Abstract
Objectives
The objective of this study was to evaluate the cost-effectiveness of quadripolar versus bipolar cardiac resynchronization defibrillator therapy systems.
Background
Quadripolar left ventricular (LV) leads for cardiac resynchronization therapy reduce phrenic nerve stimulation (PNS) and are associated with reduced mortality compared with bipolar leads.
Methods
A total of 606 patients received implants at 3 UK centers (319 Q, 287 B), between 2009 and 2014; mean follow-up was 879 days. Rehospitalization episodes were costed at National Health Service national tariff rates, and EQ-5D utility values were applied to heart failure admissions, acute coronary syndrome events, and mortality data, which were used to estimate quality-adjusted life-year differences over 5 years.
Results
Groups were matched with regard to age and sex. Patients with quadripolar implants had a lower rate of hospitalization than those with bipolar implants (42.6% vs. 55.4%; p = 0.002). This was primarily driven by fewer hospital readmissions for heart failure (51 [16%] vs. 75 [26.1%], respectively, for quadripolar vs. bipolar implants; p = 0.003) and generator replacements (9 [2.8%] vs. 19 [6.6%], respectively; p = 0.03). Hospitalization for suspected acute coronary syndrome, arrhythmia, device explantation, and lead revisions were similar. This lower health-care utilization cost translated into a cumulative 5-year cost saving for patients with quadripolar systems where the acquisition cost was <£932 (US $1,398) compared with bipolar systems. Probabilistic sensitivity analysis results mirrored the deterministic calculations. For the average additional price of £1,200 (US $1,800) over a bipolar system, the incremental cost-effective ratio was £3,692 per quality-adjusted life-year gained (US $5,538), far below the usual willingness-to-pay threshold of £20,000 (US $30,000).
Conclusions
In a UK health-care 5-year time horizon, the additional purchase price of quadripolar cardiac resynchronization defibrillator therapy systems is largely offset by lower subsequent event costs up to 5 years after implantation, which makes this technology highly cost-effective compared with bipolar systems.
Key Words: cardiac resynchronization therapy, cost-effectiveness, implantable cardiac defibrillator, left ventricular pacing, quadripolar lead
Abbreviations and Acronyms: ACS, acute coronary syndrome; CRT, cardiac resynchronization therapy; CRTD, cardiac resynchronization defibrillator therapy device; HF, heart failure; ICER, incremental cost-effectiveness ratio; LV, left ventricular; NHS, National Health Service; NICE, National Institute for Health and Care Excellence; PNS, phrenic nerve stimulation; QALY, quality-adjusted life-year
Graphical abstract
Cardiac resynchronization therapy (CRT) is an efficacious and cost-effective (1) treatment for patients with symptomatic heart failure with poor left ventricular (LV) function and prolonged QRS duration 2, 3, 4. Despite improvements in implantation delivery equipment and accumulation of user experience over the past 2 decades, approximately 30% of patients do not derive symptomatic benefit 5, 6. Post-implantation complications such as high capture thresholds, phrenic nerve stimulation (PNS), lead displacement, and infection reduce the effectiveness of this therapy 7, 8, 9, 10. The recent introduction of multipolar (quadripolar) LV leads has demonstrated a reduction in PNS through more proximal pole reprogramming, the presence of sustained lower capture thresholds, and easy deliverability (11).
However, new technology is usually provided at a higher purchase price than the conventional standard of care, which means that cost-effectiveness and affordability must be considered (12). Furthermore, the need for £22 billion in savings by 2020 in the United Kingdom (13) and an increased focus on efficiency as a result (14) further highlight the importance of cost-effective care. Multiple small clinical studies have demonstrated the clinical effectiveness of quadripolar leads at implantation and early follow-up 8, 15. Implant and 6-month follow-up data recently presented from the randomized MORE-CRT (More Options Available With a Quadripolar LV Lead Provide In-Clinic Solutions to CRT Challenges) trial (16) have confirmed the superiority of quadripolar leads, mainly from a reduction in intraoperative complications. We have previously demonstrated elimination of PNS and an associated lower all-cause mortality in patients implanted with a quadripolar lead in a large multicenter UK registry (17) (Online Figure 1).
We set out to assess the cost-effectiveness of quadripolar LV leads compared with bipolar LV leads in patients implanted with a cardiac resynchronization defibrillator therapy device (CRTD) within our previously published registry. We analyzed longer-term health-care utilization costs in terms of hospitalizations that occurred within the 5-year follow-up period to investigate whether the higher purchase price of this new technology was offset by expected reductions in cost arising from a reduction in hospitalizations. We also used mortality, acute coronary syndrome, and heart failure hospitalization data to estimate quality-adjusted life-year (QALY) differences.
Methods
Clinical data were taken from a registry of patients with conventional CRT criteria who received device implants at 3 UK centers (Guy’s and St Thomas’ NHS Foundation Trust; John Radcliffe Hospital, Oxford University Hospital NHS Foundation Trust; and Great Western Hospital, Swindon) between January 2009 and January 2014. All patients provided fully informed consent. We have previously published the results of 5-year follow-up of patients, in which we compared patients with CRTD systems with a quadripolar versus a bipolar LV lead in terms of PNS, lead complications, and all-cause mortality (17).
For the purposes of the current study, hospitalization episodes for each patient in the clinical registry were reviewed and assigned to the following categories based on diagnosis: acute coronary syndrome (ACS), arrhythmia, heart failure hospitalizations, infection requiring system explantation and reimplantation, generator replacement, and revision of any lead. These were compared between patients implanted with a CRTD incorporating a quadripolar LV lead versus those with a bipolar LV lead. Quadripolar leads in the current analysis were exclusively the Quartet lead (St. Jude Medical, Sylmar, California). Only individuals with complete hospitalization data that included coding of the cause of hospitalization were included; as such, the cohort comprised 606 patients (quadripolar, n = 319; bipolar, n = 287).
