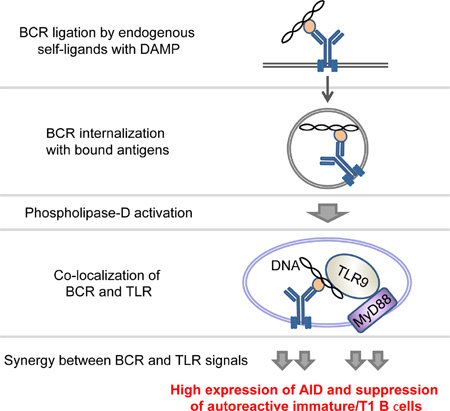
Summary
Activation-induced cytidine deaminase (AID) is required to purge autoreactive immature and transitional-1 (immature/T1) B cells at the first tolerance checkpoint but how AID selectively removes self-reactive B cells is unclear. We now show that B cell antigen receptor (BCR) and endosomal Toll-like receptor (TLR) signals synergize to elicit high levels of AID expression in immature/T1 B cells. This synergy is restricted to ligands for endocytic TLR and requires phospholipase-D activation, endosomal acidification, and MyD88. The first checkpoint is significantly impaired in AID- or MyD88-deficient mice and in mice doubly heterozygous for AID and MyD88, suggesting interaction of these factors in central B cell tolerance. Moreover, administration of chloroquine, an inhibitor of endosomal acidification, results in a failure to remove autoreactive immature/T1 B cells in mice. We propose that a BCR/TLR pathway coordinately establishes central tolerance by hyper-activating AID in immature/T1 B cells that bind ligands for endosomal TLRs.
Keywords: B cell tolerance, activation-induced cytidine deaminase, myeloid primary response gene (88), B cell receptor signaling, Toll-like receptor signaling, intracellular acidification
One-sentence summary
BCR and MyD88 signals synergize to increase AID expression greatly in autoreactive immature and transitional B cells and to mediate their loss by apoptosis.
Graphical Abstract
Introduction
Self-tolerance removes or inactivates autoreactive B cells in the bone marrow (first checkpoint) and then in peripheral tissues (second checkpoint) (Wardemann et al., 2003). The first checkpoint comprises at least three distinct mechanisms: apoptotic deletion, anergy, and receptor editing. Depending on the particulars of mouse line, one tolerance mechanism often dominates over the others (Erikson et al., 1991; Goodnow et al., 1988; Hartley et al., 1991; Tiegs et al., 1993). The mechanisms of these biases are not clear, but it is widely thought that the extent of BCR engagement provides qualitatively distinct signals that determine the tolerization pathway (Hartley et al., 1991).
We and others have shown that expression of activation-induced cytidine deaminase (AID) in immature/transitional-1 (T1) B cells is required for the first tolerance checkpoint (Cantaert et al., 2015; Kuraoka et al., 2011; Meyers et al., 2011; Umiker et al., 2014). In the absence of AID, autoreactive immature/T1 B cells are inefficiently purged and exhibit increased resistance to receptor-induced apoptosis (Kuraoka et al., 2011).
Overexpression of transgenic AID in both developing and mature B cells but not in mature B cells reduces the level of autoreactive serum IgM in 564Igi.Aicda−/− mice (Umiker et al., 2014). These results are consistent with a significant role for AID in the tolerization of developing B cells, but peak levels of AID expression by immature/T1 B cells reach only about 3% of that present in germinal center (GC) B cells (Kuraoka et al., 2011). How central B cell tolerance is effected by AID expression in immature/T1 B cells is not understood.
Given that AID expression by immature/T1 B cells is reduced in mice deficient for Bruton’s tyrosine kinase, myeloid differentiation primary response gene 88 (Myd88), or Unc93b1 (Han et al., 2007), we hypothesized that BCR- and endosomal TLR signals might intersect to regulate AID expression and tolerance in autoreactive immature/T1 B cells (Chaturvedi et al., 2008; Leadbetter et al., 2002). Indeed, the first tolerance checkpoint is impaired in humans deficient for components of endocytic TLR signaling (Isnardi et al., 2008). We investigated, therefore, whether signals by endosomal TLR and autoreactive BCR interact to purge autoreactive B cells at the first tolerance checkpoint.
We found that BCR and TLR signals synergize to elevate rapidly AID expression in immature/T1 B cells to approach that of GC B cells. This rapid synergy requires phospholipase-D (PLD) activation, endosomal acidification, and MyD88, but is not triggered by ligands for cell surface TLRs. Repertoire analyses of single B cells revealed that immature/T1 B cells from MyD88-deficient mice showed increased autoreactivity. Finally, we show that inhibition of endosomal TLR activation by chloroquine relaxes central B cell tolerance in autoreactive 3H9 and 2F5 knock-in mice (Chen et al., 1995b; Verkoczy et al., 2011). Our findings suggest that the first tolerance checkpoint is specialized for B cells that bind damage associated molecular pattern (DAMP) ligands.
Results
BCR and endosomal TLR signals synergistically activate immature/T1 B cells and elicit high levels of AID expression
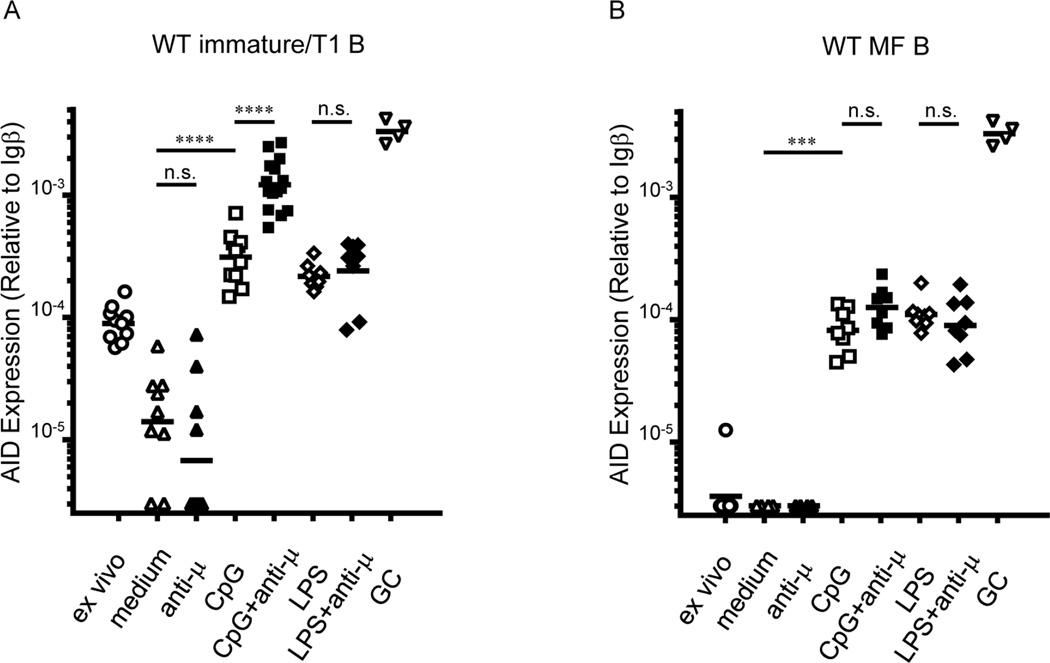
To identify signaling pathways that increase AID expression in autoreactive, immature/T1 B cells, we sorted bone marrow immature/T1 B cells from B6 mice, stimulated these cells in vitro with F(ab’)2 anti-IgM antibody (anti-µ), CpG, LPS, or combinations of these stimuli for 24 h, and quantified AID message levels (Figure 1A). Compared to cells in medium alone, addition of anti-µ did not significantly alter AID message in immature/T1 B cells; in contrast, CpG and LPS comparably elevated AID message to levels 2- to 3-fold above freshly isolated immature/T1 B cells. Co-activation of immature/T1 B cells by anti-µ+CpG synergistically increased AID mRNA expression, to levels >10-fold above ex vivo immature/T1 B cells and to levels near that of GC B cells. By contrast, no synergy was observed in immature/T1 B cells stimulated by anti-µ+LPS (Figure 1A) or in mature follicular (MF) B cells stimulated by anti-µ+CpG (Figure 1B). BCR and endocytic TLR signals rapidly and synergistically upregulate AID mRNA expression in immature/T1 B cells.
