |
| Age, yr | 62.7±11.3 | 62.8±9.2 | 60.5±9.1 | 62.9±10.9 | 62.2±10.2 | 0.27 |
| Race, n (%) | 0.33 | |||||
| American Indian or Alaska Native | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Asian | 0 (0.0) | 4 (3.8) | 2 (1.9) | 2 (1.9) | 8 (1.9) | |
| Black | 14 (13.6) | 9 (8.6) | 9 (8.7) | 7 (6.7) | 39 (9.4) | |
| Multiple | 1 (1.0) | 0 (0.0) | 2 (1.9) | 0 (0.0) | 3 (0.7) | |
| Native Hawaiian or other Pacific Islander | 1 (1.0) | 0 (0.0) | 1 (1.0) | 0 (0.0) | 2 (0.5) | |
| White | 83 (80.6) | 90 (85.7) | 84 (81.6) | 90 (85.7) | 347 (83.4) | |
| Not provided | 4 (3.9) | 2 (1.9) | 5 (4.9) | 6 (5.7) | 17 (4.1) | |
| Body mass index, kg/m2 | 34.0±6.6 | 33.4±6.0 | 34.7±7.2 | 33.3±6.8 | 33.9±6.7 | 0.41 |
| Systolic BP, mmHg | 139.8±12.3 | 137.6±14.9 | 138.2±12.5 | 136.7±13.6 | 138.1±1 3.4 | 0.4 |
| Diastolic BP, mmHg | 75.5±8.5 | 74.2±9.6 | 74.3±9.0 | 73.1±9.8 | 74.3±9.3 | 0.35 |
| Type 2 diabetes, n (%) | 93 (90.3) | 92 (87.6) | 92 (89.3) | 93 (88.6) | 370 (88.9) | 1.0 |
| Duration of diabetes, d | 7073.9 (3381.6) | 6610.1 (3324.5) | 6957.0 (3069.9) | 7110.8 (3686.5) | ||
| Duration of kidney disease, d | 1922.4 (1405.0) | 1802.3 (1655.9) | 2041.3 (1527.3) | 2133.1 (2318.4) | ||
| Hemoglobin A1c, % | 7.8±1.8 | 7.9±1.8 | 7.9±1.6 | 7.9±1.7 | 7.9±1.7 | 0.96 |
| SCr, mg/dl | 2.2±0.6 | 2.1±0.6 | 2.1±0.7 | 2.1±0.6 | 2.1±0.6 | 0.57 |
| eGFR, ml/min per 1.73 m2 | 33.8±11.1 | 35.7±11.7 | 36.4±12.5 | 35.9±12.1 | 35.5±11.9 | 0.43 |
| UPCR, g/g | 3.1±2.5 | 3.3±2.6 | 3.3±2.5 | 3.4±2.5 | 3.3±2.5 | 0.92 |
Data are means±SD unless otherwise indicated. Summary for SCr was on the basis of the imputed values. Baseline UPCR is the mean of the available values at the last visit obtained on or before the first date of administration of investigational drug. The baseline value for other parameters is the last available value obtained on or before the first date of administration of investigational drug.
P values for continuous variables were obtained using one-way ANOVA, with treatment as main effect. P values for categorical variables were obtained using Fisher exact test.
Primary and Secondary Efficacy Outcomes
SCr and eGFR
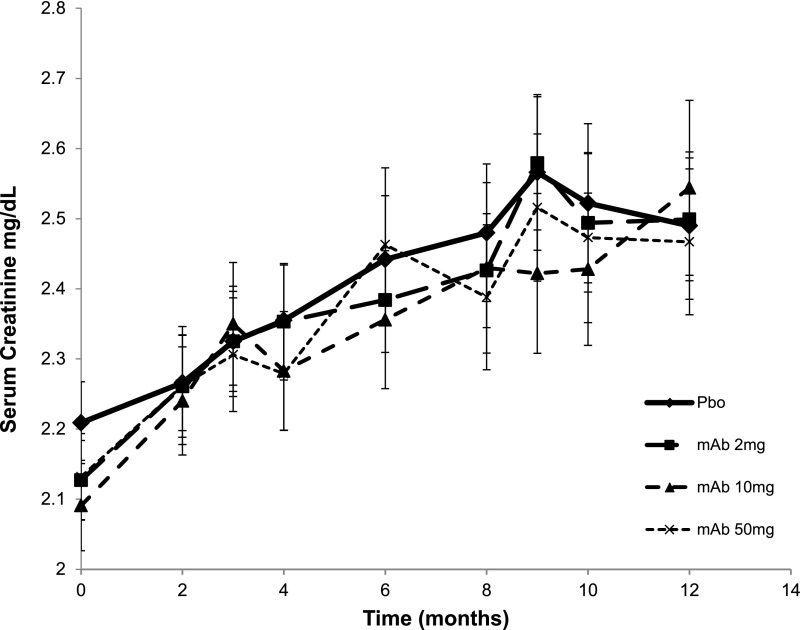
Table 2 shows the baseline results for SCr, eGFR, and UPCR for all four groups, and observed change from baseline at 6 months and at end point (12 months, or end point visit for patients who were ongoing when treatment was terminated early because of study futility). Baseline SCr and eGFR were defined as the average of the last two measurements obtained within 30 days of each other and before study treatment was initiated. The end point value was defined as the average of two samples obtained 1 week apart at 12 months or treatment termination for ongoing patients. The primary efficacy analysis of log-transformed SCr did not show a statistically significant effect for any of the TGF-β1 mAb treatments at 6 months or end point. Mean±SD change in SCr from baseline to end point for the placebo group was 0.33±0.67 mg/dl, a 15% increase from baseline, which was consistent with the study-powering assumption of a 16% rise over 1 year. Least squares mean percentage change in SCr from baseline to end point was not different between placebo (14%; 95% confidence interval [95% CI], 9.7% to 18.2%]) and TGF-β1 mAb treatments (20% [95% CI, 15.3% to 24.3%], 19% [95% CI, 14.2% to 23.0%], and 19% [95% CI, 14.0% to 23.3%] for 2, 10, and 50 mg, respectively) (Supplemental Table 1). The absence of treatment effects on changes in eGFR was consistent with the SCr results. Figure 2 shows the progressive rise in SCr over the course of the study, by treatment. The time course of SCr rise for the 2-, 10-, and 50-mg mAb treatments was similar to placebo, and there was no treatment effect on the slope of SCr or eGFR (data not shown). Thus, the progressive loss of renal function was not modified by anti–TGF-β1 therapy.
