Abstract
We report a summary of the symposium “Stress and Protists: No life without stress”, which was held in September 2015 on the VII European Congress of Protistology in partnership with the International Society of Protistologists (Seville, Spain). We present an overview on general comments and concepts on cellular stress which can be also applied to any protist. Generally, various environmental stressors may induce similar cell responses in very different protists. Two main topics are reported in this manuscript: (i) metallic nanoparticles as environmental pollutants and stressors for aquatic protists, and (ii) ultraviolet radiation – induced stress and photoprotective strategies in ciliates. Model protists such as Chlamydomonas reinhardtii and Tetrahymena thermophila were used to assess stress caused by nanoparticles while stress caused by ultraviolet radiation was tested with free living planktonic ciliates as well as with the symbiont-bearing model ciliate Paramecium bursaria. For future studies, we suggest more intensive analyses on protist stress responses to specific environmental abiotic and/or biotic stressors at molecular and genetic levels up to ecological consequences and food web dynamics.
Keywords: Environmental stress, Epigenetic, Nanoparticles, Oxidative stress, Protists, (Solar) radiation
Introduction
All living beings may have been under some kind of stress throughout their life. In fact, the existence of different environmental stress forms has been a key piece of the evolutionary machinery. Stress and life are closely connected. In general, the concept of “stress” can be considered as any harmful environmental factor that induces cellular physiological changes, disturbing the homeostasis of an organism. Therefore, it has a negative connotation because it induces damage or homeostatic disturbance on the living system. However, the initial negative connotation of stress can become a positive one after cellular acclimatization (recovering the cell homeostasis) and later selection of acclimatized cells, or in other words; “what does not kill it can make it stronger”. If acclimatization fails and homeostasis cannot be recovered, cells die (unregulated cell death) or undergo suicide (regulated cell death or apoptosis) (Galluzzi et al. 2016).
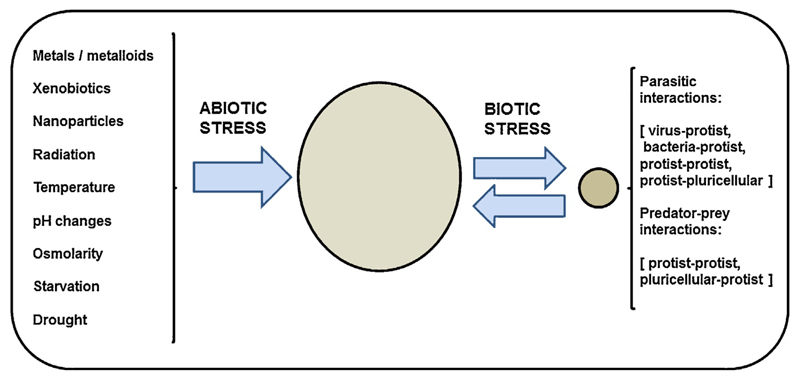
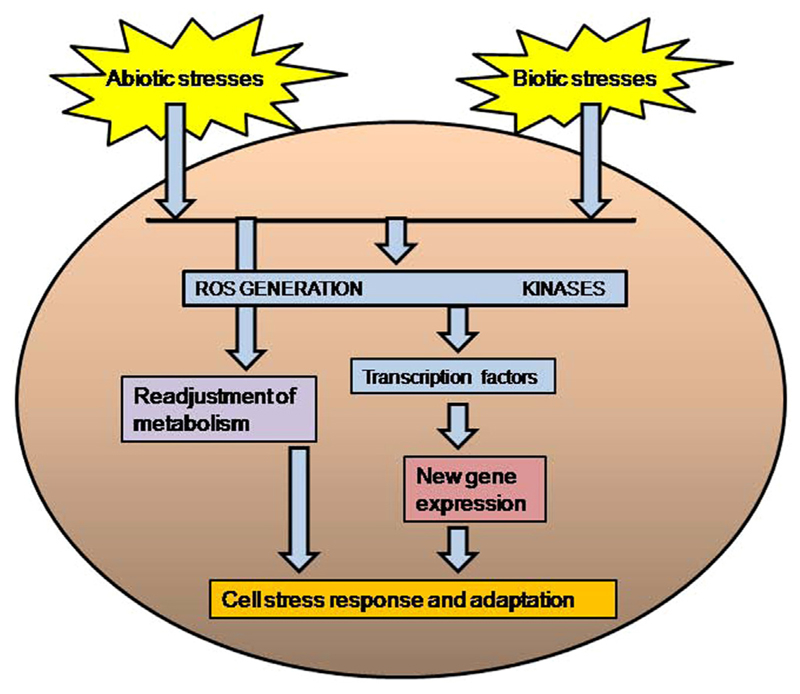
From a biological point of view, we can distinguish two types of stresses; abiotic and biotic ones (Fig. 1). Abiotic stress includes all exogenous physicochemical environmental factors that may trigger a damage to any living organism, for instance; pH, temperature, osmotic stress, (solar) radiation, inorganic (metals, metalloids, metallic nanoparticles) and organic (xenobiotic) compounds, starvation, drought, etc. On the other hand, biotic stress involves the presence of whole cells or organisms acting as the stress source, and the interaction between the living stressor and the living receptor is the real cause of the stress, and, as they are living beings, both can be stressed. Some examples of biotic stresses are parasitical interactions (virus-, bacteria, or protist-host), predator–prey interactions, or symbiotic interactions (Schwartzman and Ruby 2016). These types of stresses have convergent points in the signaling networks and overlapping gene clusters (Fujita et al. 2006) (Fig. 2). The generation of reactive oxygen species (ROS) has been considered as a key process present in both abiotic and biotic stress (Apel and Hirt 2004). Likewise, MAP-kinase cascades are another convergence point involved in the signaling network of abiotic and biotic stresses (Nakagami et al. 2005; Swiecilo 2016).
Fig. 1.
Schematic representation of the main environmental abiotic and biotic stressors on an organism (big circle). Small circle represents any parasite (living stressor). For details see text.
Fig. 2.
Schematic representation of the different strategies involved in the cellular stress response.
Cell mechanisms to respond to environmental changes are universal, so, in general, they are present in all living beings (including protists). The continuous or regular exposure to a specific stressor involves a cell acclimatization to that environmental stressor. This adaptive change can be reversible returning to the non-acclimatized cellular stage after the stressor is removed or disappears from the environment. When the stressor agent appears in the environment, a cell recognition mechanism carries out a chemical transduction by specific or unspecific receptors, indicating the cell the presence of that stressor. From this point, a complex signaling network connects the initial receptor with the molecular mechanism involved in the cell response against that specific stressor (Fig. 2). The cell response can be specific to only one stressor or general (common response) to several different stressors. In general, both cellular responses can co-exist, because cross-protection exists among different environmental stressors (Swiecilo 2016). Depending on the nature of stressor, cell adaptive responses may consist in a readjustment of metabolism or induction of new gene expression (Ruis 1997). In some cases, the new gene expression involves a cell differentiation process inducing a stressorresistant cellular stage. All these cell alterations are focused to maintain cell survival under the stress conditions.
