Abstract
Wheat stem rust (Puccinia graminis f. sp. tritici Eriks. and E. Henn.) is one of the most destructive diseases world-wide. Races belonging to Ug99 (or TTKSK) continue to cause crop losses in East Africa and threaten global wheat production. Developing and deploying wheat varieties with multiple race-specific genes or complex adult plant resistance is necessary to achieve durability. In the present study, we applied genome-wide association studies (GWAS) for identifying loci associated with the Ug99 stem rust resistance (SR) in a panel of wheat lines developed at the International Maize and Wheat Improvement Center (CIMMYT). Genotyping was carried out using the wheat 9K iSelect single nucleotide polymorphism (SNP) chip. Phenotyping was done in the field in Kenya by infection of Puccinia graminis f. sp. tritici race TTKST, the Sr24-virulent variant of Ug99. Marker-trait association identified 12 SNP markers significantly associated with resistance. Among them, 7 were mapped on five chromosomes. Markers located on chromosomes 4A and 4B overlapped with the location of the Ug99 resistance genes SrND643 and Sr37, respectively. Markers identified on 7DL were collocated with Sr25. Additional significant markers were located in the regions where no Sr gene has been reported. The chromosome location for five of the SNP markers was unknown. A BLASTN search of the NCBI database using the flanking sequences of the SNPs associated with Ug99 resistance revealed that several markers were linked to plant disease resistance analogues, while others were linked to regulatory factors or metabolic enzymes. A KASP (Kompetitive Allele Specific PCR) assay was used for validating six marker loci linked to genes with resistance to Ug99. Of those, four co-segregated with the Sr25-pathotypes while the rest identified unknown resistance genes. With further investigation, these markers can be used for marker-assisted selection in breeding for Ug99 stem rust resistance in wheat.
Introduction
Wheat stem rust, caused by a fungal pathogen Puccinia graminis f. sp. tritici, is one of the most destructive diseases of wheat. The deployment of stem rust resistance genes in modern wheat cultivars successfully controlled the disease until the new race of the pathogen named Ug99 (TTKSK, for North American nomenclature) was first reported in Uganda in 1999 [1]. Since then, the Ug99 race lineage has been observed in wheat fields in several countries in Africa and the Middle East. Due to the airborne transmission of disease, the Ug99 is expected to spread rapidly through these regions and further afield. The Ug99 pathogen is virulent against many resistance genes which have previously been applied in wheat against stem rust and can cause up to 100% crop losses [1–3]. Although efforts on developing Ug99-resistant varieties have been made, significant changes in the pathogen population and distribution are challenging as several variants have been identified within the Ug99 race lineage [2–4]. Developing new wheat varieties with diverse race-specific or durable resistance is the primary priority to mitigate the Ug99 threat worldwide.
To date, more than 60 stem rust resistance (Sr) genes have been identified and mapped to specific chromosome positions [5, 6]. However, only few of them are still effective against Ug99. Resistance to stem rust can be based on race-specific host pathogen recognition genes (R-genes) effective at all plant growth stages or multiple additive minor genes which confer adult plant resistance (APR). Durable and effective resistance to stem rust can be achieved by pyramiding multiple race-specific or slow rusting, minor resistance genes. The APR to stem rust in wheat is a complex trait and controlled by quantitative trait loci (QTL) that can provide more durable resistance better than a single, race-specific gene because of the race non-specificity of the resistance genes. To date, a total of five designated stem rust resistance genes conferring quantitative APR have been characterized. They are Sr2, Sr55, Sr56, Sr57 and Sr58. Additional QTLs associated with wheat stem rust resistance have also been reported in diverse germplasm [7].
Pyramiding of more effective rust resistance genes into wheat cultivars with common background using rust bioassays is challenging due to the lack of isolates with specific avirulence/virulence gene combinations for assigning resistance genotypes. This is particularly true for broadly effective genes [8, 9]. Furthermore, field bioassays for the Ug99 lineage can only be conducted in regions where they are already present. High throughput diagnostic markers closely linked to stem rust resistance loci are needed to facilitate selection of desirable genotype combinations.
Although molecular markers have been used for marker-assisted selection (MAS) for wheat stem rust resistance, most of them are gel-based markers using fragment size comparison (http://maswheat.ucdavis.edu/protocols/stemrust/). It is time consuming and laborious. The Single Nucleotide Polymorphic (SNP) marker is the marker of choice for MAS because of their high abundance, widespread distribution throughout the genome, and their potential for high-throughput genotyping. In the present study, we first applied genome-wide association studies (GWAS) to identify SNP makers linked to Ug99 stem rust resistance loci in wheat lines developed at CIMMYT using the wheat 9K iSelect SNP chip. We then used a high throughput assay named Kompetitive Allele Specific PCR (KASP) for validating significant markers closely linked to the Ug99 resistance loci. Our goal was to develop a robust and high throughput platform that can be used in MAS for breeding wheat cultivars with improved resistance against Ug99 stem rust.
Materials and methods
Plant materials and bioassay for disease response
A total of 277 spring wheat lines from the 2nd and 5th Stem Rust Resistance Screening Nurseries (SRRSN) from CIMMYT were used for the field evaluation of stem rust resistance (S1 and S2 Tables). They were planted at the Kenya Agricultural Research Institute (KARI), Njoro during field seasons 2008, 2009 and 2010 for the 2nd SRRSN, and 2009 and 2010 for the 5th SRRSN with two replicates. The stem rust responses of the wheat lines were assessed in field plots as previously described [10]. Briefly, an artificial rust epidemic was created by infecting the spreaders using fresh urediniospores of Puccinia graminis f. sp. tritici race TTKST collected from field plots of a Sr24 carrying spreader genotype planted at Njoro for rust increase. A suspension of freshly collected urediniospores in water was injected into individual plants (1–3 plants/m) within the border rows just prior to booting using a hypodermic syringe, on at least two occasions. Spreaders were also sprayed with urediniospore-mineral oil suspension at least twice during stem elongation. Stem rust was scored on the stem leaf sheath and true stem. Disease responses in the field were initially assessed at least twice between early to late dough stages when the susceptible control reached 80 to 100% infection and about a week later.
SNP genotyping
DNA was extracted from young leaves of wheat lines using the CTAB protocol [11]. The wheat 9K iSelect SNP chip and the Infinium assay [12] was used for genotyping 189 wheat breeding lines from the 5th SRRSN according to the manufacturer’s procedure (Illumina, San Diego, CA). The genotyping results were obtained using Illumina’s iScan instruments. Illumina’s GenomeStudio v2011.1 software was used for genotype calling and SNP clustering. To account for observed shifts in SNP clusters caused by differences in the number of duplicated gene copies detected during assays, a genotype calling algorithm was generated using an iterative procedure according to Cavanagh et al. [12].
