Abstract
Rationale: For unclear reasons, obese children with asthma have higher morbidity and reduced response to inhaled corticosteroids.
Objectives: To assess whether childhood obesity is associated with airway dysanapsis (an incongruence between the growth of the lungs and the airways) and whether dysanapsis is associated with asthma morbidity.
Methods: We examined the relationship between obesity and dysanapsis in six cohorts of children with and without asthma, as well as the relationship between dysanapsis and clinical outcomes in children with asthma. Adjusted odds ratios (ORs) were calculated for each cohort and in a combined analysis of all cohorts; longitudinal analyses were also performed for cohorts with available data. Hazard ratios (HRs) for clinical outcomes were calculated for children with asthma in the Childhood Asthma Management Program.
Measurements and Main Results: Being overweight or obese was associated with dysanapsis in both the cross-sectional (OR, 1.95; 95% confidence interval [CI], 1.62–2.35 [for overweight/obese compared with normal weight children]) and the longitudinal (OR, 4.31; 95% CI, 2.99–6.22 [for children who were overweight/obese at all visits compared with normal weight children]) analyses. Dysanapsis was associated with greater lung volumes (FVC, vital capacity, and total lung capacity) and lesser flows (FEV1 and forced expiratory flow, midexpiratory phase), and with indicators of ventilation inhomogeneity and anisotropic lung and airway growth. Among overweight/obese children with asthma, dysanapsis was associated with severe disease exacerbations (HR, 1.95; 95% CI, 1.38–2.75) and use of systemic steroids (HR, 3.22; 95% CI, 2.02–5.14).
Conclusions: Obesity is associated with airway dysanapsis in children. Dysanapsis is associated with increased morbidity among obese children with asthma and may partly explain their reduced response to inhaled corticosteroids.
Keywords: childhood asthma, airway dysanapsis, pulmonary function, childhood obesity
At a Glance Commentary
Scientific Knowledge on the Topic
Obese children with asthma have higher morbidity and reduced response to inhaled corticosteroids, but the underlying mechanisms are not fully understood.
What This Study Adds to the Field
Being overweight or obese is associated with airway dysanapsis in children with or without asthma. Among obese children with asthma, dysanapsis had a significant clinical impact and may at least partly explain the reduced response to asthma medications in this group of patients.
Obesity and asthma are major public health problems, particularly in industrialized nations. Among children and adults, obesity is a risk factor for asthma, asthma morbidity, reduced quality of life, and reduced response to inhaled corticosteroids (ICSs) (1–3). Obesity may impact asthma through multiple mechanisms, including changes in lung mechanics (4), comorbidities such as gastroesophageal reflux (5), dietary intake (6), alterations in insulin and/or glucose metabolism (7), and systemic inflammation (8).
Airway dysanapsis is a physiological incongruence between the growth of the lung parenchyma and the caliber of the airways (9), reflected by an abnormal FEV1/FVC despite normal FEV1 and FVC. Mead proposed that airway length depends on lung volume but airway caliber growth does not (10). Dysanapsis may occur in otherwise healthy subjects with expiratory flow limitation (11) and may be more pronounced in women than in men (12, 13).
Most studies have shown that obese adults (with and without asthma) have a reduced FVC but a normal FEV1/FVC (suggestive of a restrictive ventilatory deficit). On the contrary, studies in children have shown that obesity is associated with reduced FEV1/FVC (an obstructive deficit) (8, 14–17). However, rather than low FEV1, researchers in several studies have reported a normal or high FEV1 and a high FVC; thus, we hypothesized that the changes seen in obese children may be related to dysanapsis (normal flows in large lungs). We further hypothesized that obesity is associated with airway dysanapsis in children and that obesity-related dysanapsis leads to increased morbidity in obese children with asthma. We examined these hypotheses using data from pulmonary function test (PFT) clinical databases and from several research cohorts of children with and without asthma. Some of the results of this study were presented as a poster at the 2016 American Thoracic Society International Conference in San Francisco, California (18).
Methods
See online supplement for details.
Airway Dysanapsis
FVC and FEV1 z-scores (zFVC and zFEV1, respectively) were calculated using Global Lung Initiative equations (19). “Airway dysanapsis” was defined as normal to high zFVC (≥0.674 or 75th percentile), normal zFEV1 (greater than or equal to −1.645, the fifth percentile, or the lower limit of normal), and low FEV1/FVC (<80%). Control subjects were defined by the same zFVC and zFEV1 but normal FEV1/FVC (≥80%). Tests with FVC or FEV1 below those cutoffs were excluded from analysis. The dysanapsis ratio (a lower ratio means more marked dysanapsis) was calculated as maximal expiratory flow at 50% of FVC (MEF50%)/(FVC × static recoil pressure at 50% of FVC [Pst50%]) when MEF50% was available (Children’s Hospital of Pittsburgh [CHP] and Dutch study [NL] cohorts) (11). Pst50% was calculated as 6.3038–0.056 × age (20). In the Boston Children’s Hospital (BCH) cohort, only forced expiratory flow, midexpiratory phase (FEF25-75%) was available, and thus the dysanapsis ratio was calculated as FEF25-75%/FVC (21, 22).
Study Populations
CHP
All PFTs conducted at CHP between August 1991 and April 2014 in children ages 7–19 years were reviewed using a PFT clinical database. We excluded the following diagnoses: cystic fibrosis, bronchopulmonary dysplasia, vocal cord dysfunction, chronic lung disease, obstructive sleep apnea, pneumonia or acute infection, “other,” or missing. A total of 381 children with dysanapsis (783 tests) and 907 control subjects (1,523 tests) with complete data were included in the analysis. The electronic medical records of these children were also abstracted for relevant variables: race; asthma diagnosis; scheduled versus sick visit; and hospitalizations, emergency department visits, prednisone courses, use of inhaled steroids, and total number of medications for asthma.
Hartford–Puerto Rico cohort
As part of a case–control study of asthma in Puerto Rican subjects, we recruited 1,127 children (618 with asthma and 509 without asthma) living in San Juan, Puerto Rico, and Hartford, Connecticut (the HPR cohort). Details on subject recruitment and study procedures were reported previously (15, 23–25). A total of 147 children with dysanapsis (79 with and 69 without asthma) and 236 control subjects (120 with and 116 without asthma) and complete data were included in this analysis. Asthma-related outcomes and medication use were obtained by parental report.
