ABSTRACT
Post-herpetic neuralgia is the most frequent complication of herpes zoster and affects up to 30% of patients. Increased age is a well-recognized risk factor, while the role of gender is highly uncertain. Little research has been performed into a possible combined effect of age and sex in post-herpetic neuralgia.
The objective of the study was to study the role of age and sex and their combined effect in the development of post-herpetic neuralgia.
This retrospective study enrolled adult subjects with at least one episode of herpes zoster in the previous 10 y. A questionnaire on the patient's socio-demographic, anamnestic and clinical characteristics was administered by general practitioners. Multivariable logistic regression was used to detect relationships between post-herpetic neuralgia and age, sex and their interaction.
Fifty-nine of 272 patients reported post-herpetic neuralgia: a prevalence of 21.7%. Subjects with post-herpetic neuralgia (mean age 70.9 years) were significantly older (P = .001) than those without (64.2 years), the standardised mean difference being 0.5; no significant between-sex association was revealed (P = .96). A fully adjusted multivariable logistic analysis, however, revealed a highly significant (P = .007) age-sex interaction, with an odds ratio of 0.92; this also showed that older males were more likely to report post-herpetic neuralgia than younger males, while no obvious age-associated pattern was observed among females.
We discerned a significant age-by-sex interaction in the development of post-herpetic neuralgia, which suggests that the effect of age on the development of this condition may differ between men and women.
KEYWORDS: age-sex interaction, Herpes zoster, post-herpetic neuralgia, risk factors of Herpes zoster
Introduction
Herpes zoster (HZ), or shingles, a secondary infection caused by the reactivation of latent varicella zoster virus in sensory ganglia, imposes a significant public health burden. Its annual incidence is about 3–5/1,000 person-years in the general population, and increases to 6–8/1,000 and 8–12/1,000 person-years among subjects aged 60 and 80 years, respectively.1 Although HZ-related hospitalization and mortality are relatively low, the disease, and especially its complications, often seriously affect patients' quality of life, thereby engendering high individual and societal costs. Post-herpetic neuralgia (PHN) is the commonest chronic complication of HZ, and occurs as a consequence of peripheral nerve damage during an HZ episode.2 There is no a single definition of PHN, though it is most often defined as dermatomal pain that persists for at least 3 months after the onset of HZ rash.1,2 As shown by the systematic review by Kaway et al,1 the prevalence of PHN among HZ patients varies between 5% and 30%; the breadth of this range may be attributed to different study designs and populations. Several risk factors for the development of PHN in HZ patients have been cited, including age, sex, clinical characteristics of the HZ episode, chronic morbidities, immunosuppression, cancer and others. Among these, older age is the most certain and widely recognized risk factor, while the role of sex seems to be controversial. Indeed, some studies have found no association with sex, others have indicated a greater risk among females, while still others have discerned a higher risk among males.3
Almost all studies aimed at predicting PHN have used the regression approach to identify possible risk factors.3 However, only a few of these have reported testing for possible interactions between exploratory variables. Interaction occurs when the effect of one variable depends on the level of another variable, and it must be considered in any multivariable model, including PHN studies. Age-gender interaction occurs frequently; for instance, it has been suggested that the interactive effect of age and sex may make a significant contribution to pain response.4
Understanding the role of age and sex in PHN is essential, since they are the primary vaccine-targetable (i.e. may be identified before an HZ episode) patient characteristics.3 Given the inconclusive results regarding the effect of sex on PHN and the almost total lack of data on the possible interactive age-by-sex effect, the present study aimed to gain further insights into the role of age and sex and their combined effect in the development of PHN.
Results
A total of 276 HZ patients were enrolled. Four patients had no data on PHN and were therefore excluded from subsequent analyses. Females (n = 176, 64.7%) were more numerous than males (n = 96, 35.3%) and the mean age was 65.6 (SD 15.8) years. Clinical characteristics of the patients are reported in Table 1.
Table 1.
Characteristics of study participants.
| Characteristic | Level | n | % | 95% CI |
|---|---|---|---|---|
| History of HZ | No | 240 | 88.2 | 83.9–91.5 |
| Yes | 26 | 9.6 | 6.6–13.6 | |
| NA | 6 | 2.2 | — | |
| HZ localization | Thoracic | 109 | 40.1 | 34.4–46.0 |
| Cervical-facial | 42 | 15.4 | 11.6–20.2 | |
| Sacral-lumbar | 40 | 14.7 | 11.0–19.4 | |
| Abdominal | 43 | 15.8 | 12.0–20.6 | |
| Limbs | 26 | 9.6 | 6.6–13.6 | |
| Ophthalmic | 10 | 3.7 | 2.0–6.6 | |
| NA | 2 | 0.7 | — | |
| Background pathologies | Respiratory | 24 | 8.8 | 6.0–12.8 |
| Cardiovascular | 109 | 40.1 | 34.4–46.0 | |
| Endocrine | 41 | 15.1 | 11.3–19.8 | |
| Gastrointestinal | 33 | 12.1 | 8.8–16.6 | |
| Other | 74 | 27.2 | 22.3–32.8 | |
| Cancer before HZ diagnosis | No | 235 | 86.4 | 81.8–90.0 |
| Yes | 35 | 12.9 | 9.4–17.4 | |
| NA | 2 | 0.7 | — | |
| Physical trauma | No | 247 | 90.8 | 86.8–93.7 |
| Yes | 23 | 8.5 | 5.7–12.4 | |
| NA | 2 | 0.7 | — | |
| Psychological stress | No | 200 | 73.5 | 68.0–78.4 |
| Yes | 72 | 26.5 | 21.6–32.0 | |
| Systemic antiretroviral treatment | No | 34 | 12.5 | 9.1–17.0 |
| Yes | 238 | 87.5 | 83.0–90.9 |
NA = not available.