Marker-trait association
The corrected marker data and phenotypic data were used for marker-trait association. To control the possible population structure, a marker similarity matrix containing all lines (Kinship or K matrix) was generated using TASSEL v.5 [13]. Substructure within the wheat lines was also investigated using principal component analysis and the resulting covariance matrix (Q matrix) was used to correct the effect of population substructure. Both Q and K matrices were used in the mixed linear model (MLM) in TASSEL to correct for both population and family structure. A false discovery rate (FDR) of 0.05 was used as a threshold to identify significant markers associated with stem rust resistance [14].
KASP assay
SNP markers associated with stem rust resistance identified by GWAS were validated using the KASP assay. The SNP contextual sequences were obtained from Dr. Eduard Akhunov (Kansas State University) and used for designing primers. For each marker, two allele-specific primers (one for each SNP allele) and one common (reverse) primer were designed for each KASP assay using a tool provided by LGC Genomics (www.lgcgenomics.com) based on the SNP locus sequence. Table 1 presents sequence information of six markers with two allele-specific primers and one common primer for each KASP assay. The KASP assays were designed by LGC Genomics and carried out according to the company’s protocol (http://lgcgenomics.com).
Table 1. Primer sequences of SNP markers for validation in wheat lines by KASP assay.
| SNP | Allele1 | Allele2 | Allele-1 primer | Allele-2 primer | Common primer |
|---|---|---|---|---|---|
| JD_c6624_7769357 (7DL*) | A | G | CAAGAAGCCAATTGACCATGGCAAA | AAGAAGCCAATTGACCATGGCAAG | CTGATTGTTCACCAGCACTGGCATT |
| Ra_c25242_34807178 (7DL) | A | C | GGGATTCCTTCATCACAAGTTGGA | CCTTCATCACAAGTTGGC | GTTTCTCTTCTCTTGCCAAACCAGATTT |
| CAP7_c2912_1387634 (7DL*) | A | G | AACCGGTTACAAAGCCAAATCCAGA | CCGGTTACAAAGCCAAATCCAGG | ACTAGTGCTTGGTTTACCAATGTTCCTA |
| Ex_c5884_10325223 (7DL) | T | C | GTGCGCAGCTTACTTGCCAATATA | GCGCAGCTTACTTGCCAATATG | AATACCAGAATCGACAGATGTTGGAGTTT |
| Ex_c12556_19992307 (7BL) | A | G | ATTAGATCACGCTTTATCTGCTTGGAA | AGATCACGCTTTATCTGCTTGGAG | CTAGCACTRAGCTCGGCCTCAA |
| Ex_c2123_3988735 (7DS) | T | C | GTGGGGAGATAGGTGTGAAGGTA | GGGGAGATAGGTGTGAAGGTG | GTGTCACTTTTCGTTGGGAGTTACATTTT |
Note
*, marker’s position was reassigned based on the linkage disequilibrium analysis.
Results
Population structure
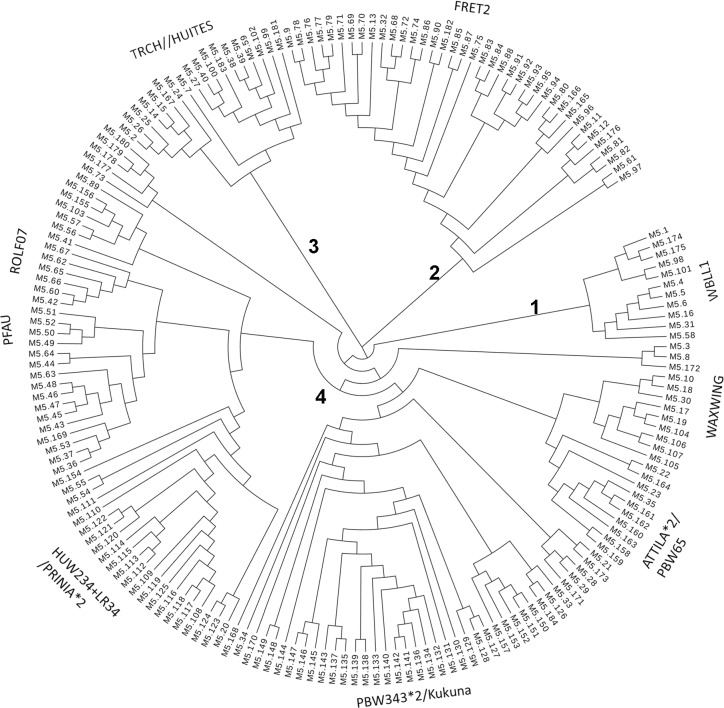
To access the population structure, a cluster analysis was performed using the SNP data and four clusters were identified. A dendrogram was produced by hierarchical clustering (Fig 1). As diverse breeding lines were used in the GWAS, a wide-range of genetic background is expected in the association panel. Wheat lines with similar pedigrees or genetic backgrounds were clustered together. Cluster 1 contained wheat lines with the common parent “WBLL1”, cluster 2 contained wheat lines with the parent “FRET2” in common and cluster 3 contained wheat lines with the parent “TRCH” in the pedigree (Fig 1, upper). The 4th cluster is a large cluster containing six subclusters with pedigrees containing “WAXWING”, “ATTILA*2/PBW65”, “PBW343*2/KUKUNA”, HUW234+LR34/PRINIA*2”, “PFAU” AND “ROLF07” (Fig 1, lower).
Fig 1. A dendrogram illustrating the clusters produced by hierarchical clustering of wheat lines in the 5th SRRSN.
Clusters with similar pedigrees and genetic backgrounds were named by their common parent.
To confirm this finding, a principal component analysis (PCA) was performed using the same SNP data set and similar population structure was identified by the PCA (Data not shown). We therefore used the covariance matrix (PC1 and PC2) as Q-matrix together with K matrix in the mixed linear model for controlling the population structure effect in association mapping.
