Abstract
Although Zika virus (ZIKV) was isolated approximately 70 years ago, few experimental studies had been published prior to 2016. The recent spread of ZIKV to countries in the Western Hemisphere is associated with reports of microcephaly, congenital malformations and Guillain-Barré syndrome. This has resulted in ZIKV being declared a public health emergency and has greatly accelerated the pace of ZIKV research and discovery. Within a short time period, useful mouse and non-human primate disease models have been established, and pre-clinical evaluation of therapeutics and vaccines has begun. Unexpectedly, ZIKV exhibits a broad tropism and persistence in body tissues and fluids, which contributes to the clinical manifestations and epidemiology that have been observed during the current epidemic. In this review, we highlight recent advances in our understanding of ZIKV pathogenesis, tissue tropism and the resulting pathology, and discuss areas for future investigation.
INTRODUCTION
Zika virus (ZIKV) is a mosquito-transmitted Flavivirus in the Flaviviridae family of positive-stranded RNA viruses, and is closely related to several other pathogens that cause disease globally including Dengue (DENV), yellow fever (YFV), West Nile (WNV), Japanese encephalitis (JEV), and tick-borne encephalitis (TBEV) viruses (Pierson and Diamond, 2013). Similar to DENV and YFV, ZIKV can circulate directly between Aedes mosquitos and humans, and thus is capable of epidemic transmission (Vasilakis and Weaver, 2017). Although ZIKV was first isolated in 1947 from a febrile rhesus macaque at the Zika Forest Research Station in Uganda (Dick et al., 1952), many of its distinguishing features have been discovered only recently after the virus spread globally and the epidemic force of infection increased. The ZIKV genome consists of an ~11 kb positive-stranded RNA molecule that encodes 3 structural and 7 non-structural proteins. The virion has a diameter of ~50 nm and contains a nucleocapsid that is surrounded by a lipid bilayer containing the structural proteins (prM/M and E), which are arranged with icosahedral symmetry on the surface (Kostyuchenko et al., 2016; Sirohi et al., 2016; Vasilakis and Weaver, 2017).
Clinical features of ZIKV infection
Historically, the clinical syndrome caused by ZIKV in humans was mild, consisting of a self-limiting flu-like febrile illness that resolved within days and occurred in an estimated ~20% of infected individuals (Duffy et al., 2009; Simpson, 1964). During the recent epidemic however, ZIKV infection has been associated with severe disease, including multi-organ failure (Swaminathan et al., 2016) and thrombocytopenia and thrombocytopenic purpura (Karimi et al., 2016). In comparison to the encephalitic flaviviruses (e.g., WNV and TBEV), ZIKV generally is less neuroinvasive in adults, and rarely causes meningitis and encephalitis (Carteaux et al., 2016). However, ZIKV preferentially infects and injures neural progenitor cells (Tang et al., 2016), which may explain its ability to impair development of the fetal brain and cause microcephaly and other neurodevelopmental injuries. ZIKV also can infect the eye and cause uveitis in adults, a potentially blinding inflammatory disease (Furtado et al., 2016; Miner et al., 2016b). ZIKV infection results in conjunctivitis in up to 15% of patients, perhaps due to direct infection of the eye (Duffy et al., 2009; Miner et al., 2016b; Sun et al., 2016).
The most alarming feature of ZIKV infection during the recent North American outbreak has been its ability to cause microcephaly, congenital malformations, and fetal demise. In a case series of symptomatic, ZIKV-infected pregnant women in Brazil, a remarkable 29% of fetuses exhibited some type of ultrasound abnormality (Brasil et al., 2016a; Brasil et al., 2016b). The clinical phenotype of congenital ZIKV infection was variable, and included cerebral calcifications, microcephaly, intrauterine growth restriction, and/or fetal demise. Retrospective assessment of the ZIKV epidemic in French Polynesia also found an increased risk of microcephaly associated with ZIKV infection, with 95 cases occurring per 10,000 women infected in the first trimester (Cauchemez et al., 2016). Computed tomography and magnetic resonance imaging of the brains of congenitally infected neonates in Brazil have demonstrated hypoplasia of the cerebellum and brainstem, ventriculomegaly, delayed myelination, enlarged cisterna magna, abnormalities of the corpus callosum, calcifications, and cortical malformations (de Fatima Vasco Aragao et al., 2016). Congenital ZIKV infection can also result in sensorineural hearing loss and blindness in humans with a variety of abnormalities of the eye including retinal mottling, lens subluxation, and optic neuritis (de Paula Freitas et al., 2016; Leal et al., 2016).
ZIKV appears to induce or mimic autoimmunity in a limited number of cases by unknown mechanisms (Oehler E et al., 2014; Zea-Vera and Parra, 2016). During the ZIKV epidemic in French Polynesia, case reports were described of ZIKV-associated Guillain-Barré syndrome, which is characterized by ascending paralysis and polyneuropathy (Oehler E et al., 2014). An association of ZIKV infection with Guillain-Barré syndrome also was reported in Brazil, Colombia, and other countries (do Rosário et al., 2016; dos Santos et al., 2016). Guillain-Barre syndrome may occur concurrently with acute ZIKV infection or in its immediate aftermath (do Rosário et al., 2016; Siu et al., 2016), suggesting that demyelination of peripheral nerves is due to direct infection and/or autoimmune-mediated targeting of neurons and glial cells. Electrophysiological studies demonstrated an acute axonal neuropathy and laboratory analysis revealed the presence of autoantibodies (e.g., anti-glycolipid and anti-GA1 antibodies), although the typical anti-ganglioside antibodies associated with Guillain-Barré syndrome were detected only rarely in these patients (Cao-Lormeau et al., 2016).
Human-to-human transmission
Unlike most other flaviviruses, a component of the spread of ZIKV may reflect its potential for human-to-human transmission. The first described case of sexual transmission involved an American, who contracted ZIKV infection while working in Senegal in 2008 and later transmitted it to his wife upon his return to the United States (Foy et al., 2011). Subsequently, multiple studies have reported suspected sexual transmission of ZIKV, and infectious virus can be detected in semen or sperm of infected males (Barzon et al., 2016; Mansuy et al., 2016). Female-to-male and male-to-female sexual transmissions of ZIKV also have been reported (Davidson et al., 2016; Musso et al., 2015). Modeling has suggested that sexual transmission contributes to approximately 3% of human cases (Gao et al., 2016), although this estimate has not yet been corroborated. In another human-to-human transmission case, ZIKV was acquired from a hospitalized patient via an unknown, non-sexual route through presumed contact with other infected body fluids (e.g., tears or saliva), which contain infectious virus as well as non-infectious viral nucleic acids (Dudley et al., 2016; Miner et al., 2016b; Sun et al., 2016; Swaminathan et al., 2016). Additionally, ZIKV has been transmitted by blood and platelet transfusion (Barjas-Castro et al., 2016; Motta et al., 2016). Although more study is warranted, the unique ability of ZIKV, relative to other flaviviruses, to spread between humans through non-insect vector routes may reflect one of several factors including its possible enhanced stability in body fluids or its broad cell and tissue tropism (Figure 1). A cryo-electron microscopy structure of ZIKV virion revealed a more compact structure than other flaviviruses, which may explain the greater stability of ZIKV at 40°C compared to DENV (Kostyuchenko et al., 2016). However, the greater physical stability of ZIKV was questioned in more detailed comparison studies with DENV and WNV (Goo et al., 2016). Further experiments are required to define how the relative stability of ZIKV in various body fluids affects its potential transmission.
