ABSTRACT
Herpes Zoster (HZ) and its main complication, post-herpetic neuralgia (PHN), represent important public health issues because of their relevant burden among older adults. However, data on the epidemiology of HZ and PHN in Italy are very limited.
A population-based study was performed by seeking for cases of HZ and PHN, occurred in the period 2013-2015, in the clinical charts of 56 General Practitioners working in 4 Italian Regions (Liguria, Puglia, Toscana and Veneto). The main objective of the study was to estimate the incidence of HZ and the proportion of PHN (at 1 and 3 mo from the onset of HZ; PHN1 and PHN3) among people aged ≥ 50 y.
Overall, 598 cases of HZ were identified over 93,146 person-years of observation, thus corresponding to an overall incidence of 6.42 (IC95%: 5.93 – 6.95) HZ cases per 1,000 person-years. The incidence of HZ increased with age and was higher in female than in male. In total, 22.7%, 12.7%, and 2.4% of HZ cases suffered PHN at 1 and 3 mo and 1 y from the onset of acute episode. The proportions of these complications significantly increased with age, with the peak occurring in people aged ≥ 85 y. Four per cent of patients suffered ophthalmic zoster.
The study provided an update of the epidemiological burden of HZ and PHN in Italy, confirming the relevant burden of the disease in the elderly population.
The study was funded by the Italian Ministry of Health, Center for Disease Prevention and Control (CCM) in 2013.
KEYWORDS: herpes zoster, post-herpetic neuralgia, epidemiology, incidence, Italy
Background
Herpes zoster (HZ), also known as shingles, is a painful disease caused by the reactivation of varicella-zoster virus (VZV) remained latent in dorsal root or cranial nerve sensory ganglia after primary infection.1,2 HZ is usually characterized by a vesicular rash and is often associated with severe pain in a single dermatome segment.1,2 The main risk factors for re-activation of VZV are represented by the aging and by other causes of decreased cellular immunity.1 As a consequence, incidence of HZ markedly increases since 50 y of age and reaches the peak of 8 to 12 HZ cases per 1000 persons annually in subjects aged 80 y or more.3 These figures correspond to a lifetime risk of nearly 25% to suffer HZ in the general population and more than 50% in the subjects aged >85 y.4,5
The most common complication of HZ is the post-herpetic neuralgia (PHN), a chronic neuropathic resilient pain that persists or develops after at least 90 d from the acute episode and can continue for months or years.6 As confirmed by several epidemiological studies, incidence and severity of PHN increase with age; further relevant risk factors for PHN are severe pain during the acute phase of the disease and the rash severity.7-9 This clinical picture is often characterized by unsatisfactory treatment management with relevant impact on the quality of life in these patients.7,10
Furthermore, HZ can be complicated by other clinical pictures (i.e. ophthalmic zoster, dermatological or neurological complications, etc.), some of which have uncommon presentations and severe sequelae. However, the frequency of these less common complications is poorly known.11
By an epidemiological standpoint, the annual incidence rates of HZ are very similar across countries, meanwhile the risk of developing PHN widely ranged from 5% to more than 30% in international studies.3 Since the Italian epidemiological data on HZ and its complications are very limited12-14 and the most recent incidence estimations date back to the period 2003-200514, the present study aims to provide updated figures on the HZ incidence in Italy and to determine the proportion of HZ cases developing PHN and other complications.
Results
Overall, 598 incident cases of HZ were identified over 93,146 person-years of observation. This corresponded to an incidence rate of 6.42 (IC95%: 5.93–6.95) HZ cases per 1,000 person-years. As showed in Fig. 1, the incidence of HZ increased markedly with age, from 3.95 (IC95%: 3.06–5.09) per 1,000 person-years in people aged 50-54 y to the peak of 9.06 (IC95%: 7.43–11.06) per 1,000 person-years occurring in subjects aged 70-74 y.
Figure 1.
