ABSTRACT
Aim: The aim of this study was to investigate whether dexmedetomidine – administered before ischemia – has protective effects against lower extremity ischemia reperfusion injury that induced by clamping and subsequent declamping of infra-renal abdominal aorta in streptozotocin-induced diabetic rats.
Material and Methods: After obtaining ethical committee approval, four study groups each containing six rats were created (Control (Group C), diabetes-control (Group DM-C), diabetes I/R (Group DM-I/R), and diabetes-I/R-dexmedetomidine (Group DM-I/R-D). In diabetes groups, single-dose (55 mg/kg) streptozotocin was administered intraperitoneally. Rats with a blood glucose level above 250 mg/dl at the 72nd hour were accepted as diabetic. At the end of four weeks, laparotomy was performed in all rats. Nothing else was done in Group C and DM-C. In Group DM-I/R, ischemia reperfusion was produced via two-hour periods of clamping and subsequent declamping of infra-renal abdominal aorta. In Group DM-I/R-D, 100 μg/kg dexmedetomidine was administered intraperitoneally 30 minutes before ischemia period. At the end of reperfusion, period biochemical and histopathological evaluation of renal tissue specimen were performed.
Results: Thiobarbituric acid reactive substance (TBARS), Superoxide dismutase (SOD), Nitric oxide synthase (NOS), Catalase (CAT) and Glutathion S transferase (GST) levels were found significantly higher in Group DM-I/R when compared with Group C and Group DM-C. In the dexmedetomidine-treated group, TBARS, NOS, CAT, and GST levels were significantly lower than those measured in the Group D-I/R. In histopathological evaluation, glomerular vacuolization (GV), tubular dilatation (TD), vascular vacuolization and hypertrophy (VVH), tubular cell degeneration and necrosis (TCDN), tubular hyaline cylinder (THC), leucocyte infiltration (LI), and tubular cell spillage (TCS) in Group DM-I/R were significantly increased when compared with the control group. Also, GV, VVH, and THC levels in the dexmedetomidine-treated group (Group DM-I/R-D) were found significantly decreased when compared with the Group DM-I/R.
Conclusion: We found that dexmedetomidine − 100 μg/kg intraperitoneally – administered 30 minutes before ischemia in diabetic rats ameliorates lipid peroxidation, oxidative stress, and I-R-related renal injury. We suggest that dexmedetomidine administration in diabetic rats before I/R has renoprotective effects.
KEYWORDS: Ischemia reperfusion, dexmedetomidine, renal, diabetes, TBARS, SOD, NOS, histopathology
Introduction
The sixth edition of Diabetes Atlas by the International Diabetes Fedaration (UDF) reported 385 million new diagnoses of diabetes mellitus in 2013, and additionally it is estimated that 592 million new diagnoses of diabetes mellitus will be determined in 2035 [1]. Besides important morbidity and mortality rates, diabetes is related to increased financial burden for both patients and governments [2]. In diabetic populations, important complications including hypertension, ischemic heart disease, nephropathy, and autonomic neuropathy are commonly seen, and physicians inevitably encounter these risks during surgical- and anesthesia-related procedures. Also, diabetic patients are at high risk of microvascular, cardiovascular complications and early mortality. Owing to these risk factors, anesthesia care in this patient group has relatively high burden [3–5]. Additionally, more careful perioperative care is needed in diabetic patients [6,7].
Ischemic injury occurs when the blood supply to an area of tissue is cut off. The incidence of ischemic injury is vast: myocardial infarction; stroke; and other thrombotic events. Ischemic injury also occurs during surgery when blood vessels are cross-clamped, and in organs for transplant.
Infra-renal abdominal aorta and lower extremity arteries clamping during surgery result in ischemia of distal body parts. Unclamping of these vascular structures after clamping result in reperfusion injury of local and distant organs/tissues [8].