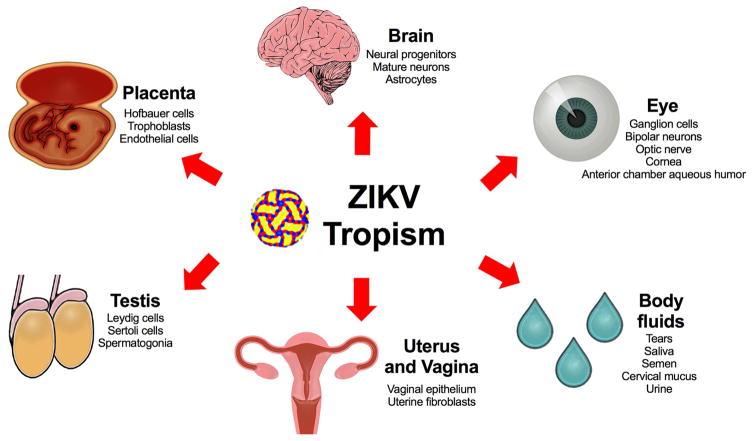
Figure 1. ZIKV tissue and cell tropism.
Human studies and animal models (mice and non-human primates) have detected ZIKV in cells of the placenta including Hofbauer cells (in vitro and in explanted human placental tissue), trophoblasts (mice, non-human primates, and humans), and endothelial cells (in vitro in explanted human placental tissue and in vivo in placenta of mice). Other ZIKV cellular targets include neuronal cell types including neural progenitor cells and mature neurons (mice, non-human primates, and humans), and astrocytes (in vitro human cell cultures). In addition, ZIKV infects ocular tissues including the cornea, neurosensory retina, and optic nerve (mice), as well as the aqueous humor of the anterior chamber (humans). ZIKV also targets cells of the reproductive tract including spermatogonia, Sertoli cells, and Leydig cells (in the testis of mice), sperm (samples from mice and humans), and the vaginal epithelium (mice) and uterine fibroblasts (in vitro infection of human samples). The extensive tropism results in ZIKV detection in multiple body fluids including conjunctival fluid or tears (mice and humans), saliva (non-human primates and humans), semen (mice, non-human primates, and humans), cervical mucus (humans), vaginal washings (mice and human) and urine (non-human primates and humans).
ZIKV pathogenesis in humans and animal models
In humans, ZIKV antagonizes the type I interferon (IFN) response, in part through its NS5 protein, which promotes proteasomal degradation of STAT2 (Grant et al., 2016; Kumar et al., 2016) a transcription factor that is activated downstream of signaling by the type I IFN receptor (Ifnar1). In comparison, ZIKV NS5 did not bind to or promote degradation of mouse Stat2 (Grant et al., 2016), which in part, may explain why immunocompetent mice are not natural hosts for the virus. To develop models of ZIKV pathogenesis in mice, some laboratories have inoculated immunocompetent animals intravenously with high titers of ZIKV (Cugola et al., 2016), which presumably bypasses innate immune responses in peripheral organs, whereas others have infected Ifnar1−/− or Ifnar1−/− Ifngr−/− double knockout (AG129) mice via subcutaneous inoculation (Aliota et al., 2016a; Lazear et al., 2016; Rossi et al., 2016).
The first ZIKV strain (MR 766) isolated, in 1947 in Uganda, pre-dated the development of tissue culture, and was passaged serially in the brains of mice ~100 times (Dick et al., 1952). Passaging of virus in a specific organ can result in adaptation of the virus to a specific tissue or cell type, attenuation of the virus in other tissues or cell types, or accumulation of mutations such that the passaged virus drifts genetically from the original clinical isolates. Thus, studies using MR 766 and other highly passaged ZIKV should be validated with other more recent clinical strains whenever possible. Multiple clinical ZIKV strains from Africa, Asia, and the Americas have been isolated and serve as important tools for studies of ZIKV pathogenesis. Many strains have been sequenced, and some developed into infectious clones for analysis of the genetic determinants of ZIKV pathogenesis (Schwarz et al., 2016; Shan et al., 2016; Tsetsarkin et al., 2016; Weger-Lucarelli et al., 2016).
Since adult wild-type immunocompetent mice are resistant to lethal ZIKV infection, one approach to study pathogenesis has been to infect neonatal mice, which generally are more vulnerable to infection. Infection of 1-week-old mice with a pathogenic ZIKV isolate from Senegal resulted in death of approximately 30% of neonatal mice (Lazear et al., 2016). A subsequent study inoculated wild-type neonatal mice with a Puerto Rican clinical ZIKV isolate and demonstrated severe neurological disease, weight loss, and death in a subset of animals (Manangeeswaran et al., 2016). Infected neonatal wild-type mice exhibited infiltration of T cells into the central nervous system, similar to that observed in other models of neuroinvasive flavivirus infection.
Mouse models of ZIKV pathogenesis using clinical isolates in immunocompromised adult mice have shown that the virus can accumulate in the blood, spleen, brain, spinal cord, kidney, and eye. Studies of ZIKV pathogenesis using Ifnar1−/− mice have recapitulated features of human disease, including placental infection and trans-placental transmission, neuroinvasive disease, and limb paralysis; the spinal cord disease in mice, however, likely does not represent autoimmune Guillain-Barre syndrome but rather is due to virus-mediated destruction of neurons. The testes of ZIKV-infected male Ifnar1−/− mice also support high levels of ZIKV infection. In another mouse model of ZIKV pathogenesis, 6-week-old mice lacking three interferon regulatory transcription factors (IRF3, IRF5, and IRF7) were more vulnerable to infection with a Cambodian ZIKV clinical isolate and developed CNS infection that resulted in apoptosis of neural progenitor cells (Li et al., 2016b).
Studies in pregnant female mice inoculated subcutaneously with a French Polynesian clinical isolate have demonstrated that ZIKV infects different trophoblasts (including glycogen trophoblasts and spongiotrophoblasts and to a lesser extent mononuclear trophoblasts and syncytiotrophoblasts) and fetal endothelial cells of the placenta and then crosses the placenta to infect the fetal head (Miner et al., 2016a). Congenital infection of immunocompromised Ifnar1−/− mice with ZIKV via a subcutaneous or intravaginal route resulted in similar abnormalities including intrauterine growth restriction and fetal demise (Miner et al., 2016a; Yockey et al., 2016). Pregnant wild-type C57BL/6 mice inoculated intravenously with a Brazilian clinical isolate did not have detectable virus in the fetus, most likely due to failure of the virus to replicate sufficiently in the placentas of these mice (Cugola et al., 2016). However, intravenous inoculation of SJL mice with unusually high doses (e.g., ~1010–1011 PFU) of the same ZIKV strain induced intrauterine growth restriction and microcephaly in these animals (Cugola et al., 2016). In addition to differing routes of infection (subcutaneous versus intravenous), a distinguishing factor between the Ifnar1−/− and the SJL models was the gestational age at which the pregnant dams were infected with ZIKV. In the initial description of the Ifnar1−/− congenital infection model, the pregnant dams were infected subcutaneously at about embryo day 7 (E7), when the placenta and fetus may be vulnerable to damage. In comparison, Cugola et al. performed intravenous inoculation with ZIKV later in gestation (E10.5 to E12.5), which corresponds to a time of significant brain development in mice. A subsequent study found that subcutaneous inoculation of pregnant Ifnar1−/− mice with a Brazilian clinical ZIKV isolate at E9.5 caused intrauterine growth restriction and reduced fetus size without demise (Miner et al., 2016b), reminiscent of the phenotype observed in the SJL model. Consistent with these different infection phenotypes in mice, a range of ZIKV-induced congenital disease has been reported in humans, including intrauterine growth restriction, microcephaly, and miscarriage (Brasil et al., 2016a; Brasil et al., 2016b; van der Eijk et al., 2016). Although more studies are required to define the effects of gestational age on ZIKV pathogenesis during pregnancy, epidemiological data suggests that infection during the first and second trimesters in humans correlates with the most severe fetal disease (Pacheco et al., 2016).