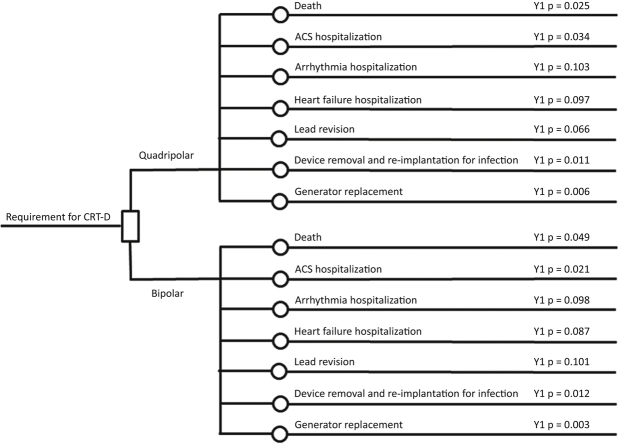
We performed an economic analysis of the registry data using all hospitalizations that occurred during the follow-up period. The rates of hospitalizations in each year from implantation were multiplied by the national tariff that pertained to the cause of hospitalization (Table 1). There was no extrapolation of data or event rates beyond the 5-year follow-up after implantation. Event rates were those that were observed to have occurred in each year; we did not derive transition probabilities that could be used for a Markov model. All events were counted, and some events occurred more than once in individual patients. A probabilistic sensitivity analysis was also undertaken to help understand the impact of parameter uncertainty and determine the probability that quadripolar CRTD was cost-effective. Probabilistic analysis was conducted by inputting data as probability (beta) distributions rather than point estimates and randomly sampling 1,000 values from these distributions. This was performed for all hospitalization episodes in addition to mortality data from our previous work (17). Comparative purchase costs were estimated between the quadripolar Quartet leads (St. Jude Medical) and the mean purchase cost of bipolar leads used in the clinical registry (QuickFlex, St. Jude Medical; AttainAbility, Medtronic; Easytrak, Boston Scientific). A National Health Service (NHS, the UK health system) perspective was used, which means that wider societal impact was not considered. Costs and effects beyond year 1 were discounted at 3.5%, following the methodology recommended by the UK’s National Institute of Health and Care Excellence (NICE) (20). A model diagram (Figure 1) demonstrates the differing probabilities of hospitalization event rates (per cause) for year 1 post-implantation in those with quadripolar and bipolar systems. The same approach was used for years 2 to 5 in the analysis, and rates for all years are shown in Online Table 1.
Table 1.
National Tariff Tables: Hospitalization Pricing by Coding Category
| Cost Item | Value (£) | Description | Source (Ref. #) |
|---|---|---|---|
| ACS hospitalization | 3,421 | EB10Z (actual or suspected MI), nonelective | ETO 2015–2016 (18) |
| Arrhythmia hospitalization | 887 | Activity-weighted average of EB07H (arrhythmia or conduction disorders with CC) and EB07I (arrhythmia or conduction disorders without CC) | ETO 2015–2016 (18) |
| Heart failure admission | 2,756 | Activity-weighted average of EB03H (heart failure or shock with CC) and EB03I (heart failure or shock without CC) | ETO 2015–2016 (18) |
| Lead revision procedure | 2,952 | Activity-weighted average of elective/nonelective HRG EA39Z (pacemaker procedure without generator implant; includes removal and reimplantation of cardiac pacemaker system) | ETO 2015–2016 (18) |
| Bipolar CRTD device | 12,615 | NICE technology appraisal (19) | |
| Additional cost of quadripolar CRTD device | 1,200 | Base-case value, varied between £0 and £2,400 in sensitivity analysis | Market estimate 2015 |
| Device removal and reimplantation for infection | 23,506 | Base value for bipolar device | NICE technology appraisal 2014 (19) |
| CRTD generator revision | 15,990 | Base value for bipolar device | NICE technology appraisal 2014 (19) |
See Online Figure 1 for equivalent cost in US dollars.
ACS = acute coronary syndrome; CC = complications and comorbidities; CRTD = cardiac resynchronization defibrillator therapy device; ETO = extended tariff option (the national tariff scheme used by most English trusts in 2015–2016); MI = myocardial infarction; NICE = National Institute for Health and Care Excellence.
Figure 1.
Model Diagram and Decision Structure Used in the Economic Model
This was used for each of the 5 years, although only year 1 is shown here. Y1 p is the probability of the event in year 1; actual data for year 1 are shown. ACS = acute coronary syndrome; CRT-D = cardiac resynchronization defibrillator therapy device.
Costs
National tariff “enhanced tariff option” prices for 2015 to 2016 (18) were applied to ACS hospitalization, arrhythmia hospitalization, heart failure hospitalization, and lead revision procedures. The base tariff price was multiplied by the local cost factor (market forces factor) for each NHS hospital that implants CRT devices, and the mean of these values was used in the model. Table 1 shows the mean unit cost data used in the calculations per hospitalization, including local cost factors. Online Table 2 shows the equivalent costs in US dollars using a simple conversion of £1 = $1.50. Where there were different tariff values for elective/nonelective procedures and different values for complication/comorbidity splits, averages weighted by the number of admissions for each were calculated. Costs for CRTD implantation, device removal and reimplantation for wound infection, and CRT generator replacement were taken from the data used to inform the economic evaluation that underpinned NICE’s 2014 Technology Appraisal Guidance (19). The additional purchase cost of quadripolar technology was estimated to be £1,200 ($1,800) for the base-case analysis (market estimate, St. Jude Medical) but varied between zero and £2,400 ($3,600) to assess sensitivity, because acquisition price may vary according to local procurement arrangements. Quadripolar device removal and reimplantation for infection was uplifted by the additional acquisition cost for the quadripolar device, on the assumption that the same type of device would be reimplanted. Quadripolar generator replacement was costed at bipolar cost plus 0.67 of additional quadripolar system costs. Quadripolar lead revision was costed at bipolar cost plus 0.33 of additional quadripolar system costs.