Identification of SNP markers associated with Ug99 resistance
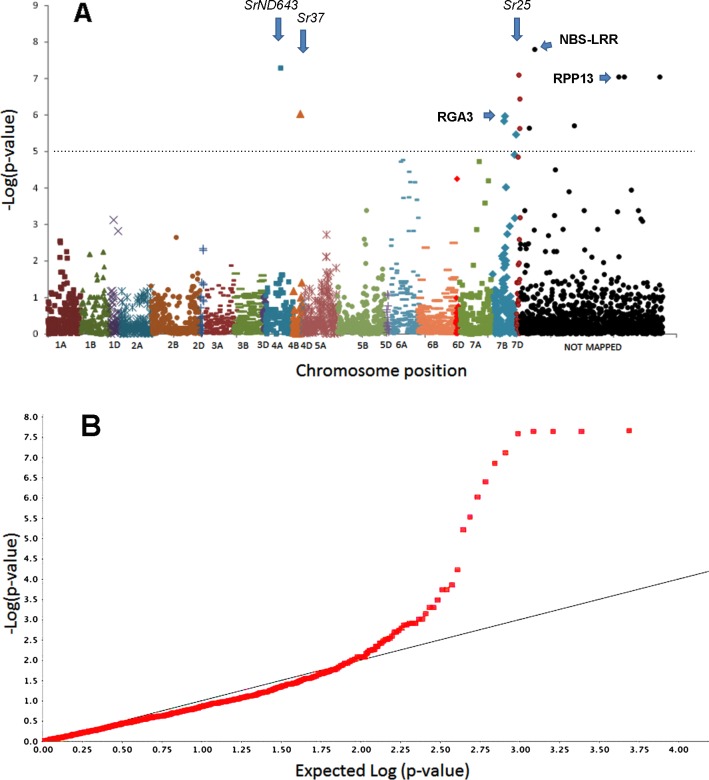
The resulting genotypic and phenotypic data were used for GWAS to identify markers associated with Ug99 resistance in the 5th SRRSN (Fig 2A). The relative positions near the known Sr genes were indicated at the top of the respective chromosomes. Fig 2B illustrated the quantile-quantile plot (QQ) using observed against expected p-values. Using a cutoff FDR rate of 0.05, 12 significant markers were detected (Fig 2A above the dotted line, Table 2). Among them, seven were located on four chromosomes based on the consensus map constructed by Cavanagh et al. [12]. Marker Ex_c1373_2628597 on chromosomes 4A was located at the position of the previously reported stem resistance gene, SrND643 [15]. Similarly, marker Ex_c30581_39482788 on chromosome 4B overlapped with the location of Sr37 [7]. On chromosome 7B, one marker (Ex_c43096_49510164) was located where no Ug99-effective genes have been reported. Although Sr17 was located on 7BS, it was reported to be ineffective to the Ug99 lineage [7]. Another marker Ex_c12556_19992307 was identified on 7BL in the same region where a QTL for stem rust resistance has been reported [16]. On 7D, a marker, Ex_c2123_3988735 was located on the short arm and coincided with the Ug99 resistance gene SrTA100171[17]. The other two, Ex_c5884_10325223 and Ra_c25242_34807178 were located on 7DL and overlapped with the location of Sr25/Sr43.
Fig 2. Manhattan and quantile-quantile (QQ) plots of association mapping of stem rust resistance in the 5th SRRSN.
A, each symbol represents a SNP. A false discovery rate of 0.05 was used for significant associations. Significant markers above the cutoff value of–log P = 5.0 (dotted line) were associated with SR resistance and they were listed in Table 2. The chromosome position was based on the 9k SNP linkage map. Each chromosome is numbered and distinguished by a color and shape of dots. The black dots represent unmapped markers. Arrows indicate the approximate position of known Sr genes and putative candidate genes for Ug99 resistance. B, The result of marker-trait association is illustrated in the QQ plot using observed against expected p-values (log transformed negatives).A mixed linear model controlled by Q and K matrices was used for analysis.
Table 2. Most significant SNP markers associated with stem rust resistance in the 5th SRRSN wheat lines.
| Trait | SNP ID | SNP name | Allele | Chr | Position | p-value | R2 | Candidate |
|---|---|---|---|---|---|---|---|---|
| SR | 1804 | Ex_c1373_2628597 | A/G | 4A | 73.0 | 7.76E-08 | 0.20 | |
| SR | 3287 | Ex_c30581_39482788 | T/C | 4B | 71.6 | 3.03E-06 | 0.15 | GLSS |
| SR | 3854 | Ex_c43096_49510164 | T/C | 7B | 65.6 | 4.10E-07 | 0.16 | TIF4A |
| SR | 1653 | Ex_c12556_19992307 | A/G | 7B | 163.9 | 9.65E-07 | 0.17 | RGA3 |
| SR | 2592 | Ex_c2123_3988735 | A/G | 7D | 56.1 | 6.29E-06 | 0.14 | IPT9 |
| SR | 4368 | Ex_c5884_10325223 | A/G | 7D | 139.7 | 1.41E-07 | 0.19 | DSDS1 |
| SR | 7785 | Ra_c25242_34807178 | T/G | 7D | 141.2 | 2.25E-08 | 0.19 | AG4 |
| SR | 6149 | JD_c6624_7769357 | A/G | NA | NA | 2.34E-08 | 0.19 | PP2C |
| SR | 8369 | RFL_Contig2671_2362005 | T/C | NA | NA | 2.34E-08 | 0.19 | MDAR6 |
| SR | 5825 | JD_c1314_1888758 | A/G | NA | NA | 2.34E-08 | 0.19 | RPP13 |
| SR | 1073 | CAP7_c2912_1387634 | T/C | NA | NA | 2.67E-08 | 0.19 | NBS-LRR |
| SR | 2175 | Ex_c1690_3206784 | A/G | NA | NA | 6.00E-05 | 0.11 |
Notes: Chr, chromosome. Known genes linked to SNPs by BLASTN in NCBI: GLS5, Brachypodium distachyon beta-galactosidase 5;, TIF4A, Triticum aestivum translation initiation factor 4A; RGA3, disease resistance gene analog RGA3; IPT9, B. distachyon importin-9; DSDS1, B. distachyon solanesyl-diphosphate synthase 1; AG4, B. distachyon enhancer of AG-4 protein 2; NBS-LRR, H. vulgare NBS-LRR resistance-like protein (RGH-2) gene; PP2C, B. distachyon protein phosphatase 2C; RPP13, putative disease resistance gene RPP13; MDAR6, T. aestivum MDAR6 gene for chloroplast protein product.
