Persistent challenges in managing pulmonary vascular disease progression and poor outcomes, despite the aggressive use of current drug therapies, highlight the need for identifying novel targets for intervention and therapeutic strategies that extend beyond pulmonary vasodilator therapy alone (1). Recent efforts to enhance clinical and molecular phenotyping of patient populations with pulmonary arterial hypertension (PAH) by incorporating “precision medicine” strategies through genetics, genomic and proteomic biomarkers, technologies that enhance right ventricular assessments and lung vascular imaging, and other methods, have generated much excitement that will likely improve patient outcomes (2–5). In addition, a key contributor to morbidity is the relatively late diagnosis in many patients with PAH, whose disease is not recognized until the onset of clinical signs and symptoms, when substantial pulmonary vascular disease is already present. Thus, a contemporary focus in PAH is on early identification and intervention of at-risk patients before the development of significant pulmonary vascular disease (2).
Conclusions from large epidemiologic studies in unselected patients cast new light on the spectrum of risk associated with cardiopulmonary hemodynamics. These reports identify a sizeable population that appears vulnerable clinically due to pulmonary arterial pressure that is minimally elevated but below the currently accepted range of abnormal (6, 7). This trend reiterates the importance of focus on pulmonary hypertension before end-stage disease and in this way parallels pivotal clinical trial data in patients with idiopathic PAH (8) and systemic sclerosis (SSc)-associated PAH (9) that favor early and aggressive therapeutic intervention to improve outcome. Furthermore, converging observations reporting on the mechanisms underlying pediatric and adult PAH have begun to identify previously unrecognized genetic, molecular, and developmental factors that predict future or adult-onset PAH, including preeclampsia (PrE), chorioamnionitis, and placental insufficiency with intrauterine growth restriction (10–13) (Figure 1). Collectively, these advances set the framework for a new and potentially transformative era in pulmonary hypertension in which preventative medicine and patient-specific therapies are a principal emphasis.
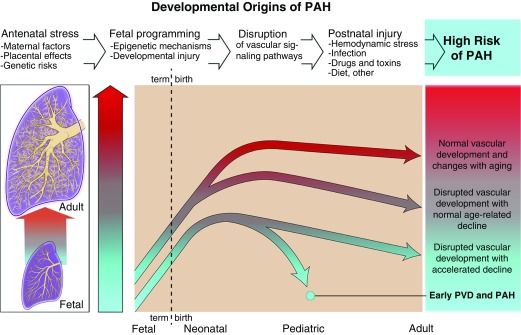
Figure 1.
Developmental origins of pulmonary arterial hypertension (PAH). This figure illustrates the concept that fetal programming and developmental events can cause sustained disruption of vascular signaling pathways and growth that increases the risk for late-onset PAH. Decreased vascular surface area due to antenatal factors, such as preeclampsia, maternal hypertension, obesity, and placental dysfunction, may limit progressive growth of the lung vascular bed, which further shortens the timing of disease onset and trajectory in response to secondary injuries, such as hemodynamic stress, inflammation and infection, toxins, dietary and metabolic factors, and others. PVD = pulmonary vascular disease.
Clinical Detection of Pulmonary Vascular Disease at Inception
New Insights on the Spectrum of Clinical Risk in Pulmonary Hypertension
The current definition of pulmonary hypertension requires a mean pulmonary artery pressure (mPAP) greater than or equal to 25 mm Hg assessed by right heart catheterization (RHC) performed supine at rest (14). The designation of 25 mm Hg as a diagnostic threshold is based on consensus opinion achieved originally during the first World Symposium on Pulmonary Hypertension in Geneva, Switzerland in 1973 (15). However, evidence in support of this was based on early-era RHC studies performed in normal volunteers. From this, it was determined that “the mean pressure in the pulmonary artery does not normally exceed 15 mm Hg . . . and never exceeds 20 mm Hg. Hypertension is defined if the pressure exceeds 25 mm Hg” (15). A number of subsequent studies have confirmed that mPAP greater than or equal to 25 mm Hg is, in fact, an independent predictor of adverse outcome in patients with PAH (16), parenchymal lung disease (17), left heart dysfunction (18), and sickle cell disease (19), among other patient subgroups. However, fresh data from large and diverse populations provides a contemporary opportunity to interpret the significance of mPAP less than 25 mm Hg (20).
In the Rochester Epidemiology Project, the relationship between pulmonary artery systolic pressure (PASP) measured noninvasively and outcome was assessed in a cross-sectional cohort (N = 2,042; mean age, 63 yr) over a median follow up of 9 years. A stepwise increase observed in age-adjusted hazard for mortality beginning at PASP greater than 23 mm Hg was 2.74 per 10-mm Hg increment (P < 0.001), which was maintained after excluding patients with established cardiopulmonary disease (hazard ratio, 2.73; P = 0.016) (21). Directionally similar findings were also observed among African Americans in the longitudinal Jackson Heart Study (N = 3,125; mean age, 56 yr), in which each 10-mm Hg incremental increase in PASP (average, 27.9 mm Hg), when assessed echocardiographically, was associated with a significant increase in the hazard for heart failure–related hospital admission at 3.5 years (hazard ratio, 2.75; P < 0.0001) (22).
Given the limited accuracy of echocardiography for estimating mildly elevated pulmonary artery pressure (23), however, sufficiently powered cardiac catheterization studies remained necessary to confirm trends from these and other noninvasive studies. Recently, RHC results from a national, unselected cohort of Veteran patients (N = 21,727) showed a continuous relationship between mPAP and adjusted hazard for all-cause mortality beginning at ∼19 mm Hg (6). Patients with mPAP 19 to 24 mm Hg, which represented ∼25% of the study population, were at significantly increased risk for mortality and hospitalization by 23 and 7%, respectively, compared with patients with mPAP less than or equal to 18 mm Hg. Furthermore, the consequences of an increase in mPAP by 1 mm Hg on mortality risk was highest in that analysis between 19 and 24 mm Hg compared with any mPAP level greater than or equal to 25 mm Hg.
A summary of reports illustrating the clinical significance of subdiagnostic mPAP level is provided in Table 1. Overall, findings from these studies suggest that unrecognized opportunity may exist to influence adverse outcome before evidence of end-stage pulmonary hypertension, although at this time data informing the consequences of treating at-risk patients by virtue of mPAP less than 25 mm Hg remain forthcoming. Early identification of pulmonary vascular disease is clearly important in the pediatric setting as well, even in the neonatal setting, especially in preterm infants. For example, mild echocardiographic evidence of pulmonary hypertension at postnatal Day 7 is highly predictive of the subsequent risk for bronchopulmonary dysplasia (BPD) and late pulmonary hypertension (24).
Table 1.
