ABSTRACT
During the influenza vaccination campaign 2014–2015, the reporting of 3 deaths within 48 hours of vaccination with Fluad brought the Italian Medicines Agency (AIFA) to pronounce the withdrawal of 2 batches of vaccine, based on the precautionary principle. Investigations by the Italian National Institute of Health, and by the Pharmacovigilance Risk Assessment Committee (PRAC), the committee at the European Medicines Agency (EMA) responsible for monitoring and assessing the safety profiles of human drugs, concluded that there was a lack of causality between the reported deaths and the vaccines administered. However, the media impact of the decision taken by AIFA, resulted in a lower influenza vaccination coverage compared with the previous years. The aim of our study was to identify possible critical points that may have led to a non-perfect management of the event. A review of the regulatory framework in place was performed, with a particular focus on the Guidelines on Good Pharmacovigilance Practices developed by the EMA to facilitate the signal management process. The management of reports involves the following steps: signal detection, its validation and confirmation, analysis and prioritization, assessment, recommendations for action and the exchange of information. In our opinion, both the signal detection phase and the phase of validation have been critical: the withdrawal of vaccine batches is possible even in case of a single suspected serious adverse reaction. However, aspects such as the biological plausibility, the presence of potential alternative causes and previous awareness should also be considered. Furthermore, the number of reported deaths was consistent with the expected background mortality rate in the vaccinated cohort. The disproportionate media coverage given to the AIFA decision resulted in a reduced vaccine confidence in the general population and in a decreased immunization coverage. Improving the communication on vaccine safety issues is crucial at this stage to restore a climate of trust in this powerful tool for primary prevention.
KEYWORDS: adverse event following immunization, FLUAD, influenza, pharmacovigilance, signal management, vaccine hesitancy
Introduction
Fluad is an inactivated trivalent influenza vaccine, adjuvanted with MF59C.1, first approved in Europe in 1997. Its use is indicated in people aged 64 y and older. During the 2014–2015 influenza vaccination campaign, about 4 million doses of this vaccine were distributed in Italy, and the vaccine was also used in Austria, Germany and Spain. The influenza strains recommended for that influenza season were: A/California/7/2009 (H1N1)pdm09-like virus; A/Texas/50/2012 (H3N2); B/Massachusetts/2/2012 – (wild type).
On November 27, 2014 the Italian Medicine Agency (AIFA) recalled 2 batches of Fluad vaccine as a precautionary measure after the reporting of 3 deaths occurred within 48 hours from vaccination.1 Although the vaccine crisis subsided in a relatively short period of time (on December 3, 2014 the Pharmacovigilance Risk Assessment Committee of the European Medicines Agency concluded that there was no evidence that Fluad had caused the reported deaths), the misinformation transmitted through the media had a negative impact on the vaccine uptake, and a significant reduction in the influenza vaccination coverage (VC) was reported for that influenza season for all Italian regions.2 This led a group of public health experts to raise the question if it was time to rethink pharmacovigilance regulations on vaccines in order to prevent serious health and economic losses for individuals and society that could be generated by possible future outbreaks of generalized panic, like the one that had just occurred.3
The aim of the present study was to perform a review of the international guidelines on post-marketing drug safety surveillance and of the European pharmacovigilance regulatory framework in order to identify possible critical points that may have led to an imperfect management of the event.
Results
The Council for International Organizations of Medical Sciences (CIOMS) and the World health Organization (WHO) Working Group on Vaccine Pharmacovigilance have defined vaccine pharmacovigilance as “the science and activities related to the detection, assessment, understanding, and communication of adverse events following immunization and other vaccine- or immunization- related issue, and to the prevention of untoward effects of vaccine.”4 An adverse event following immunization (AEFI) is “any adverse medical occurrence which follows immunization and which does not necessarily have a causal relationship with the usage of the vaccine.”5 The cause-specific definition distinguishes between:
Vaccine product-related reactions, i.e. those reactions caused or precipitated by inherent properties of the vaccine product;
Vaccine quality defect-related reactions, i.e., medical conditions caused or precipitated by quality defects of the vaccine product;
Immunization error-related reactions, i.e., events caused by inappropriate vaccine handling, prescribing or administration;
Immunization anxiety-related reactions, i.e., reactions arising from anxiety about the immunization;
Coincidental events, i.e., conditions caused by other causes.