The overall prevalence of PHN was 59/272 [21.7% (95% CI: 16.9–27.1%)]. PHN was equally (χ2 = 0, P = .96) distributed among males [21.9% (95% CI: 14.8–31.1%)] and females [21.6% (95% CI: 16.2–28.2%)]. PHN increased markedly with age, ranging from 7.7% (95% CI: 2.7–20.3%) in patients under 50 y old to 25.3% (95% CI: 17.3–35.3%) in those aged over 75 y. The mean age of subjects with PHN [70.9 (SD 12.7) years] was significantly higher t = 3.32, P = .001) than that of subjects without PHN [64.2 (SD 16.3) years] and the effect size was medium [d = 0.50 (95% CI: 0.20–0.79)].
In the first model, consisting of only sex, age and their interaction term, the main effect of increased age [OR 1.09 (95% CI: 1.04–1.15), P < .001] was a significant predictor of PHN, while sex was not [OR 1.29 (95% CI: 0.61–2.69), P = .51]. Specifically, a 1-year increase in age determined a 9.1% increase in the odds of developing PHN. Moreover, the interaction between sex and age proved highly significant [OR 0.93 (95% CI: 0.88–0.98), P = .006]. The fully adjusted model revealed the same pattern for age [OR 1.09 (95% CI: 1.03–1.16), P = .002], sex [OR 0.97 (95% CI: 0.41–2.28), P = .94] and their interaction [OR 0.92 (95% CI: 0.86–0.98), P = .007]. As shown by the effect plot (Fig. 1), gender impacted the probability of developing PHN in concomitance with age: older men, were more likely to suffer from PHN than younger ones, while no obvious age-related pattern could be seen among women.
Figure 1.
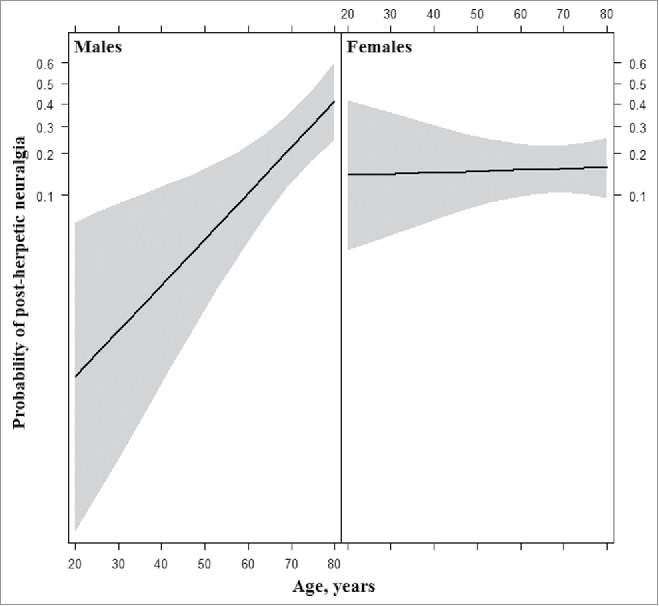
Effect plot for the interaction of age and sex in the fully adjusted logit model to predict post-herpetic neuralgia.
Discussion
To our knowledge, this is the first study to find a significant age-sex interaction in PHN patients. Indeed, in males the risk increased markedly with age, while no obvious age-related pattern was seen among females. This could partly explain previously observed inconsistencies in the relationship between PHN and sex.3
The age-by-sex interaction in PHN was explicitly tested in a prospective Icelandic study,5 which did not find a significant association. In that study, however, a much lower prevalence of PHN was recorded and the study population was significantly younger. The overall prevalence of PHN observed in our study was 21.7%; this matched estimates from the pooled analysis of 14 acyclovir trials by Crooks et al,6 who found a prevalence of 22% among placebo-treated patients. Our prevalence also fell within the range of 6.2–32.0% reported in Italian studies.7-9 Likewise, our estimate of the 1-year increase in the odds of developing PHN is in line with previously reported results (3–12%). Like most earlier studies,3 ours was not able to find a significant main effect of sex on PHN.
