ABSTRACT
Signal sequences (ss) play a critical role in the sorting of nascent secretory and membrane proteins. This function has been conserved from bacteria through eukaryotes, although ss appear diverse in length and amino acid composition. Sorting of proteins is also critical to instruct antigens for a proper immunological response. Thus, a plant ss was used to drive Human Papillomavirus (HPV) model antigens into the human secretory pathway: the HPV16 E7 oncoprotein, its chimera with the coat protein (CP) of the Potato Virus X (PVX), the first 200 amino acids of the HPV16 minor capsid protein L2 (known to harbour cross-reacting epitopes) and its chimera with E7 gene. These genes were used to transfect HEK-293 cells and to immunize C57BL/6 mice. The ss-provided genes were expressed, and proteins detected by immunofluorescence and immunoblotting. Mouse immunization with DNA constructs carrying the ss elicited a strong humoral response against both E7 and L2 and a weak cell-mediated immunity.
To our knowledge this is the first demonstration that a signal sequence derived from a plant can modulate the sorting of a heterologous protein in mammalian cells. This activity in mammalian cells may be responsible for the observed increased humoral response to DNA-based vaccines that are generally weak inducers of IgG response. This might open new perspectives in the design of DNA vaccines, especially to counteract infections where a strong humoral response is needed.
KEYWORDS: humoral immune response, HPV, infectious diseases, plant signal sequences, vaccines
Introduction
HPV is the agent behind the development of cervical squamous cell carcinoma, involved also in the pathogenesis of other cancers and in oro-pharingeal carcinogenesis. HPVs have a causative role in 5% of all tumors globally, with a deep impact on public health for the number of cases and life-time medical costs due to specific treatment missing. Current strategies for treating HPV cancers have been only moderately successful and characterized by recurrence.1 The available preventive vaccination (Gardasil, Gardasil-9 and Cervarix, based on the recombinant major capsid viral protein L1 of variously incident HPVs) will probably reduce the number of HPV cancer cases and of precancerous lesions worldwide, resulting in lives saved and related morbidity and cost-offsets.2 Nevertheless, these benefits will be visible in the next decades and, without a profound shift in rates of vaccination in men and women worldwide, prophylactic vaccination will not have great impact at least on the incidence of certain HPV infections like those causing oropharyngeal cancers, that are currently in a dramatic rise.
In the context of HPV preventive vaccines, formulations based on the induction of broadly cross-neutralizing antibodies against L2, the minor HPV capsid protein, are being developed.3 Since L2 protein neutralization epitopes only become exposed via furin cleavage when L1 interacts with heparin sulfate-proteoglycans of the host, HPV infection produces predominantly L1 antibodies with little or no L2 antibodies. The alleviated selective pressure for antigenic variance leads to structural conservation of L2 that makes this a target for the development of a ‘pan-HPV’ vaccine.4 Neutralizing epitopes of L2 were found to map at the N-terminus of the protein (mainly spanning the first 88 aa)5 and, based on this evidence, recent strategies have employed heterologous, modified L2 oligopeptides created using repeats or grafted to various scaffold antigens (difteria toxin, flagellin etc.) or assembled in virus-like display vaccines to improve the cross-neutralization range and the antibody titers (for a review see ref. 4).
Due to the presence of viral E5-E6-E7 targets (tumor-associated antigens), HPV tumors are also highly amenable to immuno-therapeutic approaches, with the possibility to set specific treatments based on vaccination eventually used in parallel with chemo-/radio-therapy and other currently available non-specific treatments, to integrate primary and secondary prevention. In this field, a strategy able to trigger E5/E6/E7-specific CTLs destroying HPV-infected and/or transformed cells is desirable but challenging, since HPV tumor-associated antigens are typically weak antigens with low immunogenicity (like many tumor antigens), active in virus immune evasion, and unable to induce inflammation.
Major goal of our work was to explore plant potentialities in increasing the immunogenicity of HPV DNA vaccines. DNA vaccines deliver genes encoding protein antigens into host cells, enabling their production to occur in vivo with many associated advantages: stimulation of both cellular and humoral immune response; possibility of manipulation to produce immunity against a specific target protein; modulation of the specificity and type of the immune response; rapid production; ease of production and storage. One approach we used to improve HPV immuno-therapy was to ‘re-invent’ vaccine design using sequences of plant origin as tools for immune function enhancement.6-8 Indeed, in the context of DNA vaccination, our first strategy was to fuse an attenuated HPV16 E7 gene to the PVX CP sequence, a carrier capable of generating CD4+ T cell responses that may enhance CD8+ T cell priming, This fusion construct was able to inhibit the growth of a HPV-expressing experimental mouse tumor.7
Plants (whole plants, plant-derived organ cultures and cell cultures) can address low-cost, high quality/safe production of proteins including antigens. In addition, plant production of candidate HPV therapeutic/prophylactic vaccines was tempted and proven.9 These studies gave evidence of immunogenicity and efficacy in animals and led also to patent release. To target the problem of low HPV16 E7 protein production yield in plants, we worked on the application of tools to tackle biomass expression like the use of sequences to improve accumulation. Exploiting the ss of the Polygalacturonase-inhibiting protein (PGIP) from Phaseolus vulgaris we were able to drive the HPV16 E7 protein in the plant secretory compartment. The protein was expressed with a 5-fold change accumulation level compared with the unfused version of the vaccine by transient plant expression. The possibility to increase the quantity of the E7 protein administered per dose, led to a higher cell-mediated immunogenicity specific to E7 of the vaccine and, ultimately, the growth inhibition of E7-expressing experimental tumors was achieved.6
In the present study, the potential effect of the PGIP ss in an expression vector for mammalian cells on the intracellular trafficking and export of HPV antigens was evaluated. DNA plasmids encoding the plant ss preceding a HPV16 E7 variant sequence (E7*) fused or not to the PVX CP (CP) carrier were constructed. In addition, ss was also genetically fused to the cross-reactive epitope of the L2 protein of HPV16 (L21–200, aa 1-200) and to the chimeric L2-E7 gene to obtain a therapeutic/preventive pan-reactive DNA vaccine.
Beyond the effects observed on the immunity triggered to the above-mentioned antigens, this ss and it may be other plant sequences could be of importance in the rational design of genetic vaccines against other tumor-associated pathogens and against emerging and re-emerging infectious agents.
Results
Expression of the ss-provided HPV genes in transfected HEK-293 cells
HEK-293 cells were transiently transfected to study the expression and the intracellular fate of the proteins encoded by the DNA constructs ss-E7*, ss-E7*-CP and ss-E7*-L-CP (Fig. 1) in a mammalian cell model. The ss-E7* construct was expressed in HEK-293 and found in the culture medium of transfected cells, whereas E7* without ss was only present in the cell lysates, as shown by immunoblotting analysis (Fig. 2A). The immunofluorescence analysis revealed a specific FITC fluorescence (green) exclusively associated to the cytosol and probably to intra-cellular membranes for ss-E7* (Fig. 2B), while E7* without ss was detected mainly in the nucleus (Fig. 2C).
Figure 1.