The causality assessment checklist developed by a subgroup of the Global Advisory Committee on Vaccine Safety to gather information on the patient-immunization-AEFI relationship, help verify whether there is evidence, in the literature, or from clinical examination and laboratory tests, for other causes, or if, on the contrary, there is evidence for a vaccine product-related reaction, a vaccine quality defect-related reaction or an immunization error-related reaction.5 Most vaccine product-related reactions, particularly those related to vaccines that have been marketed long ago, are described in the literature (e.g.: oral polio vaccine-associated paralytic poliomyelitis; idiopathic thrombocytopenic purpura related to the measles−mumps–rubella vaccine). If an AEFI is still unclassified, after thorough review of the scientific literature, the causality assessment checklist help focus the attention on the qualifying factors that need to be considered for the assessment of the relationship. These factors include the expected background rate of the event in the vaccinated cohort, biological plausibility, potential risk factors, present and past health conditions and medications. The main sections in the checklist correspond to boxes in the mandatory path of an algorithm designed to allow the reviewer to logically organize and document his observations to the appropriate conclusions, in a stepwise approach.5
The main cornerstones of the new European pharmacovigilance legislation, which aims to strengthen and rationalise the system and improve public health and patient safety, are:
the new definition for adverse drug reaction, i.e., noxious and unintended effect resulting not only “from the authorised use of a medicinal product at normal doses,” but also “from medication errors uses outside the terms of the marketing authorisation, including the misuse and abuse of the medicinal product;”
the fact that not only health care workers, but also patients are now allowed to report suspected adverse reactions directly to the competent authorities;
the definition of clear tasks and responsibilities for all parties (marketing authorisation holders, competent authorities, European Medicines Agency);
the establishment of the Pharmacovigilance Risk Assessment Committee (PRAC), at the European Medicines Agency (EMA).
In Italy, reports of spontaneous adverse reactions are collected through the National Network of Pharmacovigilance, composed of the Italian Medicines Agency (AIFA), regional authorities, regional centers of pharmacovigilance, local health authorities, hospitals, research institutes and pharmaceutical companies. The National Network of Pharmacovigilance operates in connection with Eudravigilance, the European network for pharmacovigilance that collects in a single database all data regarding AEFIs provided at national level by the EU countries. Serious reports are transmitted from the National Network of Pharmacovigilance to Eudravigilance within 15 days, while non serious reports are sent within 90 d.6
The definition for “signal” is: “information that arises from one or multiple sources (including observations and experiments), which suggests a new potentially causal association, or a new aspect of a known association, between an intervention and an event or set of related events, either adverse or beneficial, that is judged to be of sufficient likelihood to justify verificatory action.”7
In the GVP module IX, the activities to be performed in the signal management process to determine whether there are new risks associated with a medicinal product or an active substance or whether known risks have changed, are thoroughly described, from the initial signal detection, through its validation and confirmation, analysis and prioritisation and assessment to recommending action.8 The signal detection corresponds to the act, performed by the EMA, Member States and market holding authorization, of identifying signals, by reviewing individual case safety reports, the scientific literature or other sources, and by using statistical methods. In the GVP Product- or Population-Specific Considerations I: Vaccines for prophylaxis against infectious diseases9 it is pointed out that the detection of a signal during mass vaccination programmes represents a challenge, as rare or serious incident coincidental illnesses are unavoidable, given the large exposure to the vaccine over a relatively short period of observation. The priority in this phase is therefore not only to identify in real-time possible new signals, so as to inform decision-making regarding safety concerns, but also to rapidly assess the odds that the number of events observed may be consistent with the expected background incidence rate.
Signal validation consists of verifying whether the available evidence supports the existence of a new aspect of a known association or a potentially causal association. During the validation process, important aspects such as the biological plausibility, previous awareness (past experiences with the vaccine), and public information that may increase the reporting of serious AEFIs in certain periods are to be taken into account.7,8 Signals for which the validation suggests new potentially causal association are entered into the EMA's European Pharmacovigilance Issues Tracking Tool, a database aimed at tracking and sharing safety information related to the drugs or vaccines, between the national competent authorities and the EMA.