Quality-adjusted life years
The use of QALYs allows clinical effectiveness to be expressed in a common unit, to which a cost can be applied to estimate the value of health-care interventions. The EQ-5D questionnaire is commonly used to determine the quality-of-life utility values that can be translated into QALYs (21). Hawkins et al. (22) discussed this approach in the context of cardiac interventions, and it is a standard part of NICE’s methodology (20). The incremental cost-effectiveness ratio (ICER) is obtained by dividing the additional cost of using the new device by the incremental QALYs gained and can be used to estimate a value for decision-making purposes. NICE’s methods guide (20) suggests an ICER of £20,000 ($30,000) to £30,000 ($45,000) is the range in which cost-effectiveness is acceptable in terms of effective use of NHS resources; therefore, this was the benchmark used to assess the results of the current study.
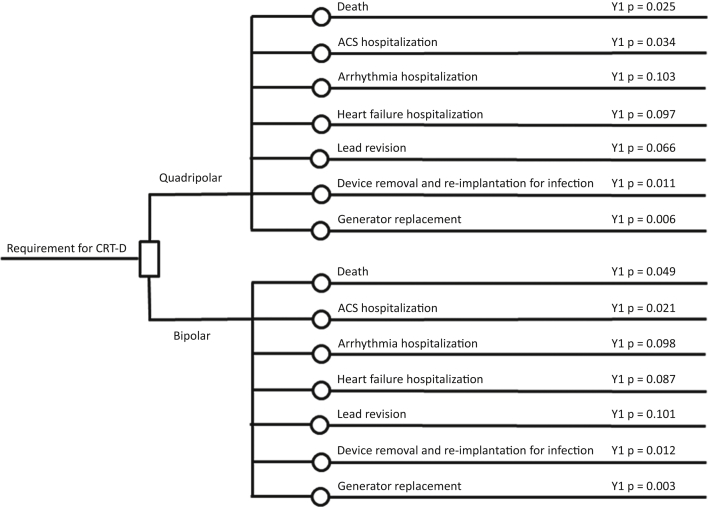
Only the mortality difference used in our previous report (17), utility loss attributable to ACS events, and utility loss attributable to heart failure hospitalizations were used to assess QALY differences between bipolar and quadripolar devices, similar to the methods used in the economic analysis that informed NICE’s recent technology appraisal of implantable cardioverter-defibrillators and CRT (19). A baseline EQ-5D utility of 0.8808 was used for a patient with heart failure and a CRT device (range: 0.85 to 0.903), with utility loss because of death being taken as a loss from this value to zero. The utility loss associated with a heart failure admission was calculated, from the work of Swinburn et al. (23) and Lewis et al. (24), to be 0.1197, persisting for 18 days (average length of stay plus 7 days post-discharge). The utility loss associated with ACS events was calculated, from the work of Lewis et al. (24) and Matza et al. (25), to be 0.1035, persisting for 10.4 days (average length of stay plus 7 days post-discharge). A range of input parameters were varied by ±95% confidence interval to show the impact of each on the base-case ICER, and the results are shown on a tornado plot (Figure 2).
Figure 2.
Tornado Plot Showing the Impact of Varying the Input Parameter Values to Their Upper and Lower 95% CIs on the Base-Case ICER
Hatched bars show the impact of using the lower 95% confidence interval (CI); solid bars show the impact of using the upper 95% CI. Data labels on each bar show the incremental cost-effectiveness ratio (ICER) resulting from the change in value. A shift to the right of the center line shows an ICER that denotes less favorable cost-effectiveness than the base case. ACS = acute coronary syndrome; c = cost of; HF = heart failure; hosp = hospitalization; QALY = quality-adjusted life-year; quad = quadripolar cardiac resynchronization therapy system; u = utility value.
Statistical analysis
Continuous variables are expressed as mean ± SD. Comparisons were made with a Student t test. Categorical data were expressed as an absolute number of occurrences and associated frequency (%); analysis was performed with a chi-squared test. A probability value of <0.05 was considered significant. Statistical analysis was performed with the Statistics Package for the Social Sciences (SPSS) version 21 (SPSS Inc., Chicago, Illinois). Economic analysis was undertaken in Microsoft Excel.
Results
A total of 606 patients were included in this analysis and were matched with regard to age and sex. Patients in the bipolar group had a higher prevalence of ischemic heart disease (quadripolar vs. bipolar: 181 [56.7%] vs. 190 [66.2%]; p = 0.02), and fewer were in sinus rhythm (quadripolar vs. bipolar: 303 [95.0%] vs. 48 [83.3%]; p < 0.001) before implantation. Mean QRS duration was similar between groups (159 ± 6.2 ms vs. 160 ± 5.1 ms, quadripolar vs. bipolar, respectively; p = 0.07), as was the proportion of patients in New York Heart Association functional class III (183 [76.9%] vs. 145 [72.1%], quadripolar vs. bipolar, respectively; p = 0.10). Mean percentage of biventricular pacing throughout the follow-up period was similar between groups (Q: 94.6 ± 1.6% vs. B: 94.4 ± 1.5%, p = 0.11). Length of stay after implantation was similar between groups, irrespective of whether they were elective admissions (1.2 ± 2.3 days vs. 1.2 ± 1.6 days, quadripolar vs. bipolar, respectively; p = 1.00) or existing inpatients (5.0 ± 8.5 days vs. 5.2 ± 7.2 days, quadripolar vs. bipolar, respectively; p = 0.76), as shown in Table 2.
Table 2.
Demographic Data
| Quadripolar (n = 319) | Bipolar (n = 287) | p Value | |
|---|---|---|---|
| Age (yrs) | 70.4 ± 11 | 68.7 ± 10 | 0.06 |
| Female | 50 (15.7) | 48 (16.7) | 0.74 |
| Ischemic heart disease | 181 (56.7) | 190 (66.2) | 0.02 |
| Sinus rhythm | 303 (95.0) | 48 (83.3) | <0.001 |
| QRS duration (ms) | 159 ± 6.2 (n = 238) |
160 ± 5.1 (n = 201) |
0.07 |
| NYHA functional class III symptoms | 183 (76.9) (n = 238) |
145 (72.1) (n = 201) |
0.10 |
| Mobitz II/complete heart block | 9 (2.8) | 14 (4.9) | 0.21 |
| % Biventricular pacing | 94.6 ± 1.6 | 94.4 ± 1.5 | 0.11 |
| LV lead upgrade | 8 (2.5) | 61 (21.3) | <0.001 |
| Length of stay post-implantation (days) (elective) | 1.2 ± 2.3 | 1.2 ± 1.6 | 1.00 |
| Length of stay post-implantation (days) (inpatient) | 5.0 ± 8.5 | 5.2 ± 7.2 | 0.76 |
Values are mean ± SD or n (%).