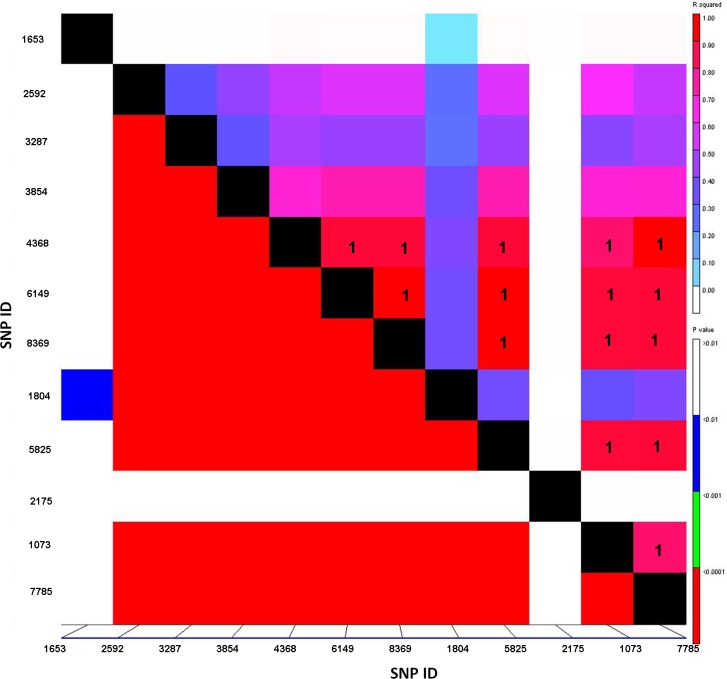
Additional significant markers could not be mapped because their chromosomal locations are unknown (Table 2). We performed pair-wise analysis using linkage disequilibrium for all significant markers and the R2 and p-values are presented in Fig 3. The R2 value of “1” was obtained between four unmapped markers JD_c6624_7769357 (SNP ID 6149), RFL_Contig2671_2362005 (ID 8369), JD_c1314_1888758 (ID5825) and CAP7_c2912_1387634 (ID 1073) and two markers on 7DL Ex_c5884_10325223 (ID4368) and Ra_c25242_34807178 (7785) (Fig 3, labeled with “1”), suggesting that the four unmapped markers are linked to the loci on 7DL.Whereas marker Ex_c1690_3206784 (Fig 3, SNP ID 2175) showed no linkage with other significant markers.
Fig 3. Linkage disequilibrium plots of significant SNP markers associated with stem rust resistance.
R square (R2) and P-value of pair-wise analyses are indicated by color in the right-side bars. The SNP pairs with R2 value of “1” are indicated. The SNP IDs are presented on the X and Y axis. The detailed information for the markers is shown in Table 1.
Assigning the loci associated with Ug99 resistance to putative candidate genes
To identify possible candidate genes linked to the resistance loci, a pairwise alignment using the BLASTN algorithm was performed using the flanking sequences of the significant markers against the NCBI (http://www.ncbi.nlm.nih.gov/) nucleotide acid databases. Three significant loci, Ex_c12556_19992307 on 7BL, CAP7_c2912_1387634 and JD_c1314_1888758 with unknown positions were linked to three plant disease resistance genes including a resistance gene analog RGA3, a member of the nucleotide-binding site (NBS)-leucine-rich repeat (LRR) gene, and a putative disease resistance gene, RPP13, respectively (Table 2). Another marker on 7BS (Ex_c43096_49510164) was linked to T.aestivum translation initiation factor 4A (TIF4A). Three loci on 7D, Ex_c2123_3988735, Ex_c5884_10325223 and Ra_c25242_34807178 were linked to importin-9 (IPT9), solanesyl-diphosphate synthase 1 (DSDS1) and enhancer of AG-4 protein 2 (AG4), respectively. Two unmapped markers, JD_c6624_7769357 and RFL_Contig2671_2362005 were linked to protein phosphatase 2C (PP2C) and a nuclear gene for chloroplast product (MDAR6), respectively (Table 2).
Validation of SNP markers using KASP assay
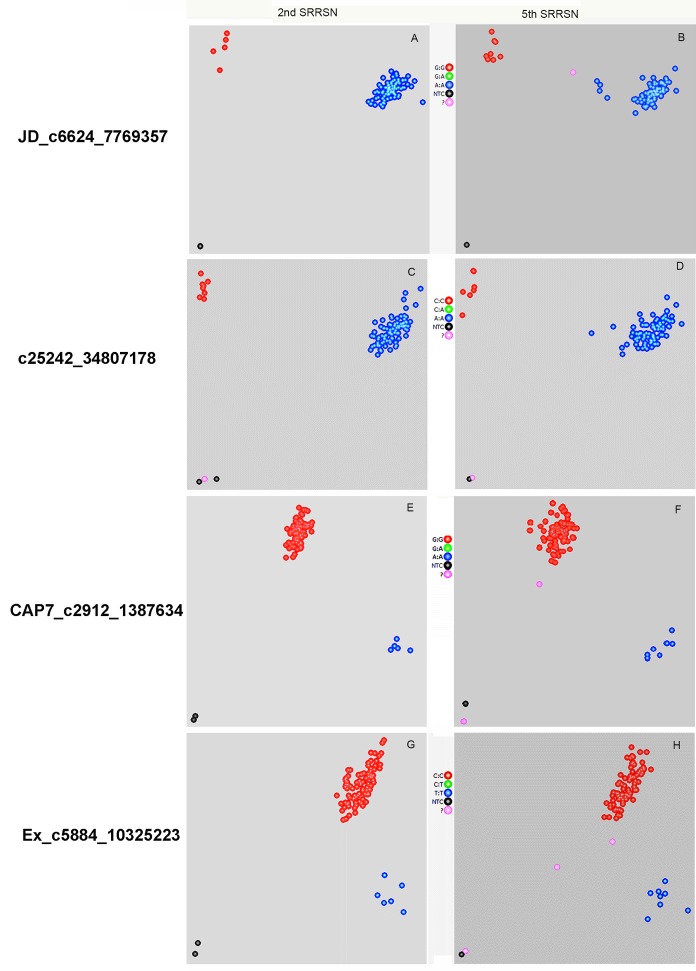
To validate marker loci identified by GWAS, a KASP assay was used for genotyping. For cross validation, we expanded the population to 277 wheat lines included in two stem rust screening nurseries, the 2nd and 5th SRRSNs. We choose six most significant markers for the KASP assay (Table 1). For each SNP, three primers were designed and two alleles are labeled with either FAM or VIC for KASP assays. Their alleles and primer sequences are shown in Table 1. As a result, the blue or red colored dots represent the respective alleles in the allelic discrimination plots (Fig 4). For instance, marker c6624_7769357 identified five and 15 lines with genotype ‘G:G’ in the 2nd and 5th SRRSNs, respectively (Table 3, Fig 4A and 4B, red). The ‘G:G’ genotype detected wheat lines carrying Sr25, while the ‘A:A’ genotype represents wheat lines without Sr25 (Fig 4A and 4B, blue). Marker Ra_c25242_34807178 identified eight and 15 lines carrying the ‘C:C’ genotype representing Sr25 carriers in the 2nd SRRN and 5th SRRSN populations, respectively (Table 3, Fig 4C and 4D, red). Marker CAP7_c2912_1387634 identified five and 14 Sr25 carriers with the ‘A:A’ genotype in the 2nd and 5th SRRSN populations, respectively (Table 3, Fig 4E and 4F, blue). The ‘G:G’ genotype represents non-Sr25 carriers (Table 3, Fig 1E and 1F red). Interestingly, the “A:A” genotype was also detected in line M5_107 with adult plant resistance (ARP-R) without Sr25. Marker Ex_c5884_10325223 identified six and 16 lines that carry genotype ‘T:T’ in the 2nd and 5th SRRSN populations, respectively (Table 3, Fig 1G and 1H, blue). Most of these lines carry Sr25. The rest of lines without Sr25 have genotype ‘C:C’ (Fig 1G and 1H, red). Among them, several lines had undetermined genotypes (Fig 1, pink). The rest two markers Ex_c2123_3988735 and Ex_c12556_19992307 were located on 7D and 7B, respectively. The former also identified some ‘C:C’ genotypes that was carried by Sr25-lines, however, genotype ‘T:T’ was also detected in two Sr25-lines. There was no significant correlation with Sr25 phenotype, although this marker is located on 7DS (56.1 cM) where an Ug99 resistance gene, SrTA10171 was mapped [17]. Marker Ex_c12556_19992307 on 7BL showed no significant cosegregation with any known Sr phenotype in the germplasm tested. It is likely to identify a novel gene for Ug99 resistance.
