SUMMARY
Micrococcal nuclease (MNase) is commonly used to map nucleosomes genome-wide, but nucleosome maps are affected by the degree of digestion. It has been proposed that many yeast promoters are not nucleosome-free but instead occupied by easily digested, unstable, “fragile” nucleosomes. We analyzed the histone content of all MNase-sensitive complexes by MNase-ChIP-seq and sonication-ChIP-seq. We find that yeast promoters are predominantly bound by non-histone protein complexes, with little evidence for fragile nucleosomes. We do detect MNase-sensitive nucleosomes elsewhere in the genome, including at transcription termination sites. However, they have high A/T content, suggesting that MNase sensitivity does not indicate instability, but rather the preference of MNase for A/T-rich DNA, such that A/T-rich nucleosomes are digested faster than G/C-rich nucleosomes. We confirm our observations by analyzing ChIP-exo, chemical mapping, and ATAC-seq data from other laboratories. Thus, histone ChIP-seq experiments are essential to distinguish nucleosomes from other DNA-binding proteins that protect against MNase.
Graphical abstract
INTRODUCTION
Nucleosomes are the basic units of DNA packaging in eukaryotes. They each comprise 147 bp of DNA wrapped around a histone octamer (two copies of each core histone: H2A, H2B, H3, and H4) in about 1.7 left-handed superhelical turns (Luger et al., 1997). In addition to their role in genome compaction, nucleosomes play a major role in gene regulation (Bai and Morozov, 2010; Han and Grunstein, 1988; Jiang and Pugh, 2009a; Li et al., 2007; Mellor, 2006; Radman-Livaja and Rando, 2010; Struhl, 1999; Wyrick et al., 1999). Most yeast genes and many genes in higher eukaryotes have a nucleosome-depleted region (NDR) just upstream of the transcription start site (TSS) flanked by arrays of regularly spaced nucleosomes (Bernstein et al., 2004; Jiang and Pugh, 2009b; Lee et al., 2004, 2007; Yuan et al., 2005). This organization has been attributed to the presence of non-histone complexes at promoters; these complexes create potential barriers that prevent nucleosome formation and organize nucleosomes into phased arrays (Chereji and Morozov, 2011; Chereji et al., 2011; Kornberg and Stryer, 1988; Mavrich et al., 2008). Additional contributions are made by poly(A) sequences, which are unfavorable for nucleosome formation (Mavrich et al., 2008; Segal and Widom, 2009; Struhl, 1985), and by multiple chromatin remodelers (Ganguli et al., 2014; Gkikopoulos et al., 2011; Musladin et al., 2014; Ocampo et al., 2016; Zhang et al., 2011). Chromatin organization is partly disrupted by transcription (Cole et al., 2014; Schwabish and Struhl, 2004; Shivaswamy et al., 2008; Studitsky et al., 2004).
Currently, nucleosome locations are typically determined by digestion of chromatin to mono-nucleosomes with micrococcal nuclease (MNase) followed by high-throughput sequencing (MNase-seq). However, analysis is complicated by the sequence preference of MNase (Hörz and Altenburger, 1981), which cleaves DNA ~30 times faster 5′ of A or T than it does 5′ of G or C (Dingwall et al., 1981). Furthermore, nucleosome maps are affected by the degree of MNase digestion. Several laboratories have reported the existence of “fragile nucleosomes” (Henikoff et al., 2011; Knight et al., 2014; Kubik et al., 2015; Weiner et al., 2010; Xi et al., 2011) located within “nucleosome-free regions” (NFRs), suggesting that promoters are associated with easily digested, unstable nucleosomes—that is, they are not nucleosome-free—and that they play a central role in gene regulation (Kubik et al., 2015; Pradhan et al., 2015). However, the existence of fragile nucleosomes is controversial, since high-resolution mapping by ChIP and tiling microarray reveals scant evidence for histones at yeast promoters (Fan et al., 2010). MNase-sensitive nucleosomes have also been reported in higher eukaryotes (Chereji et al., 2016; Ishii et al., 2015; Iwafuchi-Doi et al., 2016; Jeffers and Lieb, 2016; Mieczkowski et al., 2016; Teif et al., 2014; Vera et al., 2014; West et al., 2014).
The presence of fragile nucleosomes at promoters presents some interesting problems: how can transcription complexes form if the promoter is occupied by a fragile nucleosome? And how could a fragile nucleosome be positioned specifically to act as a nucleosome phasing barrier? Intrigued by these questions, we performed a comprehensive analysis of chromatin accessibility and MNase sensitivity in S. cerevisiae. By combining MNase digestion data with histone mapping data (MNase-ChIP-seq and sonication-ChIP-seq), we can distinguish nucleosomes from other DNA-binding proteins and analyze the histone content of fragile nucleosomes. Unexpectedly, we find that the vast majority of yeast promoters are bound by non-histone protein complexes, with little evidence for fragile nucleosomes. However, we do detect MNase-sensitive nucleosomes elsewhere in the genome, notably at transcription termination sites, consistent with the observations of Fan et al. (2010)). These MNase-sensitive nucleosomes have relatively high A/T content, suggesting that they are digested at a faster rate due to the A/T preference of the enzyme and that MNase sensitivity does not necessarily indicate an altered nucleosome structure. We obtained independent confirmation of our results by analysis of data from other laboratories obtained using complementary techniques: ChIP-exo (Rhee et al., 2014), chemical cleavage mapping (Henikoff et al., 2014), and ATAC-seq (Schep et al., 2015).
RESULTS
MNase-Sensitive Complexes at S. cerevisiae Promoters Do Not Contain Histones
We first examined how the level of MNase digestion affects the nucleosome distribution at promoters. We performed an MNase titration involving five levels of digestion and mapped nucleosomes genome-wide using paired-end sequencing. Aligning the +1 nucleosomes of all genes, we computed the average distributions of the fragment centers, using all DNA fragments with lengths in the [50, 200] bp interval. Figure 1A shows the average distributions of the fragment centers after MNase digestion. We observed a strong peak at the promoter in the least digested sample, which gradually decreased and eventually disappeared at the highest level of digestion to reveal a typical NDR flanked by arrays of phased nucleosomes. This MNase-sensitive peak has been attributed to fragile nucleosomes (Kubik et al., 2015; Weiner et al., 2010; Xi et al., 2011), which are apparently destroyed by MNase due to an altered structure.
Figure 1. MNase-Sensitive Complexes at Yeast Promoters Do Not Contain Histones.
(A) MNase-sensitive complexes at promoters contain very little H4 or H2B. Average DNA fragment center distributions are shown for all genes relative to the dyad of the +1 nucleosome for five levels of MNase digestion (inputs) for YDC439 (HA-tagged H4) and YDC443 (HA-tagged H2B) and for IPs from the most- and least-digested samples. Note that the inputs show a peak between the +1 and −1 nucleosomes that decreases with increasing MNase concentration, whereas there is no such peak in the IPs.
(B) 2D occupancies for the HA–H2B input samples (left in A; see Figure S1A for equivalent plots for HA–H4). The peak located between the +1 and −1 nucleosomes observed in the inputs in A is due to proteins that protect relatively short DNA fragments from MNase.
(C) 2D occupancies of the mildly digested H2B and H4 IP samples shown in (B) and Figure S1A.
(D) Average occupancy plots for H2B and H4 for the same strains obtained by sonication-ChIP-seq (no MNase was used); two biological replicate experiments are shown for each strain. The corresponding 2D occupancy heatmaps are shown in Figure S1D.
See also Figure S1.
We used two-dimensional (2D) occupancy plots to study the distribution of DNA fragments protected from MNase digestion. Instead of using the typical one-dimensional (1D) occupancy, which is obtained by stacking all the mapped reads regardless of their lengths, we show the relative occupancy given by DNA fragments of specific lengths in a matrix form. Figure 1B shows the 2D occupancy of the protected fragments that we obtained in our MNase digestion series (Figures 1A and S1A). In the early stages of digestion (left), the mono-nucleosomes are longer than 150 bp, indicating that they have residual linker DNA. As digestion proceeds, the protruding linker DNA is gradually trimmed away by the exonuclease activity of MNase, resulting in a series of mono-nucleosomal particles (Cole et al., 2016) that are eventually reduced to nucleosome core particles and smaller fragments (Figure 1B; rightmost). We also observed much shorter DNA fragments (~100 bp long) that are present in the early stages of digestion, but gradually disappear in the later stages. These MNase-sensitive fragments derive from the NDRs between the +1 and −1 nucleosomes and correspond to the decreasing peak in Figure 1A. This was confirmed by 2D occupancy difference plots (Figure S1B). Therefore, although the average distribution of the fragment centers (Figure 1A) suggests that an extra nucleosome is occupying the space between the +1 and −1 nucleosomes, the 2D occupancy plots (Figure 1B) reveal that promoter DNA fragments are much smaller than those expected from typical nucleosomes.
