Abstract
Hyperuricemia may contribute to endothelial dysfunction in CKD. We evaluated whether lowering serum uric acid levels with allopurinol improves endothelial dysfunction in 80 participants ≥18 years of age with stage 3 CKD and asymptomatic hyperuricemia (≥7 mg/dl in men and ≥6 mg/dl in women) randomized in a double-blinded manner to receive placebo or allopurinol for 12 weeks. Randomization was stratified according to presence or absence of diabetes mellitus. We measured vascular endothelial function by brachial artery flow-mediated dilation. No significant differences existed between groups at baseline; 61% of the participants had diabetes mellitus in both groups. The placebo and the allopurinol groups had baseline serum uric acid levels (SDs) of 8.7 (1.6) mg/dl and 8.3 (1.4) mg/dl, respectively, and baseline flow-mediated dilation values (SDs) of 6.0% (5.0%) and 4.8% (5.0%), respectively. Compared with placebo, allopurinol lowered serum uric acid significantly but did not improve endothelial function. In participants without diabetes mellitus, allopurinol associated with a trend toward improved flow-mediated dilation (+1.4% [3.9%] versus −0.7% [4.1%] with placebo), but this was not statistically significant (P=0.26). Furthermore, we did not detect significant differences between groups in BP or serum levels of markers of inflammation and oxidative stress. In conclusion, allopurinol effectively and safely lowered serum uric acid levels in adults with stage 3 CKD and asymptomatic hyperuricemia but did not improve endothelial function in this sample of patients.
Keywords: uric acid, allopurinol, CKD, flow-mediated dilation
CKD is a highly prevalent condition1; one that associates with high mortality, as patients with CKD are more likely to die than reach ESRD.2 The increased risk of death in CKD is likely due to a high prevalence of cardiovascular disease (CVD), as multiple studies have shown CKD is an independent risk factor for CVD.3–7 This is not surprising considering the high prevalence of traditional (Framingham) risk factors in this patient population.8,9 In addition, several nontraditional risk factors have been proposed to play an important role in CVD in patients with CKD, including endothelial dysfunction,10 inflammation,11 and oxidative stress.12
Endothelial dysfunction is evident in patients with CKD.13,14 In some reports, it precedes the onset of kidney function decline and is an independent predictor of incident CKD.15 Experimental evidence suggests that endothelial dysfunction plays an important role in kidney disease as inhibition of nitric oxide synthase leads to hypertension (systemic and glomerular), glomerulosclerosis, and tubulo-interstitial injury.16,17 Indeed, reduced nitric oxide (NO) is necessary for the development and progression of experimental kidney disease that mimics human disease, supporting a key role for endothelial dysfunction in human CKD.18,19 In addition, CKD itself may contribute to endothelial dysfunction via several factors, including a heightened state of inflammation and oxidative stress.20–24 One modifiable risk factor that may contribute to the triad of endothelial dysfunction, inflammation, and oxidative stress is elevated uric acid.
Uric acid is a byproduct of purine metabolism that is predominantly eliminated by the kidney.25 Serum uric acid levels are increased in CKD and have been shown to independently predict all-cause and cardiovascular mortality in patients with CKD.26 Experimental data suggest that uric acid plays a direct role in CVD as increased serum uric acid levels induce endothelial dysfunction in cells27 and in rats,28 and induce inflammation in vascular smooth muscles.29,30 In patients with CKD, increased serum uric acid levels have been associated with endothelial dysfunction,31,32 suggesting that lowering serum uric acid levels might be of therapeutic benefit. In this study, we sought to evaluate whether lowering serum uric acid levels with allopurinol in adult patients with stage 3 CKD would improve brachial artery flow-mediated dilation (BA-FMD). BA-FMD is a noninvasive assessment of endothelial function whereby brachial artery dilatation is measured in response to reactive hyperemia. The procedure has been utilized extensively in patients with CKD.13,21,33,34 Importantly, endothelial dysfunction, measured by BA-FMD, has been shown to predict CVD prospectively in healthy subjects,35–37 subjects free of clinical CVD,38 individuals with peripheral vascular disease,39 and in individuals with CKD.10 Considering the important effect of diabetes mellitus (DM) itself on vascular endothelial function,40 we ensured equal numbers of patients with DM in the placebo and treatment arms, by stratifying our randomization on the basis of DM status. We also evaluated potential physiologic mechanisms by measuring markers of inflammation and oxidative stress both systemically and in vascular endothelial cells collected from study participants.
Results
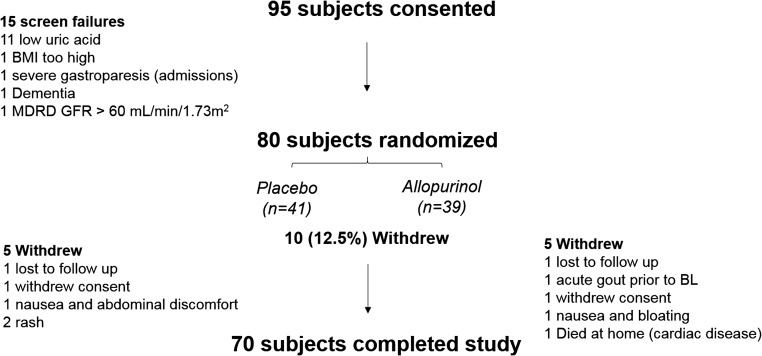
Between April 2011 and May 2015 we consented 95 participants with stage 3 CKD for participation in the study. Of those, 15 participants failed the screening process, most commonly due to lower uric acid levels than required by the inclusion criteria (Figure 1). Of the 80 subjects that were randomized, ten (12.5%) withdrew from the study, five in each group. Seventy participants completed the study measurements. Reasons for withdrawal are illustrated in the study schema in Figure 1. Of the 70 participants who completed the study, four were unable to tolerate the maximum dose of 300 mg per day. Two subjects took only 100 mg per day; one due to nausea and the other due to mildly elevated liver function tests (LFT). Another two subjects took only 200 mg per day; one due to nausea and the other due to mildly elevated LFT. Baseline characteristics for all of the participants are shown in Table 1 by study assignment. There were no significant differences between the placebo and allopurinol groups in age, gender, or race. The majority of participants in both groups had a history of DM (61% in both groups). In addition, baseline serum uric acid levels, Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) eGFR, and BA-FMD were not statistically different between both groups. Baseline characteristics for the participants who withdrew compared with those who completed the study are shown in Supplemental Table 1. Monocyte chemotactic protein-1 (MCP-1) levels were significantly lower (P=0.01) and triglyceride levels were marginally lower (P=0.07) in the participants who withdrew from the study versus those who completed it.