One possible limitation of this study is that the diagnosis of PHN was made by General Practitioners (GPs); therefore, some degree of variability was likely, since no unique definition exists. However, the very recent systematic review and meta-analysis of risk factors for PHN did not find any association between age and sex effects and PHN definition. 3 Moreover, the retrospective study design, with its known limitations, and the relatively small sample size make it essential to confirm our findings in larger prospective studies.
In conclusion, in this study, the age-by-sex interaction in the development of PHN in HZ patients was established, which suggests that the effect of age on the development of PHN may differ between males and females. Interaction terms should be considered in future risk prediction models.
Materials and methods
This retrospective study was formally approved by the Ethics Committee of S. Martino Hospital (Genoa, Italy). Adult subjects (≥ 18 years) of both sexes with a previous history of HZ, diagnosed by a GP in the previous 10 years, were eligible. Potentially eligible subjects were enrolled by 46 GPs in 2 northern Italian cities (Genoa and Turin) in 2012–2013. Once informed consent had been obtained, an ad hoc questionnaire on each patient's socio-demographic, anamnestic and clinical features was administered by GPs.
The study outcome was the presence of PHN after the last HZ episode. Potential predictors of PHN were: gender, age, history of HZ (new or relapsing case), HZ localization (thoracic, cervical-facial, sacral-lumbar, abdominal, limbs, ophthalmic), background pathologies, oncological history, traumatic physical and psychological events (grief, separation, personal or family illness, financial difficulties, job loss, etc.) in the 6 months prior to the last HZ episode, and systemic antiviral treatment.
For descriptive purposes, categorical variables were expressed as proportions with 95% confidence intervals (CIs). The univariable relationship between PHN and sex was evaluated by means of χ2 test, and that between PHN and age by means of t test. The effect size for the latter was quantified by means of Cohen's d. Subsequently, multivariable logistic modeling was undertaken in order to detect any association between PHN and age and sex. The first model utilised the independent variables of age and sex and their interaction, while the second considered all the possible confounders described above. The continuous variable of age was mean-centered. All analyses were performed in R environment.
Abbreviations
- Herpes zoster
(HZ)
- Post-herpetic neuralgia
(PHN)
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
The authors thank Dr. Bernard Patrick for his linguistic review of the manuscript.
Funding
The study belongs to a broader research program and was co-funded by the Italian Ministry of Research under project grant 2009ZPM4×4 titled “I vaccini tra nuove tecnologie e nuove applicazioni. Valutazione dell'impatto dei vaccini recentemente introdotti nei piani di prevenzione regionali e prospettive future” (Vaccines among new technologies and new applications. Assessment of impact of vaccines recently introduced in regional prevention plans and future prospects).
References
- [1].Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open 2014; 4:e004833; PMID:24916088; http://dx.doi.org/ 10.1136/bmjopen-2014-004833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Johnson RW, Rice AS. Clinical practice. Post-herpetic neuralgia. N Engl J Med 2014; 371:1526-33. [DOI] [PubMed] [Google Scholar]
- [3].Forbes HJ, Thomas SL, Smeeth L, Clayton T, Farmer R, Bhaskaran K, et al.. A systematic review and meta-analysis of risk factors for post-herpetic neuralgia. Pain 2016; 157:30-54; PMID:26218719; http://dx.doi.org/ 10.1097/j.pain.0000000000000307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sandhu SS, Sandhu J. Orthodontic pain: an interaction between age and sex in early and middle adolescence. Angle Orthod 2013; 83:966-72; PMID:23705940; http://dx.doi.org/ 10.2319/030113-174.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Helgason S, Petursson G, Gudmundsson S, Sigurdsson JA. Prevalence of postherpetic neuralgia after a first episode of herpes zoster: prospective study with long-term follow-up. BMJ 2000; 321:794-6; PMID:11009518; http://dx.doi.org/ 10.1136/bmj.321.7264.794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Crooks RJ, Jones DA, Fiddian AP. Zoster-associated chronic pain: an overview of clinical trials with acyclovir. Scand J Infect Dis Suppl 1991; 80:62-8; PMID:1803501 [PubMed] [Google Scholar]
- [7].Gialloreti LE, Merito M, Pezzotti P, Naldi L, Gatti A, Beillat M, et al.. Epidemiology and economic burden of herpes zoster and post-herpetic neuralgia in Italy: a retrospective, population-based study. BMC Infect Dis 2010; 10:230; PMID:20682044; http://dx.doi.org/ 10.1186/1471-2334-10-230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].di Luzio Paparatti U, Arpinelli F, Visonà G. Herpes zoster and its complications in Italy: an observational survey. J Infect 1999; 38:116-20; PMID:10342652; http://dx.doi.org/ 10.1016/S0163-4453(99)90079-8 [DOI] [PubMed] [Google Scholar]
- [9].Volpi A, Gatti A, Pica F, Bellino S, Marsella LT, Sabato AF. Clinical and psychosocial correlates of post-herpetic neuralgia. J Med Virol 2008; 80:1646-52; PMID:18649332; http://dx.doi.org/ 10.1002/jmv.21254 [DOI] [PubMed] [Google Scholar]


