Abstract
The structure of the caudal skeleton of extant teleost fishes has been interpreted in two different ways. In a diural interpretation, a caudal skeleton is composed of two centra articulated with one to six hypurals. Most subsequent authors have followed this interpretation. In contrast, a polyural interpretation considers the teleost fin to be derived from a fully metameristic ancestral bauplan originally composed of a one-to-one relationship between neural arches, centra (when present), and hypurals. Three different interpretations of the identity and homology of skeletal components of the caudal skeleton of the teleost fish Danio rerio have been proposed, two from a diural perspective and one from a polyural perspective. We examine each caudal skeletal component of Danio rerio from both a developmental and phylogenetic perspective. We propose that a polyural interpretation of structures is consistent with the current interpretation of the basal neopterygian caudal fin for this model organism rather than the older diural interpretation that does not take into account the metamerism observed in caudal structures during development. The polyural interpretation suggests several shared evolutionary innovations of major clades that would remain undiscovered under the older diural naming paradigm and makes the terminology of the parts of the caudal fin of Danio rerio strictly comparable to more basal fishes.
The development and morphology of the caudal skeleton of Danio rerio (Figs. 1A, 2A), a model teleost fish, has been interpreted in different ways by different authors. In this paper we offer a polyural interpretation of the phylogenetic homologies of elements of the caudal skeleton of Danio rerio differing from the diural interpretations of Bird and Mabee (2003) and Bensimon-Brito et al. (2010, 2012) but consistent with the polyural interpretation of Schultze and Arratia (2013). Diural interpretations consider the basic bauplan of the caudal fin to be composed of an anterior centrum (preural centrum 1 + ural centrum 1) supporting the parhypural and hypurals 1 and 2 and a posterior centrum supporting hypurals 3–5 or 3–6 in living species, with variations in hypural number due to fusion or loss. This interpretation dates to Nybelin (1963) and Monod (1968) and formed the basis of most, if not all, teleost caudal fin homology statements until challenged by Schultze and Arratia (1988), who offered a polyural interpretation. From the polyural perspective, the teleost caudal fin is considered an evolutionary derivation of a fully metameristic caudal fin in which each hypural is supported by a single ural centrum with its own neural arch. Thus, a fully expressed polyural caudal skeleton with six hypurals would have six ural centra and accompanying neural arches. The polyural hypothesis asserts that remnants of this bauplan exist in teleosts and that the homology of parts should be built around identification of these remnants if and when sufficient developmental information can be discovered to identify them.
Fig. 1.
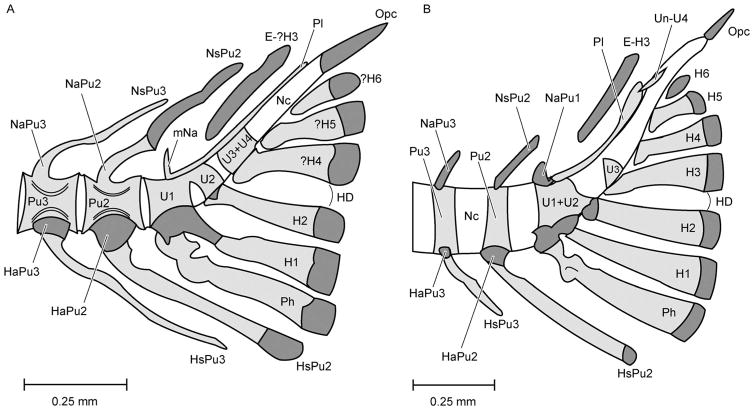
Caudal fin ossifications and terminology. Lateral view of (A) Danio rerio (28 mm SL) drawn from University of Kansas specimen KU 29144 and (B) Moxostoma hubbsi (29.1 mm SL) redrawn from Grünbaum et al. (2003). ANs, accessory neural spine; E-?H3, putative epural of hypural 3; E-H3, epural of hypural 3; H1–H6, hypurals 1–6; ?H4, ?H5, ?H6, putative hypurals 5–6; HaPu2–3, haemal arches of Pu2 and Pu3; HD, hypural diastema; HsPu2–3, haemal spines of Pu2 and Pu3; mNa, membranous neural arch; NaPu1–3, neural arches of urostyle, Pu2, and Pu3; NsPu2–3, neural spines of Pu2 and Pu3 (double spine on Pu2 in Moxostoma hubbsi); Pu2–3, preural centra 2 and 3; Ph, parhypural; Un-U4, uroneurals of ural neural arch 4; Us, urostyle (U1+U2+U3+U4+pleurostyle+membrane ural neural arch).
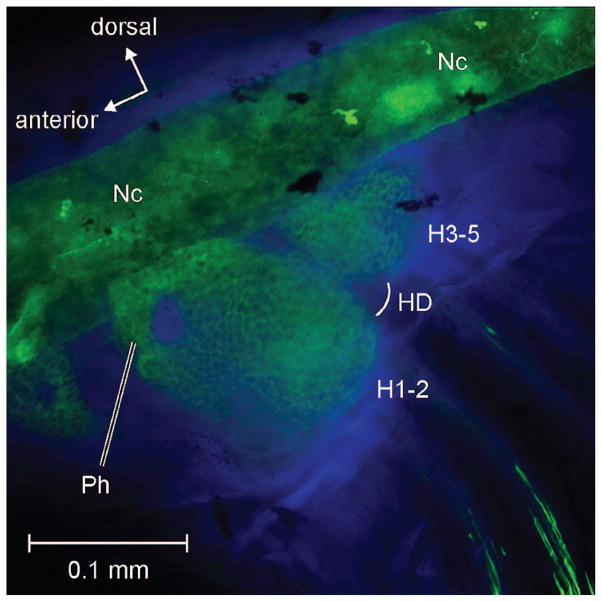
Fig. 2.
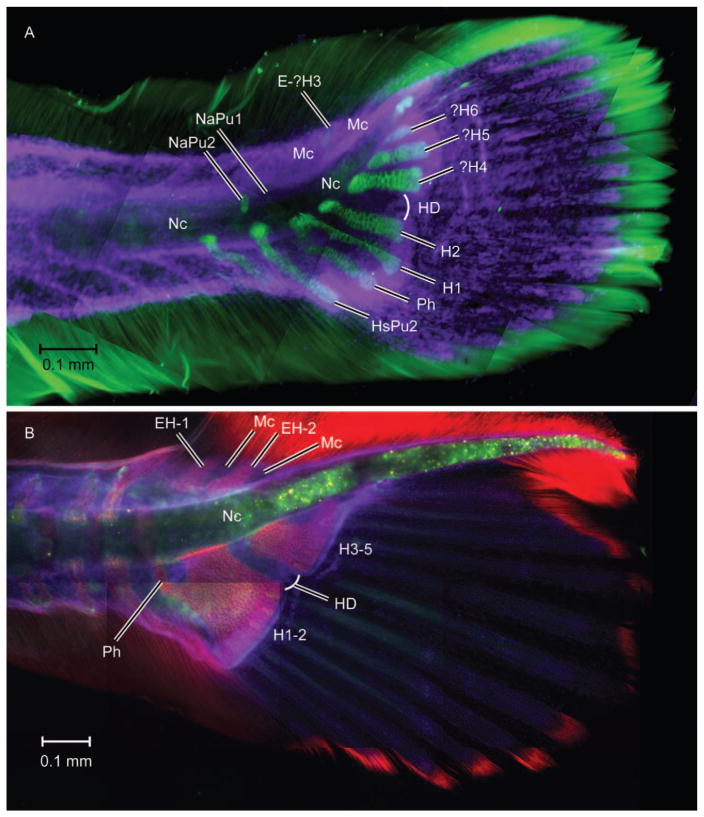
Immunostained caudal fins in early developmental stage. Lateral view of (A) Danio rerio (5.4 mm SL) and (B) Gasterosteus aculeatus (7.1 mm SL). Danio is stained with Collagen II (green) and DAPI (purple). Gasterosteus is stained with WGA (red) and DAPI (purple). EH-1, EH-2, epurals above hypurals 1 and 2; E-?H4, putative epural of H4; HD, hypural diastema; H1, H2, H4–6, hypurals 1, 2, 4, 5, and 6; H1–2, compound anterior hypural; H3–5, compound posterior hypural; Mc, mesenchyme field; NaPu1–2, neural arches of preural centra 1 and 2; Nc, notochord; Ph, parhypural. Scale bar is 0.1 mm.