BCH cohort
All PFTs conducted at BCH between January 1994 and December 2008 in children ages 6–18 years with a diagnosis of asthma or reactive airway disease were reviewed using a PFT clinical database. A total of 246 children with dysanapsis (730 PFTs) and 883 control subjects (1,700 PFTs) with complete data were included in this analysis.
National Health and Nutrition Examination Survey
Using 2007–2008 and 2009–2010 NHANES (National Health and Nutrition Examination Survey) spirometry data (26), we included results for 2,658 children (ages 6–19 yr) without asthma. A total of 1,213 children (189 with dysanapsis and 1,024 control subjects) were included in this analysis.
Childhood Asthma Management Program cohort
CAMP (Childhood Asthma Management Program) was a 4-year, multicenter, randomized clinical trial of long-term asthma medications (27). A total of 1,041 children were randomized to inhaled budesonide, inhaled nedocromil, or inhaled placebo, and followed for 48 months. A total of 195 children with dysanapsis (2,150 PFTs) and 200 control subjects (1,970 PFTs) were included in this analysis. Information on asthma exacerbations and medication use was recorded in diary cards each day during the trial (27).
Dutch study cohort
The Dutch study cohort (NL) consisted of data from a 7-year longitudinal study of 622 Caucasian children (ages 12–15 yr at enrollment) from The Hague, the Netherlands, enrolled in secondary school in 1978–1979 (28, 29). Anthropometric, spirometric, and lung function data were measured twice yearly. Nitrogen single-breath and multiple-breath techniques were used to calculate the residual volume (RVsb and RVmb) (30) and the washout phase III slope (%N2/L) (31). A total of 26 children with dysanapsis (142 PFTs) and 88 control subjects (642 PFTs) with complete data were included in this analysis.
Ethics statement
All studies were approved by the institutional review boards of the corresponding institutions and participating centers.
Statistical Analysis
In our primary analysis, we examined overweight or obesity and dysanapsis. Body mass index (BMI) z-scores (zBMI) were calculated using formulas from the CDC (32), and overweight/obesity was assessed using both continuous (zBMI) and binary (overweight [BMI in the 85th–95th percentile] or obesity [BMI ≥95th percentile]) measures. For each cohort, we performed a cross-sectional analysis including one test per subject; if more than one test was available, only the first test was selected. Multivariable logistic and linear regression models were adjusted for age, sex, race, and baseline FEV1 in all cohorts; asthma status (in HPR); treatment arm (in CAMP); and study site (in HPR and CAMP).
We also performed a random-effects longitudinal analysis in cohorts with available data (CHP, BCH, CAMP, and NL) with three comparisons: “mostly obese” versus “mostly non obese” (children with BMI >85th percentile in >50% vs. <50% of visits), “mostly obese” versus “never obese” (>50% vs. 0% of visits), and “always obese” versus “never obese” (100% vs. 0% of visits). In addition, we performed cross-sectional and longitudinal combined analyses by pooling the PFTs from all cohorts at the individual level; these analyses were additionally adjusted for study cohort and study site.
Next, we examined dysanapsis and lung function measures in a combined examination of all cohorts for which measures were available, as well as dysanapsis and clinical outcomes in children with asthma. In the NL cohort, we also examined the difference between RVsb and RVmb and the phase III slope (31), both of which are greater when airway obstruction exists (30). Lung and airway growth were evaluated using the equation MEF = c × TLCk, where k values between approximately 0.7 and approximately 1.3 are consistent with isotropic (symmetric) growth (28, 33) and TLC is total lung capacity. We calculated k50 and k75 using MEF50% and maximal expiratory flow at 75% of FVC [MEF75%], respectively. Finally, we assessed asthma-related outcomes using linear or logistic regression, as appropriate. In CAMP, survival analysis was performed in overweight/obese participants using log-rank tests of equality and Cox maximum-likelihood proportional hazards models, where each outcome (e.g., asthma exacerbation) was the “failure” event. Cumulative hazard was plotted, stratified by dysanapsis group. All analyses were performed using R v.3.2.2 (R Foundation for Statistical Computing, Vienna, Austria) and STATA v.13 software (StataCorp LP, College Station, TX).
Results
A total of 4,521 children were included in this analysis. The main characteristics of the study populations are shown in Table 1. Studies included children ages 6–20 years old. The CHP and BCH cohorts include data from children referred to pulmonary clinics for management; the HPR cohort included a population-based and a school-based sample of children with and without asthma; the NHANES cohort included a population-based sample of children without asthma; the CAMP cohort included children with asthma who participated in a clinical trial; and the NL cohort included data from a normative longitudinal study of lung growth in adolescents.
Table 1.