Figure 1.
Out of 80 randomized subjects, 70 completed the study. Study schema. MDRD, Modification of Diet in Renal Disease.
Table 1.
Baseline characteristics according to study group
| Characteristics | Placebo (n=41) | Allopurinol (n=39) |
|---|---|---|
| Age, yr | 58.9±9.3 | 55.9±13.7 |
| Male gender | 32 (78%) | 32 (82%) |
| Race | ||
| White | 34 (83%) | 25 (64%) |
| Black | 5 (12%) | 9 (23%) |
| Other | 2 (5%) | 5 (13%) |
| Hispanic ethnicity | 9 (22%) | 8 (21%) |
| History of smoking | 7 (19%) | 6 (16%) |
| History of DM | 25 (61%) | 23 (61%) |
| History of CVD | 20 (49%) | 16 (41%) |
| BMI, kg/m2 | 32.9±5.7 | 31.7±4.8 |
| SBP, mmHg | 130±16 | 127±14 |
| DBP, mmHg | 77.7±8.6 | 77.4±10.6 |
| HbA1C, % | 6.4±1.4 | 6.6±1.9 |
| Triglycerides, mg/dl | 162±111 | 166±209 |
| LDL-C, mg/dl | 84±23 | 79.5±27 |
| HDL-C, mg/dl | 37.3±8.6 | 38.9±13.8 |
| Creatinine, mg/dl | 1.75±0.42 | 1.81±0.37 |
| CKD-EPI eGFR, ml/min per 1.73m2 | 42.4±9.6 | 41.3±8.9 |
| ACR, mg/g | 452±646 | 374±581 |
| Serum urate levels, mg/dl | 8.7±1.6 | 8.3±1.4 |
| BA-FMD, % Δ | 6.0±5.0 | 4.8±5.0 |
| NMD, % Δ | 19.9±10.8 | 17.6±7.7 |
| Baseline CRP, mg/L | 3.6±3.6 | 5.4±6.7 |
| IL-6, pg/ml | 2.8±3.1 | 4.8±12.9 |
| MCP-1, pg/ml | 169.7±72.3 | 152.0±43.3 |
| Ox-LDL, U/L | 49.3±15.0 | 45.4±11 |
Values are expressed as mean±SD or n (percentage of patients). CVD was defined as history of myocardial infarction, peripheral vascular disease, angina, congestive heart failure, or arrhythmia. SBP, systolic BP; DBP, diastolic BP; LDL-C, LDL cholesterol; HDL-C, HDL cholesterol; ACR, urinary ACR; BA-FMD % Δ, % change in BA-FMD; NMD % Δ, % change in NMD.
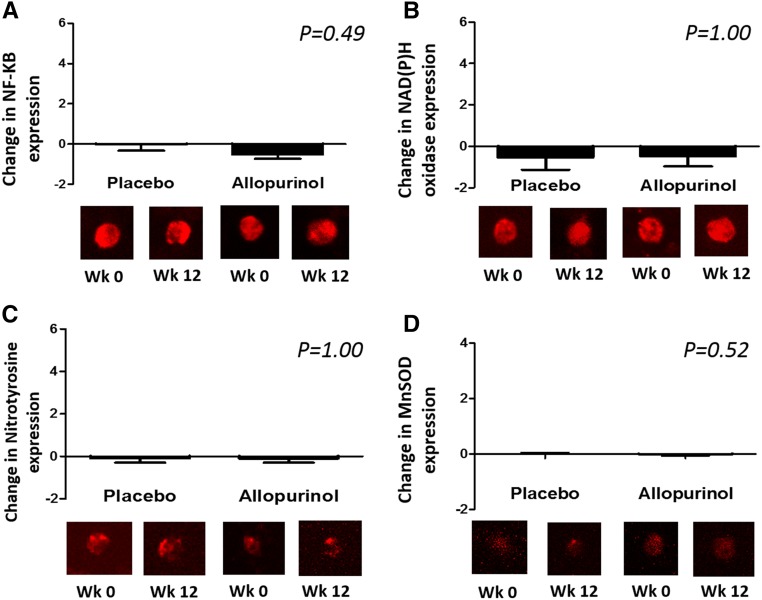
As shown in Table 2, allopurinol at 300 mg per day for 12 weeks effectively lowered serum uric acid levels by 3.24±1.35 mg/dl as compared with a change of 0.05±1.54 mg/dl in the placebo group (P<0.001). BA-FMD increased slightly and insignificantly in both groups by 0.2%±4.1% and 0.9%±3.9% in the placebo and allopurinol groups respectively (P=0.47). In the placebo group nitroglycerin-mediated dilation (NMD) tended to decrease (worsen) by 1.3%±5.3%, whereas it tended to increase (improve) by 0.9%±6.1% in the allopurinol group (P comparing change between groups =0.14). After 12 weeks, there was no significant difference between both groups in systolic or diastolic BP, high-sensitivity C-reactive protein (CRP), interleukin-6 (IL-6), MCP-1, or oxidized low-density lipoprotein (ox-LDL). Consistent with the lack of an effect on systemic markers of inflammation and oxidative stress, no significant change was noted in the vascular endothelial cell expression of nuclear factor-kappa B (NF-κB), nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase), nitrotyrosine, or manganese superoxide dismutase (MnSOD). These data are shown in Figure 2. Of note, median compliance with study drug, defined as percent number of pills taken/number of pills provided, was 99% (interquartile range, 89%–100%) in the placebo group and 95% (interquartile range, 82%–100%) in the allopurinol group.
Table 2.
Change from baseline according to treatment group
| Variable | Placebo (n=41) | Allopurinol (n=39) | P Value |
|---|---|---|---|
| Serum urate, mg/dl | 0.05±1.54 | −3.24±1.35 | <0.001 |
| BA-FMD, % Δ | 0.2±4.1 | 0.9±3.9 | 0.47 |
| NMD, % Δ | −1.3±5.3 | 0.9±6.1 | 0.14 |
| Systolic BP, mmHg | −1.63±15.51 | −1.70±17.52 | 0.85 |
| Diastolic BP, mmHg | −0.97±11.8 | 0.97±10.8 | 0.51 |
| CRP, mg/L | 0.70±3.4 | 0.42±9.5 | 0.78 |
| IL-6, pg/ml | 0.15±3.1 | 0.37±2.7 | 0.75 |
| MCP-1, pg/ml | −4.7±45.8 | 3.6±36.7 | 0.47 |
| Ox-LDL, U/L | −0.08±11.8 | −2.97±16.4 | 0.19 |
Value are expressed as absolute change from baseline±SD. BA-FMD % Δ, % change in BA-FMD; NMD % Δ, % change in NMD.