Our purpose is not to challenge the descriptive adequacy of previous studies but to review these studies, provide some additional observations, and suggest that this alternative, polyural, approach of interpreting the caudal skeleton might result in new insights into the homology of caudal fin structures within a broader comparative framework of all teleosts in our continuing efforts to place model organisms within a phylogenetic context and bridge the gap between development and macroevolution. We proceed by examining each developing structure, beginning with the notochord and its associated centra and continuing first to the lower, hypaxial, region and then to the upper, epaxial, region. Figure 1 provides an overview of caudal structures in Danio rerio as we interpret them.
MATERIALS AND METHODS
Specimens examined
Examination of the caudal skeleton development for Danio rerio is based on a series of larvae ranging from 4 to 25 days post fertilization (3.2–5.6 mm notochord length [NL]). A series of Gasterosteus aculeatus was available and used for comparison. Examination of the caudal skeleton development for Gasterosteus aculeatus is based on a series of larvae ranging from 16 to 41 days post fertilization (5.9–8.9 mm standard length [SL]). Adult Gasterosteus aculeatus were collected from Rabbit Slough (61.53591, −149.25296), Matanuska-Susitna Borough, Alaska on 12 June 2011. For Gasterosteus, Hagen’s (1967, 1973) in vitro method was followed for a single mass cross of the collected adults within 24 hours of capture. The fertilized eggs were kept at 19°C until 69.5 hours post fertilization (hpf), when eggs were cooled on ice to 5°C and transported to Stony Brook University from the University of Alaska Anchorage. Upon arrival, eggs resumed incubation at 19°C until being moved to aquaria at 11 days. Aquaria maintained an average 18.9°C and 14:10 hour (light:dark) photoperiod. Live specimens for both species were anesthetized with Tricane (MS-222) and fixed for two hours in 4% para-formaldehyde in 4°C and stored in 100% methanol at −20°C.
Immunostaining and slide preparation
Immunostaining is effective in detecting transformation of cells into cartilage at early stages in development and frequently detects transforming cartilage cells earlier than alcian blue staining. It is used in developmental studies and was first proposed by Coons et al. (1941). Seventy-six specimens of Danio rerio and 39 specimens of Gasterosteus aculeatus were stained for Collagen type II (The Developmental Studies Hybridoma Bank at University of Iowa) with DyLight 488-conjugated AffiniPure Rabbit Anti-Mouse IgG (Excitation/emission maxima = 493 nm/518 nm; Sigma) and Alexa Fluor 594 Wheat germ agglutinin (WGA; Alexa Fluor 594: Excitation/emission maxima = 590 nm/617 nm) that specifically binds to N-acetyl-D-glucosamine and Sialic acid (Life Technologies) as detailed in Table 1. Because WGA binds to N-acetyl-D-glucosamine and Sialic acid, it selectively stains cartilage structures (van Boxtel et al., 2011). Immediately after or within 48 hours of the last PBS (phosphate-buffered saline) wash, the samples were mounted on microscope slides using mounting medium with VECTASHIELD. DAPI (4′, 6-diami-dino-2-phenylindole) stain was added during slide preparation to stain cell nuclei. Before slides were made, each specimen was measured for either standard length (specimens with flexed notochords) or notochord length (specimens without flexed notochords). For specimens with flexed notochords, standard length follows that of Bird and Mabee (2003). All specimens were measured to the nearest 0.1 mm with a Lovins Microslide Field Finder #7100.
Table 1.
Cleared whole-mount antibody stain procedure for a-Collagen II and Wheat germ agglutinin (WGA).
| Step | Solution | Time |
|---|---|---|
| 1 | 4% Paraformaldehyde | Overnight |
| 2 | 1X PBS (Phosphate buffered saline) | 3 × 5 min |
| 3 | Bleach (15% hydrogen peroxide [3%]: 85% potassium hydroxide [1%]) | 10 min |
| 4 | Methanol (pre-chilled at −20°C) | Quick wash, then overnight −20°C |
| 5 | Acetone (pre-chilled at −20°C) | 7 min −20°C |
| 6 | PBST (Phosphate buffered saline+0.1% Triton X-10) | 3 × 5 min |
| 7 | 0.5% Trypsin | Until cleared (~40 min) 37°C |
| 8 | PBST | 3 × 5 min |
| 9 | 0.5% Hyaluronidase (37°C) | 1 h |
| 10 | PBST | 2 × 5 min |
| 11 | Blocking solution (10% fetal bovine serum/1% DMSO/0.1% Triton X-100/PBS) | 1 h |
| 12 | a-Collagen II (1:250 dilution in blocking solution) | Quick wash, then overnight 4°C |
| 13 | PBST | 3 × 30 min |
| 14 | Blocking solution | 30 min |
| 15 | DyLight 488-conjugated AffiniPure Rabbit Anti-Mouse IgG (1:200 dilution in blocking solution) | Overnight 4°C |
| 16 | PBST | 2 × 10 min, then 2 × 30 min |
| 17 | Wheat germ agglutinin (1:200 dilution in PBS) | 15 min |
| 18 | 1X PBS | 2 × 10 min, then 2 × 30 min |
| 19 | Glycerol 100% |
Alcian blue and alizarin red staining
Twelve specimens of Danio rerio (KU41369–41370) and 65 specimens of Gasterosteus aculeatus (KU41362–41368) were cleared and double stained to display bone and cartilage following the method of Taylor and van Dyke (1985). Specimens were measured after they were cleared and stained.
Microscopy
Immunostained specimens were visualized at 1,000X magnification on a Nikon Eclipse Ti microscope, captured using Exi Aqua camera and processed by MetaMorph imaging software. Alcian blue/alizarin red stained specimens were visualized at 400X magnification on an Olympus BH2 microscope, captured using a Canon 450 DSLR and processed in Adobe Photoshop, and at 40X magnification on a LEICA MZFLIII microscope, captured using DFC320 camera and processed by IM50 imaging software.
Anatomical nomenclature
Anatomical terms that may be unfamiliar are defined below, modified from Schultze and Arratia (2013).
Arcocentra
Paired elements of the vertebral centrum that develop from the basidorsal or the basiventral arcualia.
Autocentrum
Vertebral centrum formed by direct ossification without a cartilaginous precursor and outside the chordacentra, if present.
Chordacentrum
Vertebral centrum that forms as a result of mineralization of the middle fibrous part of the notochordal sheaths.
Epurals
Modified neural spines.