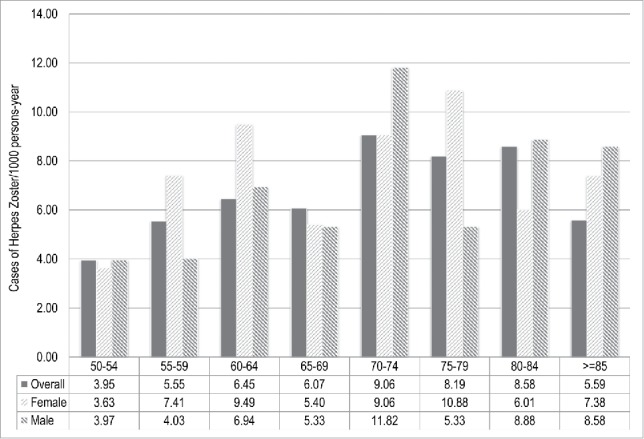
Incidence of Herpes Zoster (HZ) in adults aged ≥ 50 y stratified by age class and sex. Italy, 2013-2015.
HZ was more common among women than men, with an incidence of 7.23 (95%CI: 6.54 – 7.99) compared with 6.37 (95%CI: 5.58 – 7.28) per 1000 person-years, respectively, even though the difference resulted not statistically significant (p = 0.13). The incidence of HZ steadily increased among all age groups in both sexes (Fig. 1).
A total of 136 (22.7%) and 76 (12.7%) of HZ cases suffered PHN at 1 and 3 mo, respectively. These proportions corresponded to an incidence of 1.46 (IC95%: 1.23 – 1.73) PHN1 cases per 1,000 person-years and 0.82 (IC95%: 0.65 – 1.02) PHN3 cases per 1,000 person-years. The proportions of PHN1 and PHN3 significantly increased with age (p-value: 0.005 and 0.002, respectively), with the peak occurring in people aged ≥ 85 y (Figs. 2 and 3).
Figure 2.
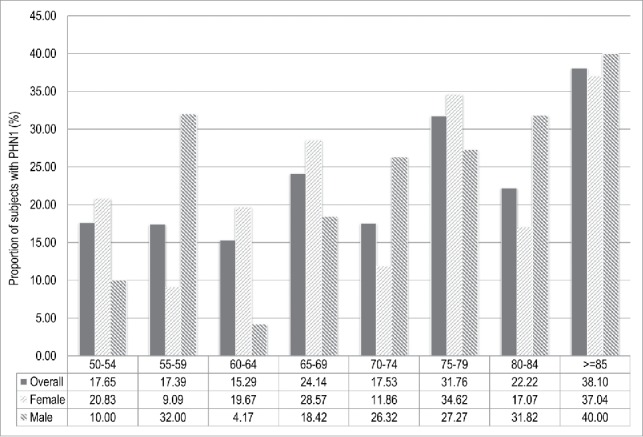
Proportion of subjects with post-herpetic neuralgia at 1 mo (PHN1) after the onset of Herpes Zoster in adults aged ≥ 50 y stratified by age class and sex. Italy, 2013-2015.
Figure 3.
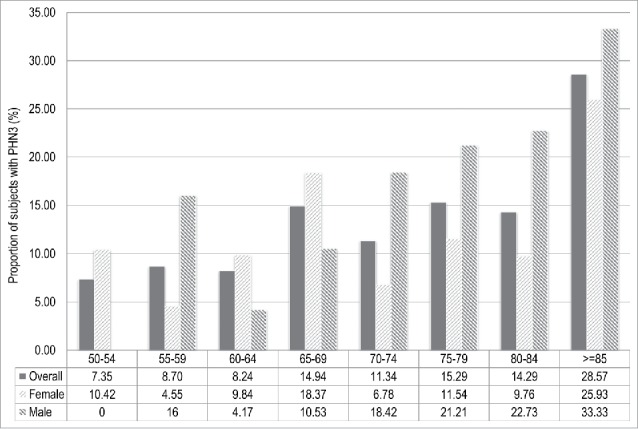
Proportion of subjects with post-herpetic neuralgia at 3 mo (PHN3) after the onset of Herpes Zoster in adults aged ≥ 50 y stratified by age class and sex. Italy, 2013-2015.
The proportions of PHN1 and PHN3 resulted higher in male (23.2% and 15.3%, respectively) than in female (21.5% and 11.3%, respectively), even though these differences were not statistically significant (p-value: 0.61 and 0.15, respectively)
Among patients with at least 1 y of follow-up, 2.4% suffered PHN at 1 y after the onset of HZ. The proportion of PHN at 1 y progressively and significantly increased among all age groups and both sexes (p-value: <0.05) (Fig. 4). Differently from PHN1 and PHN3, the proportion of PHN at 1 y resulted higher in female than in male (3,2% vs 1,8%), but the difference was not statistically significant (p-value: 0.27).
