Abstract
Background
Gamma-glutamyltransferase (GGT) is a membrane-bound enzyme involved in the metabolism of glutathione. Studies suggested that GGT played an important role in the tumor development, progression, invasion and drug resistance and prognosis. The association between GGT and prognosis of patients with nasopharyngeal carcinoma (NPC) was unknown. This study was conducted to investigate the association of pretherapeutic serum level of GGT with clinical-pathological parameters and survival in patients with NPC.
Methods
Two hundred and twenty-two patients with NPC were recruited in this study and were stratified into two GGT risk groups (≤ 34.5 U/L, > 34.5 U/L). The association of pretherapeutic serum GGT levels with clinical–pathological parameters was examined. Univariate and multivariate survival analyses were performed.
Findings
The pretherapeutic serum level of GGT was not associated with gender, age, pathology, T stage, N stage, TNM stage, chemotherapy or radiotherapy in patients with NPC. Patients in the high-risk GGT group had a poorer survival than the low-risk GGT group (3-year overall survival, 74.2% vs. 50.2%, P = 0.001; 3-year progression-free survival, 76.4% vs. 47.1%, P < 0.001; 3-year loco-regional relapse-free survival, 76.4% vs. 51.3%, P < 0.001; 3-year distant metastasis-free survival, 89.5% vs. 66.4%, P < 0.001). Multivariate analysis suggested that patients in the high-risk GGT group had 2.117 (95% confidence interval [CI], 1.225 ∼ 3.659, P = 0.007) times the risk of death, 2.836 (95% CI, 1.765 ∼ 4.557, P < 0.001) times the risk of progression, 2.551 (95% CI, 1.573 ∼ 4.138, P < 0.001) times the risk of relapse, and 3.331 (95% CI, 1.676 ∼ 6.622, P < 0.001) times the risk of metastasis compared with those in the low-risk GGT group.
Conclusion
The pretherapeutic serum level of GGT might serve as a novel independent prognostic factor for overall-survival, progression-free survival, loco-regional relapse-free survival and distant metastasis-free survival in patients with NPC.
Introduction
Nasopharyngeal carcinoma (NPC) is endemic in southern China and Southeast Asia, with high incidence rates of 20–30 cases per 100,000 population [1–3]. Chemoradiotherapy is the primary treatment modality for locoregionally advanced NPC. Although the TNM staging system is the most important prognostic indicator for NPC patients, patients with the same TNM stages and similar treatment regimens could have significantly different survival outcomes due to the tumor’s biological heterogeneity. In addition to the TNM staging system, more and more molecular biomarkers have been evaluated as potential prognosis predictors for NPC, including serum lactate dehydrogenase (LDH) [4], C-reactive protein (CRP) [5], D-dimer [6], fibrinogen [7], and plasma Epstein-Barr virus DNA (EBV DNA) [8]. Recently, pretreatment plasma EBV DNA levels have been increasingly employed for the diagnosis, risk stratification, monitoring, and prediction for the prognosis of NPC [8, 9].
Gamma-glutamyltransferase (GGT) is a cell-membrane bound enzyme involved in the metabolism of glutathione (GSH), catalyzing the degradation of extracellular GSH and subsequently promoting amino-acid recovery for intracellular GSH synthesis [10]. As GSH is the main water-soluble antioxidant within the cell, GGT has been recognized to contribute to cellular antioxidant defenses [10, 11]. Several previous studies revealed that GGT played a potentially important role in the tumor development, progression, invasion and drug resistance and prognosis [10, 12–16]. Elevated serum level of GGT was found to be associated with poorer prognosis in several human cancers, such as renal cell carcinoma [17], ovarian cancer [18], esophageal squamous cell carcinoma [19], breast cancer [20], endometrial cancer [21], as well as cervical cancer [22].
To the best of our knowledge, there have been few report about the prognostic impact of pretherapeutic serum level of GGT on patients with NPC in detail untill now. A recent study aimed to investigate the association of serum LDH and ALP with NPC showed that increased GGT level (> 50 U/L) had no significant impact on survival of patients with NPC. However, it also indicated that patients with higher GGT level had a worse survival when by using the optimal cutoff value of GGT (28.5 U/L) determined by receiver operative characteristic (ROC) curve [23]. It seemed that different cut-off values led to different conclusions. Therefore, we performed this study to further investigate the association between pretherapeutic serum level of GGT and the clinical-pathological parameters and prognosis in the patients with NPC.
Materials and methods
Patients
A total of 222 patients with primary NPC were consecutively recruited from January 2011 to December 2014 at the Cancer Center of Guangzhou Medical University, China. This study was reviewed and approved by the institutional review board and ethics committee of Cancer Center of Guangzhou Medical University. Written informed consent was obtained from all patients. Patients who presented with pre-existing comorbidities, known to be related with elevation of GGT (i.e. hepatobiliary tract, pancreatic and heart disease or alcohol abuse) were excluded from this study (number = 128).