Table 2.
Observed change from baseline in SCr, first-morning UPCR, and eGFR
| Variable | Placebo | TGF-β1 mAb | ||
|---|---|---|---|---|
| 2 mg | 10 mg | 50 mg | ||
| SCr, mg/dl | ||||
| Baseline | ||||
| N | 103 | 105 | 103 | 105 |
| Mean±SD | 2.21±0.59 | 2.13±0.58 | 2.09±0.65 | 2.13±0.63 |
| 6 months | ||||
| n | 89 | 91 | 92 | 87 |
| Mean±SD | 2.44±0.86 | 2.38±0.71 | 2.36±0.94 | 2.46±1.02 |
| Change from baseline | 0.23±0.56 | 0.28±0.40 | 0.25±0.46 | 0.33±0.60 |
| End point | ||||
| n | 85 | 90 | 91 | 82 |
| Mean±SD | 2.49±0.97 | 2.50±0.83 | 2.54±1.19 | 2.47±0.94 |
| Change from baseline | 0.33±0.67 | 0.41±0.47 | 0.47±0.76 | 0.41±0.58 |
| First-morning UPCR, g/g | ||||
| Baseline | ||||
| N | 103 | 105 | 103 | 105 |
| Mean±SD | 3.12±2.52 | 3.27±2.57 | 3.30±2.45 | 3.35±2.55 |
| 6 months | ||||
| n | 88 | 92 | 94 | 90 |
| Mean±SD | 3.03±2.77 | 3.93±6.90 | 3.21±2.54 | 3.48±3.56 |
| Change from baseline | 0.08±1.47 | 0.66±6.24 | −0.06±1.65 | 0.13±2.16 |
| End point | ||||
| n | 82 | 87 | 85 | 81 |
| Mean±SD | 3.21±2.75 | 3.50±2.71 | 3.64±3.26 | 3.45±3.42 |
| Change from baseline | 0.41±1.76 | 0.31±2.53 | 0.47±2.32 | 0.26±2.28 |
| eGFR, ml/min per 1.73 m2 | ||||
| Baseline | ||||
| N | 103 | 105 | 103 | 105 |
| Mean±SD | 33.84±11.06 | 35.71±11.72 | 36.36±12.52 | 35.95±12.09 |
| 6 months | ||||
| n | 89 | 91 | 92 | 87 |
| Mean±SD | 31.25±11.48 | 31.67±11.38 | 32.99±13.09 | 32.49±13.19 |
| Change from baseline | −2.51±5.74 | −4.23±5.92 | −3.06±5.54 | −3.90±5.78 |
| End point | ||||
| n | 85 | 90 | 91 | 82 |
| Mean±SD | 31.23±12.16 | 30.94±12.91 | 31.28±13.52 | 32.34±13.80 |
| Change from baseline | −3.39±5.47 | −5.38±6.27 | −5.38±7.55 | −4.71±8.84 |
Data are means±SD. Baseline SCr is the mean of the last two available values obtained on or before the first date of administration of investigational drug. End point SCr value is the mean of two scheduled values at the end of treatment. Baseline UPCR is the mean of the available values at the last visit obtained on or before the first date of administration of investigational drug. The mean values at each visit were used in the analysis. Baseline eGFR is the average of the last two available values obtained on or before the first date of administration of investigational drug. End point eGFR value is the mean of two scheduled values at the end of treatment.
Figure 2.
Effect of TGF-β1 mAb on SCr levels measured over time. Pbo, placebo.
Secondary subgroup analyses of change in SCr from baseline were performed, again with no apparent treatment effect in subgroups on the basis of SCr, UPCR, age, sex, whether the patient entered into the run-in period, or diabetes type. Results by diabetes type are displayed in Supplemental Table 2.
Urine Protein Excretion
Treatment had no apparent effect on proteinuria, assessed as the mean of two consecutive first-morning urine collections at baseline, 6 months, and end point (Table 2). The change in UPCR over 6 and 12 months was not different for placebo or for the three doses of TGF-β1 mAb.
