Abstract
In the kidney, formation of the functional filtration units, the nephrons, is essential for postnatal life. During development, mesenchymal progenitors tightly regulate the balance between self-renewal and differentiation to give rise to all nephron epithelia. Here, we investigated the functions of the Hippo pathway serine/threonine-protein kinases Lats1 and Lats2, which phosphorylate and inhibit the transcriptional coactivators Yap and Taz, in nephron progenitor cells. Genetic deletion of Lats1 and Lats2 in nephron progenitors of mice led to disruption of nephrogenesis, with an accumulation of spindle-shaped cells in both cortical and medullary regions of the kidney. Lineage-tracing experiments revealed that the cells that accumulated in the interstitium derived from nephron progenitor cells and expressed E-cadherin as well as vimentin, a myofibroblastic marker not usually detected after mesenchymal-to-epithelial transition. The accumulation of these interstitial cells associated with collagen deposition and ectopic expression of the myofibroblastic markers vimentin and α-smooth-muscle actin in developing kidneys. Although these myofibroblastic cells had high Yap and Taz accumulation in the nucleus concomitant with a loss of phosphorylated Yap, reduction of Yap and/or Taz expression levels completely rescued the Lats1/2 phenotype. Taken together, our results demonstrate that Lats1/2 kinases restrict Yap/Taz activities to promote nephron progenitor cell differentiation in the mammalian kidney. Notably, our data also show that myofibroblastic cells can differentiate from nephron progenitors.
Keywords: Hippo pathway, Lats1/2, Yap/Taz, MET, nephron, Myofibroblast
Kidney organogenesis is a remarkably orchestrated, reiterated process that depends on reciprocal signaling between the epithelial ureteric bud (UB) and the surrounding condensing mesenchyme (CM).1–4 Signaling from the mesenchyme induces successive rounds of UB branching, generating the collecting duct (CD) of the kidney. Surrounding the UB are self-renewing mesenchymal progenitor cells (called the CM or nephron progenitor cells [NPCs]) that express Six2 and Cited1.5,6 A subset of CM cells is reciprocally induced by the UB to form a pretubular aggregate (PA), which subsequently undergoes mesenchymal-to-epithelial transition (MET) to form the renal vesicle (RV). The RV will then undergo morphogenesis to form the comma-shaped body (CSB), followed by the S-shaped body (SSB) that will elongate to form the nephron.7 Both MET and epithelial-to-mesenchymal transition are essential during embryonic development and have been implicated in the growth of metastatic tumors.8 During MET, mesenchymal cells alter their shape and motile behavior as they differentiate into epithelial cells by acquiring an apical-basal polarity, a basement membrane, and adhesion with neighboring cells. Such events of cellular transition and movements were uncovered by means of lineage-tracing experiments in the developing kidney using, among others, the Hoxb7 (UB), Six2 (CM), and Foxd1-reporter models that label the CDs, nephrons, and stromal derivatives, respectively.9–11
The Hippo pathway is a conserved kinase cassette that controls tissue growth through the activities of the Yap and Taz effectors in both flies and mammals.12–14 These closely related transcriptional coactivators promote the expression of pro-proliferative and antiapoptotic genes. Upstream of Yap and Taz are the Hippo kinases Mst1/2 and Lats1/2, which negatively regulate Yap and Taz by causing their exclusion from the nuclear compartment. Loss of Hippo signaling (Mst1/2 or Lats1/2 inactivation) leads to unrestricted nuclear accumulation of Yap and Taz and has been linked to a variety of developmental abnormalities and cancers.15–17
In the developing mammalian kidney, Yap and Taz play different roles: Yap promotes nephron formation within the NPC population18 and Taz prevents renal cyst formation.19,20 Yap and Taz are also essential in the UB lineage for lower urinary tract development21 and branching morphogenesis.22 Finally, increased Yap and Taz have also been found to correlate with kidney fibrosis, using a unilateral ureteral obstruction model.23 From these published data, it is clear that loss of Yap and Taz are detrimental to kidney development and function in the adult. However, the roles of the core Hippo kinases in NPCs remain to be investigated.
Here we uncover a role for the Hippo kinases, Lats1 and Lats2, in nephron progenitor (NP) differentiation and demonstrate that Lats1 and Lats2 activities are critical during nephron formation. Removal of Lats1/2 from NPCs causes loss of nephron formation, accompanied with a change in cell fate and accumulation of myofibroblasts. The interstitial myofibroblastic cells also show high nuclear Yap and Taz accumulation with loss of phospho-Yap. Remarkably, the conditional loss of Lats1/2 in the NPC population is successfully rescued by depletion of Yap and/or Taz. Taken together, these data demonstrate an essential role for the core Hippo kinases (Lats1 and Lats2) in restricting Yap/Taz activity during the self-renewal of NPCs and MET and nephron formation in the mammalian kidney.