Characteristics of Study Participants by Cohort
| CHP | HPR |
BCH | NHANES | CAMP | NL | ||
|---|---|---|---|---|---|---|---|
| Cases | Controls | ||||||
| Tests, n | 2,306 | 198 | 184 | 2,430 | 1,213 | 4,120 | 784 |
| Subjects, n* | 1,288 | 198 | 184 | 1,129 | 1,213 | 395 | 114 |
| Age, yr | 10.7 (3.7) | 9.9 (2.8) | 9.8 (2.7) | 10.0 (3.2) | 12.3 (4.0) | 10.4 (2.5) | 15.3 (1.6) |
| Male sex, % | 54.9 | 46.5 | 45.1 | 53.7 | 51.0 | 59.4 | 63.0 |
| BMI z-score | 1.09 (0.98) | 1.23 (0.98) | 0.93 (1.11) | 1.13 (0.99) | 0.88 (1.06) | 0.76 (0.94) | 0.21 (0.67) |
| FEV1 | |||||||
| % predicted | 120.7 (12.9) | 108.6 (10.8) | 110.4 (12.1) | 112.0 (11.8) | 114.4 (10.1) | 105.0 (10.5) | 112.4 (7.7) |
| z-score | 1.74 (1.08) | 0.73 (0.90) | 0.88 (1.01) | 1.01 (0.97) | 1.21 (0.85) | 0.42 (0.89) | 1.07 (0.67) |
| FVC | |||||||
| % predicted | 129.6 (8.0) | 119.9 (13.4) | 121.8 (16.4) | 118.6 (7.8) | 118.1 (8.1) | 117.1 (7.3) | 114.5 (5.6) |
| z-score | 2.40 (0.63) | 1.71 (1.11) | 1.86 (1.37) | 1.51 (0.62) | 1.53 (0.70) | 1.43 (0.61) | 1.23 (0.47) |
| FEV1/FVC | |||||||
| Ratio | 81.9 (7.7) | 80.9 (7.8) | 81.5 (9.8) | 83.2 (7.6) | 85.5 (5.7) | 78.8 (7.3) | 85.0 (5.5) |
| z-score | −0.92 (1.01) | −1.28 (1.02) | −1.15 (1.27) | −0.76 (1.05) | −0.48 (0.87) | −1.38 (0.94) | −0.30 (0.83) |
| Race, % | |||||||
| White | 84.7 | 0 | 0 | 78.2 | 23.4 | 60.3 | 100 |
| Black | 14.3 | 0 | 0 | 14.8 | 19.5 | 14.1 | 0 |
| Other(s) | 1 | 100 | 100 | 7 | 57.1 | 25.6 | 0 |
| Dysanapsis, %* | |||||||
| Total study population | 33.8 | 14.3 | 15.4 | 11.7 | 7.1 | 20.2 | 4.3 |
| Sample included in the analysis | 33.9 | 39.4 | 36.9 | 30.0 | 15.6 | 52.2 | 18.1 |
| Cohort type | Clinical database | Case–control study of childhood asthma | Clinical database | U.S. population sample | Randomized clinical trial | Normative data | |
Definition of abbreviations: BCH = Boston Children’s Hospital cohort; BMI = body mass index; CAMP = Childhood Asthma Management Program cohort; CHP = Children’s Hospital of Pittsburgh cohort; HPR = Study of Asthma in Puerto Rican Children (Hartford and Puerto Rico) cohort; NHANES = National Health and Nutrition Examination Survey; NL = Dutch study cohort.
Numbers represent mean (SD) unless otherwise indicated.
CHP data were specifically queried to include only dysanapsis and control subjects.
Dysanapsis and Obesity
Table 2 summarizes the findings derived from the cross-sectional analysis of obesity and dysanapsis for each cohort. In the CHP cohort, each 1.0-point increase in zBMI was associated with 1.64 times higher odds of airway dysanapsis, and overweight or obese children had 2–2.35 times higher odds of dysanapsis than children of normal weight. Similarly, zBMI was associated with dysanapsis in all other cohorts of children (HPR, BCH, NHANES, CAMP, and NL), with odds ratios (ORs) ranging from 1.29 to 2.48 (P < 0.01 for each cohort). Overweight and obesity were also associated with dysanapsis, with ORs ranging from 1.36 to 2.48 (overweight OR, 7.95 in NL but only 14 children with dysanapsis and thus a very wide confidence interval [CI]). Moreover, zBMI and overweight/obesity were also associated with lower dysanapsis ratios by either definition (see Table E1 in the online supplement).
Table 2.
Cross-Sectional Analysis of Obesity and Airway Dysanapsis
| Dysanapsis/Total, n | BMI z-Score | Overweight/Obese | Obese Only | |
|---|---|---|---|---|
| CHP | 381/1,288 | |||
| OR (95% CI) | 1.64 (1.37–1.95) | 2.35 (1.67–3.30) | 2.00 (1.41–2.83) | |
| P value | <0.001 | <0.001 | <0.001 | |
| n | 721 | 436 | ||
| HPR* | 146/381 | |||
| OR (95% CI) | 1.29 (1.01–1.67) | 1.75 (1.05–2.92) | 1.88 (1.11–3.19) | |
| P value | 0.048 | 0.033 | 0.018 | |
| n | 207 | 136 | ||
| BCH | 246/1,128 | |||
| OR (95% CI) | 1.36 (1.11–1.66) | 2.02 (1.37–2.98) | 1.36 (0.90–2.07) | |
| P value | 0.002 | 0.001 | 0.15 | |
| n | 583 | 355 | ||
| NHANES | 189/1,213 | |||
| OR (95% CI) | 1.35 (1.11–1.65) | 1.58 (1.06–2.36) | 1.67 (1.08–2.60) | |
| P value | 0.002 | 0.024 | 0.022 | |
| n | 551 | 324 | ||
| CAMP | 195/392 | |||
| OR (95% CI) | 1.32 (0.98–1.81) | 1.42 (0.77–2.62) | 2.48 (1.5–5.35) | |
| P value | 0.075 | 0.26 | 0.021 | |
| n | 147 | 75 | ||
| NL | 26/114 | |||
| OR (95% CI) | 2.48 (1.21–5.11) | 7.95 (1.74–36.3) | 0.94 (0.06–15.4) | |
| P value | 0.013 | 0.007 | 0.96 | |
| n | 14 | 4 | ||
| All† | 1,183/4,516 | |||
| OR (95% CI) | 1.44 (1.31–1.58) | 1.95 (1.62–2.35) | 1.75 (1.44–2.14) | |
| P value | 1.4 × 10−14 | 1.3 × 10−12 | 2.6 × 10−8 | |
| n | 2,223 | 1,330 |
Definition of abbreviations: BCH = Boston Children’s Hospital cohort; BMI = body mass index; CAMP = Childhood Asthma Management Program cohort; CHP = Children’s Hospital of Pittsburgh cohort; CI = confidence interval; HPR = Study of Asthma in Puerto Rican Children (Hartford and Puerto Rico) cohort; NHANES = National Health and Nutrition Examination Survey; NL = Dutch study cohort; OR = odds ratio.
HPR combined data (cases and control subjects).
Joined analysis of all cohorts.