Hypaxial elements, hypurals
Anterior elements include hypurals 1 and 2, anterior of the hypaxial diastema. In larvae prior to flexion these comprise the anterior hypurals, after flexion they may be termed the lower hypurals. Posterior elements include hypurals 3 to 6 (with variation in number due to phenomena such as perichondral fusion) in living teleosts that are posterior of the hypural diastema. In larvae prior to flexion these comprise the posterior hypurals, after flexion they may be termed the upper hypurals.
Membranous neural arches
Membranous outgrowths of autocentra that surround the neural tube with spine-like dorsal extensions.
Neural arches
Dorsal elements developing from basidorsal arcualia.
Pleurostyle
A pair bones that may be derived from preural neural arch 1 (thus a modified uroneural) or be entirely membranous in origin.
Ural centra
Posterior-most centra of the vertebral column characterized by the absence of haemal arches and supporting hypurals.
Uroneural
Modified ural neural arch.
CHORDACENTRA OF THE NOTOCHORD
Three authors have published on the vertebral centra of Danio rerio, and each has come to a different interpretation. Bird and Mabee (2003) did not distinguish between chordacentra and autocentra. They interpreted the urostyle (Fig. 1A) as a compound structure composed of caudal vertebrae and the pleurostyle. We agree with this interpretation for adult fishes. Bensimon-Brito et al. (2012) showed the formation of a chordacentrum immediately above hypural 2 (H2) with an extension anteriorly to include the sheath immediately above hypural 1 (H1; Bensimon-Brito et al., 2012:figs. 2C, 3B). One of their histological figures shows that the anterior part is separated during at least part of development (Bensimon-Brito et al., 2012:fig. 4D). Schultze and Arratia (2013) agreed with Bensimon-Brito et al. (2012) that there are two anterior chordacentra, appearing sequentially, that fuse during development to produce a compound chordacentrum that later extends through further mineralization of the notochordal sheath to incorporate the region of the notochord dorsal to the parhypural, hypural 1, and hypural 2. This growth results in a single anterior chordacentrum by about 6 mm SL (our observations). Schultze and Arratia (2013) identified this compound element as ural centra 1+2 (U1+U2). In contrast, Bensimon-Brito et al. (2012) identified it as preural centrum 1 + ural centrum 2 (Pu1+U1), presumably following the diural nomenclature of Nybelin (1963) and Monod (1968).
Fig. 3.
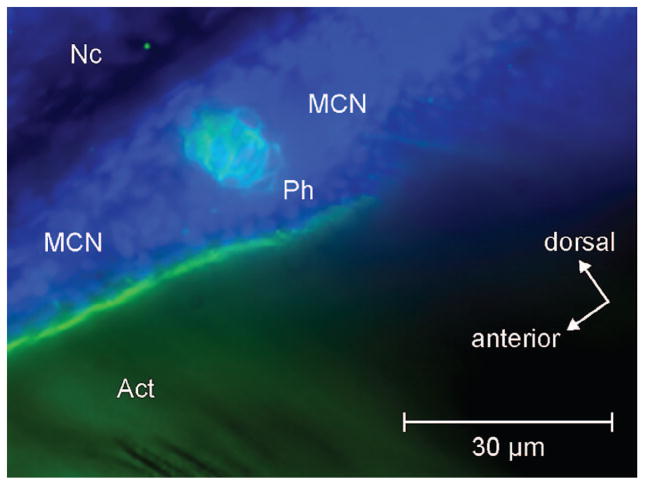
Early parhypural development in Danio. Lateral view of caudal region of Danio rerio (3.4 mm SL). Collagen II (green) and DAPI (blue) stain. Anterior left, dorsal up, axis inclined CCW about 45 degrees. Act, actinotrichia; MCN, mesenchyme cell nuclei; Nc, notochord; Ph, parhypural.
Fig. 4.
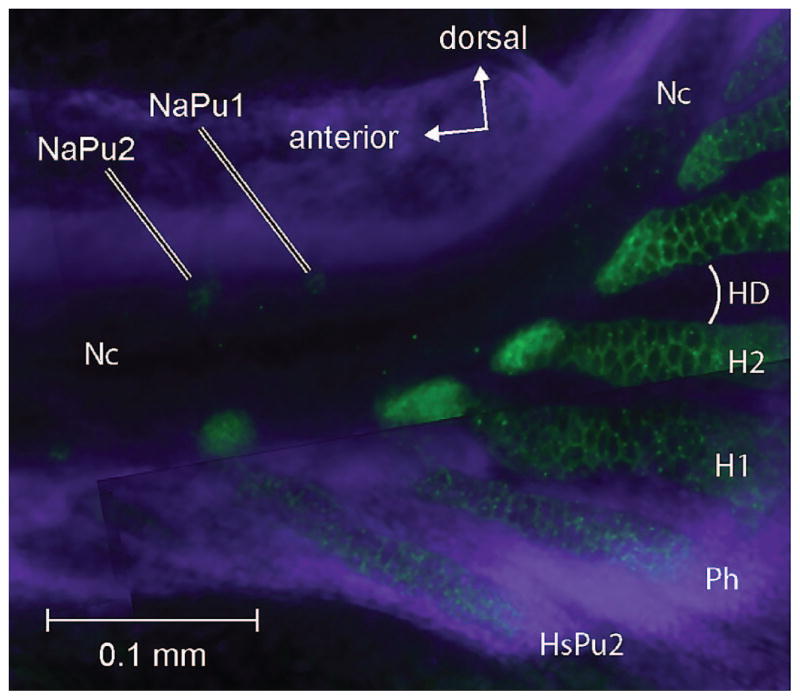
Neural arch development in Danio. Lateral view of caudal region showing formation of the neural arch of preural centrum 1 of Danio rerio (5.3 mm SL) stained with Collagen II (green) and DAPI (blue). H1, H2, hypurals 1 and 2; HD, hypural diastema; HsPu2, haemal spine of Pu2; NaPu1, NaPu2, neural arches of preural centra 1 and 2; Nc, notochord; Ph, parhypural. Anterior is left; dorsal is up.
The chordacentrum interpreted as ural centrum 2+ (U2+) by Bensimon-Brito et al. (2012) appears as a mineralization dorsal to the hypural interpreted as hypural 3+ (Hy3+). Bensimon-Brito et al. (2012) interpreted this centrum as compound based on a histological section that shows a partition between the growing mineralization, which begins as two separate elements that fuse (Bensimon-Brito et al., 2012:fig. 4). Schultze and Arratia (2013) also recognized the compound nature of the centrum but interpret it in polyural fashion as ural centrum 3 + ural centrum 4.
HYPAXIAL ELEMENTS
Danio rerio develops a complement of one parhypural and five hypurals in the hypaxial region (Figs. 1A, 2A: Bird and Mabee, 2003; Bensimon-Brito et al., 2012; Schultze and Arratia, 2013; pers. obs.). Hypaxial elements develop in a field of presumed mesenchyme cells that form a thin layer between the actinotrichia through transformation of these cells into chondrocytes (our observations). Interestingly, they do not form immediately adjacent to the notochord but more distally (Fig. 3). The parhypural (Ph) and anterior hypurals 1 and 2 (lower hypurals, H1 and H2) develop earlier while more posterior hypurals (upper hypurals, three in total) develop later. In many teleosts anterior (lower) and posterior (upper) elements are well separated in early development by the hypural diastema (HD; Figs. 1, 2), a gap that demarks the passage of the caudal arteries and veins (Schultze and Arratia, 1989; Arratia and Schultze, 1992). In Danio rerio the position of the diastema is marked during development by a gap in GFP expression in sonic hedgehog (shh) transgenic embryos as early as seven days post fertilization (Hadzhie et al., 2007). This provides a developmental marker that separates the anterior (lower) and posterior (upper) hypaxial regions of the caudal fin corresponding to the adult marker provided by the gap through which the caudal arteries and veins traverse (Schultze and Arratia, 1989).