Both abiotic and biotic environmental stressors can modify gene activities via epigenetic mechanisms, so representing a connection between environmental change and genome response. In fact, several epigenetic control events (opening or closing gene expression) have been reported in organisms undergoing environmental stress (Meyer 2015). Three main epigenetic mechanisms seem to be involved in environmental stress acclimatization; DNA methylation, histone modifications (acetylation or methylation) and non-coding microRNAs (miRNAs). Before transcription initiation the gene expression can be regulated by the chemical modification of DNA or associate histone proteins; unmethylated DNA or acetylated histones promote the gene expression, while methylated DNA or deacetylated histones block the gene expression. Likewise, after gene transcription the expression of mRNAs can be regulated by miRNAs, by blocking or inducing the transduction (Nolte-’t Hoen et al. 2015). Several examples of epigenetic processes connected to environmental stresses are: down-regulation of methyl-transferase enzymes and reduction of DNA methylation by cadmium stress (Bishak et al. 2015), different miRNAs involved in the regulation of heat stress in plants (Liu et al. 2015), or those involved in metal stresses, such as; miR398 which is essential to maintain the Cu homeostasis, or miR393 and miR717 which play an important role in the Cd stress response in plant cells (Ding and Zhu 2009). Oxidative stress (OS), hypoxia, cold stress or starvation (Hudder and Novak 2008) and stress by radiation (Josson et al. 2008) seem to be also related with post-transcriptional regulation by miRNAs. To the best of our knowledge, there are no studies involving protists, environmental stress and epigenetics.
In protists, several types of stress responses to different environmental factors have been studied, for instance; ultraviolet radiation stress in ciliates (see the section “stress responses and photoprotective strategies of ciliates exposed to ultraviolet radiation”, below), starvation stress inducing cell differentiation (encystment) in ciliates (Gutierrez and Martin-Gonzalez, 2002; Gutierrez et al. 2001), cell stress induced by metal(loid)s (Dondero et al. 2004; Ferro et al. 2015; Gutierrez et al. 2008, 2011; Kim et al. 2014; Martin-Gonzalez et al. 2005; Mendoza-Cózatl et al. 2005; Rico et al. 2009), stress by metal-containing nanoparticles (section “Engineered nanomaterials as environmental stressors and effects on aquatic protists”, below) (Gonzalo et al. 2014; Mortimer et al. 2014b; Navarro et al. 2015; Zou et al. 2013), and OS by xenobiotics (Díaz et al. 2016; Prado et al. 2012; Trielli et al. 2006), among others. Although, in the last years the study of cellular stress using different model-protists (mainly Tetrahymena thermophila, Paramecium spp., Dictyostelium discoideum or Chlamydomonas reinhardtii) has been increased, there is still much to learn about the stress response of these eukaryotic microorganisms against both abiotic and biotic stresses. Mainly, molecular and genetic (or epigenetic) studies on this topic are still in their infancy, and greater efforts are needed to cover these gaps.
The present manuscript is based on two contributions presented in the symposium “Stress and Protists: No life without stress” held in September 2015 on the VII European Congress of Protistology (ECOP) in partnership with the International Society of Protistologists (ISOP) (Seville, Spain).
Engineered nanomaterials as environmental stressors and effects on aquatic protists
Engineered nanomaterial (ENM) use in numerous applications is rapidly rising and their concomitant release into the environment is inevitable and therefore could bear important environmental implications (Nowack et al. 2012). ENM are being produced at industrial volumes with significant releases into the aquatic environment already occurring and likely to increase further. Among them the material based on inorganic nanoparticles such as metals, metal composites and metal oxides, represent about one third of all the nanomaterials (OECD 2015). The properties of ENMs that make them useful in manufacturing may also make them potentially biologically disruptive and of risk for the environment if they are in contact with biota. Therefore, ENMs can be considered as emerging stressors of anthropogenic origin with a potential to persist and to induce the detrimental alterations in the environment (Peijnenburg et al. 2015; von Moos et al. 2014). Although the increasing efforts to assess and classify the alteration potential of ENMs in the aquatic environment, the major drivers and underlaying mechanisms still need to be explored (Ivask et al. 2014). Here, we focus on the impact of ENMs on two representative aquatic protists: the photosynthetic plant-like (autotrophic) protist Chlamydomonas reinhardtii, and the ingestive animal-like (phagotrophic) protist Tetrahymena thermophyla. More specifically, the processes governing the impact of the metal containing ENMs on these protists are discussed first, and the applicability of the OS paradigm to assess the alteration potential in environmental settings and modifying factors is then illustrated with examples of metal containing nanoparticles.
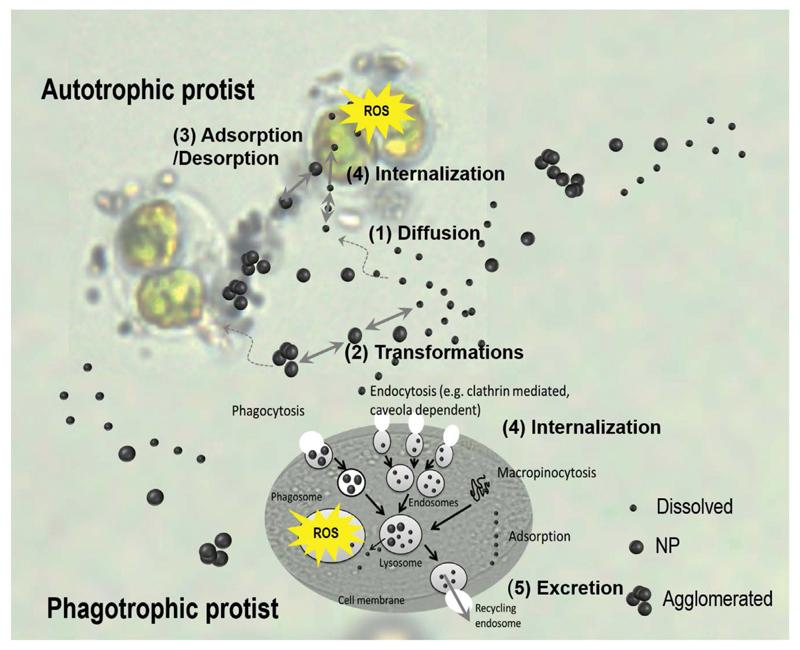
To interact with the biota (e.g. protists) and thus to induce an effect, the ENMs suspended in the water column have to reach (1, Fig. 3) the vicinity of the microorganism. In the ambient medium, ENMs can interact with different organic and inorganic components, agglomerate and release metal ions (2, Fig. 3). As a result, the biota is in contact with a complex mixture containing different ENM forms including agglomerates, free/complexed dissolved and partially dissolved ENM species. Once in the vicinity of the microorganism, different ENM forms react with sensitive receptor sites (adsorption, 3, Fig. 3) on the biological membrane, and then (but not necessarily) can diffuse through the membrane (internalization, 4, Fig. 3). Once inside the cell, ENMs can interact with different intracellular components and affect cellular processes; ENMs can be transformed or excreted (5, Fig. 3). There is a consensus that three major phenomena drive the biological effects of the ENMs (Ivask et al. 2014): (i) their dissolution, (ii) organism dependent cellular uptake of ENPs and (iii) induction of OS and consequent cellular damages.
Fig. 3.
Conceptual presentation of the key processes involved at the interface protists-medium, which govern ENMs interactions with plant-like (autotrophic) protists such as Chlamydomonas reinhardtii and particle-ingestive (phagotrophic) protists such as Tetrahymena thermophyla. For details see text.