Fig 4. Discrimination plots of KASP assays of four SNPs linked to Sr25 stem rust resistance locus.
2nd SRRSN, the 2nd Stem Rust Resistance Screen Nursery. 5th SRRSN, the 5th Stem Rust Resistance Screen Nursery (note: only the first 96-well plate samples are shown). Panels A, B, C and D, red dot = Sr25-allele; blue dot = non Sr25-allele. Panels E, F, G and H, blue dot = Sr25- allele; red dot = non Sr25-allele. For all panels, black dot = negative control, pink dot = undetermined.
Table 3. Validation of SNPs linked to stem rust resistance in the 2nd and 5th SRRSNs by KASP assay.
| Line | Rust response | JD_c6624_7769357 | CAP7_c2912_1387634 | Ex_c5884_10325223 | Ra_c25242_34807178 | Ex_c2123_3988735 | Ex_c12556_19992307 |
|---|---|---|---|---|---|---|---|
| (7DL*) | (7DL*) | (7DL) | (7DL) | (7DS) | (7BL) | ||
| H2_11 | Sr25 | G:G | A:A | T:T | C:C | C:C | A:A |
| H2_12 | Sr25 | G:G | A:A | T:T | C:C | C:C | A:A |
| H2_42 | Sr25 | G:G | A:A | T:T | C:C | C:C | G:A |
| H2_69 | Sr25 | G:G | A:A | T:T | C:C | C:C | A:A |
| H2_70 | Sr25 | G:G | A:A | T:T | C:C | C:C | G:A |
| M5_2 | Sr25 | G:G | A:A | T:T | C:C | T:T | G:A |
| M5_3 | Sr25 | G:G | A:A | T:T | C:C | C:C | G:A |
| M5_4 | Sr25 | G:G | A:A | T:T | C:C | C:C | G:A |
| M5_5 | Sr25 | G:G | A:A | T:T | C:C | C:C | G:A |
| M5_6 | Sr25 | G:G | A:A | T:T | C:C | C:C | G:A |
| M5_25 | Sr25 | G:G | A:A | T:T | C:C | C:C | A:A |
| M5_26 | Sr25 | G:G | A:A | T:T | C:C | C:C | G:A |
| M5_98 | Sr25 | G:G | A:A | T:T | C:C | C:C | A:A |
| M5_106 | Sr25 | G:G | A:A | T:T | C:C | C:C | G:A |
| M5_162 | Sr25 | G:G | A:A | T:T | C:C | C:C | A:A |
| M5_163 | Sr25 | G:G | A:A | T:T | C:C | T:T | A:A |
| M5_165 | Sr25 | G:G | A:A | T:T | C:C | C:C | G:A |
| M5_171 | Sr25 | G:G | A:A | T:T | C:C | C:C | G:A |
| M5_107 | APR-R | G:G | A:A | T:T | C:C | C:C | G:A |
| M5_81 | APR-R | G:G | ? | T:T | C:C | T:T | G:A |
| H2_28 | Sr-Sharp | A:A | G:G | T:T | C:C | T:T | G:G |
| H2_73 | S | A:A | G:G | C:C | C:C | T:T | ? |
| H2_30 | - | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_76 | - | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_57 | APR-MR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_131 | APR-MR | A:A | G:G | ? | A:A | T:T | A:A |
| M5_10 | APR-R | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_11 | APR-R | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_12 | APR-R | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_22 | APR-R | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_33 | APR-R | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_36 | APR-R | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_37 | APR-R | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_41 | APR-R | A:A | ? | ? | A:A | T:T | G:A |
| M5_45 | APR-R | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_46 | APR-R | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_47 | APR-R | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_48 | APR-R | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_49 | APR-R | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_50 | APR-R | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_52 | APR-R | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_53 | APR-R | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_66 | APR-R | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_68 | APR-R | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_69 | APR-R | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_72 | APR-R | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_83 | APR-R | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_85 | APR-R | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_99 | APR-R | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_102 | APR-R | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_126 | APR-R | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_130 | APR-R | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_137 | APR-R | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_138 | APR-R | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_142 | APR-R | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_143 | APR-R | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_147 | APR-R | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_148 | APR-R | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_149 | APR-R | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_154 | APR-R | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_164 | APR-R | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_172 | APR-R | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_173 | APR-R | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_182 | APR-R | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_7 | APR-RMR | A:A | G:G | C:C | A:A | ? | A:A |
| M5_9 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_13 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_14 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_15 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_17 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_18 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_19 | APR-RMR | A:A | ? | ? | A:A | ? | ? |
| M5_21 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_23 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_24 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_30 | APR-RMR | A:A | G:G | C:C | A:A | T:T | ? |
| M5_31 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_32 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_34 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_35 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_38 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_39 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_40 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_43 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_44 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_51 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_54 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_58 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_59 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_60 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_61 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_62 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_64 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_65 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_67 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_70 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_71 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_73 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_74 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_75 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_76 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:A |
| M5_77 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_78 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_79 | APR-RMR | A:A | G:G | C:C | A:A | T:T | ? |
| M5_80 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_82 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_84 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_86 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_87 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_88 | APR-RMR | A:A | G:G | C:C | A:A | ? | G:G |
| M5_90 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_92 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_93 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_94 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_95 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_96 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_97 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_100 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_104 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_105 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_127 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:A |
| M5_128 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_132 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_133 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_134 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_135 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_136 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_139 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_140 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_141 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_144 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_145 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_146 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_150 | APR-RMR | A:A | G:G | C:C | A:A | T:T | ? |
| M5_151 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_152 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_153 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_158 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_166 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_167 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_168 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_169 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_170 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_176 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_177 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_178 | APR-RMR | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_179 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_180 | APR-RMR | ? | G:G | C:C | A:A | T:T | A:A |