Perioperative acute kidney injury (AKI) is a consequence of sudden renal function deterioration during major cardiothoracic, vascular, and transplant surgery. AKI is related to increased mortality and elongated hospital stay at a ratio of 60%. Mortality rates are 25 times higher following heart valve surgery. Additionally, completely recovered patients with an AKI history are still at high risk of long-term mortality. Ischemia reperfusion (I/R) injury is the leading factor for perioperative AKI. Following partial or total deprivation of renal blood flow, a restored and rigorous blood flow leads to post-ischemic renal parenchyma injury characterized by vascular, tubular and inflammatory degeneration [9].
Suspected mechanisms underlying renoprotective effects of dexmedetomidine (is an agonist of α2-adrenergic receptors in certain parts of the brain) include decreased presynaptic and peripheral – increased in relation with stress – noradrenaline release. Following dexmedetomidine administration, ameliorations in glomerular filtration and renal blood flow have been shown in previous studies. Dexmedetomidine – administered during surgery – decreases plasma catecholamine levels, increases urinary output, and secures hemodynamic stability [10,11]. In animal studies, renoprotective effects of α₂-adreno-receptor agonists have been shown [9,12–15].
In this study, we aimed to investigate whether dexmedetomidine has protective effects against lower extremity ischemia reperfusion injury induced by clamping and subsequent unclamping of infra-renal abdominal aorta in streptozotocin-induced diabetic rats.
Material and methods
After obtaining ethical committee approval, 24 Wistar albino rats – 200–270 g – were randomly divided into four groups: Group C; Group DM-C; Group DM-I/R; and Group DM-I/R-D. Diabetes was induced using Streptozotocin (Sigma Chemical, St Louis. MO, USA) at a single dose of 55 mg/kg in citrate buffer (0.1 Molar, pH 4.5). Rats with a blood glucose level – determined (GlucoDr Super Sensor, Allmedicus, Korea) from blood samples drawn via tail vein – above 250 mg/dl is accepted as diabetic. No additional intervention was done in Group C and Group DM-C. After a four-week follow-up period [16], laparotomy was performed in all groups detailed below. General anesthesia induction was done using intramuscular injection of 100 mg/kg ketamine hydrochloride (Ketalar® flakon, Parke-Davis, USA). Rats were kept under a heat lamp. All procedures were performed in the supine position. After skin asepsis, midline laparotomy was performed. After removing intestines from the surgical field, the infra-renal abdominal aorta was explored. The aorta was clamped using an atraumatic microvascular clamp. The clamp was removed at the end of 120 minutes of ischemia, then reperfusion was provided for another 120 minutes. Ischemia was determined when distal aorta pulsation disappeared, while reperfusion was determined when it reappeared. In the control group, laparotomy and abdominal aorta dissection were applied during the same time period (240 minutes); however, I/R was not applied in these groups. In other groups, in order to minimize heat and fluid loss, intraperitoneal serum physiological was administered at clamping and declamping periods. Also, the abdominal incision was covered with wet gauze. In Group DM-I/R-D, 100 μg/kg dexmedetomidine was administered intraperitoneally 30 minutes before ischemia period. At the end of reperfusion period, biochemical and histopathological evaluations of renal tissue specimen were performed. Rats were decapitated at the end of experiment.
Histopathological evaluation was performed in the Kirikkkale University Medical Faculty Histology and Embryology Department. After routine fixation process, specimens were embedded in paraffin blocks, then tissue sections of 5 μ were mounted on slides for staining with hematoxylin and eosin (H&E). Histopathological evaluation under light microscopy was performed, and findings were scored using a scoring system by Bostan et al. [17]. Glomerular vacuolization (GV), tubular dilatation (TD), vascular vacuolization and hypertrophy (VVH), tubular cell degeneration and necrosis (TCDN), Bowman space dilatation (BSD), tubular hyaline cylinder (THC), leucocyte infiltration (LI), and tubular cell spillage (TCS) were scored using a scoring system: 0: no change; +1: minimal change; +2: medium; +3: severe.