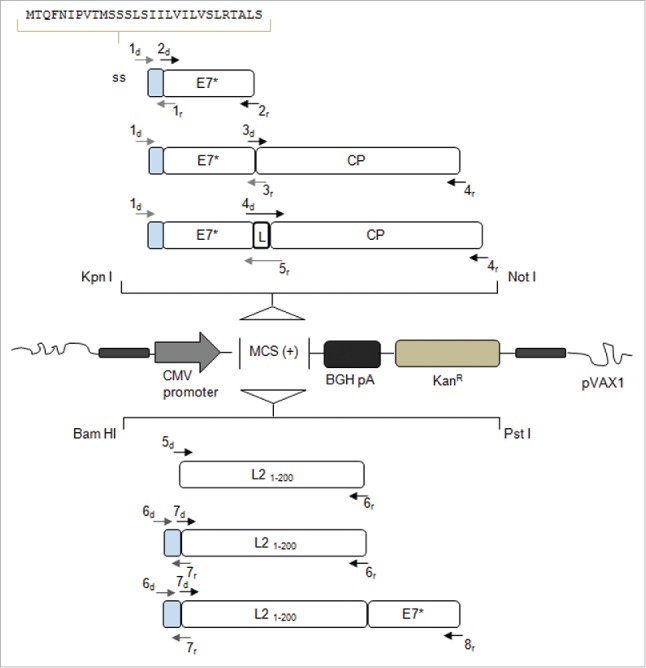
Schematic representation of the genes introduced into the mammalian vector pVAX1 with indication of the oligonucleotides used for PCR amplification and assembly (see Table 1). The recombinant genes are under the control of the CMV promoter and the BGH pA signal constituting the expression cassette, while the selectable marker is KanR. BGH pA: Bovine Growth Hormone poly-adenilation signal; KanR: Kanamycin Resistance; ss (light gray boxes): signal sequence of the gene encoding the Polygalacturonase Inhibiting Protein of Phaseolus vulgaris; E7*: attenuated E7 gene of HPV16; CP: Potato Virus-X coat protein gene; L: linker sequence encoding the Gly-Pro-Gly-Pro tetrapeptide; L21–200: nucleotide sequence corresponding to aa 1-200 of the L2 minor capsid protein of HPV type 16.
Figure 2.
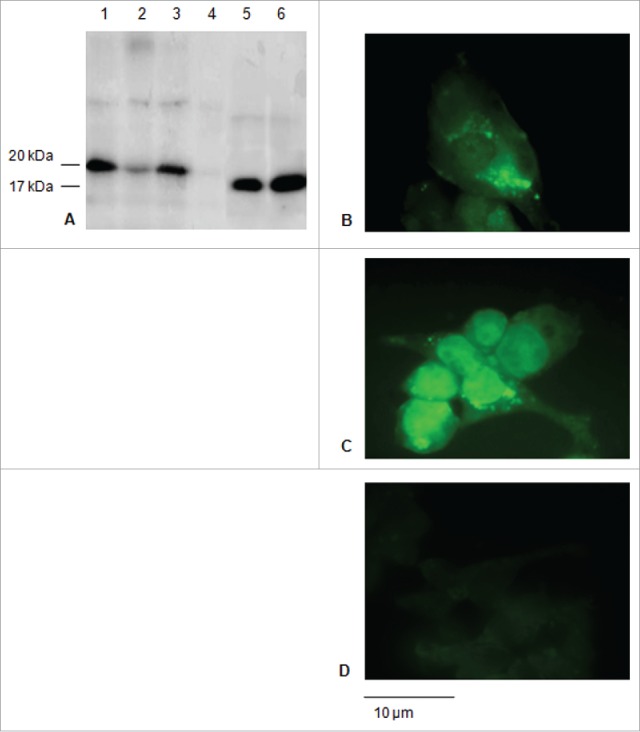
Expression of the E7* protein in HEK-293 cells upon transfection. A: Western blot of total soluble proteins from 1×106 cells transfected with the E7* +/− ss and of culture media. 1) ss-E7* (cell lysate); 2) ss-E7* culture medium; 3) E7* (cell lysate); 4) E7* culture medium; 5), 6) purified His6-E7* from E. coli 25 ng and 50 ng, respectively. The E7* protein runs as a 20 kDa protein in HEK-293 cells, while His6-E7* shows a slightly lower molecular weight in bacteria. B, C, D: Immunofluorescence (FITC) detected in cells transiently transfected with ss-E7* (b), with E7* (c) and non-transfected HEK-293 cells used as a negative control (d) (original magnification, 100x; scale bar, 10 µm).
For the ss-E7*-CP and E7*-CP constructs, expression but no secretion was observed in the culture medium. The lysates of the cells transfected with ss-provided constructs, showed presence of a double band (Fig. 3A). In the presence of ss, a specific green fluorescence that seemed to be associated to intra-cellular membranes, was observed especially for ss-E7*-CP (Fig. 3C). As already reported,7 the fusion of E7* with the CP sequence determines the re-distribution and aggregation of the E7* protein into the cytoplasm (Fig. 3B, Fig. 3D).
Figure 3.
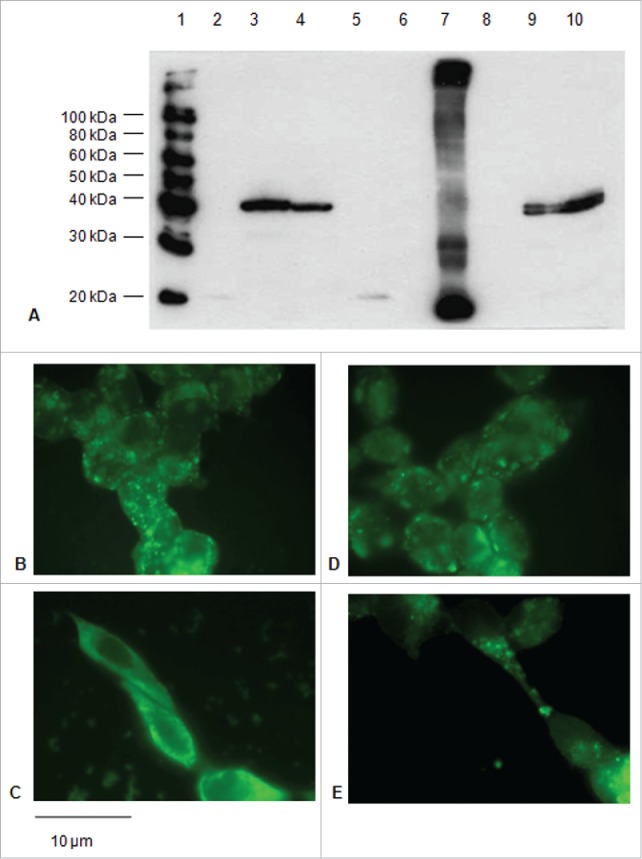
Expression of the E7*-CP proteins in HEK-293 cells upon transfection. A: Immunoblotting of proteins from lysates of 1×106 cells transfected with the E7*-CP fusions with or without ss. 1) Biotinylated Protein Ladder NEB; 2) E7* (cell lysate); 3) E7*-CP (cell lysate); 4) E7*-L-CP (cell lysate); 5) ss-E7* (cell lysate); 6) ss-E7*-CP (culture medium); 7) ss-E7 plant extract; 8) ss-E7*-L-CP (culture medium); 9) ss-E7*-CP (cell lysate); 10) ss-E7*-L-CP (cell lysate). B, C, D, E: Immunofluorescence (FITC) detected in cells transfected with E7*-CP (B, C) and E7*-L-CP (D,E) plasmids without or with the ss, respectively (original magnification, 100x; scale bar, 10 µm).
The L2 constructs were also expressed and proteins detected in the transfected cells mostly into the cytoplasm (Fig 4A, B, C).
Figure 4.