Clinical management
The pre-treatment evaluation included a complete patient history, physical examinations, haematology and biochemistry profiles, fibreoptic nasopharyngoscopy, chest X-ray, abdominal sonography, magnetic resonance imaging (MRI) of the nasopharynx and neck, and whole-body bone scan or whole-body FDG PET/CT. All patients were restaged according to the seventh American Joint Committee on Cancer (AJCC) TNM staging manual. In total, 21 (9.5%) patients were treated with three-dimensional conformal radiotherapy radiotherapy (3DCRT), and 201 (90.5%) patients were treated with intensity-modulated radiotherapy (IMRT). In addition, 209 (94.1%) patients with stage II–IV disease received platinum-based chemotherapy. A stratified multitherapeutic protocol was used. Radiation alone was administered for stage I disease, and radiation alone or with concurrent platinum-based chemotherapy was administered for stage II disease [24]. Concurrent chemoradiotherapy with or without neoadjuvant or adjuvant chemotherapy was administered for advanced-stage disease (stages III and IV). Neoadjuvant or adjuvant chemotherapy consisting of cisplatin plus 5-fluorouracil or cisplatin plus taxane was administered every 3 weeks for two or three cycles [25]. Concurrent cisplatin chemotherapy was administered every 3 weeks. All patients were treated according to the principles of treatment for NPC patients at the Cancer Center of Guangzhou Medical University.
GGT measurement
Blood samples for the evaluation of serum GGT levels were obtained by peripheral venous puncture 24–48 h prior to therapy. Gamma-glutamyltransferase was routinely determined to rule out liver damage before treatment starts. Gammaglutamyltransferase concentrations were analysed with an enzyme kinetic assay (Modular Hitachi 7600 and Hitachi 7080, Hitachi High-Technologies Corporation Tokyo, Japan).
Statistical analysis
Values were described by mean (standard deviation [SD]) when normally distributed or by median when presented with skewed distribution. The Mann–Whitney U test and chi-square test were performed to analyse the association between pretherapeutic serum level of GGT and clinical-pathological parameters. Receiver operative characteristic (ROC) curve was used to determine the optimal cutoff value of GGT for survival. According to the cutoff value, serum level of GGT was divided into two groups, high-risk group and low-risk group. Survival probabilities were calculated by the Kaplan–Meier method. Differences between groups were measured using the log-rank test. Survival times of patients still alive or dead as a result of other causes than cancer were censored with the last follow-up date. The primary end point of this study was progression-free survival (PFS). PFS was defined as the duration from the date of definite diagnosis to the date of disease progression or censored at the date of last follow-up. The secondary end points include overall survival (OS), locoregional relapse-free survival (LRRFS), distant metastasis-free survival (DMFS). OS was calculated from the time of definite diagnosis to the time of death from any cause or to the time of last follow-up (at which time data were censored). LRRFS and DMFS were also evaluated and calculated from the date of definite diagnosis until the day of first locoregional or distant relapse or until the date of the last follow-up visit. Univariate and multivariate analysis was performed using Cox’s proportional hazards regression model with a forward stepwise procedure (the entry and removal probabilities were 0.05 and 0.10, respectively). Analyses were performed using the statistical software package SPSS 20.0 (SPSS, Chicago, IL) and Graph Pad Prism for windows, version 6 (Graph Pad Prism, San Diego, CA, USA). A two-sided P-value less than 0.05 was considered statistically significant.
Results
Patients’ characteristics
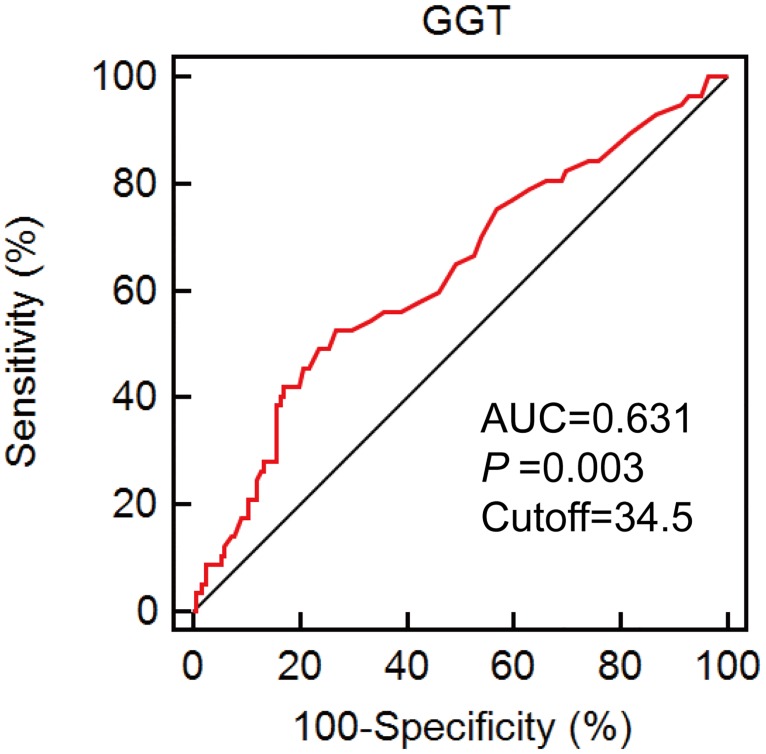
A total of 222 patients with primary NPC were included in the final analysis. The clinical–pathological characteristics of the study cohort were presented in Table 1. The optimal cutoff value of serum level of GGT with the best discriminatory power was determined to be 34.5 U/L according to ROC curve analysis (Fig 1). A total of 148 patients were assigned to the low-risk group (GGT ≤ 34.5 U/L) and 74 patients were assigned to the high-risk group (GGT > 34.5 U/L).