| M5_181 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_2 | MR | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_13 | MR | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_14 | MR | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_17 | MR | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_25 | MR | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_26 | MR | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_31 | MR | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_33 | MR | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_34 | MR | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_38 | MR | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_40 | MR | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_47 | MR | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_52 | MR | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_60 | MR | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_65 | MR | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_68 | MR | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_77 | MR | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_78 | MR | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_80 | MR | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_81 | MR | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_84 | MR | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_85 | MR | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_86 | MR | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_93 | MR | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_41 | MR-MS | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_43 | MR-MS | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_44 | MR-MS | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_45 | MR-MS | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_46 | MR-MS | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_49 | MR-MS | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_50 | MR-MS | A:A | G:G | C:C | ? | T:T | G:G |
| H2_51 | MR-MS | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_55 | MR-MS | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_56 | MR-MS | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_57 | MR-MS | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_58 | MR-MS | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_59 | MR-MS | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_62 | MR-MS | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_1 | MS | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_3 | MS | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_10 | MS | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_20 | MS | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_21 | MS | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_22 | MS | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_27 | MS | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_29 | MS | A:A | G:G | C:C | A:A | ? | G:G |
| H2_36 | MS | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_37 | MS | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_39 | MS | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_48 | MS | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_54 | MS | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_61 | MS | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_66 | MS | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_74 | MS | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_89 | MS | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_184 | MS-S | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_87 | R | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_5 | R-MR | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_6 | R-MR | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_8 | R-MR | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_16 | R-MR | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_24 | R-MR | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_35 | R-MR | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_53 | R-MR | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_63 | R-MR | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_79 | R-MR | A:A | G:G | C:C | A:A | T:T | G:A |
| H2_82 | R-MR | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_83 | R-MR | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_90 | R-MR | A:A | G:G | C:C | A:A | T:T | ? |
| H2_91 | R-MR | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_92 | R-MR | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_9 | S | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_18 | S | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_19 | S | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_32 | S | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_64 | S | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_75 | S | A:A | G:G | C:C | C:C | T:T | G:G |
| M5_91 | APR-RMR | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_63 | Sr26 | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_89 | Sr26 | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_103 | Sr26 | A:A | G:G | C:C | A:A | ? | A:A |
| M5_129 | Sr26 | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_155 | Sr26 | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_156 | Sr26 | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_157 | Sr26 | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_160 | Sr26 | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_161 | Sr26 | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_42 | Sr26? | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_159 | Sr26? | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_15 | Sr33? | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_67 | SrHUW234 | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_20 | SrHuw234 | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_55 | SrHuw234 | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_56 | SrHuw234 | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_109 | SrHuw234 | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_110 | SrHuw234 | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_111 | SrHuw234 | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_112 | SrHuw234 | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_116 | SrHuw234 | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_117 | SrHuw234 | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_118 | SrHuw234 | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_119 | SrHuw234 | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_120 | SrHuw234 | A:A | G:G | C:C | A:A | T:T | ? |
| M5_121 | SrHuw234 | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_122 | SrHuw234 | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_123 | SrHuw234 | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_124 | SrHuw234 | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_125 | SrHuw234 | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_7 | SrHUW234 | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_108 | SrHuw234/Sr26 | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_113 | SrHuw234/Sr26 | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_114 | SrHuw234/Sr26 | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_115 | SrHuw234/Sr26 | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_4 | Sr-ND643 | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_71 | SrSha7 | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_72 | SrSha7 | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_94 | SrSha7 | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_8 | SrSha7 | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_27 | SrSha7 | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_28 | SrSha7 | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_29 | SrSha7 | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_183 | SrSha7 | A:A | G:G | C:C | A:A | T:T | A:A |
| H2_88 | SrSynthetic | A:A | G:G | C:C | A:A | T:T | G:G |
| H2_23 | SrTmp | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_1 | SrTmp | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_101 | SrTnmu | A:A | G:G | C:C | A:A | T:T | A:A |
| M5_174 | SrTnmu | A:A | G:G | C:C | A:A | T:T | G:G |
| M5_175 | SrTnmu | A:A | G:G | C:C | A:A | T:T | G:G |
Notes: H2 = the 2nd SRRSN. M5 = the 5th SRRSN. SR, plant response to the stem rust pathogens. APR, adult plant resistance. R = resistance. S = susceptible. MR = moderate resistance. Yellow = Sr25 haplotypes. Other colors = non-Sr25 haplotypes.
*, marker’s position was reassigned based on the linkage disequilibrium analysis.?, result could not be determined.
Discussion
Marker loci associated with Ug99 resistance
Of markers identified in the present study, 10 were mapped and they were located on two homoeologous groups (Groups 4 and 7) based on the 9K consensus map [12]. On Group 4, marker Ex_c1373_2628597 located on 4A is in the same region where the temporarily designated gene, SrND643, was mapped [15]. This gene was identified from wheat breeding line ‘ND643/2*Webill1 (or WBLL1)’, and is effective against the Ug99 group races at both seedling and adult growth stages. “WBLL1” was also present based on pedigree in a number of wheat lines used in this study. Therefore, the marker Ex_c1373_2628597 is likely to identify the same gene as SrND643. Marker Ex_c30581_39482788 on 4B shared the same chromosome location with Sr37, a gene that is effective against the Ug99 lineage. Sr37 was originally transferred from wild Triticum timopheevii var. araraticum [5] but was not successfully utilized in wheat breeding. Of significant markers on 7B, marker Ex_c43096_49510164 located at the position (65 cM) where no Ug99 resistance gene has been reported, although Sr17 was mapped in the same region, it is ineffective against Ug99 [7]. On the other hand, marker Ex_c12556_19992307 identified on 7BL 163.9 cM) overlapped with the location of the stem rust resistance QTL reported by Bansal et al. [16]. Moreover, we identified a DArT marker associated with stem rust resistance in the same region in our previous study [10]. The consistent identification of loci associated with stem rust resistance in this region suggests that there is likely a novel Sr gene on 7BL contributing to Ug99 resistance.
Additional markers with unknown chromosomal positions were also identified with Ug99 resistance. For instance, based on the LD analysis, among five unknowns, four markers, JD_c6624_7769357, RFL_Contig2671_2362005, JD_c1314_1888758 and CAP7_c2912_1387634 were tightly linked to the Sr25 locus, a major effective gene against the Ug99 lineage (Fig 3). Two of them (JD_c6624_7769357 and CAP7_c2912_1387634) were later confirmed by the KASP assay where 90 and 95% cosegregation with Sr25 phenotypes were obtained, respectively (Table 4).