Summary of Clinical Study Data Demonstrating the Adverse Functional Consequences of Resting Pulmonary Artery Pressure Levels below the Current Diagnostic Threshold for Pulmonary Hypertension
| Study (Reference) | Patients (N) | Clinical Phenotype | PA Pressure (mm Hg) | Diagnostic Modality | Outcome Measure |
|---|---|---|---|---|---|
| Lam et al. (21) | 2,042 | Random sample, Olmsted County | PASP: 15–23 vs. 24–25; 26–29; 30–32 | ECHO | ↑Adj. mortality |
| Mutlak et al. (108) | 1,054 | Post-MI | PASP: ≤35 vs. >35 | ECHO | ↑Heart failure admission |
| Choudhary et al. (22) | 3,215 | Jackson Heart Study | PASP: ≥33 vs. <33 | ECHO | ↑Heart failure admission |
| Kovacs et al. (30) | 29 | Scleroderma-PAH/lung disease | mPAP: <17 vs. >17 | RHC | ↓po2 |
| ↓6MWD | |||||
| Hamada et al. (109) | 68 | Idiopathic pulmonary fibrosis | mPAP: <17 vs. >17 | RHC | ↑Unadj. mortality |
| Kovacs et al. (34) | 141 | Referral population at risk for PH | mPAP: <21 vs. 21–24 | RHC | ↑PVR |
| ↑mPAP/CO | |||||
| ↑TPG/CO | |||||
| ↓po2 | |||||
| ↓6MWD | |||||
| Lau et al. (31) | 290 | Referral population with unexplained dyspnea or PH risk | mPAP: <21 vs. 21–24 | RHC | ↓Exercise workload |
| ↓6MWD | |||||
| ↑PVR at peak exercise | |||||
| ↑mPAP at peak exercise | |||||
| Heresi et al. (7) | 1,491 | Referral population at risk for PH | mPAP: 10–20 vs. 21–24 | RHC | ↑Mortality |
| Maron et al. (6) | 21,727 | Referral population, Veterans Affairs | mPAP: ≤18 vs. 19–24 | RHC | ↑Adj. mortality |
| ↓Adj. event-free survival | |||||
| Assad et al. (110) | 4,343 | Referral population at risk for PH | mPAP: ≤18 vs. 19–24 | RHC | ↑Adj. mortality |
Definition of abbreviations: 6MWD = 6-minute-walk distance; Adj. = adjusted; CO = cardiac output; ECHO = echocardiography; MI = myocardial infarction; mPAP = mean pulmonary artery pressure; PA = pulmonary artery; PAH = pulmonary arterial hypertension; PASP = pulmonary artery systolic pressure; PH = pulmonary hypertension; po2 = peak volume of oxygen consumption; PVR = pulmonary vascular resistance; RHC = right heart catheterization; TPG = transpulmonary gradient; Unadj. = unadjusted.
Barriers to Early Diagnosis: Lessons from PAH
Pulmonary arterial obliteration or severe narrowing may be present in up to 70% of the pulmonary circulatory bed in patients at the time of PAH treatment initiation (2). Delayed diagnosis of pulmonary hypertension or PAH is a well-established contributor to inappropriate treatment and late referral to specialized care centers (25). This is partly due to nonspecific symptoms that are common at the time of clinical presentation, such as dyspnea, low prevalence of PAH relative to other cardiopulmonary diseases, and inadequate awareness of pulmonary vascular disease diagnostic criteria among healthcare providers (26, 27). Even in modern clinical trials, such as the AMBITION (initial use of Ambrisentan plus Tadalafil in Pulmonary Arterial Hypertension) study, which enrolled treatment-naive patients with PAH (8), baseline clinical characteristics were indicative of moderate or severe disease defined by mPAP ∼49 mm Hg, pulmonary vascular resistance (PVR) ∼10 Wood units, and World Health Organization functional class III in ∼70% of participants. This pattern is consistent across other trials assessing the early initiation of drug therapy (28) and overall underscores the importance of maintaining a high clinical index of suspicion for PAH in at-risk patients.
Exercise Testing for the Assessment of Subclinical Pulmonary Vascular Dysfunction
In SSc, approximately 20% of patients with normal resting cardiopulmonary hemodynamics ultimately develop PAH (29). Therefore, SSc status is a unique risk factor for PAH that does not hinge on symptomatology or rare genetic variants. This, in turn, identifies SSc as a target subgroup for testing methods that provoke pulmonary vascular dysfunction to develop a strategy toward early diagnosis of PAH and other forms of pulmonary vascular disease.
Kovacs and colleagues demonstrated that in patients with SSc without parenchymal lung disease or PAH (N = 29), resting mPAP greater than 17 mm Hg was associated with a decrease in 6-minute-walk distance (396 ± 71 vs. 488 ± 76 m, P < 0.001) and peak volume of oxygen extraction (76 ± 11 vs. 90 ± 24% predicted, P = 0.05) compared with matched patients with mPAP less than 17 mm Hg (30). These findings were recapitulated in a larger, mixed cohort that included patients with SSc as well as others with risk factors for primary cardiac and pulmonary disease (N = 141) (31). In that study, mPAP 20 to 25 mm Hg was present in 22% and corresponded to increased mPAP/cardiac output slope and decreased peak volume of oxygen extraction (20.9 ± 4.7 vs. 16.9 ± 4.6 ml/min/kg, P < 0.01) at peak exercise, as well as greater mortality at a median follow up of 4 years (4 vs. 19%).
On the basis of these collective findings, it is evident that classifying patients by their cardiopulmonary hemodynamic response to exercise has important ramifications on diagnosing PAH early. Yet, universally accepted criteria defining “exercise-induced pulmonary hypertension” (EI-PH) remain lacking. At the World Symposium on Pulmonary Hypertension at Dana Point in 2008 (32) and Nice in 2013 (33), it was suggested that mPAP greater than 30 mm Hg at peak exercise, which had previously been the sole EI-PH criterion, was insufficient, because this could be achieved by elevated (but physiological) cardiac output levels during exercise in well-trained athletes. Additionally, the effect of increased age on mPAP during exercise was also not considered by the traditional EI-PH definition, but several reports have since addressed these issues.
In a retrospective metaanalysis of 1,187 invasive exercise tests performed between 1948 and 2003 in patients without evident cardiopulmonary or pulmonary vascular disease, a gradient in resting mPAP was observed by age (<30 yr, 12.8 ± 3.1 vs. 30–50 yr, 12.9 ± 3.0 vs. ≥50 yr, 14.7 ± 4.0 mm Hg) (34). The magnitude of this difference across age groups exaggerated at submaximal exercise, in which the upper limit of normal for study subjects younger than 50 years and 50 years or older was 29 mm Hg and 46 mm Hg, respectively. Other reports suggest that age may also affect PVR, possibly to a greater extent than mPAP (35).
It appears that analyzing cardiopulmonary hemodynamic variables in combination improves the diagnostic accuracy of exercise testing. Total pulmonary resistance (mPAP/cardiac output) plus mPAP at peak exercise is superior to either measurement or PVR alone in characterizing cardiopulmonary hemodynamic abnormalities induced by exercise. In one study, total pulmonary resistance greater than 3 Wood units and mPAP greater than 30 mm Hg yielded a sensitivity of 0.93 and specificity of 1.0 for discriminating control subjects from patients with normal resting mPAP and PVR but pulmonary vascular disease by clinical history or left-heart disease unmasked by exercise (36). When applied retrospectively to historical datasets of healthy control subjects, use of this approach was associated with a substantial reduction in the rate of exercise-induced pulmonary hypertension false-positive diagnoses when compared with traditional criteria (34).
Basic Mechanisms of Right Ventricular–Pulmonary Arterial Uncoupling during Exercise
Data from small studies have suggested that impaired nitric oxide bioactivity (37), increased uric acid (38), or transpulmonary release of aldosterone (39) are involved in the pathophysiology of impaired exercise tolerance in pulmonary hypertension. More recently, Lewis and colleagues used a multiplexed liquid chromatography–mass spectrometry platform to identify 21 metabolites that were associated with at least two hemodynamic indicators of right ventricular (RV)–pulmonary arterial uncoupling, which describes redistribution of cardiac work from generating blood flow (i.e., oxygen delivery) to maintaining blood pressure in the pulmonary circulation (40). In patients with preserved left ventricular systolic function and dyspnea referred for invasive cardiopulmonary exercise testing, a step-up in kynurenine, anthranilate, and quinolinate levels across the pulmonary circulation (radial arterial − pulmonary arterial levels) was observed in participants with elevated PVR, decreased RV ejection fraction, and impaired pulmonary vascular compliance at peak exercise. By identifying these factors, which were also increased in peripheral plasma from patients with PAH in that study and, collectively, are indoleamine 2,3 dioxygenase–dependent tryptophan metabolites as novel biomarkers of RV–pulmonary arterial uncoupling, the authors provide critical insights into the pathogenesis of exercise dysfunction in patients. However, these findings may also shed new light on the mechanistic underpinnings of pulmonary vascular injury, as indoleamine 2,3 dioxygenase–dependent tryptophan metabolites are associated with immunomodulatory signaling in hypoxic experimental models of PAH in vivo (41).
