Abstract
Rationale: How host genetic factors affect Mycobacterium tuberculosis (Mtb) infection outcomes remains largely unknown. SP110b, an IFN-induced nuclear protein, is the nearest human homologue to the mouse Ipr1 protein that has been shown to control host innate immunity to Mtb infection. However, the function(s) of SP110b remains unclear.
Objectives: To elucidate the role of SP110b in controlling host immunity and susceptibility to tuberculosis (TB), as well as to identify the fundamental immunological and molecular mechanisms affected by SP110b.
Methods: Using cell-based approaches and mouse models of Mtb infection, we characterized the function(s) of SP110b/Ipr1. We also performed genetic characterization of patients with TB to investigate the role of SP110 in controlling host susceptibility to TB.
Measurements and Main Results: SP110b modulates nuclear factor-κB (NF-κB) activity, resulting in downregulation of tumor necrosis factor-α (TNF-α) production and concomitant upregulation of NF-κB–induced antiapoptotic gene expression, thereby suppressing IFN-γ–mediated monocyte and/or macrophage cell death. After Mtb infection, TNF-α is also downregulated in Ipr1-expressing mice that have alleviated cell death, less severe necrotic lung lesions, more efficient Mtb growth control in the lungs, and longer survival. Moreover, genetic studies in patients suggest that SP110 plays a key role in modulating TB susceptibility in concert with NFκB1 and TNFα genes.
Conclusions: These results indicate that SP110b plays a crucial role in shaping the inflammatory milieu that supports host protection during infection by fine-tuning NF-κB activity, suggesting that SP110b may serve as a potential target for host-directed therapy aimed at manipulating host immunity against TB.
Keywords: Mycobacterium tuberculosis, cell death, inflammation, tumor necrosis factor-α, nuclear factor-κB
At a Glance Commentary
Scientific Knowledge on the Subject
Host genetic factors have been shown for years to contribute to Mycobacterium tuberculosis infection outcomes in both humans and experimental animal models; however, how these factors affect host immunity and susceptibility to tuberculosis (TB) remains largely unknown. Moreover, the crucial issue of how host cells prevent excessive inflammation to reduce inflammation-associated immunopathology during M. tuberculosis infection has not been fully addressed.
What This Study Adds to the Field
We demonstrate that SP110b, via fine-tuning nuclear factor-κB activity, alleviates cell death to facilitate the host’s control of unwanted immunopathology during infection-mediated inflammation. Genetic characterization of patients with TB suggests that SP110 plays a key role in modulating TB susceptibility in concert with the nuclear factor-kB 1 and tumor necrosis factor-α genes. The findings of this study present a novel mechanism of host genetic control of immunity and susceptibility to TB, suggesting that SP110b may serve as a potential target for host-directed therapy to protect against TB.
Tuberculosis (TB), a disease caused by Mycobacterium tuberculosis (Mtb) infection, remains the leading cause of death from a single infectious agent (1). Host genetic factors have been shown to contribute to Mtb infection outcomes (2–5); however, these factors are extremely intricate and poorly understood. Using mouse models, the intracellular pathogen resistance 1 (Ipr1) gene, which is located within the supersusceptibility to tuberculosis 1 (sst1) locus (6), has been identified as a genetic determinant that confers host innate immunity to Mtb infection (7). The Ipr1 orthologous gene in humans is SP110, located on chromosome 2q37.1. Expression of both genes is intensively regulated by IFNs, suggesting that their function(s) is related to that of IFNs (8). Protein structural analyses demonstrated that both Ipr1 and SP110 are similar to nuclear proteins involved in transcriptional regulation and may function as a transcriptional coactivator and/or corepressor (9). SP110 proteins have various isoforms, including the more dominantly expressed SP110 a, b, and, c isoforms, whose functions have yet to be defined.
IFN-γ and tumor necrosis factor-α (TNF-α) are two proinflammatory cytokines that have been best characterized by genetic deficiency states for their key roles in the control of human TB (10). Both cytokines activate macrophages, thereby promoting mycobacterial killing and mediating inflammation in response to Mtb infection (11). Within Mtb-infected lesions, TNF-α (which can also be induced by IFN-γ) is produced by alveolar macrophages in an autocrine fashion or by the Th1 lymphocytes, and it synergizes with IFN-γ, thereby activating the antimycobacterial properties of macrophages (12, 13). Therefore, host cells must appropriately integrate the strength and duration of both cytokine-mediated intracellular signaling networks to ensure an appropriate milieu in response to infection.
The present study provides evidence demonstrating that SP110b, the SP110 isoform most similar to mouse Ipr1, suppresses IFN-γ–induced monocyte and/or macrophage cell death by modulating nuclear factor-κB (NF-κB) activity. The suppression was dependent at least in part on downregulating TNF-α production and upregulating antiapoptotic gene expression induced by NF-κB. Because TNF-α production can be upregulated by IFN-γ and many genes turned on by IFN-γ are also TNF-α–inducible, IFN-γ signaling synergizes the effects of TNF-α and generates a positive feedforward loop (14). Our findings indicate that SP110b plays a crucial role in regulating host immunity by functioning as a “fine-tuner” of NF-κB activity to prevent overamplifying signaling of both cytokines, thereby facilitating the host’s control of unwanted immunopathology.
Methods
A detailed description of the methods is provided in the Methods section of the online supplement.
Cell Lines
The human monocytic leukemia cell lines THP1 and U937 (Bioresource Collection and Research Center, Hsinchu, Taiwan) and the human embryonic kidney cell line HEK293T (American Type Culture Collection, Manassas, VA) were cultured at 37°C in a 5% CO2 atmosphere in RPMI 1640 medium containing penicillin-streptomycin, 2 mM l-glutamine, and 7.5% tetracycline-free fetal bovine serum (Life Technologies, Carlsbad, CA).
Microarray Analysis
The functional and biological relevance of the differentially expressed genes from the GeneChip array (Human Genome U133 Plus 2.0 Array; Affymetrix, Santa Clara, CA) was analyzed using MetaCore software (GeneGo/Thomson Reuters, New York, NY). The microarray data were deposited in the National Center for Biotechnology Information Gene Expression Omnibus database (GEO accession number GSE58096).
Study Approval
Human subject study was conducted in accordance with the terms of the informed consent forms that were provided to and received from participants prior to inclusion in the study. The study was approved by the National Taiwan University Hospital Institutional Review Board (IRB 200612009M and IRB 201003013R). Mouse experiments were performed with the full knowledge and approval of the Harvard Medical School Standing Committee on Animals (protocol 03000).
Statistical Analyses
Quantitative data were analyzed by two-tailed unpaired t test or nonparametric one-way analysis of variance using GraphPad Prism software (GraphPad Software, La Jolla, CA). Survival curves were compared by log-rank test with GraphPad Prism software. P values less than 0.05 were considered statistically significant. The associations of gene polymorphisms and gene–gene interactions with TB were analyzed using SAS software (SAS Institute, Cary, NC) in logistic regression mode.