We addressed the question of whether the MNase-sensitive complexes at promoters contain histones using strains with HA-tagged H4 or H2B (Cole et al., 2014). We used tagged strains because the N-terminal HA tag is likely to be fully exposed due to the mobile nature of the histone tail domains and because the monoclonal anti-HA antibody gave much less background than polyclonal anti-histone antibodies did in ChIP-seq experiments. For both strains, we prepared input samples at five different levels of digestion (Figures 1A, 1B, 1C, and S1A). We picked a mildly digested sample and an extensively digested sample from each series for immunoprecipitation (IP). Both the H2B and H4 IPs from the mildly digested samples lack the short DNA fragments that cover the gap between the +1 and −1 nucleosomes in the corresponding input samples (Figures 1B and S1A). Thus, the MNase-sensitive complexes occupying promoters do not contain H2B or H4.
We confirmed this result by regular ChIP-seq experiments in which chromatin was fragmented by sonication instead of by MNase digestion (“sonication-ChIP-seq”). Figure 1D shows the average occupancies obtained in these experiments (Cole et al., 2014). The inputs for the tagged H2B and H4 strains showed a peak at the promoter, which probably represents a minor PCR amplification bias for promoter sequences. In any case, IP revealed that promoters are strongly depleted of both histones (Figures 1D and S1D). Although the trough in H2B and H4 signals located between the +1 and −1 nucleosomes is deep, it does not reach zero, suggesting that a small fraction of promoters might contain H2B and H4. However, 2D occupancy heatmaps for these sonication-ChIP-seq data (Figure S1D) show that most of the residual H2B and H4 signal at promoters derives from DNA fragments >~ 150 bp originating from the flanking +1 or −1 nucleosomes. Very few short DNA fragments in the IPs originate from the gap between the +1 and −1 nucleosomes.
Since ChIP and MNase conditions vary between labs, and these methods have their limitations, we analyzed data from other labs using independent alternative techniques, including ATAC-seq, ChIP-exo, and chemical mapping of H4. ATAC-seq (Schep et al., 2015) uses Tn5 transposase to insert sequencing adaptors directly into regions of accessible chromatin, which are generally where transcription factors bind, although some insertions occur in the linker DNA on both sides of a nucleosome. Thus, DNA fragment size differentiates between nucleosomes (larger footprints) and transcription factors (smaller footprints). At promoters, ATAC-seq detects smaller footprints, consistent with transcription factor binding, rather than the larger footprint expected from a nucleosome (Figure S1C), although it is conceivable that a fragile nucleosome might not block Tn5 insertions. To confirm our critical finding that MNase-sensitive complexes at promoters lack histones, we analyzed high-resolution ChIP-exo (Rhee et al., 2014) and chemical map data (Henikoff et al., 2014). ChIP-exo involves sonication-ChIP-seq followed by exonuclease digestion, which is blocked by the first histone-DNA cross-link it encounters, resulting in a precise location. This method should remove any histone-free DNA projecting into the promoter from the flanking nucleosomes. The average distributions of histone-DNA cross-links show that promoters are indeed depleted of all four core histones (Figure S1E). Chemical mapping of nucleosome dyads requires the use of an H4-S47C mutant, in which the cysteine residue is in close proximity to the nucleosome dyad, where it mediates hydroxyl radical cleavage of the DNA; the ends of the DNA fragments represent dyad locations (Brogaard et al., 2012; Henikoff et al., 2014). The absence of a peak in the NDR confirms the absence of H4 at promoters (Figure S1F). Both of these approaches indicate that the region between the +1 and −1 nucleosomes is strongly depleted of histones.
In conclusion, our data and those of others indicate that fragile nucleosomes, if they exist, are relatively rare at yeast promoters. Accordingly, we will refer to these promoter complexes as MNase-sensitive non-histone complexes instead of fragile nucleosomes. However, fragile nucleosomes have been reported at other sites in the yeast genome, which we investigate below.
Transcription Termination Sites Are Occupied by MNase-Sensitive Nucleosomes
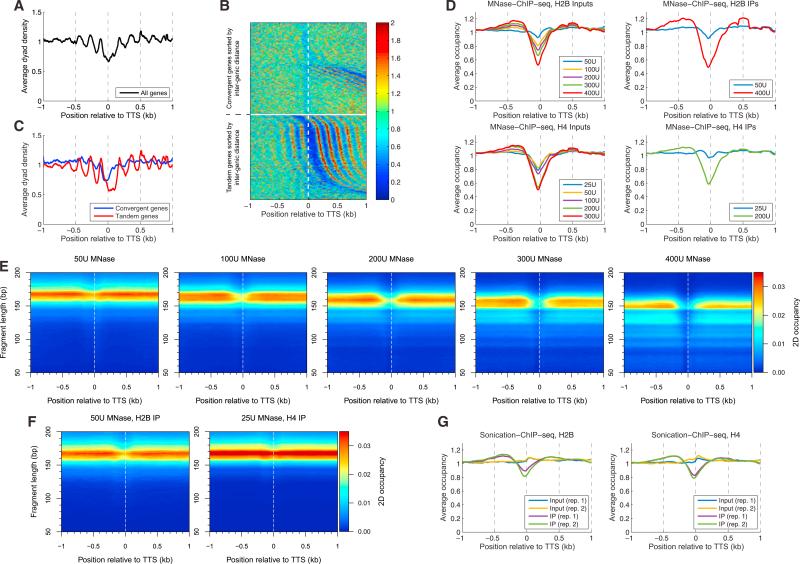
The transcription termination site (TTS) is MNase-sensitive in both S. cerevisiae (Fan et al., 2010) and Drosophila (Chereji et al., 2016). The average nucleosome organization at the TTS in yeast (Figure 2A) resembles the stereotypical organization that is observed at the TSS, with a relatively weak NDR flanked by phased nucleosome arrays. However, yeast genes are densely packed, such that intergenic regions are often very short. In the case of tandem genes, the TTS of the upstream gene is often <500 bp upstream of the TSS of the downstream gene, such that the nucleosomal arrays organized around this TSS may contribute to phasing around the TTS. We addressed this concern by separating out convergent and tandem genes (top and bottom in Figure 2B) and sorting them by intergenic distance. Clearly, the TTS is not a nucleosome phasing element at convergent genes, whereas the downstream TSS is a strong phasing element at tandem genes. Thus, the phasing observed at the TTS for all genes (Figure 2A) is actually due to the fact that about half of the TTSs of tandem genes are very close to a neighboring TSS. Separate plots of the average nucleosome distributions of tandem and convergent genes confirm that the nucleosome phasing observed at the TTS is due to the downstream promoters of tandem genes; it is not due to the TTS per se (Figure 2C).
Figure 2. Transcription Termination Sites Are Occupied by MNase-Sensitive Nucleosomes.
(A) Average nucleosome dyad distribution for all genes indicates weak nucleosome phasing relative to the TTS (data for 200U MNase).
(B) The weak phasing at the TTS is mostly due to neighboring TSSs. Heatmap of the dyad distribution at the TTS with convergent genes (top) separated from tandem genes (bottom) and sorted according to the distance between the genes.
(C) Average dyad distributions for convergent genes (blue line) and tandem genes (red line) show that nucleosome phasing due to the TTS is very poor.
(D) MNase-sensitive nucleosomes occupy the TTS. In the MNase-ChIP-seq data, average dyad distribution plots for input samples show that the complexes covering the TTS are digested relatively rapidly; similar plots for the IPs show that the complexes contain both H4 and H2B.
(E) 2D occupancy heatmaps for the H2B input data in (D).
(F) 2D occupancy heatmaps for the H2B and H4 IPs from the least-digested samples in (D).
(G) Sonication-ChIP-seq data show weak depletion of H2B and H4 at the TTS (two biological replicate experiments are shown) consistent with moderately high nucleosome occupancy at the TTS. Panels (D)–(G) contain the TTSs from all convergent genes plus tandem genes that are >1 kb from a downstream TSS.
See also Figure S2.
To study the effect of the TTS on nucleosome organization free of interference from nearby promoters, we selected the genes that have either a downstream convergent gene or a downstream tandem gene with a TSS at least 1 kb away from the reference TTS. We observed that these TTS loci are digested faster than gene bodies: the least digested samples exhibit a very weak NDR that becomes deeper as digestion proceeds (see MNase-ChIP-seq inputs in Figure 2D). It is evident from the 2D occupancy heatmaps (Figures 2E and S2A) that the DNA fragments covering the TTS are of nucleosome length but relatively MNase-sensitive because they are lost from the more digested samples, as shown by the widening gap at the TTS (Figures 2E, S2A, and S2B). Our MNase-ChIP-seq data show that the large majority of complexes covering the TTS contain H2B and H4 and that more TTS-containing nucleosomes can be immunoprecipitated from the mild digest than from the heavy digest (Figure 2D). These observations strongly suggest that the large majority of complexes covering the TTS are MNase-sensitive nucleosomes. We confirmed the presence of H4 and H2B at the TTS using our sonication-ChIP-seq data, which are unaffected by the vagaries of MNase digestion (Figures 2G and S2D). Both the mild MNase-ChIP-seq and the sonication-ChIP-seq IPs show a weak histone depletion from the TTS (Figures 2D and 2G), which may reflect the accumulation of terminating RNA polymerase II typical of yeast genes (Cole et al., 2014; Fan et al., 2010) and/or less efficient formaldehyde cross-linking to A/T-rich regions (see below).