Figure 4.
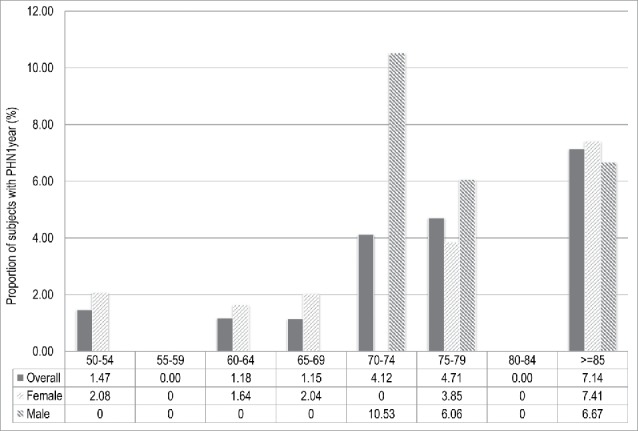
Proportion of subjects with post-herpetic neuralgia at 1 y (PHN1year) after the onset of Herpes Zoster in adults aged ≥ 50 y stratified by age class and sex. Italy, 2013-2015.
With respect to the other complications of HZ, the most frequent resulted ophthalmic zoster and dermatological complication, registered in 4% and 3.7% of patients, respectively. Neurological complications and VZV viremia were both reported in 0.7% of patients.
The proportion of patients who reported a previous episode of HZ in the year before the current episode was 2.2%, raising to 4.2% and 5.7% in the previous 5 and 10 y, respectively. Considering the entire lifetime, 14.1% of subjects reported a previous HZ, with 58.2% of subjects reporting 2 or more episodes.
Discussions
The study provided an update of the epidemiological burden of HZ and PHN in Italy. Our results showed on overall incidence of 6.42 HZ cases per 1,000 person-years among Italian people aged ≥ 50 y. This result is comparable with the findings obtained in the same age groups by Gialloreti and colleagues (6.31 per 1,000 person-years) nearly 10 y ago.14 The HZ incidence registered in our study is also consistent with the results of several international study conducted in the same age-group subjects.3 Moreover, in line with several epidemiological researches, we found an increasing incidence in older population and higher HZ incidence in women than in men.3 While the first finding can be easily explained with the decreased VZV-specific cell-mediated immunity of elderly, the reason for the higher incidence of HZ among women than among men remains controversial.15 Even though, some authors tried to explain this finding with the differences in health seeking behavior and with immunological or hormonal differences between women and men in response to reactivation of latent virus infection.16,17
In our study, the proportions of PHN1 and PHN3 resulted higher than those reported by Gialloreti and colleagues (22.7% and 12.7% vs 10.0% and 7.7%), using similar case definitions. 14 This discrepancy could be explained by possible differences in the age distribution of the 2 populations studied or by different behaviors in the prescription of neuropathic pain medication, particularly in the first month after the onset of HZ, by GPs, thus increasing the number of patients classified as suffering PHN. However, the proportions of PHN registered in our study fall in the wide range of values reported in the recent systematic review by Kawai and colleagues.3 In particular, with respect to the frequency of PHN at 3 mo from the onset of HZ, previous studies found figures widely ranging from 8.6% to 38%.3 This great heterogeneity is mainly attributable to the difference in the study design, age distribution of study populations and definitions used for PHN.
As already reported in several researches, proportions of patients with PHN increased with age reaching a peak after 80 y of age. In our study, subjects aged more than 85 y suffered PHN1 and PHN3 in 38.1% and 27.6% of cases, respectively. The high frequency of PHN, particularly in the elderly subjects, have important public health implications as its treatment has limited efficacy, patients are often refractory to these treatments, and the persistence of neuropathic pain can substantially affect patient quality of life and can determine physical disability, emotional distress, and social isolation.7,18 In this view, it is noteworthy that in our study the proportion of PHN patients that experienced persistent pain at 1 y from the onset of HZ, ranged from nearly 1% in the younger subjects to more than 7% in the patients aged more than 85 y.