The above general concept of ENM – microorganism interactions is illustrated with the example of the green alga C. reinhardtii and the ciliated protist T. thermophyla. We may anticipate that ENMs with a tendency to agglomerate will have lower biological availability to C. reinhardtii and thus reduced toxic potential, while the ENMs with a tendency to dissolve, both the dissolved and particulate fractions would contribute to the bioavailability. Additionally, ENMs and their agglomerates are expected to be taken up by T. thermophyla if they are within the phagocytosable size range. Thus knowing the cell burden of ENMs that depend on both uptake and excretion would be necessary for the prediction of bioaccumulation and possible trophic transfer of ENMs. Indeed, exposure to increasing nanomolar concentrations of carboxylic groups polymer coated CdSe/ZnS quantum dots (QDs) resulted in a significant and concentration-dependent increase of the percentage of fluorescent protozoa (i.e., protozoa associated with QDs) determined by flow cytometry and fluorescence microscopy (Mortimer et al. 2014a). However, the proportion of T. thermophyla exhibiting QD fluorescence was lower after 24 h exposure compared to 2 h exposure, suggesting QD clearance after longer exposure time. For example, approximately 60% of cells exposed to QDs for 2 h cleared their food vacuoles in 20 h, while in the 24 h exposed population only 30% of the protozoa secreted QDs from their vacuoles (Mortimer et al. 2014a). To get further insights into the QDs uptake mechanisms, T. thermophyla was pretreated with cytochalasin B and colchicine inhibitors or their mixture and then exposed to QDs. Cytochalasin B is a phagocytosis inhibitor, while colchicine is an aclathrin-mediated endocytosis inhibitor. Treatment with these inhibitors reduced significantly the percentage of the fluorescent protozoa (Mortimer et al. 2014a). However, a combination of colchicine and cytochalasin B inhibited the food vacuole formation, while the protozoan cells were still associated with QDs and exhibited QD-specific fluorescence. These results suggest that QDs can associate with or enter T. thermophila by alternative uptake pathways to phagocytosis and clathrin-mediated endocytosis (Mortimer et al. 2014a). Clearance of the food vacuoles in QD-free medium was incomplete even after 20 h, which may render them bioavailable to organisms at higher trophic levels. Similarly to QDs, other metal containing nanoparticles such as Ag, Au, CuO-NPs and TiO2-NPs, accumulated in T. thermophyla by different uptake pathways (Mortimer et al. 2014b). The ENMs agglomerates are found in food vacuoles and the cytoplasm, but the accumulation varied according to the ENM concentration and exposure time. Indeed, the amount of accumulated ENMs was higher after 2 h of exposure than at 24 h, and at higher concentrations. Moreover, the studied ENMs induced increased intracellular ROS and lipid peroxidation in protozoa as revealed by flow cytometry and staining with CellROX® Green and BODIPY®581/591 C11. Nonetheless, no quantitative relationship between ENM uptake by T. thermophila and OS and damage was found (Mortimer et al. 2014b). Therefore, the contribution of the dissolved metal fraction from NPs and the release of Ag ions form the ENMs into food vacuoles have to be taken into account. The above observations are consistent with the limited literature demonstrating higher toxicity of the dissolved species to T. thermophyla as compared with Ag-NPs (Bondarenko et al. 2013; Burkart et al. 2015) and CuO-NPs (Bondarenko et al. 2013; Mortimer et al. 2010, 2011). Furthermore, in case of TiO2-NPs exposure the level of intracellular ROS generation in Tetrahymena pyriformis was dependent on the light-illumination conditions (Zou et al. 2013).
Most of the microalgae do not exhibit cellular mechanisms such as endocytosis, for the transport of ENPs (Moore 2006), thus the role of the cell wall is significant in the modulation of their interactions with ENMs. Indeed, a comparison of the wild type of C. reinhardtii and wall-less mutant revealed a cell wall-dependent association of QDs demonstrating the important role of the cell wall as a protective barrier (Slaveykova and Startchev 2009; Worms et al. 2012).
Among different biological responses used to evaluate the potential effects of ENMs on biota, the generation of the highly ROS, disturbing the pro- and antioxidant equilibrium and thus inducing OS is currently the best-accepted paradigm to assess and compare the toxicity of different ENMs in the environment (Burello and Worth 2011; von Moos and Slaveykova 2014). Although nonspecific for a given stressor, the OS paradigm provides insights into the toxic relevance of different ENMs and is a useful paradigm for their ranking and development of the structure–reactivity and dose–response relationships. The OS (and toxicity of ENMs, in general) is a result of a complex interplay of the specific features of the target organism (e.g., particle ingesting vs. non-ingesting), the specific characteristics of the ENMs (e.g., composition, size, surface coating and function, solubility) and environmental factors (e.g., pH, water hardness, salinity, presence of organic molecules and dissolved organic matter, ultraviolet radiation, etc.) (von Moos and Slaveykova 2014; von Moos et al. 2014). Here, the influence of the ambient medium composition and a combined exposure to ENMs and other environmental stressors such as ultraviolet radiation (UVR) illustrated below are shown exemplarily for the microalga C. reinhardtii exposed to CuO nanoparticles (CuO-NPs). CuO-NPs are widely-used biocides (Ingle et al. 2014; Shi et al. 2012), however, they are more toxic to non-targeted than to targeted species (Bondarenko et al. 2013; Ivask et al. 2014). The influence of the media composition on the potential of CuO-NPs to induce OS and damage was explored at 2 h and 24 h in 3-(N-morpholino) propanesulfonic acid (MOPS) Good’s buffer, standard testing OECD medium and Lake Geneva water (von Moos et al. 2015). Oxidative stress occurred in all tested media but after 24 h exposure in OECD medium the percentage of the stressed cells increased 50 times with respect to the non-exposed controls, while in MOPS and lake water this increase was only 5 and 3 times, respectively. The medium composition modified the CuO-NPs and thus (sub)-toxic effects altering their dissolution, surface charge and aggregation, as well as their potential to generate ROS appeared. Indeed, the dissolved fraction in the 10 mg L−1 CuO-NP suspension increased from about 5% in MOPS to about 80% in OECD medium and lake water 24 h after NP suspension (von Moos et al. 2015). This observation shows the importance of the medium composition in CuO-NPs dissolution and the significant contribution of the dissolved Cu to the OS response of the algae in OECD medium and lake water. Furthermore, OS occurred also after exposure to dissolved Cu only but the response was less important in OECD medium and lake water, suggesting that CuO-NPs exerted a particle-specific effect and/or modified the bioavailability of dissolved Cu, e.g., by complexation with dissolved organic matter present in lake water (von Moos et al. 2015).
In the environment, biota are exposed to a combination of interacting stressors. For example, the interplay between solar radiation and ENMs could affect the protists by: (i) altering the ENMs dissolution, aggregation and ROS generation; (ii) modifying the chemical speciation of the released metal ions; and (iii) affecting the vital cellular functions of protists. To explore the combined effect of two stressors – ENMs and UVR, C. reinhardtii was exposed to 800 μg L−1 CuO-NPs (concentration inducing 50% effect) and to simulated solar radiation with altered PAR/UVR ratio (Cheloni et al. 2016). Synergistic interactions between the two stressors with higher OS in combined than in individual treatments were found (Cheloni et al. 2016). The above examples illustrated that the potential of CuO-NPs to induce OS was also modulated by the test medium composition (von Moos et al. 2015), as well as the interactions with other varying environmental factors, including solar radiation (Cheloni and Slaveykova, 2013; Cheloni et al. 2016).
Overall, no ENPs uptake by C. reinhardtii was observed and no clear relationship between ENP concentration and OS response was found. By contrast, all studied nanoparticles (Ag, Au, CuO, TiO2 and QDs) accumulated in T. thermophyla by different uptake pathways. The ENPs aggregates were found in food vacuoles and in the cytoplasm, but the accumulation varied according to concentration and exposure time. The OS and damage in aquatic protists induced by metalbased NPs can be triggered directly, promoted by particle properties at the nanoscale, or indirectly by dissolved, toxic metal ions from NPs. Therefore, both the oxidative potential and NP dissolution have to be taken into consideration when evaluating the toxicity potential of ENMs.
Stress responses and photoprotective strategies of ciliates exposed to ultraviolet radiation
Solar radiation is a daily stress factor for almost any free-living organism in the world. The biologically active radiation reaching the Earth’s surface comprises not only PAR (400–700 nm) that stimulates live processes but includes also the potentially harmful ultraviolet wavelengths (280–400 nm). Generally, we expect negative implications of UVR on an organism and many studies indeed showed that these short wavelengths can easily pass cell membranes and cause severe direct damages to nucleic acids and proteins. In protists, after the impact of UVR, modified shapes, reduced movements and retarded growth rates are observed. Such direct effects may indirectly affect aquatic microbial food webs; for instance, when a predator dies off the former prey can rise. Other indirect effects under the exposure to UVR are the production of ROS and consequently OS may burden an organism. Though, UVR can also have positive effects on an organism, e.g., such as longer wavelengths of UV-A radiation (315–400 nm) as well as of PAR can induce photorepair mechanisms (Sinha and Häder 2002).