LV = left ventricular; NYHA = New York Heart Association.
Patients implanted with a quadripolar lead had a significantly lower absolute number of all-cause hospitalizations (quadripolar: 191 admissions among 309 patients; bipolar: 225 admissions among 287 patients; p < 0.001), as shown in Table 3. Moreover, the proportion of patients hospitalized at least once was also significantly lower in those implanted with a quadripolar compared with a bipolar lead (42.6% vs. 55.4%, respectively; p = 0.002), as shown in Table 4. This was primarily driven by a significantly lower number of hospitalizations for heart failure (51 admissions among 309 patients with a quadripolar device vs. 75 among 287 patients with a bipolar device; p = 0.003) and CRTD generator replacement (9 admissions among 309 patients vs. 19 among 287 patients, respectively; p = 0.03). Hospitalizations for suspected ACS, arrhythmia, device explantation, and lead revisions were similar between the groups (p = NS). Each hospitalization, irrespective of cause, was counted as a separate event (Table 3); these values were multiplied by the appropriate tariff (Table 1) to produce health-care utilization costs for each group over the 5-year period.
Table 3.
Absolute Numbers of Hospitalization, Split by Cause, and Corresponding Health-Care Costs∗
| Quadripolar (n = 319) |
Bipolar (n = 287) |
p Value | |||
|---|---|---|---|---|---|
| n | Cost (£) | n | Cost (£) | ||
| ACS | 35 | 115,029 | 21 | 67,544 | 0.13 |
| Arrhythmia | 59 | 51,218 | 65 | 55,557 | 0.23 |
| Heart failure | 51 | 137,695 | 75 | 195,841 | 0.003 |
| System explantation and reimplantation | 5 | 121,122 | 6 | 136,788 | 0.76 |
| Generator replacement | 9 | 142,026 | 19 | 273,276 | 0.03 |
| RA/RV lead revision | 27 | 88,918 | 24 | 69,840 | 0.21 |
| LV lead revision | 5 | 16,466 | 15 | 43,650 | 0.02 |
| Total episodes/cost | 191 | 672,474 | 225 | 842,484 | <0.001 |
Some patients were hospitalized for the same category more than once, and some not at all. The cost of events was calculated by multiplying the number of events in each year by the cost of the event for that year (i.e., events beyond year 1 were multiplied by the discounted cost for the year in which the event occurred).
ACS = acute coronary syndrome; LV = left ventricular; RA = right atrial; RV = right ventricular.
Based on the tariff codes in Table 1.
Table 4.
Numbers and Proportions of Patients in Each Group Who Have Been Hospitalized Once (or More)
| Quadripolar (n = 319) |
Bipolar (n = 287) |
Odds Ratio (95% CI) | p Value | |
|---|---|---|---|---|
| ACS | 26 (8.2%) | 17 (5.9%) | 1.40 (0.75–2.66) | 0.34 |
| Arrhythmia | 39 (12.2%) | 45 (15.7%) | 0.75 (0.47–1.19) | 0.24 |
| Heart failure | 28 (8.8%) | 40 (13.9%) | 0.59 (0.36–0.99) | 0.05 |
| System explantation and reimplantation | 5 (1.6%) | 6 (2.1%) | 0.75 (0.23–2.47) | 0.83 |
| Generator replacement | 8 (2.5%) | 19 (6.6%) | 0.36 (0.15–0.84) | 0.02 |
| Lead revision (RA/RV/LV) | 30 (9.4%) | 32 (11.2%) | 0.83 (0.49–1.40) | 0.50 |
| Hospitalization (any cause) | 136 (42.6%) | 159 (55.4%) | 0.59 (0.43–0.83) | 0.002 |
Values are n (%).
CI = confidence interval; other abbreviations as in Table 3.
Table 4 represents the proportion of patients implanted with either quadripolar or bipolar leads who had at least 1 admission for the listed reasons. The absolute values for the hospitalization causes are therefore less than in Table 3, because each of the events are only counted once per patient. The proportions of patients hospitalized at least once for heart failure (8.8% [quadripolar] vs. 13.9% [bipolar]; p = 0.05) or generator replacement (2.5% [quadripolar] vs. 6.6% [bipolar]; p = 0.02) were significantly lower in those in whom a quadripolar lead was implanted. The average number of admissions for those who were hospitalized was similar in each group (1.40 vs. 1.42, quadripolar vs. bipolar).