Biochemical evaluation was performed in the Gazi University Medical Faculty Medical Biochemistry Department. Oxidative stress and lipid peroxidation were evaluated using Thiobarbituric acid reactive substance (TBARS) levels as Malondialdehyde (MDA) indicators in renal tissue. Also, Catalase (CAT), Glutathione s transferase (GST), Nitric oxide synthase (NOS) and Superoxide Dismutase (SOD) activities were measured.
SOD, CAT, GST, and NOS enzyme analyses were performed as described by Durak, Aebi, Habig, and Durak [respectively 18–21]. The SOD activity method is based on the measurement of absorbance increase at 560 nm due to reduction of NBT to NBTH2. One unit of SOD activity was defined as the enzyme protein amount causing 50% inhibition in NBTH2 reduction rate. The CAT activity method is based on the measurement of the absorbance decrease due to H2O2 consumption at 240 nm. The GST activity method is based on the measurement of absorbance changes at 340 nm due to formation of a GSH-CDNB complex. The NOS activity method is based on the diazotization of sulfanilic acid by nitric oxide at acid pH and subsequent coupling to N-(1-napthyl-ethylene diamine), and absorbance of the sample tube is measured against the blank tube at 540 nm. In this method, sodium nitroprusside is used as the chemical standard.
The TBARS assay was carried out to determine lipid peroxidation using the thiobarbituric acid method [22]. TBARS measurements were conducted based on the reaction of MDA with thiobarbituric acid (TBA), which form a pink pigment with an absorption maximum at 532 nm in acid pH, and 1,1,3,3-tetraethoxypropane was used as a standard MDA solution.
All procedures were performed at 4°C throughout the experiment.
Enzyme activities and TBARS levels were determined by continuously monitoring and end point change in absorbance at 25°C with a Shimadzu UV- 1601 spectrophotometer. Results were expressed IU/ mg protein for CAT and NOS, for GST and SOD mIU/mg protein and U/mg protein respectively. TBARS results were given nmol/mg protein.
Statistical analysis
We used the SPSS 20.0 packet program for statistical analysis. A p level < 0.05 determined as statistically significant. Data were expressed as mean ± standard deviation. Data were evaluated using Kruskal–Wallis variance analysis. Significant variables were analysed using the Mann–Whitney U test with Bonferroni correction.
Results
In histopathological evaluation, we found increased GV, TD, VVH, THDN, THS, Lİ, and THD in Group DM-I/R when compared with Group C. In Group DM-I/R-D GV, VVH and THS levels were significantly lower than those determined in Group DM-I/R (Figures 1–4, Table 1). In Group DM-I/R, TBARS, SOD, NOS, CAT, and GST levels were significantly higher than those measured in Group C and Group DM-C. In the Dexmedetomidine-treated group (Group DM-I/R-D), TBARS, NOS, CAT, and GST levels were significantly lower than those measured in DM-I/R (Table 2).
Figure 2.
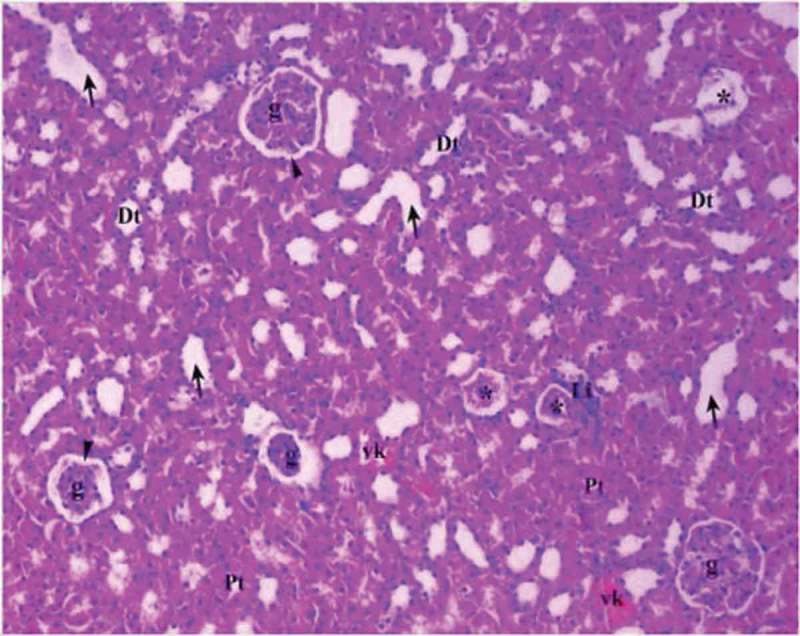
Renal tissue in diabetic control group (Group DM-C).