On the basis of the concept that early disruption of angiogenesis or vascular development reduces vascular surface area, alternate strategies that better assess microvascular growth may also prove helpful to predict changes in the lung circulation that precede changes in PAP and promote RV–pulmonary arterial uncoupling.
Early-Life Programming and Future Pulmonary Hypertension Onset
Developmental Origins Hypothesis and the Epidemiology of Cardiopulmonary Disease
An inverse relationship between birth weight and probability of coronary heart disease later in life was established first in 1907, reproduced in the modern era (42), and since expanded to include late-onset asthma and pulmonary hypertension (43). In one twin study, birth weight less than or equal to 2,500 g corresponded to a 43% increase in the hazard for developing pulmonary vascular disease of any cause after age 12 years (44). However, it is unlikely that birth weight itself is the principal determinate of these associations (45). For example, abnormal placental histology with striking vascular lesions and evidence of “underperfusion” was strongly associated with lower birth weights in preterm infants and a higher risk for pulmonary hypertension and BPD (46). Links between antenatal stress and an increase in risk of impaired lung development and/or pulmonary hypertension that persists throughout infancy have been demonstrated in animal models of intrauterine growth restriction, PrE, and chorioamnionitis, perhaps reflecting the importance of impaired angiogenesis that is common to these syndromes and critical throughout development (47–49).
Fetal or perinatal triggers of late-onset right heart dysfunction, which do not hinge on birth weight, have also been reported and include the use of assisted reproduction technology (50), detrimental patterns in maternal nutrition (51), and maternal PrE (52). More recently, echocardiogram studies of young adults who were born prematurely showed smaller RV chamber size, thicker RV mass, and lower RV ejection fraction, which were strongly related to the severity of prematurity at birth (53).
These observations imply that perturbing the normal maternal–fetal relationship or placental function may induce reprogramming of tissue-specific response pathways activated by exposure to adverse environmental stimuli encountered in the childhood and early adult phases of development. Thus, fetal/perinatal nutritional deficiency is implicated in dysregulation of processes critical to PAH pathobiology, including cellular metabolism (54), cell proliferation (55), and tissue sensitivity to hormones (56, 57). Data outlining the potential pathobiological mechanisms by which to account for the developmental origins of pulmonary hypertension in adulthood are discussed in detail next, with particular emphasis on developmental plasticity and epigenetics.
Developmental Plasticity and Epigenetic Mechanisms in PAH
Developmental plasticity describes genotype–phenotype uncoupling in response to environmental stimuli that occurs over months to years and, therefore, falls on a spectrum between homeostasis (e.g., seconds to minutes) and natural selection (e.g., generational) (58). Epigenetic mechanisms likely function as adaptive or maladaptive interfaces between genetic substrate and environmental stimuli (59, 60). Some modifications are reversible with withdrawal of the stimulus, although epigenetic inheritance has been observed in animal models through imprinting, transmission of DNA methyltransferase-deficient repetitive retrotransposons, RNA-dependent modification of the embryonic genome, and histone retention (61, 62). High rates of incomplete penetrance are reported in carriers of germline mutations associated with PAH, which further suggest that epigenetic mechanisms determine incident disease. For example, only 20% of bone morphogenetic protein receptor-2 mutation carriers express the PAH clinical syndrome (63), despite the fact that these mutations are identified in more than 70% of familial PAH (64).
Principal epigenetic mechanisms include DNA methylation, histone deacetylation, chromatin remodeling, and regulation of target gene expression post-transcriptionally by interfering noncoding RNAs. Collectively, these processes influence gene expression without affecting DNA sequence (as reviewed in Reference 65) (Figure 2). Succinctly, the structural orientation of chromosomal DNA involves the formation of nucleosomes, which comprise DNA chromatin-histone protein complexes. In contrast to heterochromatin, which is characterized by densely packed nucleosomes and transcriptionally inactive DNA, euchromatin contains spaced nucleosomes accessible to nuclear factors or repressors and, thus, is transcriptionally regulated (66). Covalent attachment of a methyl group to single-stranded regions of DNA containing the cytosine-phosphate-guanosine dinucleotide sequence (CpG) may alter gene transcription by physically inhibiting binding of factors that induce or repress transcriptional activation or by recruitment of histone deacetylase enzymes that alters the structure of chromatin to further inhibit access of transcriptional factors to genes. In turn, preprogrammed methylation induced enzymatically by DNA methyltransferases occurs during embryogenesis and appears to be necessary for ensuring normal gene coding, although methylation in response to such environmental stimuli as social stress and nutritional exposure is reported in the development of acquired cardiovascular, neurogenic, and tumorigenic diseases (66, 67).
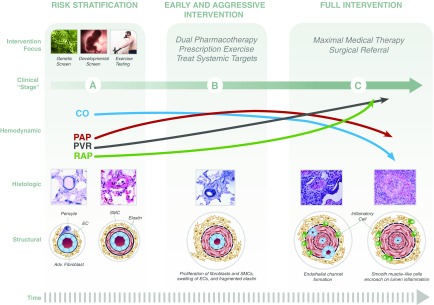
Figure 2.
Toward a comprehensive staging approach to pulmonary arterial hypertension (PAH) that emphasizes opportunities for disease prevention. An integrated approach to pulmonary vascular disease considers complex relationships between right ventricular function, pulmonary arterial pathobiology, and cardiopulmonary hemodynamics at rest and with exercise. Collectively, this permits an organization of disease staging in which stage A indicates asymptomatic status despite increased risk and histological/structural abnormalities; stage B indicates symptomatic status at the time of diagnosis; and stage C indicates patients with severe or end-stage disease. This schema may inform appropriate interventions at each stage that are based on observational and randomized clinical trial data. In particular, genetic screening, developmental screening, and exercise testing are assessments supported by empiric data for selected patient subgroups at risk for PAH. Implementation of these strategies may have important ramifications for improving PAH diagnosis timing and implementation of standard-of-care therapy prior to end-stage disease, including up-front combination PAH therapy with an endothelin receptor antagonist and phosphodiesterase type-V inhibitor in treatment-naive patients. Adv. = adventitial; CO = cardiac output; EC = endothelial cell; PAP = pulmonary artery pressure; PVR = pulmonary vascular resistance; RAP = right atrial pressure; SMC = pulmonary artery smooth muscle cell. Concepts from section on structural changes adapted from Reference 1.
Long noncoding RNAs and small noncoding RNAs (e.g., microRNAs [miRs] and small inhibitory RNAs) mediate epigenetic DNA and histone modification regulation through various mechanisms, including transposon repression via induced DNA methylation, histone deacetylation through recruitment of argonaute proteins (i.e., the catalytic component of the RNA-induced silencing complex that promotes gene silencing), and inhibition of gene transcriptional initiation by targeting coding region of DNA (e.g., the TATA-box sequences). The role of these noncanonical intermediaries in PAH pathogenesis has been described recently (68), particularly with respect to their activation after exposure to classical triggers of pulmonary vascular remodeling, such as hypoxia or endothelin-1 (69, 70). Compared with methylation reactions, the role of noncoding RNAs in the delayed development of PAH, however, remains less well defined.
Oxidant Stress, Epigenetic Modification, and Pulmonary Vascular Disease
Perturbation to the redox potential of pulmonary vascular cells is an important epigenetic mechanism linked to developmental plasticity. Decreased pulmonary arterial compliance is observed in offspring of rats exposed to chronic hypoxia during gestation (71). Rexhaj and colleagues assessed nutritional deficiency effects on pulmonary endothelial function (72). Offspring of pregnant mice exposed to FiO2 16% for 2 weeks and fed a restricted diet had impaired acetylcholine-induced vasodilation of pulmonary artery rings ex vivo compared with rats exposed to hypoxia but fed a standard diet (72). Impaired endothelium-dependent pulmonary vasodilation corresponded to increased PASP and RV mass. In that study, hypoxia-induced pulmonary hypertension in progeny from calorie-restricted mice was associated with a decrease in the number of methylated groups in lung tissue. The administration of tempol, a nonspecific antioxidant, during calorie-restricted pregnancy normalized lung methylation, prevented pulmonary endothelial dysfunction, and improved pulmonary hypertension in vivo, suggesting a redox basis for epigenetic regulation of pulmonary vascular function.