Many of the findings from mice have been recapitulated in non-human primates, which will allow further testing of candidate therapies and vaccines in models that more closely resemble human disease (Abbink et al., 2016; Aliota et al., 2016b; Li et al., 2016c; Osuna et al., 2016). In rhesus macaques, ZIKV RNA was detected in plasma one day after subcutaneous inoculation with a French Polynesian ZIKV strain, and ZIKV RNA was isolated from the brain, cerebrospinal fluid, urine, and saliva for at least 3 weeks (Dudley et al., 2016). Another study in rhesus and cynomolgus macaques found that ZIKV RNA persisted in saliva and seminal fluids for at least 3 weeks after clearance of the virus from the peripheral blood (Osuna et al., 2016). Infection of pigtail macaques during pregnancy with a Cambodian clinical ZIKV isolate resulted in the development of fetal brain lesions with white matter hypoplasia (Adams Waldorf et al., 2016).
ZIKV tissue and cell tropism
One potential mechanism for observed microcephaly is that ZIKV preferentially infects and triggers apoptosis in neural progenitor cells (Dang et al., 2016; Onorati et al., 2016; Tang et al., 2016), although it also infects mature neurons to a lesser degree (Tang et al., 2016). This capacity of ZIKV to infect and injure progenitor cells may contribute to its neurodevelopmental impact on the brain. Indeed, direct intraventricular inoculation of the brains developing mouse fetuses from wild-type mice with ZIKV resulted in cortical infection and thinning, inhibition of neuroprogenitor cell differentiation, and microcephaly (Li et al., 2016a). Although this intraventricular inoculation route is not physiological, this model confirmed the capacity for ZIKV to cause cell death and cerebral cortex disease in an animal model. As a corollary to observations from models of congenital infection, direct intracranial inoculation of ZIKV in postnatal wild-type mice caused depletion of proliferating cells in the stem cell compartment of the ventricular zone as well as disruption of corticospinal pyramidal neurons (Huang et al., 2016). Analogously, infection of human neurosphere organoid cultures in vitro with ZIKV impaired their growth and increased cell death (Garcez et al., 2016). Direct infection of neural progenitor cells may not be the only factor contributing to ZIKV-induced microcephaly. Infection of cranial neural crest cells may result in the production of inflammatory cytokines that act in a paracrine manner to deplete neural progenitor cell pools by promoting their apoptosis (Bayless et al., 2016; Dang et al., 2016).
In human studies, ZIKV RNA has been detected in both maternal and fetal tissues including cord blood, several placental cell types, amniotic fluid, and the developing fetal and neonatal human brain. ZIKV RNA also was detected in the brain and placenta of spontaneously aborted human fetuses in the first and second trimesters (Bhatnagar et al., 2017). After congenital infection of pigtail macaques with a Cambodian clinical ZIKV isolate, viral RNA was isolated from the maternal brain, eye, spleen and liver, with the highest amounts observed in the placenta (Adams Waldorf et al., 2016), suggesting that, as in mice and humans, ZIKV infects cells in the placenta of primates. In vitro infection studies of human placental cells have shown that ZIKV replicates in placental macrophages (Hofbauer cells), trophoblasts, and fetal endothelial cells and induces expression of antiviral genes (Quicke et al., 2016; Tabata et al., 2016). In one study, human trophoblasts isolated from a term placenta were relatively resistant to infection, in part due to inhibition mediated by type III IFN-λ (Bayer et al., 2016). Gestational age and genetic variation in host factors within the placenta (e.g., expression of virus attachment or immune restriction factors) may affect the relative vulnerability of placental cell types to ZIKV infection (Tabata et al., 2016).
Human and animal model studies have demonstrated that ZIKV infection can result in persistence of infectious virus and viral nucleic acid in several body fluids (e.g., semen, saliva, tears, and urine) and target organs including immune-privileged sites (e.g., eyes, brain, and testes) and the female genital tract. (a) Eyes. Studies in Ifnar1−/− mice found that ZIKV RNA is shed in tears (Miner et al., 2016b) and viral RNA can be detected in cornea, optic nerve, and neurosensory retina. Viral replication in eye-associated tissues in the mouse was corroborated with the discovery that ZIKV RNA and infectious virus can be recovered from human conjunctival fluid (Sun et al., 2016). (b) Female reproductive tract. One study found that ZIKV RNA is detectable in human cervical mucus at least 11 days after the onset of symptoms, after the virus had been cleared from blood and urine (Prisant et al., 2016). A subsequent case report described ZIKV RNA persistence in human vaginal secretions for more than 11 weeks (Murray et al., 2017). In vitro studies have shown that human uterine fibroblasts are susceptible to ZIKV infection (Chen et al., 2016), which suggests that uterine infection might contribute to impaired fetal development. (c) Male reproductive tract. Multiple studies have demonstrated infection of the male reproductive tract in mice (Chan et al., 2016; Govero et al., 2016; Ma et al., 2016). Infection of mice with a mouse-adapted African strain of ZIKV resulted in infection of spermatogonia and Sertoli cells, destruction of the testes architecture, and reduction of motile sperm count (Govero et al., 2016). Several weeks after ZIKV infection, male mice had lower levels of the sex hormones testosterone and inhibin B compared to age-matched uninfected controls, and this was associated with reduced fertility (Govero et al., 2016). A subsequent study also demonstrated damage to the testis of ZIKV-infected mice, and suggested this could result in male infertility (Ma et al., 2016). Though chronic, persistence of ZIKV RNA in sperm and semen has been described in humans even up to six months (Barzon et al., 2016; Mansuy et al., 2016), such severe testicular injury has not yet been reported and thus, warrants further longitudinal evaluation in infected men.
Host restriction factors and candidate ZIKV receptors
Similar to other flaviviruses, it is anticipated that ZIKV tropism is restricted by multiple host factors including type I and type III IFNs as well as IFN-stimulated genes (ISGs) that are activated during infection. As examples of ISGs that restrict ZIKV infection, IFITM1 and IFITM3, members of a family of transmembrane antiviral proteins that limit pathogenesis of other flaviviruses (Brass et al., 2009), also restrict ZIKV replication in vitro and limit its ability to cause cell death (Savidis et al., 2016b). The identity of other ISGs contributing to the restriction of ZIKV infection pathogenesis remains to be determined.
At present, there is no established, physiologically relevant ZIKV receptor, although in vitro studies have suggested candidates that require validation in vivo. One candidate entry factor is AXL, a member of the TAM receptor family of cell surface receptor tyrosine kinases that interact with the phosphatidylserine-binding proteins Gas6 and Protein S, which in turn bind to the surface of enveloped viruses including flaviviruses (Bhattacharyya et al., 2013). AXL has been suggested to function as an attachment or entry factor for ZIKV based on in vitro studies in 293T cells, keratinocytes, and endothelial cells (Hamel et al., 2015; Retallack et al., 2016; Savidis et al., 2016a), and also because of a correlation with its expression on target neural progenitor cells (Nowakowski et al., 2016). Others have suggested that AXL expression may correlate with infection of the testis (Ma et al., 2016). However, a comparison of ZIKV infection in AXL-deficient and wild-type mice treated with an anti-Ifnar1 blocking antibody showed equivalent levels of virus in the serum, spleen, brain, testis, or eyes (Govero et al., 2016; Miner et al., 2016b); thus, AXL was not required as an entry factor for these tissues in mice. One caveat of these findings is that AXL engagement may enhance viral infections through its signaling functions by virtue of its ability to negatively regulate the IFNAR pathway (Bhattacharyya et al., 2013); as the studies in mice were performed in the setting of blockade of Ifnar1, this signaling activity might have been missed. Alternatively, it remains possible that AXL determines tropism for a limited number of cell types in a given tissue, which does not impact the overall viral burden. Lastly, another potential explanation may be species-specific differences in requirements of attachment/entry factors by ZIKV such that other receptors have more dominant roles in mice. However, a recent study in human cells revealed that genetic ablation of AXL had no effect on ZIKV entry, replication, or cell death of iPSC-derived neural progenitor cells or cerebral organoids (Wells et al., 2016). Thus, although some studies suggest that AXL may function in cell culture as an important ZIKV entry factor, multiple in vivo and in vitro studies have demonstrated that AXL is not required for infection of many relevant tissues and cell types.