Figure 2.
Allopurinol did not significantly reduce the expression of NF-ҡB, NADPH oxidase, nitrotyrosine, or manganese superoxide dismutase (MnSOD) in the endothelial cells collected from study participants. The figures represent change in the expression of each protein from baseline by study group. Values for each sample were reported as arbitrary units and represent ratios of endothelial cell protein expression to human umbilical vein endothelial cell expression in order to account for any variation in the staining procedure. (A) NF-ҡB (n=32); (B) NADPH oxidase (n=32); (C) nitrotyrosine (n=30); (D) MnSOD (n=34).
We then stratified our analysis by presence or absence of history of DM defined a priori. Allopurinol, compared with placebo, significantly lowered serum uric acid levels in both groups (Table 3). In subjects with DM, BA-FMD increased by 0.68%±4.03% and 0.55%±4.05% in the placebo and allopurinol study groups, respectively. In contrast, BA-FMD increased (improved) by 1.4%±3.9% with allopurinol compared with declining (worsening) by −0.7%±4.1% with placebo in the participants without history of DM, but the difference between both groups was not statistically significant (P=0.26). A similar trend was noted for several of the markers of inflammation and oxidative stress. In the subjects without DM, CRP, IL-6, and ox-LDL were lower after 12 weeks of allopurinol compared with placebo, but the differences between both study groups were not statistically significant (data not shown).
Table 3.
Change in vascular function from baseline according to treatment group stratifying by DM status
| Variable | No History of DM | History of DM | ||||
|---|---|---|---|---|---|---|
| Placebo (n=16) | Allopurinol (n=15) | P Value | Placebo (n=25) | Allopurinol (n=23) | P Value | |
| Δ uric acid, mg/dl | −0.22±1.35 | −2.74±1.03 | <0.001 | 0.20±1.65 | −3.31±1.24 | <0.001 |
| BA-FMD, % Δ | −0.7±4.1 | 1.4±3.9 | 0.26 | 0.7±4.03 | 0.6±4.1 | 0.92 |
| NMD, % Δ | −0.8±5.7 | −0.1±7.3 | 0.79 | −1.6±5.2 | 1.8±5.3 | 0.13 |
Value are expressed as absolute change from baseline±SD. BA-FMD % Δ, % change in BA-FMD; NMD % Δ, % change in NMD.
In secondary analysis, we examined whether any clinical characteristics correlated with baseline BA-FMD. In univariate analysis, sex, age, history of CVD, systolic BP, and CKD-EPI eGFR associated significantly with BA-FMD at baseline. We then evaluated whether treatment with allopurinol correlated with end of study BA-FMD by linear regression. We found no association between treatment with allopurinol and end of study BA-FMD in unadjusted analysis (data not shown). After adjustment for sex, age, history of CVD, systolic BP, and CKD-EPI eGFR, there was no association between treatment with allopurinol and end of study BA-FMD. These results were unchanged when we included baseline BA-FMD and an interaction term for DM in the multivariate model (β estimate, 0.67; 95% confidence interval, −2.32 to 3.65; P=0.66). With regard to NMD, we found the following covariates correlated with baseline NMD in univariate analysis: sex, age, non-Hispanic ethnicity, history of DM, body mass index (BMI), systolic BP, hemoglobin (HbA1C), urinary albumin-to-creatinine ratio (ACR), and CKD-EPI eGFR. There was no association between treatment with allopurinol and end of study NMD in unadjusted or adjusted analysis (including sex, age, non-Hispanic ethnicity, history of DM, BMI, systolic BP, HbA1C, urinary ACR, and CKD-EPI eGFR). After including baseline NMD and an interaction term for DM in the multivariate model there was no association between treatment with allopurinol and end of study NMD (β estimate, −1.70; 95% confidence interval, −6.14 to 2.74; P=0.44).
Adverse Events
We found a low incidence of adverse events in our study participants as shown in Table 4. Notably, two gout attacks occurred during the study period, one in the placebo group and one in the allopurinol group. There was an equal number of gastrointestinal discomforts reported in both groups. In two of the participants with nausea, this led to dose reduction of the study drug (one in the placebo group and one in the allopurinol group). The other two participants who reported gastrointestinal discomfort withdrew from the study. Abnormalities in LFT were mild and led to dose reduction although these were more common in the placebo group than the allopurinol group. Three participants were noted to have mild thrombocytopenia during the study phase; two in the placebo group and one in the allopurinol group. One subject in the allopurinol group died during the study due a cardiac event and this was deemed likely not related to the study.
Table 4.
Adverse events
| Variable | Placebo (n=41) | Allopurinol (n=39) |
|---|---|---|
| Gout attack | 1 | 1 |
| Gastrointestinal adverse events | 2 | 2 |
| Rash | 2 | 0 |
| Abnormal LFT | 2 | 0 |
| Thrombocytopenia | 2 | 1 |
| Death | 0 | 1 |
Value are expressed as number of subjects per group. Gastrointestinal adverse events were defined as: nausea, vomiting, diarrhea, bloating, and abdominal pain. There was one death during the study that was attributed to a cardiovascular event.
Discussion
In this randomized placebo-controlled study, we evaluated whether lowering serum uric acid levels with allopurinol improves vascular endothelial function in a representative sample of patients with stage 3 CKD. Randomization was stratified by presence or absence of history of DM. Several of our findings stand out. First, a moderate dose of allopurinol effectively and safely lowered serum uric acid levels in this group of patients. Second, despite the effective uric acid–lowering effect of allopurinol, we did not observe a significant difference in vascular endothelial function, as measured by BA-FMD, in the allopurinol group compared with placebo. Third, there was no significant difference in any of the markers of inflammation or oxidative stress that we measured either systemically or in the vascular endothelial cells of our participants. Although there was a trend toward improved BA-FMD with allopurinol (compared with placebo) in the participants without a history of DM, this did not achieve statistical significance, likely due to the small number of patients in this group (16 randomized to placebo and 15 to allopurinol).