During mass immunization programmes, observed to expected analyses (O/E analyses) are particularly useful, and their use as a routine tool for real time surveillance is highly recommended during the phases of signal detection, signal validation and in preliminary signal evaluations.9 During the influenza season 2014–2015, the number of reported deaths was consistent with the expected background mortality rate in the vaccinated cohort3 and the increase in the number of serious AEFIs reported as possibly associated to the vaccine administration in the first 4 d following the ban of the 2 batches was the result of the impact on health care workers of the extensive media coverage of the event, which acted as a source of bias in the reporting of AEFIs.
Any confirmed signal is to be analyzed and prioritised. During signal analysis and prioritisation, the potential impact of the signal on the product's benefit-risk profile is taken into account. Signal assessment means the scientific evaluation of the available evidence.
At every step of the process, exchange of information and the need for action are to be considered, and the decision for a batch recall can be made in case a manufacturing defect is suspected. Nonetheless, in case of fatal adverse events, particularly when the cause of death is unknown, the reporting rate of the event relative to both the usage of the vaccine batch and the ‘expected’ age-specific all-cause mortality should be calculated before deciding on a recall or quarantine action, which could have a detrimental impact on the immunization program. If it is likely that a cold chain problem or a handling deviation might have occurred, health authorities are strongly advised to weigh whether taking localized action before escalating to a national recall or quarantine.9 In the vaccine crisis we are examining, the decision taken was for a national recall of the suspected batches.
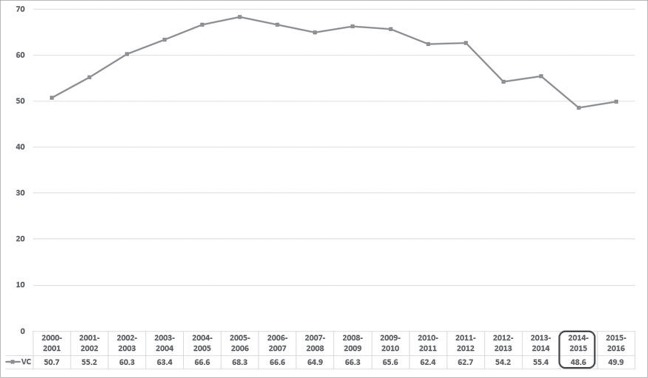
The establishment of an appropriate communication plan to address the safety concerns raised by the public and the media is another crucial aspect in the signal management. The recommendation by the WHO is for competent authorities to communicate in a proactive way the results of the signal management processes: in order to address safety concerns in an efficient way, a crisis communication team should be established during the pre-crisis period, possibly media-trained key-spokespersons should be identified, and standard texts to be subsequently adapted during the crisis should be prepared.10 During the crisis relevant background rates of signs and symptoms by age group and sex and exposure data should be kept up-to-date and communicated to the media. The decision made by AIFA had an excessive, unbalanced media coverage which resulted in a reduced vaccine confidence in the general population. As a matter of fact, despite the attempts by scientific societies to counter the panic,11 and the statement by the vaccine manufacturer, defending the vaccine safety record, that year the overall influenza vaccination coverage in people aged ≥ 65 y decreased from 55.4% to 48.6% with respect to the previous year, and the negative impact on influenza vaccine uptake was observed even in the following influenza immunization campaign (Fig. 1). These findings highlight that the communication on Fluad safety did not help prevent anxiety-related reactions and was therefore inadequate.
Figure 1.
Overall influenza vaccination coverage in people aged ≥ 65 y (per 100 inhabitants), Italy, influenza seasons 2000–2001 to 2014—2015.