Pt: Proximal tubule, Dt: Distal tubule, g: glomerular, arrow head: bowman space, arrow: dilated tubule Li: Lymphocyte infiltration, vc: vascular congestion, *: degenerated glomerulus (H&EX10).
Figure 3.
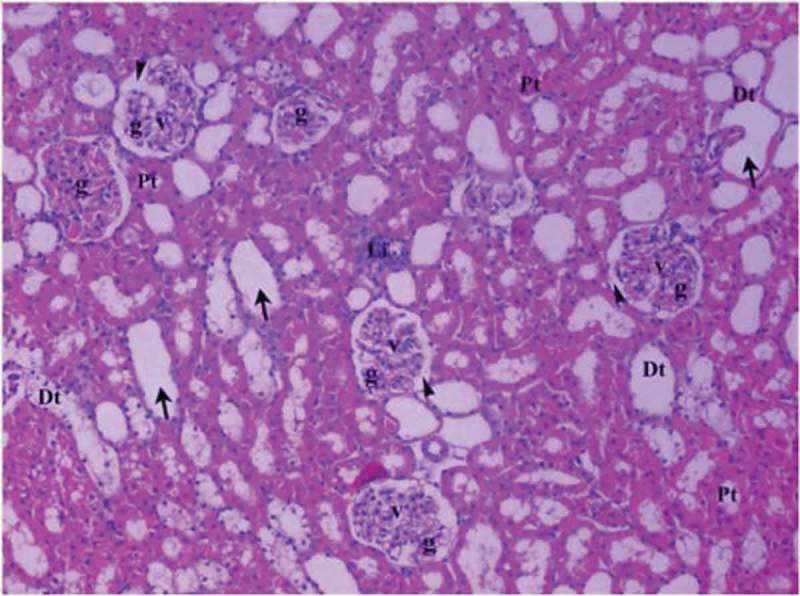
Renal tissue in diabetic ischemia reperfusion group (Group DM-I/R).
Pt: Proximal tubule, Dt: Distal tubule, v: vacuolar, Li: lymphoid infiltration, g: glomerulus, arrow head: bowman space, arrow: dilated tubule (H&EX10).
Figure 1.
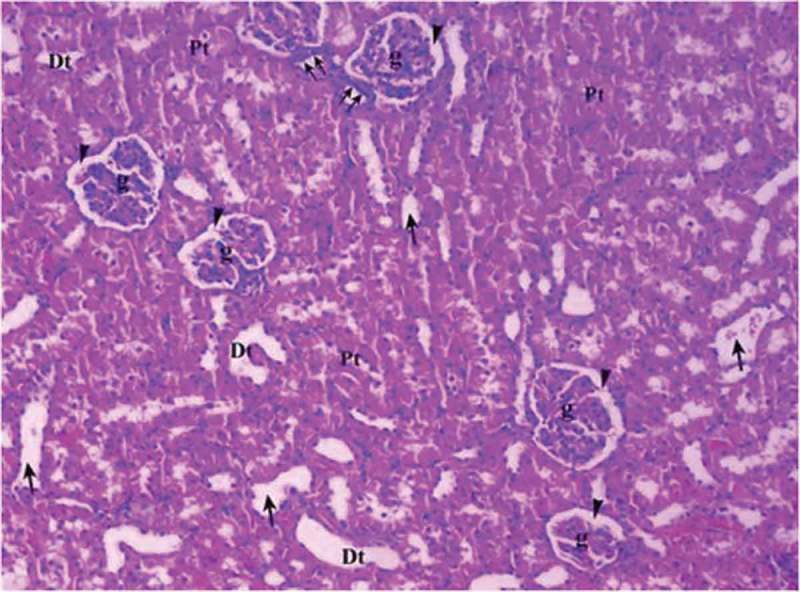
Renal tissue in control group (Group C).