Figure 1. Anti-µ+CpG co-activation synergistically elevated AID mRNA expression in immature/T1 B cells.
Quantitative PCR analysis of AID mRNA levels in bone marrow immature/T1 B cells (A) and splenic MF B cells (B) cultured for 24 h in the presence of indicated stimuli (n = 4–15). AID expression in splenic GC B cells (∇; n = 4) from NP-CGG/alum immunized mice are shown in both panels. Each point represents an individual mouse and determination from at least 4 independent experiments. n.s., not significant (P > 0.05), ***P < 0.001, ****P < 0.0001, unpaired Student’s t -test. See also Figure S4.
PLD, endosomal acidification and MyD88 are required for high levels of AID expression in immature/T1 B cells
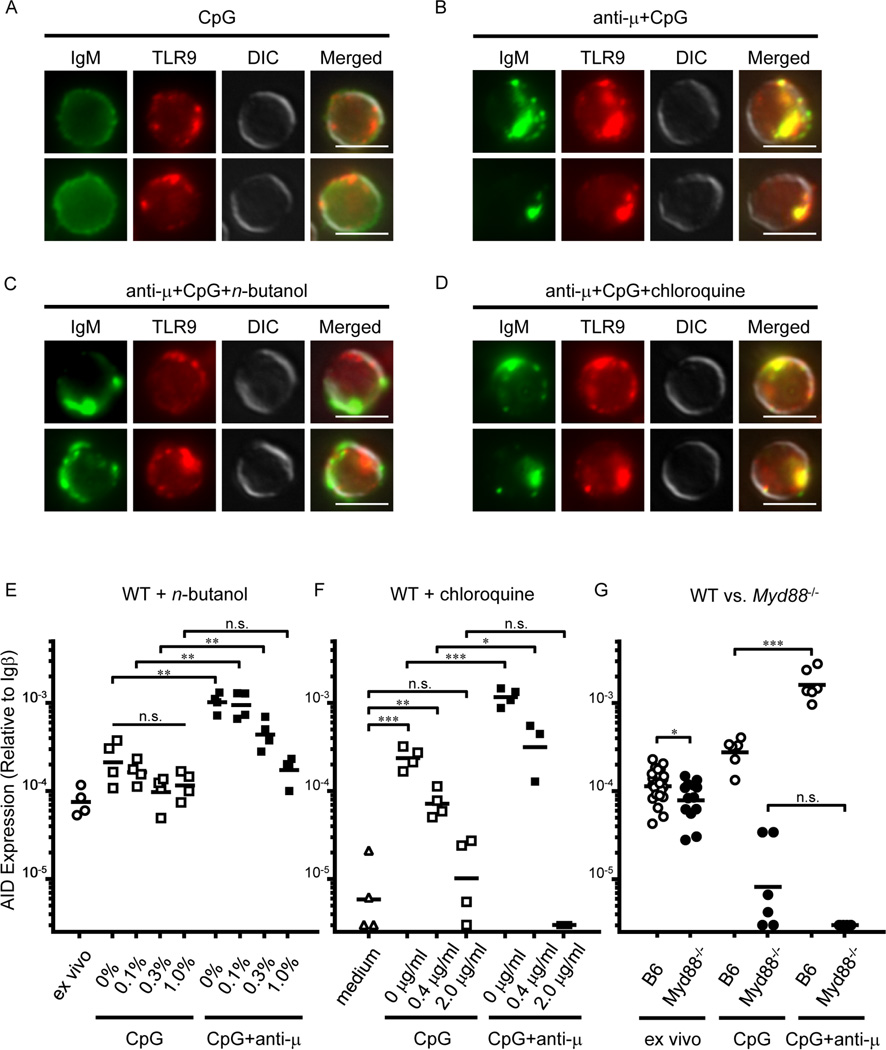
To explore the mechanism responsible for the synergy of BCR and TLR signals in AID mRNA expression, we used specific inhibitors that block specific intersections of the BCR and TLR signaling pathways (Chaturvedi et al., 2008). Given that internalized BCR and TLR9 co-localize in an autophagosome-like compartment where they synergize in downstream signaling via a PLD-dependent mechanism (Chaturvedi et al., 2008), we hypothesized that co-localization of BCR and TLR9 might direct synergistic AID up-regulation elicited by anti-µ+CpG (Figure 1A). Indeed, in immature/T1 B cells, anti-µ+CpG co-activation resulted in co-localization of BCR and TLR9 (Figures 2A and 2B). Further, addition of an inhibitor of PLD activity, normal (n)-butanol, to cultures of immature/T1 B cells (Figures 2C and 2E) inhibited this co-localization of BCR and TLR9 (Figure 2C). Whereas n-butanol did not significantly affect constitutive AID mRNA expression or its upregulation by CpG, the synergy of anti-µ+CpG on Aicda expression was inhibited in a dose-dependent manner and abrogated (to the levels of CpG alone) by 1.0% n-butanol (Figure 2E).
Figure 2. PLD activation, endosomal acidification and Myd88 are required for anti-µ+CpG-induced synergistic AID up-regulation in immature/T1 B cells.