Anterior hypaxial elements
In our series the parhypural may begin formation as early as 3.2 mm NL, followed by hypural 1 as early as 3.5 mm NL. Both elements develop within the extensive field of cells between the actinotrichia and their first appearance is within the field, not adjacent to the notochord. Only later are chondrocytes added proximally to form a common base. Whether these chondrocytes are recruited from preexisting chondrocytes or represent newly transformed cells already present between the actinotrichia and visualized by DAPI staining in earlier stages of development is unknown. We note that this ontogeny is different than that of trunk arches which appear as arcocentra. Whether Danio rerio actually forms a distinct arcocentrum in the anterior caudal region is not answered by our data. First appearance of the parhypural and hypural 1 is variable, with the parhypural consistently present in specimens 3.7 mm NL and larger and hypural 1 not consistently appearing until the specimen is over 4.0 mm NL. We attribute this variation to environmental and nutritional variables not controlled in our specimens and to differences in environment experienced in under different laboratory conditions (Arratia and Bagarinao, 2010). Hypural 2 then develops, and we have no data that would contradict the relative timing of Bird and Mabee (2003).
Posterior hypaxial elements
Posterior hypurals develop in sequence beginning about 4.5 mm NL (our observations) with the first posterior hypural usually identified as hypural 3 (Bird and Mabee, 2003; Schultze and Arratia, 2013) or hypural 3+ (Bensimon-Brito et al., 2012). This is followed by the sequential appearance of the two more posterior hypurals, usually labeled hypural 4 (H4) and hypural 5 (H5). In at least one of the figures in Bensimon-Brito et al. (2012:fig. 3I), a small and slightly elongate ball of cartilage is seen immediately above what is interpreted as hypural 5. This is probably a detached opisthural cartilage (P. E. Witten, pers. comm.). Given that the nature of the three posterior hypurals may be considered controversial, we have labeled them ?H4, ?H5, and ?H6 in Figures 1, 2, and 6.
Fig. 6.
Development of the caudal fin. Caudal structures in young specimens of (A) Danio rerio redrawn and modified from Bensimon-Brito et al. (2012) and (B) Moxostoma hubbsi redrawn and modified from Grünbaum et al. (2003). Bone is lightly shaded, cartilage heavily shaded. E-H3, epural above H3; H1–H6, hypurals 1–6; HD, hypural diastema; HaPu2–3, haemal arches of Pu2–3; HsPu2–3, haemal spines of Pu2–3; mNa, membranous neural arches; NaPu1–3, neural arches of urostyle, Pu2, and Pu3; Nc, notochord; NsPu2–3, neural spines of Pu2–3; Opc, opisthural cartilage; Ph, parhypural; Pl, pleurostyle; Pu2–3, preural centra 2–3; U1–3, uroneurals 1–3; U1+U2, fused ural centrum 1 and ural centrum 2; U3+U4, fused ural centrum 3 and ural centrum 4; Un-U4, uroneural of ural neural arch 4. Note that the neural arch of preural centrum 1 is not show on this rendering of Danio rerio.
NEURAL ARCHES AND THE PLEUROSTYLE
Danio rerio develops a single, small ball of chondrocytes (visualized by a-collagen II antibody shown in green signal in Fig. 4) in the anterior epaxial region immediately posterior to the neural arch of preural centrum 2 (Pu2; NaPu1; Fig. 4). This structure can be interpreted as the neural arch that would be associated with preural centrum 1 (Pu1), if that centrum formed. However, it does not seem to participate in centrum formation nor does it seem to be associated with pleurostyle formation as seen in Moxostoma hubbsi (Grünbaum et al., 2003). Immediately above the notochord and below the neural tube a pair of membranous bones develop that extends from the anterior to the posterior centrum forming a membranous pleurostyle extending from the anterior tip of ural centrum 1+2 (U1+U2) to ural centrum 3+4 (U3+4). As it grows it also forms one to several membranous arches (varying intraspecifically) around the neural tube anteriorly and fuses to the compound autocentrum (the urostyle), as noted by Bird and Mabee (2003), who termed it the neural arch of the urostyle. Our figures show the single arch condition.
In adults of Danio rerio, a paired uroneural is found above the dorsal-most hypural (Un-U4, Fig. 1A). Bird and Mabee (2003:fig. 11D) reported the formation of paired uroneurals, although it is not apparent to us if their figure illustrates the developing pleurostyle or a developing paired uroneural. Bensimon-Brito et al. (2010) reported a small cartilage sitting on the notochord opposite the penultimate hypural (?H4, ?H5) in at least one specimen (their fig. 2C) that can be interpreted as a neural arch, perhaps neural arch 4 or 5. The relationship of the paired uroneural to this arch is not established based on the sequence of any published studies or our observations. In the adult, the paired uroneural is not fused. We have tentatively labeled the pair as uroneural 4 (Un-U4), but further study is needed to definitely identify it as originating from neural arch 4.
EPURALS
Danio rerio usually develops a single epural (E-?H3) above what we identify as presumed hypural 4 (?H4) (or a compound H3+H4) as shown in Figure 2A. The cells appearing dorsal to the notochord stain positively with DAPI and may represent epaxial mesenchyme cells (blue DAPI staining in Fig. 2A). In some specimens two epurals may form. We have labeled the single epural illustrated for Danio in Figures 1, 2, and 6 with a question mark (E-?H3) to indicate that the association of this epural with the third hypural is a matter of conjecture relative to Moxostoma, which has a third hypural.
DISCUSSION
Phylogenetic interpretation and comparisons of chordacentrum formation
Danio rerio lacks a separate preural chordacentrum 1 (Pu1). The distribution of this loss (or replacement by anterior mineralization of ural centrum 1) is unknown pending further investigations of the development of otomorph caudal fins. (Otomorpha sensu Wiley and Johnson, 2010, includes ostariophysans and clupeiforms.) A similar lack of preural centrum 1 is reported in the smelt Mallotus villosus, a euteleost (Doosey and Domke, 2014). The two developed anterior chordacentra in D. rerio are interpreted by Schultze and Arratia (2013) as ural centra 1 and 2, which fuse to produce a compound anterior chordacentrum (U1+U2). Thus a matrix representation would reflect the fact that two chordacentra were fused as an apomorphy at some undetermined level within Otomorpha (unfused being the plesiomorphic state), and preural centrum 1 is missing.
The posterior chordacentrum also is interpreted by Bensimon-Brito et al. (2012) as compound (“U2+” following traditional diural terminology) and would represent a fused third and fourth ural centrum (U3+U4). The clupeiforms that have been studied by Schultze and Arratia (2013) also have a compound ural centrum 3+4. It would be interesting to know more about the distribution of this fusion among otomorphs and to determine whether this is a synapomorphy shared by all members of the clade. A diural interpretation implies that a posterior ural centrum 2+ is homologous with ural centrum 2 of basal neopterygians such as gars that have ural centrum 2 supporting hypural 2 in a more anterior position. Coding this compound centrum using polyural terms reveals a possible otomomorph synapomorphy. Finally, Bensimon-Brito et al. (2012) referred to the adult urostyle as a compound structure composed of many more vertebral bodies than those mentioned by Bird and Mabee (2003), but there would be only four centra (ural centra 1, 2, 3, and 4) if preural centrum 1 is missing.