Ophthalmic zoster represents a potentially dangerous localization of HZ because of its possible consequences on visual function.19 Our results evidenced that patients presenting this localization of HZ were 4%. This finding is lower than the risk of ophthalmic zoster, ranging between 10% and 15%, reported in the limited number of population-based studies that investigated the frequency of this localization.3 This difference can be explained by the behavior of GPs that register in their electronic clinical charts only the most severe form of ophthalmic zoster (i.e., those with eye involvement). Similarly, patients could tend to recall only the most severe and painful form of this localization. Therefore, the lower frequency of ophthalmic zoster measured in this study could be representative only of these more severe clinical pictures instead of the totality of HZ involving the ophthalmic division of the trigeminal nerve.
Finally, limited information are available about the frequency of HZ recurrence. Our findings demonstrated a high rate of recurrence up to 14.1% in the entire lifetime. Moreover, almost 60% of these subjects experienced 2 or more HZ episodes. Other studies reported lower rate of recurrence up to 6.2% during an 8 y of follow-up.20 However, the age characteristics of our study population and the retrospective nature of this question in our study could explained the higher rate of recurrence reported in our study.
Some major limitations of this research need to be underlined. First, the nature of the study and the source of data could lead to an underestimation of the burden of HZ and PHN. However, in Italy most patients with HZ consult the GP in order to receive a diagnosis and the drugs prescription. Therefore, our results can be considered robust. On the other hand, data about other complications of HZ and its recurrence can be affected by the quality of the information collected in the electronic clinical charts as well as by the recall bias of the patients.
In conclusion, this study provides updated information about the epidemiology of HZ and its main complications among the Italian subjects aged ≥ 50 y, confirming the relevant burden of the disease in the elderly population. In the next future, it will be important to monitor the epidemiology of HZ and PHN as, without preventive intervention, the impact of the disease could increase consequently to the aging of the Italian population.
Moreover, the epidemiological impact of HZ and its complications, together with the suboptimal treatment options to manage PHN and its effect on the quality of life, support the introduction of a safe and effective HZ vaccine in our country.21 Indeed, the introduction of HZ vaccine in national immunization program, particularly in North America and some European countries, has demonstrated to be effective in reduce the burden of the disease among vaccinated subjects.22 Therefore, the introduction of this preventive tool in Italy may substantially change the epidemiological scenario, thus requiring a continue evaluation of the epidemiology of HZ and PHN in order to assess the vaccine effectiveness also in our country.
Materials and methods
Study design
A population-based study was performed by seeking for cases of HZ and PHN, occurred in a 3-y period (from January 2013 to December 2015), among the patients aged ≥ 50 y cared by a sample of 56 General Practitioners (GPs) working in 4 Italian Regions (Liguria, Puglia, Toscana and Veneto) and covering 0.5% of the population aged ≥ 50 y indwelling in the above-mentioned Regions.
Data source
The primary data source were the electronic clinical charts used by GPs to register demographic data, medical diagnoses (coded according to the International Classification of Diseases, 9th Revision, Clinical Modification [ICD9-CM]), and drug prescriptions. Data about HZ episodes were extracted after the consent of the patients and were integrated with information regarding the complications of HZ and the occurrence of previous episodes of HZ, obtained by interviewing the patients.
Outcomes
The primary outcomes of the study were to estimate the incidence of HZ among people aged ≥ 50 y and the proportion and the incidence of PHN at 1 and 3 mo from the onset of disease (PHN1 and PHN3).
Other secondary outcomes were: the proportion of PHN at 1 y from the onset of HZ among patients with at least 1 y of follow-up, the proportion of other complications related to HZ (namely, ophthalmic zoster, neurological complications, dermatological complications and VZV viremia), and the proportion of patients referring a previous episodes of HZ.
Case definitions
In order to identify cases of HZ and PHN at 1 and 3 mo from the onset of HZ, the following case definitions were adopted:
a case of HZ was defined as a patient with a diagnosis of HZ registered in the clinical chart or a patient with the prescription of antiviral drugs for the treatment of HZ.
a case of PHN at 1 mo was defined as a patient with neuropathic pain at 1 mo after the diagnosis of HZ or a patient with the prescription of neuropathic pain medication at 1 mo after the diagnosis of HZ.
a case of PHN at 3 mo was defined as a patient with neuropathic pain at 3 mo after the diagnosis of HZ or a patient with the prescription of neuropathic pain medication at 3 mo after the diagnosis of HZ.