Table 1. Association of pretherapeutic serum level of GGT and clinical–pathological characteristics in patients with NPC.
| Characteristics | Patients N (%) | GGT (U/L) | P-value* | GGT | P-value** | |
|---|---|---|---|---|---|---|
| Median (Mean) | Low-risk group (%) | High-risk group (%) | ||||
| Gender | ||||||
| Male | 164 (73.9) | 28.0 (38.0) | 0.79 | 111 (67.7%) | 53 (32.3%) | 0.59 |
| Female | 58 (26.1) | 18.5 (39.3) | 37 (63.8%) | 21 (36.2%) | ||
| Age (years) | ||||||
| ≤ 45 | 98 (44.1) | 26.5 (38.9) | 0.81 | 60 (61.2%) | 38 (38.8%) | 0.127 |
| > 45 | 124 (55.9) | 28.0 (37.9) | 88 (71.0%) | 36 (29.0%) | ||
| Pathology (WHO type) | ||||||
| I | 1 (0.4) | 19.0 (19.0) | 0.45 | 1 (100%) | 0 (0.0%) | 0.299 |
| II | 116 (52.3) | 26.5 (34.9) | 82 (70.7%) | 34 (29.3%) | ||
| III | 105 (47.3) | 28.0 (42.3) | 65 (61.9%) | 38 (38.1%) | ||
| T stage | ||||||
| T1 | 8 (3.6) | 30.5 (39.9) | 0.21 | 5 (62.5%) | 3 (37.5%) | 0.262 |
| T2 | 85 (38.3) | 27.0 (32.7) | 61 (71.8%) | 24 (28.2%) | ||
| T3 | 77 (34.7) | 27.0 (38.7) | 53 (68.8%) | 24 (31.2%) | ||
| T4 | 52 (23.4) | 30.5 (46.6) | 29 (55.8%) | 23 (44.2%) | ||
| N stage | ||||||
| N0 | 16 (7.2) | 23.5 (38.9) | 0.16 | 12 (75.0%) | 4 (25.0%) | 0.236 |
| N1 | 74 (33.3) | 25.5 (34.3) | 53 (71.6%) | 21 (28.4%) | ||
| N2 | 103 (46.4) | 28.0 (36.7) | 68 (66.0%) | 35 (34.0%) | ||
| N3 | 29 (13.1) | 33.0 (53.9) | 15 (51.7%) | 14 (48.3%) | ||
| TNM stage | ||||||
| I | 3 (1.4) | 24.0 (28.0) | 0.08 | 2 (66.7%) | 1 (33.3%) | 0.074 |
| II | 40 (18.0) | 26.5 (34.0) | 29 (72.5%) | 11 (27.5%) | ||
| III | 106 (47.7) | 26.5 (34.2) | 77 (72.6%) | 29 (27.4%) | ||
| IV | 73 (32.9) | 32.0 (47.0) | 40 (54.8%) | 33 (45.2%) | ||
| Chemotherapy | ||||||
| Radiotherapy alone | 13 (5.9) | 34.0 (40.8) | 0.78 | 8 (61.5%) | 5 (38.5%) | 0.687 |
| Chemoradiotherapy | 209 (94.1) | 27.0 (38.2) | 140 (67.0%) | 69 (33.0%) | ||
| Radiotherapy | ||||||
| 3DCRT | 21 (9.5) | 32.0 (32.4) | 0.15 | 13 (61.9%) | 8 (38.1%) | 0.627 |
| IMRT | 201 (90.5) | 27.0 (38.9) | 135 (67.2%) | 66 (32.8%) | ||
NOTE:
*Kruskal–Wallis test.
**Chi-square test.
TNM, tumor node metastasis.
Fig 1. ROC curve using pretherapeutic serum level of GGT.
The optimal cut-off value was 34.5, with a sensitivity of 51.7% and a specificity of 73.2%.