The association between elevated serum uric acid and CVD appears to be well documented in several large epidemiologic studies in the general population,41–44 in patients with hypertension,45 and in patients with CKD.26 Experimental evidence suggests that uric acid may contribute to vascular disease by several mechanisms including endothelial dysfunction and inflammation. Uric acid has been shown to decrease NO production by endothelial cells in vitro,46 and does so in association with increased CRP expression.29 Uric acid can also react with NO irreversibly leading to the formation of 6-aminouracil and thus may lead to NO depletion.47 In addition, hyperuricemic rats develop endothelial dysfunction (as noted by reduced urinary nitrites), and early L-arginine supplementation can prevent both the systemic and glomerular hypertension in experimental hyperuricemia.28,48
Despite these data, it remains controversial whether uric acid is a mediator of endothelial dysfunction (and vascular disease in general) as opposed to a marker of disease.49 The controversy is routed in several findings. First, under physiologic concentrations, urate (the soluble form of uric acid) is a powerful antioxidant as it can scavenge superoxide, hydroxyl radicals, and singlet oxygen.50 Second, when infused intravenously, uric acid has been reported to improve endothelial function and oxidative stress in healthy adults51,52 and in patients with type 1 diabetes.53 Third, xanthine oxidase, the enzyme that converts hypoxanthine to xanthine, and xanthine to uric acid, also generates reactive oxygen species49 and may play a role in vascular disease.54 Almost all of the studies performed to evaluate the relationship between uric acid and endothelial function in humans have used allopurinol (xanthine oxidase inhibitor) in the treatment arm, and some have suggested that the benefit of allopurinol is mediated by mechanisms other than the uric acid–lowering effect.55–57 Such observations have collectively led some authors to argue that if serum uric acid levels are increased due to reduced clearance, as opposed to increased reactive oxygen species, then uric acid in this setting is cardioprotective.58
The prevalence of hyperuricemia is known to be increased in CKD.59,60 Several factors likely contribute to this, including reduced clearance due to low GFR, and the use of diuretics for BP and volume control. In addition, poor dietary habits and the high prevalence of other comorbidities likely contribute to the increased generation of uric acid.25 In this study, we sought to evaluate whether lowering uric acid levels with allopurinol might be a beneficial therapeutic strategy to improve vascular endothelial function in a representative sample of patients with stage 3 CKD. In contrast to our hypothesis, and although allopurinol effectively lowered serum uric acid levels, we found no significant improvement in vascular endothelial function with allopurinol compared with placebo. Our findings contrast with those of a recent study by Kao et al. where 67 older adults with stage 3 CKD received allopurinol or placebo.61 The primary outcome of this study was left ventricular hypertrophy, but the study also reported that BA-FMD improved slightly with allopurinol (+1.26%±3.06%) compared with placebo (−1.05%±2.84%). The differences in our findings might be explained by the fact that our study included a significantly larger number of patients with DM. Only one out of 26 participants in the placebo group and five out of 27 participants had diabetic kidney disease in the preceding study, whereas 61% of our participants had DM in both study groups. Considering that many proinflammatory and pro-oxidant pathways are activated in DM by other factors independently of hyperuricemia62 and that these pathways contribute to vascular disease also independently of hyperuricemia,63 it is possible that lowering serum uric acid levels would not affect vascular disease in these groups of patients. Consistent with this, when we evaluated our data on the basis of DM status, we found that there was a trend toward improved BA-FMD, CRP, IL-6, and ox-LDL in our nondiabetic subjects. These differences did not achieve statistical significance, nor did the interaction term for DM in the linear regression model, likely due to the small number of participants without DM. Another possible explanation for the lack of benefit in our study is that BP was well controlled before study entry. Other studies that have shown lowering serum uric levels with allopurinol improves endothelial function in patients with type 2 DM included patients with uncontrolled hypertension,56 and it is possible that the improvement in endothelial function in these studies was due to improvement in BP.64
Our study has many strengths including that it is a double-blinded randomized placebo-controlled study. In addition, we were adequately powered to detect a clinically significant difference in the primary outcome (BA-FMD), and the study was completed with a smaller than anticipated number of withdrawals. Furthermore, we included a detailed evaluation of markers of inflammation and oxidative stress including at the endothelial cellular level. Most importantly, we obtained significant separation of serum uric acid levels between the placebo and allopurinol study groups. Notwithstanding these strengths, our study has several weaknesses. First, we evaluated the potential benefit of allopurinol on vascular endothelial function, a surrogate outcome, over 12 weeks. This is a sufficient duration for the evaluation of endothelial dysfunction as several studies have shown even shorter duration of treatment with allopurinol-improved endothelial function in smokers (acutely)55 and in patients with congestive heart failure (1 week),57 but it does not allow for the evaluation of hard outcomes such as cardiovascular events and mortality. Of note, allopurinol has been shown to slow CKD progression65 and to reduce cardiovascular risk in small studies.66 It remains possible that lowering uric acid might improve such outcomes via other mechanisms than evaluated in our study such as vascular stiffness.67 Second, we were not adequately powered to detect a small difference in BA-FMD on subgroup analysis. The American College of Cardiology recommends at least 20 participants per group to enable the detection of an absolute difference of 1.5%–2.0% in flow-mediated dilation (FMD) in parallel-group studies.68 Notably, we only had 31 participants without DM; 16 in the placebo group and 15 in the allopurinol group. Thus, we cannot exclude that the use of allopurinol to lower serum uric acid levels may improve vascular endothelial function in patients with stage 3 CKD in the absence of DM.
In conclusion, allopurinol effectively and safely lowers serum uric acid levels in patients with stage 3 CKD. We did not observe, however, a significant improvement in vascular endothelial function in this representative sample of patients with stage 3 CKD. Similarly, we were unable to detect a significant difference in markers of inflammation or oxidative stress. Our data suggest that uric acid does not play a significant role in endothelial dysfunction, inflammation, or oxidative stress in this group of patients. It remains possible that lowering serum uric acid levels may improve outcomes in CKD by mechanisms other than evaluated here, and future studies are necessary to assess whether lowering serum uric acid levels might improve hard outcomes.