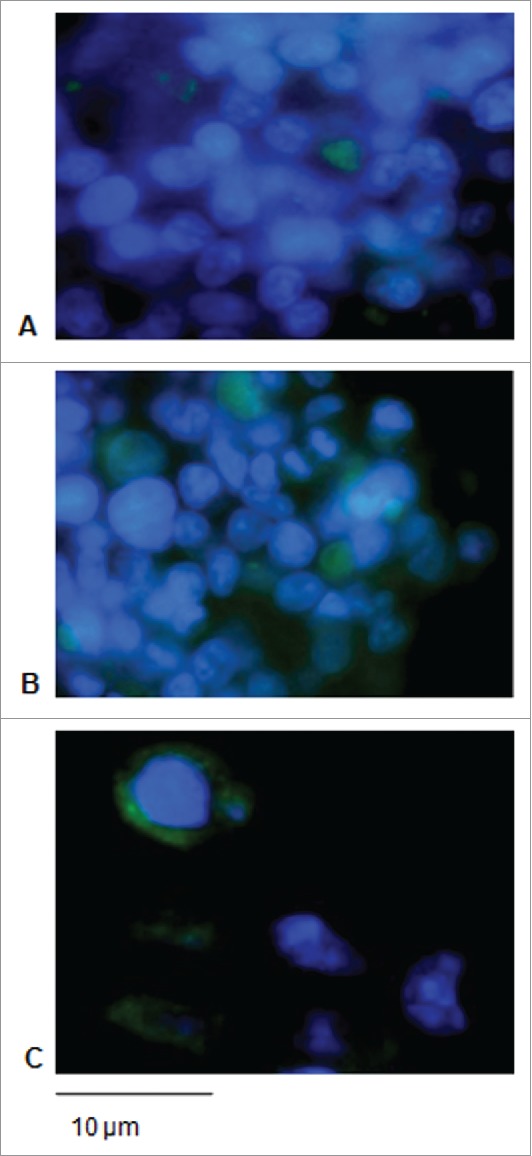
Expression of the L21–200 proteins in HEK-293 cells upon transfection. Immunofluorescence in cells transfected with L21–200 (A), ss- L21–200 (B) and ss-L21–200-E7* (C) constructs. FITC fluorescence was coupled with DAPI nuclei blue counter-staining (original magnification, 100x; scale bar, 10 µm).
Mouse immunological responses to vaccination with ss-E7*, ss-E7*-CP and ss-E7*-L-CP plasmids
The immunological effects of the ss-provided DNA vaccines were studied in C57BL/6 mice with the prime/boost schedule described in Fig. 5A.
Figure 5.
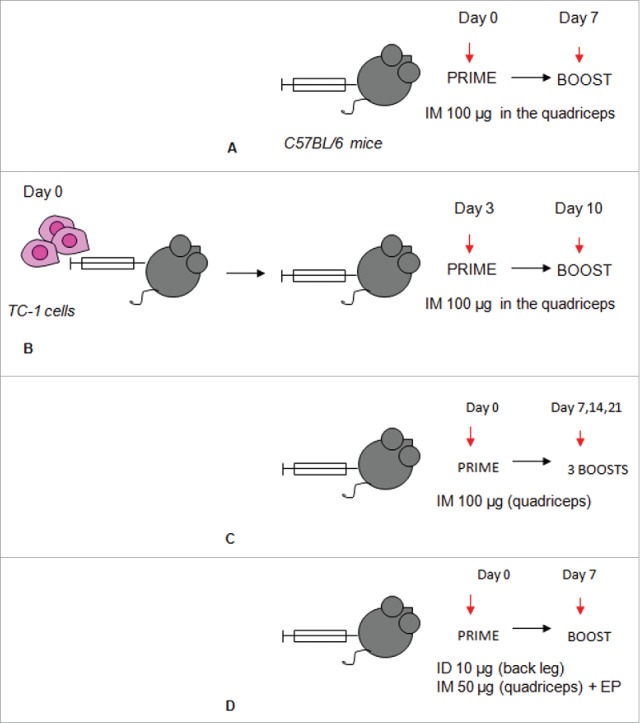
Immunization schedules. Immunization with the ss-E7* +/−CP series in absence (A) or in presence of tumor challenge (B) to evaluate immune response and tumor rejection, respectively. Long-term (C) and short-term (D) immunization protocols used to induce antibody response in mice vaccinated with the the L21–200 +/− E7* series. ID: Intra-dermal injection; IM: Intra-muscular injection; EP: Electroporation.
The effectiveness of the ss-E7*-based DNA vaccines in inducing cell-mediated responses was studied in ELISPOT assay for INF-γ secreting cells. One week after TC-1 challenge, spleens were collected from immunized mice. By the addition of ss, no enhancement of the cell-mediated immune response specifically directed against E7 was observed with respect to the non-secretory constructs. Actually, the scores obtained with the ss-series of plasmids were inferior to those recorded in mice vaccinated with the plasmids without ss. In brief, both ss-E7*-CP and ss-E7*-L-CP vaccination elicited an E7-specific CTL response approximately a 3-fold lower than E7*-CP, used for comparison. The lowest score was obtained in ss-E7* vaccinated mice, exhibiting E7-specific T cell response approximately a 7-fold lower than that recorded in mice vaccinated with E7*-CP (Fig. 6).
Figure 6.
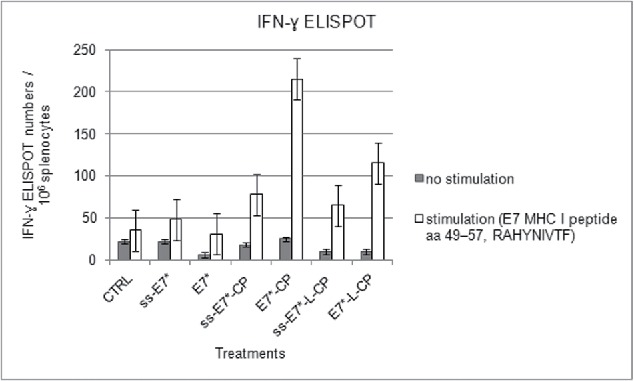
ELISPOT assay for IFN-γ-secreting splenocytes after vaccination with ss-E7* +/− CP in C57BL/6 mice. Data are presented as mean spot number ± SD per 1×106 splenocytes from triplicate wells. Cells were stimulated with an E7 specific H-2Db cytotoxic T lymphocyte MHC class I epitope. Gray columns represent non-stimulated splenocytes. The presence of IFN-γ producing E7 specific T-cell precursors was determined using an anti-IFN-γ antibody, as described in Material and Methods.
E7-specific serum antibody response was evaluated one week after the boost. Circulating antibodies were detected in ELISA assay with E.coli-produced E7 protein. Total serum specific IgGs were induced after immunization with ss-E7* and ss-E7*-CP. This anti-E7 response was significantly higher than that of DNA vaccines without ss (Fig. 7) with means and variances significantly different (pmeans < 0,05 and pvariances < 0,05).
Figure 7.
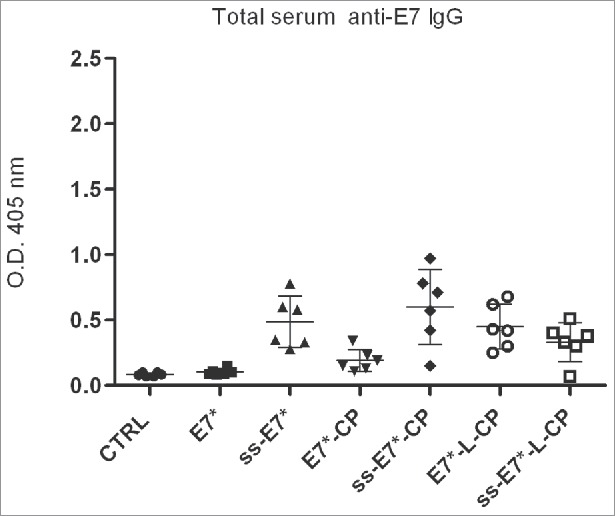
Humoral immunological response in mice after vaccination with ss-E7* +/− CP. Data for ELISA test are reported from a representative experiment. Humoral E7-specific serum IgG responses are presented as optical density values at 405 nm of 1:100 diluted sera. The mean titer value from each group is marked with a dash. Means and variances are significantly different (p< 0,05) according to the One-way ANOVA test and to the Bonferroni's multiple comparison test.
Therapeutic immunization with ss-E7*, ss-E7*-CP and ss-E7*-L-CP plasmids after challenge with TC-1-induced tumors in mice.