Results
Lats1 and Lats2 Dual Deletion Results in Loss of Nephrogenesis
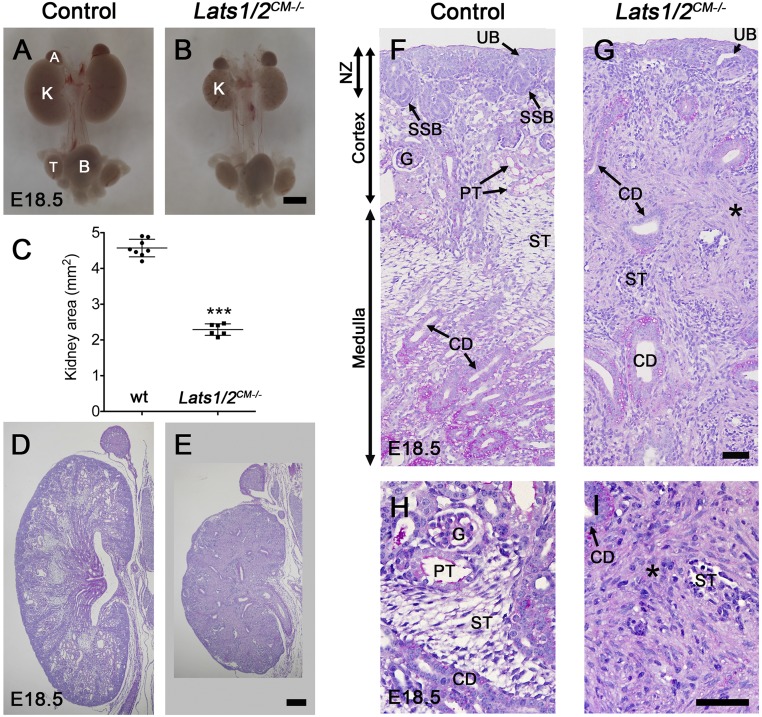
To investigate the role of Lats1 and Lats2 kinases in NPCs, both genes were conditionally inactivated using the Six2:CreTGC/+ allele.10 This system depletes Lats1 and Lats2 expression from NPCs and their epithelial derivatives. Six2:CreTGC/+ Lats1flox/flox Lats2flox/flox (called Lats1/2CM−/−) mice died within 24 hours after birth. Gross anatomic examination revealed that embryonic day 18.5 (E18.5) Lats1/2CM−/− embryos had a dramatic decrease in kidney size compared with controls (Lats1/2CM−/−: 2.3±0.1 mm2, n=6; controls: 4.5±0.2 mm2, n=8; Figure 1, A–C). Histologic examination of E18.5 kidneys revealed loss of nephrogenesis and accumulation of cells in both cortical and medullary regions (Figure 1, D–I). In control kidneys, higher magnification views identified an active nephrogenic zone, a cortex with numerous glomeruli, proximal and distal tubules, and a medulla filled with stromal cells and CDs. Remarkably, neither glomeruli nor tubules were observed in Lats1/2CM−/− kidneys at E18.5, and both the cortex and the medulla showed an accumulation of cells in the interstitium with a dense, glycoprotein-rich (periodic acid–Schiff [PAS]-positive) extracellular matrix, surrounded by dense stroma (Figure 1, F–I).
Figure 1.
Lats1/2 deletion leads to loss of nephrogenesis. (A and B) Macroscopic views of the urogenital system at E18.5 show severe kidney hypoplasia in Lats1/2CM−/− and control embryos. (C) Quantification reveals a 49% decrease in kidney size in Lats1/2CM−/− embryos compared with controls at E18.5. (D and E) PAS staining of wild-type and Lats1/2CM−/− kidneys at E18.5. (F–I) Higher magnification views reveal loss of glomeruli and tubules in Lats1/2CM−/− mutants, and accumulation of cells in the interstitium (*). A, adrenal; B, bladder; G, glomerulus; K, kidney; NZ, nephrogenic zone; PT, proximal tubule; ST, stroma; T, testis. Scale bars represent 1 mm (A and B), 200 µm (D and E), and 50 µm (F–I).
Accumulation of NP-Derived Cells in the Lats1/2CM−/− Kidney
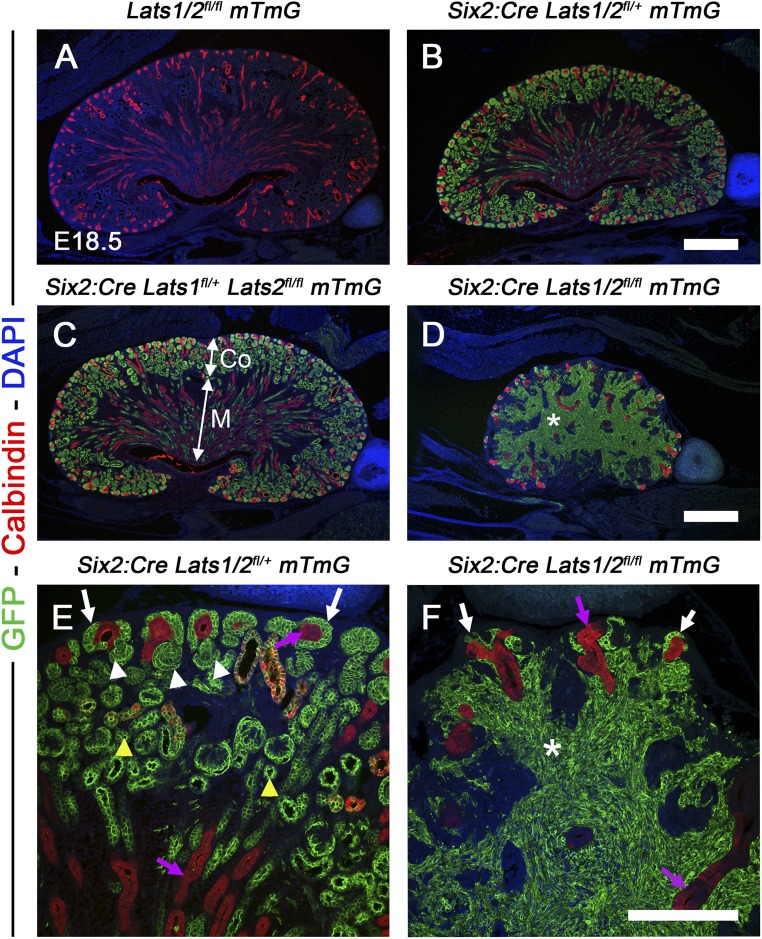
Nephrogenesis occurs in a repetitive manner, with new nephrons being formed throughout development in the nephrogenic zone. NPCs surrounding the UB tips concurrently self-renew to replenish the pool of NPCs and undergo MET to form early nephrons. To determine whether cells that accumulate in Lats1/2CM−/− kidney interstitium were derived from the NPCs, we performed lineage-tracing experiments using the Rosa-mTomato/mGFP reporter mouse line (mTmG24) which, combined with the Six2:Cre system, produces a membrane-bound green fluorescent protein (GFP) that permanently labels the NPCs and their daughter cells. We generated double conditional knockout Six2:Cre Lats1/2flox/flox mTmG mice, wherein compound mutants (Six2:Cre Lats1/2flox/+ mTmG–double heterozygous knockout, Six2:Cre Lats1flox/+ Lats2flox/flox mTmG, and Six2:Cre Lats1flox/flox Lats2flox/+ mTmG) and Lats1/2flox/flox mTmG (no Cre) were used as controls. We performed GFP and calbindin staining to mark the NPCs and daughter cells, and the UB compartment, respectively, at E18.5. As expected, no GFP staining was observed in any Cre controls (Figure 2A). In both double heterozygous knockout animals and mutants with loss of three out of four Lats alleles, we observed GFP expression in all NPCs throughout the nephrogenic zone and in their epithelial descendants (both early and more mature nephrons [Figure 2, B and C, and higher magnification in Figure 2E]). Thus, loss of three out of the four Lats alleles does not affect nephrogenesis (Figure 2C). In contrast, nephrogenesis does not occur in Lats1/2CM−/− kidneys, and all cells that accumulate in the Lats1/2CM−/− interstitium are GFP-positive, indicating that they were derived from the NPCs (Figure 2, D and F).