Association between pretherapeutic serum level of GGT and clinical–pathological characteristics
The pretherapeutic serum level of GGT was not associated with gender, age, pathology, T stage, N stage, TNM stage, chemotherapy or radiotherapy. No association between GGT and clinical–pathological characteristics was found when analying the categorical variables by chi-square test either (Table 1).
Association between pretherapeutic serum level of GGT and prognosis
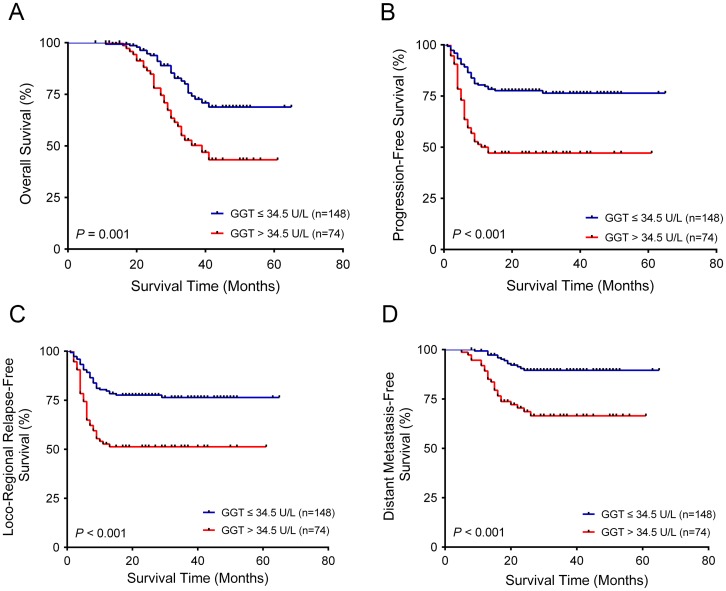
In univariate survival analysis, high-risk level of GGT, advanced T stage, advanced N stage, and advanced TNM stage were associated with poorer OS, PFS, LRRFS and DMFS (Tables 2 and 3). The 3-year OS, PFS, LRRFS and DMFS rates for patients in the low-risk GGT group and the high-risk GGT group were 74.2% vs. 50.2% (P = 0.001), 76.4% vs. 47.1% (P < 0.001), 76.4% vs. 51.3% (P < 0.001), and 89.5% vs. 66.4% (P < 0.001), respectively. Kaplan–Meier curves were shown in Fig 2 for the two groups. Multivariate analysis suggested that patients in the high-risk GGT group had 2.117 (95% confidence interval [CI], 1.225 ~ 3.659, P = 0.007) times the risk of death, 2.836 (95% CI, 1.765 ~ 4.557, P < 0.001) times the risk of progression, 2.551 (95% CI, 1.573 ~ 4.138, P < 0.001) times the risk of relapse, and 3.331 (95% CI, 1.676 ~ 6.622, P < 0.001) times the risk of metastasis compared with those in the low-risk GGT group (Tables 2 and 3). Furthermore, advanced T stage was also associated with poorer OS, PFS, LRRFS and DMFS in multivariable survival analyses (Tables 2 and 3).
Table 2. Univariate and multivariate analysis of pretherapeutic serum level of GGT associated with OS and PFS in patients with NPC.
| Variables | Univariate analysis | P value | Multivariate analysis | P value | ||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| Overall Survival | ||||||
| Gender (Female vs. Male) | 0.539 | 0.264–1.099 | 0.089 | 0.709 | 0.339–1.484 | 0.362 |
| Age (> 45y vs. ≤45y) | 0.950 | 0.565–1.599 | 0.848 | 0.722 | 0.421–1.236 | 0.234 |
| Pathology (III vs. I-II) | 0.920 | 0.547–1.547 | 0.753 | 0.597 | 0.345–1.034 | 0.066 |
| T stage (T3-4 vs. T1-2) | 3.612 | 1.870–6.978 | < 0.001 | 2.927 | 1.219–7.029 | 0.016 |
| N stage (N2-3 vs. N0-1) | 2.916 | 1.607–5.291 | < 0.001 | 2.205 | 1.046–4.646 | 0.038 |
| TNM stage (III-IV vs. I-II) | 4.004 | 1.595–10.049 | 0.003 | 0.877 | 0.215–3.568 | 0.854 |
| Radiotherapy (IMRT vs. 3D-CRT) | 7.850 | 1.086–56.739 | 0.041 | 7.502 | 1.009–55.762 | 0.049 |
| GGT expression (high vs. low) | 2.418 | 1.437–4.069 | 0.001 | 2.117 | 1.225–3.659 | 0.007 |
| Progression-Free Survival | ||||||
| Gender (Female vs Male) | 0.562 | 0.308–1.023 | 0.059 | 0.548 | 0.298–1.009 | 0.053 |
| Age (> 45y vs. ≤45y) | 0.964 | 0.608–1.529 | 0.877 | 0.882 | 0.549–1.418 | 0.605 |
| Pathology (III vs. I-II) | 1.002 | 0.633–1.585 | 0.995 | 0.773 | 0.481–1.242 | 0.287 |
| T stage (T3-4 vs. T1-2) | 3.187 | 1.829–5.553 | < 0.001 | 3.17 | 1.578–6.368 | 0.001 |
| N stage (N2-3 vs. N0-1) | 1.805 | 1.094–2.978 | 0.021 | 1.594 | 0.886–2.871 | 0.12 |
| TNM stage (III-IV vs. I-II) | 3.156 | 1.368–7.281 | 0.007 | 0.896 | 0.277–2.897 | 0.854 |
| Radiotherapy (IMRT vs. 3D-CRT) | 2.877 | 0.906–9.140 | 0.073 | 3.328 | 1.030–10.752 | 0.045 |
| GGT expression (high vs. low) | 2.865 | 1.806–4.546 | < 0.001 | 2.836 | 1.765–4.557 | < 0.001 |
NOTE: TNM, tumour node metastasis. HR, hazard ratio; CI, confidence interval.