Exploratory Biomarkers
In addition to determining the effects of anti–TGF-β1 therapy on renal function, a number of pharmacodynamic biomarkers were assessed in plasma and urine samples collected at baseline and every 3 months, with samples also collected 2 weeks after the sixth dose. No treatment-related changes occurred for any of the biomarkers, including: soluble TNF receptor 2 (sTNFR2), kidney injury molecule-1 (KIM-1), and neutrophil gelatinase-associated lipocalin (NGAL) in plasma; and fibronectin, high molecular weight type IV collagen (HMW collagen IV), monocyte chemotactic protein-1 (MCP-1), and matrix metalloproteinase-7 (MMP-7) in urine. Consistent with the SCr data, the glomerular filtration marker, plasma cystatin C, was unchanged with treatment. Results at baseline, 5.5 months (midpoint of the dosing interval), and end of treatment for the eight biomarkers are shown in Supplemental Tables 3 and 4.
Pharmacokinetic Analyses
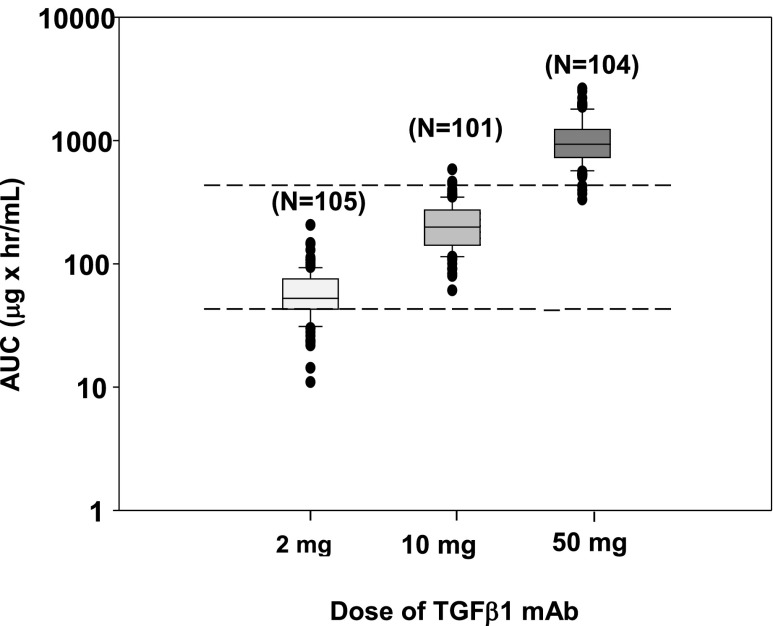
Of the 313 patients who were assigned to a TGF-β1 mAb arm and received at least one dose, three patients had incomplete data for population pharmacokinetic (PK) analysis. Thus, the final population PK analysis dataset comprised 310 patients who were comparably divided among the three active treatment groups: 105 received 2 mg, 101 received 10 mg, and 104 received 50 mg TGF-β1 mAb.
Serum drug concentrations over time were well fit with a one-compartment population PK model. The estimated apparent clearance (CL/F) and apparent volume of distribution (V/F) were 0.0458 L/h and 20.7 L, respectively. Body weight was identified as a significant covariate on both CL/F and V/F. Notably, a majority of the model-predicted human exposures were within or above the efficacious range in the uni-nephrectomy db/db mouse model of DN caused by type 2 diabetes (Figure 3).
Figure 3.
Individual post hoc steady-state exposure estimates from the population PK model by dose (box plots) compared with the estimated efficacy exposure range in uni-nephrectomy db/db mice (dashed lines). AUC, area under the concentration versus time curve.
Safety
As shown in Table 3, and as expected in this population of patients with moderate to advanced DN, during the study there were high incidences of treatment emergent adverse events (87.3%), deaths (3.8%), serious adverse events (38.5%), and discontinuations because of treatment emergent adverse events (7.7%). The overall frequencies of the various categories of adverse effects were not different between the treatment and placebo groups. Table 4 summarizes the adjudicated events of ESRD, AKI, and deaths. These episodes occurred either during the study or, in some cases, after the patient had completed the study, and each case was submitted by the investigator for adjudication. A total of 32 patients (7.7%) developed ESRD, 23 patients (5.5%) had at least one episode of AKI, and 16 patients (3.8%) died. The majority of adjudicated deaths in all study groups were from cardiovascular causes. Overall, the frequency of these events was similar between combined TGF-β1 mAb–treated patients and placebo-treated patients. Although twice as many AKI episodes occurred in the group receiving the lowest dose of TGF-β1 mAb (2 mg) as in those receiving placebo, there was no dose response for this effect, and the number of patients at each of the higher doses of 10 and 50 mg was essentially the same as for placebo.
Table 3.
Summary of adverse events and serious adverse events
| Variable | Placebo (n=103) | TGF-β1 mAb | All Active (n=313) | Total (n=416) | ||
|---|---|---|---|---|---|---|
| 2 mg (n=105) | 10 mg (n=103) | 50 mg (n=105) | ||||
| Deaths due to TEAEs | 4 (3.9) | 5 (4.8) | 3 (2.9) | 4 (3.8) | 12 (3.8) | 16 (3.8) |
| SAEa | 38 (36.9) | 44 (41.9) | 37 (35.9) | 41 (39.0) | 122 (39.0) | 160 (38.5) |
| TEAE | 93 (90.3) | 90 (85.7) | 90 (87.4) | 90 (85.7) | 270 (86.3) | 363 (87.3) |
| Study drug discontinuation due to TEAEs | 9 (8.7) | 5 (4.8) | 6 (5.8) | 12 (11.4) | 23 (7.3) | 32 (7.7) |
All data are represented as n (%). TEAE, treatment emergent adverse event; SAE, serious adverse event.