The peculiar immunological responses of these new anti-E7 vaccines led us to determine if a therapeutic potential of the DNA vaccines could still be envisaged in the TC-1 pre-clinical model. Indeed, E7 is a target for the development of therapeutic candidate HPV vaccines. The E7*-CP vaccine was utilized for comparison. After injecting TC-1 cells into naïve C57BL/6 mice, prime and boost immunizations were performed on days 3 and 10, respectively (Fig. 5B). As shown in Fig. 8, despite tumor appearance was delayed to day 30 post challenge in all anti-E7 immunized mice, at day 47, 67% of the E7*-CP treated animals was still tumor-free, while the ss-constructs induced a weaker effect: 33% of ss-E7*-L-CP vaccinated mice and 17% of ss-E7* or ss-E7*-CP vaccinated mice were tumor free, respectively. By the same day, 100% of the mice immunized with the empty vector developed tumors.
Figure 8.
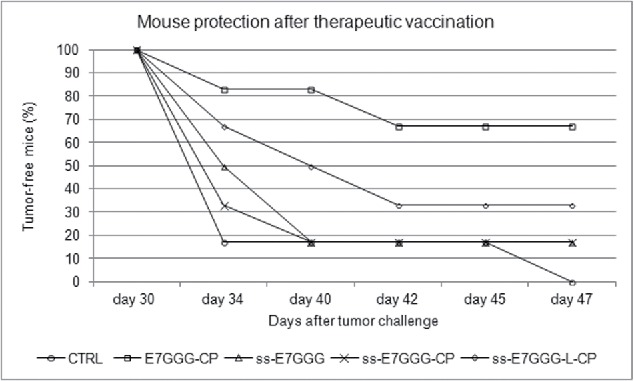
Growth inhibition of experimental TC-1-induced tumors after therapeutic vaccination of mice with ss-E7*, ss-E7*-CP and ss-E7*-L-CP. Results are presented as the percentage of tumor-free mice. The presence of tumors was monitored by palpation twice per week. The animals were sacrificed on day 47 after tumor challenge, when all the animals with tumors were euthanized for ethical reasons. E7*-CP was administered for comparison of efficacy data.
Mouse immunological responses to vaccination with L2-based DNA constructs
The structural conservation of L2 makes this capsid protein a target for the development of ‘pan-HPV’ vaccines. Based on this consideration, the plant ss was genetically fused to a cross-reactive long peptide of the L2 protein (L21–200, about 24 kDa) to produce a broad range preventive HPV vaccine. In addition, to investigate for a therapeutic/preventive HPV vaccine, fusions of L21–200 were made with the E7* sequence. C57BL/6 mice were immunized according the 2 different protocols described in Material and Methods (Fig. 5C, Fig 5D).
The L21–200 expressed and purified from E. coli (Fig. 9) was used to set-up ELISA test for antigen-specific IgG responses. Data for ELISA test were generated from sera collected from each mouse of every group and vaccination experiments were performed 3 times with similar results. One representative experiment is shown in Fig. 10. The ‘long-term’ immunization protocol (Fig. 5C) was not able to induce a significant improvement of anti-L21–200 antibody titers compare with pre-immune sera (data not shown). When the vaccination schedule implying electroporation (EP, Fig. 5C) was applied, animals were primed with DNA at time zero and boosted with DNA at 1-week interval. The repeated vaccination with the plasmids delivered by intradermal (ID) and EP immunization within a short interval, resulted in the induction of a strong humoral immune response (Fig. 10). The ss-provided constructs induced a statistically significant higher response against both L21–200 and E7 compare with DNA vaccines without ss (pmeans < 0,05 and pvariances < 0,05). The ss-L21–200-E7* plasmid was able to produce the highest titers of both anti-L21–200 (Fig. 10A) and anti-E7 IgG (Fig. 10B). The EP immunization protocol determined also a long-lasting maintenance of the acquired immunity as shown by the anti-L2 reactivity of the pooled sera collected from mice 6 months after immunization (Fig. 11).
Figure 9.
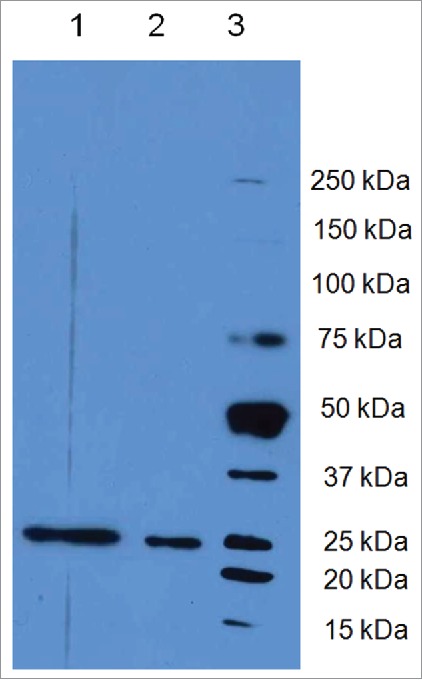
L21–200 expression in E. Coli. Lane 1: L21–200 purified under native conditions; lane 2: L21–200 purified under denaturing conditions; lane 3 Molecular weight Marker Precision Plus Protein™ Dual Color Standards (Bio-rad Milan, Italy).
Figure 10.
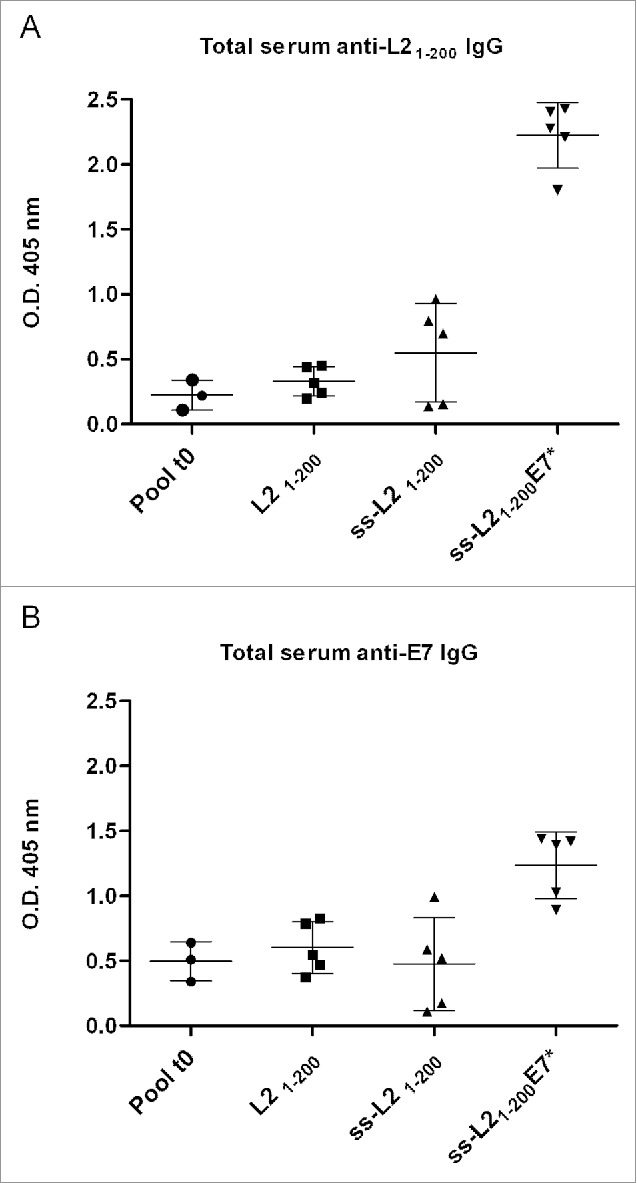
Humoral immunological response to L21–200 and E7* in mice after vaccination with the L21–200, ss-L21–200 and ss-L21–200-E7*. C57BL/6 mice were immunized as described in Fig. 5D. Data for ELISA test were generated for sera collected from every mouse (m 1-5: mouse 1-5) of each vaccination group after coating with either His6-L21–200 (Fig. 10A) or His6-E7 (Fig. 10B) and are reported from one representative experiment. Humoral specific serum IgG responses are presented as optical density values at 405 nm of 1:100 diluted sera. The mean titer value from each group is marked with a dash. Pool t0: average of pool of pre immune sera for each treatments. Means and variances are significantly different (p < 0,05) according to the One-way ANOVA test and to the Bonferroni's multiple comparison test.
