Abstract
Background and Purpose
Patient selection in clinical trials on intracerebral hemorrhage (ICH) affects overall applicability of results. We estimated eligibility for completed, ongoing, and planned clinical trials in an unselected cohort of ICH-patients.
Methods
Large clinical ICH-trials were identified using trial-registration databases. Each trial’s inclusion criteria were applied to a consecutive group of ICH-patients from the prospective hospital-based Lund Stroke Register. Survival status was obtained from the National Census Office and 90-day poor functional outcome (modified Rankin Scale ≥4) from the Swedish Stroke Register or medical files.
Results
Among 253 ICH-patients, estimated eligibility proportions ranged between 2–36% for the 11 identified clinical trials. Patients not eligible for any trial (n=96) had more intraventricular hemorrhage, lower baseline level of consciousness, higher rates of cerebellar ICH, and lower rates of lobar ICH (p≤0.001). Thirty-day case fatality for non-eligible patients was 54% vs. 18% among patients eligible in ≥1 trial (95% CI: 44–64% vs. 13–25%; p<0.001). Non-eligible ICH-patients more frequently had poor functional outcome (75% vs. 48%; 95% CI: 65–83% vs. 40–56%; p<0.001).
Conclusions
There is large variation in proportions of ICH-patients eligible for inclusion in clinical trials and over a third of ICH-patients are not eligible for any trial.
MESH search-terms: Cerebral Hemorrhage, Stroke, Clinical Trials as Topic
Introduction
Clinical treatment trials in intracerebral hemorrhage (ICH) often use several selection criteria to ensure patient safety and detect possible therapeutic benefits. This selection may reduce the trials’ overall external validity (generalizability) in a general population of ICH-patients, which is of significance as treatment trials constitute an important basis for clinical guidelines and decision-making. It is therefore important for clinicians to understand trials’ applicability and what characterizes included and non-included patients. Previous eligibility estimates for ICH-trials have been low,[1–3] and Fonville et al. reported that 17–32% of ICH-patients were not eligible for any of the 17 trials they studied.[1]
However, eligibility for several large completed and ongoing ICH-trials have not previously been estimated and an update on the subject is needed. We therefore assessed the (1) eligibility proportions for large surgical or medical clinical ICH-trials and (2) overall characteristics and outcomes for eligible and non-eligible patients, in a large consecutive and well categorized ICH-patient cohort.
Methods
Trial Selection
We identified randomized controlled trials on ICH using three online clinical trial registration databases: ClinicalTrials.gov (www.ClinicalTrials.gov), ISRCTN registry (www.isrctn.com), and the Stroke Trials Registry (www.strokecenter.org). We included large (≥300 ICH-patients) completed, ongoing, and planned phase II-IV interventional trials (see supplemental Figure I, http://stroke.ahajournals.org).
Eligibility criteria for each of the included trials were identified using abovementioned databases or each trials’ original publication (described in the Supplement). Patients with missing data (other than ictus to CT-time) were considered eligible. All eligible patients were assumed to consent to inclusion.
Study Subjects
Consecutive first-ever stroke-patients with ICH were prospectively included in the Lund Stroke Register (LSR) in 2001–2007. Registration of patients, baseline variables, and outcome follow-up, has been previously described.[4] The study was approved by the Regional Ethical Review Board.
The LSR-patients’ eligibility status for each identified trial was determined on information from prospectively registered data, medical files, and baseline-CT, as described in the online supplement. Survival status was obtained in 2011 from the National Census Office. Functional outcome (modified Rankin scale, mRS) was obtained from the Swedish Stroke Register, or review of medical records.[4] Poor outcome was defined as mRS≥4 at 90 days (range 50–150 days) or death <150 days after ICH, choosing the outcome closest to 90 days.
Statistics
Mann-Whitney U-test and Pearson’s χ2-test were used for descriptive statistics. Confidence intervals (CI) for sample proportions were calculated with Wilson’s method. Kaplan-Meier plots were used for 30- and 365-day survival analysis. IBM SPSS-statistics version 22.0 was used for statistical analyses and p-values<0.05 were considered significant.