To confirm our observations, we inspected data obtained by alternative methods (Figure S2). ATAC-seq (Schep et al., 2015) shows that very few Tn5 insertions occur at TTSs, consistent with the presence of nucleosomes rather than non-histone complexes (Figure S2C). A normal level of H4 at these TTSs is detected by chemical mapping (Henikoff et al., 2014; Figure S2F). However, ChIP-exo data (Rhee et al., 2014) suggest that all four core histones are about 50% depleted from the TTS (Figure S2E). This discrepancy may be linked to the very high A/T content of TTSs (see below). In conclusion, we present strong evidence that TTSs are occupied by fragile nucleosomes, as defined by their MNase sensitivity. However, given that TTSs are A/T rich, the MNase sensitivity of these nucleosomes may simply reflect their high A/T content, which the enzyme prefers, rather than an alternative nucleosome structure. We address this possibility below.
The MNase Sensitivity of Nucleosomes Depends on Their A/T Content
We tested whether MNase-sensitive nucleosomes are specific to the TTS or whether all A/T-rich nucleosomes are MNase sensitive. Using chemical mapping data (Henikoff et al., 2014), which are not affected by the problems associated with MNase, we identified the 5,000 nucleosomes with the highest A/T content. These nucleosomes contained between 102 and 130 A or T nucleotides (69.4% to 88.4% A/T); the average A/T content of random 147 bp sequences from the S. cerevisiae genome is 61.7%, with a standard deviation of 5.3%. A/T-rich nucleosomes are disproportionately located in intergenic regions (3,516 in intergenic regions and 1,484 in coding regions) (Figure 3A). We aligned these 5,000 nucleosomes on their dyads and sorted them according to their A/T content (Figure 3B). We followed their change in occupancy in an MNase titration (Figure 3C). While the most A/T-rich nucleosomes (top of each heatmap) are over-digested even after mild digestion, there is a progressive loss of nucleosomes with less extreme A/T content with increasing MNase concentration. Our MNase-ChIP-seq and sonication-ChIP-seq data support the identification of these 5,000 chemically mapped complexes as nucleosomes (Figures 3D and 3E). This analysis suggests that the MNase sensitivity of nucleosomes is strongly dependent on their A/T content, consistent with the preference of the enzyme for A/T-rich DNA.
Figure 3. A/T-Rich Nucleosomes Identified by Chemical Mapping Are MNase Sensitive.
(A) Heatmap analysis of the 5,000 most A/T-rich nucleosomes identified by chemical mapping (Henikoff et al., 2014) aligned on the nucleosome dyad and sorted according to A/T content.
(B) Genomic distribution of A/T-rich nucleosomes identified in A. Note that an A/T-rich nucleosome can be counted more than once; e.g., a −1 nucleosome can also be the +1 nucleosome on a neighboring divergent gene.
(C) The rate of MNase digestion depends on the A/T content of a nucleosome. MNase-ChIP-seq input occupancy (HA–H2B) for the 5,000 chemically mapped nucleosomes was aligned and sorted as in (B).
(D) MNase-sensitive A/T-rich complexes contain H4 and H2B. Average occupancy plots for the 5,000 nucleosomes are shown, with inputs and IPs for the least digested samples.
(E) Sonication-ChIP-seq: H4 and H2B are only mildly depleted from A/T-rich sites; two biological replicate experiments are shown for each strain.
See also Figure S3.
A/T-rich nucleosomes also tend to be slightly under-represented in our sonication-ChIP-seq data (Figures 3E and S3). This effect is small but clearly significant since it is observed for both H4 and H2B and in both biological replicate experiments (Figure 3E). A contributing factor may be PCR bias against extremely A/T-rich sequences. Such bias is not observed in the inputs (Figure 3E), but IP samples require more PCR cycles than input samples and any bias may be amplified. Another important factor is likely to be that thymine does not form cross-links (Lu et al., 2010), presumably because it has no amino group, which may reduce the efficiency of histone-DNA cross-linking in A/T-rich nucleosomes.
tRNA Genes Are Occupied by Transcription Factor Complexes that Are Less Accessible to MNase Than Nucleosomes
The tRNA genes are another set of loci where different degrees of MNase digestion result in variable occupancy. Our MNase titration series shows that tRNA genes are occupied by complexes that are digested more slowly than the upstream nucleosomes (Figure 4A). While relative nucleosome occupancy upstream of the tRNA genes decreased with increasing degree of digestion, the occupancy of the complex at tRNA genes showed the opposite trend, i.e., it increased relative to nucleosomes as the chromatin was digested (Figures 4B and S4A). We have shown previously that this complex contains the RNA polymerase III transcription factors TFIIIB and TFIIIC (Nagarajavel et al., 2013). The distinctive peak shape observed at tRNA genes in the MNase-ChIP-seq input samples (Figure 4A) represents TFIIIB bound at the 5′ end of the gene and TFIIIC bound at the 3′ end (Figures 4A and 4E) (Nagarajavel et al., 2013). MNase tends to cleave between the complexes and at the introns present in some tRNA genes, resulting in increased digestion of the 3′ region and a variable footprint, which is about the size of a nucleosome at most tRNA genes (Nagarajavel et al., 2013). This is clear from the 2D occupancy plots for IP of Brf1 (a TFIIIB sub-unit) and of Tfc1 (a TFIIIC sub-unit), which show that DNA fragments derived from the complete complex are of approximately nucleosomal length and that there is a major cut site between the two complexes (Figure 4E). As expected, MNase-ChIP-seq and sonication-ChIP-seq data show that the complex does not contain H2B or H4 (Figures 4A, 4C, and S4B). The TFIIIB and TFIIIC footprints are also detected by ATAC-seq (Schep et al., 2015) (Figure S4C). In conclusion, the TFIIIB-TFIIIC complex is more resistant to MNase than the nucleosome, although the junction between TFIIIB and TFIIIC is accessible to MNase and Tn5 transposase.
Figure 4. tRNA Genes Are Occupied by TFIIIB-TFIIIC Complexes, which Are Less Accessible to MNase Than Nucleosomes.
(A) tRNA genes are occupied by non-histone protein complexes that are digested more slowly than the flanking nucleosomes. Average DNA fragment center distribution plots from MNase-ChIP-seq are for all tRNA genes aligned on the tRNA gene start site. The IPs show that H4 and H2B are absent.
(B) 2D occupancy heatmaps for the H2B input data in (A).
(C) 2D occupancy heatmaps for the H2B and H4 IPs from extensively digested samples in (A). tRNA genes are bound by non-histone proteins with smaller footprints than a nucleosome.
(D) Sonication-ChIP-seq confirms the very low levels of H2B and H4 at tRNA genes.
(E) tRNA genes are occupied by a TFIIIB-TFIIIC complex: MNase-ChIP-seq for Brf1 (a TFIIIB sub-unit) and Tfc1 (a TFIIIC sub-unit). MNase first liberates the complete complex, which has a footprint similar to that of a nucleosome, and then cuts between TFIIIB, which binds upstream of the start site, and TFIIIC, which binds to the tRNA gene itself (Nagarajavel et al., 2013).
See also Figure S4.
The GAL1-GAL10 Promoter Is Bound by an MNase-Sensitive Complex that Does Not Contain Histones
The divergent promoter between the GAL1 and GAL10 genes contains four Gal4 binding sites and three putative Rsc3-binding sites within a small region of 100 bp, collectively referred to as UASg (Floer et al., 2010). Using MNase-ChIP-seq to detect the RSC ATP-dependent chromatin remodeling complex, it was proposed that UASg is occupied by a complex of RSC with a structurally altered nucleosome that has a smaller footprint (Floer et al., 2010). Our MNase titration series confirms the presence of a distinct complex covering UASg, which is digested relatively fast by MNase (Figure 5A). However, our MNase-ChIP-seq and sonication-ChIP-seq data indicate that both H4 and H2B are absent from UASg, showing that this complex does not contain a nucleosome of any kind (Figure 5A). It is possible, though unlikely, that the HA tag on both histones is masked by RSC or another protein. However, chemical mapping also indicates that H4 is absent from UASg, supporting our conclusion (Figure 5A). Henikoff et al. (2014) also noted the absence of H4 at UASg; they suggested that the putative RSC nucleosome may be insoluble, resulting in loss of H4 signal. However, the UASg complex is present in our inputs and so must be soluble under our conditions (Figure 5A). Our analysis suggests that the complex bound to UASg is a RSC-containing, MNase-sensitive, non-histone complex similar to those generally observed at promoters (Figure 1).