SAEs are treatment emergent SAEs.
Table 4.
Summary of adjudicated renal and death events
| Variable | Placebo (n=103) | TGF-β1 mAb | All Active (n=313) | Total (n=416) | ||
|---|---|---|---|---|---|---|
| 2 mg (n=105) | 10 mg (n=103) | 50 mg (n=105) | ||||
| ESRD | 9 (8.7) | 6 (5.7) | 5 (4.9) | 12 (11.4) | 23 (7.3) | 32 (7.7) |
| AKI | 4 (3.9) | 9 (8.6) | 5 (4.9) | 5 (4.8) | 19 (6.1) | 23 (5.5) |
| Deaths | 4 (3.9) | 5 (4.8) | 3 (2.9) | 4 (3.8) | 12 (3.8) | 16 (3.8) |
| Coronary ischemia | 1 (0.97) | 3 (2.9) | 1 (0.97) | 4 (1.3) | 5 (1.2) | |
| Sudden or cardiac arrest | 1 (0.95) | 1 (0.32) | 1 (0.24) | |||
| Heart failure | 2 (1.9) | 2 (1.9) | 1 (0.95) | 3 (0.96) | 5 (1.2) | |
| Stroke | 1 (0.95) | 1 (0.32) | 1 (0.24) | |||
| Cancer | 1 (0.95) | 1 (0.32) | 1 (0.24) | |||
| Hypoglycemia | 1 (0.95) | 1 (0.32) | 1 (0.24) | |||
| Respiratory failure | 1 (0.95) | 1 (0.32) | 1 (0.24) | |||
| Sepsis | 1 (0.97) | 1 (0.24) | ||||
All data are represented as n (%).
TGF-β1 mAb was not associated with induction of autoimmunity, as evident by the lack of any adjudicated autoimmune event in patients receiving TGF-β1 mAb. There were no clinically significant changes in clinical chemistry, hematology, or antinuclear antibody laboratory values, or vital signs (data not shown). Average body weight change over 12 months was <0.5 kg and did not differ among treatment groups.
Discussion
This trial was designed to assess the potential of TGF-β1 mAb, a humanized mAb that binds to and neutralizes active TGF-β1, to slow the progression of moderate to advanced CKD because of type 1 or type 2 diabetes. Treatment was assessed in patients receiving appropriate standard of care with an ACEi or ARB, and with adequate BP control. Patients with significant proteinuria and a reduced eGFR were enrolled in order to enrich the study population with patients who would be expected to demonstrate a substantial decline in renal function over 12 months, and thereby provide a suitable basis for evaluating the effect of TGF-β1 inhibition. Although the study population reflected by the placebo group demonstrated the predicted rate of decline in renal function, the trial was terminated shortly before the scheduled completion after an efficacy futility analysis that included the simulation of final results. This analysis indicated a low likelihood that a prespecified, statistically significant effect for one of the anti–TGF-β1 mAb doses would be achieved at the end of the study. Analyses of the final data confirmed the lack of efficacy of TGF-β1 mAb at all three doses, as reflected by change from baseline in SCr, eGFR, and UPCR.
Beginning with seminal work by Border and colleagues more than two decades ago, TGF-β has been implicated as a key pathogenic factor in most types of CKD associated with fibrosis, including DN.10,16,17,20–22 Among its many actions, TGF-β stimulates the synthesis of extracellular molecules such as type I and type IV collagen and fibronectin, decreases matrix degradation, and induces apoptosis of podocytes, tubular epithelial cells, and endothelial cells.10,23 Many mediators of DN, such as high glucose concentration, reactive oxygen species, advanced glycation end products, protein kinase C, renin-angiotensin II-aldosterone system components, and endothelin stimulate TGF-β production.23 Glomerular and tubulointerstitial expression of TGF-β is increased in human DN.24 Sharma et al.25 found evidence for increased renal TGF-β1 production and renal urinary excretion in patients with type 2 diabetes. ACEi and ARBs (drugs with proven efficiency in DN) lower circulating or urinary TGF-β1 levels.26–28 A key role of TGF-β in the pathogenesis of DN is further supported by rodent studies showing that inhibition of TGF-β with an mAb against all three isoforms (1D11, a pan-neutralizing mAb) is associated with renal efficacy in the db/db mouse16,17 and streptozotocin rat models of DN.29
Translating the indirect clinical and preclinical data implicating excessive TGF-β activity in human DN into a mode of therapy, although logical, has been slow and hampered, in part, by concerns that continuous TGF-β inhibition may lead to unacceptable toxicity.10,12,15 Anti–TGF-β treatment in metastatic cancer has been an active area of clinical research because of the potential benefit in terminal cancer, offsetting the potential risk associated with excessive TGF-β inhibition.12 Clinical research regarding a therapeutic role of anti–TGF-β therapy in CKD has lagged behind oncology research. One small, single-dose, phase 1, safety and PK study examined the antiproteinuria response of fresolimumab, an mAb neutralizing all three TGF-β isoforms, in patients with FSGS.30 With single-dose treatment, the drug demonstrated acceptable tolerability and minimal effects on proteinuria reduction. However, the small sample size, lack of a control group, and brief period of treatment limited any conclusion about the safety or efficacy of this treatment. A subsequent placebo-controlled, phase 2 study in 36 patients with FSGS of relatively short treatment duration was recently completed but results have not been reported (Clinicaltrials.gov identifier: NCT01665391). Pirfenidone, an antifibrotic drug reported to inhibit TGF-β activity, was observed to have beneficial changes in renal function of uncertain clinical relevance in a small trial of 77 patients with DN.31 Finally, drugs that interfere with RAS activity may inhibit TGF-β as part of their mechanism for renal efficacy.26–28