Phylogenetic interpretation and comparisons of hypaxial elements
Arcocentra are found in more basal teleosts (e.g., Schultze and Arratia, 1988). Thus the presence or absence of arcocentra in the hypaxial region is of phylogenetic interest: are they present in Danio rerio or absent? If absent, at what level in the tree is this absence synapomorphic? The presence of five hypurals, rather than the more common condition of six hypurals as seen in many otomorphs, is also found in D. albolineatus and D. kerri (Sanger and McCune, 2002) as well as a number of smaller cyprinids (Britz and Conway, 2009). Bird and Mabee (2003), Schultze and Arratia (2013), and Bensimon-Brito et al. (2012) identified the first upper hypural posterior to the hypural diastema as the third hypural (H3 or H3+). Bensimon-Brito et al. (2012) reported the appearance of a more anterior mass of chondrocytes anterior to this hypural either as a separate element (4% of their specimens), a mass connected to this hypural or a double-headed and perichondrally ossified hypural. They designated this hypural as “H3+.” Fully 15% of their specimens (their figs. 3E–G) showed this mass of chondrocytes.
We hypothesize that Danio rerio normally lacks hypural 3. Alternatively, any chondrocytes destined to form hypural 3 in other fishes either fail to form a hypural or are incorporated into the base of a compound hypural 4. If so, the hypural identified by Bensimon-Brito et al. (2012) as hypural 3+ is actually hypural 4 or a compound hypural 3+4, whereas the other posterior hypurals are phylogenetically homologous to hypural 5 and hypural 6 of ostariophysans with six hypurals. This hypothesis suggests that D. rerio and its close relatives share the synapomorphy of a loss of hypural 3 (or its incorporation into the base of hypural 4). Arguing against this hypothesis is the report of Sanger and McCune (2002:357) of a small “slip of bone remaining where the sixth hypural is found” in some specimens of D. albolineatus, the sister species to D. rerio and D. kerri in their hypothesis. This hypothesis would lead to the conclusion that D. rerio and its close relatives lost the sixth hypural and that the chondrocytes reported by Bensimon-Brito et al. (2012) are not of phylogenetic interest but individual developmental variation.
The fusion, loss, or formation of compound hypurals is common among apical teleost fishes (acanthomorphs: see figures in Fujita, 1990) and more developmental work is needed to understand the phenomenon. The usual adult pattern in the relative size of the upper hypurals is a decrease in size of the hypurals anteriorly to posteriorly. This is the case with Danio rerio (Fig. 1A). If we accept the idea that vestigial cartilaginous chondrocytes in front of “hypural 3+” actually represent chondrocytes that would form a third hypural, then we would interpret the posterior hypural as hypural 6. Arguing for this hypothesis is the fact that the posterior hypural has approximately the same relative size and orientation of hypural 6 of other cypriniforms figured by Fujita (1990) that have six hypurals and the observation that two centra are formed but only one hypural. Arguing against this interpretation is the “slip of bone” in the same position as H6 in some specimens of D. albolineatus, given that it is, in fact, the sixth hypural. Thus, there are two competing hypotheses, loss of hypural 3 or loss of hypural 6. Only further developmental studies will solve this puzzle.
Interestingly, there may be another way to evolve five hypurals. Britz and Conway (2009:406) provided a table of 19 miniaturized and three non-miniature species of cyprinids that shows a pattern of absence of what is usually labeled H6. In their description of the caudal skeleton of Paedocypris, they described the pattern of the relative size of the posterior hypurals in the following manner: “There are three upper hypurals, dorsal to the second hypural. These elements, each successively smaller in size than the ventrally adjacent element, are ossified perichondrally at the base. All hypural elements retain a cartilaginous tip.”
However, Britz and Conway’s figure (2009:fig. 7) showed that what they identify as H5 is larger than the hypural we presume they would identify as hypural 4. A pattern of a larger posterior hypural in cypriniforms with five hypurals is also shown in Fujita for the cobitid Cobitis biwae (Fujita, 1990:fig. 46) and the gonorynchiform Gonorynchus abbreviatus (Fujita, 1990:fig. 31). In contrast, the cobitid Leptobotia curta (Fujita, 1990:fig. 45) and the gonorynchiform Chanos chanos (Fujita, 1990:fig. 30) have the usual cypriniform condition of six hypurals with the sixth smaller than the fifth, and the fifth smaller than the fourth. A reduced number of hypurals is common among acanthomorphs; the condition is usually ascribed to fusion of hypurals (see Fujita, 1990; fusion implied by labeling). However, in at least some tetraodontiforms, two hypural plates develop as separate single cartilages with no evidence of either fusion or loss (Konstantinidis and Johnson, 2012). Konstantinidis and Johnson (2012) termed these plates hypurals 1 and 2 citing for their decision an inability to discriminate between fusion or loss. Aside from the homology problem (a hypural 2 posterior to the hypural diastema), the observation that both plates appear to develop as single cartilages suggests that there is been neither fusion nor loss but the transformation of an entire anterior and an entire posterior field of chondrocytes into two plates, one covering the fields of what would be hypurals 1 and 2 and the other covering the fields of the upper hypurals (3–5 by inference of the percomorph condition of five hypurals). We term this condition “compound” rather than “fused” because the pattern of chondrocyte differentiation does not show that two cartilaginous hypurals are formed followed by fusion but that the entire anterior and posterior fields of cells that transform into chondrocytes transform into a single anterior plate and a single posterior plate, forming two hypurals without fusion or loss. Our specimens of Gasterosteus aculeatus also have what appear to be compound hypurals (Fig. 5).
Fig. 5.
Hypural development in Gasterosteus. Lateral view of the caudal region of Gasterosteus aculeatus (5.9 mm SL) stained with Collagen II (green) and DAPI (blue). H1–2, anterior compound hypural comprising chondrocytes that would normally form H1 and H2 in acanthomorphs with two anterior hypurals; H3–5, posterior compound hypural formed by chondrocytes normally forming H3–H5 in acanthomorph fishes; HD, hypural diastema; Nc, notochord; Ph, parhypural. Anterior is left; dorsal is up, specimen inclined.
Although the developmental work has yet to be accomplished, these observations suggest that the posterior hypural of Paedocypris may actually be a compound hypural formed from chondrocytes normally forming hypurals 5 and 6, or alternately, by perichondral fusion of two separate hypural chondral plates. If so, this means there may be three different developmental patterns among ostariophysans, one resulting in five hypurals due to loss of hypural 3 (or incorporation of some of its chondrocytes into hypural 4; Bensimon-Brito et al., 2012), one resulting in loss of hypural 6 given the observation that Danio albolineatus occasionally shows a sixth hypural (Sanger and McCune, 2002), and a third resulting in five hypurals due to incorporation of chondrocytes into a compound H5-H6 or perichondral fusion. Further, a similar pattern is observed in relatively unrelated groups (e.g., gonorynchiforms and cobitids), suggesting homoplasy between groups and perhaps phylogenetic homology (and synapomorphy) within groups. In the absence of developmental evidence it is impossible to say whether the large terminal hypurals of some gonorynchiforms and cobitids are compound or the result of perichondral fusion, a phenomenon observed in tunas (Potthoff, 1975). In any case, simply coding of hypural elements sequentially could result in category mistakes; what is coded as hypural 5 in some species would actually be phylogenetically homologous to hypural 5, hypural 6, a compound hypural 5–6, or a perichondrally fused H5+H6 in other species.