Figure 5. The GAL1-GAL10 Regulatory Region Is Bound by an MNase-Sensitive Complex that Does Not Contain Histones.
(A) The upstream activating region in the divergent GAL1-GAL10 gene promoter (UASg) is bound by an MNase-sensitive complex with a smaller footprint than a nucleosome (120–130 bp).
(B–D) MNase-ChIP-seq (B), Sonication-ChIP-seq (C), and chemical mapping (D) show that this MNase-sensitive complex does not contain H2B or H4.
MNase-Sensitive Nucleosomes and Non-histone Complexes Have Distinct Nucleotide Compositions
We have shown that different regions of the yeast genome are digested by MNase at different rates. Some MNase-sensitive complexes are indeed nucleosomes, whereas others do not contain histones. We developed an algorithm to distinguish between these two classes of MNase-sensitive complex. To detect all of the MNase-sensitive regions in the yeast genome, we compared the occupancy profiles obtained in an MNase titration using five different levels of digestion. We identified all the peaks corresponding to nucleosomes or any other protein complexes that protect against MNase and then tracked their changes in occupancy during digestion. We defined MNase-sensitive complexes as those showing a monotonic decrease in occupancy with increasing MNase concentration.
To distinguish between nucleosomal and non-nucleosomal MNase-sensitive regions, we used chemical mapping data (Henikoff et al., 2014) as an unbiased measure of H4. We computed the average chemical cleavage density in 147 bp intervals centered on the MNase-sensitive locations. The resulting histogram of MNase-sensitive sites is bimodal (Figure 6A), which allowed us to set a threshold that separates MNase-sensitive nucleosomes (high chemical cleavage density due to H4) from MNase-sensitive non-nucleosomal complexes (low chemical cleavage density due to absence of H4). A heatmap of cleavage sites sorted by average cleavage density is shown in Figure 6B. Of the 5,738 MNase-sensitive sites identified, 2,631 are nucleosomal and 3,107 are non-nucleosomal. Both types of site are flanked by arrays of phased nucleosomes. Heatmaps representing the MNase titration experiments confirm that the occupancy of both types of MNase-sensitive site decreases as digestion increases (Figure 6C). The presence or absence of nucleosomes at these sites was confirmed by the H2B and H4 levels detected by MNase-ChIP-seq (Figure 6D) and sonication-ChIP-seq (Figure 6E). In the case of MNase-sensitive nucleosomes, the sonication-ChIP-seq IP data indicated an apparently modest histone depletion (Figure 6E) which we attribute to reduced cross-linking efficiency of A/T-rich DNA, as described above. Finally, we tested the correlation of MNase sensitivity with A/T content by aligning the DNA sequences (Figure 6F). We observed that MNase-sensitive nucleosomes are A/T rich, as expected from the analysis above. In contrast, non-nucleosomal MNase-sensitive sites tend to have a lower than average A/T content.
Figure 6. MNase-Sensitive Nucleosomes and MNase-Sensitive Non-histone Complexes Have Distinct Nucleotide Compositions.
(A) Two types of MNase-sensitive site can be distinguished by chemical cleavage (Henikoff et al., 2014). MNase-sensitive sites were identified by analysis of relative rates of MNase digestion. The histogram shows the average chemical cleavage density within these MNase-sensitive regions. The bimodal distribution separates the non-nucleosomal sites (low chemical cleavage density due to absence of H4) from nucleosomal sites (high cleavage density).
(B) Heatmap of chemical cleavage at MNase-sensitive sites. The horizontal white line indicates the separation of the two peaks in (A).
(C) Heatmaps showing an MNase digestion series for the MNase-sensitive complexes aligned and sorted as in (B).
(D and E) MNase-ChIP-seq (D) and sonication-ChIP-seq (E) confirm that there are two types of MNase-sensitive complex: non-histone complexes (top) and nucleosomes (bottom).
(F) Heatmap of the A/T content of MNase-sensitive regions aligned and sorted as in (B). MNase-sensitive nucleosomes are A/T rich, whereas MNase-sensitive non-histone complexes have much lower A/T content.
(G) MNase-sensitive non-histone-DNA complexes are hypersensitive to DNase I (Hesselberth et al., 2009), but MNase-sensitive nucleosomes are not. The non-nucleosomal MNase-sensitive regions are DNase I hypersensitive sites and are bound by transcription factors such as TBP (Paul et al., 2015; Zentner and Henikoff, 2013; Zentner et al., 2015), Abf1 (Paul et al., 2015; Zentner and Henikoff, 2013; Zentner et al., 2015), and Reb1 (Paul et al., 2015; Zentner and Henikoff, 2013; Zentner et al., 2015).
See also Figure S5.
Using DNase-seq data (Hesselberth et al., 2009), we found that non-nucleosomal MNase-sensitive loci are generally hypersensitive to DNase I, whereas MNase-sensitive nucleosomes are insensitive to DNase I; this suggests that these nucleosomes are not necessarily unusual (Figure 6G). We also analyzed the binding of various transcription factors and remodelers at MNase-sensitive regions using ChIP-seq and ChEC-seq data published by others. We found that TBP (Zentner and Henikoff, 2013), Abf1 (Paul et al., 2015), and Reb1 (Zentner et al., 2015) all bind to non-nucleosomal MNase-sensitive sites, but not to MNase-sensitive nucleosomes (Figure 6G), consistent with the fact that the large majority (80%) of non-nucleosomal MNase-sensitive sites are in promoters.
A general analysis of all nucleosome sequences as a function of MNase digestion revealed that their average A/T content decreases as digestion proceeds (Figure 7). This observation suggests that the rate of internal digestion (endonuclease activity of MNase) or invasion (exonuclease activity of MNase) of a nucleosome depends on its A/T content, such that A/T-rich nucleosomes are digested faster than G/C-rich nucleosomes.
Figure 7. The Extent of MNase Digestion Affects the Nucleotide Composition of Mono-nucleosomal DNA.
(A) Probability distribution function for the G/C content of the mono-nucleosomes obtained in an MNase titration (50 to 400U). The G/C content of mono-nucleosomes increases during digestion as G/C-rich nucleosomes are released from oligo-nucleosomes and A/T-rich nucleosomes are destroyed.
(B) Cumulative distribution function for nucleosomal G/C content.
DISCUSSION
We have shown that different regions in the S. cerevisiae genome are digested by MNase at different rates. In particular, promoters and transcription termination regions are digested faster than the rest of the genome, whereas tRNA genes are digested more slowly. We performed a comprehensive analysis using data from all available histone-mapping techniques (MNase-ChIP-seq, sonication-ChIP-seq, ChIP-exo, and chemical mapping) in order to distinguish between nucleosomes and other DNA-binding protein complexes. We showed that the vast majority of promoters are bound by MNase-sensitive non-histone protein complexes. Unexpectedly, we found no evidence for fragile nucleosomes occupying the gap between the +1 and −1 nucleosomes; none of the histone maps support this hypothesis. The data strongly suggest that promoter NDRs are substantially free of nucleosomes. Instead, they may be occupied by a Pol II transcription complex akin to the Pol III transcription complex (TFIIIB-TFIIIC), which is stably bound to tRNA genes and acts as a strong nucleosome phasing barrier. Another possibility is that promoter NDRs are occupied by large chromatin remodeling complexes such as RSC. Although many transcription factors have been mapped to Pol II promoters by ChIP-seq, their occupancies relative to nucleosomes are unknown. It is important to identify the MNase-sensitive, relatively stable, high-occupancy complexes that act as nucleosome phasing barriers at Pol II promoters. It is conceivable that their composition varies from gene to gene and that inactive promoters may be occupied by a repressive complex.