Cost-effectiveness analysis
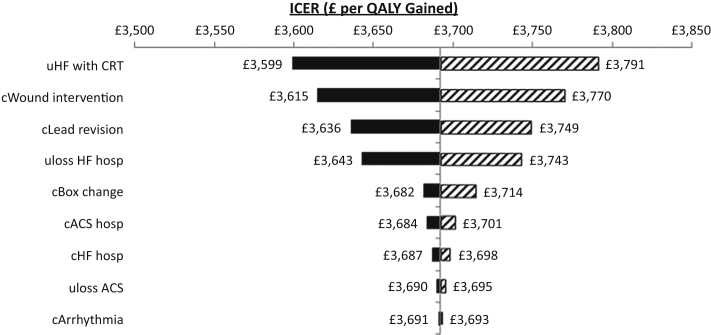
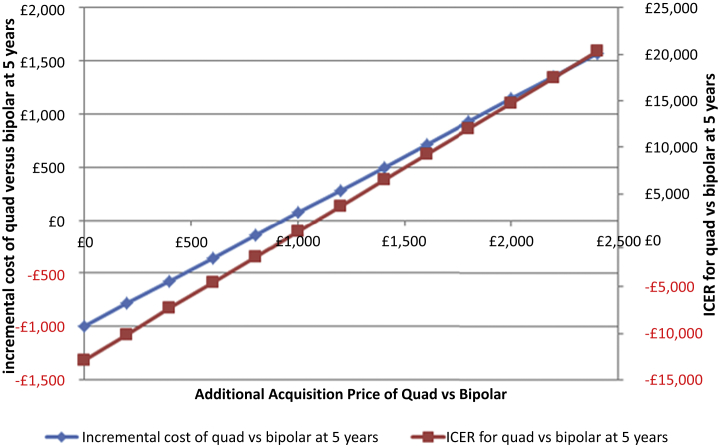
The base-case ICER was £3,692 ($5,538) in the deterministic model (i.e., based on point estimates) and £3,835 ($5,753) in the probabilistic model. Up to an additional purchase cost of £932 ($1,398), quadripolar leads translated into a cumulative cost saving compared with bipolar leads because of the higher health-care utilization costs associated with the latter (Figure 3). The cost saving was up to £1,000 ($1,500) for purchasing a quadripolar system for the same price as a bipolar system (Table 5). Beyond £932 ($1,398), the additional ICER was up to £20,288 ($30,432) (Figure 3). Figure 2 shows the impact of varying a range of input parameters by ± 95% confidence intervals. The analysis was most sensitive to the utility of patients with heart failure, because death resulted in a loss of 0.8808 QALYs in each patient who died. All resulting ICERs remained <£4,000 per QALY gained.
Figure 3.
Incremental Cost and Cost-Effectiveness of Implanting a Quadripolar Versus Bipolar CRT-D System, Varied by the Additional Acquisition Cost of the Quadripolar System
Quadripolar (quad) leads that cost up to £932 ($1,398) more than bipolar leads result in either a cost-neutral outcome or a cost saving because of reduced health-care utilization events. CRT-D = cardiac resynchronization defibrillator therapy device; ICER = incremental cost-effectiveness ratio.
Table 5.
Cumulative Total Cost of Implanting a Quadripolar Versus Bipolar CRTD for Different Acquisition Prices and Associated ICERs
| Additional Acquisition Cost of Quadripolar CRTD (£) | 5-Yr Incremental Cost of Quadripolar vs. Bipolar CRTD (£) | ICER of Quadripolar vs. Bipolar CRTD |
|---|---|---|
| 0 | −1,000 | Quadripolar dominates |
| 200 | −786 | Quadripolar dominates |
| 400 | −571 | Quadripolar dominates |
| 600 | −357 | Quadripolar dominates |
| 800 | −143 | Quadripolar dominates |
| 1,000 | 72 | £926 |
| 1,200 | 286 | £3,692 |
| 1,400 | 501 | £6,458 |
| 1,600 | 715 | £9,224 |
| 1,800 | 929 | £11,990 |
| 2,000 | 1,144 | £14,756 |
| 2,200 | 1,358 | £17,522 |
| 2,400 | 1,572 | £20,288 |
CRTD = cardiac resynchronization defibrillator therapy device; ICER = incremental cost-effectiveness ratio; Quadripolar dominates = quadripolar CRTD is less costly and more effective than bipolar CRTD at 5 years. In this situation, ICERs are negative and not conventionally shown.
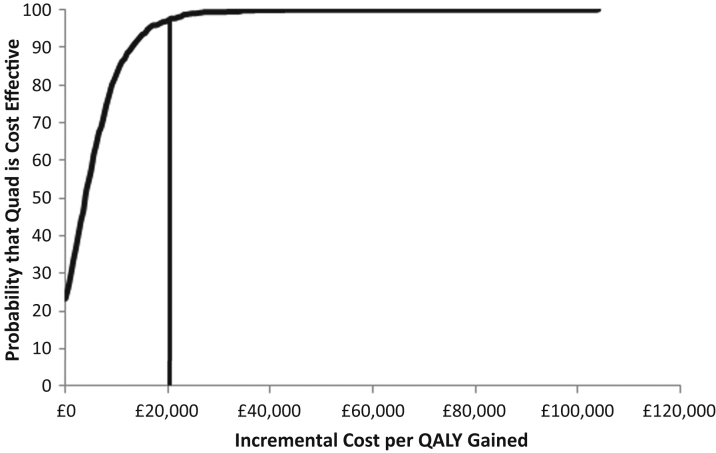
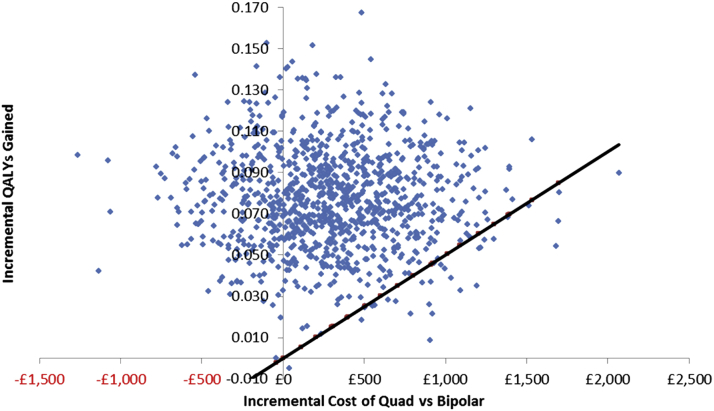
In the probabilistic sensitivity analysis, quadripolar CRTD was 97.1% likely to be cost-effective at £20,000 per QALY gained and 99.3% likely to be cost-effective at £30,000 per QALY gained (Figure 4). A cost-effectiveness panel showing the results of each of the 1,000 simulations provides a visualization of the proportion of cases for which quadripolar systems were more effective and more expensive and for which they were more effective and less expensive (Figure 5).
Figure 4.