Phylogenetic interpretation and comparisons of the pleurostyle and uroneurals
In basal teleosts such as †Leptolepis (Arratia, 1991), elopomorphs, and osteoglossomorphs (Schultze and Arratia, 1988, 1989), as well as in salmonids (Arratia and Schultze, 1992), uroneurals are derived from ural neural arches, either as direct extensions of the cartilage or as membranous bone extending from the ural neural arch cartilages that grow from posterior to anterior. Further, ural neural arches are formed from arcocentra, again cartilaginous structures. The small cartilage interpreted herein as NaPu1 (Figs. 2A, 4) does not seem to contribute to the formation of the neural arch nor the pleurostyle observed in adult Danio rerio. The neural arches and pleurostyle of adults appear to be membranous in origin. If so, they are not phylogenetically or developmentally homologous with the neural arches or uroneurals of more basal teleosts that form ural neural arches from arcocentra and uroneurals as membranous outgrowths of neural arches. Accordingly, we refer to the uroneurals of Bensimon-Brito et al. (2012) as a pair of membranous pleurostyles and the neural arches as membranous neural arches (mNa) with no particular association with endochondral elements.
What appear to be a pleurostyle and an arch are commonly observed in other adult otomorphs. However, the development appears to be different. In Moxostoma hubbsi, the pleurostyle begins in cartilage, not membrane bone (Grünbaum et al., 2003). Arratia (2010) demonstrated that the clupeiforms she analyzed have a membranous pleurostyle, whereas the neural arches associated with the pleurostyle develop separately in cartilage and fuse to the pleurostyle via perichondral ossification. Further, in some species there is a single neural arch formed in association with ural centrum 1 while the neural arch of preural centrum 1 atrophies during growth. Many clupeiform species figured by Fujita (1990:e.g., figs. 23, 24) have very large processes labeled “NPU1” (neural arch of preural centrum 1). Similar structures are seen in cypriniforms (e.g., Fujita, 1990:figs. 37, 38), characiforms (e.g., Fujita, 1990:figs. 47, 48), and siluriforms (e.g., Fujita, 1990:figs. 49, 51). Whether these are neural arches of preural centrum 1, or of ural centrum 1, or of purely membrane origin is unknown. Obviously, the “pleurostyle” is not a single structure but a complex structure potentially rich in phylogenetic information.
There are many phylogenetic implications for the development of the pleurostyle. If the pleurostyle of Danio rerio is actually a membrane structure with no association with ural neural arches, then coding it is as “uroneural,” implying a phylogenetic homology with an uroneural of basal teleosts, is a category mistake. Further, even if they are associated with the small Pu1 cartilage (an arcocentrum?), their formation is different than that of basal teleosts, as the growth is anterior to posterior in Danio, not posterior to anterior as in basal teleosts. In fact, what is usually coded uroneural 1(Un1) in teleosts is, itself, a misidentification under polyural terminology; uroneural 1 in other teleosts is associated with the arch of the fourth ural centrum (U4; Arratia and Schultze, 1992) and not the arch of a segment anterior to the hypural diastema. Thus uroneural 1 in other teleosts should be coded as uroneural 4 (Arratia and Schultze, 1992) if serial homology in the epaxial region is hypothesized. That is, labeling the uroneural as “Un1” implies an ontogenetic origin of the structure from the first ural neural arch, not the fourth. Further, coding a membranous neural arch as homologous to the arcocentra and any membranous outgrowths associated with the arcocentra of basal teleosts is a category mistake. Instead, membranous pleurostyles and membranous neural arches may be counted as possible synapomorphies at some level in the phylogeny that is unknown pending evidence as to the distribution of these characters and their states.
Phylogenetic interpretation and comparisons of the epural
The number of epurals in Danio rerio varies from one to two. Usually a single epural is formed, and no epural is formed anterior to the hypural diastema. Association of epurals with neural arches and hypurals is problematic. In basal teleosts there may be a direct association with neural arches, as with the epurals of Elops and Hiodon (Schultze and Arratia, 2013). However, Danio rerio develops its epural/s in the apparent absence of a neural arch. Complicating the assessment of homology is the evidence, suggested by studies of the trunk, that arcocentra and their associated neural spines are sclerotomal/somatic elements while chordacentra are notochordal elements that do not form from cartilage (Morin-Kensicki et al., 2002) and that, at least in the trunk, control of metamerism of sclerotomal and notochordal elements may be under different genetic control (van Eeden et al., 1996; Fleming et al., 2004). Research on the medaka, Oryzias latipes, suggests a similar pattern (Mise et al., 2008). Further, mutant analysis and knockout experiments on Oryzias reported by Ohtsuka et al. (2004) demonstrate that the hypaxial and epaxial regions express genes differently. One can get an incomplete “mirror image” duplication of caudal fin structures across the midline in the Oryzias Da mutant, implying that differential gene expression is at work in the epaxial and hypaxial regions in wild-type (normal) individuals. In light of these observations, making identity statements that directly associate epurals with a particular, more ventral, structure (a particular centrum or hypural) may be problematic, unless there is a direct association with a particular neural arch. However, associating an epural with a particular region (anterior or posterior to the hypural diastema) and labeling it by its relative position to underlying structures without implying direct serial homology with the underlying hypaxial elements is not problematic. It is a simple statement of relative position without the underlying assumption of hypaxialepaxial serial homology. The question is: are the relative positions of epurals phylogenetically informative?
Like most Danio rerio, Moxostoma hubbsi also forms a single epural dorsal to posterior hypurals (Grünbaum et al., 2003). Adult otomorphs generally, except for siluriforms (Schultze and Arratia, 1989; Arratia and Schultze, 1992) have epurals that appear to be in posterior position relative to the hypural diastema (see Fujita, 1990:figs. 15–39). In contrast, elopiforms, hiodontid osteoglossomorphs, and salmonids (summary in Arratia and Schultze, 1992) form epurals in the anterior region of the caudal skeleton, and even in the case of some salmonids, in preural body segments. The exception is the grayling, Thymallus, which also forms posterior epurals. Sequential numbering of epurals, like that of other structures, can lead to category mistakes in phylogenetic homology statements. The first epural of the elopomorph Elops is not topologically homologous with the first/only epural of Danio rerio, if the epurals of Elops are formed in the anterior field of the caudal fin (the epaxial region anterior of the hypural diastema), whereas the single epural (and occasionally second epural) of Danio rerio is formed in the posterior field. Why D. rerio forms only a single epural (or occasionally two) and does so in spite of the fact that there are cells anterior to the epural from which chondrocytes (Fig. 2A) might be developed to form more anterior epurals is not known. Why other teleosts do not form posterior epurals is also not known, but our preliminary work with Gasterosteus aculeatus (a percomorph distantly related to Danio, see Wiley and Johnson, 2010) provides a possible answer: there are few to no cells to form epurals posterior to the second epural, and the base of this epural forms anterior to the diastema (Fig. 2B). Recognizing that epurals form in different regions of the caudal fin relative to hypurals and ural centra suggests that the relative positions of epurals may be phylogenetically informative.