Table 4. Validation of SNP markers linked to Sr25 in wheat breeding lines with known gene resources.
| SNP marker | Sr25 allele | Other allele | No. of Sr25 genotype | No. of Sr25 phenotype | Accuracy % |
|---|---|---|---|---|---|
| JD_c6624_7769357 | G:G | A:A | 20 | 18 | 90 |
| Ra_c25242_34807178 | C:C | A:A | 23 | 18 | 78 |
| CAP7_c2912_1387634 | A:A | G:G | 19 | 18 | 95 |
| Ex_c5884_10325223 | T:T | C:C | 21 | 18 | 86 |
Markers associated with Ug99 resistance linked to known disease resistance genes
Among the resistance loci identified in the present study, three markers (Ex_c12556_19992307, CAP7_c2912_1387634 and JD_c1314_1888758) were linked to three disease resistance genes (RGA3, NBS-LRR and RPP13). The RGA13 is a member of resistance gene analogs in plants that triggers a defense system to restrict the pathogen growth. The RGA3 was linked to marker Ex_c12556_19992307 which is located on 7BL where a QTL and a DArT marker associated with stem rust resistance has been reported [10, 16]. The RGA3 may be a putative candidate underlying the QTL interval. The NBS-LRR genes play roles in disease resistance in plants through similar mechanisms as other RGAs [18]. The identification of the NBS-LRR linked to the marker CAP7_c2912_1387634 at the Sr25 locus suggests that the NBS-LRR is a putative candidate underlying Sr25. Furthermore, at the same locus, marker JD_c1314_1888758 was linked to RPP13, another member of the NBS-LRR gene family. It has been reported that RPP13 contributes plant resistance to downy mildew in Arabidopsis [19]. Therefore, RPP13 may be another candidate for Sr25 and play a role in plant resistance to Ug99.
Marker loci linked to Sr25
Gene Sr25 is one the few race-specific genes effective against all races belonging to the Ug99 lineage [8, 9]. Sr25 was transferred into wheat in a translocation on 7DL from Thinopyrum (Th) ponticum (Podp.) by Barkworth and Dewey [20]. However, the use of germplasm containing Sr25 was limited due to the linkage of Sr25 with another Th. ponticum derived gene resulting in undesirable yellow flour. Later on, mutant lines, Agatha-28 and Agatha-235, with reduced levels of yellow pigment in flour were produced [21]. One of the mutant lines containing Sr25 was backcrossed into the Australian wheat backgrounds and has been used in the CIMMYT breeding program where it is present in the variety ‘Wheatear’ [22].
Diagnostic markers for Sr25 reported in this study can facilitate MAS for Ug99 resistance in wheat since Sr25 is effective against races of the Ug99 lineage [1, 8, 23]. In the present study, four SNP markers were found to be closely linked to Sr25 and were able to predict wheat lines carrying Sr25 with marker CAP7_c2912_1387634 having the highest accuracy (95%) (Table 4). The other two markers, Ex_c5884_10325223 and JD_c6624_7769357 were able to detect Sr25-cariers at 86 and 90% accuracies, respectively (Table 4), while marker Ra_c25242_34807178 had the lowest accuracy (78%).
Our results show that wheat lines that possess the Sr25 resistance locus fall into the haplotype of ‘T-C-G-A’ in the 2nd and 5th SRRSN populations with the marker combination of Ex_c5884_10325223- Ra-c25242_34807178-JD_c6624_7769357- CAP7_c2912_1387634 (Table 3, yellow highlighted). The Sr25-haplotype detected all Sr25-lines in 277 wheat lines tested in the present study. Most of the Sr25-lines with the ‘T-C-G-A’ haplotype had ‘Wheatear’ parent in their pedigrees, which is consistent with the introduction of gene Sr25 in this variety. Although a similar haplotype was found in line M5_107, This line neither responded to Sr25 nor was ‘Wheatear’ in the pedigree. However, M5_107 showed “APR-R” response against Ug99 (S1 and S2 Tables). A possible explanation is that Sr25 allele might be accidently introduced to this line or a mistake in phenotyping. However, the use of haplotypes with the combination of four markers increased the diagnostic accuracy.
Among the 277 lines screened with markers reported in this study, only 18 lines carried the Sr25-genotype. This is in agreement with the limited use of this resistance gene in breeding programs [5]. However, we anticipate the use of Sr25 will increase since increasing efforts have been made to develop cultivars with stem rust resistance due to the threat posed by races of the Ug99 lineage. Gene Sr25 is among a few race-specific genes effective against these races [8, 9]. Furthermore, Sr25 without yellow flour has been recently transferred into Australian and CIMMYT wheat backgrounds [21], and there is evidence to suggest that the Th. ponticum segment carrying Sr25 can increase yield potential under irrigated conditions [24, 25].
Conclusion
In the present study, we applied genome-wide association studies to identify SNP makers linked to Ug99 stem rust resistance loci using the 9K SNP chip. Marker-trait association analysis identified 12 SNPs significantly associated with the stem rust resistance. They were located on 4 chromosomes (4A, 4B, 7B and 7D). Markers located on 4A, 4B and 7D were overlapped with the reported genes SrND643, Sr37 and Sr25, respectively. Whereas, markers identified on 7B were located in the regions where no Sr gene has been reported, although a QTL has been reported in the same region as the marker Ex_c12556_19992307 identified in the present study. Several markers associated with stem rust resistance were linked to putative candidate genes that play roles in plant disease resistance. Six markers linked to the resistance were validated in 277 breeding lines using a high-throughput KASP assay. The result indicated that four of them cosegregated with Sr25 genotypes while the other two are likely to be linked to other genes. The diagnostic ability of these markers and the high throughput platform characterized in the present study may be used for marker-assisted selection, especially for Sr25, and would be beneficial for accelerating breeding programs to improve wheat resistance to stem rust such as Ug99.
Supporting information
(XLSX)
(XLSX)
Acknowledgments
This work was supported by funds provided by a grant from the Bill & Melinda Gates Foundation to Cornell University for the Borlaug Global Rust Initiative (BGRI) Durable Rust Resistance in Wheat (DRRW) Project, partial support from the USDA-ARS Cooperative Project 2090-21000-031-05-A and Hatch Project 149–430.
Data Availability
The sequences reported in this paper are deposited in the NCBI SRA database (accession nos. SRA059240, SRA012746, and GSM632785–GSM632791).