Our observations are inconsistent with reports of fragile nucleosomes at yeast promoters (Kubik et al., 2015; Weiner et al., 2010; Xi et al., 2011). We all agree that promoters are occupied by MNase-sensitive complexes that have footprints similar in size to a nucleosome. The crucial evidence comes from histone ChIP-seq data, which establish the presence or absence of histones. Weiner et al. (2010) did not do histone ChIP-seq experiments but noted that some MNase-protected DNA fragments may not be nucleosomal. Xi et al. (2011) reported fragile nucleosomes located within NDRs at a small number of Pol II promoters and also at many tRNA genes, although whether they contain histone is unclear. Kubik et al. (2015) performed paired-end sequencing and reported fragile nucleosomes located within NDRs that are wide enough to form a nucleosome. However, analysis of the fragile nucleosomes reported by Kubik et al. (2015) using our data and those of others clearly show that histones are absent from these MNase-sensitive complexes (Figure S5). A possible concern could be that other proteins at promoters may mask the N-terminal HA tag on both histones in our ChIP experiments, but not the epitopes recognized by the antibodies used by Kubik et al. (2015). However, we do detect MNase-sensitive nucleosomes elsewhere in the genome that act essentially as positive controls, suggesting that epitope masking is very unlikely. Furthermore, their histone ChIP-seq data also show histone depletion (Figure S5). Their sonicated, mildly digested chromatin is largely composed of DNA fragments much longer than a nucleosome, with consequent poor resolution between the NDR and the flanking nucleosomes (Figure S5). We believe that long histone-bound DNA fragments originating from the +1 and −1 nucleosomes that overlap the NDR explain the discrepancy between our data and theirs. Our MNase-ChIP-seq and sonication-ChIP-seq data have shorter average lengths, facilitating the distinction between NDR and flanking nucleosome fragments (Figure S6). We also performed biological replicate experiments, including input samples, which gave consistent results. In conclusion, our data clearly show that H2B and H4 are generally heavily depleted from promoters. In addition, our H3 ChIP-seq data (Qiu et al., 2016) and those of Fan et al. (2010), obtained using a polyclonal antibody, confirm that H3 is also depleted from promoter NDRs.
On the other hand, our data are consistent with the presence of fragile nucleosomes elsewhere in the genome (Henikoff et al., 2011; Weiner et al., 2010; Xi et al., 2011). We detected MNase-sensitive nucleosomes at TTSs and other A/T-rich sites. Multiple histone mapping techniques confirmed that these sites are mostly occupied by nucleosomes, although there is a weak depletion that can be explained by the presence of terminating Pol II on some genes in some cells. In general, the rate of digestion of a nucleosome increases with its A/T content. This is not surprising, given that MNase prefers to cut A/T-rich DNA, since G/C-rich nucleosomes have fewer potential cut sites. Essentially, each nucleosome has its own digestion kinetics, which are at least partly determined by the nucleosome's A/T content. Overall, this effect is relatively minor because otherwise nucleosome occupancies determined by MNase-seq would not be as even as they generally appear in individual gene maps. However, care is needed when comparing the occupancies of nucleosomes with very different A/T contents because their relative levels will change as digestion proceeds.
In summary, we confirm the presence of MNase-sensitive nucleosomes at A/T-rich sites in yeast, but not generally at promoter NDRs. The term “fragile” suggests instability, but MNase-sensitive nucleosomes are not necessarily less thermodynamically stable than more resistant nucleosomes. This is because sensitivity most likely reflects the preference of MNase for A/T-rich sequences rather than altered nucleosome structure. Of course, nucleosome stability does depend on DNA sequence, but there is no evidence that MNase-sensitive nucleosomes are structurally altered. Their biological significance is therefore questionable, and the term “fragile” may be misleading. Interestingly, the relatively unstable, salt-labile nucleosomes containing H3.3 and H2A.Z located at regulatory regions in higher organisms are not MNase-sensitive (Jin and Felsenfeld, 2007; Jin et al., 2009).
Our study reveals that paired-end histone ChIP-seq experiments are essential in order to distinguish nucleosomes from other DNA-binding proteins that offer protection against MNase. MNase-sensitive nucleosomes with relatively high A/T content have been identified and confirmed in Drosophila (Chereji et al., 2016), but fragile nucleosomes reported in higher organisms with very large genomes (mouse and human) must be verified by expensive, high-coverage histone ChIP-seq experiments.
STAR*METHODS
Detailed methods are provided in the online version of this paper and include the following:
KEY RESOURCES TABLE
CONTACT FOR REAGENT AND RESOURCE SHARING
EXPERIMENTAL MODEL AND SUBJECT DETAILS
- METHOD DETAILS
- ○ MNase-ChIP-seq
- ○ Bioinformatics
- ○ Two-Dimensional Occupancy Plots
- ○ Detection of MNase-Sensitive Loci
QUANTIFICATION AND STATISTICAL ANALYSIS
- DATA AND SOFTWARE AVAILABILITY
- ○ Data Resources
- ○ Software Resources
STAR*METHODS
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-HA Agarose | Roche | Cat#11815016001; RRID: AB_390914 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| cOmplete, EDTA-free Protease Inhibitor Cocktail | Roche | 05056489001 |
| Ampure beads | Beckman Coulter | A63880 |
| Proteinase K | Invitrogen | 25530049 |
| Micrococcal nuclease | Worthington | LS004798 |
| Formaldehyde | Sigma-Aldrich | F8775-500ML |
| T4 polynucleotide kinase | Affymetrix | 70031X |
| Klenow-exo | NEB | M0210L |
| Quick ligation kit | NEB | M220L |
| dNTPs | NEB | N0447L |
| Phusion High Fidelity PCR Master Mix | NEB | M0531L |
| Centrifugal filter Millipore Ultrafree-MC, 0.45-μm | Millipore | UFC30HV25 |
| HA-peptide | Roche | 11666975001 |
| NEBNext Multiplex Oligos | NEB | E7335L |
| PreCR Repair Mix | NEB | M0309L |
| NEBNext Ultra DNA library Prep Kit | NEB | E7370L |
| Deposited Data | ||
| MNase-ChIP-seq for histones H2B and H4 | This paper | GEO: GSE83123 |
| Sonication-ChIP-seq for H2B and H4 | Cole et al., 2014 | GEO: GSE54524 |
| ATAC-seq | Schep et al., 2015 | GEO: GSE66386 |
| H4-S47C chemical cleavage | Henikoff et al., 2014 | GEO: GSE51949 |
| ChEC-seq | Zentner et al., 2015 | GEO: GSE67453 |
| MNase-ChIP-seq for TFIIIB and TFIIIC | Nagarajavel et al., 2013 | GEO: GSE44586 |
| MNase-ChIP-seq | Zentner and Henikoff, 2013 | GEO: GSE44200 |
| Sonication-ChIP-seq | Parnell et al., 2015 | GEO: GSE65594 |
| ChIP-exo | Rhee et al., 2014 | SRA: SRA059355 |
| DNase-seq | Hesselberth et al., 2009 | SRA: SRP000620 |
| Experimental Models: Organisms/Strains | ||
| YDC439, S. cerevisiae: MATa ade2-1 leu2-3,112 trp1-1 ura3-1 hht1-hhf1Δ::LEU2 URA3::HHT2-HA-HHF2 | David Clark lab | Cole et al., 2014 |
| YDC443, S. cerevisiae: MATa can1-100 his3-11 leu2-3,112 lys2Δ trp1-1 ura3-1 hta2-htb2Δ::TRP1 HA-6His-HTB1::HIS3 | David Clark lab | Cole et al., 2014 |
| YDC111, S. cerevisiae: MATa ade2-1 can1-100 leu2-3,112 trp1-1 ura3-1 | David Clark lab | Kim et al., 2006 |
| Software and Algorithms | ||
| Bowtie2 | Langmead and Salzberg, 2012 | http://bowtie-bio.sourceforge.net/bowtie2/index.shtml |
| Samtools | Li et al., 2009 | http://samtools.sourceforge.net/ |
| MATLAB | MathWorks, Inc. | https://www.mathworks.com/products/matlab.html |
| Custom script for plotting the 2D occupancy heatmaps | This paper | https://github.com/rchereji/plot2DO |
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for reagents may be directed to and will be fulfilled by the Lead Contact, David J. Clark (clarkda@mail.nih.gov).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
S. cerevisiae strains used in this study were grown using standard methods and are listed in the Key Resources Table.
METHOD DETAILS
MNase-ChIP-seq
Strains YDC111 (no tag control), YDC439 (HA-tagged H4) and YDC443 (HA-tagged H2B) (Cole et al., 2014) were grown to log-phase in synthetic complete (SC) medium. Cells were fixed with 1% formaldehyde (15 min at room temperature) and glycine was added to 0.5 M for 5 min. Cells were collected by filter, resuspended in the same medium, aliquoted in batches of ~200 OD600 units and stored at −80°C. Nuclei were prepared as described (Cole et al., 2011) and divided into seven aliquots of 400 μL in Digestion Buffer, which were digested with increasing amounts of MNase. The digestion was stopped by adding 100 μL of Stop buffer (5 mM Na-EDTA, pH 7.5, 5 mM Na-EGTA, 0.05% NP40). Nuclear debris was removed by centrifugation (1 min at 12,000 g and 4°C). The supernatant was passed through a centrifugal filter (Millipore Ultrafree-MC, 0.45-μm; UFC30HV25). The extent of digestion was verified by analysis in an agarose gel (Figure S6) after reversing the cross-links (Cole et al., 2014). The remainder of each sample was stored at −80°C prior to IP. Samples were thawed on ice and incubated with 20 μL anti-HA agarose (Roche 11815016001) that had been washed three times with PBS containing BSA (5 mg/ml). Binding was performed in an Eppendorf Thermomixer for 30 min at 1,400 rpm and 4°C. The supernatant was removed. The agarose was washed three times with 0.15 M NaCl, 10 mM Tris-HCl pH 8.0, 0.05% NP40, 1 mM Na-EGTA and protease inhibitors (10 min each at 4°C). Bound nucleosomes were eluted with 100 mL 0.1 μg/ml HA-peptide (Roche 11666975001) in PBS in a Thermomixer (30 min, 1,000 rpm, 4°C). The eluate was removed, the cross-links were reversed and DNA was purified (Cole et al., 2014). Paired-end libraries were prepared from IP and input DNA either as described (Cole et al., 2014) or by using Illumina paired-end kits from New England Biolabs (E7370 and E7335). Agencourt AMPure XP beads (Beckman-Coulter A63880) were used to purify the DNA after adaptor ligation and the PCR products. IP-DNA from YDC111 (no tag control) was prepared for sequencing in parallel, but little final product was obtained.