A polyural interpretation of caudal structures in Danio rerio and Moxostoma hubbsi
Figures 6A and B graphically summarize our conclusions by comparing the juvenile caudal skeletons of Danio rerio and Moxostoma hubbsi. There are two anterior caudal ural centra that fuse during development (U1+U2) and no indication of a separately developing preural chordacentrum 1 in either species. Danio rerio is similar to some clupeomorphs in having two posterior chordacentra that fuse (ural centrum 3+4), whereas M. hubbsi appears to lack ural centrum 4. Ventrally D. rerio has five hypurals, and, if the third is lost (which we conclude is the case) then the three posterior hypurals represent hypurals 4–6 of M. hubbsi. Dorsally D. rerio differs from M. hubbsi in having membranous elements making up the pleurostyle and the neural arch, while M. hubbsi begins to develop these structures from cartilage with membranous outgrowth. Both species have uroneurals (Un-U4), although our figured specimen of D. rerio lacks uroneurals at this stage of development. The adult urostyle of D. rerio comprises four ural centra, the pleurostyle, and the membranous neural arch (U1+U2+U3+U4+Pl+mNa).
CONCLUSIONS
Progress in understanding the evolution and development of the teleost caudal skeleton has, in our opinion, been hampered by ichthyologists fitting teleost caudal nomenclature into a single common set of structural labels commonly termed diural nomenclature and the tendency to simply name structures in sequence. Diural terminology has a long history dating from the works of Nybelin (1963) and Monod (1968). The original intention of diural labeling was to provide a descriptively accurate account of teleost caudal morphology pending information about the development of this complex (Nybelin, 1963). It served this purpose admirably in the absence of developmental information, which is now becoming more readily accessible. Under the diural paradigm, two adult (or late juvenile) caudal centra are usually identified as preural centrum 1 + ural centrum 1 and ural centrum 2, in spite of the fact that “ural centrum 2” develops above hypural 3 and in spite of the fact that the first preural segment may entirely lack an independent chordacentrum. The compound centrum that develops in the posterior of Danio rerio is universally termed “ural centrum 2,” but it is posterior to the hypural diastema, a region associated with more posterior hypurals (hypurals 3–6), and thus we surmise is not homologous with ural centrum 2 of basal, non-teleost actinopterygians.
The alternative to the diural paradigm is the polyural paradigm advocated by Schultze and Arratia (1989, 2013). A polyural interpretation begins with the observation that teleosts below Leptolepis coryphaenoides (Arratia, 2013) and the sister group of teleosts (Holostei) are characterized by a polyural caudal skeleton, in which there is a more-or-less regular metamerism of parts, each anterior segment being associated with serially homologous centra, neural arches, neural spines/epurals, and haemal arches/hypurals. Holosteans such as gars and fossil groups show this pattern, as do acipenseriforms albeit without centra. Teleosts begin to lose this one-to-one serial homology when they develop the synapomorphy of flexing of the caudal fin to produce the homocercal tail. This is signaled, developmentally, by differential gene control, but the pattern does not disappear completely. Developmental remnants of past structures persist and should be accounted for in our efforts to meld evolution and development.
The ontogeny of the teleost caudal skeleton is a complex phenomenon. Caudal flexion in many species is a nonlinear phenomenon and may occur at different stages in development relative to the appearance of skeletal structures. Consider caudal flexion and the appearance of skeletal elements. A series of papers by Potthoff and colleagues (e.g., 1975, 1980, 1988) demonstrated that the apical acanthomorphs studied by them form epurals anterior to the hypural diastema before caudal flexion, like Gasterosteus aculeatus (our observations). In contrast Danio rerio forms an epural posterior to the diastema after flexion. Hilton and Johnson (2007) demonstrated that loss of epurals in the carangid jack Caranx crysos involves the disappearance during ontogeny of the second epural by the time individual reaches about 40 mm SL. Konstantinidis and Johnson (2012) provided evidence (our interpretation) that tetraodontiforms do not fuse their hypurals but rather have compound hypurals, and our observations of Gasterosteus aculeatus suggest that they develop in a similar manner, but other teleosts reduce the number of their hypurals differently. In the tuna, Thunnus atlanticus, hypural reduction proceeds via perichondral fusion of five separate cartilaginous hypurals (Potthoff, 1975). These studies suggest that progress in morphological analysis to untangle the “bush at the top” of teleost phylogeny (reflected in Wiley and Johnson, 2010; an alphabetical listing of some 24 orders in one vast polytomy based on present morphological evidence) may require increasing attention to the developmental dynamics of structures such as the teleost caudal skeleton.
Acknowledgments
Our sincere thanks to Michael Bell (Stony Brook), not only for alerting EOW to the potential benefits of using COII staining techniques but also for providing specimens of Gasterosteus aculeatus used in this study. Gloria Arratia (Kansas) not only critically read the manuscript but she also provided advice and guidance to EOW for several years on polyural nomenclature and the interpretation of structures. G. Arratia and Hans-Peter Schultze (Kansas) were kind to allow us to read and incorporate information from their paper on caudal skeletons (2013) while still in press. David Moore and Heather Singleton provided both the training and facilities of the University of Kansas Microscopy and Analytical Imaging Laboratory that allowed us to image many of our specimens. We thank the organizers of the symposium, Gloria Arratia and G. David Johnson, for inviting our participation and making the symposium a success. The protocol for the zebrafish usage was approved by the University of Kansas, Lawrence Institutional Animal Care and Use Committee (IACUC; Animal use statement: Permit Number #197-01). All of the zebrafish were maintained and humanely euthanized following the protocols. This research was funded in part by the Euteleost Tree of Life Project (NSF-DEB 0732819) to EOW, NIH grant P20RR016475/P20GM103418 and P20GM103638 to MA, and the University of Kansas Endowment Association.