Table 3. Univariate and multivariate analysis of pretherapeutic serum level of GGT associated with LRRFS and DMFS in patients with NPC.
| Variables | Univariate analysis | P value | Multivariate analysis | P value | ||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| Loco-regional Relapse-Free Survival | ||||||
| Gender (Female vs. Male) | 0.475 | 0.250–0.905 | 0.024 | 0.438 | 0.228–0.843 | 0.013 |
| Age (> 45y vs. ≤45y) | 1.074 | 0.669–1.724 | 0.768 | 0.987 | 0.606–1.605 | 0.957 |
| Pathology (III vs. I-II) | 1.036 | 0.648–1.655 | 0.884 | 0.854 | 0.528–1.383 | 0.521 |
| T stage (T3-4 vs. T1-2) | 3.238 | 1.828–5.735 | < 0.001 | 3.310 | 1.593–6.875 | 0.001 |
| N stage (N2-3 vs. N0-1) | 1.681 | 1.014–2.786 | 0.044 | 1.461 | 0.808–2.640 | 0.209 |
| TNM stage (III-IV vs. I-II) | 2.989 | 1.293–6.906 | 0.010 | 0.837 | 0.254–2.763 | 0.771 |
| Radiotherapy (IMRT vs. 3D-CRT) | 2.731 | 0.859–8.685 | 0.089 | 3.000 | 0.930–9.677 | 0.066 |
| GGT expression (high vs. low) | 2.580 | 1.612–4.128 | < 0.001 | 2.551 | 1.573–4.138 | < 0.001 |
| Distant Metastasis-Free Survival | ||||||
| Gender (Female vs. Male) | 0.549 | 0.229–1.317 | 0.179 | 0.590 | 0.243–1.428 | 0.242 |
| Age (> 45y vs. ≤45y) | 0.588 | 0.307–1.127 | 0.109 | 0.525 | 0.268–1.031 | 0.061 |
| Pathology (III vs. I-II) | 1.163 | 0.610–2.216 | 0.647 | 0.761 | 0.388–1.490 | 0.425 |
| T stage (T3-4 vs. T1-2) | 4.285 | 1.786–10.280 | 0.001 | 3.063 | 1.165–8.051 | 0.023 |
| N stage (N2-3 vs. N0-1) | 4.048 | 1.688–9.708 | 0.002 | 2.914 | 1.107–7.676 | 0.030 |
| TNM stage (III-IV vs. I-II) | 10.240 | 1.403–74.734 | 0.022 | 1.940 | 0.183–20.547 | 0.582 |
| Radiotherapy (IMRT vs. 3D-CRT) | 4.253 | 0.583–31.028 | 0.153 | 4.949 | 0.656–37.33 | 0.121 |
| GGT expression (high vs. low) | 3.876 | 1.993–7.536 | < 0.001 | 3.331 | 1.676–6.622 | 0.001 |
NOTE: TNM, tumour node metastasis. HR, hazard ratio; CI, confidence interval.
Fig 2. Kaplan–Meier curves for OS, PFS, LRRFS and DMFS regarding the pretherapeutic serum level of GGT.
Kaplan-Meier survival estimates and log-rank tests were used to analyze the prognostic significance of GGT in all patients. (a) OS; (b) PFS; (c) LRRFS; (d) DMFS.