Concise Methods
Study Population
Recruitment for the study commenced in April 2011. Patients were recruited from three locations: the CKD clinics at the University of Colorado Hospital (Anschutz Medical Campus), the Denver Veteran’s Administration Hospital, and Denver Health Medical Center. Individuals were considered eligible for participation if they were 18 years of age or older, had stage 3 CKD on the basis of Modification of Diet in Renal Disease (MDRD) eGFR of 30–59 ml/min per 1.73m2,1 had elevated serum uric acid levels (defined as >7.0 mg/dl for men and >6.0 mg/dl for women), and were able to give informed consent. Exclusion criteria included the following: life expectancy <1 year; uncontrolled hypertension; history of severe liver disease or congestive heart failure; active infection or on antibiotics; pregnant, breastfeeding, or unwilling to use adequate birth control; history of hospitalization within the last 3 months; expected to undergo living related kidney transplant in the next 6 months; history of immunosuppressive therapy in the last 6 months; history of warfarin use; BMI≥40 kg/m2; or serum albumin <3.0 mg/dl. In addition, subjects were excluded if they had acute gout attacks, were receiving allopurinol, or if they reported an adverse reaction to allopurinol in the past. The study was approved by the Colorado Multiple Institutional Review Board. The nature, benefits, and risks of the study were explained to the volunteers and their written informed consent was obtained before participation. Notably, all study visits and procedures were conducted at one location, that being The Clinical and Translational Research Center (CTRC) at the University of Colorado Anschutz Medical Campus. The study was registered at ClinicalTrials.gov (NCT01228903).
Study Intervention
Upon randomization, and after the baseline visit, participants were provided with 100 mg tablets of allopurinol or placebo. Participants were instructed to take one tablet of the study drug (either placebo or allopurinol) for 1 week and if tolerated to increase to two tablets for another week and subsequently to three tablets once a day (300 mg per day). Participants were instructed to remain on this maximum dose until the end of the study.
Follow-Up
In addition to the baseline and end of study visits, participants were seen at week 4 and at week 6. These study visits included a questionnaire to ascertain adverse events and to review medications. Safety labs were drawn at the week 4 visit including a complete blood count and LFT. A pill count was conducted at the week 6 and end of study visits.
Outcomes
Study participants were followed for 12 weeks. The primary outcome was change in BA-FMD from the week 0 to week 12 visits. BA-FMD was measured at the CTRC by a trained technician using high-resolution ultrasonography (GE Vivid 7 Dimension) as described originally by Celermajer et al.69 and subsequently by our group.33,70 Electrocardiogram-gated end-diastolic ultrasound images and Doppler flow of the artery were acquired during baseline and FMD conditions. For FMD, reactive hyperemia was produced by inflating a pediatric forearm cuff around the forearm to 250 mmHg for 5 minutes followed by rapid deflation. NMD was determined by measuring brachial artery dilation for 10 minutes after administration of sublingual nitroglycerin (0.4 mg). A commercially available software package (Vascular Analysis Tools 5.8.1, Medical Imaging Applications) was used to concurrently acquire electrocardiogram-gated brachial artery diameters. Brachial artery dilation was determined as the percentage change from baseline. Doppler flow of the brachial artery was also measured and peak shear rate was calculated as a potential covariate. The images were analyzed by an independent research assistant who was blinded to the study groups.
High sensitivity CRP, IL-6, and MCP-1 were measured as markers of inflammation. Ox-LDL was measured as a marker of systemic oxidative stress. These markers were measured on the collected serum by the Colorado Clinical Translational Sciences Institute core laboratory on the University of Colorado Anschutz Medical Campus. CRP was measured by an immunoturbidimetry method on the SYNCHRON Lx System. IL-6 was measured by Quantikine HS (R&D Systems, Minneapolis, MN) IL-6 ELISA kit. MCP-1 was measured via a commercially available Luminex multiplex assay (R&D Systems). Ox-LDL was measured by ALPCO Diagnostics ox-LDL ELISA kit.
To evaluate endothelial cell markers of inflammation and oxidative stress, endothelial cells were collected from the antecubital vein of study participants as published previously by our group.33,71–74 Collected cells were fixed with 3.7% formaldehyde and plated on slides. After blocking nonspecific binding sites with 5% normal donkey serum (Jackson ImmunoResearch Laboratories, West Grove, PA), cells were incubated with the primary antibody. Then, cells were incubated with CY3-conjugated secondary antibodies (Life Technologies, Carlsbad, CA). Slides were systematically scanned to identify endothelial cells (positive VE-Cadherin), and nuclear integrity was confirmed using 4',6'-diamidino-2-phenylindole hydrochloride staining. Once endothelial cells with intact nuclei were identified, images were captured and then analyzed using NIS Elements AR Software (Laboratory Imaging) to quantify the intensity of CY3 staining (i.e., average pixel intensity). Values for each sample were reported as ratios of endothelial cell protein expression to human umbilical vein endothelial cell expression to account for any variation in the staining procedure. Technicians were blinded to subject identity during the staining and analysis procedures. NF-κB was evaluated as a marker of proinflammatory signaling in 32 subjects (Santa Cruz Biotechnology, Santa Cruz, CA).71,75 NADPH oxidase72,73,76 (Upstate), nitrotyrosine72,73,76 (Abcam, Inc., Cambridge, MA), and manganese superoxide dismutase33 (EMD Millipore, Billerica, MA) were evaluated as markers of oxidative stress in 32, 30, and 34 subjects, respectively.
Other Variables
Race/ethnicity were evaluated by questionnaire. Similarly, smoking was evaluated by questionnaire as history of smoking (current or former) or no history of smoking. DM status was defined as history of DM according to the chart, current treatment with oral hypoglycemic agents or with insulin, or fasting glucose ≥126 mg/dl. Preexisting cardiovascular disease was defined as history of myocardial infarction, stroke, congestive heart failure, or arrhythmia. BP was measured via automated cuff after 10 minutes of rest at the beginning of each visit. Weight and height were measured, and BMI was calculated and expressed as kg/m2. We measured clinical labs including fasting lipid profile, fasting glucose and HbA1C, serum creatinine, and ACR at the University of Colorado Hospital clinical lab. eGFR was calculated on the basis of the recently described CKD-EPI formula.78 Compliance data were collected at the end of the study in the form of a pill count. Patient were asked to bring their pill bottles to the end of study visit. The number of pills patients actually took was calculated as: number of pills provided − number of pills returned. Percent compliance was then calculated as: actual pills taken/number of pills provided ×100%.