Funding Statement
This work was supported by funds provided by a grant from the Bill & Melinda Gates Foundation to Cornell University for the Borlaug Global Rust Initiative (BGRI) Durable Rust Resistance in Wheat (DRRW) Project, partial support from the USDA-ARS Cooperative Project 2090-21000-031-05-A and Hatch Project 149-430. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Pretorius ZA, Bender CM, Visser B, Terefe T (2010) First report of a Puccinia graminis f. sp. tritici race virulent to the Sr24 and Sr31 wheat stem rust resistance genes in South Africa. Plant Dis 94(6):784–785. [DOI] [PubMed] [Google Scholar]
- 2.Jin Y, Szabo LJ, Pretorius ZA, Singh RP, Ward R, Fetch T Jr (2008) Detection of virulence to resistance gene Sr24 within race TTKS of Puccinia graminis f sp. tritici. Plant Dis 92:923–926 [DOI] [PubMed] [Google Scholar]
- 3.Singh RP, Hodson DP, Huerta-Espino J, Jin Y, Bhavani S, Njau P, et al. (2011) The emergence of Ug99 races of the stem rust fungus is a threat to world wheat production. In: Van Alfen NK, Bruening G, Leach JE (eds) Annual review of phytopathology, vol 49. Palo Alto, pp 465–481. [DOI] [PubMed]
- 4.Wanyera R, Kinyua MG, Jin Y, Singh RP (2006) The spread of stem rust caused by Puccinia graminis f. sp. tritici, with virulence on Sr31 in wheat in Eastern Africa. Plant Dis 90:113. [DOI] [PubMed] [Google Scholar]
- 5.McIntosh RA, Wellings CR, Park RF (1995) Wheat rusts, an atlas of resistance genes. CSIRO Publications, East Melbourne, pp 93–99 [Google Scholar]
- 6.McIntosh RA, Dubcovsky J, Rogers WJ, Morris CF, Appels R, Xia XC (2012) Catalogue of gene symbols for wheat: 2012 supplement. Ann Wheat Newsl 58:259–279. [Google Scholar]
- 7.Yu LX, Barbier H, Rouse MN, Singh S, Singh RP, Bhavani S, et al. (2014) A consensus map for Ug99 stem rust resistance loci in wheat. Theor Appl Genet. 127:1561–81. 10.1007/s00122-014-2326-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh RP, Hodson DP, Jin Y, Huerta-Espino J, Kinyua MG, Wanyera R, et al. (2006) Current status, likely migration and strategies to mitigate the threat to wheat production from race Ug99 (TTKS) of stem rust pathogen. CAB Rev Perspect Agric Vet Sci Nutr Nat Resour 1(054):13 [Google Scholar]
- 9.Jin Y, Singh RP, Ward RW, Wanyera R, Kinyua M, Njau P, Pretorius ZA (2007) Characterization of seedling infection types and adult plant infection responses of monogenic Sr gene lines to race TTKS of Puccinia graminis f sp. tritici. Plant Dis 91:1096–1099 [DOI] [PubMed] [Google Scholar]
- 10.Yu LX, Lorenz A, Rutkoski J, Singh RP, Bhavani S, Huerta-Espino J, et al. (2011) Association mapping and gene-gene interaction for stem rust resistance in CIMMYT spring wheat germplasm. Theor Appl Genet. 123:1257–68. 10.1007/s00122-011-1664-y [DOI] [PubMed] [Google Scholar]
- 11.Doyle JJ. and Doyle J.L (1987) A rapid DNA isolation procedure from small quantities of fresh leaf tissues. Phytochem Bull. 19:11–15 [Google Scholar]
- 12.Cavanagh CR., Chao S, Wang S, Huang B E, Stephen S, Kiani S, et al. (2013) Genome-wide comparative diversity uncovers multiple targets of selection for improvement in hexaploid wheat landraces and cultivars. Proceedings of the National Academy of Sciences of the United States of America, 110 (20), 8057–8062. 10.1073/pnas.1217133110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradbury PJ, Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, Buckler ES. (2007) TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics. 1;23:2633–2635. 10.1093/bioinformatics/btm308 [DOI] [PubMed] [Google Scholar]
- 14.Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 57:289–300. [Google Scholar]
- 15.Basnet BR, Singh S, Lopez-Vera EE, Huerta-Espino J, Bhavani S, Jin Y, et al. (2015)Molecular Mapping and Validation of SrND643: A New Wheat Gene for Resistance to the Stem Rust Pathogen Ug99 Race Group. Phytopath.105:470–6. [DOI] [PubMed] [Google Scholar]
- 16.Bansal UK, Bossolini E, Miah H, Keller B, Park RF, Bariana HS (2008) Genetic mapping of seedling and adult plant stem rust resistance in two European winter wheat cultivars. Euphytica, 164:821–828 [Google Scholar]
- 17.Olson EL, Rouse MN, Pumphrey MO, Bowden RL, Gill BS, Poland JA (2013) Introgression of stem rust resistance genes SrTA10187 and SrTA10171 from Aegilops tauschii to wheat. Theor Appl Genet. [DOI] [PubMed] [Google Scholar]
- 18.Sekhwal MK, Li P, Lam I, Wang X, Cloutier S, You FM (2015) Disease Resistance Gene Analogs (RGAs) in Plants. Int J Mol Sci.16:19248–90. 10.3390/ijms160819248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bittner-Eddy PD, Crute IR, Holub EB, Beynon JL (2000) RPP13 is a simple locus in Arabidopsis thaliana for alleles that specify downy mildew resistance to different avirulence determinants in Peronospora parasitica. Plant J 21:177–188. [DOI] [PubMed] [Google Scholar]
- 20.Friebe B, Jiang J, Knott DR, Gill BS (1994) Compensation indexes of radiation-induced wheat Agropyron elongatum translocations conferring resistance to leaf rust and stem rust. Crop Sci 34:400–404 [Google Scholar]
- 21.Knott DR (1980) Mutation of a gene for yellow pigment linked to Lr19 in wheat. Can J Genet Cytol 22:651–654 [Google Scholar]
- 22.Bariana HS, Brown GN, Bansal UK, Miah H, Standen GE, Lu M (2007) Breeding triple rust resistant wheat cultivars for Australia using conventional and marker-assisted selection technologies. Aust J Agric Res 58:576–587 [Google Scholar]
- 23.Jin Y, Szabo LJ, Rouse MN, Fetch T Jr, Pretorius ZA, Wanyera R, et al. (2009) Detection of virulence to resistance gene Sr36 within the TTKS race lineage of Puccinia graminis f sp. tritici. Plant Dis 93:367–370 [DOI] [PubMed] [Google Scholar]
- 24.Singh RP, Huerta-Espino J, Rajaram S, Crossa J (1998) Agronomic effects from chromosome translocations 7DL.7Ag and 1BL.1RS in spring wheat. Crop Sci 38:27–33 [Google Scholar]
- 25.Monneveux P, Reynolds MP, Aguilar JG, Singh RP (2003) Effects of the 7DL.7Ag translocation from Lophopyrum elongatum on wheat yield and related morphophysiological traits under different environments. Plant Breed 122:379–384 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
Data Availability Statement
The sequences reported in this paper are deposited in the NCBI SRA database (accession nos. SRA059240, SRA012746, and GSM632785–GSM632791).