Results
SP110b Suppresses IFN-γ–induced Cell Death
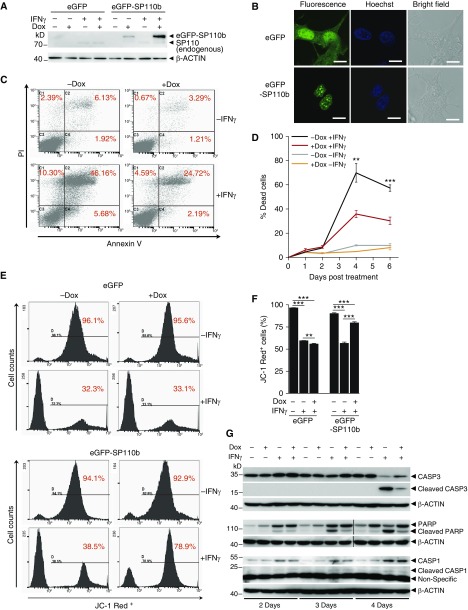
To define SP110b functions, stable THP1 cell lines that could be induced with doxycycline (Dox) to overexpress enhanced green fluorescent protein (eGFP)–SP110b fusion protein were generated and validated (Figures 1A and 1B; see Figure E1A in the online supplement). As shown in Figure 1B, expressed eGFP-SP110b protein was localized to the nucleus, forming nuclear speckles as previously reported (9), while expression of eGFP alone was present in both the nucleus and the cytoplasm. Because IFN-γ, which plays a critical role in TB immunity, has been shown to regulate the cell death features of monocytes and/or macrophages (11, 15, 16), we examined the response of eGFP-SP110b–expressing THP1 cells to IFN-γ stimulation. These experiments demonstrated that THP1 cell death increased between 2 and 6 days post–IFN-γ treatment but that Dox-induced eGFP-SP110b expression decreased IFN-γ–induced cell death (Figures 1C and 1D). Cell death suppression was not observed in the eGFP-expressing THP1 cells upon Dox induction (see Figure E1B). Moreover, eGFP-SP110b expression prevented cell death caused by either low (1–10 U ml−1) or high (50–100 U ml−1) IFN-γ concentrations (see Figure E1C). Similar suppressive effects of SP110b on cell death were also observed for two additional THP1-eGFP-SP110b clones, in differentiated THP1-eGFP-SP110b cells, in U937-eGFP-SP110b cells, and in human monocyte-derived macrophages (see Figures E1D–E1G). Our data indicate that prolonged IFN-γ stimulation caused monocyte and/or macrophage cell death; however, these effects were mitigated by SP110b upregulation.
Figure 1.
SP110b suppresses IFN-γ–induced cell death. (A) Western blot analysis of SP110 expression in THP1-enhanced green fluorescent protein (THP1-eGFP) and THP1-eGFP-SP110b clonal cell lines at 2 days post-treatment. (B) Confocal microscopy was used to visualize the cellular distribution of eGFP-SP110b and eGFP in the THP1 clonal cell lines at 2 days after doxycycline (Dox) induction. Scale bars: 10 μm. (C) Fluorescence-activated cell sorting (FACS) analysis was used to examine IFN-γ–induced cell death of a THP1-eGFP-SP110b clonal cell line (with or without eGFP-SP110b induction) using annexin V/propidium iodide (PI) staining 4 days post-treatment. (D) Kinetic analysis of the cell death frequency of a THP1-eGFP-SP110b clonal cell line by FACS using annexin V/PI staining at the indicated time points post-treatment. Statistical analyses were conducted between the −Dox+IFN-γ groups and the +Dox+IFN-γ groups. (E and F) The mitochondrial health status of cells of the THP1-eGFP and THP1-eGFP-SP110b clonal cell lines was determined using JC-1 staining, followed by (E) FACS analysis and (F) high-content imaging at 4 days post-treatment. (G) Caspase-3 (CASP3), poly(adenosine diphosphate)-ribose polymerase (PARP), and CASP1 expression and cleavage in a THP1-eGFP-SP110b clonal cell line was characterized by Western blot analysis at the indicated time points post-treatment. Data in D and F are presented as the mean ± SD of three cultures. Statistical significance was calculated using a two-tailed, unpaired t test. **P < 0.01; ***P < 0.001. In D, for multiple-comparisons adjustments, the P values less than 0.0125 were considered to be statistically significant. All data represent at least three independent experiments.
Dox-induced eGFP-SP110b expression protected cells against mitochondrial injury (recognized as an early stage of apoptosis) that was elicited by the IFN-γ treatment (Figures 1E and 1F), indicating that SP110b rescued the cells from the early stage of apoptosis that was induced by IFN-γ stimulation. We further demonstrated that SP110b decreased the cleaved caspase-3 (CASP3) (17) and poly(adenosine diphosphate)-ribose polymerase (18) levels (both represent hallmarks of an apoptotic pathway) that were upregulated by the IFN-γ treatment at Day 4 and Day 3 to Day 4, respectively (Figure 1G). In contrast to apoptosis, CASP1-mediated pyroptosis, which occurs during inflammation (19), was barely affected by SP110b (Figure 1G). These data indicate that SP110b expression suppressed cell death mediated by CASP3 but not by CASP1.
SP110b Reduces TNF-α, Resulting in Suppression of Cell Death
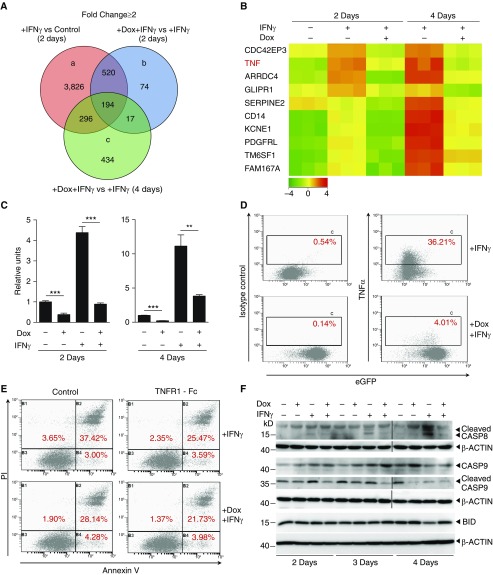
A genome-wide microarray analysis was used to identify genetic determinants that are associated with IFN-γ–induced cell death and regulated by SP110b (see Figures E2A and E2B). This analysis identified three primary sets of genes whose expression levels were at least statistically twofold different (Figure 2A). Among the 194 probes (see Table E1 in the online supplement) that were present in all three sets, TNF-α expression was most significantly inhibited by Dox-induced SP110b expression at both time points post–IFN-γ treatment (Figure 2B and Table 1). The SP110b expression–mediated decreases in IFN-γ–induced TNF-α expression were further validated at both the mRNA and protein levels (Figures 2C and 2D). In addition, expression of a panel of genes as well as a few other key cytokines upregulated by IFN-γ treatment and differentially regulated by SP110b expression was determined by real-time quantitative polymerase chain reaction (see Figures E2C and E2D). To determine whether SP110b suppressed IFN-γ–induced cell death by downregulating TNF-α, we demonstrated that the extent of cell death suppression that was mediated by conditioned medium generated from IFN-γ–treated THP1 cells with eGFP-SP110b expression was similar to that mediated by TNF-α–neutralized conditioned medium from IFN-γ–treated THP1 cells without eGFP-SP110b expression (Figure 2E; see Figure E2E). Moreover, CASP8 (20) activation was inhibited by SP110b (Figure 2F). Because cross-talk exists between CASP8 and mitochondria-mediated cell death via BH3-interacting domain death agonist and CASP9 (21), we further demonstrated that SP110b inhibited CASP9 activation and CASP8-mediated cleavage of BH3-interacting domain death agonist (Figure 2F). These data indicate that SP110b represents a critical regulator of TNF-α production and signaling and that SP110b suppresses IFN-γ–induced cell death by downregulating TNF-α.
Figure 2.