Figure 11.
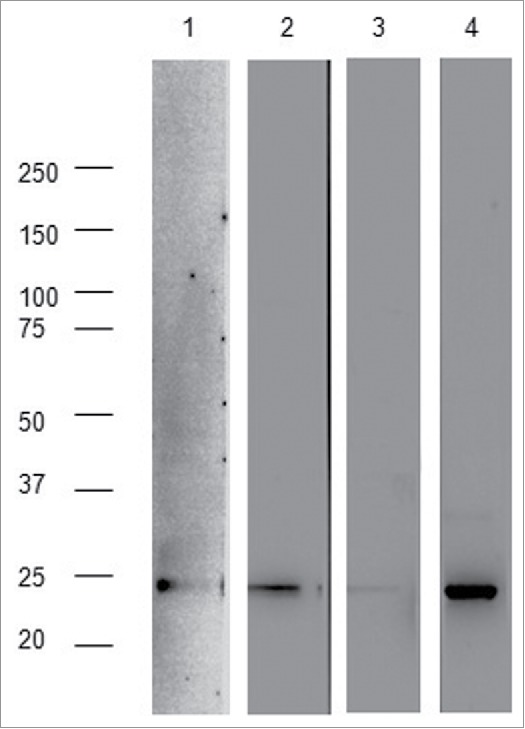
Reactivity of sera of immunized mice against the L21–200 peptide 6 months post-immunization, as described in Fig. 5D. 50ng of L21–200 were loaded for each lane and revealed with RG-1 monoclonal antibody (strip 1) or 1:100 dilutions of pooled sera from ss-L21–200 (strip 2), L21–200 (strip 3), and ss-L21–200-E7* (strip 4) mouse groups.
Discussion
In previous reports, we showed that immunization against the E7 protein of HPV16 is able to inhibit the growth of E7-expressing tumors in different mouse models in the form of both genetic vaccines fused to plant-derived sequences and plant-produced recombinant proteins.6,7,10,11
To optimize plant production yield, signal sequences can be used. They may serve to target products to specific compartments of the plant cell for accumulation, like the endoplasmic reticulum (very suitable for protein folding and assembly) from where the proteins can be secreted to the apoplast, offering a natural way to concentrate proteins.12 Indeed, in a previous study, we demonstrated the expression of the E7 protein in the secretory compartment and its efficient accumulation in the apoplast of Nicotiana benthamiana by the plant-derived signal sequence of PGIP from bean, the same ss used in the present work. Moreover, targeting of the E7 protein to the plant secretory pathway enhanced plant protein production and improved the immunotherapeutic activity of E7-containing plant extracts.6
DNA vaccines can be rapidly produced with high purity and low production cost. They consist of recombinant plasmids expressing in vivo a vaccine immunogen that may stimulate both cellular and humoral immune responses. They can be designed to modulate the specificity of the immunity or to ‘enhance’ responses by inserting sequences that can act as ‘adjuvants’. Even though theoretical possibility of integration into the host genome exists, they are generally considered safe.13
In the present study, we evaluated the potential of using the PGIP ss in the context of DNA immunization to enhance immunity responses to the encoded E7 antigen. In our previous studies, mouse DNA immunization with E7 fused to PVX CP generated CD4+ T cell responses that increased CD8+ T cell priming through the presentation within MHC class II pathway. These fusion constructs were able to determine the growth inhibition of E7-expressing experimental tumors upon therapeutic vaccination.7 Various DNA vaccination studies indicate that secreted antigens can induce better immune responses than those expressed in the cytosol (for a review seeref. 14), thus we decided to manipulate the antigen expression by the PGIP ss to design HPV DNA vaccines.
DNA plasmids harbouring PGIP ss preceding the E7* antigen fused or not to the PVX CP gene were constructed. Upon immunization, the translated signal peptide should have directed the nascent E7* protein into the endoplasmic reticulum and Golgi pathway of the transfected host cells, facilitating secretion of E7*. Therefore, the expression of the proteins was characterized in vitro by transfection of HEK-293 cells. Indeed, the E7* protein was found in culture media of transfected cells, suggesting that it was targeted in the secretory pathway by the signal peptide that, ultimately, determined its secretion. In effect, immunofluorescence data show that the ss shifts E7* from a mainly nuclear to a cytoplasmic location. The lysates of the transfected cells showed that the produced fusion antigens separated in a double band in immunoblotting, probably because ss is not always properly recognized. However, to our best knowledge, this is the first demonstration that a signal sequence of a plant protein may exert a biological activity in mammalian cells.
The effectiveness of the secretory DNA vaccines in inducing humoral and cell-mediated responses was studied. In general, DNA vaccines are considered strong inducers of Th1 responses. In the current study, it was observed that the secretory constructs elicited both humoral and cell-mediated immunity even if the latter response was weaker with respect to the non-secretory counterparts used as control, as shown by IFN-γ production in re-stimulated spleen cells from vaccinated mice. This poor response affected outcomes of tumor challenged mice because induction of a strong cell-mediated immunity is strictly linked to protection against HPV tumors. Indeed, animals that did not develop tumors after challenge, showed higher levels of IFN-γ secreting splenocytes (data not shown). On the other hand, although not the crucial one for HPV tumor resolution, antibody response was astonishingly high compare with that of non-secretory constructs.
Thus, this activity of the plant ss was utilized for designing a DNA ‘pan’-reactive therapeutic/preventive HPV vaccine. The plant ss was genetically fused to a cross-reactive epitope of the L2 protein from HPV16 (L21–200) and fusions were made also with the E7* sequence. The L2 peptide was successfully expressed in E. coli to set up ELISA experiments and protein expression in mammalian cells was demonstrated, confirming that the plant protein signal is active in mammalian cells.
One of the issues regarding DNA vaccine efficiency is the way of its delivery. Conventional injection of DNA is considered a key limitation due to inefficient uptake by cells. Electroporation appears a promising approach for improving immunogenicity of DNA vaccines for its ability to increase cellular permeability resulting in a high level of protein expression and improved immune response.15 Recently, clinical trials have showed the efficiency of EP in humans.16,17 Our data confirmed that EP in more efficient than IM injection. Indeed, quite disappointing results were obtained after using a conventional IM vaccination schedule (data not shown), whereas a short vaccination program by EP and ID injection15 gave rise to strong humoral response by ss-linked constructs. Moreover, EP was able to induce a longstanding humoral immune response against L21–200, at least persisting 6 months in the utilized mouse model.
In conclusion, results from this study demonstrate for the first time that a plant signal sequence can exert an activity of targeting to secretion also in mammalian cells. We identified that the ss of PGIP is an optimal signal peptide for E7 secretion. Moreover, this ss plays a critical role in inducing a strong humoral response against E7GGG and L21–200 antigens of HPV16. In addition, ss-constructs delivered by EP are able to induce a rapid, robust, and durable humoral response. This result is important because DNA vaccines suffer from poor IgG induction and were believed not efficacious as prophylactic vaccines. Preliminary experiments seem to indicate the neutralizing nature of the anti-L2 antibodies (data not shown), making this a very important step in the field of HPV preventive/therapeutic vaccination against HPV.