To our knowledge, this is the first clinical trial assessing the effects of direct TGF-β modulation in slowing the progression of DN. To minimize potential serious target-mediated adverse effects from prolonged and excessive TGF-β inhibition, we sought to neutralize only the TGF-β1 isoform for a portion of the dosing interval, and leave unopposed the β2 and β3 isoforms. Preclinical toxicology testing in monkeys demonstrated a superior safety profile with TGF-β1 mAb versus a humanized mAb that neutralized all three TGF-β isoforms (Eli Lilly and Company, unpublished data). Furthermore, we found that in the db/db mouse model of DN, TGF-β1 inhibition with a murine surrogate mAb of the TGF-β1 mAb used in this study was equally efficacious to an mAb against all three TGF-β isoforms.19 These data were consistent with data from Yu et al.18 demonstrating that TGF-β1 was the highest expressed TGF-β isoform in the kidney, and that the β2 and β3 isoforms appear to mediate part of their effects through the upregulation of β1 expression.18 Continuous neutralization of TGF-β1 might also increase the risk of serious toxicity and result in the loss of renal efficacy, as large doses of TGF-β inhibitor have been associated with loss of efficacy in several experimental CKD models, including the db/db DN mouse model.19,32–34 The monthly dosing interval used in this study is two to three times longer than the serum TGF-β1 mAb half-life, and this regimen minimized drug accumulation. We also explored a 25-fold dose range to account for a possible nonmonotonic dose response, and to assess doses and systemic drug exposures that were below and well above the dose and exposure ranges associated with efficacy in the db/db experiments. The chosen dose regimens and corresponding systemic drug exposures appeared to be tolerated, although we did observe a higher frequency of AKI episodes in the 2-mg TGF-β1 mAb group than placebo or the two higher mAb groups. The low incidence and lack of a dose response suggest this observation to be a chance finding, but require additional human data to determine if this adverse effect is caused by anti–TGF-β1 therapy. However, given the absence of evidence for renal efficacy on the basis of changes in SCr, eGFR, and UPCR, and no identified pharmacodynamic biomarker, it is unclear how to modulate this target and safely achieve renal efficacy in DN. The target-mediated adverse effects noted in cynomolgus monkeys (Eli Lilly and Company, unpublished data), although providing evidence that TGF-β1 mAb is a pharmacologically active agent, limit the intensity that patients with DN can be chronically dosed with this mAb.
Although this trial failed to demonstrate renal efficacy with TGF-β1 mAb treatment, there were limitations that confound drawing definitive conclusions that TGF-β has no role in the pathogenesis of DN. For example, the trial was comprised mainly of nonblack patients with type 2 diabetes. Although analyses did not demonstrate significant differential treatment effects on the basis of race or diabetes type, the small number of black patients and patients with type 1 diabetes substantially limits the power to assess treatment effects in these subgroups. Another limitation of this phase 2, dose-ranging study is that, by necessity, the trial used a change in SCr as a surrogate measure for renal efficacy. Mean change in SCr over such a short treatment period may not be sensitive enough to detect a benefit.35,36 The challenge of detecting a treatment effect during a short follow-up period may have been exacerbated by (1) a substantial fraction of patients with little or no renal disease progression in the placebo group (mean decline in eGFR from baseline to end of treatment was 3.39 ml/min per 1.73 m2; Table 2), and (2) possible nonrandom dropouts leading to informative censoring (38% of randomized patients did not complete the 12 months of treatment; Figure 1). In longer-term trials, time-to-event analyses on the basis of designated changes in SCr or eGFR can, in some cases, provide greater statistical power than analyses with a basis of mean change in SCr or eGFR as a continuous variable, especially if the treatment effect is proportional to the rate of disease progression.36 In addition, the latter approach would not be as influenced by small changes in SCr related to events other than the natural progression of the chronic disease. It is possible that assessing measured GFR using exogenous markers, such as iohexol, would provide greater precision in estimating the longitudinal loss of renal function. However, it is unlikely that their use would change the overall conclusions about the lack of a beneficial treatment effect. Finally, treating less severe disease or providing more complete TGF-β inhibition (producing corresponding changes in early response pharmacodynamic biomarkers) might be required to slow renal progression. Methods to more fully inhibit this pathway include a more intensive TGF-β1 mAb dose regimen (i.e., use of higher doses or more frequent administration) or use of a pan–TGF-β inhibitor. These approaches likely increase the risk of serious toxicity from chronic and excessive TGF-β inhibition, and are of uncertain clinical benefit. What is apparent is that the current attempt to modulate TGF-β1 activity by intermittently dosing with an mAb, while appearing to be tolerated, did not slow the progression of DN.