Almost nothing is known about the reactions and strategies of free-living protists how they cope with UVR as an environmental stress factor. Ciliated protists, in general, play a key role in the microbial food webs of oceans and lakes because they are one of the major consumers of phytoplankton and bacteria and hence an essential link to higher trophic levels (e.g., Azam et al. 1983; Sommer et al. 2012). Their abundance and diversity in freshwater plankton ranges between 2.0 and 130 ind. mL−1, comprising either a few or wide more than 100 morphospecies (e.g., Müller et al. 1991; Sonntag et al. 2006, 2011a; Van Wichelen et al. 2013; Wille et al. 1999). Moreover, recent phylogenetic investigations revealed a generally high genetic protist diversity in lakes (Kammerlander et al. 2015; Stoeck et al. 2014; Triadó-Margarit and Casamajor 2012).
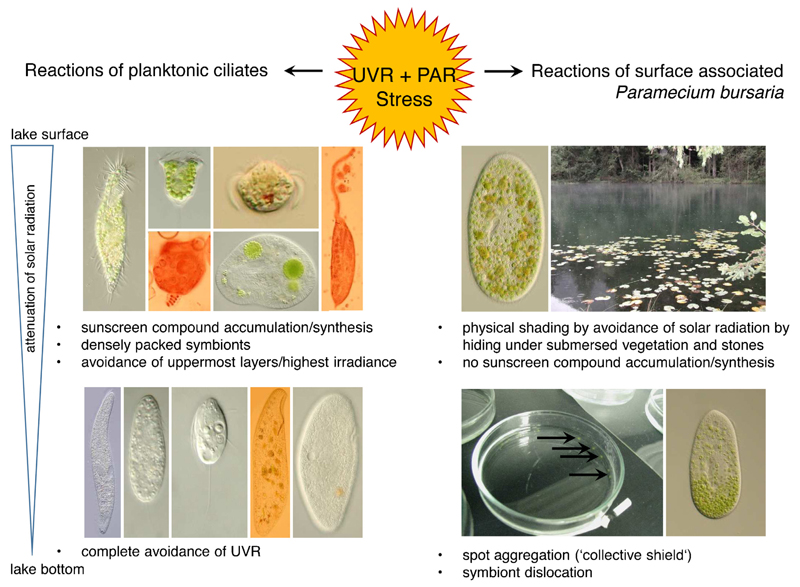
Taken into consideration the available studies so far, we see that effects of UVR on protists reveal species-specific reactions and strategies. Difficulties in studying natural protist communities from lakes arise because virtually no cultivated species are available in public culture collections to perform relevant experiments in the laboratory. This lack of, for example, limnetic planktonic species of the common genera Balanion, Rimostrombidium, Urotricha or Askenasia force scientists to cultivate the desired species in their laboratories often including multitudinous approaches to optimize growing conditions in terms of medium, food and temperature; alternatively, commercially available (model) species such as Paramecium or Tetrahymena are used in experiments. As these model ciliates derive from other habitats than the pelagic, different stress responses and photoprotective mechanisms can be expected from UVR experiments. How different the impact of UVR on varying ciliate species can be is elucidated in the following examples on Paramecium bursaria and investigations on free-living planktonic communities (Fig. 4).
Fig. 4.
Overview on the stress reactions and photoprotective strategies of planktonic and surface associated ciliated protists under UVR and PAR exposure. For details see text. The two pictures on the bottom on the right (petridish and ciliate with dislocated symbionts) were kindly provided by M. Summerer.
Paramecium bursaria has a mixotrophic lifestyle, i.e. the species lives in symbiosis with autotrophic green algae and also grazes phagotrophically on bacteria and other smaller protists. Paramecium bursaria is wide-spread and found in the littoral zone of lakes or in stagnant waters such as ponds where it commonly lives attached to surfaces such as submersed leaves and stones. This species is a suitable model ciliate because it can be relatively easily maintained in the laboratory, symbiont-free cell lines can be established, and many populations and strains from all over the world are available. To test for the stress responses of P. bursaria to UVR, the ciliates were exposed under an artificial UVR and PAR source in laboratory experiments. In contrast to their aposymbiotic (algal-free) counterparts, symbiont-bearing individuals of P. bursaria were more resistant to UVR and PAR, and also (photo-) oxidative stress was lower (Hörtnagl and Sommaruga 2007; Summerer et al. 2007, 2009). Apparently, the algal symbionts are somehow involved in the photoprotection of the species. In a second approach, two interesting phenomena emerged: under UVR and PAR individual ciliates accumulated to dense green spots that were even visible to the naked eye and formed a kind of ‘collective shield’ (Sommaruga and Sonntag 2009). When taking a closer look onto these aggregated individuals, the algal symbionts within the cells were dislocated to the posterior cell end. The assumption was that the individuals adjusted their rear end that was densely stuffed with algae against the irradiation source and that the ciliates’ nuclear material was effectively shaded from harmful UVR (Summerer et al. 2009). The fact that several algal layers significantly reduce the transmission of UVR at 320 nm can be calculated with an optical model developed by Garcia-Pichel (1994). Taken together, the algal symbionts obviously play a major role in the stress balance of these mixotrophic ciliates when they are exposed to UVR and PAR. Though, several questions remain unsolved as it is still unknown what triggers the rapid dislocation of the algae within the host under exposure to UVR and PAR. Besides, the phenomenon is reversible within minutes as soon as P. bursaria is not exposed to the irradiation source any more. Another question is how the individual ciliates manage their spot aggregation under the irradiation source. A photosensitive area in P. bursaria might be involved somehow (Nakaoka 1989).
Commonly, another stress factor is accelerated by UVR and PAR, which is (photo-)oxidative stress by inducing the formation of ROS. Photo-oxidative stress is defined as the production and accumulation of ROS beyond the capacity of an organism to quench them (Lesser 2006). All respiring cells and especially photosynthetically active cells such as mixotrophic ciliates are in principle susceptible to OS. Symbiotic algae produce singlet oxygen, superoxide and hydrogen peroxide. As hydrogen peroxide is uncharged it can easily diffuse across biological membranes, as is the case here from the algal symbiont into the host cell where again ROS are produced (Kawano et al. 2004). Interestingly, the photo-oxidative stress in symbiotic P. bursaria was lower in comparison to their aposymbiotic counterparts (Hörtnagl and Sommaruga 2007). Hörtnagl and Sommaruga (2007) further screened both symbiotic and aposymbiotic P. bursaria for their antioxidant defenses by measuring the activity of the enzymes catalase, superoxide dismutase and glutathione reductase. These antioxidants are known to quench the harmful effects of ROS. After exposure to UVR, ROS levels were highest in the aposymbiotic individuals although superoxide dismutase activity was increased. The catalase activity was strongly decreased in both symbiotic and aposymbiotic individuals and glutathione reductase was undetectable. Hörtnagl and Sommaruga (2007) proposed that not only the high UVR screening capacity of the symbionts were responsible for this result but also the activity of antioxidant enzymes originating from the algae. Overall, these results suggest that in this ciliate symbiosis, the presence of symbionts minimizes photo-oxidative stress in P. bursaria. The effective physical shading strategies of P. bursaria were suggested to be responsible not only for low photo-oxidative stress but also for an overall photoprotection: (i) by compact symbiont layers (consisting of dislocated algae within the ciliate cell) that significantly reduce the transition of UVR reaching the sensitive nuclear material and (ii) the accumulation into tight assemblages forming a kind of ‘collective shield’ in dense cultures (>500 ind. mL−1). Considering aggregation, this strategy might not be relevant for planktonic ciliates of oligo- or mesotrophic lakes as the natural density of a ciliate species is simply too low.