Results
Trial Identification and Patient Selection
Of 59 unique interventional ICH-trials identified, 11 matched our selection criteria (Table 1); exclusion causes were: phase 0/I-trial (n=6) or phase II-trial succeeded by a phase III-trial (n=5), trial inactive/suspended (n=13), <300 ICH-patients (n=23), and unclear inclusion criteria (n=1). For eligibility estimation, we included 253 LSR-patients with spontaneous ICH (supplemental Figure II). Survival status was available for all patients; functional outcome (functional status for survivors or death) was obtainable for 224 patients (89%; 139 eligible for ≥1 trial), with a median follow-up time of 94 days (IQR 90–115) for patients who survived up to 150 days.
Table 1.
Trial characteristics and distribution of eligibility assessments for 253 ICH-patients (204 supratentorial ICH)
| Trial Characteristics | LSR findings | ||||
|---|---|---|---|---|---|
| Eligibility criteria (n) |
Ictus to inclusion time (hours) |
Eligible n (%)* |
Eligible supra- tentorial ICH, n (%) |
Eligible but missing information on ≥1 criteria (n) |
|
| Medical trials | |||||
| CHANT (2004–2005) | 20 | <6 | 38 (15) | 36 (18) | 5 |
| FAST (2005–2007) | 12 | <3 | 44 (17) | 41 (20) | 4 |
| INTERACT-2 (2008–2012) | 18 | <6 | 41 (16) | 36 (18) | 11 |
| ATACH-II (2011–2015) | 21 | <4.5 | 26 (10) | 26 (13) | 7 |
| RESTART (2013-ongoing) | 15 | Min>24 | 41 (15†) | 32 (15‡) | 3 |
| TICH-2 (2013-ongoing) | 11 | <8 | 91 (36) | 75 (37) | 10 |
| Surgical trials | |||||
| STICH-I (1995–2003) | 11 | <72 | 89 (35) | 89 (44) | 11 |
| STICH-II (2007–2012) | 12 | <48 | 23 (9) | 23 (11) | 14 |
| CLEAR-III (2009–2015) | 24 | <72 | 9 (4) | 9 (4) | 2 |
| MISTIE-III (2013-ongoing) | 35 | <72 | 14 (6) | 14 (7) | 1 |
| SWITCH (2014-ongoing) | 27 | <66 | 4 (2) | 4 (2) | 2 |
Based on 271 ICH-patients
219 supratentorial
see supplemental Figure II which contains numbers on patients considered non-eligible due to missing time.
CHANT=Cerebral Hemorrhage And NXY Treatment[5]; FAST=Recombinant Factor VIIa in Acute ICH[6]; INTERACT-2=The Second Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial[7]; ATACH-II=Antihypertensive Treatment of Acute Cerebral Hemorrhage (ClinicalTrials.gov NCT01176565); RESTART=Restart or Stop Antithrombotics Randomised Trial (ISRCTN-registry ISRCTN71907627); TICH-2=Tranexamic acid for ICH (ISRCTN-registry ISRCTN93732214); STICH-I=The International Surgical Trial in ICH[8]; STICH-II=Surgical Trial in Lobar ICH[9]; CLEAR-III=Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage Phase III[10]; MISTIE-III=Minimally Invasive Surgery Plus rt-PA for ICH Evacuation Phase III (ClinicalTrials.gov NCT01827046); SWITCH=Swiss Trial of Decompressive Craniectomy Versus Best Medical Treatment of Spontaneous Supratentorial ICH (ClinicalTrials.gov NCT02258919)
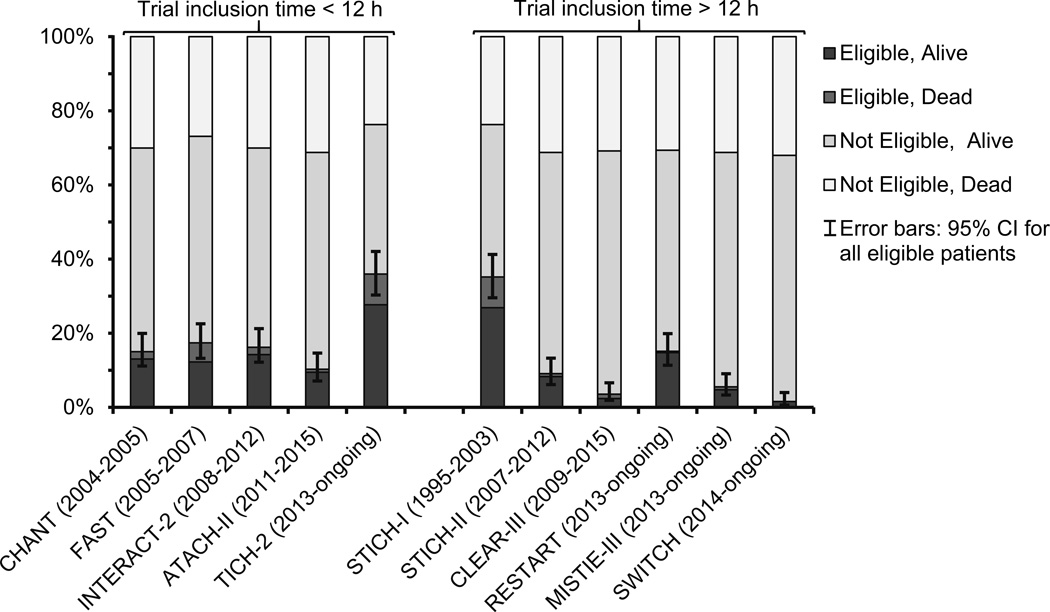
Eligibility proportions ranged from 2–36% between included trials and 96 (38%) ICH-patients were ineligible for all of the included trials. Individual trial characteristics are shown in Figure 1 and Table 1.