Randomization and Blinding
Randomization was stratified by presence or absence of history of DM to ensure an equal number of patients with DM in both study groups.40 Randomizations were generated by the statistician using blocks of four with a random number generator which was then forwarded to Belmar Pharmacy (Lakewood, Colorado). Research pharmacy staff were aware of the randomization and were responsible for the packaging and labeling of the study drug.
Statistical Analyses
Baseline characteristics are reported as mean±SD for continuous variables and as a number and percentage for categoric variables. The Wilcoxon two-sample rank-sum test, chi-squared test, and Fisher exact test were applied to compare continuous, categoric, and dichotomous variables, respectively, between both study groups. For the study outcomes, we compared change from baseline for each outcome between both study groups using the Wilcoxon two-sample rank-sum test. This statistical approach was also employed to evaluate change in secondary outcomes. α for the primary end point of BA-FMD was set at 0.05, with no adjustment for secondary outcomes.
For the secondary linear regression analysis, we examined whether there was an association between treatment with allopurinol and end of study BA-FMD or NMD respectively. We then evaluated whether any of the baseline variables correlated with baseline BA-FMD or NMD. Variables that were found to associate significantly with the vascular outcomes were then included in the linear regression models. An interaction term was subsequently applied to both linear regression models (BA-FMD and NMD) for DM. SAS software (version 6.3; SAS Institute Inc., Cary, NC) was used to conduct all analyses.
Sample Size
The primary end point for sample size calculation was BA-FMD. At the time of the study design, no study existed in the literature on the use of allopurinol and endothelial function in patients with CKD. Thus, we relied on published data in groups at similarly high risk of CVD to patients with CKD. Doehner et al.57 found that congestive heart failure patients receiving allopurinol (n=14) had higher (improved) BA-FMD=10.6±2.0 (mean±SEM) compared with placebo (n=14; FMD=6.7±0.1), with a difference in means of 3.9. Yiginer et al.77 found a similar difference in FMD between allopurinol and placebo among patients with metabolic syndrome (mean±SEM: 11.8±0.6 versus 8.8±0.9). We anticipated a similar response in CKD patients and estimated a sample size of 34 in each group will have 80% power to detect a difference in means of 3.9 (the difference between a group 1 mean, μ1, of 10.6 and a group 2 mean, μ2, of 6.7) assuming that the common SD is 5.6 using a two group t test with a 0.050 two-sided significance level. In order to account for a potential drop-out rate of approximately 17%, we determined that n=40 per group for a total of n=80 should be recruited.
Disclosures
R.J.J. has several patent applications with the University of Florida (Gainesville, FL) related to lowering uric acid as a means to prevent or treat metabolic complications of obesity.
Supplementary Material
Acknowledgments
We are thankful to all of our patients who participated in the clinical study. We would like to thank Dr. Kevin Deane who served as the safety officer for the study. We would also like to thank Dr. Jessica Kendrick for her help with patient recruitment from the Denver Health Medical Center.
The study was supported by a career development awards, K23DK088833 and K01DK103678, from the National Institutes of Health by the National Institute of Diabetes and Digestive and Kidney Diseases.
Findings from this study were previously presented during a poster session for Late-Breaking Abstracts at the annual meeting for the American Society of Nephrology (November 7, 2015, SA-PO1107).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016050521/-/DCSupplemental.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH: Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 164: 659–663, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Brantsma AH, Bakker SJ, Hillege HL, de Zeeuw D, de Jong PE, Gansevoort RT; PREVEND Study Group : Cardiovascular and renal outcome in subjects with K/DOQI stage 1-3 chronic kidney disease: the importance of urinary albumin excretion. Nephrol Dial Transplant 23: 3851–3858, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 5.McCullough PA, Jurkovitz CT, Pergola PE, McGill JB, Brown WW, Collins AJ, Chen SC, Li S, Singh A, Norris KC, Klag MJ, Bakris GL; KEEP Investigators : Independent components of chronic kidney disease as a cardiovascular risk state: results from the Kidney Early Evaluation Program (KEEP). Arch Intern Med 167: 1122–1129, 2007 [DOI] [PubMed] [Google Scholar]
- 6.McCullough PA, Li S, Jurkovitz CT, Stevens L, Collins AJ, Chen SC, Norris KC, McFarlane S, Johnson B, Shlipak MG, Obialo CI, Brown WW, Vassalotti J, Whaley-Connell AT, Brenner RM, Bakris GL; KEEP Investigators : Chronic kidney disease, prevalence of premature cardiovascular disease, and relationship to short-term mortality. Am Heart J 156: 277–283, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Weiner DE, Tighiouart H, Stark PC, Amin MG, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ: Kidney disease as a risk factor for recurrent cardiovascular disease and mortality. Am J Kidney Dis 44: 198–206, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Whaley-Connell AT, Sowers JR, Stevens LA, McFarlane SI, Shlipak MG, Norris KC, Chen SC, Qiu Y, Wang C, Li S, Vassalotti JA, Collins AJ; Kidney Early Evaluation Program Investigators : CKD in the United States: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) 1999-2004. Am J Kidney Dis 51[Suppl 2]: S13–S20, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Rao MV, Qiu Y, Wang C, Bakris G: Hypertension and CKD: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES), 1999-2004. Am J Kidney Dis 51[Suppl 2]: S30–S37, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Yilmaz MI, Stenvinkel P, Sonmez A, Saglam M, Yaman H, Kilic S, Eyileten T, Caglar K, Oguz Y, Vural A, Çakar M, Altun B, Yenicesu M, Carrero JJ: Vascular health, systemic inflammation and progressive reduction in kidney function; clinical determinants and impact on cardiovascular outcomes. Nephrol Dial Transplant 26: 3537–3543, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Muntner P, He J, Astor BC, Folsom AR, Coresh J: Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities study. J Am Soc Nephrol 16: 529–538, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Zoccali C, Bode-Böger S, Mallamaci F, Benedetto F, Tripepi G, Malatino L, Cataliotti A, Bellanuova I, Fermo I, Frölich J, Böger R: Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet 358: 2113–2117, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Ghiadoni L, Cupisti A, Huang Y, Mattei P, Cardinal H, Favilla S, Rindi P, Barsotti G, Taddei S, Salvetti A: Endothelial dysfunction and oxidative stress in chronic renal failure. J Nephrol 17: 512–519, 2004 [PubMed] [Google Scholar]