Living in the plankton of clear lakes evidently implies a permanent exposure to UVR without any possibility of physical shading. Moreover, in the catchment of remote habitats over the tree line, only scarce vegetation exists and consequently the input of dissolved chromophoric organic matter attenuating UVR is low and UVR transparency is high. As studies on P. bursaria showed, a mutualistic relationship with symbionts obviously provided an advantage for ciliates when exposed to UVR. Though, irrespective of a mixotrophic or heterotrophic lifestyle results from a ‘transplantation’ study where the ciliate assemblage of a less UV transparent lake was exposed in a highly UV transparent high mountain lake revealed that mortality under different UVR intensities was species-specific (Sonntag et al. 2011b). Consequently, not only single cells are affected under UVR exposure but also whole food webs (Mostajir et al. 1999; Sommaruga et al. 1999; Wickham and Carstens 1998). Following the exposure to (elevated) UVR, generally lower division and growth rates, retarded swimming or cell death have been observed (e.g., Giese et al. 1963; Sanders et al. 2005).
Life under daily UVR exposure as it is the case for planktonic organisms in lakes or oceans certainly leads to enormous stress and demands for effective photoprotection and -repair strategies. For example, phyto- and zooplankton in clear alpine lakes perform vertical downward migrations around noontime (e.g., Tilzer 1973; Alonso et al. 2004). Interestingly, this kind of UV avoidance strategy was not observed for the (only) three ciliate species colonizing such an extreme environment in summer (Sonntag et al. 2011a). The UV sensitive Balanion planctonicum persisted in the deepest area near the sediment whereas the algal-bearing mixotrophic Askenasia chlorelligera thrived all over the water column and was obviously well protected by specific sunscreen compounds (mycosporine-like amino acids; MAAs) synthesized by its symbionts. Besides, in an A. chlorelligera population from a less UV transparent lake, none of these photoprotective compounds were detected (Summerer et al. 2008). MAAs, in general, are water-soluble, colorless sunscreens that absorb UVR between 309 and 362 nm (Carreto and Carignan 2011). Biochemical pathways involved in MAAs synthesis have been recently identified but are unknown for ciliates (Balskus and Walsh 2010; Osborn et al. 2015; Singh et al. 2010). The MAAs detected in several algal-bearing freshwater ciliates originated from the synthesis by the symbionts but the uptake from algal food was also demonstrated in a heterotrophic species (Sonntag et al. 2007; Summerer et al. 2008; Sonntag unpubl.). Sunscreen compounds (MAAs and mycosporine–glutaminol–glucoside) were neither synthesized by the algal symbionts of P. bursaria nor accumulated in aposymbiotic individuals from food (Pérez et al. 2006; Summerer et al. 2009). Preliminary experiments with B. planctonicum also showed that this heterotrophic species was not able to accumulate MAAs from offered MAA-rich algal food (Sonntag and Pöll unpubl.).
Other efficient mechanisms to cope with stress caused by UVR are DNA-repair strategies including photoenzymatic repair (PER) and nucleotide excision repair (‘dark repair’) that can be stimulated by UV-A and PAR (e.g., Karentz et al. 1991; Mitchell and Karentz 1993). Both strategies are widespread and found in bacteria, phyto- and zooplankton and in ciliated protists (Buma et al. 1997; Joux et al. 1999; Malloy et al. 1997; Sanders et al. 2005). For example, two ciliates from an oligotrophic lake responded differently to UVR exposure: Glaucoma sp. was able to recover under photoreactive radiation whereas Cyclidium sp. was not. Moreover, the efficiency of PER in Glaucoma sp. was temperature-dependent and significantly reduced at low temperatures (Sanders et al. 2005). Yet, only some few ciliate species were tested for the presence of PER. Future studies will have to elucidate which DNA-repair strategies are generally present in (planktonic) ciliates.
Concluding remarks
From the foregoing, we can highlight some important general points. Oxidative stress (from the production of excessive ROS) seems to be a general cellular response to manifold environmental stressors (UVR or inorganic nanoparticles, among others) in very diverse protists. Therefore, an increase in ROS can be considered as a key process found in different types of stress. A general updated view from studies related to “environmental stress and protists” leads us to several conclusions: (i) the scarcity of stress-related studies, (ii) the absence of molecular approaches and genetic analyses and (iii) the necessity to increase physiological analyses. Still many open questions on how ciliates cope with UVR as an environmental stressor remain and future investigations from single species up to food webs are needed. Model protists such as Chlamydomonas reinhardtii (among photosynthetic protists), Tetrahymena thermophila or Paramecium species (among free living phagotrophic protists) are up to now the most manageable and well known protists (with already sequenced genomes). Although they are very good candidates to carry out analyses on environmental stress-protist interactions, studies have to consider other common protist species that are relevant for example in lake food web studies (see also Weisse et al. 2016). Overall, we would like to encourage scientists to use protists as eukaryotic model organisms more often for investigating the cellular responses to environmental stress at any level.
Acknowledgements
The authors wish thank the president of the congress organizing committee Dr. Aurelio Serrano for giving us the opportunity to celebrate this symposium. The research carried out by VS was supported by Swiss National Science Foundation grants 406440-131280 and 200021-134627, as well as the Sciex grant 11.270. The contribution of Giulia Cheloni, Monika Mortimer, Nadia von Moos and Isabelle Worms, is warmly acknowledge by VS. Research carried out by BS was supported by the Austrian Science Fund (FWF), grants P21013-B03, I2238-B25, P28333-B25 and P16559-B06 (PI Ruben Sommaruga). BS thanks Barbara Kammerlander for constructive input on the ‘stress response and photoprotection of ciliates exposed to UVR’ chapter.