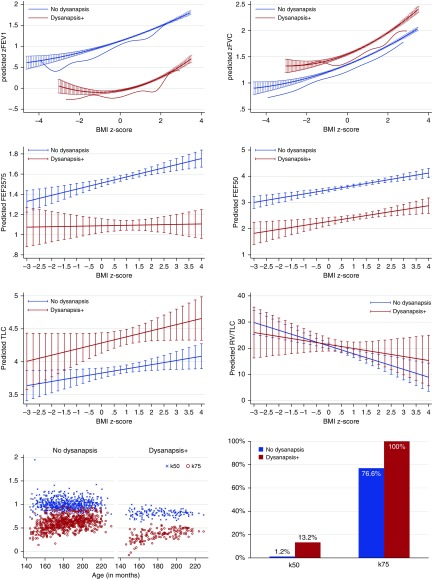
We then performed a combined analysis of PFTs for all cohorts (Table 2). In this analysis (which was additionally adjusted for cohort and study site), both zBMI (OR, 1.44; 95% CI, 1.31–1.58) and overweight or obesity (OR, 1.95; 95% CI, 1.62–2.35) were associated with dysanapsis. Figure 1 shows the behavior of FEV1 and FVC by zBMI in children with and without dysanapsis: FEV1 and FVC had similarly increasing slopes with zBMI regardless of dysanapsis, but in the dysanapsis group FVC was slightly higher (β = +0.18 z-score, or +2.1% of predicted; P < 0.001) and FEV1 was markedly lower (β = −1.24 z-score, or −14.9% of predicted; P < 0.001). Figure E1 shows these results by individual cohort. Boys had slightly higher odds of dysanapsis than girls (Figure E2); there were no significant differences by race or ethnicity.
Figure 1.
Body mass index (BMI) z-score, airway dysanapsis, and lung function. The top six panels show predicted lung function (FEV1; FVC; forced expiratory flow, midexpiratory phase [FEF25-75%]; expiratory rate at 50% of FVC [FEF50]; total lung capacity [TLC]; and residual volume [RV]/TLC) and 95% confidence intervals, by BMI z-score and airway dysanapsis status (with dysanapsis in red; no dysanapsis in blue). Children with dysanapsis had lower FEV1, FEF25-75%, and FEF50 but higher FVC and TLC. Bottom left panel shows values for k50 and k75. Bottom right panel shows the percentage of children with k50 or k75 values consistent with anisotropic (asymmetric) growth of the lungs and airways. Children with dysanapsis had lower k50 and k75 values, which was more consistent with anisotropic lung and/or airway growth.
Longitudinal Analysis
The results of the longitudinal analysis of overweight or obesity measures and dysanapsis are shown in Table 3. In CHP, approximately 20% of children had at least two tests (maximum of 18 tests over a 9-yr span); each 1-point increase in zBMI was associated with a 2.25 times increased odds of dysanapsis, and children who were “always overweight or obese” had 4.34 higher odds of dysanapsis than those who were “never overweight or obese.” In BCH, 50% of children had more than 2 tests, and 10% had more than 6 tests (maximum of 20 tests over an approximately 11-yr span); each 1-point increase in zBMI was associated with a 1.82 higher odds of dysanapsis, and children who were “always overweight/obese” had 3.48 times higher odds of dysanapsis than those who were “never overweight/obese.” In CAMP, children had an average of 9.5 tests over approximately 48 months; each 1-point increment in zBMI was significantly associated with a 1.62 times increased odds of dysanapsis, and children who were “always overweight/obese” had 4.95 times higher odds of dysanapsis than those who always had normal weight. In NL, children had an average of 6.5 tests (range, 1–13 over 7 yrs of follow-up); each 1-point increment in zBMI was associated with a 3.24 times increased odds of dysanapsis, while the dichotomous analyses were not statistically significant. The combined analysis that each 1-point increment in zBMI was associated with a 1.93 times increased odds of dysanapsis, a 3.41 times increased odds of dysanapsis for the comparison of “mostly” obese versus “mostly not” obese, and a 4.18–4.31 times increased odds of dysanapsis for the comparison of “mostly” or “always” obese versus “never” obese.
Table 3.
Longitudinal Analysis of Obesity and Airway Dysanapsis
| BMI z-Score | “Mostly” vs. “Mostly Not” Obese* | “Mostly” vs. “Never” Obese† | “Always” vs. “Never” Obese‡ | |
|---|---|---|---|---|
| CHP | ||||
| OR (95% CI) | 2.25 (1.49–3.42) | 3.49 (1.55–7.86) | 4.76 (1.81–12.6) | 4.34 (1.71–11.0) |
| P value | 0.001 | 0.003 | 0.002 | 0.002 |
| n | 915 | 915 | 869 | 781 |
| BCH | ||||
| OR (95% CI) | 1.82 (1.32–2.44) | 2.78 (1.54–5.02) | 2.86 (1.54–5.31) | 3.48 (1.72–7.02) |
| P value | 0.001 | 0.001 | 0.001 | 0.001 |
| n | 2,429 | 2,429 | 2,308 | 2,014 |
| CAMP | ||||
| OR (95% CI) | 1.62 (1.23–2.13) | 2.94 (1.61–5.41) | 4.77 (2.31–9.85) | 4.95 (2.25–10.9) |
| P value | 0.001 | 0.001 | 0.001 | 0.001 |
| n | 4,412 | 4,120 | 3,105 | 2,370 |
| NL | ||||
| OR (95% CI) | 3.24 (1.33–7.90) | 3.03 (0.33–28.3) | 3.42 (0.38–31.0) | 6.18 (0.35–108) |
| P value | 0.01 | 0.33 | 0.27 | 0.21 |
| n | 783 | 783 | 735 | 715 |
| All | ||||
| OR (95% CI) | 1.93 (1.67–2.23) | 3.41 (2.45–4.73) | 4.18 (2.94–5.98) | 4.31 (2.99–6.22) |
| P value | 3.1 × 10−19 | 3.0 × 10−13 | 3.0 × 10−15 | 5.0 × 10−15 |
| n | 10,060 | 8,856 | 7,594 | 6,336 |
Definition of abbreviations: BCH = Boston Children’s Hospital cohort; BMI = body mass index; CAMP = Childhood Asthma Management Program cohort; CHP = Children’s Hospital of Pittsburgh cohort; CI = confidence interval; NL = Dutch study cohort; OR = odds ratio.
Overweight or obese at more than 50% of visits versus less than 50% of visits.
Overweight or obese at more than 50% of visits versus 0% of visits.
Overweight or obese at 100% of visits versus 0% of visits.
Dysanapsis and Other Lung Function Measures
We analyzed other lung function measures jointly for the cohorts for which each measure was available. Children with dysanapsis had lower FEF25-75% (β = −1.89 z-score or −43% of predicted; P < 0.001 [combined analysis of CHP, BCH, and NL]) and MEF50% percent predicted (β = −41.4% of predicted; P < 0.001 [CHP]), and higher TLC (β = +457 ml; P < 0.001 [CHP, BCH, and NL combined analysis]) than those without dysanapsis (Figure 1), with no significant differences in residual volume (RV)/TLC. In NL, dysanapsis was associated with higher VC (β = +322 ml; P = 0.034), lower MEF75% (β = −0.281 L/s; P = 0.018), and no changes in closing volume or closing capacity.