Figure 2.
Accumulation of NP-derived cells in the Lats1/2CM−/− kidney. (A–D) GFP and calbindin staining in (A) negative control; (B) Six2:Cre Lats1/2flox/+ mTmG; (C) Six2:Cre Lats1flox/+ Lats2flox/flox mTmG; and (D) Six2:Cre Lats1/2flox/flox mTmG E18.5 kidneys, where GFP staining labels the Six2 expressing NPCs and their progeny, and calbindin the UB-derived structures (purple arrows). Note that calbindin is also expressed in distal tubules. (E) High magnification views of the cortical zone of double heterozygous knockout showed GFP staining is observed in NPCs (arrows), early nephrons (white arrowheads) and differentiated tubules (yellow arrowheads). (F) In contrast, nephrogenesis does not occur in Lats1/2CM−/− kidneys, and all cells that accumulate in the interstitium are GFP-positive (*), indicating they were derived from NPCs. Note the reduced pool of NPCs in Lats1/2CM−/− kidneys (white arrows). Co, cortex; M, medulla. Scale bars represent 500 µm (A–D) and 200 µm (E and F).
Lats1/2 Deletion Impaired Maintenance and Differentiation of NPCs
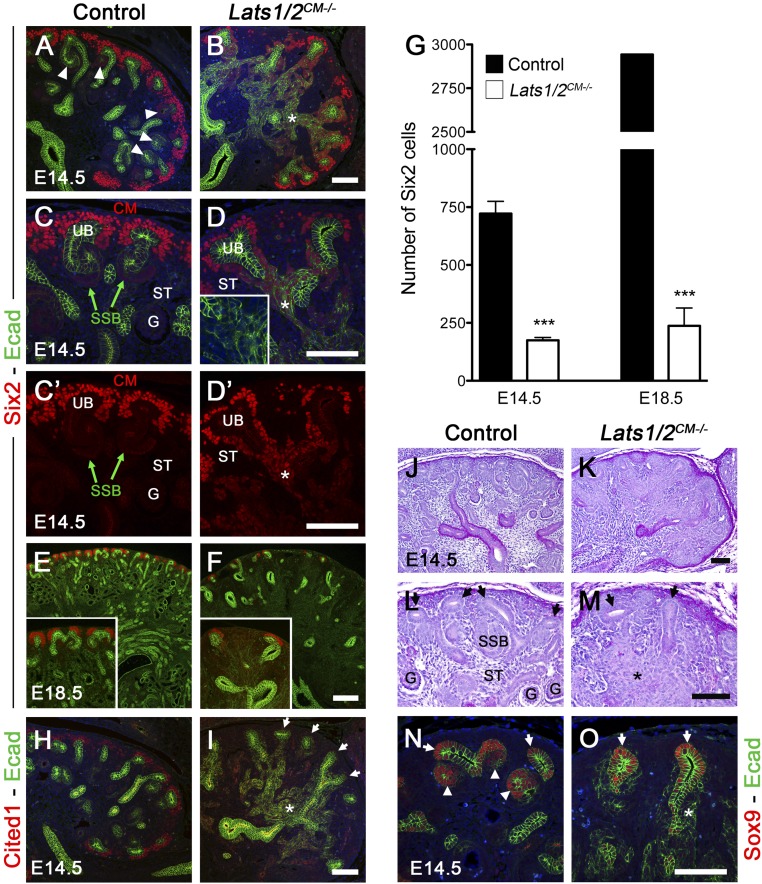
To determine the developmental origin of the abnormal nephrogenesis observed in Lats1/2CM−/− kidneys, we examined earlier time points. In E14.5 control kidneys, 2–3 layers of Six2-positive self-renewing NPCs surround the dorsal side of the UB, with early nephron structures (PA, RV, CSB and SSB) forming on the ventral side of the UB. Six2 immunofluorescence (Figure 3, A–G) and lineage tracing (Figure 2F) revealed a reduced pool of Six2-positive cells capping UB tips (quantification in Figure 3G), indicating that Lats1/2 deletion leads to poor maintenance of the NPCs. Moreover, Lats1/2 deletion also abolished expression of the NPC marker Cited1, suggesting loss of progenitor potential (Figure 3, H and I). The reduced maintenance of NPCs in Lats1/2CM−/− is accompanied by a complete absence of early nephron structures. No RV, CSB, SSB, or glomerulus were observed in Lats1/2CM−/− E14.5 kidneys compared with controls as seen by histology or Sox9 staining, a marker for UB tips and early nephrons25 (Figure 3, J–O and the absence of SSB in Figure 3D).
Figure 3.