(A–D) Representative images of immature/T1 B cells (IgM, TLR9, DIC and merged images) cultured with indicated stimuli. Top and bottom represents two independent cells. Scale bars: 5 µm. (E–G) AID mRNA levels in immature/T1 B cells stimulated with CpG or anti-µ+CpG in the presence of various concentrations of (E) n-butanol (v/v, n = 4) or (F) chloroquine (n = 3–4). (G) AID mRNA levels in immature/T1 B cells from B6 and B6.Myd88 −/− mice before (n = 13) and after culture (n = 4) in the presence of CpG or anti-µ+CpG. Each point represents an individual mouse and determination from at least 2 independent experiments. n.s.: not significant, P > 0.05; *P < 0.05, **P < 0.01, ***P < 0.001, unpaired Student’s t test. See also Figure S1.
To determine whether endosomal acidification, which is essential for the functional maturation of TLR3, −7, −8, and −9 (Blasius and Beutler, 2010), mediates anti-µ+CpG-induced synergistic AID expression, we added chloroquine, to cultures of immature/T1 B cells (Figures 2D and 2F). Chloroquine, an inhibitor of endosomal acidification, suppressed both CpG- and anti-µ+CpG-induced AID expression in immature/T1 B cells without blocking BCR and TLR9 co-localization (Figures 2D and 2F). Chloroquine did not affect LPS-induced AID mRNA up-regulation (Figure S1), indicating that inhibition of endosomal acidification, not general toxicity, blocked the synergistic increase in AID message elicited by anti-µ+CpG.
As MyD88 is required for all endosomal TLR signaling (Blasius and Beutler, 2010), we next compared constitutive and induced AID expression in Myd88+/+ and Myd88−/− immature/T1 B cells. AID expression in freshly isolated Myd88−/− immature/T1 B cells was modestly (~30%) lower (P <0.05) than in Myd88+/+ B6 controls (Han et al., 2007) (Figure 2G). As expected, Myd88−/− immature/T1 B cells cultured with CpG or anti-µ+CpG showed no increase in AID expression (Figure 2G). These findings indicate that PLD activation by BCR signaling, co-localization of BCR and TLR9, and concomitant MyD88 signaling are necessary for the synergistic expression of AID in immature/T1 B cells activated by anti-µ+CpG.
First tolerance checkpoint is relaxed in MyD88-deficient mice
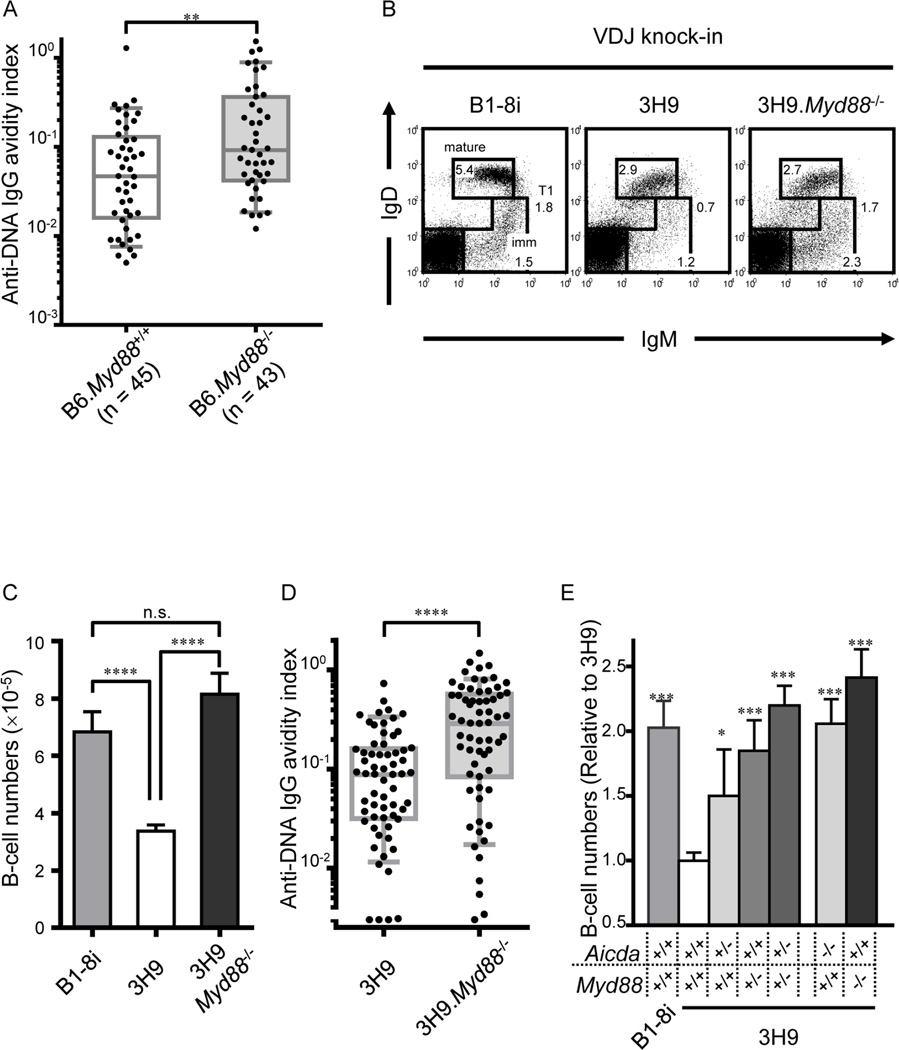
AID expression in immature/T1 B cells is required for central B cell tolerance (Kuraoka et al., 2011; Meyers et al., 2011). To determine whether MyD88 is crucial to this AID dependent tolerization, we compared the frequency and BCR avidity of immature/T1 B cells specific for double-strand DNA (dsDNA) in MyD88 sufficient and -deficient mice by sorting single immature/T1 B cells from bone marrow into Nojima cultures (Kuraoka et al., 2016). Whereas the frequencies of immature/T1 B cells reactive with dsDNA (dsDNA IgG+/total IgG+) were not significantly different between Myd88+/+ (45/194, 23%) and Myd88−/− (43/168, 26%) mice, the mean dsDNA avidity index (AvIn) (Kuraoka et al., 2016) for Myd88−/− immature/T1 B cells was ~2-fold (0.12 vs 0.05; P <0.001) higher than for Myd88+/+ controls (Figure 3A).
Figure 3. Autoreactive immature/T1 B cells are not efficiently purged in the absence of Myd88.