Discussion
During the “Fluad case” in Italy, all reported deaths had occurred between 12th and 18th of November. The first fatal event occurred in Sicily, where a man aged 68, one hour after been vaccinated by his general practitioner, came back to the GP's office reporting chest pain, rapidly lost consciousness and died, despite resuscitation attempts.12 The other 2 deaths, of an 87-year-old man in Sicily and a 79-year-old woman in Molise, occurred within 48 hours from immunization and were both linked to an acute inflammation of the central nervous system.13 Both men were already affected by comorbidities.14 In the days following the reporting of the suspension of the 2 batches, more reports of possible illnesses or deaths related to the vaccine were investigated: on November 30, in the Italian National Network of Pharmacovigilance (RNF) the number of the deaths reported as possibly related to the administration of Fluad were as many as 13, distributed among 8 different regions.15 On December 1, the Italian National Institute of Health announced the first test results, which excluded evidence of contamination, as well as defects in the production: the content and the characteristics of the vaccine strains were compliant with the quality standards.16 On December 4, the Pharmacovigilance Risk Assessment Committee of the European Medicines Agency (EMA) excluded a causal link between the administration of FLUAD and the serious adverse events reported.17 Finally, the abnormal toxicity test and sterility test conducted by the Italian National Institute of Health, which were released on December 23, 2014, confirmed the vaccine safety profile and the 2 batches were re-introduced on the market.18
According to our review of the guidelines and of the regulatory framework for pharmacovigilance, the most critical phases were those of identification and validation of the signal: the withdrawal of a batch suspected to be contaminated is foreseen even in the case of a single suspected serious adverse reaction. However, other aspects, such as the biological plausibility or the presence of risk factors or underlying medical conditions, as well as previous awareness, should always also be considered. Decision making on a quarantine or a precautionary recall during a mass vaccination program is difficult if no vaccine quality-defect is known, as a cause-and-effect relationship between a particular AEFI and the use of a vaccine can seldom be identified when an initial decision is needed. It is useful to keep in mind that certain events, particularly if unexpected, severe and happening shortly after the administration of the vaccine, are more likely to be reported and to be perceived as a signal, while mild, expected AEFIs occurring sometime after the immunization are less likely to be reported and considered as possibly causally-related. In order to prevent unsubstantiated rumours to undermine the very success of the vaccination campaign and past achievements, a temporally-related AEFI must quickly be differentiated from a causal-related AEFI. Coincidental events need to be considered, particularly during mass immunization programmes, when, especially if the cause of a serious or fatal event is unknown, observed to expected analyses help distinguish coincidental from causal associations. The number of deaths temporally associated with the vaccine administration was consistent with the expected background mortality rate in the vaccinated cohort: it has been estimated that by pure statistical chance, every day in Italy, during the seasonal influenza campaign, from 15 to 20 individuals aged 65 y old or older die within 48 hours of vaccination.3 Decisions both in the signal detection and signal validation phases should have been based on such contextual information. Furthermore, if chain or handling deviation was hypothesized, localized action should have been considered before escalating to a national recall.
The extensive media coverage of the event acted as a source of bias in the reporting of AEFIs on both private citizens and health care workers, with the latter starting to report fatal events that occurred within the first few days after vaccination, even if most of those deaths were clearly linked to the extremely precarious medical conditions of their patients. Even if representatives of the Italian Medicines Agency stated that a full picture could be formed “only after a full analysis of all aspects, including the general health of the patients, their ages and probable conditions they might have had”19 and distinguished public health officials tried and reassured the public that the vaccine posed no threat,11 the media played a crucial role in keeping public distrust alive. As a matter of fact, immediately after the decision on the withdrawal of the 2 batches, the health authorities in Liguria and in Veneto regions and in the cities of Rome and Campobasso, decided to suspend the flu vaccinations until all implied batches were retired, and the most important and representative Italian consumers' association, Codacons, asked the Ministry of health to suspend the immunization campaign. Data on influenza vaccination coverage (VC) released by the Italian National Institute of Health, showed that the overall VC in people aged ≥ 65 y in the influenza season 2014–2015 decreased by 12.3% with respect to the previous one, falling below the levels achieved in the 2000–2001 national influenza campaign.2 Moreover, the number of severe influenza