SP110b expression downregulates tumor necrosis factor-α (TNF-α) production, resulting in suppression of IFN-γ–induced cell death. (A) Venn diagram showing the number of genes that were up- or downregulated following doxycycline (Dox)-induced SP110b expression in a THP1-enhanced green fluorescent protein (eGFP)-SP110b clonal cell line following microarray analyses at 2 and 4 days after IFN-γ treatment. (B) Heat map representing the set of differentially expressed genes regulated by SP110b in the THP1-eGFP-SP110b clonal cell line at 2 and 4 days post–IFN-γ treatment. −4 to 4 refer to log2 ratio values (log2 fold change). Orange indicates upregulated genes, and green indicates downregulated genes. (C and D) SP110b-mediated suppression of TNF-α expression in the THP1-eGFP-SP110b clonal cell line was validated (C) at the RNA level by quantitative polymerase chain reaction 2 and 4 days after treatment and (D) at the protein level by intracellular staining 2 days after treatment. (E) THP1 cell death was measured using fluorescence-activated cell sorting analysis of annexin V/propidium iodide (PI)-stained cells after 3 days of culture in the presence of conditioned medium. THP1 cells were cultured in conditioned medium (in the presence or absence of TNF receptor 1 [TNFR1]-Fc) that was generated from THP1-eGFP-SP110b cells that had been treated for 2 days with either IFN-γ alone or IFN-γ + Dox. See also Figure E2E. (F) Caspase-8 (CASP8), CASP9, and BH3-interacting domain death agonist (BID) expression was characterized with Western blot analyses at the indicated time points post-treatment. Data in C are presented as the mean ± SD of three cultures. Statistical significance was calculated using a two-tailed, unpaired t test. **P < 0.01; ***P < 0.001. The results represent one experiment in A and B and three independent experiments in C–F.
Table 1.
Differentially Expressed Genes Regulated by SP110b in THP1-eGFP-SP110b Cells 2 and 4 Days Post–IFN-γ Treatment
| Gene Symbol | Probe Set ID | Fold Change (+Dox+IFN-γ vs. +IFN-γ 4 d) | Regulation | Fold Change (+Dox+IFN-γ vs. +IFN-γ 2 d) | Regulation | Fold Change (+IFN-γ vs. Control 2 d) | Regulation |
|---|---|---|---|---|---|---|---|
| TNF | 207113_s_at | −10.89 | Down | −4.26 | Down | 9.99 | Up |
| LOC284801 | 225762_x_at | −9.54 | Down | −2.11 | Down | 3.36 | Up |
| RCAN2 | 203498_at | −8.46 | Down | −3.28 | Down | 3.60 | Up |
| PDGFRL | 205226_at | −8.40 | Down | −3.72 | Down | 5.48 | Up |
| CD14 | 201743_at | −7.92 | Down | −4.84 | Down | 4.91 | Up |
| TRIM2 | 202341_s_at | −7.33 | Down | −3.44 | Down | 3.43 | Up |
| KCNE1 | 236407_at | −6.51 | Down | −4.45 | Down | 5.84 | Up |
| ARRDC4 | 225283_at | −6.09 | Down | −11.48 | Down | 6.15 | Up |
| CCL2 | 216598_s_at | −5.77 | Down | −2.33 | Down | 29.96 | Up |
| CYP19A1 | 203475_at | −5.60 | Down | −3.07 | Down | 3.25 | Up |
Definition of abbreviations: Dox = doxycycline; eGFP = enhanced green fluorescent protein.
SP110b/Ipr1 Downregulates TNF-α in Mice Infected with Mtb
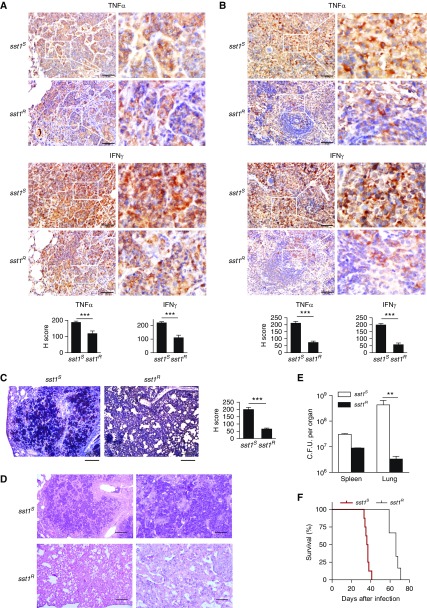
To determine the effects of SP110b/Ipr1 on TNF-α production in vivo, we characterized TNF-α expression levels by performing immunohistochemistry in sst1-susceptible (sst1S) C3HeB/FeJ mice (which do not express the mouse orthologue of SP110 [i.e., Ipr1]) and sst1-resistant (sst1R) C3H.B6-sst1 mice (expressing Ipr1) following Mtb infection. Our results demonstrated that higher TNF-α production levels were detected in the lungs and spleens of the sst1S mice than in the sst1R mice (Figures 3A and 3B; see Figure E3A). In addition, higher IFN-γ production levels were observed in the lungs and spleens of the Mtb-infected sst1S mice (Figures 3A and 3B; see Figure E3A). Moreover, a higher level of active Casp3 was also detected in the lung lesions of the Mtb-infected sst1S mice (Figure 3C). These results indicate that cell death was more strongly induced in the lung lesions of the Mtb-infected sst1S mice and support our data showing that cell death was suppressed in the SP110b-expressing cells during inflammation (Figure 1). Moreover, the Mtb-infected sst1S mice presented with more severe necrotic lung lesions (Figure 3D; see Figure E3B), which correlated with less efficient control of Mtb growth in the lungs (Figure 3E) and shorter survival time (sst1S vs. sst1R; P = 0.0003) (Figure 3F). Given that excessive TNF-α induces a hyperinflammatory milieu that results in immunopathology (22–24), our data suggested that Ipr1-mediated suppression of TNF-α contributed to reduced cell death, alleviated tissue damage, limited Mtb replication in the lungs, and increased survival duration in the infected sst1R mice by preventing excessive inflammation. These findings indicate that SP110b/Ipr1 plays a crucial role in host immunity against Mtb.
Figure 3.
The effect of SP110b/Ipr1 on tumor necrosis factor-α (TNF-α) is associated with reduced tuberculosis susceptibility in mice. (A) Lung and (B) spleen tissues harvested from C3HeB/FeJ (supersusceptibility to tuberculosis 1–susceptible [sst1S]) and C3H.B6-sst1 (supersusceptibility to tuberculosis 1–resistant [sst1R]) mice infected with Mycobacterium tuberculosis (Mtb) were subjected to immunohistochemical analysis using anti–TNF-α and anti–IFN-γ antibodies, respectively. Scale bars: 50 μm. Bottom: Graphs of the immunohistochemistry scores (H scores). Ten fields in four replicates were counted at ×400 magnification. See the Methods section in the online supplement for details. (C) Lungs harvested from the Mtb-infected sst1S and sst1R mice were subjected to immunohistochemical analysis with anti–caspase-3 antibody. Scale bars: 200 μm. Right: Graph of the H scores. Ten fields in four replicates were counted at ×400 magnification. (D) The results of histological analysis (hematoxylin and eosin staining) of the lungs that were harvested from the Mtb-infected sst1S and sst1R mice are shown. Scale bars: 200 μm (left panels), 50 μm (right panels). (E) Mtb burden was determined using the indicated organs that were harvested from the sst1S and sst1R mice by plating organ homogenates at 3 weeks after intravenous infection with Mtb. The data are expressed as the mean ± SD of 10 fields in A–C and 4 samples in E. Statistical significance was calculated using a two-tailed, unpaired t test. **P < 0.01; ***P < 0.001. (F) Survival curves of the sst1S and sst1R mice after intravenous infection with Mtb. The results are representative of one experiment with at least four independent biological replicates (four animals per group in A–E, eight sst1S mice and six sst1R mice in F).