Hypermethylation of a CpG island within the enhancer region of the superoxide dismutase-2 (SOD2) promoter down-regulates SOD2, which is an antioxidant enzyme via its catalyzing the dismutase of superoxide anion to the weaker oxidant hydrogen peroxide (H2O2). This finding parallels observations indicating decreased expression of mitochondrial SOD2 (in the absence of DNA mutations) in pulmonary artery smooth muscle cells (PASMCs) harvested from patients with PAH (73) and demonstrates a decrease in H2O2 that is due to epigenetic regulation of SOD2. In other disease models, however, an increase in superoxide anion accumulation is a precipitating event that induces disrupted methylation of SOD (74). Thus, it appears that alterations to the redox potential of pulmonary vascular cells may be a cause or consequence of epigenetics.
Convergence of Epigenetic Mechanisms and PAH Pathobiology
The bromodomain (BRD) is a highly conserved 110–amino acid motif consisting of four α-helices linked by two loops and is present in proteins that interact with transcription factors, histone acetylases, and nucleosomes to induce epigenetic modification of DNA (75). Up-regulation of BRD4, in part through miR-204, promotes transcription of the protooncogene MYC (76) in selected tumorigenic cell lines (76). However, there is important overlap in the molecular basis of many cancers and the vasculopathy of PAH (77–79). In both conditions, up-regulation of survivin (80), nuclear factor of activated T cells (81), hypoxia inducible factor-1α (82), and protein kinase B–mammalian target of rapamycin complex signaling (55, 83) are observed and alter cellular oxygen sensing to disrupt normal cellular bioenergetics and induce cell survival, growth, and proliferation.
On the basis of these associations, Meloche and colleagues investigated miR-204-BRD4 signaling in PASMCs harvested from patients with PAH (68, 84). They observed that BRD4 overexpression is increased by sevenfold in distal pulmonary arterioles (and increased as well in RV cardiomyocytes) in PAH, which was contingent on down-regulation of miR-204 (68). In turn, molecular inhibition of BRD4 prevented up-regulation of nuclear factor of activated T cells-c2, Bcl-2, and survivin in cultured PAH-PASMCs. Treatment with miR-204 mimics and/or pharmacological inhibition of BRD4, in turn, reversed angioproliferation in Sugen-5416/hypoxia-PAH rats to improve pulmonary hypertension and RV hypertrophy in vivo.
Others have demonstrated convergence of epigenetic mechanisms in pulmonary artery endothelial cells (PAECs), including impaired transcription factor myocyte enhancer factor 2 signaling that is due to excess nuclear accumulation of histone deacetylase (HDAC) 4 and HDAC5. The functional consequences of HDAC4/5-induced inhibition of myocyte enhancer factor 2 includes a decrease in miR-424 and miR-503 that promotes endothelial cell apoptosis and the development of plexogenic lesions in experimental PAH in vivo (85). The possibility of HDAC function in the development of an abnormal phenotype in PASMCs and fibroblasts, and as a potential treatment target in PAH, is supported by findings from other groups as well (86–88).
Pulmonary Vascular Disease Inception through the Lens of Developmental Biology
The relevance of genetic, epigenetic, and developmental origins of pulmonary vascular disease is illustrated by the natural history of BPD. In the “postsurfactant era,” BPD most often affects premature infants born 24 to 28 weeks post-menstrual age during the late canalicular or early saccular phase of lung development (89). Key triggers for BPD in at-risk infants include exposure to hyperoxia, ventilator-induced lung injury, inflammation, and sepsis. Improved respiratory care, surfactant administration, antenatal steroids, aggressive nutritional support, and other measures have improved survival, yet the prevalence of BPD continues to increase. Although abnormal lung development is a cornerstone feature of BPD, dysregulated vascular growth and structure play an increasingly recognized role in the pathogenesis of pulmonary hypertension (90, 91), which occurs in ∼25% of cases (92).
Normal embryonic vascular development requires vascular endothelial cell–specific vascular endothelial growth factor (VEGF) and its target receptors Flt-1 and Flk-1 (90). For example, tetraploid embryos transfected with VEGF homozygous null embryonic stem cells result in a midgestation lethal phenotype due, in part, to failed lung development (93). Brief pharmacological inhibition of VEGF signaling in the late fetus or after birth causes sustained impairment of alveolarization as well as severe pulmonary hypertension (90, 94, 95). Recent studies suggest that the developing endothelium plays a key role in enhancing alveolar type II cell growth and maturation through angiocrine signaling involving nitric oxide, hepatocyte growth factor, retinoids, and other vasoactive factors (96, 97), Similarly, postnatal intratracheal adenovirus–mediated VEGF gene therapy that restores VEGF and VEGF receptor 2 expression promotes normal lung capillary formation and alveolarization and improves survival in hyperoxia experimental BPD in vivo (97). These and other similar observations (98–100) support a connection between impaired VEGF bioactivity, vascular development, and pulmonary hypertension in BPD.
Clinical data suggest that early echocardiogram findings of pulmonary vascular disease in preterm infants at postnatal Day 7 were strongly associated with the risk of subsequent pulmonary hypertension, severe BPD, and late respiratory events during infancy (101). Interestingly, offspring to mothers with PrE are at high risk for developing BPD and pulmonary hypertension. In PrE, oversynthesis of placental-derived Flt-1 increases VEGF–Flt-1 binding to inhibit VEGF bioavailability (102), which likely impairs fetal lung vascular development due to direct effects on fetal angiogenesis or indirectly through impaired placental vascular structure and function (48).
Vertical transmission of signaling intermediaries that affects disease risk in offspring is also observed in models of persistent pulmonary hypertension of the newborn, which is characterized by failure of pulmonary vascular resistance to attenuate after fetal transition to postnatal circulation. In PAECs harvested from fetal sheep undergoing partial ligation of the ductus arteriosus in utero, for example, decreased VEGF and endothelial nitric oxide synthase expression is observed compared with controls (103, 104). These findings correspond to impaired vascular tube formation and PAEC growth in vitro (105) via increased Ras homolog gene family member A (RhoA) activation (106).
Given the key role of abnormal RhoA-dependent signal transduction in adult-onset PAH (107), these data from pediatric patients seem to illustrate a problem in the larger pulmonary vascular disease field in which the current pulmonary hypertension classification system, tied closely to clinical risk factors and demographics (e.g., pediatric vs. adult, hypoxia vs. vascular congestion), may limit the recognition of common molecular pathways across subphenotypes. Greater efforts are warranted to bridge knowledge gaps between developmental origins of disease and late-onset pulmonary hypertension along these lines.
Conclusions
Findings demonstrating the importance of environmental factors during gestation for determining pulmonary vascular function “across the lifespan” have exposed an important connection between fetal health, epigenetics, and pulmonary hypertension (Figure 2). Overlap in abnormal signaling pathways between pediatric and adult PAH, particularly VEGF and RhoA, indicate potential commonality of disease origins across clinical phenotypes that seem unrelated currently. Clinically, a number of large population studies now show a consistent signal toward increased risk for adverse outcome in patients with cardiopulmonary hemodynamics below the current diagnostic threshold for pulmonary hypertension. Overall, these findings permit a transition toward focus on factors underling disease inception for the purpose of innovating and ultimately implementing preventative strategies for pulmonary vascular disease.
Acknowledgments
Acknowledgment
The authors acknowledge the numerous important works and contributions to the field of pulmonary vascular medicine that, due to space limitations, could not be referenced in the current work.
Footnotes
Funded by National Institutes of Health grant 1K08HL11207-01A1, American Heart Association grant 15GRNT25080016, the Pulmonary Hypertension Association, the Cardiovascular Medical Research and Education Fund, and the Klarman Foundation at Brigham and Women’s Hospital (B.A.M.); and NHLBI grants U01 HL12118, R01 HL085703, and R01 HL68702 (S.H.A.).