We also evaluated markers of ventilation inhomogeneity and found that dysanapsis was not associated with the phase III nitrogen slope, but the RVsb − RVmb difference was more pronounced in the dysanapsis group (β = 26.9 ml; P = 0.002). Children with dysanapsis had lower mean values of k50 (0.79 vs. 1.01) and k75 (0.35 vs. 0.61) (Figure 1). After adjustment for age, sex, and FEV1, children with dysanapsis had 4.6 times increased odds (95% CI, 1.9–7.3; P = 0.001) of having k50 values consistent with anisotropic lung and/or airway growth.
Clinical Outcomes
We assessed airway dysanapsis and clinical outcomes in children with asthma. In CHP, children with dysanapsis were more likely to use at least three medications for asthma (OR, 1.72; 95% CI, 1.02–2.90; P = 0.04). In HPR, children with dysanapsis were more likely to use daily albuterol (OR, 8.3; 95% CI, 1.1–64.0; P = 0.04) and miss school due to asthma (OR, 10.5; 95% CI, 1.9–58.5; P = 0.007).
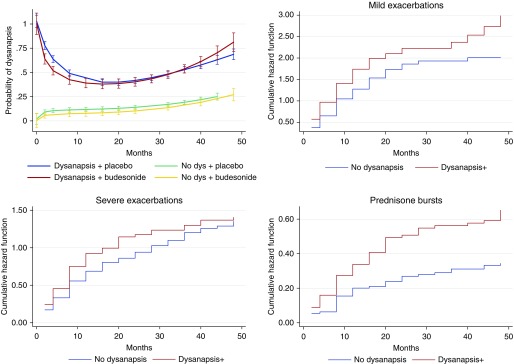
At the randomization visit in CAMP, children with dysanapsis (n = 197) had higher odds of reporting at least one hospitalization for asthma in the year prior to the trial (OR, 3.03; 95% CI, 1.10–8.37; P = 0.033) than those without dysanapsis. Figure 2 shows the proportion of children with dysanapsis by treatment arm during the trial; there was no significant differences in the proportion of children with dysanapsis between budesonide and nedocromil/placebo at either the beginning or the end of the trial. After adjustment for treatment arm and other covariates, dysanapsis was associated with shorter time to a severe asthma exacerbation requiring emergency department visit, hospitalization, or systemic corticosteroids (β = −3.29 mo; 95% CI, −5.70 to −0.89; P = 0.007). Compared with overweight/obese children without dysanapsis, those with dysanapsis had an increased risk of at least one severe exacerbation (hazard ratio, 1.40; 95% CI, 1.07–1.75; P = 0.014) and of having at least two prednisone bursts between study visits (hazard ratio, 2.53; 95% CI, 1.71–3.73; P < 0.001) (Figure 2). The effects were all more pronounced in overweight/obese children than in children of normal weight (Table E2). We found no significant modification of the effect of dysanapsis (or the association between obesity and dysanapsis) by treatment arm.
Figure 2.
Dysanapsis (dys) and clinical outcomes in children with asthma in CAMP (Childhood Asthma Management Program). Top left panel shows the probability of dysanapsis during 48 months in CAMP by treatment arm in children with dysanapsis at randomization. There were no significant differences between the budesonide or nedocromil and/or placebo arms. Error bars represent 95% confidence intervals. The other three panels show cumulative hazard function of dysanapsis for mild exacerbations (top right), severe exacerbations (bottom left), and requiring more than two prednisone bursts between study visits (bottom right). Children with dysanapsis had a higher risk of exacerbations and prednisone courses during the trial.
Sensitivity Analyses
We repeated the analysis using different FEV1, FVC, and FEV1/FVC cutoffs to define dysanapsis, with essentially unchanged results (Table 4). In addition, we performed sensitivity analyses with other FEV1/FVC or FVC cutoffs (Tables E3 and E4), indicating that the associations did not depend solely on our cutoff selection. Finally, we repeated the analysis after excluding subjects with significant bronchodilator response (>12%) or using only post-bronchodilator spirometry (in cohorts with available data), with similar results (data not shown), suggesting that the associations were likely not due to unrecognized bronchoconstriction.
Table 4.
Sensitivity Analysis Using Different Cutoffs to Define Dysanapsis
| Cutoff Values | BMI z-Score |
|||
|---|---|---|---|---|
| FVC | FEV1 | FEV1/FVC* | OR (95% CI) | P Value |
| >75th percentile | >LLN | <80% vs. >80% | 1.44 (1.31–1.58) | 1.4 × 10−14 |
| >75th percentile | >100% predicted | <80% vs. >80% | 1.48 (1.34–1.63) | 4.9 × 10−15 |
| >100% predicted | >100% predicted | <80% vs. >80% | 1.54 (1.41–1.69) | 8.8 × 10−21 |
| >100% predicted | >100% predicted | <LLN vs. >LLN | 1.54 (1.38–1.72) | 3.4 × 10−14 |
| >LLN | >LLN | <LLN vs. >LLN | 1.44 (1.34–1.53) | 7.3 × 10−26 |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; LLN = lower limit of normal; OR = odds ratio.
Odds ratios (and confidence intervals) for the association between BMI z-score and dysanapsis.
The same FEV1 and FVC criteria were used for both groups, but the dysanapsis group was defined as low FEV1/FVC (e.g., <80% or <LLN) and the control subjects as normal FEV1/FVC (e.g., >80% or >LLN). The first line represents the definition used in the main analysis (see Table 2).
Discussion
In this report, we show that overweight or obesity are associated with airway dysanapsis in children with and without asthma. Moreover, we demonstrate that airway dysanapsis is associated with symptoms, medication use, and severe disease exacerbations in children with asthma. To our knowledge, this is the first report of an association between obesity and airway dysanapsis in childhood, as well as the first report linking dysanapsis to worse disease severity or control in children with asthma.