SP110b Downregulates TNF-α via Modulating NF-κB Activity
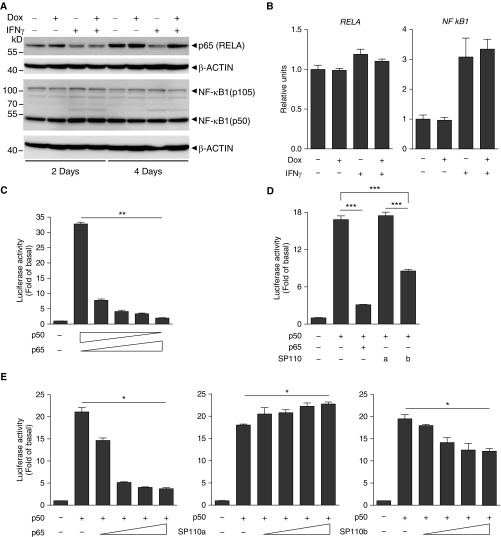
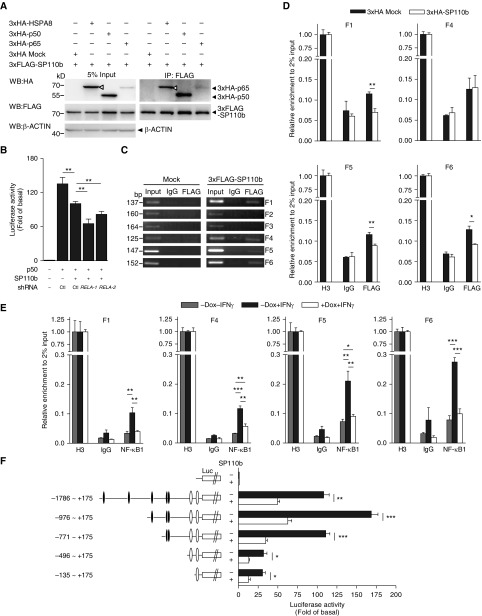
SP110b-mediated downregulation of TNF-α was not mediated through the JAK-STAT pathway (25), because STAT1 expression and phosphorylation levels were not downregulated by SP110b expression (see Figures E4A and E4B). Next, we determined whether TNF-α downregulation was mediated via NF-κB, which has been shown to play an essential role in preventing and suppressing TNF-α–induced cell death (26, 27). In the presence of IFN-γ, SP110b expression increased RELA (p65), but not NF-κB1 (p105/p50), protein levels (Figure 4A), while the mRNA levels of both RELA (the gene that encodes NF-κB p65 subunit) and NFκB1 were not significantly affected (Figure 4B). The effects of SP110b on NF-κB–driven TNF-α transcription were examined subsequently using luciferase reporter assays with the TNF-α promoter region. These results showed that p50 overexpression induced the highest TNF-α promoter activity levels, whereas this activity decreased when the p65/p50 ratio was increased (Figure 4C). The p50-driven TNF-α promoter activity was suppressed by SP110b and p65 but enhanced by the expression of SP110a, which is the other SP110 isoform (Figures 4D and 4E). However, neither SP110a nor SP110b significantly influenced p65- or p50/p65-driven TNF-α promoter activity (see Figure E4C). Using a different reporter assay system with a basic promoter element linked to the five κB sites, we demonstrated that neither p50- nor p65-driven transcriptional activation was significantly affected by SP110a or SP110b (see Figure E4D). These results indicate that SP110b expression selectively downregulated the observed p50-driven TNF-α promoter activity.
Figure 4.
SP110b downregulates tumor necrosis factor-α (TNF-α) production by modulating nuclear factor-κB (NF-κB) activity. (A) Western blot analysis of RELA and NF-κB1 expression in a THP1-enhanced green fluorescent protein (eGFP)-SP110b clonal cell line at 2 and 4 days after treatment. (B) RELA and NFκB1 gene expression in a THP1-eGFP-SP110b clonal cell line was analyzed by quantitative polymerase chain reaction at 2 days post-treatment. (C–E) The relative firefly (F.)/Renilla (R.) luciferase (Luc) values associated with the TNF-α promoter were measured 2 days after transient cotransfection with the pGL3-TNF-α promoter F.Luc, pSV40-R.Luc, and the indicated constructs into HEK293T cells. An empty vector is included in the left column as a negative control. In D, a and b indicate SP110a and SP110b, respectively. Data in B–E are presented as the mean ± SD of three cultures. Statistical significance was calculated using (C and E) nonparametric one-way analysis of variance (Kruskal-Wallis test) or (D) a two-tailed, unpaired t test. *P < 0.05; **P < 0.01; ***P < 0.001. All data represent three independent experiments. Dox = doxycycline; RELA = the gene that encodes NF-κB p65 subunit.
SP110b Interacts with NF-κB–Binding Sites in TNF-α Promoter
Using a coimmunoprecipitation assay, we further demonstrated that SP110b interacted with either p65 or p50 (Figure 5A) and that phosphorylation facilitated these interactions (see Figure E5A). Knocking down endogenous RELA expression did not restore the p50-driven TNF-α promoter activity in the presence of SP110b (Figure 5B; see Figure E5B), suggesting that it is unlikely that SP110b expression inhibited the p50-driven TNF-α promoter activity by enhancing endogenous p65 protein levels. Because SP110b is regarded as a transcriptional corepressor (9), we further demonstrated that SP110b interacted with several NF-κB–binding regions within the TNF-α promoter (Figure 5C; see Figure E5C). Moreover, quantitative chromatin immunoprecipitation and reporter assays demonstrated that the interaction between the p50 complex and several DNA elements containing κB-binding sites in the TNF-α promoter was inhibited in the presence of SP110b (Figures 5D–5F; see Figures E5D and E5E). These data suggest that SP110b inhibits p50-driven TNF-α promoter activity by interacting with NF-κB–binding regions in the TNF-α promoter, thus suppressing TNF-α production.
Figure 5.
SP110b interacts with nuclear factor-κB (NF-κB) binding sites in the tumor necrosis factor-α (TNF-α) promoter region. (A) Whole-cell HEK293T extracts were subjected to immunoprecipitation (IP) using an anti-FLAG antibody and analyzed by Western blotting (WB) with the indicated antibodies at 2 days post-transfection with the respective constructs. Open triangles indicate 3×hemagglutinin–heat shock 70 kDa protein 8 (3×HA-HSPA8). HSPA8 was used as a positive control (see the Methods section in the online supplement). (B) Relative luciferase (Luc, L.) values of the TNF-α promoter were measured 2 days after transient cotransfection of the HEK293T cells with the pGL3-TNF-α promoter-F.Luc, pSV40-R.Luc, and the indicated constructs. An empty vector control is shown in the left column. Ctl indicates a vector that expressed shLacZ (used as a control). (C and D) Nuclear extracts from HEK293T cells at 2 days post-transfection (C) with the empty vector (mock) or the 3×FLAG-SP110b–expressing vector or (D) with the 3×FLAG-p50–expressing vector plus an empty vector or plus the 3×HA-SP110b–expressing vector were subjected to chromatin IP using anti-FLAG, anti–histone H3 (H3), or anti-IgG antibodies, respectively. Precipitated DNA fragments were amplified by (C) polymerase chain reaction (PCR) and (D) quantitative PCR with the primer pairs indicated in Figure E5C. (E) Nuclear extracts from THP1-eGFP-SP110b clonal cell lines at 2 days post-treatment were subjected to chromatin IP using anti-NF-κB1, anti–histone H3 (H3) or anti-IgG antibodies, respectively. Precipitated DNA fragments were amplified by quantitative PCR with the primer pairs indicated in Figure E5C. (F) The relative Luc values of the full-length and serial deletion TNF-α promoter constructs were measured 2 days after transient cotransfection of HEK293T cells with pGL3-TNF-α promoter-F.Luc, pSV40-R.Luc, and the indicated constructs. An empty vector control is included in the top row. Solid ovals indicate putative NF-κB binding sites, and open ovals indicate NF-κB binding sites, as described in Figure E5C. (B, D, E, and F) Data are presented as the mean ± SD of three cultures. Statistical significance was calculated using a two-tailed, unpaired t test. *P < 0.05; **P < 0.01; ***P < 0.001. All data are representative of at least two independent experiments. Dox = doxycycline; eGFP = enhanced green fluorescent protein; RELA = the gene that encodes NF-κB p65 subunit; shRNA = short hairpin RNA.