Figure 1.
Estimated eligibility and 30-day case fatality rates for 11 ICH-trials. Based on 253 ICH-patients (except for RESTART where n=271) from LSR. Acronyms are explained in Table 1.
Baseline Characteristics Eligible vs. Non-eligible ICH-patients
Compared to ICH-patients not eligible for any trial, ICH-patients eligible for ≥1 trial had higher admission level of consciousness (using Glasgow Coma Scale, p<0.001), less severe intraventricular hemorrhage (using modified Graeb Scale, p=0.001), more often lobar and less often cerebellar ICH (p<0.001), see supplemental Table I. No differences in age, sex, or ICH-volume were observed (p>0.05).
Outcome Eligible vs. Non-eligible ICH-patients
Eligible patients had 30- and 365-day case fatality rates of 18% (n=29) and 28% (n=44) (95% CI: 13–25% and 22–36%), while corresponding rates among non-eligible patients were 54% (n=52) and 59% (n=57) (95% CI: 44–64% and 49–69%). Survival plots illustrate these differences (supplemental Figure III; p<0.001 for both end-points). The eligible ICH-patients less frequently had poor functional outcome compared to the non-eligible ICH-patients (n=67/139=48% vs. 64/85=75%; 95% CI: 40–56% vs. 65–83%; p<0.001).
Discussion
Eligibility for clinical trials on ICH differ greatly and even in trials with the broadest inclusion criteria a minority was estimated to be eligible. Compared to an unselected cohort, clinical trials generally include ICH-patients with less severe baseline characteristics and better outcomes which should be considered when translating trial results into clinical practice and guidelines. However, many non-eligible patients would likely also be ineligible for future trials due to serious prognosis with non-survivable hemorrhages or hematomas requiring life-saving surgery.
We present novel eligibility and survival estimates for seven trials, of which four are ongoing and two are recently completed. Eligibility proportions ranged from 2–36% with current surgical trials being the least inclusive, possibly due to negative results from the previous, more inclusive, surgical trials STICH-I and STICH-II.[8,9] In our cohort 9% (95% CI: 6–13%) of ICH-patients were potentially eligible for STICH-II, which is higher than reported in a previous study (4%)[1] but in line with another (8%).[2] Our eligibility estimate for FAST (17%; 95% CI: 13–23%) was similar to an eligibility study on recombinant factor VIIa-therapy (13–18%)[3] but higher than an all-inclusive population-based study (7%)[1] with somewhat different design compared to LSR. The latter study had a lower eligibility estimate for INTERACT-2 (7% vs. 16%, 95% CI: 12–21%) but a similar estimate for STICH-I (40% vs. 35%, 95% CI: 30–41%) compared to our study.[1] Our eligibility proportions were higher than the screening to enrollment ratios for FAST (9%)[6] and INTERACT-2 (10%)[7], but similar to screening results from STICH-II (8%)[9], CLEAR-III (5%, see Supplement), and ATACH-II (12%)[11].