Obesity measures were consistently associated with airway dysanapsis in all cohorts of children, in both cross-sectional and longitudinal analyses. Furthermore, lower FEV1, FEF25-75%, MEF50%, and/or MEF75% in the dysanaptic groups strongly suggests that the relative airflow obstruction is likely present throughout airways of all sizes; however, a normal RV/TLC suggests the absence of significant air trapping. These children had larger vital capacity, RV, and TLC, indicating they have larger lungs; this is in sharp contrast to studies in adults that have described a restrictive deficit in obesity, with lower lung volumes and a preserved FEV1/FVC (4). Our analysis of the NL data (19) evaluating the exponential relationship between flows and volumes (28, 33) further supports that children with dysanapsis have anisotropic (asymmetric) growth of the lungs and airways.
Dysanapsis is merely a reflection of the incongruence between a (faster) growth in lung volume and airway length, and the (slower) increase in airway caliber. Such incongruence may happen physiologically and may be accentuated by several mechanisms, including in utero exposures such as tobacco smoke or vitamin D deficiency (34, 35), although it is unclear whether these effects would persist later in childhood. Chronic hypoxemia has been associated with a higher risk of dysanaptic lung growth (36); dysanapsis may also be present in breathhold divers, who use glossopharyngeal insufflation to force their lung volumes above TLC (providing extra oxygen and preventing compression when diving) (37). Compensatory lung growth after pneumonectomy may also be dysanaptic (38).
There is evidence that lung function may “track” from birth throughout life, and this may be influenced by weight from early on. As early as 1990, Barker and coworkers described that low birth weight was associated with low lung function in adults approximately 59–70 years old and with death caused by chronic obstructive pulmonary disease (39). More recently, den Dekker and colleagues (40) performed a metaanalysis of 25,000 children from 24 birth cohorts and found that greater birth weight and infant weight gain were associated with higher FEV1 and FVC at school age (independent of gestational age); the increases were greater for FVC than for FEV1. Moreover, infant weight gain was associated with lower FEV1/FVC and FEF25-75%. Therefore, the changes we observed may be established very early in life. Further studies are needed to discern whether these changes are fixed or vary, particularly in the transition from childhood to adulthood.
Obesity-related airway dysanapsis was associated with worse clinical outcomes in children with asthma. In CAMP, ICSs had no effect on the association between BMI and dysanapsis, and, despite ICS treatment, children with dysanapsis remained at higher risk of severe asthma exacerbations. Our findings thus suggest that airway dysanapsis partly explains previous reports of a strong link between obesity and increased morbidity (8, 15, 41) or reduced response to treatment (2, 42) in children with asthma. We speculate that obese children with dysanapsis may have airflow obstruction that is anatomical and/or developmental and thus at least partly not related to bronchospasm or airway inflammation.
We found evidence of sex differences, with higher odds of dysanapsis among boys. This could partly explain prior reports that obesity is more consistently associated with worse asthma control in boys than in girls (41). Some of the initial studies on airway dysanapsis (not related to obesity) described that women have smaller airways than men relative to their lung volumes (10, 43). Later studies have reported that boys have smaller airways than girls (44, 45) and described changes in dysanapsis ratios before and after puberty (46). Moreover, a longitudinal population-based study of children with asthma reported that dysanapsis was more pronounced in boys, but this became less significant with age (47).
Our study has several limitations. None of the study cohorts was specifically designed to assess obesity and airway dysanapsis; however, we show consistent replication in independent and diverse populations. We cannot completely exclude reversible airflow obstruction, but this is unlikely because we included cohorts of children without asthma, and our results did not change when we used post-bronchodilator measurements (when available) or excluded children with a bronchodilator response. Moreover, budesonide treatment had no effect on the association between obesity and dysanapsis. There is evidence that using an arbitrary cutoff may not be the best way to identify “abnormal” lung function; however, we used cutoffs (e.g., FEV1/FVC <0.80) that are widely accepted and used in research and clinical practice. Furthermore, we performed sensitivity analyses using different cutoffs for FVC, FEV1, and/or FEV1/FVC to define dysanapsis, and the results remained essentially unchanged. We cannot assess whether children with high BMI are able to exhale more forcibly, resulting in higher intrathoracic pressure, more airway compression, and relatively reduced FEV1. We lack imaging of the airways or other measurements of airway diameter to confirm findings derived from lung function testing. Finally, we cannot determine whether dysanapsis was solely responsible for the observed differences in asthma outcomes among obese children.
In summary, obesity is associated with airway dysanapsis in children with or without asthma. Among obese children with asthma, dysanapsis has significant clinical impact and may at least partly explain the decreased response to asthma medications in this group of patients.
Acknowledgments
Acknowledgment
The authors thank the participants of the corresponding cohorts and their families, and the members of the local field teams. The authors also thank Prof. Philip Quanjer for his helpful input and for sharing the data from the NL study for our analysis.
Footnotes
Supported by National Institutes of Health (NIH) grant HL125666 and a grant from Children’s Hospital of Pittsburgh of UPMC (E.F.); NIH grants HL079966 and HL117191 (J.C.C.); The Heinz Endowments; and NIH grant HL066289 (S.T.W. and A.A.L.).