Originally Published in Press as DOI: 10.1164/rccm.201604-0882PP on November 17, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest. 2012;122:4306–4313. doi: 10.1172/JCI60658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Austin ED, Kawut SM, Gladwin MT, Abman SH. Pulmonary hypertension: NHLBI Workshop on the primary prevention of chronic lung diseases. Ann Am Thorac Soc. 2014;11:S178–S185. doi: 10.1513/AnnalsATS.201312-443LD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang R-S, Maron BA, Loscalzo J. Systems medicine: evolution of systems biology from bench to bedside. Wiley Interdiscip Rev Syst Biol Med. 2015;7:141–161. doi: 10.1002/wsbm.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brittain EL, Chan SY. Integration of complex data sources to provide biologic insight into pulmonary vascular disease (2015 Grover Conference Series) Pulm Circ. 2016;6:251–260. doi: 10.1086/686995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Austin ED, West J, Loyd JE, Hemnes AR.Molecular medicine of pulmonary arterial hypertension: from population genetics to precision medicine and gene editing Am J Respir Crit Care Med [online ahead of print]11 Jul 2016. DOI: 10.1164/rccm.201605-0905PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maron BA, Hess E, Maddox TM, Opotowsky AR, Tedford RJ, Lahm T, Joynt KE, Kass DJ, Stephens T, Stanislawski MA, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the Veterans Affairs Clinical Assessment, Reporting and Tracking Program. Circulation. 2016;133:1240–1248. doi: 10.1161/CIRCULATIONAHA.115.020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heresi GA, Minai OA, Tonelli AR, Hammel JP, Farha S, Parambil JG, Dweik RA. Clinical characterization and survival of patients with borderline elevation in pulmonary artery pressure. Pulm Circ. 2013;3:916–925. doi: 10.1086/674756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galiè N, Barberá JA, Frost AE, Ghofrani HA, Hoeper MM, McLaughlin VV, Peacock AJ, Simonneau G, Vachiery JL, Grünig E, et al. AMBITION Investigators. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015;373:834–844. doi: 10.1056/NEJMoa1413687. [DOI] [PubMed] [Google Scholar]
- 9.Hassoun PM, Zamanian RT, Damico R, Lechtzin N, Khair R, Kolb TM, Tedford RJ, Hulme OL, Housten T, Pisanello C, et al. Ambrisentan and tadalafil up-front combination therapy in scleroderma-associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2015;192:1102–1110. doi: 10.1164/rccm.201507-1398OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, Hanna BD, Rosenzweig EB, Raj JU, Cornfield D, et al. American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; and the American Thoracic Society. Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation. 2015;132:2037–2099. doi: 10.1161/CIR.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 11.Austin ED, Loyd JE. The genetics of pulmonary arterial hypertension. Circ Res. 2014;115:189–202. doi: 10.1161/CIRCRESAHA.115.303404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Austin ED, Ma L, LeDuc C, Berman Rosenzweig E, Borczuk A, Phillips JA, III, Palomero T, Sumazin P, Kim HR, Talati MH, et al. Whole exome sequencing to identify a novel gene (caveolin-1) associated with human pulmonary arterial hypertension. Circ Cardiovasc Genet. 2012;5:336–343. doi: 10.1161/CIRCGENETICS.111.961888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma L, Roman-Campos D, Austin ED, Eyries M, Sampson KS, Soubrier F, Germain M, Trégouët DA, Borczuk A, Rosenzweig EB, et al. A novel channelopathy in pulmonary arterial hypertension. N Engl J Med. 2013;369:351–361. doi: 10.1056/NEJMoa1211097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galié N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. The joint task force for the diagnosis a treatment of pulmonary hypertension of the European Society of Cardiology and the European Respiratory Society: Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015;46:903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 15.Hatano S, Strasser T. World Health Organization. 1975 [Google Scholar]
- 16.Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Koerner SK, et al. Primary pulmonary hypertension: a national prospective study. Ann Intern Med. 1987;107:216–223. doi: 10.7326/0003-4819-107-2-216. [DOI] [PubMed] [Google Scholar]
- 17.Nadrous HF, Pellikka PA, Krowka MJ, Swanson KL, Chaowalit N, Decker PA, Ryu JH. The impact of pulmonary hypertension on survival in patients with idiopathic pulmonary fibrosis. Chest. 2005;128:616S–617S. doi: 10.1378/chest.128.6_suppl.616S-a. [DOI] [PubMed] [Google Scholar]
- 18.Aronson D, Eitan A, Dragu R, Burger AJ. Relationship between reactive pulmonary hypertension and mortality in patients with acute decompensated heart failure. Circ Heart Fail. 2011;4:644–650. doi: 10.1161/CIRCHEARTFAILURE.110.960864. [DOI] [PubMed] [Google Scholar]
- 19.Mehari A, Gladwin MT, Tian X, Machado RF, Kato GJ. Mortality in adults with sickle cell disease and pulmonary hypertension. JAMA. 2012;307:1254–1256. doi: 10.1001/jama.2012.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torbicki A. Hypertension: definition of pulmonary hypertension challenged? Nat Rev Cardiol. 2016;13:250–251. doi: 10.1038/nrcardio.2016.44. [DOI] [PubMed] [Google Scholar]
- 21.Lam CSP, Borlaug BA, Kane GC, Enders FT, Rodeheffer RJ, Redfield MM. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation. 2009;119:2663–2670. doi: 10.1161/CIRCULATIONAHA.108.838698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choudhary G, Jankowich M, Wu WC. Elevated pulmonary artery systolic pressure predicts heart failure admissions in African Americans: Jackson Heart Study. Circ Heart Fail. 2014;7:558–564. doi: 10.1161/CIRCHEARTFAILURE.114.001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farber HW, Foreman AJ, Miller DP, McGoon MD. REVEAL Registry: correlation of right heart catheterization and echocardiography in patients with pulmonary arterial hypertension. Congest Heart Fail. 2011;17:56–64. doi: 10.1111/j.1751-7133.2010.00202.x. [DOI] [PubMed] [Google Scholar]
- 24.Mourani PM, Sontag MK, Younoszai A, Miller JI, Kinsella JP, Baker CD, Poindexter BB, Ingram DA, Abman SH. Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2015;191:87–95. doi: 10.1164/rccm.201409-1594OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deaño RC, Glassner-Kolmin C, Rubenfire M, Frost A, Visovatti S, McLaughlin VV, Gomberg-Maitland M. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral study. JAMA Intern Med. 2013;173:887–893. doi: 10.1001/jamainternmed.2013.319. [DOI] [PubMed] [Google Scholar]
- 26.Brown LM, Chen H, Halpern S, Taichman D, McGoon MD, Farber HW, Frost AE, Liou TG, Turner M, Feldkircher K, et al. Delay in recognition of pulmonary arterial hypertension: factors identified from the REVEAL Registry. Chest. 2011;140:19–26. doi: 10.1378/chest.10-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan JJ, Butrous G, Maron BA. The heterogeneity of clinical practice patterns among an international cohort of pulmonary arterial hypertension experts. Pulm Circ. 2014;4:441–451. doi: 10.1086/677357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sitbon O, Channick R, Chin KM, Frey A, Gaine S, Galiè N, Ghofrani H-A, Hoeper MM, Lang IM, Preiss R, et al. GRIPHON Investigators. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015;373:2522–2533. doi: 10.1056/NEJMoa1503184. [DOI] [PubMed] [Google Scholar]