Another candidate receptor for ZIKV is TIM1, a glycoprotein that interacts with phosphatidylserine displayed on the viral membrane (Tabata et al., 2016). Indeed, TIM1 is expressed broadly on several ZIKV-infected cell types (e.g., Hofbauer macrophages, endothelial cells, and cytotrophoblasts) in the human placenta (Tabata et al., 2016). Whether TIM1 functions as a physiologically important attachment factor remains to be determined. Once confirmed as physiologically relevant, essential attachment or entry receptor(s) might serve as therapeutic targets to block ZIKV infection and pathogenesis.
Humoral immunity to ZIKV
ZIKV immune sera effectively neutralizes virus strains from both the Asian and African lineages (Dowd et al., 2016a), and prior infection with an Asian-lineage strain of ZIKV protects against heterologous infection with an African ZIKV strain (Aliota et al., 2016b), making it plausible that an effective vaccine can be developed against divergent ZIKV strains. Functional studies, epitope mapping, and cryo-EM structures of neutralizing anti-ZIKV antibodies bound to E protein have revealed multiple targets for vaccine design (Sapparapu et al., 2016; Wang et al., 2016; Zhao et al., 2016) (Figure 2A). Cross-reactive anti-DENV antibodies recognizing the envelope dimer epitope (EDE) (Dejnirattisai et al., 2015; Rouvinski et al., 2015) bind to and neutralize ZIKV infection (Barba-Spaeth et al., 2016) and protect AG129 mice from lethal ZIKV infection (Dai et al., 2016). Treatment with neutralizing human and mouse anti-ZIKV antibodies recognizing epitopes on the E protein dimer or in domain III can neutralize ZIKV infection and protect susceptible mice from lethal ZIKV challenge (Sapparapu et al., 2016; Zhao et al., 2016) (Figure 2A). Similarly, treatment of Ifnar1−/− mice with human monoclonal antibodies binding to distinct epitopes in E protein domains I, II, or III prevented weight loss and lethality during ZIKV infection (Wang et al., 2016). Neutralizing human antibodies also protected against congenital disease in a mouse model of in utero ZIKV infection (Sapparapu et al., 2016). Consistent with these findings, DNA plasmid adenovirus-vectored, and inactivated virus vaccines protected rhesus macaques from ZIKV infection (Abbink et al., 2016; Dowd et al., 2016b) (Figure 2B).
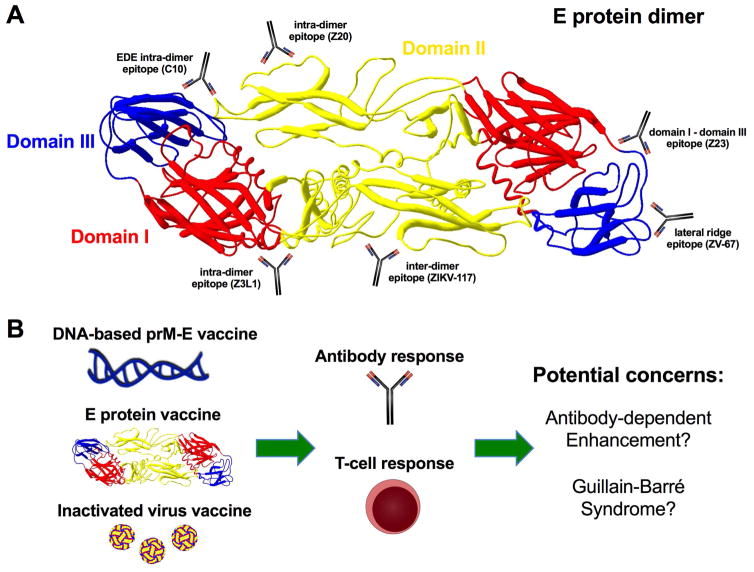
Figure 2. Targets for ZIKV vaccine design.
A. Highly neutralizing mouse and human anti-ZIKV monoclonal antibodies bind to distinct epitopes in the E protein including the lateral ridge of domain III (e.g., ZV-67) (Zhao et al., 2016), a domain I–III interface epitope (e.g., Z23) (Wang et al., 2016), an EDE intra-dimer epitope (e.g., C10) (Zhang et al., 2016), domain I–II and domain II intra-dimer epitopes (e.g., Z3L1 and Z20, respectively) (Wang et al., 2016), and a domain II inter-dimer epitope (e.g., ZIKV-117) (Sapparapu et al., 2016). These epitopes represent candidate regions for ZIKV vaccine design. B. Vaccine approaches that are being developed for ZIKV include DNA plasmids encoding prM-E or E genes (Abbink et al., 2016; Dowd et al., 2016b), soluble E based proteins or peptides (Alam et al., 2016), or inactivated viral particles (Abbink et al., 2016). Vaccines should generate protective B and T cell responses for greatest efficacy. Potential concerns of ZIKV vaccines that will need to be resolved prior to deployment include possible induction of Guillian-Barré syndrome or sensitizing individuals to more severe future DENV infection due to the generation of cross-reactive antibodies that promote ADE.
During secondary DENV infection with a heterologous DENV serotype in humans, pre-existing cross-reactive anti-DENV antibodies from the first infection can promote the phenomenon of antibody-dependent enhancement (ADE) of infection (Dowd and Pierson, 2011), which is believed to promote greater infection in myeloid cells and more severe DENV disease (Halstead, 1979). Because of the high degree of structural and sequence similarity between ZIKV and DENV, antibodies produced to these flaviviruses can cross-react with one another (Dejnirattisai et al., 2016; Priyamvada et al., 2016; Stettler et al., 2016). These cross-reactive antibodies have implications for ZIKV vaccine design, as they could result in exacerbated disease with subsequent DENV infection. Indeed, cross-reactive anti-DENV antibodies can enhance ZIKV infection in cell culture (Charles and Christofferson, 2016; Dejnirattisai et al., 2016; Priyamvada et al., 2016) and reciprocally, cross-reactive human anti-ZIKV antibodies can enhance DENV infection in cell culture and in mice (Stettler et al., 2016). However, at present, it remains unknown whether ADE of ZIKV infection by anti-DENV antibodies occurs in humans or other experimental animal models. Although ADE is readily demonstrable in cell culture with many flaviviruses, more detailed animal studies and epidemiological evidence are necessary to confirm whether clinically relevant antibody-dependent enhancement of ZIKV pathogenesis occurs in vivo.
Conclusions
Animal and human studies of ZIKV pathogenesis have revealed broad tissue and cell tropism for ZIKV as well as the capacity for the virus to cause severe end-organ disease in addition to placental and congenital infection. Established mouse and non-human primate models now serve as useful platforms to study ZIKV pathogenesis and to test candidate vaccines and therapies. Nevertheless, many unanswered questions remain with regard to optimal ZIKV antigens, the viral genetics of virulence, mechanisms of host restriction and immune evasion, the potential for ADE of ZIKV and DENV pathogenesis, as well as the long-term neurodevelopmental implications of congenital infection in humans. Given the exceptionally rapid pace of ZIKV research, we expect several of these questions to be answered soon.