Abbreviations
- ENM
engineered nanomaterial
- ENPs
engineerednanoparticles
- miRNA
microRNA
- MOPS
3-(N-morpholino) propanesul-fonic acid
- NPs
nanoparticles
- OS
oxidative stress
- PAR
photosyntheticallyactive radiation
- PER
photoenzymatic repair
- QDs
quantum dots
- ROS
reactive oxygen species
- UVR
ultraviolet radiation
References
- Alonso C, Rocco V, Barriga JP, Battini MA, Zagarese H. Surface avoidance by freshwater zooplankton: field evidence on the role of ultraviolet radiation. Limnol Oceanogr. 2004;49:225–232. [Google Scholar]
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Azam FT, Fenchel T, Field JG, Gray JS, Meyer-Reil LA, Thingstad F. The ecological role of water-column microbes in the sea. Mar Ecol Prog Ser. 1983;10:257–263. [Google Scholar]
- Balskus EP, Walsh CT. The genetic and molecular basis for sunscreen biosynthesis in cyanobacteria. Science. 2010;329:1653–1656. doi: 10.1126/science.1193637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishak YK, Payahoo L, Osatdrahimi A, Nourazarian A. Mechanisms of cadmium carcinogenicityin the gastrointestinal tract. Asian Pac J Cancer Prev. 2015;16:9–21. doi: 10.7314/apjcp.2015.16.1.9. [DOI] [PubMed] [Google Scholar]
- Bondarenko O, Juganson K, Ivask A, Kasemets K, Mortimer M, Kahru A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Arch Toxicol. 2013;87:1181–1200. doi: 10.1007/s00204-013-1079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buma AGJ, Engelen AH, Gieskes WWC. Wavelength-dependent induction of thymine-dimers and growth rate reduction in the marine diatom Cyclotella sp. exposed to ultraviolet radiation. Mar Ecol Progr Ser. 1997;153:91–97. [Google Scholar]
- Burello E, Worth AP. A theoretical framework for predicting the oxidative stress potential of oxide nanoparticles. Nanotoxicology. 2011;5:228–235. doi: 10.3109/17435390.2010.502980. [DOI] [PubMed] [Google Scholar]
- Burkart C, Tümpling W, Berendonk T, Jungmann D. Nanoparticles in wastewater treatment plants: a novel acute toxicity test for ciliates and its implementation in risk assessment. Environ Sci Pollut Res. 2015;22:7485–7494. doi: 10.1007/s11356-014-4057-3. [DOI] [PubMed] [Google Scholar]
- Carreto JI, Carignan MO. Mycosporine-like amino acids: relevant secondary metabolites. Chemical and ecological aspects. Mar Drugs. 2011;9:387–446. doi: 10.3390/md9030387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheloni G, Slaveykova VI. Optimization of the C11-BODIPY581/591 dye for the determination of lipid oxidation in Chlamydomonas reinhardtii by flow cytometry. Cytometry A. 2013;83A:952–961. doi: 10.1002/cyto.a.22338. [DOI] [PubMed] [Google Scholar]
- Cheloni G, Marti E, Slaveykova VI. Interactive effects of copper oxide nanoparticles and light to green alga Chlamydomonas reinhardtii. Aquat Toxicol. 2016;170:120–128. doi: 10.1016/j.aquatox.2015.11.018. [DOI] [PubMed] [Google Scholar]
- Díaz S, Martin-Gonzalez A, Cubas LL, Ortega R, Amaro F, Rodriguez-Martin D, Gutierrez JC. High resistance of Tetrahymena thermophila to paraquat: mitochondrial alterations, oxidative stress and antioxidant genes expression. Chemosphere. 2016;144:909–917. doi: 10.1016/j.chemosphere.2015.09.010. [DOI] [PubMed] [Google Scholar]
- Ding Y-F, Zhu C. The role of microRNAs in copper and cadmium homeostasis. Biochem Biophys Res Commun. 2009;386:6–10. doi: 10.1016/j.bbrc.2009.05.137. [DOI] [PubMed] [Google Scholar]
- Dondero F, Cavaletto M, Ghezzi AR, La Terza A, Banni M, Viarengo A. Biochemical characterization and quantitative gene expression analysis of the multi-stress inducible metallothionein from Tetrahymena thermophila. Protist. 2004;155:157–168. doi: 10.1078/143446104774199565. [DOI] [PubMed] [Google Scholar]
- Ferro D, Bakiu R, De Pittà C, Boldrin F, Cattalini F, Pucciarelli S, Miceli C, Santovito G. Cu, Zn superoxide dismutases from Tetrahymena thermophila: molecular evolution and gene expression of the first line of antioxidant defenses. Protist. 2015;166:131–145. doi: 10.1016/j.protis.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Schinozaki K. Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol. 2006;9:436–442. doi: 10.1016/j.pbi.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Bravo-San Pedro JM, Kepp O, Kroemer G. Regulated cell death and adaptive stress responses. Cell Mol Life Sci. 2016 doi: 10.1007/s00018-016-2209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Pichel F. A model for internal self-shading in plank-tonic organisms and its implications for the usefulness of ultraviolet sunscreens. Limnol Oceanogr. 1994;39:1704–1717. [Google Scholar]
- Giese AC, McCaw B, Cornell R. Retardation of division of three ciliates by intermittent and continuous ultraviolet radiations at different temperatures. J Gen Physiol. 1963;46:1095–1108. doi: 10.1085/jgp.46.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo S, Llaneza V, Pulido-Reyes G, Fernández-Piñas F, Bonzongo JC, Leganes F, Rosal R, Garcia-Calvo E, RodeaPalomares I. A colloidal singularity reveals the crucial role of colloidal stability for nanomaterials in vitro toxicity testing: nZVI-Microalgae colloidal system as a case study. PLOS ONE. 2014;9(10):e109645. doi: 10.1371/journal.pone.0109645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez JC, Martin-Gonzalez A. Ciliate encystment–excystment cycle: a response to environmental stress. In: Gutierrez JC, editor. Microbial Development Under Environmental Stress. Research Signpost; India: 2002. pp. 29–49. [Google Scholar]
- Gutierrez JC, Amaro F, Díaz S, de Francisco P, Cubas LL, Martín-González A. Ciliate metallothioneins: unique microbial eukaryotic heavy-metal-binder molecules. J Biol Inorg Chem. 2011;16:1025–1034. doi: 10.1007/s00775-011-0820-9. [DOI] [PubMed] [Google Scholar]
- Gutierrez JC, Callejas S, Borniquel S, Benitez L, Martin-Gonzalez A. Ciliate cryptobiosis: a microbial strategy against environmental starvation. Int Microbiol. 2001;4:151–157. doi: 10.1007/s10123-001-0030-3. [DOI] [PubMed] [Google Scholar]
- Gutierrez JC, Martin-Gonzalez A, Díaz S, Amaro F, Ortega R, Gallego A, de Lucas MP. Ciliates as cellular tools to study the eukaryotic cell-heavy metal interactions. In: Brown SE, Welton WC, editors. Heavy Metal Pollution. Nova Science Publishers Inc; USA: 2008. pp. 1–44. [Google Scholar]
- Hoertnagl PH, Sommaruga R. Photo-oxidative stress in symbiotic and aposymbiotic strains of the ciliate Paramecium bursaria Photochem. Photobiol Sci. 2007;6:842–847. doi: 10.1039/b703119j. [DOI] [PubMed] [Google Scholar]
- Hudder A, Novak RF. miRNAs: effectors of environmental influences on gene expression and disease. Toxicol Sci. 2008;103:228–240. doi: 10.1093/toxsci/kfn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle A, Duran N, Rai M. Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: a review. Appl Microbiol Biotechnol. 2014;98:1001–1009. doi: 10.1007/s00253-013-5422-8. [DOI] [PubMed] [Google Scholar]
- Ivask A, Juganson K, Bondarenko O, Mortimer M, Aruoja V, Kasemets K, Blinova I, Heinlaan M, Slaveykova V, Kahru A. Mechanisms of toxic action of Ag, ZnO and CuO nanoparticles to selected ecotoxicological test organisms and mammalian cells in vitro: a comparative review. Nanotoxicology. 2014;8(Suppl. 1):57–71. doi: 10.3109/17435390.2013.855831. [DOI] [PubMed] [Google Scholar]