Bioinformatics
Paired-end reads were aligned to the S. cerevisiae reference genome sacCer3, using Bowtie2 (Langmead and Salzberg, 2012) with parameters -X 1000–very-sensitive, to map sequences up to 1 kb with maximum accuracy. Insert fragments of 50 - 200 bp were selected for analysis. Raw genome-wide occupancy profiles were computed in MATLAB by counting the number of DNA fragments that overlapped with every bp, using the bioinformatics toolbox, and normalized to an average occupancy of 1 for each chromosome. Distributions of DNA fragment centers were constructed by stacking only the nucleotides corresponding to the fragment centers. The 2D occupancy heatmaps were generated in R using the plot2DO package described below. Heatmaps where multiple loci are shown as different rows were generated in MATLAB using the bioinformatics toolbox to import data and the heatmap plotting function (http://www.mathworks.com/matlabcentral/fileexchange/24253-customizable-heat-maps). To visualize specific loci, igvtools was used to create tracks (tdf files) for viewing with the IGV browser (Robinson et al., 2011). Transcript end coordinates (TSS and TTS) were obtained from (Park et al., 2014). Functional regions (Figure 3B) were defined using S288C_reference_genome_R64-2-1_20150113.tgz, available at http://downloads.yeastgenome.org/sequence/S288C_reference/genome_releases/.
Two-Dimensional Occupancy Plots
To study nucleosome distributions more precisely, instead of using the typical one-dimensional (1D) occupancy (or coverage) obtained by stacking all the mapped reads, regardless of their lengths (Figure S7A), we distinguished the contribution to occupancy of DNA fragments having different lengths, using two-dimensional (2D) plots. Specifically, for each fragment size, we computed the relative occupancy due to all DNA fragments of this size. Then we created a matrix with the rows representing the relative occupancy corresponding to each DNA fragment size, and the columns representing the number of fragments of different sizes covering each base pair in promoter regions. This 2D representation of the occupancy (Figure S7B) allowed us to distinguish nucleosomes from other DNA-binding proteins, which have different footprints, as shown by the lengths of the MNase-protected DNA fragments. The columns mimic the separation of DNA fragments in a gel electrophoresis experiment, while the rows provide information on their genomic locations. The marginal distribution obtained by summing all values from each column of this matrix will generate the usual 1D occupancy (Figure S7A), while the marginal distribution obtained by summing all values from each row of this matrix will generate the DNA fragment length histogram (Figure S7C).
The software used for computing and visualizing the 2D occupancies is freely available on the GitHub repository page: https://github.com/rchereji/plot2DO. Plot2DO is an R script that can be launched from the terminal. For more information on usage options, consult the online documentation from the GitHub page.
Detection of MNase-Sensitive Loci
Different regions of the S. cerevisiae genome are digested by MNase at different rates. Although MNase-sensitive regions are easily detectable in an MNase titration, they are not necessarily nucleosomes, as other proteins can also protect against MNase. To distinguish between the two classes of MNase-sensitive complexes (nucleosomal or non-histone proteins), we used the following algorithm. Using an MNase titration with five different levels of digestion of the HA-tagged H2B strain (YDC443), we first pooled all sequencing reads from the five samples with lengths between 50 and 200 bp, we trimmed all reads to 51 bp, by extending the read center by 25 bp in both directions, and then we constructed the occupancy/coverage profile by stacking all trimmed reads. We normalized this occupancy such that its average is 1 for every chromosome, and we smoothed this profile by a moving average filter with a window size of 25 bp. Then we identified all the peaks corresponding to nucleosomes or any other protein complexes that protect against MNase digestion, using the findpeaks MATLAB function, with a minimum peak-to-peak distance of 120 bp, and a minimum peak height of 0.5 (50% of the genomic average occupancy). To identify the MNase-sensitive regions, we selected only those peaks for which, during the four increases in MNase concentration, the occupancy decreased at least three out of four times, and the overall occupancy decrease during the titration was at least 50% of the initial occupancy. These strong requirements resulted in identification of 5738 MNase-sensitive sites. To distinguish between nucleosomal and non-nucleosomal MNase-sensitive regions, we used the chemical mapping data (Henikoff et al., 2014) as an unbiased measure for the probability of finding histone H4 bound to DNA. We computed the average chemical cleavage density in 147 bp intervals centered on the MNase-sensitive peak locations. The histogram of average density of chemical cleavages at these sites is bimodal (Figure 6A), which allowed us to set a threshold that separates MNase-sensitive nucleosomes (sites with a high chemical cleavage density due to H4) from MNase-sensitive non-histone complexes (sites with a low chemical cleavage density). Out of the 5738 MNase-sensitive sites, we identified 2631 nucleosomal sites and 3107 sites that are occupied by non-histone complexes. Note that the procedure of trimming the reads to a reduced footprint of 51 bp, described above, was only used for the purpose of enhancing the linker regions and to simplify the identification of MNase-sensitive region centers, and everywhere else in this study we used the original fragment sizes, as obtained from the sequencer. Also, the strong requirements of monotonic decrease in occupancy and an overall occupancy decrease of at least 50% could be relaxed (e.g., by just comparing the occupancies obtained in the mildest and the most extensive digestion levels), in which case more MNase-sensitive regions would be detected. MNase-sensitivity should not be understood as a binary property of nucleosomes, but as a continuous measure of the rate of digestion by MNase of the specific DNA sequence that is contained by each nucleosome.
QUANTIFICATION AND STATISTICAL ANALYSIS
The detection of NDR and flanking nucleosome positions was done as in (Ocampo et al., 2016). Average dyad densities were computed by aligning all yeast genes at their +1 nucleosome dyad positions, and averaging the corresponding 2kb genomic regions, centered at the +1 nucleosome dyad. The 2D occupancy heatmaps were generated using the R package plot2DO. In Figure 7, the GC-content distributions were obtained by first computing the fraction of G and C nucleotides of every mono-nucleosomal paired-end read, and then computing the probability distribution functions (PDF, Figure 7A) and cumulative distribution functions (CDF, Figure 7B) for these fractions.
DATA AND SOFTWARE AVAILABILITY
Data Resources
The accession number for the MNase-ChIP-seq data reported in this paper is GEO: GSE83123. GEO accession numbers for the other datasets used in this study are: GSE54524 – sonication-ChIP-seq (Cole et al., 2014); GSE66386 – ATAC-seq (Schep et al., 2015); GSE51949 – chemical cleavage (Henikoff et al., 2014); GSE67453 – ChEC-seq (Zentner et al., 2015); GSE44586 – MNase-ChIP-seq (Nagarajavel et al., 2013); GSE44200 – MNase-ChIP-seq (Zentner and Henikoff, 2013); GSE65594 – sonication-ChIP-seq (Parnell et al., 2015). ChIP-exo (Rhee et al., 2014) (SRA059355) and DNase-seq data (Hesselberth et al., 2009) (SRP000620) are available at the NCBI Sequence Read Archive (SRA, http://www.ncbi.nlm.nih.gov/sra).
Software Resources
For generating 2D occupancy heatmaps, we developed an R package, plot2DO, which is freely available on the GitHub repository page: https://github.com/rchereji/plot2DO.
Supplementary Material
Highlights.
MNase-sensitive complexes at yeast promoters do not contain histones
Transcription termination sites are occupied by MNase-sensitive nucleosomes
The MNase sensitivity of nucleosomes depends on their A/T content
A/T-rich nucleosomes are digested faster than G/C-rich nucleosomes
ACKNOWLEDGMENTS
We thank Alan Hinnebusch, Gary Felsenfeld, Natalia Petrenko, and Peter Eriksson for discussion and helpful comments on the manuscript. We thank Ho-Sung Rhee and Bongsoo Park for helpful discussions regarding the ChIP-exo experiments. We thank the NHLBI Core Facility (Yan Luo, Poching Liu, and Jun Zhu) for paired-end sequencing. This study utilized the computational resources of the NIH HPC Biowulf cluster (https://hpc.nih.gov). This work was supported by the Intramural Research Program of the National Institutes of Health (NICHD).