Discussion
In the present study, we investigated the associations of the serum level of pretherapeutic GGT with the clinical–pathological parameters and prognosis of NPC. To the best of our knowledge, there have been few report on the prognostic impact of pretherapeutic serum level of GGT on patients with NPC in detail up to now. We stratified the patients into high-risk group and low-risk group, according to the best cutoff value determined by ROC curve. We demonstrated that high-risk group of GGT was associated with poorer prognosis in patients with NPC. Pretherapeutic serum level of GGT was an independent prognostic factor for patients with NPC. Patients in the high-risk GGT group had significant worse 5-year OS, PFS, LRRFS and DMFS than patients in the low-risk GGT group. Patients in the high-risk GGT group had higher risks of death, progression, relapse and metastasis than those in the low-risk GGT group. Our finding was similar to a recent study which showed that NPC patients with higher GGT level (> 28.5 U/L) had a worse survival except that the cut-off value was different [23]. Furthermore, our finding was also consistent with findings of the previous studies on prognostic relevance of pretherapeutic serum level of GGT in several other cancers, such as esophageal squamous cell carcinoma [19, 26], cervical cancer [22], ovarian cancer [18], renal cell carcinoma [17], and endometrial cancer [21]. Higher level of GGT was indicated to be associated with poorer prognosis in cervical cancer [22].
Although previous studies have indicated that GGT might play a meaningful role in tumor cell biology, the exact functional mechanisms remain unclear. Several potential mechanisms through which GGT impacts cancer biology have been postulated. GGT was demonstrated to participate in the important redox-sensitive processes, such as antioxidant/antitoxic defenses and cellular proliferative/apoptotic balance [27, 28], thereby function as an antioxidative role, as well as a prooxidative role within the tumour microenvironment. On the one hand, GGT was found to play a crucial role in the metabolism of glutathione which was the major thiol antioxidant in the body, consequently protecting cells against further oxidative stress [10, 29, 30]. GGT could generate an additional source of reactive oxygen species (ROS) during glutathione metabolism, which was implicated to modulate a series of biological reactions involving cellular growth, proliferation and apoptosis [14, 28, 31], and the ROS-related genes redox regulation seemed to modulate GGT expression in reflect [12]. Therefore, the continuous production of ROS generated by increased GGT expression in tumor cells may contribute to tumor progression and invasion. Moreover, GGT was indicated to be upregulated after oxidative stress through the Ras–mitogen-activated protein kinase (MAPK) pathways in rat colon carcinoma cell [32].
On the other hand, GGT and GSH were regarded as essential components of the antioxidant defence by quenching free radicals on DNA [10, 11]. The previous study revealed that GGT act as pro-oxidant functions, impairing cellular proliferative/apoptotic balance, sequently modulating tumour formation and progression [12]. GSH was indicated to mediate the reduction of phosphatase and tensin homolog (PTEN), which act as a tumour suppressor by inhibiting phosphoinositide 3-kinasedependent activation of AKT [33].
Furthermore, evidences indicated that GGT mRNA might be induced by several cytokines, such as tumor necrosis factor alpha (TNF-alpha) [34], and interferon (IFN)-alpha and–beta [35]. TNF-alpha was implicated to induce GGT expression via nuclear factor-kappaB (NF-κB)-dependent signaling, in cooperation with specificity protein 1 (Sp1) transcription factor and RNA polymerase II recruitment to the GGT promoter [36]. The results above perhaps imply that GGT may participate in the inflammation processes mediated by specific inflammatory cytokines [12].
As a retrospective study, our study was limited by biases such as lack of random assignment, and patient’s incomplete data acquisition. Nonetheless, patients with clinically relevant comorbidities known to be associated with elevated GGT, such as hepatobiliary tract, pancreatic and heart disease or alcohol abuse were excluded from the study. Despite the potential limitations, our results were clinically valuable.
We conclude that pretherapeutic serum level of GGT might be a novel independent prognostic factor for OS, PFS, LRRFS and DMFS in patients with NPC. Patients with higher level of GGT have poorer prognosis. However, whether GGT itself has a direct etiological role in carcinogenesis or may just be a marker of an underlying etiology needs further research.