- 14.Schmidt RJ, Baylis C: Total nitric oxide production is low in patients with chronic renal disease. Kidney Int 58: 1261–1266, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perticone F, Maio R, Perticone M, Sciacqua A, Shehaj E, Naccarato P, Sesti G: Endothelial dysfunction and subsequent decline in glomerular filtration rate in hypertensive patients. Circulation 122: 379–384, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Zatz R, Baylis C: Chronic nitric oxide inhibition model six years on. Hypertension 32: 958–964, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang DH, Nakagawa T, Feng L, Johnson RJ: Nitric oxide modulates vascular disease in the remnant kidney model. Am J Pathol 161: 239–248, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakagawa T, Johnson RJ: Endothelial nitric oxide synthase. Contrib Nephrol 170: 93–101, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Muller V, Tain YL, Croker B, Baylis C: Chronic nitric oxide deficiency and progression of kidney disease after renal mass reduction in the C57Bl6 mouse. Am J Nephrol 32: 575–580, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, Himmelfarb J: Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int 65: 1009–1016, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Yilmaz MI, Saglam M, Caglar K, Cakir E, Sonmez A, Ozgurtas T, Aydin A, Eyileten T, Ozcan O, Acikel C, Tasar M, Genctoy G, Erbil K, Vural A, Zoccali C: The determinants of endothelial dysfunction in CKD: oxidative stress and asymmetric dimethylarginine. Am J Kidney Dis 47: 42–50, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP, Furberg CD, Psaty BM: Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation 107: 87–92, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Landray MJ, Wheeler DC, Lip GY, Newman DJ, Blann AD, McGlynn FJ, Ball S, Townend JN, Baigent C: Inflammation, endothelial dysfunction, and platelet activation in patients with chronic kidney disease: the chronic renal impairment in Birmingham (CRIB) study. Am J Kidney Dis 43: 244–253, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Dounousi E, Papavasiliou E, Makedou A, Ioannou K, Katopodis KP, Tselepis A, Siamopoulos KC, Tsakiris D: Oxidative stress is progressively enhanced with advancing stages of CKD. Am J Kidney Dis 48: 752–760, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Jalal DI, Chonchol M, Chen W, Targher G: Uric acid as a target of therapy in CKD. Am J Kidney Dis 61: 134–146, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madero M, Sarnak MJ, Wang X, Greene T, Beck GJ, Kusek JW, Collins AJ, Levey AS, Menon V: Uric acid and long-term outcomes in CKD. Am J Kidney Dis 53: 796–803, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi YJ, Yoon Y, Lee KY, Hien TT, Kang KW, Kim KC, Lee J, Lee MY, Lee SM, Kang DH, Lee BH: Uric acid induces endothelial dysfunction by vascular insulin resistance associated with the impairment of nitric oxide synthesis. FASEB J 28: 3197–3204, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, Lan HY, Kivlighn S, Johnson RJ: Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 38: 1101–1106, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Kang DH, Park SK, Lee IK, Johnson RJ: Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol 16: 3553–3562, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Kang DH, Han L, Ouyang X, Kahn AM, Kanellis J, Li P, Feng L, Nakagawa T, Watanabe S, Hosoyamada M, Endou H, Lipkowitz M, Abramson R, Mu W, Johnson RJ: Uric acid causes vascular smooth muscle cell proliferation by entering cells via a functional urate transporter. Am J Nephrol 25: 425–433, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Kanbay M, Yilmaz MI, Sonmez A, Turgut F, Saglam M, Cakir E, Yenicesu M, Covic A, Jalal D, Johnson RJ: Serum uric acid level and endothelial dysfunction in patients with nondiabetic chronic kidney disease. Am J Nephrol 33: 298–304, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang Z, Cheng LT, Li HY, Wang T: Serum uric acid and endothelial dysfunction in continuous ambulatory peritoneal dialysis patients. Am J Nephrol 29: 368–373, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Jablonski KL, Decker E, Perrenoud L, Kendrick J, Chonchol M, Seals DR, Jalal D: Assessment of vascular function in patients with chronic kidney disease [published online ahead of print June 16, 2014]. J Vis Exp doi:10.3791/51478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caglar K, Yilmaz MI, Saglam M, Cakir E, Acikel C, Eyileten T, Yenicesu M, Oguz Y, Vural A, Carrero JJ, Axelsson J, Lindholm B, Stenvinkel P: Short-term treatment with sevelamer increases serum fetuin-a concentration and improves endothelial dysfunction in chronic kidney disease stage 4 patients. Clin J Am Soc Nephrol 3: 61–68, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shechter M, Shechter A, Koren-Morag N, Feinberg MS, Hiersch L: Usefulness of brachial artery flow-mediated dilation to predict long-term cardiovascular events in subjects without heart disease. Am J Cardiol 113: 162–167, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Shechter M, Issachar A, Marai I, Koren-Morag N, Freinark D, Shahar Y, Shechter A, Feinberg MS: Long-term association of brachial artery flow-mediated vasodilation and cardiovascular events in middle-aged subjects with no apparent heart disease. Int J Cardiol 134: 52–58, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM: Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation 115: 2390–2397, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM: Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation 120: 502–509, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brevetti G, Silvestro A, Schiano V, Chiariello M: Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: additive value of flow-mediated dilation to ankle-brachial pressure index. Circulation 108: 2093–2098, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Avogaro A, Albiero M, Menegazzo L, de Kreutzenberg S, Fadini GP: Endothelial dysfunction in diabetes: the role of reparatory mechanisms. Diabetes Care 34[Suppl 2]: S285–S290, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bengtsson C, Lapidus L, Stendahl C, Waldenström J: Hyperuricaemia and risk of cardiovascular disease and overall death. A 12-year follow-up of participants in the population study of women in Gothenburg, Sweden. Acta Med Scand 224: 549–555, 1988 [PubMed] [Google Scholar]
- 42.Lehto S, Rönnemaa T, Pyörälä K, Laakso M: Predictors of stroke in middle-aged patients with non-insulin-dependent diabetes. Stroke 27: 63–68, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Klein R, Klein BE, Cornoni JC, Maready J, Cassel JC, Tyroler HA: Serum uric acid. Its relationship to coronary heart disease risk factors and cardiovascular disease, Evans County, Georgia. Arch Intern Med 132: 401–410, 1973 [DOI] [PubMed] [Google Scholar]