(A and D) Single immature/T1 B cells isolated from indicated mice were grown in Nojima cultures. Culture supernatants containing 1- to 3 µg/ml IgG were screened for DNA reactivity by ELISA. Anti-DNA IgG AvIn of individual cultures of immature/T1 B cells (B6, n = 45; B6.Myd88 −/−, n = 43; 3H9, n = 63; 3H9, Myd88 −/−, n = 64) are shown. Each point represents an individual immature/T1 B cell culture and determination from at least 2 independent experiments. Boxes extend from the 25th to 75th percentiles and lines in the box represent median. Error bars represent 10th-90th percentile. **P < 0.01, ****P < 0.0001, Mann-Whitney’s U test. (B) Representative flow diagrams for IgM/IgD expression by bone marrow cells of B1-8i, 3H9 and 3H9.Myd88 −/− mice. Numbers near boxes represent frequencies of immature B cells (imm; IgMloIgD−), T1 B cells (T1; IgMhiIgD+/−) and mature B cells (mature; IgMintIgDhi). (C) Absolute cell numbers of immature/T1 B cells in bone marrow of B1-8i (n = 9), 3H9 (n = 29) and 3H9.Myd88 −/− (n = 12) mice. n.s.: not significant, P > 0.05; ****P < 0.0001, unpaired Student’s t test; error bars, s.e.m. (E) Number of immature/T1 B cells (relative to 3H9) in bone marrow of B1-8i (n = 9), 3H9 (n = 29), 3H9.Aicda +/− (n = 5), 3H9.Myd88 +/− (n = 7), 3H9.Aicda +/− Myd88 +/− (n = 18), 3H9.Aicda −/− (n = 10), and 3H9.Myd88 −/− (n = 12) mice. Error bars, s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, Unpaired Student’s t test. See also Figure S2.
In addition, the developmental blockade of immature/T1 B cells that characterizes autoreactive 3H9 mice (Chen et al., 1995a; Kuraoka et al., 2011) was reversed in 3H9.Myd88−/− mice. Numbers of immature/T1 B cells in the bone marrow of 3H9 mice was significantly lower (48%; P <0.001) than in non-autoreactive B1-8i controls (Sonoda et al., 1997) (Figures 3B and 3C). In 3H9.Myd88−/− mice, immature/T1 B cell numbers were significantly higher (P <0.001), reaching levels comparable to those in B1-8i controls (Figures 3B and 3C). Moreover, mean dsDNA AvIn were 2.7-fold higher (P <0.001) in 3H9.Myd88−/− mice than in 3H9.Myd88+/+ mice (Figure 3D). We conclude that Myd88 is crucial for purging autoreactive immature/T1 B cells at the first tolerance checkpoint and that this tolerization is guided by BCR avidity.
Aicda and Myd88 coordinately mediate central B cell tolerance
Analysis of 3H9 mice heterozygous for Aicdanull or Myd88null alleles (3H9.Aicda+/− or 3H9.Myd88+/−) revealed gene dosage effects on the loss of autoreactive immature/T1 B cells (Figure 3E). Compared to Aicda+/+ 3H9 mice, the number of immature/T1 B cells increased by 55% (P <0.05) and 112% (P <0.001) in 3H9.Aicda+/− and 3H9.Aicda−/− mice, respectively. Similarly, immature/T1 B cell numbers increased by 91% (P <0.001) in 3H9.Myd88+/− and by 148% (P <0.001) in 3H9.Myd88−/− mice. Significantly, the number of immature/T1 B cells in 3H9.Aicda+/−Myd88+/− doubly heterozygous mice was no different (P ≥0.43) from that in 3H9.Aicda−/− or 3H9.Myd88−/− mice. Aicda and Myd88 cooperate in a common genetic pathway to regulate the first checkpoint of B cell tolerance.
Chloroquine relaxes central B cell tolerance
To assess whether inhibition of endosomal acidification could reduce central B cell tolerance in vivo, we treated autoreactive 3H9 and 2F5 (Verkoczy et al., 2011) mice with chloroquine or PBS for 7–8 days (Figure S3) and subsequently enumerated immature/T1 B cells in the bone marrow.
Chloroquine indiscriminately diminished pre-B cell numbers in 3H9, 2F5 and B1-8i mice (data not shown). To identify, therefore, any sparing of autoreactive cells by the chloroquine treatment, we determined the ratio of immature/T1 B cells to their immediate precursors, small pre-B cells. As there is no proliferation between these developmental compartments, increased ratios of immature/T1 to small pre-B cells would reflect improved survival and/or retention of immature/T1 B cells across the first tolerance checkpoint.
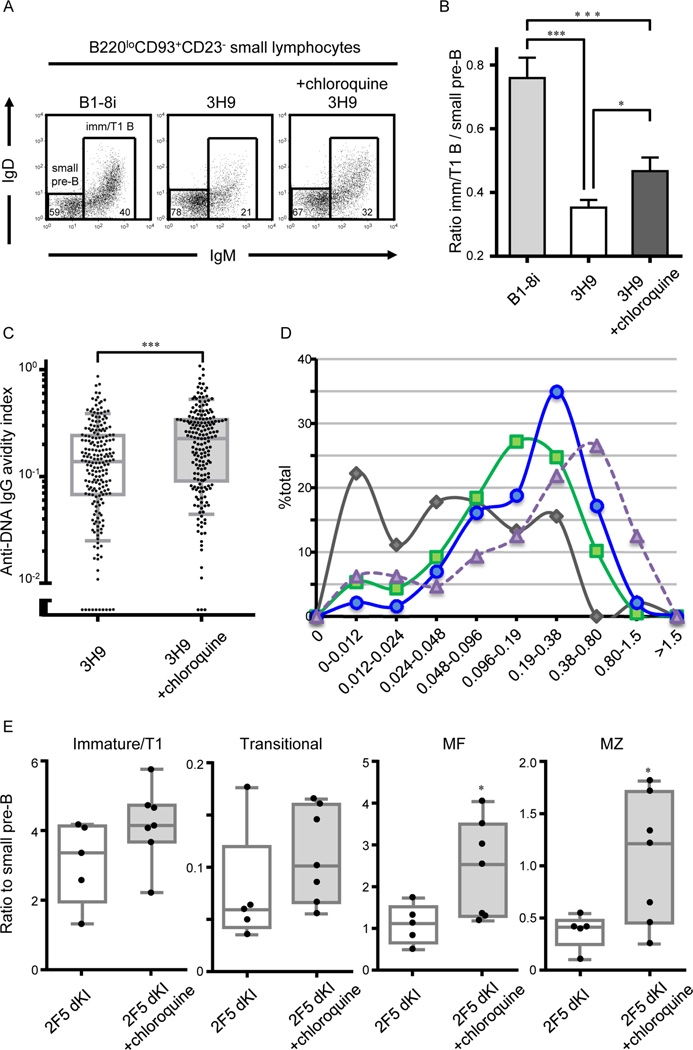
Chloroquine significantly (P <0.05) increased the ratio of immature/T1 to small pre-B cells in 3H9 mice (Figures 4A and 4B). The increase was associated with the accumulation of immature/T1 B cells that avidly bound DNA (Figures 4C and 4D). Mean dsDNA AvIn values for clonal IgGs from chloroquine-treated 3H9 mice was significantly higher (P <0.001) than from control 3H9 mice (Figure 4C). Indeed, chloroquine shifted the distribution of dsDNA AvIn values nearly to that of 3H9.Myd88−/− mice (Figure 4D) by preferentially increasing the frequencies of immature/T1 B cells with higher avidity BCR (Figure S3). Thus, chloroquine treatment relaxes central B cell tolerance in 3H9 knock-in mice and allows the primary B cell repertoire to shift towards increasingly avid autoreactivity.