This study, highlights also the important interplay between a plant signal peptide and mammalian intracellular trafficking that may lead to the induction of humoral response upon immunization. One explanation for this surprising results can be found in the structure and function of ss. The secretion function has been conserved from bacteria through eukaryotes and, though diverse in length/composition, ss share a conserved tripartite structure: 20–30 residues in length with a basic “N domain,” a 7-13 residue hydrophobic “H domain” and a slightly polar “C domain.”18 In addition to this common structure, conservation of residues in particular positions of the mammalian signal peptide sequences is emerging. In this sense, our results are corroborated by very recent findings showing that in mammalian ss, small neutral amino acids are prevalent in −1 (last amino acid of the signal peptide) and −3 positions, whereas bulky aromatic amino acids are often found at −2 position and proline residues are virtually absent from all positions from −3 to +1 (first amino acid of the mature peptide) positions.19 Interestingly, these features of mammalian ss are found also in PGIP ss where −1 position corresponds to serine and −3 position to alanine and position −2 corresponds indeed to leucine. Moreover, proline residues are absent from all positions from −3 to +1 both in the fusion with E7* and with L21–200.
The potential of using heterologous signal peptides to target proteins might be significant to many areas of biological research as well as the biopharmaceutical industry. In particular, since the ability to express a protein of interest of a certain quality upon DNA immunization is critical for building reliable vaccinogens able to trigger an adequate immune response, the possibility to use signal sequences of plant origin to drive antigen trafficking in different compartments of mammalian cells offers new perspectives in the design of new preventive and therapeutic HPV vaccines (chimeric ss- L21–200-E7 vaccine). In addition, this approach may be promising for designing next-generation DNA candidate vaccines against other tumor or infectious causative agents with possible implications in the management of pandemics and bioterrorism.
Material & methods
Plasmids
The attenuated E7GGG gene (E7*)7 was obtained from the E7 gene of HPV16 (HPV16 genome NCBI Reference Sequence: K02718). The E7*-CP and the E7*-L-CP previously constructed by fusing the E7* sequence to the 3′ end of the gene of the PVX CP (CP, CoreNucleotide accession number D00344) without or with a linker interposition, respectively, were used as templates to obtain the ss-E7*, ss-E7*-CP and the ss-E7*-L-CP. In these constructs, the E7* gene was N-terminally fused with the PGIP ss from bean (corresponding to the aa sequence MTQFNIPVTMSSSLSIILVILVSLRTALS)20 by SOE-PCR performed using the Pfu polymerase (Stratagene, La Jolla, CA, USA). The template for PGIP ss was a plant expression vector containing the ss sequence fused to the 3′ end of HPV16 E7.6
The synthetic sequence L21–200–E7* cloned into the pUC vector was already available in the laboratory as a codon-optimized gene for expression in Chlamydomonas reinhardtii (purchased by GenScript, Piscataway, NJ, USA). This construct was used as a template to obtain the L21–200, the ss-L21–200 and the ss-L21–200-E7* constructs. L21–200 was N-terminally fused with the PGIP ss.
All utilized primers are indicated in Fig. 1 and listed in Table 1 (note that all upstream primers had a Kozak sequence for expression regulation in mammals). After digestion with either the restriction enzymes KpnI / NotI (for ss-E7*, ss-E7*-CP and ss-E7*-L-CP) or BamHI / PstI (for L21–200, ss-L21–200 and ss-L21–200-E7*), all fusion fragments were cloned into the expression vector (Fig. 1).
Table 1.
DNA primer list with sequences, templates and PCR products.
| primer id. | sequence 5′ – 3′ | template | PCR and assembly product |
|---|---|---|---|
| 1d | G GCC GGT ACCATC ATG ACT CAA TTC AAT ATC CAA G KpnI site | PGIP ss | ss-E7* |
| 1r | TGT AGG TGT ATC TCC ATG CAT TGA GAG TGC AGT TCT CAA upstream nucleotides of E7* + downstream nucleotides of ss | ||
| 2d | TTG AGA ACT GCA CTC TCA ATG CAT GGA GAT ACA CCT ACA downstream nucleotides of ss + upstream nucleotides of E7* | E7* | |
| 2r | TTC TCG ACT TGC GGC CGC TTA TGG TTT CTG AGA ACA GAT NotI site | ||
| 1d | G GCC GGT ACCATC ATG ACT CAA TTC AAT ATC CAA G (see above) | ss-E7* | ss-E7*-CP |
| 3r | CTG TGT TGT GCT AGC TGG TGG TTT CTG AGA ACA GAT GGG upstream nucleotides of CP + downstream nucleotides of E7* | ||
| 3d | ATC TGT TCT CAG AAA CCA CCA GCT AGC ACA ACA CAG CCC downstream nucleotides of E7* + upstream nucleotides of CP | CP | |
| 4r | TTC TCG ACT TGC GGC CGC TTA TGG TGG TGG TAG AGT GAC NotI site | ||
| 1d | G GCC GGT ACCATC ATG ACT CAA TTC AAT ATC CAA G (see above) | ss-E7* | ss-E7*-L-CP |
| 5r | GCT AGC TGG AGG TCC GGG TCC TGG TTT CTG AGA ACA GAT GGG upstream nucleotides of CP + linker sequence + downstream nucleotides of E7* | ||
| 4d | CAG AAA CCA GGA CCC GGA CCT CCA GCT AGC ACA ACA CAG CCC downstream nucleotides of E7*+ linker sequence + upstream nucleotides of CP | CP | |
| 4r | TTC TCG ACT TGC GGC CGC TTA TGG TGG TGG TAG AGT GAC (see above) | ||
| 5d | GGCCGGATCCATCATGCGTCACAAACGTTCAG BamHI site | synthetic L21-200–E7* | L21-200 |
| 6r | GGCCCTGCAGTTAAAATGTATCCATCGGAATT PstI site | ||
| 6d | GGCCGGATCCATCATGACTCAATTCAATATCCAAG BamHI site | PGIP ss | ss- L21-200 |
| 7r | TGAACGTTTGTGGACGCATTGAGAGTGCAGTTCTCAA upstream nucleotides of L21-200 + downstream nucleotides of ss | ||
| 7d | TTGAGAACTGCACTCTCAATGCGTCACAAACGTTCA downstream nucleotides of ss + upstream nucleotides of HPV16 L2 | synthetic L21-200–E7* | |
| 6r | GGCCCTGCAGTTAAAATGTATCCATCGGAATT (see above) | ||
| 6d | GGCCGGATCCATCATGACTCAATTCAATATCCAAG (see above) | PGIP ss | ss- L21-200-E7* |
| 7r | TGAACGTTTGTGGACGCATTGAGAGTGCAGTTCTCAA (see above) | ||
| 7d | TTGAGAACTGCACTCTCAATGCGTCACAAACGTTCA (see above) | synthetic L21-200–E7* | |
| 8r | GGCCCTGCAGTTATGGTTTCTGAGAACAGATG PstI site |
Primer identification (id.) is as shown in Figure 1; restriction sites are underlined and Kozak sequence is in bold (d: direct, r: reverse).
Plasmids integrity was verified by DNA sequencing. In each experiment, all plasmid preparations were purified by CsCl gradient to obtain endotoxin-free products.
Cell lines
The E7-expressing TC-1 tumor cells were kindly provided by Prof. T.C. Wu (Johns Hopkins Medical Institutions, Baltimore, MD) and were cultured in RPMI with 10% fetal bovine serum (Gibco) supplemented with G418 400 µg/ml. Before inoculation with 5 × 104 cells, TC-1 cells were harvested by trypsinization, washed twice, and resuspended in saline solution.
Human embryonic kidney cells 293 (HEK-293) were grown in D-MEM (INVITROGEN, Paisley, UK) supplemented with 10% fetal calf serum, 2 mM L-glutamine, 100 IU/ml penicillin and 100 µg/ml streptomycin.
Mammalian cell transfection, immunofluorescence staining and immunoblot analysis
The expression of the different genes upon transfection was analyzed both by immunoblotting and immunofluorescence.