Pt: Proximal tubule, Dt: Distal tubule, g: glomerular, arrow head: bowman space, arrow: dilated tubule, double arrow: macula densa (H&EX10).
Figure 4.
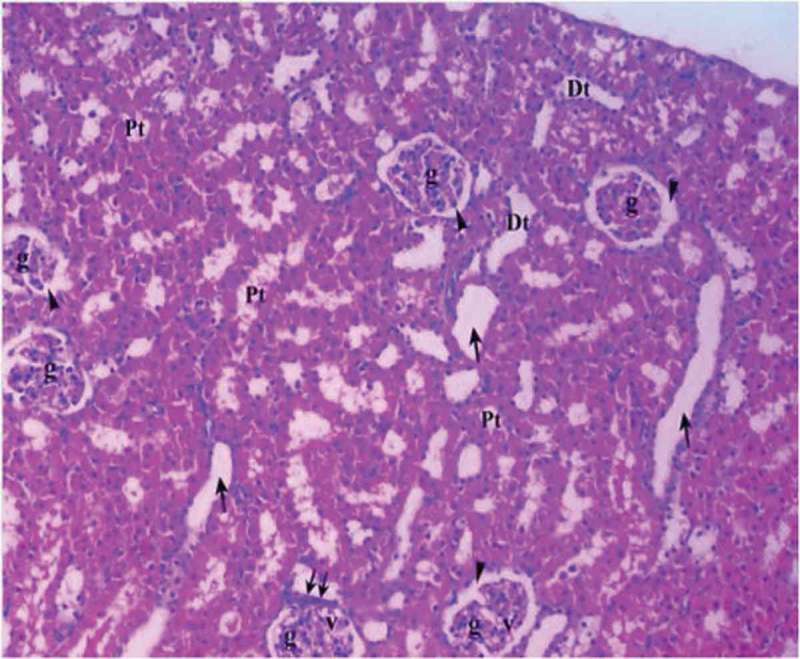
Renal tissue in diabetic ischemia reperfusion treated with dexmedetomidine group (Group DM-I/R-D).
Pt: Proximal tubule, Dt: Distal tubule, g: glomerulus, v: vacuole, arrow head: bowman space, arrow: dilated tubule, double arrow: macula densa (H&EX10).
Table 1.
Histopathological findings of renal tissue [mean ± SD].
| Group C (n = 6) |
Group DM-C (n = 6) |
Group DM-I/R (n = 6) |
Group DM-I/R-D (n = 6) |
P** | |
|---|---|---|---|---|---|
| Glomerular vacuolization (GV) | 0.33 ± 0.21 | 1.00 ± 0.00* | 1.33 ± 0.33* | 0.67 ± 0.21& | 0.029 |
| Tubular dilatation (TD) | 0.33 ± 0.21 | 0.83 ± 0.17 | 1.83 ± 0.31*,? | 1.33 ± 0.21* | 0.001 |
| Vascular vacuolization and hypertrophy (VVH) | 0.33 ± 0.21 | 0.83 ± 0.31 | 1.33 ± 0.66* | 0.67 ± 0.21& | 0.042 |
| Tubular cell degeneration and necrosis (TCDN) | 0.33 ± 0.21 | 0.83 ± 0.17* | 1.00 ± 0.00* | 0.83 ± 0.17* | 0.040 |
| Bowman space dilatation (BSD) | 0.00 ± 0.00 | 0.33 ± 0.21 | 0.67 ± 0.21 | 0.33 ± 0.21 | 0.117 |
| Tubular hyaline cylinders (THC) | 0.17 ± 0.17 | 0.17 ± 0.17 | 1.33 ± 0.21*,? | 0.67 ± 0.21& | 0.001 |
| Lymphocyte infiltration (Lİ) | 0.33 ± 0.21 | 0.67 ± 0.21 | 1.33 ± 0.21*,? | 1.00 ± 0.00* | 0.006 |
| Tubular cell spillage (TCS) | 0.50 ± 0.22 | 1.00 ± 0.00* | 1.00 ± 0.00* | 1.00 ± 0.00* | 0.010 |
*p < 0.05: compared with Group C; &p < 0.05: compared with Group DM-I/R; ?p < 0.05: compared with Group DM-C.