Concise Methods
Design Overview
All patients provided informed consent before any investigational procedures, and the trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. The trial is registered with Clinicaltrials.gov (identifier: NCT01113801).
This study was a randomized, double-masked, placebo-controlled, multicenter, multicountry, phase 2 study that included three periods: a screening and optional run-in period, a 12-month treatment period, and a 2-month follow-up period. The optional run-in period was up to 4 months when necessary, and was principally used to equilibrate patients on appropriate RAS inhibitor treatment (defined as the maximal US Food and Drug Administration labeled dose of the agent, or a lesser dose that the investigator considered appropriate for the patient). For patients without documentation who indicated an intolerance of RAS treatment, treatment with a single agent was required for at least 3 months. Patients also had to be on the same dose for at least 2 months and receiving a dose that was not expected to change during the study period.
Participants
Eligible patients were women (of nonchildbearing potential) and men ≥25 years of age, with a diagnosis of type 1 diabetes or type 2 diabetes, according to the American Diabetes Association definition.37 Patients were required to have proteinuric CKD and reduced renal function believed to be caused by diabetes (with or without superimposed hypertension-induced nephrosclerosis), defined as follows: (1) an SCr concentration of 1.3‒3.3 mg/dl (115‒291 mmol/L) for women and 1.5‒3.5 mg/dl (132‒309 mmol/L) for men, or an eGFR of 20–60 ml/min per 1.73 m2, according to the simplified Modified Diet in Renal Disease formula38; and (2) a 24-hour UPCR of at least 800 mg/g (91 mg/mmol) while receiving RAS treatment. As described above, patients who could tolerate RAS treatment required treatment for at least 3 months and a stable dose for at least 2 months. Stable BP of ≤150/90 mmHg and stable doses of BP medications were also required. Patients entered the run-in period if they needed an equilibration period to stabilize on the RAS inhibitor treatment, other BP treatment, or for any other reason. To qualify for randomization after the run-in period, SCr had to be stable, as indicated by two consecutive measurements obtained 10–21 days apart immediately before randomization, and were within 25% of each other. For drugs that antagonize renin-angiotensin-aldosterone system activity, only single-agent ACEi or ARB treatment was allowed.
Patients who met the study entry criteria were randomly assigned to placebo or a TGF-β1 mAb dose of 2, 10, or 50 mg. Masked, trained study site personnel administered study medication monthly by subcutaneous injection, beginning at the randomization visit and ending with the 12th dose administered at month 11. Patients were seen at least monthly at the research site during the 12-month treatment period (ending 1 month after the final dose), and a final visit occurred at month 14 (3 months after the final dose). Study treatment was discontinued for patients requiring dialysis.
Measurements
SCr was measured at Covance Central Laboratory (Princeton, NJ) using an automated enzymatic chemistry panel performed on Roche Modular/Cobas instruments. The SCr assay demonstrated an interassay variability (percentage coefficient of variation) of <2% at an SCr concentration of 0.85–6.7 mg/dl, and intra-assay precision of 0.5%–1.1% coefficient of variation. The SCr levels were determined at baseline, months 2, 3, and 4, and then at least every 2 months for the duration of the study, ending at month 14. In addition, baseline and end-of-treatment SCr values were on the basis of the average of two samples. Baseline was established from two samples obtained no more than 30 days apart, with the second sample obtained just before randomization. End-of-treatment SCr was calculated from samples obtained 1 week apart, at the end of the 12-month treatment period. Duplicate first-morning urine collections for UPCR were obtained on consecutive mornings at baseline and 5.5, 6, 12, and 14 months. Safety data, including data on adverse events, standard clinical chemistry parameters, complete blood count with differential counts, and antinuclear antibody titers were obtained at screening and months 1, 3, 9, and 12.
After the decision to prematurely stop the study, sites were notified and instructed to have patients complete the visit procedures associated with the end-of-treatment visit, as well as the procedures associated with the follow-up visits occurring 1 and 3 months after the final dose of study medication.
Exploratory Biomarkers
Plasma and urine biomarkers were measured from stored samples collected at baseline and 1, 3, 5.5, 6, 9, and 12 months after treatment initiation. All but the 5.5-month postbaseline time point represented effects at trough drug concentrations. The 5.5-month sample corresponded to the middle of the dosing interval and was included to minimize the chance of not detecting a biomarker response that was not sustained over the entire dosing interval. Sample donation was optional for patients. The following biomarkers were measured in those patients providing samples: cystatin C, sTNFR2, KIM-1, and NGAL in plasma; and fibronectin, HMW IV, MCP-1, and MMP-7 in urine. Urine biomarkers were normalized to measured urine creatinine. All biomarkers were measured in duplicate with commercially available ELISAs. Reported values were averaged across replicate measurements, and replicate deviations were averaged across all measured values to assess assay variability.
PK Analyses
A population PK analysis was conducted on all patients randomized to mAb who received at least one dose of investigational drug and contributed samples for analysis. All mAb serum concentration data were pooled for population analysis using the nonlinear mixed-effect modeling program NONMEM (version 7.3, ICON PLC., Hanover, MD). A series of one-compartment structural models with linear absorption and first-order elimination were evaluated. Interindividual variability terms on absorption rate constant, CL/F, and V/F were tested, as were proportional, additive/proportional, and additive residual error structures.