Transfection of HEK-293 mammalian cells with all the constructs was accomplished with a chemical reagent (Effectene transfection kit; Qiagen) in the presence of 25 µg of MG132 proteasome inhibitor (carbobenzoxy-L-leucyl-L-leucyl-L-leucinal in dimethyl sulfoxide [DMSO]; Calbiochem, Darmstadt, Germany) added to culture medium 24 h post-transfection. Forty-eight hours after transfection, cells were washed twice with ice-cold PBS, scraped, resuspended in gel loading buffer, and boiled for 10 min. Proteins from about 1×106 cells were analyzed by immunoblotting according to standard procedures. Briefly, lysates were separated by SDS–PAGE and then transferred onto polyvinylidene difluoride (PVDF) membrane.
The immuno-reactive E7* protein in the lysates was analyzed by immunoblotting assay with a mouse polyclonal antibody specific for HPV16 E7 protein (pAb α-E7, gift of P. Di Bonito, National Institute of Health, Rome, Italy) diluted 1:1,000 and, subsequently, with a goat anti-mouse peroxidase-labeled secondary antibody (diluted 1:10,000) purchased by GE Healthcare Life Sciences (Uppsala, Sweden). Bound antibody was detected with the ECL Plus system (GE Healthcare Life Sciences).
To detect the possible secretion of the antigens encoded in the different plasmids provided with the ss, cell culture media were centrifuged at 800 × g to eliminate residual detached cells, ultra-centrifuged at 136.000 × g and concentrated (Millipore centrifugal filter units, with appropriate molecular weight cut off).
For immunofluorescence staining, HEK-293 cells were grown on multi-chamber glass slides and, 48 h after transfection, they were washed twice with ice-cold PBS, fixed with 4% paraformaldehyde for 10 min, and permeabilized with 0.1% Triton X-100 in PBS. Samples were blocked with 5% non-fat dry milk in PBS. E7* and L21–200 antigens were stained with either a 1:1,000 diluted mouse pAb α-E7 or a 1:250 diluted mAb RG-1 directed to HPV16 L2 aa 17 to 36 (kind gift of Prof. R. Kirnbauer, Medical University of Wien, Austria)5,21 followed by a 1:300 dilution of an anti-mouse biotinylated secondary antibody (RPN 1021, GE Healthcare Life Sciences, Uppsala, Sweden) and a 1:50 dilution of fluorescein isothiocyanate (FITC)-conjugated streptavidin (Sigma-Aldrich, Steinheim, Germany). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich, Steinheim, Germany). DAPI emits blue fluorescence upon binding to AT regions of DNA. Slides were examined with a fluorescence Axiolab microscope (Carl Zeiss, Oberkochen, Germany) interfaced with a CoolSNAP CCD camera (Photometrics/Roper Scientific, Tucson, AZ).
Bacterial expression of the L21–200 peptide
The L21–200 was expressed in E. coli, after cloning into the pQE30 vector, and purified both as a soluble His6-L21–200 peptide under native conditions and also under denaturing conditions, by means of Ni-NTA resin. After dialysis against phosphate-buffered saline (PBS), Coomassie blue-stained 12% sodium dodecyl sulfate (SDS)–polyacrylamide gels were used to determine the purity of samples. The amount of immunoreactive His6-L21–200 in the purification fractions was analyzed by immunoblotting using 1:250 mAb RG-1 and, subsequently, with a goat anti-mouse peroxidase-labeled secondary antibody (GE Healthcare Life Sciences, Uppsala, Sweden) diluted 1:10,000).
Animals and vaccination schedules
Charles River Laboratories supplied 6–8 weeks female C57BL/6 mice. All procedures involving handling and sacrifice of animals were performed under specific pathogen-free conditions at the Animal House of the Regina Elena National Cancer Institute. The study was approved by the Government Committee of National Minister of Health (85/2016-PR) and was conducted according to EU Directive 2010/63/EU for animal experiments
Two immunization protocols were followed (Fig. 5): the immune response protocol, in which the mice received vaccinations prior immune response analysis, and the therapeutic immunization protocol, in which the mice were challenged with TC-1 tumor cells before administration of the vaccines. In the immunization protocol, animals were primed with the recombinant constructs (100 µg/mouse, IM into the quadriceps), and boosted 2 times at 1 week intervals with the same preparations. In the therapeutic protocol, mice received an injection of 200 µl of saline solution containing 5 × 104 TC-1 tumor cells and, 3 d after tumor challenge, mice were primed with the same preparations by the same time schedule. Tumor growth was monitored by visual inspection and palpation 2 times a week. Animals were scored as tumor bearing when tumors reached a size of approximately 1 to 2 mm in diameter.
In a first immunization protocol for the L2-based plasmids, groups of 5 mice were primed with the constructs (100 µg/mouse, IM into the quadriceps) and boosted 3 times once a week using the same plasmid preparations. In a second immunization protocol performed to improve response,15 the L2-based plasmids were administered ID in back leg (10 µg/mouse) and IM injection in the other leg (50 µg/mouse into the quadriceps) followed by square wave electroporation at the injection site (100 V of positive and negative polarity at a rate of 1 pulse lasting 50 ms delivered by ECM830 electroporation system BTX Harvard Apparatus tweezer electrodes) using the same plasmid preparations.
Enzyme-linked immune-sorbent assay (ELISA)
The anti-E7 and the anti-L21–200 serum antibodies were determined by a direct ELISA assay. A 96-well plate was coated at 4 °C overnight with 200 ng of either E.coli-derived HPV16 His6-E7 or His6-L21–200 and then blocked with 150 µl of 10% (w/v) no-fat dry milk in PBS. Sera were collected on day 7 after the last boost, diluted in 2% blocking buffer (data are shown for 1:100 dilution), and incubated for 2 h at 37 °C. Total IgGs were detected with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG(H-L) (GE Healthcare Life Sciences, Uppsala, Sweden).
IFN-gamma enzyme-linked immuno-spot assay (ELISPOT)
HPV16 E7-specific T cell precursors were detected by enzyme-linked immunospot assay (ELISPOT)22 one week after the last boost, according to previous reported protocols. A single-cell suspension of splenocytes (1×106 cells per well) from each group of vaccinated mice, was added to microtiter wells coated overnight at 4°C with anti-mouse IFN-γ antibody (5µg/ml; BD Biosciences PharMingen, San Diego, CA), along with interleukin-2 (50 units/ml; Sigma-Aldrich Italia, Milan, Italy). Sample in triplicates were incubated at 37°C for 24–48 hr with the E7-specific H-2Db (10µg/ml) cytotoxic T lymphocyte (CTL) MHC class I epitope (amino acids 49–57, RAHYNIVTF) for detecting E7-specific CD8+ T cell precursors.23 Plates were incubated with a biotinylated anti-mouse IFN-γ antibody (2 µg/ml) for 4 h at room temperature. Streptavidin–HRP was then used for 1 h at room temperature, and the cell spots stained by addition of the filtered 3-amino-9-ethylcarbazole substrate (BD Biosciences PharMingen, San Diego, CA), for 1–5 min. The reaction was terminated once the formation of discrete purple-colored spots was detected. Spots were counted using a dissecting microscope.
Statistical analysis
Statistical analyses of ELISA data were performed using the one-way ANOVA parametric test and Bonferroni's analysis of variance, using GraphPad Prism 5 software (www.graphpad.com). The statistical significance was set as p < 0,05.
Abbreviations
- ss
signal sequence
- HPV
Human Papilloma Virus
- CP
coat protein
- PVX
Potato Virus X
- HEK 293
Human Embryonic Kidney cells 293
- PGIP
Polygalacturonase-inhibiting protein
- aa
amino acids
- IM
intramuscular
- ID
intradermal
- EP
electroporation
- ELISPOT
Enzime-Linked Immuno spot
- ELISA
Enzyme-linked immunosorbent assay
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Authors thank all the staff of IRE animal house for mouse stabling and maintenance.