cases and of hospitalizations increased throughout the national territory. In Italy, since the pandemic season 2009/10, the monitoring of serious and complicated influenza cases is in place.20 In accordance with the circular from the Ministry of Health, the mandatory monitoring entails that the Regions and Autonomous Provinces report to the Ministry of Health and to the Italian National Institute of Health all severe and complicated cases that require admission to the intensive care unit and/or the use of extracorporeal membrane oxygenation.20 During the 2014/15 influenza season, 485 severe influenza-related cases and 160 influenza-related deaths were reported by 19 Regions and autonomous provinces: after the pandemic season 2009–2010 (in which 592 serious cases and 204 deaths were recorded) the 2014/15 influenza season has been the influenza season with the highest number of severe cases, even higher than the post-pandemic 2010–2011 season.21 Even if such increase can be partly related to the mismatch that occurred between the A(H3N2) strain selected for the vaccine and the main A(H3N2) strain that circulated in the northern hemisphere that influenza season, it is evident that if the immunization uptake did not drop to those disastrous levels, many cases could have been prevented and deaths would have been averted. The quantitative study performed by Odone A. et al.22 confirmed that, in the most popular Italian newspaper, the largest share of articles focused on influenza and immunization-related issues was published exactly in the days following the first reporting of the deaths suspected to be associated with the administration of Fluad, but prior to the announcement of the first test results by Italian National Institute of Health, i.e., the most critical days of the Fluad vaccine crisis. On the contrary, the release of the official safety results received very poor media coverage, and no coverage at all was given to the EMA's Pharmacovigilance Risk Assessment Committee statement. In this regard, our opinion is that the establishment of a task force for the management of vaccine safety crises would be very valuable to guarantee an appropriate communication on vaccine safety with the public and, in particular, with the news media, given the important role of the latter in influencing the public's perceptions regarding this topic. Nowadays unsubstantiated rumours about vaccine safety can be very easily amplified and reach the most vulnerable groups that need to be protected precisely by being immunized. It is therefore crucial that the key messages, conveyed through the most appropriate communication channels, are tailored to the different audiences and transmitted in a clear and timely manner. Improving communication on safety aspects of vaccines is very important, at this stage, to restore a climate of confidence in this powerful tool for primary prevention.
Material and methods
The review first took into account the manual issued in 2013 by the World health Organization (WHO) for the causality assessment of adverse events following immunization.5 Subsequently, a comprehensive review of the existing EU and Italian pharmacovigilance regulations was performed. In the first half of the 2010 the European pharmacovigilance legislation was subject to major amendments that lead to the adoption of Regulation No 1235/201023 and Directive 2010/84/EU24 (both became applicable in July 2012), and of Regulation No 1027/201225 and Directive 2012/26/EU26 (which started to apply from June and October 2013). In Italy, the pharmacovigilance system is regulated by the provisions contained in Title IX of Italian Legislative Decree 219/200627 and by those contained in Italian Legislative Decree 42/201428.
To facilitate the performance of the pharmacovigilance activities, the EMA has released good pharmacovigilance practice guidelines (GVP); our review focused particularly to those specifically dedicated to the vaccine safety signal management system, i.e.:
GVP Module IX – Signal management,8 which describes in detail the signal management process and defines mandatory additional monitoring;
GVP Product- or Population-Specific Considerations I: Vaccines for prophylaxis against infectious diseases,9 which focuses on those aspects and challenges that should be borne in mind when designing and implementing pharmacovigilance activities for vaccines.
Questions and answers on signal management,7 in which the crucial phases of the signal management process are highlighted;
Interim guidance on enhanced safety surveillance for seasonal influenza vaccines in the EU,29 in which the steps that marketing authorization holders had to take to enhance safety surveillance during the influenza immunization campaign 2014–2015 are reported.
Abbreviations
- AEFI
Adverse Event Following Immunization
- AIFA
Italian Medicines Agency
- CIOMS
Council for International Organizations of Medical Sciences
- EMA
European Medicines Agency
- GVP
Guideline on Good Pharmacovigilance Practices
- O/E analyses
Observed to Expected Analyses
- PRAC
Pharmacovigilance Risk Assessment Committee
- VC
Vaccination Coverage
- WHO
World Health Organization
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Italian Medicines Agency Comunicato stampa 401. AIFA dispone il divieto di utilizzo per due lotti del vaccino antinfluenzale FLUAD. 2014. Available from: http://www.agenziafarmaco.gov.it/it/content/aifa-dispone-il-divieto-di-utilizzo-due-lotti-del-vaccino-antinfluenzale-fluad.