SP110b Upregulates Antiapoptotic Genes Induced by NF-κB
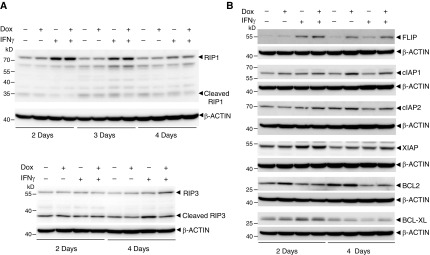
The receptor-interacting protein (RIP), a marker that reflects a divergence in the TNF receptor 1 pathway and that is implicated as collaborating with death receptor proteins to regulate the balance between cell survival and death (28), is cleaved by CASP8 following TNF-α–induced apoptosis, and RIP cleavage further abolishes TNF-α–induced NF-κB activation (29). The cleaved RIP (RIP1 and RIP3) levels decreased in the presence of SP110b (Figure 6A), suggesting that the SP110b-mediated downregulation of cleaved RIP may lead to NF-κB activation. NF-κB activation promotes the expression of a number of antiapoptotic genes, including members of the inhibitor of apoptosis protein family, the Bcl-2 family, and c-FLIP (Fas-associated death domain–like IL-1β–converting enzyme–inhibitory protein) (30). We demonstrated that the expression of several antiapoptotic genes was enhanced concomitant with SP110b induction (Figure 6B). These results indicate that SP110b not only modulates NF-κB activity to suppress TNF-α production but also enhances the expression of antiapoptotic genes, resulting in a reduction in cell death during proinflammation.
Figure 6.
SP110b upregulates the expression of antiapoptotic genes that are induced by nuclear factor κB. (A) Receptor-interacting protein (RIP1 and RIP3) expression and cleavage levels in the THP1-eGFP-SP110b clonal cell line were characterized by Western blot analysis at the indicated time points post-treatment. (B) FLIP (Fas-associated death domain–like IL-1β–converting enzyme–inhibitory protein), cellular inhibitor of apoptosis protein 1 (cIAP1), cIAP2, X-linked inhibitor of apoptosis protein (XIAP), B-cell lymphoma 2 (BCL2), and B-cell lymphoma–extra large (BCL-XL) expression levels in the THP1-eGFP-SP110b clonal cell line were characterized by Western blot analysis with the indicated antibodies at the respective time points post-treatment. All data are representative of at least two independent experiments. Dox = doxycycline; eGFP = enhanced green fluorescent protein.
SP110 Regulates TB Susceptibility in Concert with NFκB1-TNFα
We examined polymorphisms in the SP110, NFκB1, and TNFα genes for associations with Mtb infection control by performing genetic characterization of patients with TB. In total, 12 single-nucleotide polymorphisms (SNPs) in SP110, 23 in NFκB1, and 9 in TNFα were analyzed in 296 pulmonary patients with TB and 231 healthy household contacts (including family members and nurses of the patients) (see Tables E2–E5). These results demonstrated that epistatic interactions of SP110 with NFκB1 and TNFα gene polymorphisms produced much more noticeable associations with the genetic susceptibility to TB infection than the effects of the NFκB1–TNFα interaction and individual genes (Table 2; see Tables E6 and E7). In addition, the association of NFκB1–TNFα interaction with TB susceptibility is determined by the SP110 alleles. These genetic studies in patients with TB suggest that SP110 plays a key role in modulating TB susceptibility in concert with NFκB1 and TNFα genes. Altogether, our findings indicate that SP110 controls TB susceptibility by regulating NFκB1–TNFα interaction–mediated responses.
Table 2.
Odds Ratios for Epistatic Interactions of Single-Nucleotide Polymorphisms in SP110, NFκB1, and TNFα Genes
| NFκB1: rs230487 | TNFα: rs1800629 | Controls (%) | Cases (%) | OR (95% CI) | P Value | |
|---|---|---|---|---|---|---|
| |
CC |
GG |
68 (33) |
75 (28) |
1.000 |
N/A |
| |
CC |
GA/AA |
12 (6) |
20 (7) |
1.603 (0.657–3.91) |
0.299 |
| |
CA/AA |
GG |
114 (55) |
146 (54) |
1.187 (0.739–1.907) |
0.479 |
| |
CA/AA |
GA/AA |
15 (7) |
31 (11) |
1.756 (0.813–3.794) |
0.152 |
| SP110: rs7580900 | NFκB1: rs230487 | TNFα: rs1800629 | Controls (%) | Cases (%) | OR (95% CI) | P Value |
|---|---|---|---|---|---|---|
| TT |
CC |
GG |
27 (13) |
18 (7) |
1.000 |
N/A |
| TT |
CC |
GA/AA |
4 (2) |
9 (3) |
4.524 (1.033–19.824) |
0.045 |
| TT |
CA/AA |
GG |
38 (18) |
54 (20) |
2.919 (1.244–6.85) |
0.014 |
| TT |
CA/AA |
GA/AA |
9 (4) |
10 (4) |
2.52 (0.752–8.445) |
0.134 |
| CT/CC |
CC |
GG |
41 (20) |
57 (21) |
3.026 (1.304–7.025) |
0.010 |
| CT/CC |
CC |
GA/AA |
8 (4) |
11 (4) |
2.885 (0.823–10.116) |
0.098 |
| CT/CC |
CA/AA |
GG |
75 (36) |
90 (33) |
2.319 (1.064–5.052) |
0.034 |
| CT/CC |
CA/AA |
GA/AA |
6 (3) |
21 (8) |
5.442 (1.646–17.993) |
0.006 |
| SP110: rs7580912 | NFκB1: rs230487 | TNF-α: rs1800629 | Controls (%) | Cases (%) | OR (95% CI) | P Value |
|---|---|---|---|---|---|---|
| TT | CC | GG | 32 (15) | 27 (10) | 1.000 | N/A |
| TT | CC | GA/AA | 6 (3) | 12 (4) | 3.666 (1.039–12.931) | 0.043 |
| TT | CA/AA | GG | 51 (24) | 66 (24) | 2.135 (1.011–4.51) | 0.047 |
| TT | CA/AA | GA/AA | 11 (5) | 14 (5) | 2.067 (0.712–5.999) | 0.182 |
| CT/CC | CC | GG | 36 (17) | 48 (18) | 2.235 (1.014–4.926) | 0.046 |
| CT/CC | CC | GA/AA | 6 (3) | 8 (3) | 1.695 (0.444–6.469) | 0.440 |
| CT/CC | CA/AA | GG | 63 (30) | 78 (29) | 1.706 (0.836–3.479) | 0.142 |
| CT/CC | CA/AA | GA/AA | 4 (2) | 17 (6) | 4.644 (1.27–16.977) | 0.020 |
Definition of abbreviations: CI = confidence interval; N/A = not applicable; NFκB1 = nuclear factor-κ B1; OR = odds ratio; TNF-α = tumor necrosis factor-α.