LITERATURE CITED
- Arratia G. The caudal skeleton of Jurassic teleosts: a phylogenetic analysis. In: Chang M-M, Liu Y-H, Zhang G-R, editors. Early Vertebrates and Related Problems in Evolutionary Biology. Science Press; Beijing: 1991. pp. 249–340. [Google Scholar]
- Arratia G. The Clupeocephala re-visited: analysis of characters and homologies. Revista de Biología Marina Oceanografía. 2010;45:635–657. [Google Scholar]
- Arratia G. Morphology, taxonomy, and phylogeny of Triassic pholidophorid fishes (Actinopterygii, Teleostei) Journal of Vertebrate Paleontology. 2013;33(Supplement 6):1–138. Memoir 13. [Google Scholar]
- Arratia G, Bagarinao T. Developmental morphology of the skeleton of Chanos chanos (Ostario-physi, Gonorynchiformes) In: Grande T, Poyato-Ariza F-J, Diogo R, editors. A Comprehensive Review of Gonorynchiformes and of Ostariophysan Relationships. Science Publishers Inc; Enfield, New Hampshire: 2010. pp. 73–106. [Google Scholar]
- Arratia G, Schultze HP. Reevaluation of the caudal skeleton of certain actinopterygian fishes: III. Salmonidae. Homologization of caudal skeletal structures. Journal of Morphology. 1992;214:187–249. doi: 10.1002/jmor.1052140209. [DOI] [PubMed] [Google Scholar]
- Bensimon-Brito A, Cancela ML, Huysseune A, Witten PE. The zebrafish (Danio rerio) caudal complex—a model to study vertebral fusion. Journal of Applied Ichthyology. 2010;26:235–238. [Google Scholar]
- Bensimon-Brito A, Cancela ML, Huysseune A, Witten PE. Vestiges, rudiments and fusion events: the zebrafish caudal fin endoskeleton in an evo-devo perspective. Evolution and Development. 2012;14:116–127. doi: 10.1111/j.1525-142X.2011.00526.x. [DOI] [PubMed] [Google Scholar]
- Bird NC, Mabee PM. Developmental morphology of the axial skeleton of the zebrafish, Danio rerio (Ostariophysi: Cyprinidae) Developmental Dynamics. 2003;228:337–357. doi: 10.1002/dvdy.10387. [DOI] [PubMed] [Google Scholar]
- Britz R, Conway KW. Osteology of Paedocypris, a miniature and highly developmentally truncated fish (Teleostei: Ostariophysi: Cyprinidae) Journal of Morphology. 2009;270:389–412. doi: 10.1002/jmor.10698. [DOI] [PubMed] [Google Scholar]
- Coons AH, Creech HJ, Jones RN. Immunological properties of an antibody containing a fluorescent group. Proceedings of the Society of Experimental Biology and Medicine. 1941;47:200–202. [Google Scholar]
- Doosey MH, Domke ND. Early development of the caudal fin skeleton of capelin Mallotus villosus (Osmeridae) Copeia. 2014;2014:355–365. [Google Scholar]
- Fleming A, Keynes R, Tannahill D. A central role for the notochord in vertebral patterning. Development. 2004;131:873–880. doi: 10.1242/dev.00952. [DOI] [PubMed] [Google Scholar]
- Fujita K. The Caudal Skeleton of Teleostean Fishes. Tokai University Press; Tokyo: 1990. [Google Scholar]
- Grünbaum T, Cloutier R, Dumont P. Congruence between chondrification and ossification sequences during caudal skeleton development: a Moxostomatini case study. In: Browman HI, Skiftesvik AB, editors. The Big Fish Bang: Proceedings of the 26th Annual Larval Fish Conference. The Norwegian Institute of Marine Research; Bergen: 2003. pp. 161–176. [Google Scholar]
- Hadzhie Y, Lele Z, Schindler S, Wilson SW, Ahlberg P, Strähle U, Müller F. Hedgehog signaling patterns the outgrowth of unpaired skeletal appendages in zebrafish. BMC Developmental Biology. 2007;7:75. doi: 10.1186/1471-213X-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen DW. Isolating mechanisms in threespine sticklebacks (Gasterosteus) Canadian Journal of Fisheries and Aquatic Sciences. 1967;24:1637–1692. [Google Scholar]
- Hagen DW. Inheritance of numbers of lateral plates and gill rakers in Gasterosteus aculeatus. Heredity. 1973;30:303–312. [Google Scholar]
- Hilton EJ, Johnson GD. When two equals three: developmental osteology and homology of the caudal skeleton in carangid fishes (Perciformes: Carangidae) Evolution and Development. 2007;9:178–189. doi: 10.1111/j.1525-142X.2007.00148.x. [DOI] [PubMed] [Google Scholar]
- Konstantinidis P, Johnson GD. A comparative ontogenetic study of the tetraodontiform caudal complex. Acta Zoologica. 2012;93:98–114. [Google Scholar]
- Mise T, Iijima M, Inohaya K, Kudo A, Wada H. Function of Pax1 and Pax9 in the sclerotome of medaka fish. Genesis. 2008;46:185–192. doi: 10.1002/dvg.20381. [DOI] [PubMed] [Google Scholar]
- Monod T. Le complexe urophore des poisons téléostéens. Mémoires de l’Institut Fondamental d’Afrique Noire. 1968;81:1–705. [Google Scholar]
- Morin-Kensicki EM, Melancon E, Eisen JS. Segmental relationship between somites and vertebral column in zebrafish. Development. 2002;129:3851–3860. doi: 10.1242/dev.129.16.3851. [DOI] [PubMed] [Google Scholar]
- Nybelin O. Zur Morphologie und Terminologie des Schwanzskelettes der Actinopterygier. Arkiv fur Zoologi. 1963;15:485–516. [Google Scholar]
- Ohtsuka M, Kikuchi N, Yokoi H, Kinoshita M, Wakamatsu Y, Ozato K, Takeda H, Inoko H, Kimura M. Possible roles of zic1 and zic4, identified within the medaka Double anal fin (Da) locus, in dorsoventral patterning of the trunk-tail region (related to phenotypes of the Da mutant) Mechanisms of Development. 2004;121:873–882. doi: 10.1016/j.mod.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Potthoff T. Development and structure of the caudal complex, the vertebral column, and the pterygiophores in the blackfin tuna (Thunnus atlanticus, Pisces, Scombridae) Bulletin of Marine Science. 1975;25:205–231. [Google Scholar]
- Potthoff T. Development and structure of fins and fin supports in dolphin fishes Coryphaena hippurus and Coryphaena equiselis (Coryphaenidae) Fisheries Bulletin. 1980;78:277–312. [Google Scholar]
- Potthoff T, Kelly S, Collins LA. Osteological development of the red snapper, Lutjanus campechanus (Lutjanidae) Bulletin of Marine Science. 1988;43:1–40. [Google Scholar]
- Sanger TJ, McCune AR. Comparative osteology of the Danio (Cyprinidae: Ostariophysi) axial skeleton with comments on Danio relationships based on molecules and morphology. Zoological Journal of the Linnean Society. 2002;135:529–546. [Google Scholar]
- Schultze HP, Arratia G. Reevaluation of the caudal skeleton of some actinopterygian fishes: II. Hiodon, Elops and Albula. Journal of Morphology. 1988;195:257–303. doi: 10.1002/jmor.1051950304. [DOI] [PubMed] [Google Scholar]
- Schultze HP, Arratia G. The composition of the caudal skeleton of teleosts (Actinopterygii, Osteichthyes) Zoological Journal of the Linnean Society. 1989;97:189–231. [Google Scholar]
- Schultze H-P, Arratia G. The caudal skeleton of basal teleosts, its conventions, and some major evolutionary novelties in a temporal dimension. In: Arratia G, Schultze H-P, Wilson MVH, editors. Mesozoic Fishes 5: Global Diversity and Evolution. Verlag Dr. Friedrich Pfeil; München, Germany: 2013. pp. 187–246. [Google Scholar]
- Taylor WR, van Dyke GC. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium. 1985;9:107–119. [Google Scholar]
- van Boxtel AL, Gansner JM, Hakvoort HW, Snell H, Legler J, Gitlin JD. Lysyl oxidase-like 3b is critical for cartilage maturation during zebrafish craniofacial development. Matrix Biology. 2011 Apr;30:178–187. doi: 10.1016/j.matbio.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eeden FJM, Granato M, Schach U, Brand M, Furutani-Seiki M, Haffter P, Hammerschmidt M, Heisenberg CP, Jiang Y-J, Kane DA, Kelsh RN, Mullins MC, Odenthal J, Warga RM, Allende ML, Weinberg ES, Nüsslein-Volhard C. Genetic analysis of fin formation in the zebrafish, Danio rerio. Development. 1996;123:255–262. doi: 10.1242/dev.123.1.255. [DOI] [PubMed] [Google Scholar]
- Wiley EO, Johnson GD. A teleost classification based on monophyletic groups. In: Nelson JS, Schultze H-P, Wilson MVH, editors. Origin and Phylogenetic Relationships of Teleosts. Verlag Dr. Friedrich Pfeil; München, Germany: 2010. pp. 123–182. [Google Scholar]