Lats1/2 deletion affect NPC maintenance and nephron formation. (A–F) Six2 and E-cadherin staining at E14.5 and E18.5 showed poor maintenance of the population of NPCs throughout kidney development in Lats1/2CM−/− compared with controls. (C–D’) Note that early nephron structures (SSB) are absent in Lats1/2CM−/− mutants and cells that accumulate in the interstitium still express Six2. (D) Note the discontinuous E-cadherin staining in accumulated interstitial cells in Lats1/2CM−/− kidneys (inset in D). (G) Quantification of all Six2 cells (E-cadherin–negative) per entire kidney section at E14.5 and E18.5 in both genotypes. Quantification of the total number of Six2 cells was made in six kidneys, except for the control at E18.5 where only one kidney section was used to quantify the number of Six2 cells. (H and I) Lats1/2 deletion leads to loss of Cited1 expression from the NPCs (arrows) and accumulation of E-cadherin–positive cells in the interstitium (*). (J–M) PAS staining of E14.5 kidneys from wild-type and Lats1/2CM−/− kidneys. Higher magnification views reveal absence of early nephrons in Lats1/2CM−/− kidneys (compare M to L). Arrows point to the UB tips. (N and O) Sox9 and E-cadherin staining at E14.5 showed loss of early nephron structures in Lats1/2CM−/− kidneys compared with controls (arrows point to the UB tips, white arrowheads to early nephrons). G, glomerulus; ST, stroma. Asterisk points to accumulated cells. Scale bars represent 100 µm (A–D’, H–O) and 200 µm (E and F).
Lats1 and Lats2 Deletion Induces Loss of Epithelial Characteristics
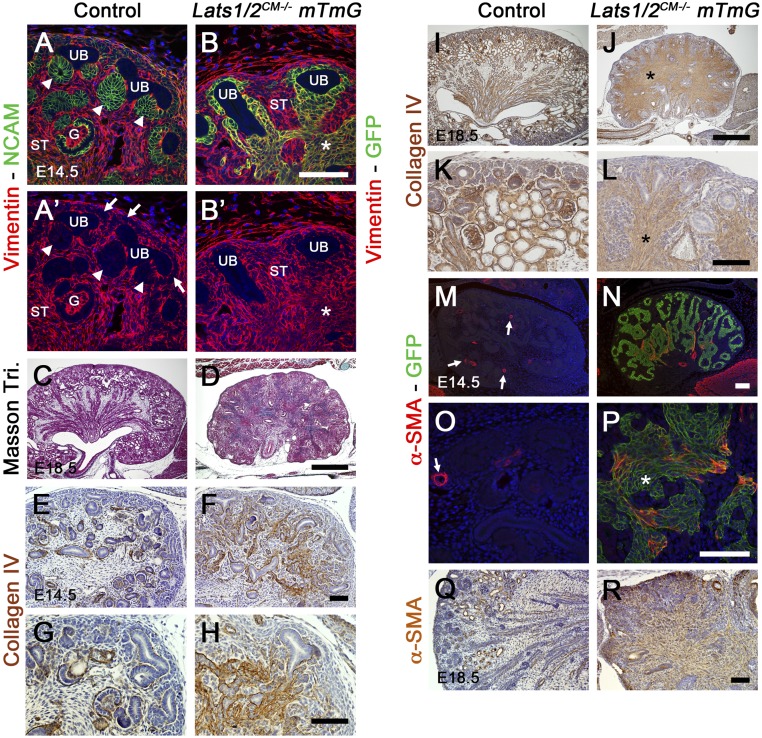
We next characterized the accumulated interstitial cells observed in Lats1/2CM−/− kidneys. Since controls (Lats1/2flox/flox mTmG) do not have the Six2:Cre allele, we used either Neural Cell Adhesion Molecule (NCAM) or E-cadherin to mark the CM and/or early nephron structures and GFP staining for NPs and their epithelial derivatives in Lats1/2CM−/− kidneys (Six2:Cre Lats1/2flox/flox mTmG). At E14.5, the accumulated interstitial cells in Lats1/2CM−/− still expressed Six2 (Figure 3D’). In addition, cells that accumulate in Lats1/2CM−/− kidneys expressed NCAM and β-catenin, as in control early nephrons (Supplementary Figure 1, A–B’ and E–F’). Discontinuous E-cadherin staining was observed in accumulated interstitial cells in Lats1/2CM−/− kidneys and, consistent with the loss of tubule formation, we observed loss of the apical marker Crumbs3 and reduced expression of the basement membrane marker laminin (Supplemental Figure 1, C, D, G, and H). Expression of E-cadherin, NCAM, and β-catenin gradually reduced as cells located further away from the nephrogenic zone (boxes in Supplemental Figure 1), and by E18.5, the level of E-cadherin expression in the cells that accumulate in the interstitium was greatly reduced (Figure 3F).
During MET, expression of vimentin, a type-III intermediate filament protein normally expressed in mesenchymal cells, becomes downregulated as cells adopt epithelial characteristics.26 In control kidneys, vimentin protein is strongly expressed in stromal cells and, to a smaller extent, in NPCs, but is absent from all epithelial structures (UB and CM-derived PA, RV, CSB, and SSB; Figure 4, A and A’). Surprisingly, Six2-derived cells (GFP-positive) in Lats1/2CM−/− mTmG kidneys showed high vimentin expression (Figure 4, B and B’) while still expressing E-cadherin (Figure 3, A and B), suggesting that they do not undergo complete MET. Accordingly, Masson trichrome staining revealed collagen deposition in Lats1/2CM−/− kidneys (further validated by collagen IV expression at E14.5 and E18.5), suggesting that Lats1/2 deletion led to accumulation of cells with fibrotic characteristics (Figure 4, C–L). Moreover, in control kidneys, no expression of the myofibroblast marker α-smooth muscle actin (α-SMA) could be detected (apart from the vascular smooth-muscle cells [Figure 4, M and O]). In contrast, a fraction of the more medullary accumulated interstitial cells in Lats1/2CM−/− kidneys ectopically expressed α-SMA at E14.5 (Figure 4, N and P), whereas most of them expressed α-SMA at E18.5 (Figure 4, Q and R). Altogether, these data suggest that Lats1/2 deletion converts NPCs into myofibroblasts.
Figure 4.