- [2].Italian Ministry of Health, Istituto Superiore di Sanità Vaccinazione antinfluenzale in Italia: coperture vaccinali negli anziani (età >= 65 anni) (per 100 abitanti) Stagioni 2000-2001/2015-2016. 2016. Available from: http://www.salute.gov.it/imgs/C_17_tavole_19_allegati_iitemAllegati_0_fileAllegati_itemFile_3_file.pdf.
- [3].Signorelli C, Odone A, Conversano M, Bonanni P. Deaths after Fluad flu vaccine and the epidemic of panic in Italy. Br Med J 2015; 350:116; http://dx.doi.org/ 10.1136/bmj.h116 [DOI] [PubMed] [Google Scholar]
- [4].CIOMS. Definition and application of terms for vaccine pharmacovigilance Report of CIOMS/WHO working group on vaccine pharmacovigilance. Geneva; 2012. Available from: http://www.who.int/vaccine_safety/initiative/tools/CIOMS_report_WG_vaccine.pdf. [Google Scholar]
- [5].World Health Organization Causality assessment of adverse event following immunization (AEFI): user manual for the revised WHO classification. Geneva, Switzerland: WHO Library Cataloguing-in-Publication Data; 2013. Available from: http://www.who.int/vaccine_safety/publications/aefi_manual.pdf?ua=1. [Google Scholar]
- [6].Russo E, Capuano A, De Francesco A, De Sarro G, Esposito S, Mazzitello C. Pharmacovigilance in Italy: An overview. J Pharmacol Pharmacother. 2013; 4(5):20 Available from: http://www.jpharmacol.com/text.asp?2013/4/5/20/120942; http://dx.doi.org/ 10.4103/0976-500X.120942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Inspections and Human Medicines Pharmacovigilance Division Questions & answers on signal managementEMA/261758/2013 Rev 1*. London; 2016. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Other/2013/09/WC500150743.pdf.
- [8].Heads of Medicines Agency (HMA) and European Medicines Agency (EMA). Guideline on good pharmacovigilance practices (GVP). Module IX – Signal management. Doc. Ref. EMA/827661/2011. London: EMA; 2012. [Google Scholar]
- [9].Heads of Medicines Agency (HMA) and European Medicines Agency (EMA) Guideline on good pharmacovigilance practices (GVP) Product- or Population-Specific Considerations I: Vaccines for prophylaxis against infectious diseases. Doc. Ref. EMA/488220/2012. London: EMA; 2013. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/12/WC500157839.pdf. [Google Scholar]
- [10].World Health Organization Regional Office for Europe Vaccine Safety Events: managing the communications response A Guide for Ministry of Health EPI Managers and Health Promotion Units, Copenhagen, Denmark; 2013. Available from: http://www.euro.who.int/__data/assets/pdf_file/0007/187171/Vaccine-Safety-Events-managing-the-communications-response-final.pdf?ua = 1. [Google Scholar]
- [11].Cricelli (Simg) “Ogni giorno muoiono 800 anziani che si sono vaccinati per l'influenza, senza che vi sia alcuna correlazione con il vaccino.” 2014. Available from: http://www.agenziafarmaco.gov.it/it/content/cricelli-simg-ogni-giorno-muoiono-800-anziani-che-si-sono-vaccinati-linfluenza-senza-che-vi-.
- [12].Vaccino antinfluenzale Parla il medico del paziente deceduto ad Augusta: “Tutto fa presupporre morte cardiaca.” Quotidianosanità.it Quotidiano on line di informazione Sanitaria. 2014; Available from: http://www.quotidianosanita.it/scienza-e-farmaci/articolo.php?articolo_id=24621.
- [13].Intini E. Vaccini anti-influenzali e morti sospette: che cosa c'è da sapere. Focus.it. 2014; Available from: http://www.focus.it/scienza/salute/vaccini-anti-influenzali-e-morti-sospette-che-cosa-ce-da-sapere.
- [14].Tre morti sospette, AIFA blocca vaccino antinfluenzale ANSA. 2014; Available from: http://www.ansa.it/saluteebenessere/notizie/rubriche/salute/2014/11/27/ansa-tre-morti-sospette-aifa-blocca-vaccino-antinfluenzale_f9ac8b74-99b8-48cf-ae98-74155808f6c0.html.