Acknowledgments
NIH grants (R01 AI073755 and R01 AI104972) to M.S.D supported this work. J.J.M. was supported by a scientist development award from the Rheumatology Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbink P, Larocca RA, De La Barrera RA, Bricault CA, Moseley ET, Boyd M, Kirilova M, Li Z, Ng’ang’a D, Nanayakkara O, et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science. 2016;353:1129–1132. doi: 10.1126/science.aah6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams Waldorf KM, Stencel-Baerenwald JE, Kapur RP, Studholme C, Boldenow E, Vornhagen J, Baldessari A, Dighe MK, Thiel J, Merillat S, et al. Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat Med. 2016;22:1256–1259. doi: 10.1038/nm.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam A, Ali S, Ahamad S, Malik MZ, Ishrat R. From ZikV genome to vaccine: in silico approach for the epitope-based peptide vaccine against Zika virus envelope glycoprotein. Immunology. 2016;149:386–399. doi: 10.1111/imm.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliota MT, Caine EA, Walker EC, Larkin KE, Camacho E, Osorio JE. Characterization of Lethal Zika Virus Infection in AG129 Mice. PLoS Negl Trop Dis. 2016a;10:e0004682. doi: 10.1371/journal.pntd.0004682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliota MT, Dudley DM, Newman CM, Mohr EL, Gellerup DD, Breitbach ME, Buechler CR, Rasheed MN, Mohns MS, Weiler AM, et al. Heterologous Protection against Asian Zika Virus Challenge in Rhesus Macaques. PLOS Neglected Tropical Diseases. 2016b;10:e0005168. doi: 10.1371/journal.pntd.0005168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barba-Spaeth G, Dejnirattisai W, Rouvinski A, Vaney MC, Medits I, Sharma A, Simon-Lorière E, Sakuntabhai A, Cao-Lormeau VM, Haouz A, et al. Structural basis of potent Zika–dengue virus antibody cross-neutralization. Nature. 2016;536:48–53. doi: 10.1038/nature18938. [DOI] [PubMed] [Google Scholar]
- Barjas-Castro ML, Angerami RN, Cunha MS, Suzuki A, Nogueira JS, Rocco IM, Maeda AY, Vasami FGS, Katz G, Boin IFSF, et al. Probable transfusion-transmitted Zika virus in Brazil. Transfusion. 2016;56:1684–1688. doi: 10.1111/trf.13681. [DOI] [PubMed] [Google Scholar]
- Barzon L, Pacenti M, Franchin E, Lavezzo E, Trevisan M, Sgarabotto D, Palù G. Infection dynamics in a traveller with persistent shedding of Zika virus RNA in semen for six months after returning from Haiti to Italy. Euro Surveill. 2016;21:30316. doi: 10.2807/1560-7917.ES.2016.21.32.30316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer A, Lennemann Nicholas J, Ouyang Y, Bramley John C, Morosky S, Marques, Ernesto Torres De A, Cherry S, Sadovsky Y, Coyne Carolyn B. Type III Interferons Produced by Human Placental Trophoblasts Confer Protection against Zika Virus Infection. Cell Host & Microbe. 2016;16:30100–30107. doi: 10.1016/j.chom.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayless Nicholas L, Greenberg Rachel S, Swigut T, Wysocka J, Blish Catherine A. Zika Virus Infection Induces Cranial Neural Crest Cells to Produce Cytokines at Levels Detrimental for Neurogenesis. Cell Host & Microbe. 2016;20:423–428. doi: 10.1016/j.chom.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar J, Rabeneck DB, Martines RB, Reagan-Steiner S, Ermias Y, Estetter LBC, Suzuki T, Ritter J, Keating MK, Hale G, et al. Zika Virus RNA Replication and Persistence in Brain and Placental Tissue. Emerging Infectious Disease journal. 2017:23. doi: 10.3201/eid2303.161499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Zagórska A, Lew Erin D, Shrestha B, Rothlin Carla V, Naughton J, Diamond Michael S, Lemke G, Young John AT. Enveloped Viruses Disable Innate Immune Responses in Dendritic Cells by Direct Activation of TAM Receptors. Cell Host & Microbe. 2013;14:136–147. doi: 10.1016/j.chom.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil P, Pereira J, Jose P, Raja Gabaglia C, Damasceno L, Wakimoto M, Ribeiro Nogueira RM, Carvalho de Sequeira P, Machado Siqueira A, Abreu de Carvalho LM, Cotrim da Cunha D, et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro — Preliminary Report. New England Journal of Medicine. 2016a doi: 10.1056/NEJMoa1602412. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil P, Pereira JPJ, Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, Rabello RS, Valderramos SG, Halai UA, Salles TS, et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. New England Journal of Medicine. 2016b;375:2321–2334. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass AL, Huang IC, Benita Y, John SP, Krishnan MN, Feeley EM, Ryan BJ, Weyer JL, van der Weyden L, Fikrig E, et al. The IFITM Proteins Mediate Cellular Resistance to Influenza A H1N1 Virus, West Nile Virus, and Dengue Virus. Cell. 2009;139:1243–1254. doi: 10.1016/j.cell.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao-Lormeau VM, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. The Lancet. 2016;387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carteaux G, Maquart M, Bedet A, Contou D, Brugières P, Fourati S, Cleret de Langavant L, de Broucker T, Brun-Buisson C, Leparc-Goffart I, et al. Zika Virus Associated with Meningoencephalitis. New England Journal of Medicine. 2016;374:1595–1596. doi: 10.1056/NEJMc1602964. [DOI] [PubMed] [Google Scholar]
- Cauchemez S, Besnard M, Bompard P, Dub T, Guillemette-Artur P, Eyrolle-Guignot D, Salje H, Van Kerkhove MD, Abadie V, Garel C, et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. The Lancet. 2016;387:2125–2132. doi: 10.1016/S0140-6736(16)00651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JF-W, Zhang AJ, Chan CC-S, Yip CC-Y, Mak WW-N, Zhu H, Poon VK-M, Tee K-M, Zhu Z, Cai J-P, et al. Zika Virus Infection in Dexamethasone-immunosuppressed Mice Demonstrating Disseminated Infection with Multi-organ Involvement Including Orchitis Effectively Treated by Recombinant Type I Interferons. EBioMedicine. 2016 doi: 10.1016/j.ebiom.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles A, Christofferson R. Utility of a Dengue-Derived Monoclonal Antibody to Enhance Zika Infection In Vitro. PLOS Currents Outbreaks. 2016 doi: 10.1371/currents.outbreaks.4ab8bc87c945eb41cd8a49e127082620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Wang Z, Huang H, Weitz SH, Wang A, Qiu X, Baumeister MA, Uzgiris A. Infection of human uterine fibroblasts by Zika virus in vitro: implications for viral transmission in women. International Journal of Infectious Diseases. 2016;51:139–140. doi: 10.1016/j.ijid.2016.07.015. [DOI] [PubMed] [Google Scholar]
- Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JLM, Guimarães KP, Benazzato C, Almeida N, Pignatari GC, Romero S, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016 doi: 10.1038/nature18296. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L, Song J, Lu X, Deng YQ, Musyoki Abednego M, Cheng H, Zhang Y, Yuan Y, Song H, Haywood J, et al. Structures of the Zika Virus Envelope Protein and Its Complex with a Flavivirus Broadly Protective Antibody. Cell Host & Microbe. 2016;19:696–704. doi: 10.1016/j.chom.2016.04.013. [DOI] [PubMed] [Google Scholar]