Supporting information
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Adham M, Kurniawan AN, Muhtadi AI, Roezin A, Hermani B, Gondhowiardjo S, et al. Nasopharyngeal carcinoma in Indonesia: epidemiology, incidence, signs, and symptoms at presentation. Chin J Cancer. 31(4):185–96. 10.5732/cjc.011.10328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao SM, Simons MJ, Qian CN. The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer. 30(2):114–9. 10.5732/cjc.010.10377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wee JT, Ha TC, Loong SL, Qian CN. Is nasopharyngeal cancer really a "Cantonese cancer"? Chin J Cancer. 29(5):517–26. [DOI] [PubMed] [Google Scholar]
- 4.Zhou GQ, Tang LL, Mao YP, Chen L, Li WF, Sun Y, et al. Baseline serum lactate dehydrogenase levels for patients treated with intensity-modulated radiotherapy for nasopharyngeal carcinoma: a predictor of poor prognosis and subsequent liver metastasis. Int J Radiat Oncol Biol Phys. 82(3):e359–65. 10.1016/j.ijrobp.2011.06.1967 [DOI] [PubMed] [Google Scholar]
- 5.Xia WX, Zhang HB, Shi JL, Lu X, Wang L, Ye YF, et al. A prognostic model predicts the risk of distant metastasis and death for patients with nasopharyngeal carcinoma based on pre-treatment serum C-reactive protein and N-classification. Eur J Cancer. 49(9):2152–60. 10.1016/j.ejca.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 6.Chen WH, Tang LQ, Wang FW, Li CP, Tian XP, Huang XX, et al. Elevated levels of plasma D-dimer predict a worse outcome in patients with nasopharyngeal carcinoma. BMC Cancer. 14:583 10.1186/1471-2407-14-583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang LQ, Chen QY, Guo SS, Chen WH, Li CF, Zhang L, et al. The impact of plasma Epstein-Barr virus DNA and fibrinogen on nasopharyngeal carcinoma prognosis: an observational study. Br J Cancer. 111(6):1102–11. 10.1038/bjc.2014.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin JC, Wang WY, Chen KY, Wei YH, Liang WM, Jan JS, et al. Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med. 2004;350(24):2461–70. 10.1056/NEJMoa032260 [DOI] [PubMed] [Google Scholar]
- 9.Leung SF, Chan AT, Zee B, Ma B, Chan LY, Johnson PJ, et al. Pretherapy quantitative measurement of circulating Epstein-Barr virus DNA is predictive of posttherapy distant failure in patients with early-stage nasopharyngeal carcinoma of undifferentiated type. Cancer. 2003;98(2):288–91. 10.1002/cncr.11496 [DOI] [PubMed] [Google Scholar]
- 10.Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38(4):263–355. 10.1080/20014091084227 [DOI] [PubMed] [Google Scholar]
- 11.Pastore A, Federici G, Bertini E, Piemonte F. Analysis of glutathione: implication in redox and detoxification. Clin Chim Acta. 2003;333(1):19–39. [DOI] [PubMed] [Google Scholar]
- 12.Corti A, Franzini M, Paolicchi A, Pompella A. Gamma-glutamyltransferase of cancer cells at the crossroads of tumor progression, drug resistance and drug targeting. Anticancer Res. 30(4):1169–81. [PubMed] [Google Scholar]
- 13.Hanigan MH, Gallagher BC, Townsend DM, Gabarra V. Gamma-glutamyl transpeptidase accelerates tumor growth and increases the resistance of tumors to cisplatin in vivo. Carcinogenesis. 1999;20(4):553–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pompella A, Corti A, Paolicchi A, Giommarelli C, Zunino F. Gamma-glutamyltransferase, redox regulation and cancer drug resistance. Curr Opin Pharmacol. 2007;7(4):360–6. 10.1016/j.coph.2007.04.004 [DOI] [PubMed] [Google Scholar]
- 15.Pompella A, De Tata V, Paolicchi A, Zunino F. Expression of gamma-glutamyltransferase in cancer cells and its significance in drug resistance. Biochemical pharmacology. 2006;71(3):231–8. 10.1016/j.bcp.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 16.Van Hemelrijck M, Jassem W, Walldius G, Fentiman IS, Hammar N, Lambe M, et al. Gamma-glutamyltransferase and risk of cancer in a cohort of 545,460 persons—the Swedish AMORIS study. Eur J Cancer. 47(13):2033–41. 10.1016/j.ejca.2011.03.010 [DOI] [PubMed] [Google Scholar]
- 17.Hofbauer SL, Stangl KI, de Martino M, Lucca I, Haitel A, Shariat SF, et al. Pretherapeutic gamma-glutamyltransferase is an independent prognostic factor for patients with renal cell carcinoma. Br J Cancer. 111(8):1526–31. 10.1038/bjc.2014.450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimm C, Hofstetter G, Aust S, Mutz-Dehbalaie I, Bruch M, Heinze G, et al. Association of gamma-glutamyltransferase with severity of disease at diagnosis and prognosis of ovarian cancer. Br J Cancer. 109(3):610–4. 10.1038/bjc.2013.323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang F, Zhang S, Yang H, Luo K, Wen J, Hu Y, et al. Prognostic significance of gamma-glutamyltransferase in patients with resectable esophageal squamous cell carcinoma. Dis Esophagus. 28(5):496–504. 10.1111/dote.12227 [DOI] [PubMed] [Google Scholar]