Author Contributions: Study conception and design: E.F., D.J.W., and J.C.C.; data acquisition and/or interpretation: all authors; data analysis and writing of first draft of manuscript: E.F.; manuscript revision for important intellectual content: all authors; and approval of the final version for submission: all authors.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as 10.1164/rccm.201605-1039OC on August 23, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Dixon AE, Holguin F, Sood A, Salome CM, Pratley RE, Beuther DA, Celedón JC, Shore SA American Thoracic Society Ad Hoc Subcommittee on Obesity and Lung Disease. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc. 2010;7:325–335. doi: 10.1513/pats.200903-013ST. [DOI] [PubMed] [Google Scholar]
- 2.Forno E, Lescher R, Strunk R, Weiss S, Fuhlbrigge A, Celedón JC Childhood Asthma Management Program Research Group. Decreased response to inhaled steroids in overweight and obese asthmatic children. J Allergy Clin Immunol. 2011;127:741–749. doi: 10.1016/j.jaci.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, Griffes LA, Garudathri J, Raymond D, Poynter ME, Bunn JY, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol. 2011;128:508–515.e1–515.e2. doi: 10.1016/j.jaci.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest. 2006;130:827–833. doi: 10.1378/chest.130.3.827. [DOI] [PubMed] [Google Scholar]
- 5.Beuther DA, Weiss ST, Sutherland ER. Obesity and asthma. Am J Respir Crit Care Med. 2006;174:112–119. doi: 10.1164/rccm.200602-231PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Marcos L, Canflanca IM, Garrido JB, Varela AL, Garcia-Hernandez G, Guillen Grima F, Gonzalez-Diaz C, Carvajal-Urueña I, Arnedo-Pena A, Busquets-Monge RM, et al. Relationship of asthma and rhinoconjunctivitis with obesity, exercise and Mediterranean diet in Spanish schoolchildren. Thorax. 2007;62:503–508. doi: 10.1136/thx.2006.060020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forno E, Han YY, Muzumdar RH, Celedón JC. Insulin resistance, metabolic syndrome, and lung function in US adolescents with and without asthma. J Allergy Clin Immunol. 2015;136:304–311.e8. doi: 10.1016/j.jaci.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han YY, Forno E, Celedón JC. Adiposity, fractional exhaled nitric oxide, and asthma in U.S. children. Am J Respir Crit Care Med. 2014;190:32–39. doi: 10.1164/rccm.201403-0565OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green M, Mead J, Turner JM. Variability of maximum expiratory flow-volume curves. J Appl Physiol. 1974;37:67–74. doi: 10.1152/jappl.1974.37.1.67. [DOI] [PubMed] [Google Scholar]
- 10.Mead J. Dysanapsis in normal lungs assessed by the relationship between maximal flow, static recoil, and vital capacity. Am Rev Respir Dis. 1980;121:339–342. doi: 10.1164/arrd.1980.121.2.339. [DOI] [PubMed] [Google Scholar]
- 11.Smith JR, Rosenkranz SK, Harms CA. Dysanapsis ratio as a predictor for expiratory flow limitation. Respir Physiol Neurobiol. 2014;198:25–31. doi: 10.1016/j.resp.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Martin TR, Castile RG, Fredberg JJ, Wohl ME, Mead J. Airway size is related to sex but not lung size in normal adults. J Appl Physiol (1985) 1987;63:2042–2047. doi: 10.1152/jappl.1987.63.5.2042. [DOI] [PubMed] [Google Scholar]
- 13.Sheel AW, Guenette JA, Yuan R, Holy L, Mayo JR, McWilliams AM, Lam S, Coxson HO. Evidence for dysanapsis using computed tomographic imaging of the airways in older ex-smokers. J Appl Physiol (1985) 2009;107:1622–1628. doi: 10.1152/japplphysiol.00562.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tantisira KG, Litonjua AA, Weiss ST, Fuhlbrigge AL Childhood Asthma Management Program Research Group. Association of body mass with pulmonary function in the Childhood Asthma Management Program (CAMP) Thorax. 2003;58:1036–1041. doi: 10.1136/thorax.58.12.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forno E, Acosta-Pérez E, Brehm JM, Han YY, Alvarez M, Colón-Semidey A, Canino G, Celedón JC. Obesity and adiposity indicators, asthma, and atopy in Puerto Rican children. J Allergy Clin Immunol. 2014;133:1308–1314.e1–1314.e5. doi: 10.1016/j.jaci.2013.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu YT, Chen WY, Wang TN, Tseng HI, Wu JR, Ko YC. Extreme BMI predicts higher asthma prevalence and is associated with lung function impairment in school-aged children. Pediatr Pulmonol. 2009;44:472–479. doi: 10.1002/ppul.21023. [DOI] [PubMed] [Google Scholar]
- 17.Weinmayr G, Forastiere F, Büchele G, Jaensch A, Strachan DP, Nagel G ISAAC Phase Two Study Group. Overweight/obesity and respiratory and allergic disease in children: international study of asthma and allergies in childhood (ISAAC) phase two. PLoS One. 2014;9:e113996. doi: 10.1371/journal.pone.0113996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forno E, Weiner D, Mullen J, Kurland G, Sawicki GS, Acosta-Perez E, Colon-Semidey A, Alvarez M, Canino G, Celedon JC. Obesity and airway dysanapsis in children and adolescents with and without asthma [abstract] Am J Respir Crit Care Med. 2016;193:A3828. doi: 10.1164/rccm.201605-1039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MS, Zheng J, et al. ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner JM, Mead J, Wohl ME. Elasticity of human lungs in relation to age. J Appl Physiol. 1968;25:664–671. doi: 10.1152/jappl.1968.25.6.664. [DOI] [PubMed] [Google Scholar]
- 21.Vilozni D, Lavie M, Sarouk I, Efrati O. Progressive flow-to-volume dysanapsis in cystic fibrosis: a predictor for lung transplantation? Am J Respir Crit Care Med. 2012;186:82–87. doi: 10.1164/rccm.201202-0272OC. [DOI] [PubMed] [Google Scholar]
- 22.Tager IB, Weiss ST, Muñoz A, Welty C, Speizer FE. Determinants of response to eucapneic hyperventilation with cold air in a population-based study. Am Rev Respir Dis. 1986;134:502–508. doi: 10.1164/arrd.1986.134.3.502. [DOI] [PubMed] [Google Scholar]