- 29.Condliffe R, Kiely DG, Peacock AJ, Corris PA, Gibbs JS, Vrapi F, Das C, Elliot CA, Johnson M, DeSoyza J, et al. Connective tissue disease-associated pulmonary arterial hypertension in the modern treatment era. Am J Respir Crit Care Med. 2009;179:151–157. doi: 10.1164/rccm.200806-953OC. [DOI] [PubMed] [Google Scholar]
- 30.Kovacs G, Maier R, Aberer E, Brodmann M, Scheidl S, Tröster N, Hesse C, Salmhofer W, Graninger W, Gruenig E, et al. Borderline pulmonary arterial pressure is associated with decreased exercise capacity in scleroderma. Am J Respir Crit Care Med. 2009;180:881–886. doi: 10.1164/rccm.200904-0563OC. [DOI] [PubMed] [Google Scholar]
- 31.Lau EM, Godinas L, Sitbon O, Montani D, Savale L, Jaïs X, Lador F, Gunther S, Celermajer DS, Simonneau G, et al. Resting pulmonary artery pressure of 21-24 mmHg predicts abnormal exercise haemodynamics. Eur Respir J. 2016;47:1436–1444. doi: 10.1183/13993003.01684-2015. [DOI] [PubMed] [Google Scholar]
- 32.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, Langleben D, Manes A, Satoh T, Torres F, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D42–D50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 34.Kovacs G, Avian A, Olschewski H. Proposed new definition of exercise pulmonary hypertension decreases false-positive cases. Eur Respir J. 2016;47:1270–1273. doi: 10.1183/13993003.01394-2015. [DOI] [PubMed] [Google Scholar]
- 35.Oliveira RK, Agarwal M, Tracy JA, Karin AL, Opotowsky AR, Waxman AB, Systrom DM. Age-related upper limits of normal for maximum upright exercise pulmonary haemodynamics. Eur Respir J. 2016;47:1179–1188. doi: 10.1183/13993003.01307-2015. [DOI] [PubMed] [Google Scholar]
- 36.Herve P, Lau EM, Sitbon O, Savale L, Montani D, Godinas L, Lador F, Jaïs X, Parent F, Günther S, et al. Criteria for diagnosis of exercise pulmonary hypertension. Eur Respir J. 2015;46:728–737. doi: 10.1183/09031936.00021915. [DOI] [PubMed] [Google Scholar]
- 37.Kane DW, Tesauro T, Koizumi T, Gupta R, Newman JH. Exercise-induced pulmonary vasoconstriction during combined blockade of nitric oxide synthase and beta adrenergic receptors. J Clin Invest. 1994;93:677–683. doi: 10.1172/JCI117020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagaya N, Uematsu M, Satoh T, Kyotani S, Sakamaki F, Nakanishi N, Yamagishi M, Kunieda T, Miyatake K. Serum uric acid levels correlate with the severity and the mortality of primary pulmonary hypertension. Am J Respir Crit Care Med. 1999;160:487–492. doi: 10.1164/ajrccm.160.2.9812078. [DOI] [PubMed] [Google Scholar]
- 39.Maron BA, Stephens TE, Farrell LA, Oldham WM, Loscalzo J, Leopold JA, Lewis GD. Elevated pulmonary arterial and systemic plasma aldosterone levels associate with impaired cardiac reserve capacity during exercise in left ventricular systolic heart failure patients: a pilot study. J Heart Lung Transplant. 2016;35:342–351. doi: 10.1016/j.healun.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis GD, Ngo D, Hemnes AR, Farrell L, Domos C, Pappagianopoulos PP, Dhakal BP, Souza A, Shi X, Pugh ME, et al. Metabolic profiling of right ventricular-pulmonary vascular function reveals circulating biomarkers of pulmonary hypertension. J Am Coll Cardiol. 2016;67:174–189. doi: 10.1016/j.jacc.2015.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao Y, Christou H, Liu L, Visner G, Mitsialis SA, Kourembanas S, Liu H. Endothelial indoleamine 2,3-dioxygenase protects against development of pulmonary hypertension. Am J Respir Crit Care Med. 2013;188:482–491. doi: 10.1164/rccm.201304-0700OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hovi P, Andersson S, Eriksson JG, Järvenpää AL, Strang-Karlsson S, Mäkitie O, Kajantie E. Glucose regulation in young adults with very low birth weight. N Engl J Med. 2007;356:2053–2063. doi: 10.1056/NEJMoa067187. [DOI] [PubMed] [Google Scholar]
- 43.Steffensen FH, Sørensen HT, Gillman MW, Rothman KJ, Sabroe S, Fischer P, Olsen J. Low birth weight and preterm delivery as risk factors for asthma and atopic dermatitis in young adult males. Epidemiology. 2000;11:185–188. doi: 10.1097/00001648-200003000-00018. [DOI] [PubMed] [Google Scholar]
- 44.Class QA, Rickert ME, Lichtenstein P, D’Onofrio BM. Birth weight, physical morbidity, and mortality: a population-based sibling-comparison study. Am J Epidemiol. 2014;179:550–558. doi: 10.1093/aje/kwt304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mestan KK, Check J, Minturn L, Yallapragada S, Farrow KN, Liu X, Su E, Porta N, Gotteiner N, Ernst LM. Placental pathologic changes of maternal vascular underperfusion in bronchopulmonary dysplasia and pulmonary hypertension. Placenta. 2014;35:570–574. doi: 10.1016/j.placenta.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rozance PJ, Seedorf GJ, Brown A, Roe G, O’Meara MC, Gien J, Tang JR, Abman SH. Intrauterine growth restriction decreases pulmonary alveolar and vessel growth and causes pulmonary artery endothelial cell dysfunction in vitro in fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2011;301:L860–L871. doi: 10.1152/ajplung.00197.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang JR, Karumanchi SA, Seedorf G, Markham N, Abman SH. Excess soluble vascular endothelial growth factor receptor-1 in amniotic fluid impairs lung growth in rats: linking preeclampsia with bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2012;302:L36–L46. doi: 10.1152/ajplung.00294.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang JR, Markham NE, Lin YJ, McMurtry IF, Maxey A, Kinsella JP, Abman SH. Inhaled nitric oxide attenuates pulmonary hypertension and improves lung growth in infant rats after neonatal treatment with a VEGF receptor inhibitor. Am J Physiol Lung Cell Mol Physiol. 2004;287:L344–L351. doi: 10.1152/ajplung.00291.2003. [DOI] [PubMed] [Google Scholar]
- 50.Scherrer U, Rimoldi SF, Rexhaj E, Stuber T, Duplain H, Garcin S, de Marchi SF, Nicod P, Germond M, Allemann Y, et al. Systemic and pulmonary vascular dysfunction in children conceived by assisted reproductive technologies. Circulation. 2012;125:1890–1896. doi: 10.1161/CIRCULATIONAHA.111.071183. [DOI] [PubMed] [Google Scholar]
- 51.Han H, Hansen TR, Berg B, Hess BW, Ford SP. Maternal undernutrition induces differential cardiac gene expression in pulmonary hypertensive steers at high elevation. Am J Physiol Heart Circ Physiol. 2008;295:H382–H389. doi: 10.1152/ajpheart.01272.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jayet PY, Rimoldi SF, Stuber T, Salmòn CS, Hutter D, Rexhaj E, Thalmann S, Schwab M, Turini P, Sartori-Cucchia C, et al. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation. 2010;122:488–494. doi: 10.1161/CIRCULATIONAHA.110.941203. [DOI] [PubMed] [Google Scholar]
- 53.Lewandowski AJ, Bradlow WM, Augustine D, Davis EF, Francis J, Singhal A, Lucas A, Neubauer S, McCormick K, Leeson P. Right ventricular systolic dysfunction in young adults born preterm. Circulation. 2013;128:713–720. doi: 10.1161/CIRCULATIONAHA.113.002583. [DOI] [PubMed] [Google Scholar]