Figure 4. Endosomal acidification is required for central B cell tolerance.
Representative flow plots for IgM/IgD expression by B220loCD93+CD43−CD23− small bone marrow lymphocytes (A), and Ratio of immature/T1 B cells to small pre-B cells (B) in B1–8 (n = 9), and PBS-treated (n = 7) or chloroquine-treated (n = 10) 3H9 mice from 5 independent experiments. (C and D) Single immature/T1 B cells isolated from PBS- or chloroquine-treated 3H9 mice were grown in Nojima cultures (see legend for Figure 3A and 3D). (C) Anti-DNA IgG AvIn of individual immature/T1 B cell cultures [n = 206 and 186 for PBS- (n = 5) and chloroquine-treated (n = 5) 3H9 mice, respectively]. Boxes extend from the 25th to 75th percentiles and lines in the box represent median. Error bars represent 10th-90th percentile. (D) Anti-DNA IgG AvIn of immature/T1 B cell cultures of B6 (Figure 2A, gray), 3H9 (Figure 4C; PBS-treated, green; chloroquine-treated, blue), and 3H9.Myd88 −/− mice (Figure 3D; purple) were compartmentalized by binning into 2-fold intervals. (E) Ratio of indicated B cell compartments to small pre-B cells were compared between control- (n = 5) and chloroquine-tread (n = 7) 2F5 dKI mice. Boxes extend from the 25th to 75th percentiles and lines in the box represent median. Bars represent the range (minimum/maximum) of values. Each symbol represents individual mice from 2 independent experiments. *P < 0.05, ***P < 0.001, Mann-Whitney’s U test. See also Figure S3.
To ascertain whether endosomal acidification plays any role in tolerizing immature/T1 B cells that recognize self-antigens other than dsDNA, we also treated 2F5 double knock-in (dKI) mice (Verkoczy et al., 2011) with chloroquine. The 2F5 antibody binds a neutralizing epitope in the membrane proximal external region (MPER) of HIV-1 envelope glycoprotein but cross-reacts with mammalian autoantigens including cardiolipin and kynureninase (Haynes et al., 2005; Yang et al., 2013); B cell development is blocked in 2F5 dKI mice at the first tolerance checkpoint (Verkoczy et al., 2011). Relative to small pre-B cells numbers, chloroquine treatment resulted in increased numbers of immature/T1 B cells in the bone marrow, and transitional (T1+T2), MF, and marginal zone (MZ) B cells in the spleen (Figure 4E). In vivo, endosomal acidification is necessary for purging immature/T1 B cells bearing poly- or autoreactive BCR that do not bind dsDNA.
Discussion
The first tolerance checkpoint in mice (Kuraoka et al., 2011) and humans (Meyers et al., 2011) requires AID and is correlated with early Aicda expression that peaks in the immature/T1 B cells (Kuraoka et al., 2009; Kuraoka et al., 2011). Whereas the coincidence of peak AID expression and impaired tolerance at the first checkpoint implied causality, the low levels of AID transcripts in immature/T1 B cells (Kuraoka et al., 2009; Kuraoka et al., 2011) were difficult to reconcile with the substantial efficiency for central B cell tolerance.
This apparent conundrum can now be resolved. Immature/T1 B cells expressing autoreactive and/or polyreactive BCR endocytose ligands for endosomal TLRs (Leadbetter et al., 2002), generating synergistic BCR+TLR signals that rapidly increase AID expression to nearly that of GC B cells (Figure 1). If factors that mitigate apoptotic responses to AID-induced DNA damage (Hasham et al., 2010; Liu et al., 2008) in GC B cells are reduced or absent in immature/T1 B cells, those immature/T1 B cells that bind DAMPs or are polyreactive would be purged at the first tolerance checkpoint by overwhelming genomic toxicity (Cantaert et al., 2015). Our own studies, in contrast to Duy et al. (Duy et al., 2010), show that immature/T1 B cells contain significantly lower quantities of BCL6 mRNA (Ueda et al., 2007) and immunoreactive protein (Figure S5) than do GC B cells. Given that BCL6 supports GC B cell survival and proliferation by modulating DNA damage-induced apoptotic responses (Phan and Dalla-Favera, 2004), BCL6lo immature/T1 B cells may be overwhelmed by the accumulated genotoxic stress induced by both AID and RAG (Cantaert et al., 2015). Indeed, while only a minor fraction of AID-expressing GC B cells in human tonsil express activated caspase-3, AID+CD34−CD19+ B cells in fetal liver and adult bone marrow frequently co-express activated caspase-3, indicating that in humans, many AID+ immature B cells are fated for apoptosis (Cantaert et al., 2015).
If AID drives the first checkpoint, shouldn’t the immature/T1 B cells of 3H9 mice or other autoimmune prone strains uniformly express elevated levels of AID? In vivo, we propose that poly-/autoreactive immature/T1 B cells that up-regulate AID expression initiate apoptosis and are rapidly and efficiently removed by local phagocytes in the same way that apoptotic T cells are rapidly cleared thymus (Surh and Sprent, 1994). In consequence, immature/T1 B cells that survive the first checkpoint should express low levels of AID; indeed, live immature/T1 B cells from 3H9 mice express lower levels of AID mRNA than immature/T1 B cells from B6 mice (Kuraoka et al., 2011). If tolerized immature/T1 B cells are lost in this way, we reasoned that analysis of whole bone marrow - without exclusion of dying or phagocytosed cells – would demonstrate the cryptic AIDhi immature/T1 B cells. Consequently, we compared AID mRNA expression in equal numbers of bone marrow cells isolated from B1-8i and 3H9 mice and found that AID mRNA expression was 4-fold higher in 3H9 mice compared to B1-8i control (Figure S2). This increase likely reflects even greater differences at the level of individual cells as the number of immature/T1 B cells in 3H9 bone marrow is about half that of B1-8i mice (Figures 3 and S2). This observation directly links our in vitro data and the fates of poly-/autoreactive immature/T1 B cells in vivo.