**p significance level with Kruskal-Wallis test p < 0.05.
Table 2.
Oxidative status parameters [mean ± SD].
| Group C (n = 6) |
Group DM-C (n = 6) |
Group DM-I/R (n = 6) |
Group DM-I/R-D (n = 6) |
P** | |
|---|---|---|---|---|---|
| GST (mIU/mg.protein) | 95.77 ± 7.63 | 276.27 ± 7.46* | 937.98 ± 7.83*,& | 816.42 ± 7.73*,&,? | < 0.0001 |
| CAT (IU/mg protein) | 9122.23 ± 1701.14 | 12516.44 ± 1644.18 | 18407.05 ± 2438.32*,& | 10346.95 ± 1915.87? | 0.015 |
| NOS (IU/mg protein) | 38.72 ± 11.72 | 104.19 ± 21.57* | 500.76 ± 163.82*,& | 175.80 ± 35.73*,? | 0.002 |
| TBARS (nmol/mg protein) | 7.17 ± 1.19 | 13.84 ± 1.67* | 28.24 ± 4.80*,& | 15.42 ± 2.51*,? | < 0.0001 |
| SOD (U/mg protein) | 9.79 ± 2.29 | 11.83 ± 2.03 | 24.09 ± 3.16*,& | 16.84 ± 3.80 | 0.013 |
*p < 0.05: compared with Group C; &p < 0.05: compared with Group DM-I/R; ?p < 0.05: compared with Group DM-C.
**p significance level with Kruskal-Wallis test p < 0.05.
Discussion
Mechanisms responsible for increased inflammatory response in Type-2 DM and the effects of these mechanisms on severe renal I/R injury encountered in Type-DM are unclear. Reactive oxygen products (ROS) and Nitric Oxide (NO) play an important role in cellular damage development during I/R processes. Inflammation significantly contributes to I/R pathogenesis, in addition to adhesion molecules, cytokines, and other various cells. Neutrophils are inflammatory cells that produce high levels of ROS. Diabetic patients may need renal transplantation secondary to diabetic nephropathy in their advanced lifetime. Ischemia reperfusion injury is a dangerous complication that can be encountered in this period. A short period of ischemia (30 minutes) may lead to progressive renal injury in diabetics; however, it was shown that in such circumstance renal failure may be reversed. Renal susceptibility is increased in streptozotocin-induced diabetic rats [23].
Ischemia Reperfusion injury is a well-known triggering factor in AKI. Renal tubules are very susceptible to ischemia, and prolonged ischemia results in epithelial cellular death in proximal tubular regions. Even though encountered with prolonged ischemia periods, normal structure and functions of the kidney may be recovered. Following I/R injury, pro-inflammatory/inflammatory, injury/recovery processes are all engaged, and damaged the kidney may be recovered after I/R injury [24].