ORs are adjusted for age and sex. The significant ORs are shown in italic type.
Discussion
Most humans susceptible to Mtb infection are not immunodeficient, and susceptible humans with active TB indeed develop Mtb-specific immunity (11). One explanation for why this immunity fails to protect susceptible humans from infection is that overactivity of the immune response elicited by Mtb infection causes immunopathology that further contributes to TB susceptibility in these individuals. Among various host-protective immune factors, TNF-α plays an important role in controlling Mtb infection and disease reactivation; however, overproduction of TNF-α may cause pathology (22, 31). Our studies demonstrated that SP110b, whose expression is upregulated by IFNs, downregulated TNF-α production of cells activated by IFN-γ, thereby preventing cell death, and that Ipr1 (homologue of SP110b) prevented overproduction of TNF-α and further development of necrotic lung lesions during Mtb infection. Wu and colleagues found that, consistent with our findings, Ipr1 overexpression downregulated TNF-α production in mouse macrophages infected with Mtb in vitro (32). These studies indicate that SP110b/Ipr1 acted as a regulator of proinflammatory cytokines of host immunity, contributing to the reduction of tissue damage caused by excessive inflammation during Mtb infection.
SP110 is a single-copy gene that is located on chromosome 2 in humans; however, the mouse Ipr1 gene is part of a rearranged and repeat cluster containing 60–2,000 copies of Ipr1 and 20 neighboring genes on chromosome 1 in all inbred experimental mouse strains (33, 34), making the generation of an Ipr1-knockout mouse strain using gene-targeting approaches currently infeasible. Therefore, we used C3HeB/FeJ mice with a natural deficiency in the Ipr1 gene, which mediates innate immunity to TB, and demonstrated that necrotic lesions that incapacitated the control of Mtb growth specifically developed in the lungs of the mice following intravenous infection with either high (1 × 105) (35) or low (3.3 × 104) Mtb doses (Figure 3D; see Figure E3B). Our immunohistochemical results further demonstrated that the absence of the Ipr1 gene in the susceptible mice was associated with increased TNF-α production in necrotic lung lesions and spleens compared with the levels observed in resistant mice (Figures 3A and 3B). These results are consistent with, and represent an extension of, our in vitro observations demonstrating that SP110b expression downregulated TNF-α production, which contributed to cell death (Figure 2). In our mouse models of infection, high IFN-γ production levels were detected in the spleens (Figure 3B), indicating that T-cell priming still occurred in the susceptible mice. This finding is consistent with our previous observation that Mtb infection in other organs is more efficiently controlled than in the lungs (35), suggesting that TB progression in these susceptible mice is not due to systemic failure of host immunity, but rather that increased TNF-α production contributed to the severe necrotic lung lesion development, thereby resulting in rapid multiplication of Mtb within the lesions. Taken together, the disease features of our susceptible mouse model resemble those of immunocompetent, susceptible humans with a systemic defense mechanism that is able to control the disease in organs other than the lungs, suggesting that an imbalanced, but not a deficient, immune response causes disease development in the lungs.
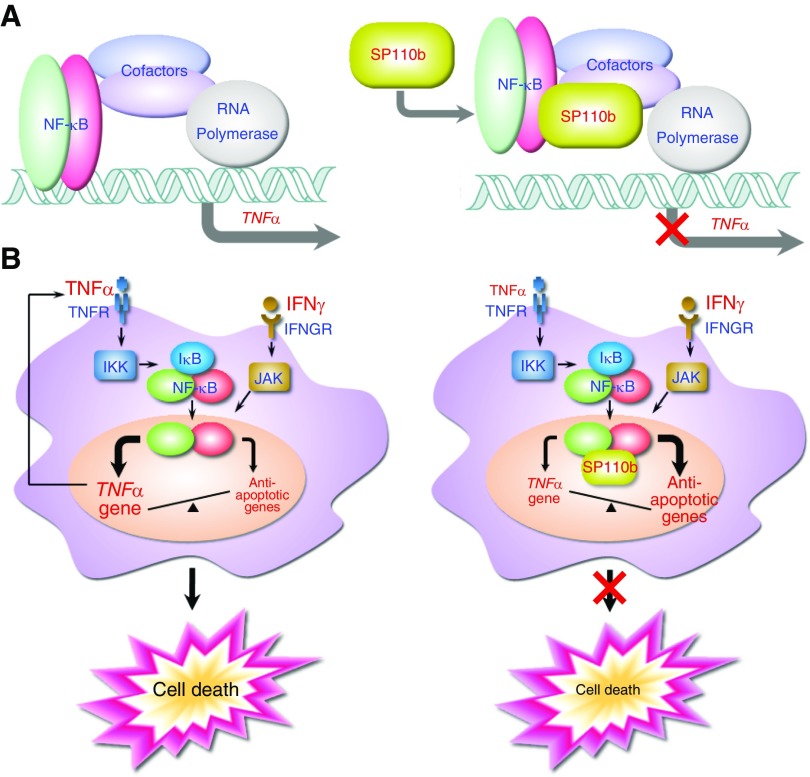
NF-κB proteins are sequence-specific transcription factors that are capable of inducing hundreds of genes with extremely diverse, and even apparently opposing, biological functions, and NF-κB activity is integrated with that of many other transcriptional regulators (36–38). Although p50 does not possess potential transactivation domains, in vitro studies have shown that p50 dimers induce κB-site–dependent transcriptional activation (39), most likely by cooperating with other transcription factors and coregulators (40, 41). Using a luciferase reporter system, we demonstrated that p50 overexpression induced the highest TNF-α promoter transcriptional activity, with a region containing seven potential NF-κB binding sites (Figure 4C; see Figure E5C) and that this activity was suppressed by SP110b or p65 (Figures 4D and 4E). Because NF-κB dimers induced specific transcriptional activity at each promoter following binding to the κB DNA response elements (42, 43), our data presented in Figure 4C support the notion that TNF-α promoter activity can be determined by a distinctive combination of NF-κB dimers. In the presence of SP110b, NFκB1 mRNA and protein levels were not significantly affected, while p65 protein levels increased (Figures 4A and 4B). Therefore, we speculate that SP110b downregulated the TNF-α promoter activity by regulating the ratio of p50/p50 to p50/p65, which are two of the most abundant NF-κB dimers that are present in cells (44). Moreover, the quantitative chromatin immunoprecipitation analysis data and reporter assays (Figures 5D–5F; see Figures E5D and E5E) suggested that SP110b suppressed the p50-mediated transcriptional activity of the TNF-α promoter possibly by interrupting the formation of a complex composed of p50 (and other components) at the κB sites or by altering the composition of the complex (Figure 7A). Future studies are required to determine whether SP110b competes with p50 for direct binding to the κB sites or blocks the DNA-binding activity of p50, thereby inhibiting p50-mediated TNF-α transcription.
Figure 7.