The AvIns of clonal IgGs from single-cell Nojima cultures effectively map the parameters of the first tolerance checkpoint for dsDNA reactivity. AvIns were determined relative to a monoclonal antibody standard, HYB331-01, and are highly correlated (R2 ≥0.90) to direct measures of BCR avidity (Kuraoka et al., 2016). Distributions of AvIn values for individual immature/T1 B cells that bind dsDNA from B6 (Figure 3) and 3H9 mice (Figures 3 and 4) show that central tolerance does not act by the exhaustive depletion of autoreactive B cells or by selectively purging cells that bind self-ligands with avidities above some threshold. Instead, the mechanisms of central B cell tolerance determine “avidity set-points” about which autoreactive BCRs cluster (Figures 3 and 4). We can visualize the shape of B cell tolerance for a native DAMP self-ligand. In principle, the capacity of Nojima cultures for identifying large number of antigen-specific B cells and the determination of BCR avidities enable us to define the effects of self-tolerance to any endogenous ligand expressed at physiological levels and sites on a naturally diverse BCR repertoire.
The in vitro efficacy of chloroquine in reducing AID message up-regulation by BCR+TLR synergy (Figure 2) suggested that blocking endosomal acidification in vivo might provide a workable strategy to regulate central B cell tolerance (Figure 4). In fact, treatment of 3H9 mice with chloroquine resulted in the generation of immature/T1 B cells exhibiting significantly higher BCR avidities (AvIn values) for dsDNA (Figure 4). The effect of chloroquine on the immature/T1 repertoire was intermediate to the loss of MyD88 (Figure 4D) but consistent with our hypothesis that the first checkpoint controls DAMP-specific autoreactivity by enforcing lower avidity set-points rather than by eliminating autoreactive cells. A consequence of this control method is that the maximal clonal BCR avidities for dsDNA do not much differ between B6 and B6.Myd88−/− or 3H9 and 3H9.Myd88−/− or even B6 and 3H9 mice (compare Figures 3A and 3D). The first checkpoint appears to act by reducing the probability of autoreactivity, not by eliminating it (Wardemann et al., 2003; Wardemann and Nussenzweig, 2007). That difference is surely fundamental in the initiation of systemic autoimmune disease.
Chloroquine treatment of 2F5 dKI mice resulted in substantial increases in the numbers of immature/T1 B cells in the bone marrow and transitional (T1+T2), MF, and MZ B cells in the spleen (Figure 4). The 2F5 antibody does not bind dsDNA or other known DAMPs (Haynes et al., 2005; Yang et al., 2013), indicating that endosomal acidification plays a crucial role not only in tolerizing B cells that bind DAMPs, but also those B cells that indirectly focus DAMPs into the endosomal compartments. Presumably, this indirect recruitment occurs through polyreactivity, e.g., the reactivity of the 2F5 antibody for lipids (Haynes et al., 2005), or by binding ligands physically linked to DAMPs. We note that the rescue of B cell development in 2F5 dKI mice suggests a pathway for generating HIV-1 broadly neutralizing antibody responses curtailed by central B cell tolerance (Holl et al., 2014; Kelsoe et al., 2014; Verkoczy et al., 2010; Verkoczy et al., 2011); it is plausible that transient treatment with chloroquine might benefit humoral responses to HIV-1 vaccines.
How might BCR polyreactivity or autoreactivity effect the synergistic BCR+TLR up-regulation of AID in immature/T1 B cells? We propose that cellular debris, apoptotic blebs, extruded nuclei, etc. containing ligands for endosomal TLRs are endocytosed by specific and polyreactive BCRs into endosomal compartments shared by TLRs. Coincident signaling by BCR and endosomal TLR synergize to up-regulate AID expression and the ensuing genomic damage leads to apoptotic cell death. In this model, the first checkpoint is driven by direct and indirect recognition of DAMPs. Wardemann and her colleagues have made exactly this observation; in studies of human B cell development, the majority of autoreactive BCRs lost at the first checkpoint are either specific for cell nucleus epitopes or are polyreactive (Wardemann et al., 2003; Wardemann and Nussenzweig, 2007).
If DAMPs are the self-ligands that drive the first tolerance checkpoint, how would this affect B-cell responses to sterile tissue damage or microbial infection? Removal of higher affinity BCRs that focus and transport TLR-ligands to endocytic TLRs (Figure 2), in effect, detunes B-cell activation to DAMPs. Whereas apoptotic debris, including autologous DNA- or RNA-complexes on dying cells and apoptotic blebs is normally ignored by the immune system, in excess, e.g., when cell debris is not efficiently cleared, it elicits B-cell activation and autoantibody production (Sharma et al., 2015). The physiological level for B-cell activation by DAMPs is set, we believe, by reducing the ability of B cells to capture and concentrate DAMPs via avid BCR binding. The first checkpoint does not so much reduce the frequency of DNA-binding immature/T1 B cells as it lowers mean BCR affinity for this TLR9 ligand (Figures 3 and 4). Presumably, this set-point is permissive for physiological levels of DAMPs. In contrast, the primary BCR repertoire for microbial pathogens would retain its full sensitivity and capacity to accumulate microbial DNA or RNA by BCR-mediated endocytosis.
Finally, our model provides a novel explanation for the different effects of soluble versus membrane-bound autoantigens on B cell development (Goodnow et al., 1988; Hartley et al., 1991). In a classic study, mice transgenic for hen egg lysozyme (HEL) BCR and either soluble or membrane-bound HEL exhibited very different modes of tolerization to the HEL neo-autoantigen. Despite expression of identical BCRs, B cell tolerance in mice expressing soluble HEL was predominated by anergy whereas membrane-bound HEL induced clonal deletion (Goodnow et al., 1988; Hartley et al., 1991). The different tolerance outcomes have long been explained as the consequence of quantitatively distinct BCR signals provided by mono- (soluble) or oligomeric (membrane) HEL (Hartley et al., 1991). While this may be the case, the HyHEL-10 antibody used to construct the HEL BCR transgenic mouse line exhibits a very high affinity (~1.5 × 109 M−1) for HEL (Padlan et al., 1989). This affinity is characteristic of BCRs that have undergone affinity maturation in GCs but unusual in BCRs expressed by immature B cells. If central tolerance could only induce apoptosis when immature B cells expressed a BCR with nanomolar affinity for a ubiquitous, membrane-bound autoantigen, how could physiological, deletional tolerance be efficient? In our model, BCR recognition of membrane-bound HEL would result in the internalization of the DAMP-containing cellular debris and the transmission of a qualitatively distinct activation signal than in those B cells that endocytosed soluble HEL alone.
Our findings provide a genetic pathway and mechanism for the first tolerance checkpoint that will be useful in understanding the fundamental nature of B cell tolerance, the origins of systemic autoimmune disease, and the develop novel immunization strategies to promote humoral responses normally limited by B cell tolerance.