- [15].Italian Medicines Agency Press release. Segnalato oggi in RNF nuovo decesso con FLUAD. 2014; Available from: http://www.agenziafarmaco.gov.it/it/content/segnalato-oggi-rnf-nuovo-decesso-con-fluad.
- [16].Istituto Superiore di Sanità COMUNICATO STAMPA N° 21/2014 - Vaccino Fluad: negative prime analisi ISS su lotti bloccati dall'AIFA. 2014. Available from: http://www.iss.it/pres/?lang = 1&id=1469&tipo=1.
- [17].European Medicines Agency Press release 3 December 2014. No evidence that Fluad vaccine caused deaths in Italy. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2014/12/WC500177992.pdf.
- [18].Istituto Superiore di Sanità COMUNICATO STAMPA N° 23/2014 - Vaccino Fluad: favorevole esito finale analisi ISS. AIFA annuncia sblocco lotti. 2014. Available from: http://www.iss.it/pres/?lang=1&id=1481&tipo=1. [Google Scholar]
- [19].Redazione ansa Flu vaccine raises concerns across Italy. ANSA: Rome, Italy; 2014; Available from: http://www.ansa.it/english/news/general_news/2014/11/28/flu-vaccine-raises-concerns-across-italy_dec930b3-20b8-42aa-8587-f963866dc4a9.html. [Google Scholar]
- [20].Italian Ministry of Health - Directorate General of Prevention Monitoraggio dell'andamento delle forme gravi e complicate di influenza confermata, stagione 2014-2015.. Italy; 2015. Available from: http://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=0&codLeg=50960&parte=1&serie=.
- [21].Rizzo C, Bella A. La sorveglianza dei casi gravi e decessi da influenza confermata in Italia nella stagione 2014/15. 2015. Available from: http://www.epicentro.iss.it/temi/vaccinazioni/CasiGravi2015.asp.
- [22].Odone A, Chiesa V, Ciorba V, Cella P, Pasquarella C, Signorelli C. Influenza and immunization : a quantitative study of media coverage in the season of the « Fluad case » Informazione : risultati di un indagine di monitoraggio nella stagione del « caso Fluad ». Epidemiol Prev 2015; 39(4):139-45; PMID:26499432. [PubMed] [Google Scholar]
- [23].The European Parliament and the Council Regulation (EU) No 1235/2010 of 15 December 2010 amending, as regards pharmacovigilance of medicinal products for human use, Regulation (EC) No 726/2004 laying down Community procedures for the authorisation a. 2010. [Google Scholar]
- [24].The European Parliament and the Council Directive 2010/84/EU of 15 December 2010 amending, as regards pharmacovigilance, Directive 2001/83/EC on the Community code relating to medicinal products for human use. 2010. [Google Scholar]
- [25].The European Parliament and the Council of the European Union Regulation (EU) No 1027/2012 of 25 October 2012 amending Regulation (EC) No 726/2004 as regards pharmacovigilance. 2012. [Google Scholar]
- [26].The European Parliament and the Council of the European Union Directive 2012/26/EU of 25 October 2012 amending Directive 2001/83/EC as regards pharmacovigilance. 2012. [Google Scholar]
- [27].Italian legislative decree April 24, 2006, n. 219 (GU Serie Generale n.142 del 21-6-2006 - Suppl. Ordinario n. 153). 2006. Available from: http://www.gazzettaufficiale.it/eli/id/2006/06/21/006G0237/sg.
- [28].Italian Legislative Decree march 4, 2014, n. 42 Attuazione dell'articolo 1, paragrafi 1, 5 e 12 della direttiva 2012/26/UE, che modifica la direttiva 2001/83/CE, per quanto riguarda la farmacovigilanza. (14G00053) (GU Serie Generale n.69 del 24-3-2014). 2014. [Google Scholar]
- [29].European Medicines Agency Pharmacovigilance Risk Assessment Committee (PRAC) Interim guidance on enhanced safety surveillance for seasonal influenza vaccines in the EU EMA/PRAC/222346/2014. United Kingdom; 2014. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/04/WC500165492.pdf. [Google Scholar]