- 44.Fang J, Alderman MH: Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971-1992. National Health and Nutrition Examination Survey. JAMA 283: 2404–2410, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Alderman MH, Cohen H, Madhavan S, Kivlighn S: Serum uric acid and cardiovascular events in successfully treated hypertensive patients. Hypertension 34: 144–150, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Zharikov S, Krotova K, Hu H, Baylis C, Johnson RJ, Block ER, Patel J: Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. Am J Physiol Cell Physiol 295: C1183–C1190, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gersch C, Palii SP, Kim KM, Angerhofer A, Johnson RJ, Henderson GN: Inactivation of nitric oxide by uric acid. Nucleosides Nucleotides Nucleic Acids 27: 967–978, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sánchez-Lozada LG, Tapia E, López-Molina R, Nepomuceno T, Soto V, Avila-Casado C, Nakagawa T, Johnson RJ, Herrera-Acosta J, Franco M: Effects of acute and chronic L-arginine treatment in experimental hyperuricemia. Am J Physiol Renal Physiol 292: F1238–F1244, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, Tuttle KR, Rodriguez-Iturbe B, Herrera-Acosta J, Mazzali M: Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension 41: 1183–1190, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Ames BN, Cathcart R, Schwiers E, Hochstein P: Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A 78: 6858–6862, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waring WS, Convery A, Mishra V, Shenkin A, Webb DJ, Maxwell SR: Uric acid reduces exercise-induced oxidative stress in healthy adults. Clin Sci (Lond) 105: 425–430, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Waring WS, Webb DJ, Maxwell SR: Systemic uric acid administration increases serum antioxidant capacity in healthy volunteers. J Cardiovasc Pharmacol 38: 365–371, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Waring WS, McKnight JA, Webb DJ, Maxwell SR: Uric acid restores endothelial function in patients with type 1 diabetes and regular smokers. Diabetes 55: 3127–3132, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Boueiz A, Damarla M, Hassoun PM: Xanthine oxidoreductase in respiratory and cardiovascular disorders. Am J Physiol Lung Cell Mol Physiol 294: L830–L840, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Guthikonda S, Sinkey C, Barenz T, Haynes WG: Xanthine oxidase inhibition reverses endothelial dysfunction in heavy smokers. Circulation 107: 416–421, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Butler R, Morris AD, Belch JJ, Hill A, Struthers AD: Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension 35: 746–751, 2000 [DOI] [PubMed] [Google Scholar]
- 57.Doehner W, Schoene N, Rauchhaus M, Leyva-Leon F, Pavitt DV, Reaveley DA, Schuler G, Coats AJ, Anker SD, Hambrecht R: Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation 105: 2619–2624, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Reyes AJ, Leary WP: The ALLHAT and the cardioprotection conferred by diuretics in hypertensive patients: a connection with uric acid? Cardiovasc Drugs Ther 16: 485–487, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Krishnan E: Chronic kidney disease and the risk of incident gout among middle-aged men: a seven-year prospective observational study. Arthritis Rheum 65: 3271–3278, 2013 [DOI] [PubMed] [Google Scholar]
- 60.Wang W, Bhole VM, Krishnan E: Chronic kidney disease as a risk factor for incident gout among men and women: retrospective cohort study using data from the Framingham Heart Study. BMJ Open 5: e006843, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kao MP, Ang DS, Gandy SJ, Nadir MA, Houston JG, Lang CC, Struthers AD: Allopurinol benefits left ventricular mass and endothelial dysfunction in chronic kidney disease. J Am Soc Nephrol 22: 1382–1389, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kayama Y, Raaz U, Jagger A, Adam M, Schellinger IN, Sakamoto M, Suzuki H, Toyama K, Spin JM, Tsao PS: Diabetic Cardiovascular Disease Induced by Oxidative Stress. Int J Mol Sci 16: 25234–25263, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Santilli F, D’Ardes D, Davì G: Oxidative stress in chronic vascular disease: From prediction to prevention. Vascul Pharmacol 74: 23–37, 2015 [DOI] [PubMed] [Google Scholar]
- 64.Feig DI, Soletsky B, Johnson RJ: Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA 300: 924–932, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Siu YP, Leung KT, Tong MK, Kwan TH: Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis 47: 51–59, 2006 [DOI] [PubMed] [Google Scholar]
- 66.Goicoechea M, de Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, Rincón A, Arroyo D, Luño J: Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol 5: 1388–1393, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mehta T, Nuccio E, McFann K, Madero M, Sarnak MJ, Jalal D: Association of Uric Acid With Vascular Stiffness in the Framingham Heart Study. Am J Hypertens 28: 877–883, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R; International Brachial Artery Reactivity Task Force : Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265, 2002 [DOI] [PubMed] [Google Scholar]
- 69.Celermajer DS, Sorensen K, Ryalls M, Robinson J, Thomas O, Leonard JV, Deanfield JE: Impaired endothelial function occurs in the systemic arteries of children with homozygous homocystinuria but not in their heterozygous parents. J Am Coll Cardiol 22: 854–858, 1993 [DOI] [PubMed] [Google Scholar]
- 70.Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR: 25-Hydroxyvitamin D Deficiency Is Associated With Inflammation-Linked Vascular Endothelial Dysfunction in Middle-Aged and Older Adults. Hypertension 57: 63–69, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jalal DI, Jablonski KL, McFann K, Chonchol MB, Seals DR: Vascular endothelial function is not related to serum uric acid in healthy adults. Am J Hypertens 25: 407–413, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR: Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res 100: 1659–1666, 2007 [DOI] [PubMed] [Google Scholar]
- 73.Silver AE, Christou DD, Donato AJ, Beske SD, Moreau KL, Magerko KA, Seals DR: Protein expression in vascular endothelial cells obtained from human peripheral arteries and veins. J Vasc Res 47: 1–8, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jablonski KL, Racine ML, Geolfos CJ, Gates PE, Chonchol M, McQueen MB, Seals DR: Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. J Am Coll Cardiol 61: 335–343, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Donato AJ, Pierce GL, Lesniewski LA, Seals DR: Role of NFkappaB in age-related vascular endothelial dysfunction in humans. Aging (Albany, NY) 1: 678–680, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR: Nuclear factor-kappaB activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation 119: 1284–1292, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yiginer O, Ozcelik F, Inanc T, Aparci M, Ozmen N, Cingozbay BY, Kardesoglu E, Suleymanoglu S, Sener G, Cebeci BS: Allopurinol improves endothelial function and reduces oxidant-inflammatory enzyme of myeloperoxidase in metabolic syndrome. Clin Res Cardiol 97: 334–340, 2008 [DOI] [PubMed] [Google Scholar]
- 78.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI: A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.