Footnotes
AUTHOR CONTRIBUTIONS
Conceptualization, R.V.C. and D.J.C.; Methodology, R.V.C., J.O., and D.J.C.; Software, R.V.C.; Validation, R.V.C. and J.O.; Formal Analysis, R.V.C.; Investigation, J.O.; Writing – Original Draft, R.V.C.; Writing – Review & Editing, R.V.C., J.O., and D.J.C.; Visualization, R.V.C.; Supervision, D.J.C.
SUPPLEMENTAL INFORMATION
Supplemental Information includes seven figures and can be found with this article online at http://dx.doi.org/10.1016/j.molcel.2016.12.009.
REFERENCES
- Bai L, Morozov AV. Gene regulation by nucleosome positioning. Trends Genet. 2010;26:476–483. doi: 10.1016/j.tig.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Liu CL, Humphrey EL, Perlstein EO, Schreiber SL. Global nucleosome occupancy in yeast. Genome Biol. 2004;5:R62. doi: 10.1186/gb-2004-5-9-r62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogaard K, Xi L, Wang J-P, Widom J. A map of nucleosome positions in yeast at base-pair resolution. Nature. 2012;486:496–501. doi: 10.1038/nature11142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chereji RV, Morozov AV. Statistical mechanics of nucleosomes constrained by higher-order chromatin structure. J. Stat. Phys. 2011;144:379–404. doi: 10.1007/s10955-011-0214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chereji RV, Tolkunov D, Locke G, Morozov AV. Statistical mechanics of nucleosome ordering by chromatin-structure-induced two-body interactions. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2011;83:050903. doi: 10.1103/PhysRevE.83.050903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chereji RV, Kan T-W, Grudniewska MK, Romashchenko AV, Berezikov E, Zhimulev IF, Guryev V, Morozov AV, Moshkin YM. Genome-wide profiling of nucleosome sensitivity and chromatin accessibility in Drosophila melanogaster. Nucleic Acids Res. 2016;44:1036–1051. doi: 10.1093/nar/gkv978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole HA, Howard BH, Clark DJ. Activation-induced disruption of nucleosome position clusters on the coding regions of Gcn4-dependent genes extends into neighbouring genes. Nucleic Acids Res. 2011;39:9521–9535. doi: 10.1093/nar/gkr643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole HA, Ocampo J, Iben JR, Chereji RV, Clark DJ. Heavy transcription of yeast genes correlates with differential loss of histone H2B relative to H4 and queued RNA polymerases. Nucleic Acids Res. 2014;42:12512–12522. doi: 10.1093/nar/gku1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole HA, Cui F, Ocampo J, Burke TL, Nikitina T, Nagarajavel V, Kotomura N, Zhurkin VB, Clark DJ. Novel nucleosomal particles containing core histones and linker DNA but no histone H1. Nucleic Acids Res. 2016;44:573–581. doi: 10.1093/nar/gkv943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C, Lomonossoff GP, Laskey RA. High sequence specificity of micrococcal nuclease. Nucleic Acids Res. 1981;9:2659–2673. doi: 10.1093/nar/9.12.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Moqtaderi Z, Jin Y, Zhang Y, Liu XS, Struhl K. Nucleosome depletion at yeast terminators is not intrinsic and can occur by a transcriptional mechanism linked to 3′-end formation. Proc. Natl. Acad. Sci. USA. 2010;107:17945–17950. doi: 10.1073/pnas.1012674107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floer M, Wang X, Prabhu V, Berrozpe G, Narayan S, Spagna D, Alvarez D, Kendall J, Krasnitz A, Stepansky A, et al. A RSC/nucleosome complex determines chromatin architecture and facilitates activator binding. Cell. 2010;141:407–418. doi: 10.1016/j.cell.2010.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguli D, Chereji RV, Iben JR, Cole HA, Clark DJ. RSC-dependent constructive and destructive interference between opposing arrays of phased nucleosomes in yeast. Genome Res. 2014;24:1637–1649. doi: 10.1101/gr.177014.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkikopoulos T, Schofield P, Singh V, Pinskaya M, Mellor J, Smolle M, Workman JL, Barton GJ, Owen-Hughes T. A role for Snf2-related nucleosome-spacing enzymes in genome-wide nucleosome organization. Science. 2011;333:1758–1760. doi: 10.1126/science.1206097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M, Grunstein M. Nucleosome loss activates yeast downstream promoters in vivo. Cell. 1988;55:1137–1145. doi: 10.1016/0092-8674(88)90258-9. [DOI] [PubMed] [Google Scholar]
- Henikoff JG, Belsky JA, Krassovsky K, MacAlpine DM, Henikoff S. Epigenome characterization at single base-pair resolution. Proc. Natl. Acad. Sci. USA. 2011;108:18318–18323. doi: 10.1073/pnas.1110731108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S, Ramachandran S, Krassovsky K, Bryson TD, Codomo CA, Brogaard K, Widom J, Wang J-P, Henikoff JG. The budding yeast Centromere DNA Element II wraps a stable Cse4 hemisome in either orientation in vivo. eLife. 2014;3:e01861. doi: 10.7554/eLife.01861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselberth JR, Chen X, Zhang Z, Sabo PJ, Sandstrom R, Reynolds AP, Thurman RE, Neph S, Kuehn MS, Noble WS, et al. Global mapping of protein-DNA interactions in vivo by digital genomic footprinting. Nat. Methods. 2009;6:283–289. doi: 10.1038/nmeth.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörz W, Altenburger W. Sequence specific cleavage of DNA by micrococcal nuclease. Nucleic Acids Res. 1981;9:2643–2658. doi: 10.1093/nar/9.12.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii H, Kadonaga JT, Ren B. MPE-seq, a new method for the genome-wide analysis of chromatin structure. Proc. Natl. Acad. Sci. USA. 2015;112:E3457–E3465. doi: 10.1073/pnas.1424804112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwafuchi-Doi M, Donahue G, Kakumanu A, Watts JA, Mahony S, Pugh BF, Lee D, Kaestner KH, Zaret KS. The pioneer transcription factor FoxA maintains an accessible nucleosome configuration at enhancers for tissue-specific gene activation. Mol. Cell. 2016;62:79–91. doi: 10.1016/j.molcel.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers TE, Lieb JD. Nucleosome fragility is associated with future transcriptional response to developmental cues and stress in C. elegans. Genome Res. 2016 doi: 10.1101/gr.208173.116. Published online November 14, 2016. http://dx.doi.org/10.1101/gr.208173. [DOI] [PMC free article] [PubMed]
- Jiang C, Pugh BF. Nucleosome positioning and gene regulation: advances through genomics. Nat. Rev. Genet. 2009a;10:161–172. doi: 10.1038/nrg2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Pugh BF. A compiled and systematic reference map of nucleosome positions across the Saccharomyces cerevisiae genome. Genome Biol. 2009b;10:R109. doi: 10.1186/gb-2009-10-10-r109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Felsenfeld G. Nucleosome stability mediated by histone variants H3.3 and H2A.Z. Genes Dev. 2007;21:1519–1529. doi: 10.1101/gad.1547707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Zang C, Wei G, Cui K, Peng W, Zhao K, Felsenfeld G. H3.3/H2A.Z double variant-containing nucleosomes mark ‘nucleosome-free regions’ of active promoters and other regulatory regions. Nat. Genet. 2009;41:941–945. doi: 10.1038/ng.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, McLaughlin N, Lindstrom K, Tsukiyama T, Clark DJ. Activation of Saccharomyces cerevisiae HIS3 results in Gcn4p-dependent, SWI/SNF-dependent mobilization of nucleosomes over the entire gene. Mol. Cell. Biol. 2006;26:8607–8622. doi: 10.1128/MCB.00678-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight B, Kubik S, Ghosh B, Bruzzone MJ, Geertz M, Martin V, Dénervaud N, Jacquet P, Ozkan B, Rougemont J, et al. Two distinct promoter architectures centered on dynamic nucleosomes control ribosomal protein gene transcription. Genes Dev. 2014;28:1695–1709. doi: 10.1101/gad.244434.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg RD, Stryer L. Statistical distributions of nucleosomes: nonrandom locations by a stochastic mechanism. Nucleic Acids Res. 1988;16(14A):6677–6690. doi: 10.1093/nar/16.14.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubik S, Bruzzone MJ, Jacquet P, Falcone J-L, Rougemont J, Shore D. Nucleosome stability distinguishes two different promoter types at all protein-coding genes in yeast. Mol. Cell. 2015;60:422–434. doi: 10.1016/j.molcel.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C-K, Shibata Y, Rao B, Strahl BD, Lieb JD. Evidence for nucleosome depletion at active regulatory regions genome-wide. Nat. Genet. 2004;36:900–905. doi: 10.1038/ng1400. [DOI] [PubMed] [Google Scholar]