Cost-Effectiveness Acceptability Curve for Quadripolar Versus Bipolar CRTD
The x-axis shows the willingness-to-pay threshold (i.e., the incremental cost per QALY gained). Quadripolar (Quad) CRTD is 97.1% likely to be cost-effective at £20,000 ($30,000) per QALY gained and 99.3% likely to be cost-effective at £30,000 ($45,000) per QALY gained. Abbreviations as in Figure 2.
Figure 5.
Cost-Effectiveness Plane
Each point represents the result of 1 of the 1,000 simulations. Points to the left of the vertical axis are simulation results in which quadripolar CRTD was more effective and less expensive than bipolar CRTD. Points to the right of the vertical axis are simulation results in which quadripolar CRTD was more effective and more expensive than bipolar CRTD. The diagonal black line is the £20,000 ($30,000) per QALY gained line (i.e., all points above this are simulation results in which the incremental cost per QALY gained was <£20,000 [$30,000]). Abbreviations as in Figure 2.
Discussion
This is the first comprehensive health economic analysis to use real-world UK clinical data from hospitalization events and mortality to produce an accurate comparison of cumulative cost differences between implanting quadripolar versus bipolar CRTD systems.
The main findings were as follows:
-
1.
There was a lower absolute number of hospitalizations in patients in whom quadripolar CRTD systems were implanted, predominantly driven by a reduction in readmissions for heart failure and generator replacements.
-
2.
Quadripolar CRTD systems, if purchased for up to £932 ($1,398) more than bipolar systems, yielded a cost saving over a 5-year period after health-care utilization costs were considered.
-
3.
Quadripolar CRTD systems with an additional purchase price of £933 to £2,400 ($1,400 to $3,600) compared with bipolar systems remained cost-effective, with ICER values well within the range of acceptability used by NICE.
-
4.
The calculated cost-effectiveness using real-world clinical data (deterministic model) was closely mirrored by the probabilistic sensitivity analysis, which reaffirms confidence in the results.
Multipolar LV leads for CRT delivery have demonstrated high implant success, good capture thresholds at implantation and follow-up, and a low rate of lead displacement 11, 26. Rates of intraprocedural lead complications appear lower than with conventional bipolar leads (27). Reduction or even elimination in PNS during medium-term follow-up provides invaluable utility in CRT delivery 9, 15. We have recently shown a reduction in all-cause mortality associated with quadripolar leads compared with a bipolar lead (17). Furthermore, rates of reintervention for lead repositioning were lower in those implanted with a quadripolar compared with a bipolar lead (2% vs. 5.2%; p = 0.03), and the radiation dose during implantation was almost one-half (1,028 cGy·cm2 vs. 1,950 cGy·cm2; p < 0.001).
The lower rates of hospitalization associated with a quadripolar lead in the current study could be driven by the improved efficacy in CRT delivery (attributable to PNS reduction and fewer reinterventions for lead displacement). Our previous study (17) also demonstrated lower implantation capture energy with quadripolar than with bipolar leads (0.95 μJ vs. 1.08 μJ; p = 0.003). Pacing systems consistently delivering higher-output voltages to capture the LV will have a reduced longevity (28), and this could explain the current findings of a significantly lower number of generator replacements among those implanted with a quadripolar system. This might be of significant clinical importance given the higher prevalence of device-related infections after generator replacement (29), which might contribute to greater morbidity and mortality in this group. Furthermore, from an economic modeling perspective, the lower proportion of generator changes in the quadripolar group was contributory to the lower overall health-care utilization costs over the follow-up period. The presence of 4 poles on the quadripolar LV lead allows greater programmability and the ability to choose multiple vectors for CRT delivery 30, 31. This provides the implanting physician with more choices for implantation locations, which might allow leads to be implanted more distally in posterolateral or lateral veins for stability purposes 8, 17, with the ability to stimulate the LV more basally from the proximal poles, which in turn could contribute to more optimal CRT delivery and could result in fewer heart failure hospitalizations and reduced mortality.
Comparison with previous studies
Forleo et al. (32) have reported reduced rates of heart failure hospitalizations and LV lead revision among patients implanted with a quadripolar lead in a single-center Italian registry (events per patient per year: 0.15 vs. 0.32, quadripolar vs. bipolar; p = 0.04). Non–heart failure hospitalization rates were similar among groups. This study demonstrated lower health-care utilization costs associated with the quadripolar group (434 euros/patient-year vs. 1136 euros/patient-year; p = 0.02). However, the study by Forleo et al. (32) used Italian cost data that cannot be directly translated into the UK health-care setting, used only single-center data, had a much shorter follow-up period, included a smaller number of patients (193 vs. 606), and did not include as wide a range of clinical events in the follow-up costing. In the present study, the time to each event was calculated individually from the time of original implantation. In addition, the present study recorded and coded for all relevant acute and elective hospitalizations, not just heart failure and LV lead revisions, including admissions with ACS, arrhythmia, generator replacements, device extractions/reimplantation, and all right atrial and right ventricular lead revisions. Our calculated cumulative 5-year cost analysis was paralleled by the 5-year follow-up data, which provides confidence in the clinical relevance and accuracy of this study. Furthermore, we undertook a probabilistic sensitivity analysis similar to that by Forleo et al. (32), and the results for the base-case ICER closely mirrored the value calculated in the deterministic model. The only other contemporary UK-specific cost-effectiveness analysis was published recently by NICE (33). Recommendations made by NICE in a 2014 review of CRT and implantable defibrillators for heart failure were based on plausible ICERs in the range of £11,000 ($16,500) to £31,000 ($46,500) per QALY gained (19). By way of comparison, our results showed ICERs for quadripolar systems of up to £20,288 ($30,432) per QALY gained. Had data been available to include QALY adjustments for arrhythmia admissions, device removal and reimplantation for wound infection, generator changes, and lead revisions, it is likely that the ICERs would have been lower, because the rates of these events favor the quadripolar system. This analysis is therefore conservative with respect to the cost-effectiveness of quadripolar CRTD.