Lats1/2 deletion leads to myofibroblast formation. (A and A’) In controls, vimentin staining at E14.5 shows that NP and derived cells (marked by NCAM) are vimentin-negative. (B and B’) In Lats1/2CM−/− mTmG kidneys, NP-derived cells (*, marked by GFP) are vimentin-positive. White arrowheads point to early nephrons. White arrows point to NPCs. (C and D) Masson trichrome staining at E18.5 reveals collagen deposition in Lats1/2CM−/− kidneys. (E–L) Immunohistochemistry for collagen IV staining demonstrates collagen deposition at E14.5 (E–H) and E18.5 (I–L). (M–P) α-SMA staining at E14.5 identifies myofibroblasts in Lats1/2CM−/− kidneys, whereas α-SMA expression is only observed in smooth-muscle vascular cells (arrows) in controls. (Q and R) At E18.5, all NP-derived cells in Lats1/2CM−/− kidneys are α-SMA–positive. Asterisk points to accumulated cells. G, glomerulus; ST, stroma. Scale bars represent 100 µm (A–B’, E–H, and K–R) and 500 µm (C, D, I, and J).
Unrestricted Yap/Taz Activity Accounts for Myofibroblast Differentiation Observed in Lats1/2CM−/− Embryos
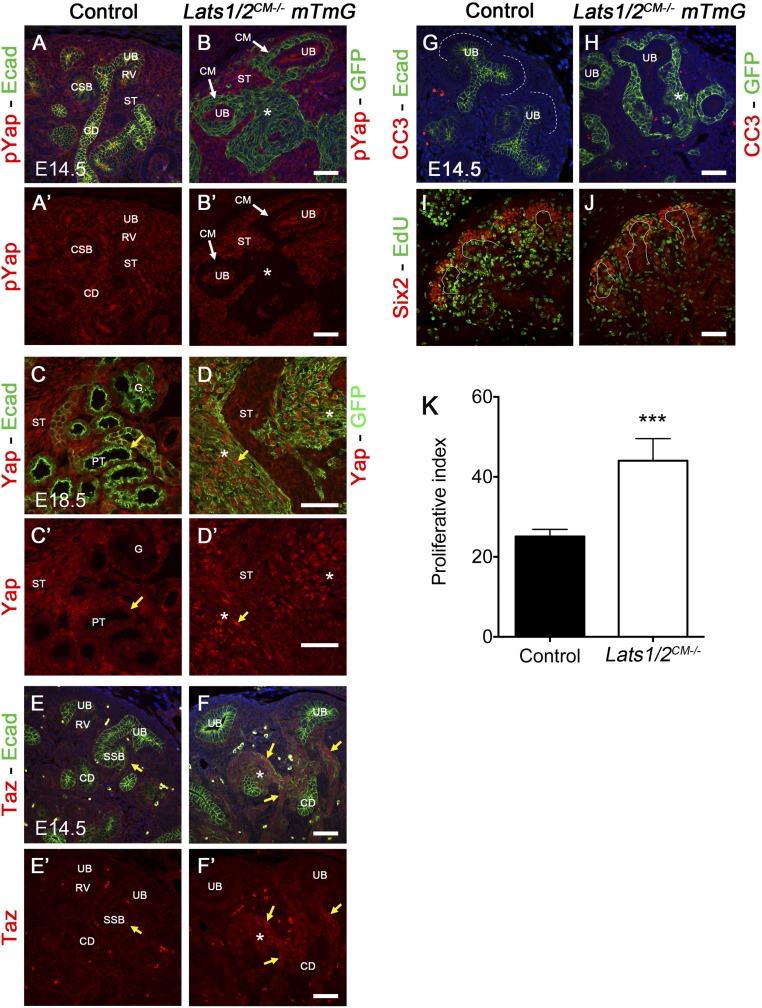
To investigate whether Lats1/2 functions upstream of Yap/Taz in NPCs, we stained E14.5 control and Lats1/2CM−/− mTmG kidneys using phospho-Yap, Yap, and Taz antibodies. Immunostaining showed loss of phospho-Yap and strong nuclear Yap and Taz in NP-derived cells of Lats1/2CM−/− kidneys compared with early nephron structures in controls (Figure 5, A–F’).
Figure 5.
Lats1/2 restricts Yap/Taz expression in NPC. (A–F’) Lats1/2 deletion leads to loss of phospho-Yap (A–B’), increased nuclear Yap (C–D’), and increased nuclear Taz (E–F’) in Lats1/2CM−/− mTmG NP-derived cells compared with early nephrons in controls. Staining with E-cadherin and GFP marks early nephron structures in controls and NP-derived cells in Lats1/2CM−/− mTmG kidneys, respectively. (G and H) Cleaved caspase 3 staining in control and Lats1/2CM−/− kidneys at E14.5. (I and J) EdU incorporation in Lats1/2CM−/− and control NPCs at E14.5 shows an overall increase in the percentage of proliferating Six2 cells in Lats1/2CM−/− kidneys compared with controls. (See quantification in [K]. For the quantification, 3611 and 875 Six2Pos-E-cadherinNeg cells were counted in total in controls and Lats1/2CM−/− embryos, respectively [n=5].) G, glomerulus; PT, proximal tubule; ST, stroma. Asterisk points to accumulated cells. Scale bars represent 50 µm.