A series of continuous covariates (body weight, body mass index, and age) and categorical covariates (sex, race, and ethnicity) were assessed by standard forward inclusion and backward elimination methodology to determine their significance in further characterizing interindividual differences in PK parameters. Parameters were included in the forward inclusion step if they resulted in a drop of ≥6.635 points in objective function value, assuming a chi-squared distribution and 1 degree of freedom (P≤0.01), and retained in the backward elimination step if they resulted in ≥10.828 increase in objective function value (P≤0.001) upon removal.
Statistical Analyses
The primary outcome was the change in SCr from baseline to 12 months, and the study was powered on the basis of an assumed 16% rise in SCr for the placebo group. Primary outcome was assessed by analyzing log-transformed SCr change from baseline in all patients using a mixed-effects repeated-measures analysis of covariance, with treatment, visit (categorical), and interaction as fixed effects, patient as a random effect, and baseline SCr and baseline first-morning UPCR as covariates. An overall treatment effect on the basis of contrasts at last visit was tested using a maximum trend test with one-sided type 1 error, controlled at 5%. If an eGFR value was <8 ml/min per 1.73 m2 then it was imputed to 8 ml/min per 1.73 m2, and the corresponding SCr was back-calculated on the basis of that imputation.
Secondary efficacy analyses included analysis of covariance of change in SCr from baseline to 12 months, with last observation carried forward (treatment as main effect and baseline SCr and baseline first-morning UPCR as covariates) for subgroups (sex, race, ethnicity, age group, geographic region, baseline UPCR, baseline SCr, need for study run-in period, and type of diabetes). Median values of continuous variables were used to define subgroups. Secondary end points, such as change in first-morning UPCR and eGFR, were analyzed in a similar manner to SCr. Maximum trend test analyses on the linear slope of change were also conducted for SCr and eGFR.
An independent DMC, including two nephrologists, a cardiologist, and a statistician, oversaw the study and provided unmasked safety reviews at intervals of approximately 6 months. Deaths, major cardiovascular events, ESRD events, suspected AKI events, cancer, and suspected autoimmune events were adjudicated by an independent Event Adjudication Committee using prespecified case definitions.
Disclosures
J.V., P.H.B., M.S., K.D., T.S., and B.M. are employees and/or stockholders of Eli Lilly and Co. (Indianapolis, IN). J.B.L. has received research and travel support from Eli Lilly and Co. T.G. has received salary support from Eli Lilly and Co.
Supplementary Material
Acknowledgments
The authors thank the trial investigators (see Supplemental Table 5), trial staff, and trial participants for their contributions, without which this study could not have been performed. We further acknowledge the efforts of Drs. Gil Rubin, Carole Salaun Martin, Terezie Skokanova, and Reka Veress for country-level study coordination activities in their respective countries; Alexandria Gunnell, James Knight, and Jozef Milata for clinical trial study management; Dennis Laska for exploratory biomarker analyses; and Eric Nantz and Joseph Haas for statistical support. We thank ICON, which functioned as the clinical research organization for the study, and Covance, for laboratory analyses. In addition, we would like to thank the members of the respective Data Monitoring Committee and Event Adjudication Committee. Finally, we thank Deborah Swartz-Basile, of Eli Lilly and Co., for medical writing support.
This trial was sponsored by Eli Lilly and Co. (Indianapolis, IN).
Partial results from this study were presented at the meeting of the American Society of Nephrology on November 15, 2014, in Philadelphia, PA.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015111230/-/DCSupplemental.
References
- 1.Anand S, Bitton A, Gaziano T: The gap between estimated incidence of end-stage renal disease and use of therapy. PLoS One 8: e72860, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United States Renal Data System : 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2014 [Google Scholar]
- 3.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J: Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 305: 2532–2539, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Diabetes Federation : IDF Diabetes Atlas, 6th Ed., Brussels, Belgium, International Diabetes Federation, 2013 [Google Scholar]
- 5.Hu FB: Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 34: 1249–1257, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I; Collaborative Study Group : Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S; RENAAL Study Investigators : Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group : KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 1–150, 2013 [Google Scholar]
- 9.Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, Williams DE, Geiss L: Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med 370: 1514–1523, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Böttinger EP: TGF-β in renal injury and disease. Semin Nephrol 27: 309–320, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Miyazono K, Kusanagi K, Inoue H: Divergence and convergence of TGF-β/BMP signaling. J Cell Physiol 187: 265–276, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Massagué J: TGFbeta in Cancer. Cell 134: 215–230, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massagué J: TGF-β signal transduction. Annu Rev Biochem 67: 753–791, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Attisano L, Wrana JL: Signal transduction by the TGF-β superfamily. Science 296: 1646–1647, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Pohlers D, Brenmoehl J, Löffler I, Müller CK, Leipner C, Schultze-Mosgau