- Dang J, Tiwari SK, Lichinchi G, Qin Y, Patil Veena S, Eroshkin Alexey M, Rana Tariq M. Zika Virus Depletes Neural Progenitors in Human Cerebral Organoids through Activation of the Innate Immune Receptor TLR3. Cell Stem Cell. 2016;19:258–265. doi: 10.1016/j.stem.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected Female-to-Male Sexual Transmission of Zika Virus — New York City. Morb Mortal Wkly Rep. 2016;65:716–717. doi: 10.15585/mmwr.mm6528e2. [DOI] [PubMed] [Google Scholar]
- de Fatima Vasco Aragao M, van der Linden V, Brainer-Lima AM, Coeli RR, Rocha MA, Sobral da Silva P, Durce Costa Gomes de Carvalho M, van der Linden A, Cesario de Holanda A, Valenca MM. Clinical features and neuroimaging (CT and MRI) findings in presumed Zika virus related congenital infection and microcephaly: retrospective case series study. BMJ. 2016:353. doi: 10.1136/bmj.i1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paula Freitas B, de Oliveira Dias J, Prazeres J, et al. Ocular findings in infants with microcephaly associated with presumed Zika virus congenital infection in Salvador, Brazil. JAMA Ophthalmology. 2016;134:529–535. doi: 10.1001/jamaophthalmol.2016.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejnirattisai W, Supasa P, Wongwiwat W, Rouvinski A, Barba-Spaeth G, Duangchinda T, Sakuntabhai A, Cao-Lormeau VM, Malasit P, Rey FA, et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol. 2016;17:1102–1108. doi: 10.1038/ni.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejnirattisai W, Wongwiwat W, Supasa S, Zhang X, Dai X, Rouvinski A, Jumnainsong A, Edwards C, Quyen NTH, Duangchinda T, et al. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat Immunol. 2015;16:170–177. doi: 10.1038/ni.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick G, Kitchen S, Haddow A. Zika virus. I. Isolations and serologically specifity. Trans R Soc Trop Med Hyg. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- do Rosário MS, de Jesus PAP, Vasilakis N, Farias DS, Novaes MAC, Rodrigues SG, Martins LC, Vasconcelos PFdC, Ko AI, Alcântara LCJ, et al. Guillain–Barré Syndrome After Zika Virus Infection in Brazil. The American Journal of Tropical Medicine and Hygiene. 2016;95:1157–1160. doi: 10.4269/ajtmh.16-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos T, Rodriguez A, Almiron M, Sanhueza A, Ramon P, de Oliveira WK, Coelho GE, Badaró R, Cortez J, Ospina M, et al. Zika Virus and the Guillain–Barré Syndrome — Case Series from Seven Countries. New England Journal of Medicine. 2016;375:1598–1601. doi: 10.1056/NEJMc1609015. [DOI] [PubMed] [Google Scholar]
- Dowd Kimberly A, DeMaso Christina R, Pelc Rebecca S, Speer Scott D, Smith Alexander RY, Goo L, Platt Derek J, Mascola John R, Graham Barney S, Mulligan Mark J, et al. Broadly Neutralizing Activity of Zika Virus-Immune Sera Identifies a Single Viral Serotype. Cell Reports. 2016a;16:1485–1491. doi: 10.1016/j.celrep.2016.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd KA, Ko S-Y, Morabito KM, Yang ES, Pelc RS, DeMaso CR, Castilho LR, Abbink P, Boyd M, Nityanandam R, et al. Rapid development of a DNA vaccine for Zika virus. Science. 2016b doi: 10.1126/science.aai9137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd KA, Pierson TC. Antibody-mediated neutralization of flaviviruses: A reductionist view. Virology. 2011;411:306–315. doi: 10.1016/j.virol.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley DM, Aliota MT, Mohr EL, Weiler AM, Lehrer-Brey G, Weisgrau KL, Mohns MS, Breitbach ME, Rasheed MN, Newman CM, et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nature Communications. 2016;7:12204. doi: 10.1038/ncomms12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, et al. Zika Virus Outbreak on Yap Island, Federated States of Micronesia. New England Journal of Medicine. 2009;360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- Foy B, Kobylinski K, Foy J, Blitvich B, Travassos da Rosa A, Haddow A. Probable Non–Vector-borne Transmission of Zika Virus, Colorado, USA. Emerging infectious diseases. 2011;17:880–882. doi: 10.3201/eid1705.101939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtado JM, Espósito DL, Klein TM, Teixeira-Pinto T, da Fonseca BA. Uveitis Associated with Zika Virus Infection. New England Journal of Medicine. 2016;375:394–396. doi: 10.1056/NEJMc1603618. [DOI] [PubMed] [Google Scholar]
- Gao D, Lou Y, He D, Porco TC, Kuang Y, Chowell G, Ruan S. Prevention and Control of Zika as a Mosquito-Borne and Sexually Transmitted Disease: A Mathematical Modeling Analysis. Scientific Reports. 2016;6:28070. doi: 10.1038/srep28070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcez PP, Loiola EC, Madeiro da Costa R, Higa LM, Trindade P, Delvecchio R, Nascimento JM, Brindeiro R, Tanuri A, Rehen SK. Zika virus impairs growth in human neurospheres and brain organoids. Science. 2016;352:816. doi: 10.1126/science.aaf6116. [DOI] [PubMed] [Google Scholar]
- Goo L, Dowd KA, Smith ARY, Pelc RS, DeMaso CR, Pierson TC. Zika Virus Is Not Uniquely Stable at Physiological Temperatures Compared to Other Flaviviruses. mBio. 2016:7. doi: 10.1128/mBio.01396-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govero J, Esakky P, Scheaffer SM, Fernandez E, Drury A, Platt DJ, Gorman MJ, Richner JM, Caine EA, Salazar V, et al. Zika virus infection damages the testes in mice. Nature. 2016 doi: 10.1038/nature20556. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A, Ponia Sanket S, Tripathi S, Balasubramaniam V, Miorin L, Sourisseau M, Schwarz Megan C, Sánchez-Seco Mari P, Evans Matthew J, Best Sonja M, et al. Zika Virus Targets Human STAT2 to Inhibit Type I Interferon Signaling. Cell Host & Microbe. 2016;19:882–890. doi: 10.1016/j.chom.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead SB. In Vivo Enhancement of Dengue Virus Infection in Rhesus Monkeys by Passively Transferred Antibody. Journal of Infectious Diseases. 1979;140:527–533. doi: 10.1093/infdis/140.4.527. [DOI] [PubMed] [Google Scholar]
- Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, Perera-Lecoin M, Surasombatpattana P, Talignani L, Thomas F, et al. Biology of Zika Virus Infection in Human Skin Cells. Journal of Virology. 2015;89:8880–8896. doi: 10.1128/JVI.00354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WC, Abraham R, Shim BS, Choe H, Page DT. Zika virus infection during the period of maximal brain growth causes microcephaly and corticospinal neuron apoptosis in wild type mice. Scientific Reports. 2016;6:34793. doi: 10.1038/srep34793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi O, Goorhuis A, Schinkel J, Codrington J, Vreden SGS, Vermaat JS, Stijnis C, Grobusch MP. Thrombocytopenia and subcutaneous bleedings in a patient with Zika virus infection. The Lancet. 2016;387:939–940. doi: 10.1016/S0140-6736(16)00502-X. [DOI] [PubMed] [Google Scholar]