Moreover a case of PHN at 1 y from the onset of HZ was defined as a patient with neuropathic pain at 1 y after the diagnosis of HZ or a patient with the prescription of neuropathic pain medication at 1 mo after the diagnosis of HZ.
Statistical analysis
Annual incidences of HZ with their 95% confidence intervals (95%CI) were calculated as the number of events per 1,000 patient-years and stratified by 5-y age groups and sex.
Proportion of PHN1 and PHN3 were calculated as number of events per 100 cases of HZ. Annual incidences of PHN1 and PHN3 with their 95%CI were calculated as the number of events per 1,000 patient-years. Proportion of PHN1 and PHN3 were also stratified by 5-y age groups and sex.
A Chi square test for linear trend was used to assess the change in the proportions of HZ, PHN1 and PHN3 over the 5-y age groups.
All the analyses were performed using Epi-Info 7.0 (Centers for Disease Control and Prevention, CDC, Atlanta, GA, USA).
Ethics
Eligible subjects were informed by a physician about the rationale and aims of the study and all subjects provided a written informed consent. The study was approved by the Regional Ethics Committee of Liguria Region.
Abbreviations
- 95%CI
95% confidence interval
- GPs
General Practitioners
- HZ
Herpes Zoster
- PHN
post-herpetic neuralgia
- PHN1
post-herpetic neuralgia at 1 mo
- PHN3
post-herpetic neuralgia at 3 mo
- VZV
varicella-zoster virus
Disclosure of interest
G.I., R.P. e P.B. have previously participated at speaker's bureaus and advisory board meetings sponsored and have received research funding as investigator or principal investigator from GlaxoSmithKline Biologicals SA, Sanofi Pasteur MSD, Novartis, Crucell/Janssen e Pfizer. C.A., C.T., C.P., I.B., S.B., D.M., B.P., A.B., A.O., S.I. declare that they have no conflict of interest.
Acknowledgments
The authors wish to thank all the General Practitioners that participated to the study.
Funding
The study was funded by the Italian Ministry of Health, Center for Disease Prevention and Control (CCM) in 2013.
References
- [1].Johnson RW. Herpes zoster and postherpetic neuralgia. Expert Rev Vaccines 2010; 9(3 Suppl):21-6; PMID:20192714; http://dx.doi.org/ 10.1586/erv.10.30 [DOI] [PubMed] [Google Scholar]
- [2].Cohen JI. Clinical practice: Herpes zoster. N Engl J Med 2013; 369(3):255-63; PMID:23863052; http://dx.doi.org/ 10.1056/NEJMcp1302674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open 2014; 4:e004833; PMID:24916088; http://dx.doi.org/ 10.1136/bmjopen-2014-004833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Miller E, Marshall R, Vurdien J. Epidemiology, outcome and control of varicella-zoster infection. Rev Med Microbiol 1993; 4:222-30; http://dx.doi.org/ 10.1097/00013542-199310000-00006 [DOI] [Google Scholar]
- [5].Schmader K. Herpes zoster in older adults. Clin Infect Dis 2001; 32:1481-6; PMID:11317250; http://dx.doi.org/ 10.1086/320169 [DOI] [PubMed] [Google Scholar]
- [6].Schmader K, Jr Gnann JW, Watson CP. The epidemiological, clinical, and pathological rationale for the herpes zoster vaccine. J Infect Dis 2008; 197:S207-15; PMID:18419399; http://dx.doi.org/ 10.1086/522152 [DOI] [PubMed] [Google Scholar]
- [7].Johnson RW, Bouhassira D, Kassianos G, Leplège A, Schmader KE, Weinke T. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med 2010; 8:37; PMID:20565946; http://dx.doi.org/ 10.1186/1741-7015-8-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Whitley RJ, Shukla S, Crooks RJ. The identification of risk factors associated with persistent pain following Herpes Zoster. J Infect Dis 1998; 178:S71-5; PMID:9852979; http://dx.doi.org/ 10.1086/514274 [DOI] [PubMed] [Google Scholar]
- [9].Jung BF, Johnson RW, Griffin DR, Dworkin RH. Risk factors for postherpetic neuralgia in patients with herpes zoster. Neurology 2004; 62:1545-51; PMID:15136679; http://dx.doi.org/ 10.1212/01.WNL.0000123261.00004.29 [DOI] [PubMed] [Google Scholar]