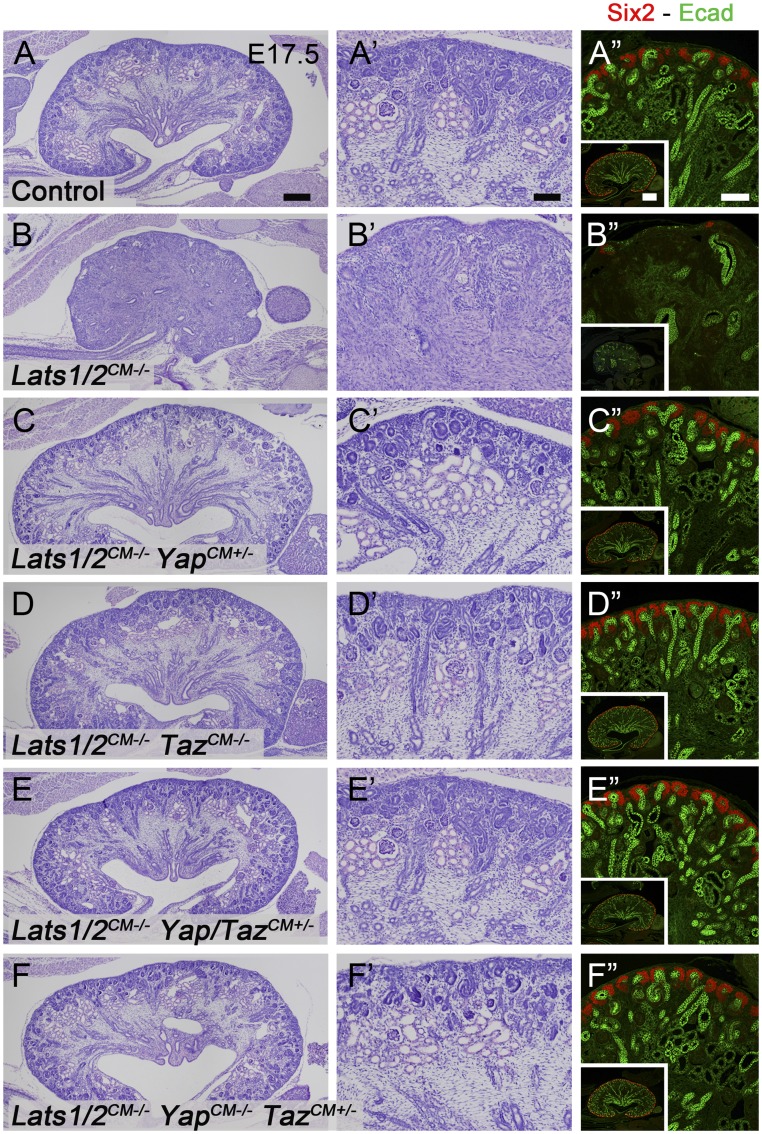
To test whether unrestricted Yap/Taz expression is responsible for the cell fate change observed in Lats1/2CM−/− kidneys, we attempted to rescue the Lats1/2CM−/− phenotype by reducing Yap and/or Taz levels by generating Six2:CreTGC/+ Lats1flox/flox Lats2flox/flox Yapflox/(flox/+) Tazflox/(flox/+) embryos. We then performed PAS and Six2/E-cadherin staining in all genotypes. Strikingly, the abnormal nephrogenesis seen in Lats1/2CM−/− kidneys was largely rescued by reducing Yap and/or Taz levels (Figure 6). These data indicate that Lats1/2 kinases inhibit Yap/Taz in NPCs to promote epithelialization and nephron formation.
Figure 6.
Rescue of the Lats1/2CM−/− phenotype by loss of Yap and/or Taz. PAS and Six2/E-cadherin staining of kidneys from (A–A”) control, Lats1/2CM−/− (n=6 kidneys) (B–B”), Lats1/2CM−/− YapCM+/− (n=2) (C–C”), Lats1/2CM−/− TazCM−/− (n=2) (D–D”), Lats1/2CM−/− Yap/TazCM+/− (n=4) (E–E”), and Lats1/2CM−/− YapCM−/− TazCM+/− (n=2) (F–F”) at E17.5. Scale bars represent 250 µm (A–F), 100 µm (A’–F’ and A”–F”), and 500 µm (insets in A”–F”).
Discussion
Here, we demonstrate that Lats1/2 kinases regulate Yap/Taz in NPCs, as Lats1/2 deletion leads to increased nuclear Yap and Taz and loss of phosphorylated Yap. Moreover, increased Yap/Taz activity is responsible for the Lats1/2 phenotype, as a complete rescue is observed upon genetic ablation of a single allele of Yap or Taz, or by the deletion of one copy of both Yap and Taz. Ablation of Lats1/2 impaired MET, which is essential for nephron formation, and induced a phenotypic change from epithelial (nephron) to myofibroblast. These findings demonstrate that myofibroblasts can derive from NPCs and bring new insights into the regulation of self-renewal and differentiation of NPCs.
During development, NPCs self-renew and undergo MET to form nephrons. During this process, cells decrease in motility and acquire epithelial characteristics. However, in Lats1/2CM−/− kidneys, NP-derived cells never become fully epithelial, as seen by the absence of Crumbs3, strong vimentin expression, and the absence of tubule organization. Proximal NP-derived cells in Lats1/2CM−/− kidneys express β-catenin, NCAM, discontinued E-cadherin, and reduced laminin. This expression profile suggests that accumulated interstitial cells in Lats1/2CM−/− might be in a transient state. Nevertheless, as NP descendants localize further away from the nephrogenic zone in Lats1/2CM−/− kidneys, expression of NCAM, β-catenin, and E-cadherin is gradually lost, and they ectopically express the myofibroblast marker α-SMA. We speculate that complete myofibroblast conversion, observed in the more medullary accumulated interstitial cells, depends on the extent of Yap/Taz activation. Altogether, these results strongly suggest that increased Yap/Taz activities in the NPCs as a consequence of Lats1/2 deletion is sufficient to directly drive epithelial-to-myofibroblast cell fate change.
Upon Lats1/2 deletion, the pool of NPs is greatly reduced. Loss of NPCs could not be explained by increased cell death, as little or no apoptosis is observed in Lats1/2CM−/− NPCs as seen in controls (Figure 5, G and H). We found increased proliferation of NPCs in Lats1/2CM−/− compared with controls, consistent with the overall increased nuclear Yap and Taz (Figure 5, I–K). An appealing hypothesis is that NPCs with higher nuclear Yap/Taz (Hippo inactive) are more likely to exit the NP niche and accumulate in the cortical and medullary parts of the kidney. Consistent with this model is our finding that NPCs depleted for Lats1/2 show loss of the progenitor marker Cited1. NPCs treated with Bmp7 also lose Cited1, which results in a population of ‘Six2-only’ progenitors more likely to respond to Wnt signaling, and p-Smad signaling has been shown to promote the transition of progenitors out of the Cited1 positive compartment.27 Consistent with this, we found increased p-Smad1/5 staining in Lats1/2CM−/− kidneys (Supplemental Figure 2). Interestingly, Yap depletion from the NPCs also led to the loss of Cited1 expression,18 suggesting that Yap levels in NPCs need to be tightly controlled for commitment to the nephron lineage.