- Kostyuchenko VA, Lim EXY, Zhang S, Fibriansah G, Ng TS, Ooi JSG, Shi J, Lok SM. Structure of the thermally stable Zika virus. Nature. 2016;533:425–428. doi: 10.1038/nature17994. [DOI] [PubMed] [Google Scholar]
- Kumar A, Hou S, Airo AM, Limonta D, Mancinelli V, Branton W, Power C, Hobman TC. Zika virus inhibits type I interferon production and downstream signaling. EMBO reports. 2016;17:1766–1775. doi: 10.15252/embr.201642627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, Diamond MS. A Mouse Model of Zika Virus Pathogenesis. Cell Host & Microbe. 2016;19:720–730. doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal MdC, Muniz LF, Caldas Neto SdS, van der Linden V, Ramos RCF. Sensorineural hearing loss in a case of congenital Zika virus. Brazilian Journal of Otorhinolaryngology. 2016 doi: 10.1016/j.bjorl.2016.06.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Xu D, Ye Q, Hong S, Jiang Y, Liu X, Zhang N, Shi L, Qin CF, Xu Z. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell. 2016a;19:120–126. doi: 10.1016/j.stem.2016.04.017. [DOI] [PubMed] [Google Scholar]
- Li H, Saucedo-Cuevas L, Regla-Nava Jose A, Chai G, Sheets N, Tang W, Terskikh Alexey V, Shresta S, Gleeson Joseph G. Zika Virus Infects Neural Progenitors in the Adult Mouse Brain and Alters Proliferation. Cell Stem Cell. 2016b;19:593–598. doi: 10.1016/j.stem.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XF, Dong HL, Huang XY, Qiu YF, Wang HJ, Deng YQ, Zhang NN, Ye Q, Zhao H, Liu ZY, et al. Characterization of a 2016 Clinical Isolate of Zika Virus in Non-human Primates. EBioMedicine. 2016c;12:170–177. doi: 10.1016/j.ebiom.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Li S, Ma S, Jia L, Zhang F, Zhang Y, Zhang J, Wong G, Zhang S, Lu X, et al. Zika Virus Causes Testis Damage and Leads to Male Infertility in Mice. Cell. 2016;167:1511–1524. e1510. doi: 10.1016/j.cell.2016.11.016. [DOI] [PubMed] [Google Scholar]
- Manangeeswaran M, Ireland DDC, Verthelyi D. Zika (PRVABC59) Infection Is Associated with T cell Infiltration and Neurodegeneration in CNS of Immunocompetent Neonatal C57Bl/6 Mice. PLOS Pathogens. 2016;12:e1006004. doi: 10.1371/journal.ppat.1006004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansuy JM, Dutertre M, Mengelle C, Fourcade C, Marchou B, Delobel P, Izopet J, Martin-Blondel G. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? The Lancet Infectious Diseases. 2016;16:405. doi: 10.1016/S1473-3099(16)00138-9. [DOI] [PubMed] [Google Scholar]
- Miner J, Cao B, Govero J, Smith Amber M, Fernandez E, Cabrera Omar H, Garber C, Noll M, Klein Robyn S, Noguchi Kevin K, et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell. 2016a;165:1081–1091. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JJ, Sene A, Richner JM, Smith AM, Santeford A, Ban N, Weger-Lucarelli J, Manzella F, Ruckert C, Govero J, et al. Zika Virus Infection in Mice Causes Panuveitis with Shedding of Virus in Tears. Cell Reports. 2016b;16:3208–3218. doi: 10.1016/j.celrep.2016.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta IJF, Spencer BR, Cordeiro da Silva SG, Arruda MB, Dobbin JA, Gonzaga YBM, Arcuri IP, Tavares RCBS, Atta EH, Fernandes RFM, et al. Evidence for Transmission of Zika Virus by Platelet Transfusion. New England Journal of Medicine. 2016;375:1101–1103. doi: 10.1056/NEJMc1607262. [DOI] [PubMed] [Google Scholar]
- Murray K, Gorchakov R, Carlson A, Berry R, Lai L, Natrajan M, Garcia M, Correa A, Patel S, Aagaard K, et al. Prolonged detection of Zika virus in vaginal secretions and whole blood. Emerg Infect Dis. 2017 doi: 10.3201/eid2301.161394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau V. Potential sexual transmission of Zika virus. Emerg Infect Dis. 2015;21:552. doi: 10.3201/eid2102.141363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakowski Tomasz J, Pollen Alex A, Di Lullo E, Sandoval-Espinosa C, Bershteyn M, Kriegstein Arnold R. Expression Analysis Highlights AXL as a Candidate Zika Virus Entry Receptor in Neural Stem Cells. Cell Stem Cell. 2016;18:591–596. doi: 10.1016/j.stem.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehler E, Watrin L, Larre P, Leparc-Goffart I, Lastère S, Valour F, Baudouin L, Mallet HP, Musso D, FG Zika virus infection complicated by Guillain-Barré syndrome – case report, French Polynesia. Euro Surveill. 2014;19:20720. doi: 10.2807/1560-7917.es2014.19.9.20720. [DOI] [PubMed] [Google Scholar]
- Onorati M, Li Z, Liu F, Sousa André MM, Nakagawa N, Li M, Dell’Anno Maria T, Gulden Forrest O, Pochareddy S, Tebbenkamp Andrew TN, et al. Zika Virus Disrupts Phospho-TBK1 Localization and Mitosis in Human Neuroepithelial Stem Cells and Radial Glia. Cell Reports. 2016;16:2576–2592. doi: 10.1016/j.celrep.2016.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuna CE, Lim SY, Deleage C, Griffin BD, Stein D, Schroeder LT, Omange R, Best K, Luo M, Hraber PT, et al. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat Med. 2016;22:1448–1455. doi: 10.1038/nm.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco O, Beltrán M, Nelson CA, Valencia D, Tolosa N, Farr SL, Padilla AV, Tong VT, Cuevas EL, Espinosa-Bode A, et al. Zika Virus Disease in Colombia — Preliminary Report. New England Journal of Medicine. 2016 doi: 10.1056/NEJMoa1604037. [DOI] [PubMed] [Google Scholar]
- Pierson T, Diamond M. Flaviviruses. Wolter; Kluwer: 2013. [Google Scholar]
- Prisant N, Bujan L, Benichou H, Hayot PH, Pavili L, Lurel S, Herrmann C, Janky E, Joguet G. Zika virus in the female genital tract. The Lancet Infectious Diseases. 2016;16:1000–1001. doi: 10.1016/S1473-3099(16)30193-1. [DOI] [PubMed] [Google Scholar]
- Priyamvada L, Quicke KM, Hudson WH, Onlamoon N, Sewatanon J, Edupuganti S, Pattanapanyasat K, Chokephaibulkit K, Mulligan MJ, Wilson PC, et al. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proceedings of the National Academy of Sciences. 2016;113:7852–7857. doi: 10.1073/pnas.1607931113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quicke Kendra M, Bowen James R, Johnson Erica L, McDonald Circe E, Ma H, O’Neal Justin T, Rajakumar A, Wrammert J, Rimawi Bassam H, Pulendran B, et al. Zika Virus Infects Human Placental Macrophages. Cell Host & Microbe. 2016;20:83–90. doi: 10.1016/j.chom.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retallack H, Di Lullo E, Arias C, Knopp KA, Laurie MT, Sandoval-Espinosa C, Mancia Leon WR, Krencik R, Ullian EM, Spatazza J, et al. Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proceedings of the National Academy of Sciences. 2016 doi: 10.1073/pnas.1618029113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi SL, Tesh RB, Azar SR, Muruato AE, Hanley KA, Auguste AJ, Langsjoen RM, Paessler S, Vasilakis N, Weaver SC. Characterization of a Novel Murine Model to Study Zika Virus. The American Journal of Tropical Medicine and Hygiene. 2016;94:1362–1369. doi: 10.4269/ajtmh.16-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvinski A, Guardado-Calvo P, Barba-Spaeth G, Duquerroy S, Vaney MC, Kikuti CM, Navarro Sanchez ME, Dejnirattisai W, Wongwiwat W, Haouz A, et al. Recognition determinants of broadly neutralizing human antibodies against dengue viruses. Nature. 2015;520:109–113. doi: 10.1038/nature14130. [DOI] [PubMed] [Google Scholar]