- 54.Dunham-Snary KJ, Hong ZG, Xiong PY, Del Paggio JC, Herr JE, Johri AM, Archer SL. A mitochondrial redox oxygen sensor in the pulmonary vasculature and ductus arteriosus. Pflugers Arch. 2016;468:43–58. doi: 10.1007/s00424-015-1736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goncharov DA, Kudryashova TV, Ziai H, Ihida-Stansbury K, DeLisser H, Krymskaya VP, Tuder RM, Kawut SM, Goncharova EA. Mammalian target of rapamycin complex 2 (mTORC2) coordinates pulmonary artery smooth muscle cell metabolism, proliferation, and survival in pulmonary arterial hypertension. Circulation. 2014;129:864–874. doi: 10.1161/CIRCULATIONAHA.113.004581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maron BA, Zhang YY, White K, Chan SY, Handy DE, Mahoney CE, Loscalzo J, Leopold JA. Aldosterone inactivates the endothelin-B receptor via a cysteinyl thiol redox switch to decrease pulmonary endothelial nitric oxide levels and modulate pulmonary arterial hypertension. Circulation. 2012;126:963–974. doi: 10.1161/CIRCULATIONAHA.112.094722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ventetuolo CE, Baird GL, Barr RG, Bluemke DA, Fritz JS, Hill NS, Klinger JR, Lima JA, Ouyang P, Palevsky HI, et al. Higher estradiol and lower dehydroepiandrosterone-sulfate levels are associated with pulmonary arterial hypertension in men. Am J Respir Crit Care Med. 2016;193:1168–1175. doi: 10.1164/rccm.201509-1785OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hanson MA, Gluckman PD. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol Rev. 2014;94:1027–1076. doi: 10.1152/physrev.00029.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schones DE, Leung A, Natarajan R. Chromatin modifications associated with diabetes and obesity. Arterioscler Thromb Vasc Biol. 2015;35:1557–1561. doi: 10.1161/ATVBAHA.115.305041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim GH, Ryan JJ, Marsboom G, Archer SL. Epigenetic mechanisms of pulmonary hypertension. Pulm Circ. 2011;1:347–356. doi: 10.4103/2045-8932.87300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holliday R. Epigenetics: an overview. Dev Genet. 1994;15:453–457. doi: 10.1002/dvg.1020150602. [DOI] [PubMed] [Google Scholar]
- 62.van Otterdiik SD, Michels KB. Transgenerational epigenetic inheritance in mammals: how good is the evidence? FASEB J. 2016;30:2457–2465. doi: 10.1096/fj.201500083. [DOI] [PubMed] [Google Scholar]
- 63.Newman JH, Wheeler L, Lane KB, Loyd E, Gaddipati R, Phillips JA, III, Loyd JE. Mutation in the gene for bone morphogenetic protein receptor II as a cause of primary pulmonary hypertension in a large kindred. N Engl J Med. 2001;345:319–324. doi: 10.1056/NEJM200108023450502. [DOI] [PubMed] [Google Scholar]
- 64.Cogan JD, Pauciulo MW, Batchman AP, Prince MA, Robbins IM, Hedges LK, Stanton KC, Wheeler LA, Phillips JA, III, Loyd JE, et al. High frequency of BMPR2 exonic deletions/duplications in familial pulmonary arterial hypertension. Am J Respir Crit Care Med. 2006;174:590–598. doi: 10.1164/rccm.200602-165OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim GH, Ryan JJ, Archer SL. The role of redox signaling in epigenetics and cardiovascular disease. Antioxid Redox Signal. 2013;18:1920–1936. doi: 10.1089/ars.2012.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Handy DE, Castro R, Loscalzo J. Epigenetic modifications: basic mechanisms and role in cardiovascular disease. Circulation. 2011;123:2145–2156. doi: 10.1161/CIRCULATIONAHA.110.956839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 68.Meloche J, Potus F, Vaillancourt M, Bourgeois A, Johnson I, Deschamps L, Chabot S, Ruffenach G, Henry S, Breuils-Bonnet S, et al. Bromodomain-containing protein 4: the epigenetic origin of pulmonary arterial hypertension. Circ Res. 2015;117:525–535. doi: 10.1161/CIRCRESAHA.115.307004. [DOI] [PubMed] [Google Scholar]
- 69.Bertero T, Cottrill KA, Annis S, Bhat B, Gochuico BR, Osorio JC, Rosas I, Haley KJ, Corey KE, Chung RT, et al. A YAP/TAZ-miR-130/301 molecular circuit exerts systems-level control of fibrosis in a network of human diseases and physiologic conditions. Sci Rep. 2015;5:18277. doi: 10.1038/srep18277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parikh VN, Jin RC, Rabello S, Gulbahce N, White K, Hale A, Cottrill KA, Shaik RS, Waxman AB, Zhang YY, et al. MicroRNA-21 integrates pathogenic signaling to control pulmonary hypertension: results of a network bioinformatics approach. Circulation. 2012;125:1520–1532. doi: 10.1161/CIRCULATIONAHA.111.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jones RD, Morice AH, Emery CJ. Effects of perinatal exposure to hypoxia upon the pulmonary circulation of the adult rat. Physiol Res. 2004;53:11–17. [PubMed] [Google Scholar]
- 72.Rexhaj E, Bloch J, Jayet PY, Rimoldi SF, Dessen P, Mathieu C, Tolsa JF, Nicod P, Scherrer U, Sartori C. Fetal programming of pulmonary vascular dysfunction in mice: role of epigenetic mechanisms. Am J Physiol Heart Circ Physiol. 2011;301:H247–H252. doi: 10.1152/ajpheart.01309.2010. [DOI] [PubMed] [Google Scholar]
- 73.Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, Dyck JR, Gomberg-Maitland M, Thébaud B, Husain AN, et al. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapuetic target. Circulation. 2010;121:2261–2271. doi: 10.1161/CIRCULATIONAHA.109.916098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cyr AR, Hitchler MJ, Domann FE. Regulation of SOD2 in cancer by histone modifications and CpG methylation: closing the loop between redox biology and epigenetics. Antioxid Redox Signal. 2013;18:1946–1955. doi: 10.1089/ars.2012.4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Belkina AC, Denis GV. BET domain co-regulators in obesity, inflammation and cancer. Nat Rev Cancer. 2012;12:465–477. doi: 10.1038/nrc3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mertz JA, Conery AR, Bryant BM, Sandy P, Balasubramanian S, Mele DA, Bergeron L, Sims RJ., III Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc Natl Acad Sci USA. 2011;108:16669–16674. doi: 10.1073/pnas.1108190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sobulo OM, Borrow J, Tomek R, Reshmi S, Harden A, Schlegelberger B, Housman D, Doggett NA, Rowley JD, Zeleznik-Le NJ. MLL is fused to CBP, a histone acetyltransferase, in therapy-related acute myeloid leukemia with a t(11;16)(q23;p13.3) Proc Natl Acad Sci USA. 1997;94:8732–8737. doi: 10.1073/pnas.94.16.8732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sutendra G, Michelakis ED. The metabolic basis of pulmonary arterial hypertension. Cell Metab. 2014;19:558–573. doi: 10.1016/j.cmet.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 79.Paulin R, Michelakis ED. The metabolic theory of pulmonary arterial hypertension. Circ Res. 2014;115:148–164. doi: 10.1161/CIRCRESAHA.115.301130. [DOI] [PubMed] [Google Scholar]
- 80.Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J. 2005;19:1178–1180. doi: 10.1096/fj.04-3261fje. [DOI] [PubMed] [Google Scholar]