A model of SP110b functions. (A) A model of the SP110b-mediated downregulation of tumor necrosis factor-α (TNF-α) production. SP110b suppresses the nuclear factor-κB (NF-κB)–mediated transcriptional activity of the TNF-α promoter, possibly by inhibiting the interaction between NF-κB complex and DNA elements containing κB-binding sites in the TNF-α promoter. (B) A model of the SP110b-mediated mechanisms that are associated with cell death suppression. SP110b modulates NF-κB activity, resulting in downregulation of TNF-α production and concomitant upregulation of NF-κB–induced antiapoptotic gene expression, thereby suppressing IFN-γ–mediated monocyte and/or macrophage cell death. IFNGR = IFN-γ receptor; IκB = inhibitor of κB; IKK = inhibitor of κB kinase; JAK = Janus kinase; TNFR = tumor necrosis factor receptor.
Although genetic defects in the SP110 gene have been found to be responsible for hepatic venoocclusive disease and immunodeficiency (45), the role that SP110 plays in controlling TB in humans remains controversial (46–50). After screening Taiwanese populations for polymorphisms in SP110, NFκB1, and TNFα genes that are associated with TB susceptibility, one SNP (rs11556887) in SP110 and one SNP (rs13117745) in NFκB1 were found to be associated with TB (see Tables E3–E5). The logistic regression analysis further revealed that two SNPs (rs7580900 and rs7580912) in SP110 significantly interacted with three NFκB1 SNPs (rs230487, rs230519, and rs1599961) and one TNFα SNP (rs1800629) and that the epistatic interactions produce much more noticeable associations with the control of TB disease than the NFκB1–TNFα interaction effects (Table 2; see Tables E6 and E7). Moreover, subjects with a TT genotype in SP110 rs7580900 and rs7580912, a CC genotype in NFκB1 rs230487, and a GA/AA genotype in TNFα rs1800629 have a higher risk of developing TB than those with the same genotype in SP110 and NFκB1 but a GG genotype in TNFα. On the contrary, subjects carrying a CT/CC genotype in SP110, a CC genotype in NFκB1, and a GA/AA genotype in TNFα exhibit a lower risk than those carrying the same genotype in SP110 and NFκB1 but a GG genotype in TNFα, indicating that the C alleles of SP110 SNPs change the trend of the effect of NFκB1–TNFα SNP interaction (Table 2). Our study indicates that, in combination with other genetic factors, genomic variations in the SP110 region contributed to TB susceptibility in humans. These results not only support that the interaction of SP110 with NFκB1 and TNFα genes plays a crucial role in the pathogenesis of the disease but also suggest that the combinations of SP110 with the NFκB1 and TNFα gene variants may provide novel predictive markers for TB disease outcomes.
In summary, the results presented in this report provide novel data regarding the role of SP110b in controlling cell death and host immunity as well as a mechanistic explanation for the observations that SP110b regulates NF-κB activity. By fine-tuning NF-κB activity, SP110b suppressed TNF-α production and enhanced expression of antiapoptotic genes, resulting in alleviation of cell death due to IFN-γ–mediated cytotoxicity and prevention of overamplifying signaling of both cytokines (Figure 7B). Our findings define a previously unprecedented function of SP110b/Ipr1 proteins in integrating the cellular signals generated during inflammation with mechanisms regulating gene expression and cell death of host cells. These studies not only provide novel mechanistic insights into TB pathogenesis but also disclose potential new molecular targets for developing host-directed therapeutic treatments for TB.
Acknowledgments
Acknowledgment
The authors thank Prof. Pan-Chyr Yang for his supervision and support of this study, Dr. Min-Shu Hsieh for providing expert assistance in pathology, and Hsiang-Ting Hsu and Bo-Wen Chen for technical assistance. In addition, the authors are grateful for the expert assistance provided by the Microarray Core Facility of the National Research Program for Genomic Medicine of the National Science Council in Taiwan.
Footnotes
Supported by the National Science Council, Taiwan (NSC 97-2320-B-010-006-MY3, NSC 99-2321-B-002-022, NSC 100-2321-B-002-009, NSC 100-2320-B-002-106, NSC 101-2321-B-002-001, NSC 101-2320-B-002-022, and NSC 103-2320-B-002-044-MY3 [B.-S.Y.]; NSC 99-2320-B-002-078-MY3, NSC 103-2314-B-002-083, and 104-2314-B-002-075 [M.-L.C.]); the Institute for Biotechnology and Medicine Industry, Taiwan; National Taiwan University Hospital, Taiwan (UN105-034); and National Institutes of Health grant 5R01 HL059836 (I.K.).
Author Contributions: Conception and design: J.-S.L., M.-L.C., and B.-S.Y.; data acquisition: J.-S.L., S.-Y.C., C.-W.L., H.W., W.-C.C., and B.-S.Y.; data analysis and interpretation: J.-S.L., M.-L.C., S.-L.Y., C.-H.C., and B.-S.Y.; patient recruitment and specimen processing: J.-Y.W., L.-N.L., and C.-J.Y.; and drafting of the manuscript for important intellectual content: M.-L.C., I. K., and B.-S.Y.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201601-0103OC on November 18, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.World Health Organization (WHO) Global tuberculosis report 2015. 20th ed. Geneva, Switzerland: WHO; 2015. [Google Scholar]
- 2.Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20:581–620. doi: 10.1146/annurev.immunol.20.081501.125851. [DOI] [PubMed] [Google Scholar]
- 3.Hill AV. The genomics and genetics of human infectious disease susceptibility. Annu Rev Genomics Hum Genet. 2001;2:373–400. doi: 10.1146/annurev.genom.2.1.373. [DOI] [PubMed] [Google Scholar]
- 4.Apt A, Kramnik I. Man and mouse TB: contradictions and solutions. Tuberculosis (Edinb) 2009;89:195–198. doi: 10.1016/j.tube.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fortin A, Abel L, Casanova JL, Gros P. Host genetics of mycobacterial diseases in mice and men: forward genetic studies of BCG-osis and tuberculosis. Annu Rev Genomics Hum Genet. 2007;8:163–192. doi: 10.1146/annurev.genom.8.080706.092315. [DOI] [PubMed] [Google Scholar]
- 6.Kramnik I, Dietrich WF, Demant P, Bloom BR. Genetic control of resistance to experimental infection with virulent Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2000;97:8560–8565. doi: 10.1073/pnas.150227197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan H, Yan BS, Rojas M, Shebzukhov YV, Zhou H, Kobzik L, Higgins DE, Daly MJ, Bloom BR, Kramnik I. Ipr1 gene mediates innate immunity to tuberculosis. Nature. 2005;434:767–772. doi: 10.1038/nature03419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadereit S, Gewert DR, Galabru J, Hovanessian AG, Meurs EF. Molecular cloning of two new interferon-induced, highly related nuclear phosphoproteins. J Biol Chem. 1993;268:24432–24441. [PubMed] [Google Scholar]
- 9.Bloch DB, Nakajima A, Gulick T, Chiche JD, Orth D, de La Monte SM, Bloch KD. Sp110 localizes to the PML-Sp100 nuclear body and may function as a nuclear hormone receptor transcriptional coactivator. Mol Cell Biol. 2000;20:6138–6146. doi: 10.1128/mcb.20.16.6138-6146.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filipe-Santos O, Bustamante J, Chapgier A, Vogt G, de Beaucoudrey L, Feinberg J, Jouanguy E, Boisson-Dupuis S, Fieschi C, Picard C, et al. Inborn errors of IL-12/23- and IFN-γ-mediated immunity: molecular, cellular, and clinical features. Semin Immunol. 2006;18:347–361. doi: 10.1016/j.smim.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 11.North RJ, Jung YJ. Immunity to tuberculosis. Annu Rev Immunol. 2004;22:599–623. doi: 10.1146/annurev.immunol.22.012703.104635. [DOI] [PubMed] [Google Scholar]