- Lee W, Tillo D, Bray N, Morse RH, Davis RW, Hughes TR, Nislow C. A high-resolution atlas of nucleosome occupancy in yeast. Nat. Genet. 2007;39:1235–1244. doi: 10.1038/ng2117. [DOI] [PubMed] [Google Scholar]
- Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128:707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K, Ye W, Zhou L, Collins LB, Chen X, Gold A, Ball LM, Swenberg JA. Structural characterization of formaldehyde-induced cross-links between amino acids and deoxynucleosides and their oligomers. J. Am. Chem. Soc. 2010;132:3388–3399. doi: 10.1021/ja908282f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- Mavrich TN, Ioshikhes IP, Venters BJ, Jiang C, Tomsho LP, Qi J, Schuster SC, Albert I, Pugh BF. A barrier nucleosome model for statistical positioning of nucleosomes throughout the yeast genome. Genome Res. 2008;18:1073–1083. doi: 10.1101/gr.078261.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor J. Dynamic nucleosomes and gene transcription. Trends Genet. 2006;22:320–329. doi: 10.1016/j.tig.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Mieczkowski J, Cook A, Bowman SK, Mueller B, Alver BH, Kundu S, Deaton AM, Urban JA, Larschan E, Park PJ, et al. MNase titration reveals differences between nucleosome occupancy and chromatin accessibility. Nat. Commun. 2016;7:11485. doi: 10.1038/ncomms11485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musladin S, Krietenstein N, Korber P, Barbaric S. The RSC chromatin remodeling complex has a crucial role in the complete remodeler set for yeast PHO5 promoter opening. Nucleic Acids Res. 2014;42:4270–4282. doi: 10.1093/nar/gkt1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajavel V, Iben JR, Howard BH, Maraia RJ, Clark DJ. Global ‘bootprinting’ reveals the elastic architecture of the yeast TFIIIB-TFIIIC transcription complex in vivo. Nucleic Acids Res. 2013;41:8135–8143. doi: 10.1093/nar/gkt611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocampo J, Chereji RV, Eriksson PR, Clark DJ. The ISW1 and CHD1 ATP-dependent chromatin remodelers compete to set nucleosome spacing in vivo. Nucleic Acids Res. 2016;44:4625–4635. doi: 10.1093/nar/gkw068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Morris AR, Battenhouse A, Iyer VR. Simultaneous mapping of transcript ends at single-nucleotide resolution and identification of widespread promoter-associated non-coding RNA governed by TATA elements. Nucleic Acids Res. 2014;42:3736–3749. doi: 10.1093/nar/gkt1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell TJ, Schlichter A, Wilson BG, Cairns BR. The chromatin remodelers RSC and ISW1 display functional and chromatin-based promoter antagonism. eLife. 2015;4:e06073. doi: 10.7554/eLife.06073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul E, Zhu ZI, Landsman D, Morse RH. Genome-wide association of mediator and RNA polymerase II in wild-type and mediator mutant yeast. Mol. Cell. Biol. 2015;35:331–342. doi: 10.1128/MCB.00991-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan SK, Xue Y, Carey MF. Fragile nucleosomes influence pol II promoter function. Mol. Cell. 2015;60:342–343. doi: 10.1016/j.molcel.2015.10.035. [DOI] [PubMed] [Google Scholar]
- Qiu H, Chereji RV, Hu C, Cole HA, Rawal Y, Clark DJ, Hinnebusch AG. Genome-wide cooperation by HAT Gcn5, remodeler SWI/SNF, and chaperone Ydj1 in promoter nucleosome eviction and transcriptional activation. Genome Res. 2016;26:211–225. doi: 10.1101/gr.196337.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radman-Livaja M, Rando OJ. Nucleosome positioning: how is it established, and why does it matter? Dev. Biol. 2010;339:258–266. doi: 10.1016/j.ydbio.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee HS, Bataille AR, Zhang L, Pugh BF. Subnucleosomal structures and nucleosome asymmetry across a genome. Cell. 2014;159:1377–1388. doi: 10.1016/j.cell.2014.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. Integrative genomics viewer. Nat. Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schep AN, Buenrostro JD, Denny SK, Schwartz K, Sherlock G, Greenleaf WJ. Structured nucleosome fingerprints enable high-resolution mapping of chromatin architecture within regulatory regions. Genome Res. 2015;25:1757–1770. doi: 10.1101/gr.192294.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabish MA, Struhl K. Evidence for eviction and rapid deposition of histones upon transcriptional elongation by RNA polymerase II. Mol. Cell. Biol. 2004;24:10111–10117. doi: 10.1128/MCB.24.23.10111-10117.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal E, Widom J. Poly(dA:dT) tracts: major determinants of nucleosome organization. Curr. Opin. Struct. Biol. 2009;19:65–71. doi: 10.1016/j.sbi.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivaswamy S, Bhinge A, Zhao Y, Jones S, Hirst M, Iyer VR. Dynamic remodeling of individual nucleosomes across a eukaryotic genome in response to transcriptional perturbation. PLoS Biol. 2008;6:e65. doi: 10.1371/journal.pbio.0060065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K. Naturally occurring poly(dA-dT) sequences are upstream promoter elements for constitutive transcription in yeast. Proc. Natl. Acad. Sci. USA. 1985;82:8419–8423. doi: 10.1073/pnas.82.24.8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K. Fundamentally different logic of gene regulation in eukaryotes and prokaryotes. Cell. 1999;98:1–4. doi: 10.1016/S0092-8674(00)80599-1. [DOI] [PubMed] [Google Scholar]
- Studitsky VM, Walter W, Kireeva M, Kashlev M, Felsenfeld G. Chromatin remodeling by RNA polymerases. Trends Biochem. Sci. 2004;29:127–135. doi: 10.1016/j.tibs.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Teif VB, Beshnova DA, Vainshtein Y, Marth C, Mallm J-P, Höfer T, Rippe K. Nucleosome repositioning links DNA (de)methylation and differential CTCF binding during stem cell development. Genome Res. 2014;24:1285–1295. doi: 10.1101/gr.164418.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera DL, Madzima TF, Labonne JD, Alam MP, Hoffman GG, Girimurugan SB, Zhang J, McGinnis KM, Dennis JH, Bass HW. Differential nuclease sensitivity profiling of chromatin reveals biochemical footprints coupled to gene expression and functional DNA elements in maize. Plant Cell. 2014;26:3883–3893. doi: 10.1105/tpc.114.130609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A, Hughes A, Yassour M, Rando OJ, Friedman N. High-resolution nucleosome mapping reveals transcription-dependent promoter packaging. Genome Res. 2010;20:90–100. doi: 10.1101/gr.098509.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JA, Cook A, Alver BH, Stadtfeld M, Deaton AM, Hochedlinger K, Park PJ, Tolstorukov MY, Kingston RE. Nucleosomal occupancy changes locally over key regulatory regions during cell differentiation and reprogramming. Nat. Commun. 2014;5:4719. doi: 10.1038/ncomms5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrick JJ, Holstege FC, Jennings EG, Causton HC, Shore D, Grunstein M, Lander ES, Young RA. Chromosomal landscape of nucleosome-dependent gene expression and silencing in yeast. Nature. 1999;402:418–421. doi: 10.1038/46567. [DOI] [PubMed] [Google Scholar]
- Xi Y, Yao J, Chen R, Li W, He X. Nucleosome fragility reveals novel functional states of chromatin and poises genes for activation. Genome Res. 2011;21:718–724. doi: 10.1101/gr.117101.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan G-C, Liu Y-J, Dion MF, Slack MD, Wu LF, Altschuler SJ, Rando OJ. Genome-scale identification of nucleosome positions in S. cerevisiae. Science. 2005;309:626–630. doi: 10.1126/science.1112178. [DOI] [PubMed] [Google Scholar]
- Zentner GE, Henikoff S. Mot1 redistributes TBP from TATA-containing to TATA-less promoters. Mol. Cell. Biol. 2013;33:4996–5004. doi: 10.1128/MCB.01218-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentner GE, Kasinathan S, Xin B, Rohs R, Henikoff S. ChEC-seq kinetics discriminates transcription factor binding sites by DNA sequence and shape in vivo. Nat. Commun. 2015;6:8733. doi: 10.1038/ncomms9733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wippo CJ, Wal M, Ward E, Korber P, Pugh BF. A packing mechanism for nucleosome organization reconstituted across a eukaryotic genome. Science. 2011;332:977–980. doi: 10.1126/science.1200508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.