- 23.Brehm JM, Ramratnam SK, Tse SM, Croteau-Chonka DC, Pino-Yanes M, Rosas-Salazar C, Litonjua AA, Raby BA, Boutaoui N, Han YY, et al. Stress and bronchodilator response in children with asthma. Am J Respir Crit Care Med. 2015;192:47–56. doi: 10.1164/rccm.201501-0037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs TS, Forno E, Brehm JM, Acosta-Pérez E, Han YY, Blatter J, Colón-Semidey A, Alvarez M, Canino G, Celedón JC. Underdiagnosis of allergic rhinitis in underserved children. J Allergy Clin Immunol. 2014;134:737–739.e6. doi: 10.1016/j.jaci.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han YY, Forno E, Brehm JM, Acosta-Pérez E, Alvarez M, Colón-Semidey A, Rivera-Soto W, Campos H, Litonjua AA, Alcorn JF, et al. Diet, interleukin-17, and childhood asthma in Puerto Ricans. Ann Allergy Asthma Immunol. 2015;115:288–293.e1. doi: 10.1016/j.anai.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National Health and Nutrition Examination Survey: plan and operations, 1999–2010. Vital Health Stat 1. 2013;(56) [PubMed] [Google Scholar]
- 27.The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343:1054–1063. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 28.Merkus PJ, Borsboom GJ, Van Pelt W, Schrader PC, Van Houwelingen HC, Kerrebijn KF, Quanjer PH. Growth of airways and air spaces in teenagers is related to sex but not to symptoms. J Appl Physiol (1985) 1993;75:2045–2053. doi: 10.1152/jappl.1993.75.5.2045. [DOI] [PubMed] [Google Scholar]
- 29.Schrader PC, Quanjer PH, van Zomeren BC, Wise ME. Changes in the FEV1-height relationship during pubertal growth. Bull Eur Physiopathol Respir. 1984;20:381–388. [PubMed] [Google Scholar]
- 30.Sterk PJ, Quanjer PH, van der Maas LL, Wise ME, van der Lende R. The validity of the single-breath nitrogen determination of residual volume. Bull Eur Physiopathol Respir. 1980;16:195–213. [PubMed] [Google Scholar]
- 31.Mikamo M, Shirai T, Mori K, Shishido Y, Akita T, Morita S, Asada K, Fujii M, Tsuchiya T, Suda T. Predictors of phase III slope of nitrogen single-breath washout in COPD. Respir Physiol Neurobiol. 2013;189:42–46. doi: 10.1016/j.resp.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention A SAS program for the CDC growth charts 2011[accessed 2013 Jan 10]. Available from: http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm
- 33.Martin TR, Feldman HA, Fredberg JJ, Castile RG, Mead J, Wohl ME. Relationship between maximal expiratory flows and lung volumes in growing humans. J Appl Physiol (1985) 1988;65:822–828. doi: 10.1152/jappl.1988.65.2.822. [DOI] [PubMed] [Google Scholar]
- 34.Lieberman E, Torday J, Barbieri R, Cohen A, Van Vunakis H, Weiss ST. Association of intrauterine cigarette smoke exposure with indices of fetal lung maturation. Obstet Gynecol. 1992;79:564–570. [PubMed] [Google Scholar]
- 35.Zosky GR, Hart PH, Whitehouse AJ, Kusel MM, Ang W, Foong RE, Chen L, Holt PG, Sly PD, Hall GL. Vitamin D deficiency at 16 to 20 weeks’ gestation is associated with impaired lung function and asthma at 6 years of age. Ann Am Thorac Soc. 2014;11:571–577. doi: 10.1513/AnnalsATS.201312-423OC. [DOI] [PubMed] [Google Scholar]
- 36.Llapur CJ, Martínez MR, Grassino PT, Stok A, Altieri HH, Bonilla F, Caram MM, Krowchuk NM, Kirby M, Coxson HO, et al. Chronic Hypoxia Accentuates Dysanaptic Lung Growth. Am J Respir Crit Care Med. 2016;194:327–332. doi: 10.1164/rccm.201509-1851OC. [DOI] [PubMed] [Google Scholar]
- 37.Lemaître F, Clua E, Andréani B, Castres I, Chollet D. Ventilatory function in breath-hold divers: effect of glossopharyngeal insufflation. Eur J Appl Physiol. 2010;108:741–747. doi: 10.1007/s00421-009-1277-1. [DOI] [PubMed] [Google Scholar]
- 38.Hsia CC. Lessons from a canine model of compensatory lung growth. Curr Top Dev Biol. 2004;64:17–32. doi: 10.1016/S0070-2153(04)64002-6. [DOI] [PubMed] [Google Scholar]
- 39.Barker DJ, Godfrey KM, Fall C, Osmond C, Winter PD, Shaheen SO. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ. 1991;303:671–675. doi: 10.1136/bmj.303.6804.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.den Dekker HT, Sonnenschein-van der Voort AM, de Jongste JC, Anessi-Maesano I, Arshad SH, Barros H, Beardsmore CS, Bisgaard H, Phar SC, Craig L, et al. Early growth characteristics and the risk of reduced lung function and asthma: A meta-analysis of 25,000 children. J Allergy Clin Immunol. 2016;137:1026–1035. doi: 10.1016/j.jaci.2015.08.050. [DOI] [PubMed] [Google Scholar]
- 41.Borrell LN, Nguyen EA, Roth LA, Oh SS, Tcheurekdjian H, Sen S, Davis A, Farber HJ, Avila PC, Brigino-Buenaventura E, et al. Childhood obesity and asthma control in the GALA II and SAGE II studies. Am J Respir Crit Care Med. 2013;187:697–702. doi: 10.1164/rccm.201211-2116OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGarry ME, Castellanos E, Thakur N, Oh SS, Eng C, Davis A, Meade K, LeNoir MA, Avila PC, Farber HJ, et al. Obesity and bronchodilator response in black and Hispanic children and adolescents with asthma. Chest. 2015;147:1591–1598. doi: 10.1378/chest.14-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brooks LJ, Byard PJ, Helms RC, Fouke JM, Strohl KP. Relationship between lung volume and tracheal area as assessed by acoustic reflection. J Appl Physiol (1985) 1988;64:1050–1054. doi: 10.1152/jappl.1988.64.3.1050. [DOI] [PubMed] [Google Scholar]
- 44.Taussig LM, Cota K, Kaltenborn W. Different mechanical properties of the lung in boys and girls. Am Rev Respir Dis. 1981;123:640–643. doi: 10.1164/arrd.1981.123.6.640. [DOI] [PubMed] [Google Scholar]
- 45.Dockery DW, Berkey CS, Ware JH, Speizer FE, Ferris BG., Jr Distribution of forced vital capacity and forced expiratory volume in one second in children 6 to 11 years of age. Am Rev Respir Dis. 1983;128:405–412. doi: 10.1164/arrd.1983.128.3.405. [DOI] [PubMed] [Google Scholar]
- 46.Smith JR, Emerson SR, Kurti SP, Gandhi K, Harms CA. Lung volume and expiratory flow rates from pre- to post-puberty. Eur J Appl Physiol. 2015;115:1645–1652. doi: 10.1007/s00421-015-3149-1. [DOI] [PubMed] [Google Scholar]
- 47.Weiss ST, Tosteson TD, Segal MR, Tager IB, Redline S, Speizer FE. Effects of asthma on pulmonary function in children. A longitudinal population-based study. Am Rev Respir Dis. 1992;145:58–64. doi: 10.1164/ajrccm/145.1.58. [DOI] [PubMed] [Google Scholar]