- Sapparapu G, Fernandez E, Kose N, Cao B, Fox JM, Bombardi RG, Zhao H, Nelson CA, Bryan AL, Barnes T, et al. Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature. 2016;540:443–447. doi: 10.1038/nature20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savidis G, McDougall William M, Meraner P, Perreira Jill M, Portmann Jocelyn M, Trincucci G, John Sinu P, Aker Aaron M, Renzette N, Robbins Douglas R, et al. Identification of Zika Virus and Dengue Virus Dependency Factors using Functional Genomics. Cell Reports. 2016a;16:232–246. doi: 10.1016/j.celrep.2016.06.028. [DOI] [PubMed] [Google Scholar]
- Savidis G, Perreira Jill M, Portmann Jocelyn M, Meraner P, Guo Z, Green S, Brass Abraham L. The IFITMs Inhibit Zika Virus Replication. Cell Reports. 2016b;15:2323–2330. doi: 10.1016/j.celrep.2016.05.074. [DOI] [PubMed] [Google Scholar]
- Schwarz MC, Sourisseau M, Espino MM, Gray ES, Chambers MT, Tortorella D, Evans MJ. Rescue of the 1947 Zika Virus Prototype Strain with a Cytomegalovirus Promoter-Driven cDNA Clone. mSphere. 2016;1 doi: 10.1128/mSphere.00246-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan C, Xie X, Muruato Antonio E, Rossi Shannan L, Roundy Christopher M, Azar Sasha R, Yang Y, Tesh Robert B, Bourne N, Barrett Alan D, et al. An Infectious cDNA Clone of Zika Virus to Study Viral Virulence, Mosquito Transmission, and Antiviral Inhibitors. Cell Host & Microbe. 2016;19:891–900. doi: 10.1016/j.chom.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DIH. Zika virus infection in man. Transactions of The Royal Society of Tropical Medicine and Hygiene. 1964;58:335–337. [PubMed] [Google Scholar]
- Sirohi D, Chen Z, Sun L, Klose T, Pierson TC, Rossmann MG, Kuhn RJ. The 3.8 Å resolution cryo-EM structure of Zika virus. Science. 2016;352:467–470. doi: 10.1126/science.aaf5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu R, Bukhari W, Todd A, Gunn W, Huang QS, Timmings P. Acute Zika infection with concurrent onset of Guillain-Barré Syndrome. Neurology. 2016;87:1623–1624. doi: 10.1212/WNL.0000000000003038. [DOI] [PubMed] [Google Scholar]
- Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, Vanzetta F, Minola A, Jaconi S, Mele F, et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science. 2016;353:823–826. doi: 10.1126/science.aaf8505. [DOI] [PubMed] [Google Scholar]
- Sun J, Wu D, Zhong H, et al. Presence of zika virus in conjunctival fluid. JAMA Ophthalmology. 2016 doi: 10.1001/jamaophthalmol.2016.3417. [DOI] [PubMed] [Google Scholar]
- Swaminathan S, Schlaberg R, Lewis J, Hanson KE, Couturier MR. Fatal Zika Virus Infection with Secondary Nonsexual Transmission. New England Journal of Medicine. 2016;375:1907–1909. doi: 10.1056/NEJMc1610613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata T, Petitt M, Puerta-Guardo H, Michlmayr D, Wang C, Fang-Hoover J, Harris E, Pereira L. Zika Virus Targets Different Primary Human Placental Cells, Suggesting Two Routes for Vertical Transmission. Cell Host & Microbe. 2016;20:155–166. doi: 10.1016/j.chom.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Hammack C, Ogden Sarah C, Wen Z, Qian X, Li Y, Yao B, Shin J, Zhang F, Lee Emily M, et al. Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell Stem Cell. 2016;18:587–590. doi: 10.1016/j.stem.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsetsarkin KA, Kenney H, Chen R, Liu G, Manukyan H, Whitehead SS, Laassri M, Chumakov K, Pletnev AG. A Full-Length Infectious cDNA Clone of Zika Virus from the 2015 Epidemic in Brazil as a Genetic Platform for Studies of Virus-Host Interactions and Vaccine Development. mBio. 2016;7 doi: 10.1128/mBio.01114-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Eijk AA, van Genderen PJ, Verdijk RM, Reusken CB, Mögling R, van Kampen JJA, Widagdo W, Aron GI, GeurtsvanKessel CH, Pas SD, et al. Miscarriage Associated with Zika Virus Infection. New England Journal of Medicine. 2016;375:1002–1004. doi: 10.1056/NEJMc1605898. [DOI] [PubMed] [Google Scholar]
- Vasilakis N, Weaver SC. Flavivirus transmission focusing on Zika. Current Opinion in Virology. 2017;22:30–35. doi: 10.1016/j.coviro.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Yang H, Liu X, Dai L, Ma T, Qi J, Wong G, Peng R, Liu S, Li J, et al. Molecular determinants of human neutralizing antibodies isolated from a patient infected with Zika virus. Science Translational Medicine. 2016;8:369ra179–369ra179. doi: 10.1126/scitranslmed.aai8336. [DOI] [PubMed] [Google Scholar]
- Weger-Lucarelli J, Duggal NK, Bullard-Feibelman K, Veselinovic M, Romo H, Nguyen C, Rückert C, Brault AC, Bowen RA, Stenglein M, et al. Development and Characterization of Recombinant Virus Generated from a New World Zika Virus Infectious Clone. Journal of Virology. 2016 doi: 10.1128/JVI.01765-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells Michael F, Salick Max R, Wiskow O, Ho Daniel J, Worringer Kathleen A, Ihry Robert J, Kommineni S, Bilican B, Klim Joseph R, Hill Ellen J, et al. Genetic Ablation of AXL Does Not Protect Human Neural Progenitor Cells and Cerebral Organoids from Zika Virus Infection. Cell Stem Cell. 2016;19:703–708. doi: 10.1016/j.stem.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Yockey Laura J, Varela L, Rakib T, Khoury-Hanold W, Fink Susan L, Stutz B, Szigeti-Buck K, Van den Pol A, Lindenbach Brett D, Horvath Tamas L, et al. Vaginal Exposure to Zika Virus during Pregnancy Leads to Fetal Brain Infection. Cell. 2016;166:1247–1256. e1244. doi: 10.1016/j.cell.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zea-Vera AF, Parra B. Zika virus (ZIKV) infection related with immune thrombocytopenic purpura (ITP) exacerbation and antinuclear antibody positivity. Lupus. 2016 doi: 10.1177/0961203316671816. [DOI] [PubMed] [Google Scholar]
- Zhang S, Kostyuchenko VA, Ng TS, Lim XN, Ooi JSG, Lambert S, Tan TY, Widman DG, Shi J, Baric RS, et al. Neutralization mechanism of a highly potent antibody against Zika virus. Nature Communications. 2016;7:13679. doi: 10.1038/ncomms13679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Fernandez E, Dowd Kimberly A, Speer Scott D, Platt Derek J, Gorman Matthew J, Govero J, Nelson Christopher A, Pierson Theodore C, Diamond Michael S, et al. Structural Basis of Zika Virus-Specific Antibody Protection. Cell. 2016;166:1016–1027. doi: 10.1016/j.cell.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]