- [10].Gabutti G, Valente N, Sulcaj N, Stefanati A. Evaluation of efficacy and effectiveness of live attenuated zoster vaccine. J Prev Med Hyg 2014; 55:130-6; PMID:26137786 [PMC free article] [PubMed] [Google Scholar]
- [11].Volpi A. Severe complications of herpes zoster. Herpes 2007; 14(Suppl 2):35-9. [PubMed] [Google Scholar]
- [12].Di Luzio Paparatti U, Arpinelli F, Visona G. Herpes zoster and its complications in Italy: an observational survey. J Infect 1999; 38:116-119; PMID:10342652; http://dx.doi.org/ 10.1016/S0163-4453(99)90079-8 [DOI] [PubMed] [Google Scholar]
- [13].Di Legami V, Gianino MM, Atti MCD, Massari M, Migliardi A, Tomba GS, Zotti C, Zoster Study Group . Epidemiology and costs of herpes zoster: background data to estimate the impact of vaccination. Vaccine 2007; 25:7598-7604; PMID:17889410; http://dx.doi.org/ 10.1016/j.vaccine.2007.07.049 [DOI] [PubMed] [Google Scholar]
- [14].Gialloreti LE, Merito M, Pezzotti P, Naldi L, Gatti A, Beillat M, Serradell L, di Marzo R, Volpi A. Epidemiology and economic burden of herpes zoster and post-herpetic neuralgia in Italy: a retrospective, population-based study. BMC Infect Dis 2010; 10:230; PMID:20682044; http://dx.doi.org/ 10.1186/1471-2334-10-230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Takao Y, Miyazaki Y, Okeda M, Onishi F, Yano S, Gomi Y, Ishikawa T, Okuno Y, Mori Y, Asada H, et al.. Incidences of Herpes Zoster and Postherpetic Neuralgia in Japanese adults aged 50 years and older from a community-based prospective cohort study: The SHEZ study. J Epidemiol 2015; 25:617-25; PMID:26399445; http://dx.doi.org/ 10.2188/jea.JE20140210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fleming DM, Cross KW, Cobb WA, Chapman RS. Gender difference in the incidence of shingles. Epidemiol Infect 2004; 132:1-5; PMID:14979582; http://dx.doi.org/ 10.1017/S0950268803001523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Johnson BH, Palmer L, Gatwood J, Lenhart G, Kawai K, Acosta CJ. Annual incidence rates of herpes zoster among an immunocompetent population in the United States. BMC Infect Dis 2015; 15:502; PMID:26546419; http://dx.doi.org/ 10.1186/s12879-015-1262-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kawai K, Rampakakis E, Tsai TF, Cheong HJ, Dhitavat J, Covarrubias AO, Yang L, Cashat-Cruz M, Monsanto H, Johnson K, et al.. Predictors of postherpetic neuralgia in patients with herpes zoster: a pooled analysis of prospective cohort studies from North and Latin America and Asia. Int J Infect Dis 2015; 34:126-31; PMID:25841633; http://dx.doi.org/ 10.1016/j.ijid.2015.03.022 [DOI] [PubMed] [Google Scholar]
- [19].Tran KD, Falcone MM, Choi DS, Goldhardt R, Karp CL, Davis JL, Galor A. Epidemiology of Herpes Zoster Ophthalmicus: Recurrence and chronicity. Ophthalmology 2016; 123:1469-75; PMID:27067924; http://dx.doi.org/ 10.1016/j.ophtha.2016.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yawn BP, Wollan PC, Kurland MJ, St Sauver JL, Saddier P. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc 2011; 86:88-93; PMID:21220354; http://dx.doi.org/ 10.4065/mcp.2010.0618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Paganino C, Alicino C, Trucchi C, Albanese E, Sticchi L, Icardi G. Herpes Zoster: the rationale for the introduction of vaccination in Italy. J Prev Med Hyg 2015; 56:E33-6; PMID:26789830 [PMC free article] [PubMed] [Google Scholar]
- [22].Ansaldi F, Trucchi C, Alicino C, Paganino C, Orsi A, Icardi G. Real-world effectiveness and safety of a live-attenuated herpes zoster vaccine: a comprehensive review. Adv Ther 2016; 33:1094-104; PMID:27262452; http://dx.doi.org/ 10.1007/s12325-016-0355-0 [DOI] [PMC free article] [PubMed] [Google Scholar]