- 20.Staudigl C, Concin N, Grimm C, Pfeiler G, Nehoda R, Singer CF, et al. Prognostic relevance of pretherapeutic gamma-glutamyltransferase in patients with primary metastatic breast cancer. PLoS One. 10(4):e0125317 10.1371/journal.pone.0125317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seebacher V, Polterauer S, Grimm C, Rahhal J, Hofstetter G, Bauer EM, et al. Prognostic significance of gamma-glutamyltransferase in patients with endometrial cancer: a multi-centre trial. Br J Cancer. 106(9):1551–5. 10.1038/bjc.2012.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polterauer S, Hofstetter G, Grimm C, Rahhal J, Mailath-Pokorny M, Kohl M, et al. Relevance of gamma-glutamyltransferase—a marker for apoptotic balance—in predicting tumor stage and prognosis in cervical cancer. Gynecol Oncol. 122(3):590–4. 10.1016/j.ygyno.2011.05.027 [DOI] [PubMed] [Google Scholar]
- 23.Li G, Gao J, Tao YL, Xu BQ, Tu ZW, Liu ZG, et al. Increased pretreatment levels of serum LDH and ALP as poor prognostic factors for nasopharyngeal carcinoma. Chin J Cancer. 31(4):197–206. 10.5732/cjc.011.10283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen QY, Wen YF, Guo L, Liu H, Huang PY, Mo HY, et al. Concurrent chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal carcinoma: phase III randomized trial. J Natl Cancer Inst. 103(23):1761–70. 10.1093/jnci/djr432 [DOI] [PubMed] [Google Scholar]
- 25.Chen L, Hu CS, Chen XZ, Hu GQ, Cheng ZB, Sun Y, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol. 13(2):163–71. 10.1016/S1470-2045(11)70320-5 [DOI] [PubMed] [Google Scholar]
- 26.Takemura K, Kawachi H, Eishi Y, Kitagaki K, Negi M, Kobayashi M, et al. gamma-Glutamylcyclotransferase as a novel immunohistochemical biomarker for the malignancy of esophageal squamous tumors. Hum Pathol. 45(2):331–41. 10.1016/j.humpath.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 27.Dominici S, Valentini M, Maellaro E, Del Bello B, Paolicchi A, Lorenzini E, et al. Redox modulation of cell surface protein thiols in U937 lymphoma cells: the role of gamma-glutamyl transpeptidase-dependent H2O2 production and S-thiolation. Free Radic Biol Med. 1999;27(5–6):623–35. [DOI] [PubMed] [Google Scholar]
- 28.Franzini M, Corti A, Lorenzini E, Paolicchi A, Pompella A, De Cesare M, et al. Modulation of cell growth and cisplatin sensitivity by membrane gamma-glutamyltransferase in melanoma cells. Eur J Cancer. 2006;42(15):2623–30. 10.1016/j.ejca.2006.04.016 [DOI] [PubMed] [Google Scholar]
- 29.Lim JS, Yang JH, Chun BY, Kam S, Jacobs DR Jr., Lee DH. Is serum gamma-glutamyltransferase inversely associated with serum antioxidants as a marker of oxidative stress? Free Radic Biol Med. 2004;37(7):1018–23. 10.1016/j.freeradbiomed.2004.06.032 [DOI] [PubMed] [Google Scholar]
- 30.Choi J, Liu RM, Forman HJ. Adaptation to oxidative stress: quinone-mediated protection of signaling in rat lung epithelial L2 cells. Biochemical pharmacology. 1997;53(7):987–93. [DOI] [PubMed] [Google Scholar]
- 31.Cotgreave IA, Gerdes RG. Recent trends in glutathione biochemistry—glutathione-protein interactions: a molecular link between oxidative stress and cell proliferation? Biochemical and biophysical research communications. 1998;242(1):1–9. 10.1006/bbrc.1997.7812 [DOI] [PubMed] [Google Scholar]
- 32.Pandur S, Pankiv S, Johannessen M, Moens U, Huseby NE. Gamma-glutamyltransferase is upregulated after oxidative stress through the Ras signal transduction pathway in rat colon carcinoma cells. Free Radic Res. 2007;41(12):1376–84. 10.1080/10715760701739488 [DOI] [PubMed] [Google Scholar]
- 33.Kim Y, Song YB, Kim TY, Kim I, Han SJ, Ahn Y, et al. Redox regulation of the tumor suppressor PTEN by glutathione. FEBS Lett. 584(16):3550–6. 10.1016/j.febslet.2010.07.006 [DOI] [PubMed] [Google Scholar]
- 34.Daubeuf S, Accaoui MJ, Pettersen I, Huseby NE, Visvikis A, Galteau MM. Differential regulation of gamma-glutamyltransferase mRNAs in four human tumour cell lines. Biochim Biophys Acta. 2001;1568(1):67–73. [DOI] [PubMed] [Google Scholar]
- 35.Bouman L, Sanceau J, Rouillard D, Bauvois B. gamma-Glutamyl transpeptidase expression in Ewing's sarcoma cells: up-regulation by interferons. Biochem J. 2002;364(Pt 3):719–24. 10.1042/BJ20011854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reuter S, Schnekenburger M, Cristofanon S, Buck I, Teiten MH, Daubeuf S, et al. Tumor necrosis factor alpha induces gamma-glutamyltransferase expression via nuclear factor-kappaB in cooperation with Sp1. Biochemical pharmacology. 2009;77(3):397–411. 10.1016/j.bcp.2008.09.041 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.