- 12.Fenton MJ, Vermeulen MW, Kim S, Burdick M, Strieter RM, Kornfeld H. Induction of γ interferon production in human alveolar macrophages by Mycobacterium tuberculosis. Infect Immun. 1997;65:5149–5156. doi: 10.1128/iai.65.12.5149-5156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flesch IE, Kaufmann SH. Activation of tuberculostatic macrophage functions by γ interferon, interleukin-4, and tumor necrosis factor. Infect Immun. 1990;58:2675–2677. doi: 10.1128/iai.58.8.2675-2677.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-γ: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 15.Munn DH, Beall AC, Song D, Wrenn RW, Throckmorton DC. Activation-induced apoptosis in human macrophages: developmental regulation of a novel cell death pathway by macrophage colony-stimulating factor and interferon γ. J Exp Med. 1995;181:127–136. doi: 10.1084/jem.181.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J, Kornfeld H. Interferon-γ regulates the death of M. tuberculosis-infected macrophages. J Cell Death. 2010;3:1–11. doi: 10.4137/jcd.s2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salvesen GS, Dixit VM. Caspases: intracellular signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 18.Tewari M, Quan LT, O’Rourke K, Desnoyers S, Zeng Z, Beidler DR, Poirier GG, Salvesen GS, Dixit VM. Yama/CPP32β, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 19.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 22.Bekker LG, Moreira AL, Bergtold A, Freeman S, Ryffel B, Kaplan G. Immunopathologic effects of tumor necrosis factor α in murine mycobacterial infection are dose dependent. Infect Immun. 2000;68:6954–6961. doi: 10.1128/iai.68.12.6954-6961.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roca FJ, Ramakrishnan L. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell. 2013;153:521–534. doi: 10.1016/j.cell.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dorhoi A, Kaufmann SH. Tumor necrosis factor α in mycobacterial infection. Semin Immunol. 2014;26:203–209. doi: 10.1016/j.smim.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 26.Beg AA, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 27.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-α-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 28.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 29.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karin M, Lin A. NF-κB at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 31.Ehlers S, Benini J, Held HD, Roeck C, Alber G, Uhlig S. αβ T cell receptor-positive cells and interferon-γ, but not inducible nitric oxide synthase, are critical for granuloma necrosis in a mouse model of mycobacteria-induced pulmonary immunopathology. J Exp Med. 2001;194:1847–1859. doi: 10.1084/jem.194.12.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Y, Guo Z, Yao K, Miao Y, Liang S, Liu F, Wang Y, Zhang Y. The transcriptional foundations of Sp110-mediated macrophage (RAW264.7) resistance to Mycobacterium tuberculosis H37Ra. Sci Rep. 2016;6:22041. doi: 10.1038/srep22041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weichenhan D, Kunze B, Winking H, van Geel M, Osoegawa K, de Jong PJ, Traut W. Source and component genes of a 6–200 Mb gene cluster in the house mouse. Mamm Genome. 2001;12:590–594. doi: 10.1007/s00335-001-3015-9. [DOI] [PubMed] [Google Scholar]
- 34.Agulnik S, Plass C, Traut W, Winking H. Evolution of a long-range repeat family in chromosome 1 of the genus Mus. Mamm Genome. 1993;4:704–710. doi: 10.1007/BF00357793. [DOI] [PubMed] [Google Scholar]
- 35.Yan BS, Pichugin AV, Jobe O, Helming L, Eruslanov EB, Gutiérrez-Pabello JA, Rojas M, Shebzukhov YV, Kobzik L, Kramnik I. Progression of pulmonary tuberculosis and efficiency of bacillus Calmette-Guérin vaccination are genetically controlled via a common sst1-mediated mechanism of innate immunity. J Immunol. 2007;179:6919–6932. doi: 10.4049/jimmunol.179.10.6919. [DOI] [PubMed] [Google Scholar]
- 36.Vallabhapurapu S, Karin M. Regulation and function of NF-κB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 37.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 38.Smale ST. Hierarchies of NF-κB target-gene regulation. Nat Immunol. 2011;12:689–694. doi: 10.1038/ni.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin R, Gewert D, Hiscott J. Differential transcriptional activation in vitro by NF-κB/Rel proteins. J Biol Chem. 1995;270:3123–3131. doi: 10.1074/jbc.270.7.3123. [DOI] [PubMed] [Google Scholar]
- 40.Sheppard KA, Rose DW, Haque ZK, Kurokawa R, McInerney E, Westin S, Thanos D, Rosenfeld MG, Glass CK, Collins T. Transcriptional activation by NF-κB requires multiple coactivators. Mol Cell Biol. 1999;19:6367–6378. doi: 10.1128/mcb.19.9.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann A, Baltimore D. Circuitry of nuclear factor κB signaling. Immunol Rev. 2006;210:171–186. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 42.Saccani S, Pantano S, Natoli G. Modulation of NF-κB activity by exchange of dimers. Mol Cell. 2003;11:1563–1574. doi: 10.1016/s1097-2765(03)00227-2. [DOI] [PubMed] [Google Scholar]
- 43.Wang VY, Huang W, Asagiri M, Spann N, Hoffmann A, Glass C, Ghosh G. The transcriptional specificity of NF-κB dimers is coded within the κB DNA response elements. Cell Rep. 2012;2:824–839. doi: 10.1016/j.celrep.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 45.Roscioli T, Cliffe ST, Bloch DB, Bell CG, Mullan G, Taylor PJ, Sarris M, Wang J, Donald JA, Kirk EP, et al. Mutations in the gene encoding the PML nuclear body protein Sp110 are associated with immunodeficiency and hepatic veno-occlusive disease. Nat Genet. 2006;38:620–622. doi: 10.1038/ng1780. [DOI] [PubMed] [Google Scholar]
- 46.Tosh K, Campbell SJ, Fielding K, Sillah J, Bah B, Gustafson P, Manneh K, Lisse I, Sirugo G, Bennett S, et al. Variants in the SP110 gene are associated with genetic susceptibility to tuberculosis in West Africa. Proc Natl Acad Sci USA. 2006;103:10364–10368. doi: 10.1073/pnas.0603340103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thye T, Browne EN, Chinbuah MA, Gyapong J, Osei I, Owusu-Dabo E, Niemann S, Rüsch-Gerdes S, Horstmann RD, Meyer CG. No associations of human pulmonary tuberculosis with Sp110 variants. J Med Genet. 2006;43:e32. doi: 10.1136/jmg.2005.037960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Babb C, Keet EH, van Helden PD, Hoal EG. SP110 polymorphisms are not associated with pulmonary tuberculosis in a South African population. Hum Genet. 2007;121:521–522. doi: 10.1007/s00439-007-0335-1. [DOI] [PubMed] [Google Scholar]
- 49.Szeszko JS, Healy B, Stevens H, Balabanova Y, Drobniewski F, Todd JA, Nejentsev S. Resequencing and association analysis of the SP110 gene in adult pulmonary tuberculosis. Hum Genet. 2007;121:155–160. doi: 10.1007/s00439-006-0293-z. [DOI] [PubMed] [Google Scholar]
- 50.Fox GJ, Sy DN, Nhung NV, Yu B, Ellis MK, Van Hung N, Cuong NK, Thi Lien L, Marks GB, Saunders BM, et al. Polymorphisms of SP110 are associated with both pulmonary and extra-pulmonary tuberculosis among the Vietnamese. PLoS One. 2014;9:e99496. doi: 10.1371/journal.pone.0099496. [DOI] [PMC free article] [PubMed] [Google Scholar]