S, Stallmach A, Kinne RW, Wolf G: TGF-β and fibrosis in different organs - molecular pathway imprints. Biochim Biophys Acta 1792: 746–756, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Ziyadeh FN, Hoffman BB, Han DC, Iglesias-De La Cruz MC, Hong SW, Isono M, Chen S, McGowan TA, Sharma K: Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-β antibody in db/db diabetic mice. Proc Natl Acad Sci U S A 97: 8015–8020, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S, Iglesias-de la Cruz MC, Jim B, Hong SW, Isono M, Ziyadeh FN: Reversibility of established diabetic glomerulopathy by anti-TGF-β antibodies in db/db mice. Biochem Biophys Res Commun 300: 16–22, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Yu L, Border WA, Huang Y, Noble NA: TGF-β isoforms in renal fibrogenesis. Kidney Int 64: 844–856, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Rowlinson S, Brault P, Galbreath E, Jones B, Mcentire J, McKinney T, Shanafelt A, Syed S, Voelker J, Zhang T, Dunn S, Heuer J, Sharma K: An anti-TGF-β1 specific mAb demonstrates renal efficacy equivalent to a pan neutralizing mAb in the rat anti-Thy1.1 and mouse db/db models [Abstract]. J Am Soc Nephrol 18: 414A, SA-PO329, 2007
- 20.Border WA, Okuda S, Languino LR, Sporn MB, Ruoslahti E: Suppression of experimental glomerulonephritis by antiserum against transforming growth factor β 1. Nature 346: 371–374, 1990 [DOI] [PubMed] [Google Scholar]
- 21.Okuda S, Languino LR, Ruoslahti E, Border WA: Elevated expression of transforming growth factor-beta and proteoglycan production in experimental glomerulonephritis. Possible role in expansion of the mesangial extracellular matrix. J Clin Invest 86: 453–462, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Border WA, Noble NA: Transforming growth factor beta in tissue fibrosis. N Engl J Med 331: 1286–1292, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Ziyadeh FN: Mediators of diabetic renal disease: the case for tgf-Beta as the major mediator. J Am Soc Nephrol 15[Suppl 1]: S55–S57, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto T, Noble NA, Cohen AH, Nast CC, Hishida A, Gold LI, Border WA: Expression of transforming growth factor-beta isoforms in human glomerular diseases. Kidney Int 49: 461–469, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Sharma K, Ziyadeh FN, Alzahabi B, McGowan TA, Kapoor S, Kurnik BR, Kurnik PB, Weisberg LS: Increased renal production of transforming growth factor-beta1 in patients with type II diabetes. Diabetes 46: 854–859, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Sharma K, Eltayeb BO, McGowan TA, Dunn SR, Alzahabi B, Rohde R, Ziyadeh FN, Lewis EJ: Captopril-induced reduction of serum levels of transforming growth factor-beta1 correlates with long-term renoprotection in insulin-dependent diabetic patients. Am J Kidney Dis 34: 818–823, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Esmatjes E, Flores L, Iñigo P, Lario S, Ruilope LM, Campistol JM: Effect of losartan on TGF-beta1 and urinary albumin excretion in patients with type 2 diabetes mellitus and microalbuminuria. Nephrol Dial Transplant 16[Suppl 1]: 90–93, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Agarwal R, Siva S, Dunn SR, Sharma K: Add-on angiotensin II receptor blockade lowers urinary transforming growth factor-beta levels. Am J Kidney Dis 39: 486–492, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Benigni A, Zoja C, Campana M, Corna D, Sangalli F, Rottoli D, Gagliardini E, Conti S, Ledbetter S, Remuzzi G: Beneficial effect of TGFbeta antagonism in treating diabetic nephropathy depends on when treatment is started. Nephron, Exp Nephrol 104: e158–e168, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Trachtman H, Fervenza FC, Gipson DS, Heering P, Jayne DR, Peters H, Rota S, Remuzzi G, Rump LC, Sellin LK, Heaton JP, Streisand JB, Hard ML, Ledbetter SR, Vincenti F: A phase 1, single-dose study of fresolimumab, an anti-TGF-β antibody, in treatment-resistant primary focal segmental glomerulosclerosis. Kidney Int 79: 1236–1243, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma K, Ix JH, Mathew AV, Cho M, Pflueger A, Dunn SR, Francos B, Sharma S, Falkner B, McGowan TA, Donohue M, Ramachandrarao S, Xu R, Fervenza FC, Kopp JB: Pirfenidone for diabetic nephropathy. J Am Soc Nephrol 22: 1144–1151, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma LJ, Jha S, Ling H, Pozzi A, Ledbetter S, Fogo AB: Divergent effects of low versus high dose anti-TGF-beta antibody in puromycin aminonucleoside nephropathy in rats. Kidney Int 65: 106–115, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Khanna AK, Plummer MS, Hilton G, Pieper GM, Ledbetter S: Anti-transforming growth factor antibody at low but not high doses limits cyclosporine-mediated nephrotoxicity without altering rat cardiac allograft survival: potential of therapeutic applications. Circulation 110: 3822–3829, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Yu L, Border WA, Anderson I, McCourt M, Huang Y, Noble NA: Combining TGF-beta inhibition and angiotensin II blockade results in enhanced antifibrotic effect. Kidney Int 66: 1774–1784, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Levey AS, Inker LA, Matsushita K, Greene T, Willis K, Lewis E, de Zeeuw D, Cheung AK, Coresh J: GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis 64: 821–835, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Gassman JJ, Greene T, Wright JT Jr, Agodoa L, Bakris G, Beck GJ, Douglas J, Jamerson K, Lewis J, Kutner M, Randall OS, Wang SR: Design and statistical aspects of the African American Study of Kidney Disease and Hypertension (AASK). J Am Soc Nephrol 14[Suppl 2]: S154–S165, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.American Diabetes Association : Diagnosis and classification of diabetes mellitus. Diabetes Care 33[Suppl 1]: S62–S69, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration : Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.