Study limitations
The data used as the basis of this economic evaluation were derived from a multicenter clinical registry, and the choice of whether quadripolar or bipolar leads were implanted was not subject to a randomization process. However, the approach we have taken reflects current demands in which real-world data are becoming more important to assess the impact that new technologies have actually had on patients and health systems. We took real clinical events that occurred in NHS practice and applied NHS tariffs to them to determine the actual charge and cost-effectiveness. This was an in-study cost-effectiveness analysis, not an extrapolation to a lifetime horizon. We therefore did not assume event rates and did not model beyond the time for which we had gathered follow-up data. We did not perform a Markov model. Wider societal benefit was also not taken into account, which might be a further limitation.
As might be expected, the incremental acquisition cost of quadripolar technology is a strong determinant of the overall incremental cost-effectiveness of the 2 therapies. We therefore made an estimate of base cost and performed an analysis either side of the additional purchase cost to account for the variation in procurement acquisition costs. With respect to QALYs, the mortality difference was the strongest driver of the QALY gain associated with quadripolar CRTD. There was a significant difference in the proportions of patients with ischemic heart disease and those not in sinus rhythm (with more such patients in the bipolar group); however, this was corrected for in the multivariate analysis, and mortality remained significantly different.
Conclusions
In a 5-year time horizon calculated from a UK health-care system perspective, the additional purchase price of quadripolar CRTD systems is substantially offset by lower health-care utilization costs, which suggests this technology is highly cost-effective compared with bipolar systems.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Quadripolar leads are associated with a lower rate of hospitalization than bipolar leads, primarily driven by fewer heart failure readmissions and generator replacements. This translates into a lower health-care utilization cost over a 5-year follow-up period and offsets the additional purchase price of quadripolar CRTD systems, which makes this technology highly cost-effective.
TRANSLATIONAL OUTLOOK: Given the near elimination of PNS, the reduction in hospital readmissions for generator replacement and heart failure, and the observed lower mortality in patients implanted with quadripolar leads, quadripolar CRTD systems may be considered the standard of care for CRT delivery.
Footnotes
Drs. Behar and Claridge have received fellowship support from the National Institute of Health Research (NIHR) Biomedical Research Centre (BRC) at Guy’s and St Thomas’ NHS Foundation Trust. Dr. Behar has received support from the Rosetrees trust. Dr. Fearn is a salaried employee of St. Jude Medical. Drs. Gamble and Claridge have received research fellowship funding from St. Jude Medical. Drs. Gamble and Betts have received support from Heart Research UK. Dr. Jackson has received research fellowship funding from Medtronic Inc. Dr. Sohal has received an educational grant from St. Jude Medical. Dr. Betts has received research funding from St. Jude Medical; honoraria for product development and speaker fees from Boston Scientific Ltd. and Medtronic Ltd.; and has received support from the UK NIHR Oxford BRC. Dr. Herring is a British Heart Foundation (BHF) Intermediate Fellow; and acknowledges support from the BHF Centre of Research Excellence (RE/08/004), Oxford. Dr. Rinaldi is a consultant to St. Jude Medical, Medtronic, and Spectranetics; and receives research funding from St. Jude Medical and Medtronic. All other authors have reported that they have no relationships relevant to the content of this article to disclose. Drs. Herring and Rinaldi are joint senior authors.
For a supplemental table and figures, please see the online version of this article.
Appendix
References
- 1.Boriani G., Biffi M., Martignani C. Is cardiac resynchronization therapy cost-effective? Europace. 2009;11(Suppl 5):v93–v97. doi: 10.1093/europace/eup274. [DOI] [PubMed] [Google Scholar]
- 2.Cleland J.G.F., Daubert J.-C., Erdmann E. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 3.Brignole M., Auricchio A., Baron-Esquivias G. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association. Eur Heart J. 2013;34:2281–2329. doi: 10.1093/eurheartj/eht150. [DOI] [PubMed] [Google Scholar]
- 4.Bristow M.R., Saxon L.A., Boehmer J. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 5.Daubert J.-C., Saxon L., Adamson P.B. 2012 EHRA/HRS expert consensus statement on cardiac resynchronization therapy in heart failure: implant and follow-up recommendations and management. Europace. 2012;14:1236–1286. doi: 10.1093/europace/eus222. [DOI] [PubMed] [Google Scholar]
- 6.Mullens W., Grimm R.A., Verga T. Insights from a cardiac resynchronization optimization clinic as part of a heart failure disease management program. J Am Coll Cardiol. 2009;53:765–773. doi: 10.1016/j.jacc.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 7.Sohal M., Chen Z., Sammut E. New developments in the delivery of cardiac resynchronization therapy: targeted lead placement, multi-site and endocardial pacing. Expert Rev Med Devices. 2014;11:295–304. doi: 10.1586/17434440.2014.885320. [DOI] [PubMed] [Google Scholar]
- 8.Forleo G.B., Mantica M., Di Biase L. Clinical and procedural outcome of patients implanted with a quadripolar left ventricular lead: early results of a prospective multicenter study. Heart Rhythm. 2012;9:1822–1828. doi: 10.1016/j.hrthm.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Biffi M., Exner D.V., Crossley G.H. Occurrence of phrenic nerve stimulation in cardiac resynchronization therapy patients: the role of left ventricular lead type and placement site. Europace. 2013;15:77–82. doi: 10.1093/europace/eus237. [DOI] [PubMed] [Google Scholar]
- 10.Gras D., Böcker D., Lunati M. Implantation of cardiac resynchronization therapy systems in the CARE-HF trial: procedural success rate and safety. Europace. 2007;9:516–522. doi: 10.1093/europace/eum080. [DOI] [PubMed] [Google Scholar]