Fat4 mutants have an enlarged CM.28–30 It has been proposed that increased nuclear Yap is responsible for the increased CM thickness observed in Fat4 mutants.31 This implies that increased nuclear Yap would favor self-renewal, rather than differentiation of NPCs. In contrast, our data demonstrate that Yap/Taz nuclear accumulation in Lats1/2-deficient NPCs leads to rapid exhaustion of the NPCs that differentiate into myofibroblasts. It is possible that Lats1/2 deletion may lead to a stronger level of Yap activation compared with Fat4 deletion, possibly explaining the opposite observations. Alternatively, Yap might not be the primary cause of increased CM in Fat4 mutants, as suggested by the lack of suppression of the expanded CM in both Fat4−/− YapCM−/− and Fat4−/− Taz−/− mutants.29,30 The different outcomes observed upon Yap activation are inconsistent and will therefore require further analysis.
Is the sole function of Lats1 and Lats2 kinases in the NPCs to restrict Yap/Taz activities? Our rescue experiments suggest that this is the case, since lowering the level of Yap, Taz, or both is enough to rescue the nephrogenesis defects observed in Lats1/2CM−/− kidneys. Intriguingly, we also found that loss of Lats1/2 suppresses the cyst formation normally observed in Taz mutants,18–20 as there were no cysts in Lats1/2CM−/− TazCM−/− compound mutants. Thus, the levels of Yap and Taz in NPCs are important to drive proper nephrogenesis.
In conclusion, our data indicates that Lats1/2 function is necessary to inhibit Yap/Taz activity during nephron formation and to allow self-renewal and differentiation of NPCs. Remarkably, the effects of deletion of the core Hippo kinases Lats1 and Lats2 is to impair epithelialization of NPCs, efficiently converting all NPC into myofibroblasts.
Concise Methods
Mouse Lines
The Six2:CreTGC/+, Lats1flox, Lats2flox Tazflox, Yapflox, and mTmG mouse strains were generously provided and have been described elsewhere.10,18,24,32 All mice were bred on a mixed genetic background. Husbandry and ethical handling of mice were conducted according to guidelines approved by the Canadian Council on Animal Care. Genotyping was done by PCR using genomic DNA prepared from mouse-ear punches.
Histologic and Immunologic Analyses
Embryonic samples from timed matings (day of vaginal plug = E0.5) were collected, fixed in 4% paraformaldehyde overnight at 4°C, serially dehydrated, and then embedded in paraffin. Microtome sections of 7 µm thickness were examined histologically via PAS staining. For immunofluorescent analysis, paraffin sections were dewaxed and rehydrated via ethanol series. Antigen retrieval was performed for 20 minutes in boiling Antigen Unmasking Solution (H-3300; Vector Laboratories, Burlingame, CA). Sections were incubated for 1 hour in blocking solution (3% BSA, 10% goat serum, and 0.1% Tween20 in PBS) at room temperature. Blocking solution was replaced with a solution of primary antibodies diluted in 3% BSA, 3% goat serum, and 0.1% Tween20 in PBS. Relevant conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) were used for primary antibody detection. Slides were mounted using Vectashield with or without 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories). The following primary antibodies (1:300 dilution if not specified) were used: β-Catenin (#9562S; Cell Signaling Technology, Danvers, MA), calbindin (PC253C; Calbiochem, San Diego, CA), Cited1 (RB-9219-P0; Neomarkers), cleaved caspase 3 (#9661; Cell Signaling Technology), Collagen IV (#681241; MP Biomedicals), Crumbs3 (HPA013835; Sigma-Aldrich, St. Louis, MO), E-cadherin (610181, BD Transduction Laboratories; #3195, Cell Signaling Technology), GFP (ab13970; Abcam Inc., Cambridge, MA), HA (11 867 423 001; Roche Diagnostics, Indianapolis, IN), laminin (L9393; Sigma-Aldrich), NCAM (C9672; Sigma-Aldrich), Pax2 (PRB-276P; Covance), Phospho-Smad1/5 (#9516; Cell Signaling Technology), Phospho-Yap (#4911, Cell Signaling Technology; 1:150 dilution), Six2 (11562–1-AP; Proteintech), α-SMA (A2547, Sigma-Aldrich; 1:500 dilution), Sox9 (AB5535; Chemicon), Taz (560235, BD Transduction; 1:200 dilution), Vimentin (ab92547; Abcam), Yap (sc-101199, Santa Cruz Biotechnology, Santa Cruz, CA; 1:150 dilution).
5-Ethynyl-2'-Deoxyuridine Incorporation
A solution containing 5-ethynyl-2'-deoxyuridine (EdU) (10 mg/ml) was injected intraperitoneally into pregnant mice (50 mg EdU/kg of mice) 10–15 minutes before embryonic dissection. The samples were prepared and sectioned as described above before using the Click-iT EdU Alexa Fluor 488/455 Imaging Kit (Life Technologies, Carlsbad, CA).
Statistical Analyses
All data are expressed as mean values with SD. An unpaired two-tailed t test was used to determine differences between two groups (e.g., wild-type versus mutant). All statistical analyses were conducted using GraphPad Prism 5.0a software (GraphPad Software, La Jolla, CA).
Each experiment was performed using littermate control and mutant embryos, and the observations are representative of n≥4 embryos (8 kidneys), except for the rescue experiments were n values are mentioned in the figure legend (Figure 6).
Disclosures
None
Supplementary Material
Acknowledgments
We thank Randy Johnson and Jeff Wrana for kindly providing the Lats1/2 flox and Yap/Taz flox mouse strains.
H.M. is a Tier 1 Canada Research Chair in Coordinating Growth and Polarity.
A.R. designed, performed, and analyzed all experiments. A.R. and H.M. wrote the manuscript.