- 81.Bonnet S, Rochefort G, Sutendra G, Archer SL, Haromy A, Webster L, Hashimoto K, Bonnet SN, Michelakis ED. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proc Natl Acad Sci USA. 2007;104:11418–11423. doi: 10.1073/pnas.0610467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol. 2008;294:H570–H578. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 83.Aghamohammadzadeh R, Zhang YY, Stephens TE, Arons E, Zaman P, Polach KJ, Matar M, Yung LM, Yu PB, Bowman FP, et al. Up-regulation of the mammalian target of rapamycin complex 1 subunit Raptor by aldosterone induces abnormal pulmonary artery smooth muscle cell survival patterns to promote pulmonary arterial hypertension. FASEB J. 2016;30:2511–2527. doi: 10.1096/fj.201500042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ranchoux B, Meloche J, Paulin R, Boucherat O, Provencher S, Bonnet S. DNA damage and pulmonary hypertension. Int J Mol Sci. 2016;17:E990. doi: 10.3390/ijms17060990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim J, Hwangbo C, Hu X, Kang Y, Papangeli I, Mehrotra D, Park H, Ju H, McLean DL, Comhair SA, et al. Restoration of impaired endothelial myocyte enhancer factor 2 function rescues pulmonary arterial hypertension. Circulation. 2015;131:190–199. doi: 10.1161/CIRCULATIONAHA.114.013339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang Q, Dahl MJ, Albertine KH, Ramchandran R, Sun M, Raj JU. Role of histone deacetylases in regulation of phenotype of ovine newborn pulmonary arterial smooth muscle cells. Cell Prolif. 2013;46:654–664. doi: 10.1111/cpr.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao L, Chen CN, Hajji N, Oliver E, Cotroneo E, Wharton J, Wang D, Li M, McKinsey TA, Stenmark KR, et al. Histone deacetylation inhibition in pulmonary hypertension: therapeutic potential of valproic acid and suberoylanilide hydroxamic acid. Circulation. 2012;126:455–467. doi: 10.1161/CIRCULATIONAHA.112.103176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang D, Zhang H, Li M, Frid MG, Flockton AR, McKeon BA, Yeager ME, Fini MA, Morrell NW, Pullamsetti SS, et al. MicroRNA-124 controls the proliferative, migratory, and inflammatory phenotype of pulmonary vascular fibroblasts. Circ Res. 2014;114:67–78. doi: 10.1161/CIRCRESAHA.114.301633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Charafeddine L, D’Angio CT, Phelps DL. Atypical chronic lung disease patterns in neonates. Pediatrics. 1999;103:759–765. doi: 10.1542/peds.103.4.759. [DOI] [PubMed] [Google Scholar]
- 90.Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, Abman SH. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol. 2000;279:L600–L607. doi: 10.1152/ajplung.2000.279.3.L600. [DOI] [PubMed] [Google Scholar]
- 91.Stenmark KR, Abman SH. Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol. 2005;67:623–661. doi: 10.1146/annurev.physiol.67.040403.102229. [DOI] [PubMed] [Google Scholar]
- 92.An HS, Bae EJ, Kim GB, Kwon BS, Beak JS, Kim EK, Kim HS, Choi JH, Noh CI, Yun YS. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circ J. 2010;40:131–136. doi: 10.4070/kcj.2010.40.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 94.Grover TR, Parker TA, Balasubramaniam V, Markham NE, Abman SH. Pulmonary hypertension impairs alveolarization and reduces lung growth in the ovine fetus. Am J Physiol Lung Cell Mol Physiol. 2005;288:L648–L654. doi: 10.1152/ajplung.00288.2004. [DOI] [PubMed] [Google Scholar]
- 95.Chettimada S, Joshi SR, Alzoubi A, Gebb SA, McMurtry IF, Gupte R, Gupte SA. Glucose-6-phosphate dehydrogenase plays a critical role in hypoxia-induced CD133+ progenitor cells self-renewal and stimulates their accumulation in the lungs of pulmonary hypertensive rats. Am J Physiol Lung Cell Mol Physiol. 2014;307:L545–L556. doi: 10.1152/ajplung.00303.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seedorf G, Metoxen AJ, Rock R, Markham N, Ryan S, Vu T, Abman SH. Hepatocyte growth factor as a downstream mediator of vascular endothelial growth factor-dependent preservation of growth in the developing lung. Am J Physiol Lung Cell Mol Physiol. 2016;310:L1098–L1110. doi: 10.1152/ajplung.00423.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Thébaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, et al. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation. 2005;112:2477–2486. doi: 10.1161/CIRCULATIONAHA.105.541524. [DOI] [PubMed] [Google Scholar]
- 98.Kunig A, Balasubramaniam V, Markham N, Morgan D, Montgomery G, Grover TR, Abman SH. Recombinant human VEGF treatment enhances alveolarization after hyperoxic lung injury in neonatal rats. Am J Physiol Lung Cell Mol Physiol. 2005;289:L529–L535. doi: 10.1152/ajplung.00336.2004. [DOI] [PubMed] [Google Scholar]
- 99.Kunig AM, Balasubramaniam V, Markham NE, Seedorf G, Gien J, Abman SH. Recombinant human VEGF treatment transiently increases lung edema but enhances lung structure after neonatal hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1068–L1078. doi: 10.1152/ajplung.00093.2006. [DOI] [PubMed] [Google Scholar]
- 100.Abman SH, Baker C, Gien J, Mourani P, Galambos C. The Robyn Barst Memorial Lecture. Differences between the fetal, newborn, and adult pulmonary circulations: relevance for age-specific therapies (2013 Grover Conference series) Pulm Circ. 2014;4:424–440. doi: 10.1086/677371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mourani PM, Ivy DD, Gao D, Abman SH. Pulmonary vascular effects of inhaled nitric oxide and oxygen tension in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2004;170:1006–1013. doi: 10.1164/rccm.200310-1483OC. [DOI] [PubMed] [Google Scholar]
- 102.Shibuya M. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct Funct. 2001;26:25–35. doi: 10.1247/csf.26.25. [DOI] [PubMed] [Google Scholar]
- 103.Villamor E, Le Cras TD, Horan MP, Halbower AC, Tuder RM, Abman SH. Chronic intrauterine pulmonary hypertension impairs endothelial nitric oxide synthase in the ovine fetus. Am J Physiol. 1997;272:L1013–L1020. doi: 10.1152/ajplung.1997.272.5.L1013. [DOI] [PubMed] [Google Scholar]
- 104.Grover TR, Parker TA, Markham NE, Abman SH. rhVEGF treatment preserves pulmonary vascular reactivity and structure in an experimental model of pulmonary hypertension in fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2005;289:L315–L321. doi: 10.1152/ajplung.00038.2005. [DOI] [PubMed] [Google Scholar]
- 105.Gien J, Seedorf GJ, Balasubramaniam V, Markham N, Abman SH. Intrauterine pulmonary hypertension impairs angiogenesis in vitro: role of vascular endothelial growth factor nitric oxide signaling. Am J Respir Crit Care Med. 2007;176:1146–1153. doi: 10.1164/rccm.200705-750OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gien J, Seedorf GJ, Balasubramaniam V, Tseng N, Markham N, Abman SH. Chronic intrauterine pulmonary hypertension increases endothelial cell Rho kinase activity and impairs angiogenesis in vitro. Am J Physiol Lung Cell Mol Physiol. 2008;295:L680–L687. doi: 10.1152/ajplung.00516.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lu A, Zuo C, He Y, Chen G, Piao L, Zhang J, Xiao B, Shen Y, Tang J, Kong D, et al. EP3 receptor deficiency attenuates pulmonary hypertension through suppression of Rho/TGF-β1 signaling. J Clin Invest. 2015;125:1228–1242. doi: 10.1172/JCI77656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mutlak D, Lessick J, Carasso S, Kapeliovich M, Dragu R, Hammerman H, Agmon Y, Aronson D. Utility of pulmonary hypertension for the prediction of heart failure following acute myocardial infarction. Am J Cardiol. 2012;109:1254–1259. doi: 10.1016/j.amjcard.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 109.Hamada K, Nagai S, Tanaka S, Handa T, Shigematsu M, Nagao T, Mishima M, Kitaichi M, Izumi T. Significance of pulmonary arterial pressure and diffusion capacity of the lung as prognosticator in patients with idiopathic pulmonary fibrosis. Chest. 2007;131:650–656. doi: 10.1378/chest.06-1466. [DOI] [PubMed] [Google Scholar]
- 110.Assad TR, Robbins IM, Hemnes AR, Xu M, Maron BA, Choudhary G, Wells QS, Farber-Eger E, Hess E, Baron AE, et al. Subclinical pulmonary hypertension is common and associated with increased mortality in a large electronic medical record-based cohort [abstract] J Heart Lung Transplant. 2016;35:S118. [Google Scholar]