Funding
Work partially supported by AIRC IG 12916; Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq/PVE 2014); Fundação de Amparo a Ciência e Tecnologia do Estado de Pernambuco (FACEPE/PRONEM APQ-0562–2.02/14).
References
- [1].Vici P, Mariani L, Pizzuti L, Sergi D, Di Lauro L, Vizza E, Tomao F, Tomao S, Mancini E, Vincenzoni C, et al.. Emerging biological treatments for uterine cervical carcinoma. J Cancer 2014; 5:86-97; PMID:24494026; http://dx.doi.org/ 10.7150/jca.7963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mariani L, Venuti A. HPV vaccine: an overview of immune response, clinical protection, and new approaches for the future. J Transl Med 2010; 8:105; PMID:20979636; http://dx.doi.org/ 10.1186/1479-5876-8-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tyler M, Tunban E, Chackerian B. Second-generation prophylactic HPV vaccines: successes and challenges. Expert Rev Vaccines 2014; 13:247-55; PMID:24350614; http://dx.doi.org/ 10.1586/14760584.2014.865523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Whang DW, Zhihai L, Jieqiong X, Junqi W, Li Z, Yajing L, Fei F, Lu X, Minxi W, Zhibo K, et al.. Identification of broad-genotype HPV L2 neutralization site for pan-HPV vaccine development by a cross-neutralizing antibody. PLoS One 2015; 10:1-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ghambira R, Karanam B, Jagu S, Roberts JN, Buck CB, Bossis I, Alphs H, Culp T, Christensen ND, Roden RB. A protective and broadly cross-neutralizing epitope of human papillomavirus L2. J Virol 2007; 81:13927-31; PMID:17928339; http://dx.doi.org/ 10.1128/JVI.00936-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Franconi R, Massa S, Illiano E, Muller A, Cirilli A, Accardi L, Di Bonito P, Giorgi C, Venuti A. International J Immunopathol Pharmacol 2006; 19:785-95 [PubMed] [Google Scholar]
- [7].Massa S, Simeone P, Muller A, Benvenuto E, Venuti A, Franconi R. Antitumor activity of DNA vaccines based on the human papillomavirus-16 E7 protein genetically fused to a plant virus coat protein. Hum Gene Ther 2008; 19:354-64; PMID:18439124; http://dx.doi.org/ 10.1089/hum.2007.122 [DOI] [PubMed] [Google Scholar]
- [8].Massa S, Paolini F, Spanò L, Franconi R, Venuti A. Mutants of plant genes for developing cancer vaccines. Human Vaccines 2011; 7:147-55; PMID:21266841; http://dx.doi.org/ 10.4161/hv.7.0.14577 [DOI] [PubMed] [Google Scholar]
- [9].Franconi R, Demurtas OC, Massa S. Plant-derived vaccines and other therapeutics produced in contained systems. Expert Rev Vaccines 2010; 9:877-92; PMID:20673011; http://dx.doi.org/ 10.1586/erv.10.91 [DOI] [PubMed] [Google Scholar]
- [10].Massa S, Franconi R, Brandi R, Muller A, Mett V, Yusibov V, Venuti A. Anti-cancer activity of plantproduced HPV16 E7 vaccine. Vaccine 2007; 25:3018-21; PMID:17280752; http://dx.doi.org/ 10.1016/j.vaccine.2007.01.018 [DOI] [PubMed] [Google Scholar]
- [11].Venuti A, Massa S, Mett V, Vedova LD, Paolini F, Franconi R, Yusibov V. An E7-based therapeutic vaccine protects mice against HPV16 associated cancer. Vaccine 2009; 27:3395-97; PMID:19200826; http://dx.doi.org/ 10.1016/j.vaccine.2009.01.068 [DOI] [PubMed] [Google Scholar]
- [12].Schillberg S, Zimmermann S, Voss A, Fischer R. Apoplastic and cytosolic expression of full-size antibodies and antibody fragments in Nicotiana tabacum. Transgenic Res 1999; 8:255-63; PMID:10621973; http://dx.doi.org/ 10.1023/A:1008937011213 [DOI] [PubMed] [Google Scholar]
- [13].Vici P, Pizzuti L, Mariani L, Zampa G, Santini D, Di Lauro L, Gamucci T, Natoli C, Marchetti P, Barba M, et al.. Targeting immune response with therapeutic vaccines in premalignant lesions and cervical cancer: hope or reality from clinical studies. Expert Rev Vaccines 2016; 9:1-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat Rev Genet 2008; 9:776-88; PMID:18781156; http://dx.doi.org/ 10.1038/nrg2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hallengärd D, Haller BK, Maltais A-K, Gelius E, Nihlmark K, Wahren B, Bråve1 A. Comparison of plasmid vaccine immunization schedules using intradermal in vivo electroporation. Clin Vaccine Immunol 2011; 18:1577-81; http://dx.doi.org/ 10.1128/CVI.05045-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Low L, Mander A, McCann K, Dearnaley D, Tjelle T, Mathiesen I, Stevenson F, Ottensmeier CH. DNA vaccination with electroporation induces increased antibody responses in patients with prostate cancer. Hum Gene Ther 2009; 20:1269-78; PMID:19619001; http://dx.doi.org/ 10.1089/hum.2009.067 [DOI] [PubMed] [Google Scholar]
- [17].Vasan S, Hurley A, Schlesinger SJ, Hannaman D, Gardiner DF, Dugin DP, Boente-Carrera M, Vittorino R, Caskey M, Andersen J, et al.. In vivo electroporation enhances the immunogenicity of a HIV-1 DNA vaccine candidate in healthy volunteers. PLoS One 2011; 6:e19252; PMID:21603651; http://dx.doi.org/ 10.1371/journal.pone.0019252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Güler-Gane G, Kidd S, Sridharan S, Vaughan TJ, Wilkinson TCI, Tigue NJ. Overcoming the refractory expression of secreted recombinant proteins in mammalian cells through modification of the signal peptide and adjacent amino acids. PLoS One 2016; 11:e0155340; PMID:27195765; http://dx.doi.org/ 10.1371/journal.pone.0155340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Von Heijne G. The signal peptide. J Membrane Biol 1990; 115:195-201; http://dx.doi.org/ 10.1007/BF01868635 [DOI] [PubMed] [Google Scholar]
- [20].Toubart P, Desiderio A, Salvi G, Cervone F, Daroda L, De Lorenzo G, Bergmann C, Darvill AG, Albersheim P. Cloning and characterization of the gene encoding the endopolygalacturonase-inhibiting protein (PGIP) of Phaseoulus vulgaris L. Plant J 1992; 2:367-73; PMID:1303801 [DOI] [PubMed] [Google Scholar]
- [21].Kirnbauer Schellenbacher C, Roden R, Kirnbauer R. Chimeric L1-L2 virus-like particles as potential broad-spectrum human papillomavirus vaccines. J Virol 2009; 83:10085-95; PMID:19640991; http://dx.doi.org/ 10.1128/JVI.01088-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Miyahira Y, Murata K, Rodriguez D, Rodriguez JR, Esteban M, Rodrigues MM, Zavala F. Quantification of antigen specific CD8+ T cells using an ELISPOT assay. J Immunol Methods 1995; 181:45-54; PMID:7537312; http://dx.doi.org/ 10.1016/0022-1759(94)00327-S [DOI] [PubMed] [Google Scholar]
- [23].Hsu KF, Hung CF, Cheng WF, He L, Slater LA, Ling M, Wu TC. Enhancement of suicidal DNA vaccine potency by linking Mycobacterium tuberculosis heat shock protein 70 to an antigen. Gene Ther 2001; 8:376-83; PMID:11313814; http://dx.doi.org/ 10.1038/sj.gt.3301408 [DOI] [PubMed] [Google Scholar]