- 11.Sperzel J., Dänschel W., Gutleben K.-J. First prospective, multi-centre clinical experience with a novel left ventricular quadripolar lead. Europace. 2012;14:365–372. doi: 10.1093/europace/eur322. [DOI] [PubMed] [Google Scholar]
- 12.NHS England . Specialised Commissioning, NHS England; Redditch, UK: 2015. Consultation Guide: Investing in Specialised Services. [Google Scholar]
- 13.NHS England . Specialised Commissioning, NHS England; Redditch, UK: 2014. Five Year Forward View. [Google Scholar]
- 14.Carter P.R. National Archives; London, UK: 2013. Review of Operational Productivity in NHS Providers: An Independent Report for the Department of Health by Lord Carter of Coles. [Google Scholar]
- 15.Mehta P.A., Shetty A.K., Squirrel M., Bostock J., Rinaldi C.A. Elimination of phrenic nerve stimulation occurring during CRT: follow-up in patients implanted with a novel quadripolar pacing lead. J Interv Card Electrophysiol. 2012;33:43–49. doi: 10.1007/s10840-011-9598-5. [DOI] [PubMed] [Google Scholar]
- 16.Boriani G., Connors S., Kalarus Z. Cardiac resynchronization therapy with a quadripolar electrode lead decreases complications at 6 months: results of the MORE-CRT randomized trial. J Am Coll Cardiol EP. 2016;2:212–220. doi: 10.1016/j.jacep.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Behar J.M., Bostock J., Zhu Li A.P. Cardiac resynchronization therapy delivered via a multipolar left ventricular lead is associated with reduced mortality and elimination of phrenic nerve stimulation: long-term follow-up from a multicenter registry. J Cardiovasc Electrophysiol. 2015;26:540–546. doi: 10.1111/jce.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NHS England . NHS England; London, UK: 2015. Guide to the Enhanced Tariff Option for 2015/16. 2015. Gateway publication reference No. 03237. Available at: https://www.england.nhs.uk/wp-content/uploads/2015/05/eto-guidance-15-16.pdf. Accessed May 2016. [Google Scholar]
- 19.National Institute for Health and Care Excellence . NICE; London, UK: 2014. Implantable cardioverter defibrillators and cardiac resynchronisation therapy for arrhythmias and heart failure (review of TA95 and TA120) [Google Scholar]
- 20.National Institute for Health and Care Excellence. Guide to the Methods of Technology Appraisal 2013. 2013:1–93. Available at: https://www.nice.org.uk/process/pmg9/chapter/foreword. Accessed February 3, 2017. [PubMed]
- 21.Kind P. The EuroQoL instrument: an index of health related quality of life. In: Spiker B., editor. Quality of Life and Pharmacoeconomics in Clinical Trials. Lippincott-Raven; Philadelphia, PA: 1996. [Google Scholar]
- 22.Hawkins N., Sculpher M.R.M. Modelling the cost-effectiveness of cardiac interventions: the case of sirolimus-eluting stents. Br J Cardiol Acute Interv Cardiol. 2005;12:83–91. [Google Scholar]
- 23.Swinburn P., Shingler S., Ong S.H., Lecomte P., Lloyd A. Assessing the health-related quality of life in patients hospitalised for acute heart failure. Br J Cardiol. 2013;20:72–76. [Google Scholar]
- 24.Lewis E.F., Li Y., Pfeffer M.A. Impact of cardiovascular events on change in quality of life and utilities in patients after myocardial infarction: a VALIANT study (valsartan in acute myocardial infarction) J Am Coll Cardiol HF. 2014;2:159–165. doi: 10.1016/j.jchf.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Matza L.S., Stewart K.D., Gandra S.R. Acute and chronic impact of cardiovascular events on health state utilities. BMC Health Serv Res. 2015;15:173. doi: 10.1186/s12913-015-0772-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forleo G.B., Della Rocca D.G., Papavasileiou L.P., Molfetta A.D., Santini L., Romeo F. Left ventricular pacing with a new quadripolar transvenous lead for CRT: early results of a prospective comparison with conventional implant outcomes. Heart Rhythm. 2011;8:31–37. doi: 10.1016/j.hrthm.2010.09.076. [DOI] [PubMed] [Google Scholar]
- 27.Forleo G.B., Di Biase L., Panattoni G. Improved implant and postoperative lead performance in CRT-D patients implanted with a quadripolar left ventricular lead: a 6-month follow-up analysis from a multicenter prospective comparative study. J Interv Card Electrophysiol. 2015;42:59–66. doi: 10.1007/s10840-014-9956-1. [DOI] [PubMed] [Google Scholar]
- 28.Behar J.M., Bostock J., Ginks M. Limitations of chronic delivery of multi-vein left ventricular stimulation for cardiac resynchronization therapy. J Interv Card Electrophysiol. 2015 doi: 10.1007/s10840-014-9971-2. [DOI] [PubMed] [Google Scholar]
- 29.Johansen J.B., Jørgensen O.D., Møller M., Arnsbo P., Mortensen P.T., Nielsen J.C. Infection after pacemaker implantation: infection rates and risk factors associated with infection in a population-based cohort study of 46299 consecutive patients. Eur Heart J. 2011;32:991–998. doi: 10.1093/eurheartj/ehq497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shetty A.K., Duckett S.G., Bostock J. Initial single-center experience of a quadripolar pacing lead for cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2011;34:484–489. doi: 10.1111/j.1540-8159.2010.03003.x. [DOI] [PubMed] [Google Scholar]
- 31.Crossley G.H., Biffi M., Johnson B. Performance of a novel left ventricular lead with short bipolar spacing for cardiac resynchronization therapy: primary results of the Attain Performa quadripolar left ventricular lead study. Heart Rhythm. 2015;12:751–758. doi: 10.1016/j.hrthm.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 32.Forleo G.B., Di Biase L., Bharmi R. Hospitalization rates and associated cost analysis of cardiac resynchronization therapy with an implantable defibrillator and quadripolar vs. bipolar left ventricular leads: a comparative effectiveness study. Europace. 2015;17:101–107. doi: 10.1093/europace/euu290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.NICE. Equality impact assessment: Guidance development MTA Implantable cardioverter defibrillators and cardiac resynchronisation therapy for arrhythmias and heart failure (review of TA95 and TA120). 2014. Available at: https://www.nice.org.uk/guidance/ta314/documents/arrhythmias-icds-heart-failure-cardiac-resynchronisation-equality-impact-assessment-guidance-development2. Accessed May 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.