A meta-analysis showed that unchanged mortality rates (50%) related to AKI in the last five decades. AKI may have occurred in 1% of surgical patients; however, mortality rates may be as high as 83% in patients undergoing cardiac surgery. In a retrospective study, conducted with 2,672 patients undergoing coronary artery bypass surgery, it was shown that AKI resulted in a 14-fold increased mortality rate [25]. Also, vascular interventions, urological procedures, and kidney and liver transplantations are related to AKI. High-risk patients – with advanced age, hypertension, diabetes, decreased cardiac functions, or known renal disease – undergoing cardiac, vascular or transplantation surgeries – which I/R is inevitable – are more susceptible to AKI occurrence and high mortality rates.
Khajuria et al. [26] indicated decreased renal injury after dexmedetomidine treatment in rats with I/R injury. Normal glomerulus structures were seen in dexmedetomidine-treated rats, while swelling of tubular cells, medullar congestion, and renal cell necrosis were determined in non-treated animals [26].
Collino et al. [27] showed – in conjunction with previous studies [28,29] – increased susceptibility to I/R injury in diabetic rats [28,29].
Malondialdehyde is an end product of lipid peroxidation and is accepted as a marker of cell wall peroxidation. Plasma and tissue levels of MDA are accepted as good markers of systemic response following I/R and oxidative stress [30].
Several enzymes have intracellular antioxidant functions against I/R-induced oxidative stress. High activity levels of such enzymes are accepted as markers of inflammation. Catalase (CAT) catalases degredation of hydrogen peroxide and high blood levels of CAT indicate antioxidant effectiveness [31].
Several studies indicated protective effects of dexmedetomidine against I/R injury in different organs, such as lung, heart, brain, and kidney, through its anti-inflammatory and antioxidant properties [9,32–36]. Kucuk et al. [37] showed increased MDA and SOD levels decreased CAT activity in hepatic I/R group of rats. On the other hand, dexmedetomidine administration in this experiment resulted in normalization of MDA and SOD levels. However, they couldn’t find any change in GST levels. The authors concluded that dexmedetomidine prevented lipid peroxidation following hepatic I/R injury.
There is no clear recommendation regarding dexmedetomidine dose range in order to determine effectiveness of the agent in experimental I/R or inflammatory response models. Hanci et al. [38] used dexmedetomidine at a dose of 10 μg/kg in testicular I/R model in rats. Bektaş et al. [39] found higher MDA and NO levels in the I/R group when compared with those measured in the control group, I/R + 50 μg/kg dexmedetomidine group and I/R + 100 μg/kg dexmedetomidine group. Also, they found lower MDA levels in rats treated with 100 μg/kg dexmedetomidine when compared with the 50 μg/kg dexmedetomidine-treated group. However, NO levels were similar in the two dexmedetomidine treated groups.
Cakir et al. [40] showed extensive cortical damage and increased interstitial bleeding in the I/R group when compared with the control group. They found decreased tubular damage in the 10 μg/kg dexmedetomidine treated group when compared with other study groups. Interestingly, they found similar histological findings between the control group and the dexmedetomidine 100 μg/kg treated group. These results indicate that 100 μg/kg dexmedetomidine administration is more effective in recovery of renal I/R injury.
Göksedef et al. [41] conducted a randomized, double–blinded, and placebo-controlled study in 87 patients undergoing coronary artery bypass surgery. They compared renal effects of placebo and low-dose intravenous dexmedetomidine. They reported unchanged renal functions secondary to dexmedetomidine administration, and they supposed that a low dose of the agent that was used in the study may result in such a consequence. In our study, we used the maximal safe dose of dexmedetomidine – 100 μg/kg [42,43] –reported in the literature.
Gonullu et al. showed that the preconditioning or postconditioning with the administration of dexmedetomidine could both reduce the renal IRI histomorphologically, as the renal histopathological score in the IRI group was significantly higher than the groups with dexmedetomidine [44].
Liu et al. showed that before ischemia administration of dexmedetomidine long term protective effect against renal IRI through the alleviation of inflammation [45].