- Josson S, Sung S-Y, Lao K, Chung LWK, Johnstone PAS. Radiation modulation of microRNA in prostate cancer cell lines. Prostate. 2008;68:1599–1606. doi: 10.1002/pros.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joux E, Jeffrey WH, Lebaron P, Mitchell D. Marine bacterial isolates display diverse responses to UV-B radiation. Appl Environ Microbiol. 1999;65:3820–3827. doi: 10.1128/aem.65.9.3820-3827.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammerlander B, Breiner H-W, Filker S, Sommaruga R, Sonntag B, Stoeck T. High diversity of protistan plankton communities in remote high mountain lakes in the European Alps and the Himalaya mountains. FEMS Microbiol Ecol. 2015;91 doi: 10.1093/femsec/fiv010. fiv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karentz D, Cleaver JE, Mitchell DL. DNA damage in the Antarctic. Nature. 1991;350:28. [Google Scholar]
- Kawano T, Kadono T, Kosaka T, Hosoya H. Green paramecia as an evolutionary winner of oxidative symbiosis: a hypothesis and supportive data. Z Naturforsch. 2004;59:538–542. doi: 10.1515/znc-2004-7-816. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kim SJ, Lee JS, Lee YM. Acute effects of heavy metals on the expression of glutathione-related antioxidant genes in the marine ciliate Euplotes crassus. Mar Pollut Bull. 2014;85:455–462. doi: 10.1016/j.marpolbul.2014.05.025. [DOI] [PubMed] [Google Scholar]
- Lesser MP. Oxidative stress in marine environment: biochemistry and physiological ecology. Annu Rev Physiol. 2006;68:278–583. doi: 10.1146/annurev.physiol.68.040104.110001. [DOI] [PubMed] [Google Scholar]
- Liu J, Feng L, Li J, He Z. Genetic and epigenetic control of plant heat responses. Front Plant Sci. 2015;6:267. doi: 10.3389/fpls.2015.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy KD, Holman MA, Mitchell D, Dietrich HW. Solar UV-B induced DNA damage and photoenzymatic DNA repair in Antarctic zooplankton. Proc Natl Acad Sci U S A. 1997;94:1258–1263. doi: 10.1073/pnas.94.4.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Gonzalez A, Borniquel S, Díaz S, Ortega R, Gutierrez JC. Ultrastructural alterations in ciliated protozoa under heavy metal exposure. Cell Biol Int. 2005;29:119–126. doi: 10.1016/j.cellbi.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Mendoza-Cózatl D, Loza-Tavera H, Hernández-Navarro A, Moreno-Sanchez R. Sulfur assimilation and glutathione metabolism under cadmium stress in yeast, protists and plants. FEMS Microbiol Rev. 2005;29:653–671. doi: 10.1016/j.femsre.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Meyer P. Epigenetic variation and environmental change. J Exp Bot. 2015;66:3541–3548. doi: 10.1093/jxb/eru502. [DOI] [PubMed] [Google Scholar]
- Mitchell DL, Karentz D. The induction and repair of DNA photodamage in the environment. In: Young AR, Björn LO, Moan J, Nultsch W, editors. Environmental UV Photobiology. Plenum Press; New York: 1993. pp. 345–377. [Google Scholar]
- Moore MN. Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ Int. 2006;32:967–976. doi: 10.1016/j.envint.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Mortimer M, Kasemets K, Kahru A. Toxicity of ZnO and CuO nanoparticles to ciliated protozoa Tetrahymena thermophila. Toxicology. 2010;269:182–189. doi: 10.1016/j.tox.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Mortimer M, Kasemets K, Vodovnik M, Marinšek-Logar R, Kahru A. Exposure to CuO nanoparticles changes the fatty acid composition of protozoa Tetrahymena thermophila. Environ Sci Technol. 2011;45:6617–6624. doi: 10.1021/es201524q. [DOI] [PubMed] [Google Scholar]
- Mortimer M, Kahru A, Slaveykova VI. Uptake, localization and clearance of quantum dots in ciliated protozoa Tetrahymena thermophila. Environ Pollut. 2014a;190:58–64. doi: 10.1016/j.envpol.2014.03.021. [DOI] [PubMed] [Google Scholar]
- Mortimer M, Gogos A, Bartolome N, Kahru A, Bucheli TD, Slaveykova VI. Potential of hyperspectral imaging microscopy for semi-quantitative analysis of nanoparticle uptake by protozoa. Environ Sci Technol. 2014b;48:8760–8767. doi: 10.1021/es500898j. [DOI] [PubMed] [Google Scholar]
- Mostajir B, Demers S, de Mora S, Belzile C, Chanut J-P, Gosselin M, Roy S, Villegas PZ, Fauchot J, Bouchard J, Bird D, et al. Experimental test of the effect of ultraviolet-B radiation in a planktonic community. Limnol Oceanogr. 1999;44:586–596. [Google Scholar]
- Müller H, Schöne A, Pinto-Coelho RM, Schweizer A, Weisse T. Seasonal succession of ciliates in Lake Constance. Microb Ecol. 1991;21:119–138. doi: 10.1007/BF02539148. [DOI] [PubMed] [Google Scholar]
- Nakagami H, Pitzschke A, Hirt H. Emerging MAP kinase pathways in plant stress signaling. Trends Plant Sci. 2005;10:339–346. doi: 10.1016/j.tplants.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Nakaoka Y. Localization of photosensitivity in Paramecium bursaria. J Comp Physiol A. 1989;165:637–641. [Google Scholar]
- Navarro E, Wagner B, Odzak N, Sigg L, Behra R. Effects of differently coated silver nanoparticles on the photosynthesis of Chlamydomonas reinhardtii. Environ Sci Technol. 2015:8041–8047. doi: 10.1021/acs.est.5b01089. [DOI] [PubMed] [Google Scholar]
- Nolte-’t Hoen ENM, Van Rooij E, Bushell M, Zhang C-Y, Dashwood RH, James WPT, Harris C, Baltimore D. The role of microRNA in nutritional control. J Intern Med. 2015 doi: 10.1111/joim.12372. [DOI] [PubMed] [Google Scholar]
- Nowack B, Ranville JF, Diamond S, Gallego-Urrea JA, Met-calfe C, Rose J, Horne N, Koelmans AA, Klaine SJ. Potential scenarios for nanomaterial release and subsequent alteration in the environment. Environ Toxicol Chem. 2012;31:50–59. doi: 10.1002/etc.726. [DOI] [PubMed] [Google Scholar]
- OECD. Database on Research into the Safety of Manufactured Nanomaterials. 2015 www.oecd.org/env/nanosafety/database.
- Osborn AR, Almabruk KH, Holzwarth G, Asamizu S, LaDu J, Kean KM, Karplus PA, Tanguay RL, Bakalinsky AT, Mahmud T. De novo synthesis of a sunscreen compound in vertebrates. eLife. 2015;4:e05919. doi: 10.7554/eLife.05919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peijnenburg WJGM, Baalousha M, Chen J, Chaudry Q, Von der Kammer F, Kuhlbusch TAJ, Lead J, Nickel C, Quik JTK, Renker M, Wang Z, et al. A review of the properties and processes determining the fate of engineered nanomaterials in the aquatic environment. Crit Rev Environ Sci Technol. 2015;45:2084–2134. [Google Scholar]
- Pérez P, Libkind D, Diéguez MdC, Summerer M, Sonntag B, Sommaruga R, van Broock M, Zagarese HE. Mycosporines from freshwater yeasts: a trophic cul-de-sac? Photochem Photobiol Sci. 2006;5:25–30. doi: 10.1039/b509764a. [DOI] [PubMed] [Google Scholar]
- Prado R, Rioboo C, Herrero C, Suarez-Bregua P, Cid A. Flow cytometry analysis to evaluate physiological alterations in herbicide-exposed Chlamydomonas moewusii cells. Ecotoxicology. 2012;21:409–420. doi: 10.1007/s10646-011-0801-3. [DOI] [PubMed] [Google Scholar]
- Rico D, Martin-Gonzalez A, Díaz S, de Lucas P, Gutierrez JC. Heavy metals generate reactive oxygen species in terrestrial and aquatic ciliated protozoa. Comp Biochem Physiol Part C. 2009;149:90–96. doi: 10.1016/j.cbpc.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Ruis H. Yeast stress responses: achievements, goals and a look beyond yeast. In: Hohmann S, Mager WH, editors. Yeast Stress Responses. R.G. Landes Company; California: 1997. pp. 231–247. [Google Scholar]