Experimental procedures
Mice and immunizations
Female C57BL/6 (B6) mice were purchased from Jackson laboratory. Congenic AID-deficient mice (Aicda−/−) (Muramatsu et al., 2000), MyD88-deficient mice (Myd88−/−) (Adachi et al., 1998), B1-8i (Sonoda et al., 1997), 3H9 (Chen et al., 1995a), 3H9.Aicda−/− mice (Kuraoka et al., 2011), 3H9.Myd88−/− mice, and 2F5 (d)KI mice (Verkoczy et al., 2010; Verkoczy et al., 2011) were bred and maintained under specific pathogen-free conditions at the Duke University Animal Care Facility. Mice used in experiments were 7–12 weeks of age. All experiments involving animals were approved by the Duke University Institutional Animal Care and Use Committee.
In some experiments, 3H9 and 2F5 mice were injected i.p. with 100 µl PBS with or without 1.2 mg of chloroquine daily from day 0 to day 4, and then twice a day from day 4 to day 7 or day 8. After final injection, bone marrow cells and splenocytes were harvested from these mice for subsequent analyses.
Flow cytometry and definition of hematopoietic populations
Specific B-lineage developmental compartments were identified as described (Kuraoka et al., 2011) and are detailed in SI Supplemental Experimental Procedures.
Cell cultures
Sorted bone marrow immature/T1 B cells and splenic MF B cells (2.5 × 104 cells/well) were cultured in IMDM (Invitrogen) containing 10% HyClone FBS (Thermo scientific), 2-mercaptoethanol (5.5 × 10−5 M), penicillin (100 units/ml), streptomycin (100 µg/ml; all Invitrogen) and recombinant BAFF (250 ng/ml; R & D systems), in the presence or absence of F(ab’)2 fragment of anti-IgM (anti-µ) Ab (10 µg/ml; Jackson Immunoresearch), CpG (ODN1826, 0.5 and 5 µg/ml; InvivoGen), LPS (0127:B8, 0.5 and 5 µg/ml; Sigma) or combinations of these stimuli. In some cultures, we also added n-butanol (0.1, 0.3, and 1.0%; Sigma) or chloroquine (0.4 and 2.0 µg/ml; Sigma). Twenty-four hours after culture, B cells were harvested in TRIzol-LS reagent for AID mRNA quantification.
Single immature/T1 B cells and MF B cells were expanded in the presence of NB-21.2D9 feeder cells (Nojima cultures) (Kuraoka et al., 2016). These procedures are detailed in SI Supplemental Experimental Procedures.
Quantitative RT-PCR and quantification of AID expression
Expression of AID mRNA was determined by a quantitative RT-PCR (Kuraoka et al., 2009). These procedures are detailed in SI Supplemental Experimental Procedures.
Immunofluorescence
Sorted B220loCD93+CD43−CD23−FSClo bone marrow developing B cells were attached to poly-D-lysine coated 12-well slides (MP Biomedicals), treated with combinations of 0.5 µg/ml CpG, 10 µg/ml of AlexaFluor488-conjugated F(ab’)2 goat anti-mouse IgM (Southern Biotech), 1% n-butanol, and 2 µg/ml chloroquine at 37°C for 1.5 hours, and then fixed with 4% paraformaldehyde for 20 min at room temperature. After blocking with PBS containing 0.5% BSA and 5% goat serum, cells were incubated with AlexaFluor488-conjugated F(ab’)2 goat anti-mouse IgM for 1 hour at room temperature. After washing, cells were permeabilized with 0.5% Triton-X100/0.5% Saponin in PBS, blocking with PBS containing 0.5% BSA, 5% goat serum and 0.1% Triton-X100, and incubated with PE-labeled mouse anti-mouse TLR9 (J1547, BD Biosciences) at 4°C for overnight. Coverslips were mounted in Fluoromount-G (Southern Biotech). Representative images were photographed using an Axiovert 200M microscope with AxioCam MRm and AxioVision AC 4.5 software (Zeiss).
ELISA determinations
Concentrations of total IgG and anti-DNA IgG and the DNA AvIn in culture supernatants were determined by ELISA. These procedures are detailed in SI Supplemental Experimental Procedures
Statistical analyses of data
Statistical significance (P < 0.05) was determined by unpaired, two-tailed Student’s t test and Mann-Whitney’s U test.
Supplementary Material
Acknowledgments
We thank D. Liao, W. Zhang, C. Bowman, and E. Obremski for assistance, and X. Liang, M. Cunningham, S. Sanders, and X. Nie for mouse colonies. We thank Dr. M.J. Shlomchik for advice. This work was supported in part by the Bill and Melinda Gates Foundation to G.K. and by US NIH grants and contracts to G.K. (AI117892, HHSN272201000053C) and to B.F.H. (AI100645).
Abbreviations
- AID
activation-induced cytidine deaminase
- AvIn
avidity index
- BCR
B cell antigen receptor
- bNAb
broadly neutralizing antibody
- DAMP
damage associated molecular pattern
- dsDNA
double-stranded DNA
- HEL
hen egg lysozyme
- Ig
immunoglobulin
- MZ
marginal zone
- MyD88
myeloid differentiation primary response gene (88)
- PLD
phospholipase-D
- T1
transitional 1
- T2
transitional 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Information includes Supplemental Experimental Procedures, Supplemental References, and 4 figures.
Author contributions
M.K. and G.K. designed the experiments. M.K., P.S. and T.N. performed the experiments. M.K., B.F.H., and G.K. analyzed data and wrote the manuscript. L.V. and D.K. provided reagents. G.K. conceived and directed the study.
The authors have no financial conflicts of interest.