This work was supported by grants from the Canadian Institutes of Health Research (MOP 136924, 84468), March of Dimes (1-FY11-506), and US Department of Defense (W81XWH-15-1-0461) to H.M.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016060611/-/DCSupplemental.
References
- 1.Saxén L, Sariola H, Lehtonen E: Sequential cell and tissue interactions governing organogenesis of the kidney. Anat Embryol (Berl) 175: 1–6, 1986 [DOI] [PubMed] [Google Scholar]
- 2.Schedl A: Renal abnormalities and their developmental origin. Nat Rev Genet 8: 791–802, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Little MH, McMahon AP: Mammalian kidney development: principles, progress, and projections. Cold Spring Harb Perspect Biol 2012. Available at http://cshperspectives.cshlp.org/content/4/5/a008300. Accessed 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costantini F, Kopan R: Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev Cell 18: 698–712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Self M, Lagutin OV, Bowling B, Hendrix J, Cai Y, Dressler GR, Oliver G: Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J 25: 5214–5228, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyle S, Misfeldt A, Chandler KJ, Deal KK, Southard-Smith EM, Mortlock DP, Baldwin HS, de Caestecker M: Fate mapping using Cited1-CreERT2 mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Dev Biol 313: 234–245, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopan R, Chen S, Little M: Nephron progenitor cells: shifting the balance of self-renewal and differentiation. Curr Top Dev Biol 107: 293–331, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Larue L, Bellacosa A: Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene 24: 7443–7454, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Yu J, Carroll TJ, McMahon AP: Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development 129: 5301–5312, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP: Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3: 169–181, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi A, Mugford JW, Krautzberger AM, Naiman N, Liao J, McMahon AP: Identification of a multipotent self-renewing stromal progenitor population during mammalian kidney organogenesis. Stem Cell Rep 3: 650–662, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staley BK, Irvine KD: Hippo signaling in Drosophila: recent advances and insights. Dev Dyn 241: 3–15, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enderle L, McNeill H: Hippo gains weight: added insights and complexity to pathway control. Sci Signal 2013. Available at http://stke.sciencemag.org/content/6/296/re7. Accessed 2013 [DOI] [PubMed] [Google Scholar]
- 14.Halder G, Johnson RL: Hippo signaling: growth control and beyond. Development 138: 9–22, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan D: The hippo signaling pathway in development and cancer. Dev Cell 19: 491–505, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harvey K, Tapon N: The Salvador-Warts-Hippo pathway - an emerging tumour-suppressor network. Nat Rev Cancer 7: 182–191, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Varelas X: The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development 141: 1614–1626, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Reginensi A, Scott RP, Gregorieff A, Bagherie-Lachidan M, Chung C, Lim DS, Pawson T, Wrana J, McNeill H: Yap- and Cdc42-dependent nephrogenesis and morphogenesis during mouse kidney development. PLoS Genet 9: e1003380, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hossain Z, Ali SM, Ko HL, Xu J, Ng CP, Guo K, Qi Z, Ponniah S, Hong W, Hunziker W: Glomerulocystic kidney disease in mice with a targeted inactivation of Wwtr1. Proc Natl Acad Sci U S A 104: 1631–1636, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makita R, Uchijima Y, Nishiyama K, Amano T, Chen Q, Takeuchi T, Mitani A, Nagase T, Yatomi Y, Aburatani H, Nakagawa O, Small EV, Cobo-Stark P, Igarashi P, Murakami M, Tominaga J, Sato T, Asano T, Kurihara Y, Kurihara H: Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. Am J Physiol Renal Physiol 294: F542–F553, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Reginensi A, Hoshi M, Boualia SK, Bouchard M, Jain S, McNeill H: Yap and Taz are required for Ret-dependent urinary tract morphogenesis. Development 142: 2696–2703, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reginensi A, Enderle L, Gregorieff A, Johnson RL, Wrana JL, McNeill H: A critical role for NF2 and the Hippo pathway in branching morphogenesis. Nat Commun 7: 12309, 2016 [DOI] [PMC free article] [PubMed]
- 23.Szeto SG, Narimatsu M, Lu M, He X, Sidiqi AM, Tolosa MF, Chan L, De Freitas K, Bialik JF, Majumder S, Boo S, Hinz B, Dan Q, Advani A, John R, Wrana JL, Kapus A, Yuen DA: YAP/TAZ Are Mechanoregulators of TGF-β-Smad Signaling and Renal Fibrogenesis [published online ahead of print March 9, 2016]. J Am Soc Nephrol doi:10.1681/ASN.2015050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L: A global double-fluorescent Cre reporter mouse. Genesis 45: 593–605, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Reginensi A, Clarkson M, Neirijnck Y, Lu B, Ohyama T, Groves AK, Sock E, Wegner M, Costantini F, Chaboissier MC, Schedl A: SOX9 controls epithelial branching by activating RET effector genes during kidney development. Hum Mol Genet 20: 1143–1153, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendez MG, Kojima S, Goldman RD: Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J 24: 1838–1851, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown AC, Muthukrishnan SD, Guay JA, Adams DC, Schafer DA, Fetting JL, Oxburgh L: Role for compartmentalization in nephron progenitor differentiation. Proc Natl Acad Sci U S A 110: 4640–4645, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, Quaggin SE, Harrison R, Mount R, McNeill H: Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet 40: 1010–1015, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Bagherie-Lachidan M, Reginensi A, Pan Q, Zaveri HP, Scott DA, Blencowe BJ, Helmbacher F, McNeill H: Stromal Fat4 acts non-autonomously with Dchs1/2 to restrict the nephron progenitor pool. Development 142: 2564–2573, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Mao Y, Francis-West P, Irvine KD: Fat4/Dchs1 signaling between stromal and cap mesenchyme cells influences nephrogenesis and ureteric bud branching. Development 142: 2574–2585, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Das A, Tanigawa S, Karner CM, Xin M, Lum L, Chen C, Olson EN, Perantoni AO, Carroll TJ: Stromal-epithelial crosstalk regulates kidney progenitor cell differentiation. Nat Cell Biol 15: 1035–1044, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF: Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 332: 458–461, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.