- Sanders RW, Macaluso AL, Sardina TJ, Mitchell DL. Photoreactivation in two freshwater ciliates: differential responses to variations in UV-B flux and temperature. Aquat Microb Ecol. 2005;40:283–292. [Google Scholar]
- Shi M, Kwon HS, Peng Z, Elder A, Yang H. Effects of surface chemistry on the generation of reactive oxygen species by copper nanoparticles. ACS Nano. 2012;6:2157–2164. doi: 10.1021/nn300445d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzman JA, Ruby EG. Stress as a normal cue in the symbiotic environment. Trends Microbiol. 2016 doi: 10.1016/j.tim.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SP, Klisch M, Sinha RP, Haeder DP. Genome mining of mycosporine-like amino acids (MAA) synthesizing and non-synthesizing cyanobacteria: a bioinformatics study. Genomics. 2010;95:120–128. doi: 10.1016/j.ygeno.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Sinha RP, Häder D-P. UV-induced DNA damage and repair: a review. Photochem Photobiol Sci. 2002;1:225–236. doi: 10.1039/b201230h. [DOI] [PubMed] [Google Scholar]
- Slaveykova VI, Startchev K. Effect of natural organic matter and green microalga on carboxyl-polyethylene glycol coated CdSe/ZnS quantum dots stability and transformations under freshwater conditions. Environ Pollut. 2009;157:3445–3450. doi: 10.1016/j.envpol.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Sommaruga R, Sattler B, Oberleiter A, Wille A, Wögrath-Sommaruga S, Psenner R, Felip M, Camarero L, Pina S, Gironés R, Catalán J. An in situ enclosure experiment to test the solar UVB impact on plankton in a high-altitude mountain lake. II. Effects on the microbial food web. J Plankton Res. 1999;21:859–876. [Google Scholar]
- Sommaruga R, Sonntag B. Photobiological aspects of the mutualistic association between Paramecium bursaria and Chlorella. In: Fujishima M, editor. Endosymbionts of Paramecium Microbiology Monographs 12. Springer Verlag; Berlin, Germany: 2009. pp. 111–130. [Google Scholar]
- Sommer U, Adrian R, De Senerpont Domis L, Elser JJ, Gaedke U, Ibelings B, Jeppesen E, Lurling M, Molinero JC, Mooij WM, van Donk E, et al. Beyond the Plankton Ecology Group (PEG) model: mechanisms driving plankton succession. Annu Rev Ecol Evol Syst. 2012;43:429–448. [Google Scholar]
- Sonntag B, Posch T, Klammer S, Teubner K, Psenner R. Phagotrophic ciliates and flagellates in an oligotrophic deep alpine lake: contrasting variability with seasons and depths. Aquat Microb Ecol. 2006;43:193–207. [Google Scholar]
- Sonntag B, Summerer M, Sommaruga R. Sources of mycosporine-like amino acids in planktonic Chlorella-bearing ciliates (Ciliophora) Freshw Biol. 2007;52:1476–1485. [Google Scholar]
- Sonntag B, Summerer M, Sommaruga R. Factors involved in the distribution pattern of ciliates in the water column of a transparent alpine lake. J Plankton Res. 2011a;33:541–546. doi: 10.1093/plankt/fbq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag B, Summerer M, Sommaruga R. Are freshwater mixotrophic ciliates less sensitive to solar UV radiation than heterotrophic ones? J Euk Microbiol. 2011b;58:196–202. doi: 10.1111/j.1550-7408.2011.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeck T, Breiner H-W, Filker S, Ostermaier V, Kammerlander B, Sonntag B. A morpho-genetic survey on ciliate plankton from a mountain lake pinpoints the necessity of lineage-specific barcode markers in microbial ecology. Environ Microbiol. 2014;16:430–444. doi: 10.1111/1462-2920.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerer M, Sonntag B, Sommaruga R. An experimental test of the symbiosis specificity between the ciliate Paramecium bursaria and strains of the unicellular green alga Chlorella. Environ Microbiol. 2007;9:2117–2122. doi: 10.1111/j.1462-2920.2007.01322.x. [DOI] [PubMed] [Google Scholar]
- Summerer M, Sonntag B, Sommaruga R. Host-symbiont specificity of freshwater endosymbiotic Chlorella (Trebouxiophyceae, Chlorophyta) J Phycol. 2008;44:77–87. doi: 10.1111/j.1529-8817.2007.00455.x. [DOI] [PubMed] [Google Scholar]
- Summerer M, Sonntag B, Hoertnagl P, Sommaruga R. Symbiotic ciliates receive protection against UV damage from their algae: a test with Paramecium bursaria and Chlorella. Protist. 2009;160:233–243. doi: 10.1016/j.protis.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Swiecilo A. Cross-stress resistance in Saccharomyces cerevisiae yeast-new insight into an old phenomenon. Cell Stress Chaperones. 2016;21:187–200. doi: 10.1007/s12192-016-0667-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilzer MM. Diurnal periodicityin the phytoplankton assemblage of a high mountain lake. Limnol Oceanogr. 1973;18:15–30. [Google Scholar]
- Triadó-Margarit X, Casamayor EO. Genetic diversity of planktonic eukaryotes in high mountain lakes (Central Pyrenees, Spain) Environ Microbiol. 2012;14:2445–2456. doi: 10.1111/j.1462-2920.2012.02797.x. [DOI] [PubMed] [Google Scholar]
- Trielli F, Chessa MG, Amaroli A, Ognibene M, Delmonte Corrado MU. Effects of organophosphate compounds on a soil protist, Colpoda inflata (Ciliophora, Colpodidae) Chemosphere. 2006;65:1731–1737. doi: 10.1016/j.chemosphere.2006.04.081. [DOI] [PubMed] [Google Scholar]
- Van Wichelen J, Johansson LS, Vanormelingen P, Declerck SAJ, Lauridsen TL, De Meester L, Jeppesen E, Vyverman W. Planktonic ciliate community structure in shallow lakes of lowland Western Europe. Eur J Protistol. 2013;49:538–551. doi: 10.1016/j.ejop.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Von Moos N, Bowen P, Slaveykova VI. Bioavailability of inorganic nanoparticles to planktonic bacteria and aquatic microalgae in freshwater. Environ Sci: Nano 1. 2014:214–232. [Google Scholar]
- Von Moos N, Maillard L, Slaveykova VI. Dynamics of sub-lethal effects of nano-CuO on the microalga Chlamydomonas reinhardtii during short-term exposure. Aquat Toxicol. 2015;161:267–275. doi: 10.1016/j.aquatox.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Von Moos N, Slaveykova VI. Oxidative stress induced by inorganic nanoparticles in bacteria and aquatic microalgae – state of the art and knowledge gaps. Nanotoxicology. 2014;8:605–630. doi: 10.3109/17435390.2013.809810. [DOI] [PubMed] [Google Scholar]
- Weisse T, Anderson R, Arndt H, Calbet A, Hansen PJ, Montagnes DJS. Functional ecology of aquatic phagotrophic protists – Concepts, limitations, and perspectives. Eur J Protistol. 2016 doi: 10.1016/j.ejop.2016.03.003. this issue. [DOI] [PubMed] [Google Scholar]
- Wickham S, Carstens M. Effects of ultraviolet-B radiation on two arctic microbial food webs. Aquat Microb Ecol. 1998;16:163–171. [Google Scholar]
- Wille A, Sonntag B, Sattler B, Psenner R. Abundance, biomass and size structure of the microbial assemblage in the high mountain lake Gossenköllesee (Tyrol, Austria) during the ice-free period. J Limnol. 1999;58:117–126. [Google Scholar]
- Worms IAM, Boltzman J, Garcia M, Slaveykova VI. Cell-wall-dependent effect of carboxyl-CdSe/ZnS quantum dots on lead and copper availability to green microalgae. Environ Pollut. 2012;167:27–33. doi: 10.1016/j.envpol.2012.03.030. [DOI] [PubMed] [Google Scholar]
- Zou X-Y, Xu B, Yu C-P, Zhan H-W. Imbalance between oxidative and antioxidative systems: toward an understanding of visible light-induced titanium dioxide nanoparticles toxicity. Chemosphere. 2013;93:2451–2457. doi: 10.1016/j.chemosphere.2013.08.076. [DOI] [PubMed] [Google Scholar]