References
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1-and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32:305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Cantaert T, Schickel JN, Bannock JM, Ng YS, Massad C, Oe T, Wu R, Lavoie A, Walter JE, Notarangelo LD, et al. Activation-Induced Cytidine Deaminase Expression in Human B Cell Precursors Is Essential for Central B Cell Tolerance. Immunity. 2015;43:884–895. doi: 10.1016/j.immuni.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi A, Dorward D, Pierce SK. The B cell receptor governs the subcellular location of Toll-like receptor 9 leading to hyperresponses to DNA-containing antigens. Immunity. 2008;28:799–809. doi: 10.1016/j.immuni.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Nagy Z, Prak EL, Weigert M. Immunoglobulin heavy chain gene replacement: a mechanism of receptor editing. Immunity. 1995a;3:747–755. doi: 10.1016/1074-7613(95)90064-0. [DOI] [PubMed] [Google Scholar]
- Chen C, Nagy Z, Radic MZ, Hardy RR, Huszar D, Camper SA, Weigert M. The site and stage of anti-DNA B-cell deletion. Nature. 1995b;373:252–255. doi: 10.1038/373252a0. [DOI] [PubMed] [Google Scholar]
- Duy C, Yu JJ, Nahar R, Swaminathan S, Kweon SM, Polo JM, Valls E, Klemm L, Shojaee S, Cerchietti L, et al. BCL6 is critical for the development of a diverse primary B cell repertoire. J Exp Med. 2010;207:1209–1221. doi: 10.1084/jem.20091299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson J, Radic MZ, Camper SA, Hardy RR, Carmack C, Weigert M. Expression of anti-DNA immunoglobulin transgenes in non-autoimmune mice. Nature. 1991;349:331–334. doi: 10.1038/349331a0. [DOI] [PubMed] [Google Scholar]
- Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- Han JH, Akira S, Calame K, Beutler B, Selsing E, Imanishi-Kari T. Class switch recombination and somatic hypermutation in early mouse B cells are mediated by B cell and Toll-like receptors. Immunity. 2007;27:64–75. doi: 10.1016/j.immuni.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley SB, Crosbie J, Brink R, Kantor AB, Basten A, Goodnow CC. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991;353:765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- Hasham MG, Donghia NM, Coffey E, Maynard J, Snow KJ, Ames J, Wilpan RY, He Y, King BL, Mills KD. Widespread genomic breaks generated by activation-induced cytidine deaminase are prevented by homologous recombination. Nat Immunol. 2010;11:820–826. doi: 10.1038/ni.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- Hoffmann R, Seidl T, Neeb M, Rolink A, Melchers F. Changes in gene expression profiles in developing B cells of murine bone marrow. Genome Res. 2002;12:98–111. doi: 10.1101/gr.201501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holl TM, Yang G, Kuraoka M, Verkoczy L, Alam SM, Moody MA, Haynes BF, Kelsoe G. Enhanced Antibody Responses to an HIV-1 Membrane-Proximal External Region Antigen in Mice Reconstituted with Cultured Lymphocytes. J Immunol. 2014;192:3269–3279. doi: 10.4049/jimmunol.1302829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isnardi I, Ng YS, Srdanovic I, Motaghedi R, Rudchenko S, von Bernuth H, Zhang SY, Puel A, Jouanguy E, Picard C, et al. IRAK-4- and MyD88-dependent pathways are essential for the removal of developing autoreactive B cells in humans. Immunity. 2008;29:746–757. doi: 10.1016/j.immuni.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsoe G, Verkoczy L, Haynes BF. Immune System Regulation in the Induction of Broadly Neutralizing HIV-1 Antibodies. Vaccines (Basel) 2014;2:1–14. doi: 10.3390/vaccines2010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraoka M, Liao D, Yang K, Allgood SD, Levesque MC, Kelsoe G, Ueda Y. Activation-induced cytidine deaminase expression and activity in the absence of germinal centers: insights into hyper-IgM syndrome. J Immunol. 2009;183:3237–3248. doi: 10.4049/jimmunol.0901548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraoka M, Holl TM, Liao D, Womble M, Cain DW, Reynolds AE, Kelsoe G. Activation-induced cytidine deaminase mediates central tolerance in B cells. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1102571108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraoka M, Schmidt AG, Nojima T, Feng F, Watanabe A, Kitamura D, Harrison SC, Kepler TB, Kelsoe G. Complex Antigens Drive Permissive Clonal Selection in Germinal Centers. Immunity. 2016;44:542–552. doi: 10.1016/j.immuni.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- Liu M, Duke JL, Richter DJ, Vinuesa CG, Goodnow CC, Kleinstein SH, Schatz DG. Two levels of protection for the B cell genome during somatic hypermutation. Nature. 2008;451:841–845. doi: 10.1038/nature06547. [DOI] [PubMed] [Google Scholar]
- Meyers G, Ng YS, Bannock JM, Lavoie A, Walter JE, Notarangelo LD, Kilic SS, Aksu G, Debre M, Rieux-Laucat F, et al. Activation-induced cytidine deaminase (AID) is required for B-cell tolerance in humans. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1102600108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Padlan EA, Silverton EW, Sheriff S, Cohen GH, Smith-Gill SJ, Davies DR. Structure of an antibody-antigen complex: crystal structure of the HyHEL-10 Fab-lysozyme complex. Proc Natl Acad Sci U S A. 1989;86:5938–5942. doi: 10.1073/pnas.86.15.5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan RT, Dalla-Favera R. The BCL6 proto-oncogene suppresses p53 expression in germinal-centre B cells. Nature. 2004;432:635–639. doi: 10.1038/nature03147. [DOI] [PubMed] [Google Scholar]
- Sharma S, Fitzgerald KA, Cancro MP, Marshak-Rothstein A. Nucleic Acid-Sensing Receptors: Rheostats of Autoimmunity and Autoinflammation. J Immunol. 2015;195:3507–3512. doi: 10.4049/jimmunol.1500964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda E, Pewzner-Jung Y, Schwers S, Taki S, Jung S, Eilat D, Rajewsky K. B cell development under the condition of allelic inclusion. Immunity. 1997;6:225–233. doi: 10.1016/s1074-7613(00)80325-8. [DOI] [PubMed] [Google Scholar]
- Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372:100–103. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Liao D, Yang K, Patel A, Kelsoe G. T-independent activation-induced cytidine deaminase expression, class-switch recombination, and antibody production by immature/transitional 1 B cells. J Immunol. 2007;178:3593–3601. doi: 10.4049/jimmunol.178.6.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umiker BR, McDonald G, Larbi A, Medina CO, Hobeika E, Reth M, Imanishi-Kari T. Production of IgG autoantibody requires expression of activation-induced deaminase in early-developing B cells in a mouse model of SLE. Eur J Immunol. 2014;44:3093–3108. doi: 10.1002/eji.201344282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkoczy L, Diaz M, Holl TM, Ouyang YB, Bouton-Verville H, Alam SM, Liao HX, Kelsoe G, Haynes BF. Autoreactivity in an HIV-1 broadly reactive neutralizing antibody variable region heavy chain induces immunologic tolerance. Proc Natl Acad Sci U S A. 2010;107:181–186. doi: 10.1073/pnas.0912914107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkoczy L, Chen Y, Bouton-Verville H, Zhang J, Diaz M, Hutchinson J, Ouyang YB, Alam SM, Holl TM, Hwang KK, et al. Rescue of HIV-1 Broad Neutralizing Antibody-Expressing B Cells in 2F5 VH x VL Knockin Mice Reveals Multiple Tolerance Controls. J Immunol. 2011;187:3785–3797. doi: 10.4049/jimmunol.1101633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- Wardemann H, Nussenzweig MC. B-cell self-tolerance in humans. Adv Immunol. 2007;95:83–110. doi: 10.1016/S0065-2776(07)95003-8. [DOI] [PubMed] [Google Scholar]
- Yang G, Holl TM, Liu Y, Li Y, Lu X, Nicely NI, Kepler TB, Alam SM, Liao HX, Cain DW, et al. Identification of autoantigens recognized by the 2F5 and 4E10 broadly neutralizing HIV-1 antibodies. J Exp Med. 2013;210:241–256. doi: 10.1084/jem.20121977. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.