Our well categorized consecutive hospital-based ICH-patient cohort from a setting with high hospitalization and CT rates for stroke-patients[4] provides validity to our findings. Eligibility might have been over-estimated because patients with missing data were included, and consent from all patients was assumed.
Conclusion
Our study highlights the importance of understanding how eligibility criteria affect patient selection in trial design and clinical implementation of trial results.
Supplementary Material
Acknowledgments
Dr Hanley is principal investigator for the trials CLEAR-III (NINDS-grant U01NS062851) and MISTIE-III (NINDS-grant U01NS080824). Dr Lindgren reports honoraria from Bristol-Myers Squibb for seminar presentations, and Boehringer Ingelheim, Bayer, and Astra Zeneca for medical advisory board participation.
Footnotes
Disclosures
Hansen, Ullman, and Norrving have no disclosures.
References
- 1.Fonville AF, Samarasekera N, Hutchison A, Perry D, Roos YB, Al-Shahi Salman R. Eligibility for randomized trials of treatments specifically for intracerebral hemorrhage: community-based study. Stroke. 2013;44:2729–2734. doi: 10.1161/STROKEAHA.113.001493. [DOI] [PubMed] [Google Scholar]
- 2.Adeoye O, Woo D, Haverbusch M, Tao H, Sekar P, Moomaw CJ, et al. Eligibility for the surgical trial in intracerebral hemorrhage II study in a population-based cohort. Neurocrit Care. 2008;9:237–241. doi: 10.1007/s12028-007-9045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flaherty ML, Woo D, Haverbusch M, Moomaw CJ, Sekar P, Sauerbeck L, et al. Potential applicability of recombinant factor VIIa for intracerebral hemorrhage. Stroke. 2005;36:2660–2664. doi: 10.1161/01.STR.0000189634.08400.82. [DOI] [PubMed] [Google Scholar]
- 4.Hansen BM, Morgan TC, Betz JF, Sundgren PC, Norrving B, Hanley DF, et al. Intraventricular extension of supratentorial intracerebral hemorrhage: the modified Graeb scale improves outcome prediction in Lund Stroke Register. Neuroepidemiology. 2016;46:43–50. doi: 10.1159/000442575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyden PD, Shuaib A, Lees KR, Davalos A, Davis SM, Diener HC, et al. Safety and tolerability of NXY-059 for acute intracerebral hemorrhage: the CHANT Trial. Stroke. 2007;38:2262–2269. doi: 10.1161/STROKEAHA.106.472746. [DOI] [PubMed] [Google Scholar]
- 6.Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–2137. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
- 7.Anderson CS, Heeley E, Huang Y, Wang J, Stapf C, Delcourt C, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368:2355–2365. doi: 10.1056/NEJMoa1214609. [DOI] [PubMed] [Google Scholar]
- 8.Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365:387–397. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- 9.Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013;382:397–408. doi: 10.1016/S0140-6736(13)60986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziai WC, Tuhrim S, Lane K, McBee N, Lees K, Dawson J, et al. A multicenter, randomized, double-blinded, placebo-controlled phase III study of Clot Lysis Evaluation of Accelerated Resolution of Intraventricular Hemorrhage (CLEAR III) Int J Stroke. 2014;9:536–542. doi: 10.1111/ijs.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qureshi AI, Palesch YY, Barsan WG, Hanley DF, Hsu CY, Martin RL, et al. Intensive blood-pressure lowering in patients with acute cerebral hemorrhage. [published online ahead of print June 8, 2016] [Accessed June 9, 2016];N Engl J Med. doi: 10.1056/NEJMoa1603460. http://www.nejm.org/doi/full/10.1056/NEJMoa1603460. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.