In our study, we used a model representing diabetic patient group – that may be encountered with high ARI – and a cardiac, vascular surgical procedure that inevitably consists I/R. Diabetes was induced using streptozotocin, and rats were housed for one month in order to determine chronic effects of diabetes. Following lower extremity I/R, protective effects of dexmedetomidine were investigated. We found that 100 μg/kg dexmedetomidine administered 30 minutes before ischemia resulted in decreased lipid peroxidation and oxidative stress, and ameliorated histopathological findings of renal injury. In conclusion, we suggest that dexmedetomidine administration before I/R has renoprotective effects.
RESPONSIBLE EDITOR
Omran Bakoush, University of Lund
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- IDF diabetes atlas. 6th ed. 2013 doi: 10.1016/j.diabres.2015.05.037. https://www.google.com.tr/webhp?sourceid=chrome-instant&ion=1&espv=2&ie=UTF-8#q=international%20diabetes%20federation%20diabetes%20atlas%206th%20edition%202013 [DOI] [PubMed]
- Turkey Ministry of Health, Institute of Public Health . Turkish diabetes program 2015-2020. Ankara: 2014. pp. 1–7.http://beslenme.gov.tr/content/files/diyabet/turkiyediyabetprogrami.pdf [Google Scholar]
- McAnulty GR, Robertshaw HJ, Hall GM. Anaesthetic management of patients with diabetes mellitus. Br J Anesth. 2000;85:80–90. doi: 10.1093/bja/85.1.80. [DOI] [PubMed] [Google Scholar]
- Giquel J, Rodriguez-Blanco YF, Matadial C. Diabetes mellitus in anaesthesia. Br J Diabetes Vasc Dis. 2012;12:60–64. doi: 10.1177/1474651412441223. [DOI] [Google Scholar]
- Miller RD. Miller’s anesthesia. In: Roizen MF, Fleisher LA, editors. Anesthesia implications of current disease. 7th. Chicago: Year Book Medical Publishers; 2010. pp. 1067–1149. [Google Scholar]
- Robertshaw HJ, Hall GM. Diabetes mellitus: anaesthetic management. Anaesthesia. 2006;61:1187–1190. doi: 10.1111/ana.2006.61.issue-12. [DOI] [PubMed] [Google Scholar]
- Kadoi Y. Anesthetic considerations in diabetic patients. Part I: preoperative considerations of patients with diabetes mellitus. J Anesth. 2010;24:739–747. doi: 10.1007/s00540-010-0987-1. [DOI] [PubMed] [Google Scholar]
- Gökşin İ, Akbulut M, Baltalarlı A. The effect of normovolomic hemodilution on lung injury after ischemia-reperfusion of lower extremities. Turkish J Thorac Cardiovasc Surg. 2006;14(1):54–58. [Google Scholar]
- Gu J, Sun P, Zhao H. Dexmedetomidine provides renoprotection against ischemia-reperfusion injury in mice. Critical Care. 2011;15:R153. doi: 10.1186/cc10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinin M, Kallio A, Koulu M. Sedative and cardiovascular effects of medetomidine, a novel selective alpha 2-adrenoceptor agonist, in healthy volunteers. Br J Clin Pharmacol. 1987;24:443–451. doi: 10.1111/j.1365-2125.1987.tb03196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalonen J, Hynynen M, Kuitunen A. Dexmedetomidine as an anesthetic adjunct in coronary artery bypass grafting. Anesthesiology. 1997;86:331–345. doi: 10.1097/00000542-199702000-00009. [DOI] [PubMed] [Google Scholar]
- Frumento RJ, Logginidou HG, Wahlander S. Dexmedetomidine infusion is associated with enhanced renal function after thoracic surgery. J Clin Anesth. 2006;18:422–426. doi: 10.1016/j.jclinane.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Billings FT, Chen SW, Kim M. Alpha 2-adrenergic agonists protect against radiocantrast-induced nephropathy in mice. Am J Physiol Renal Physiol. 2008;295:741–748. doi: 10.1152/ajprenal.90244.2008. [DOI] [PubMed] [Google Scholar]
- Solez K, Ideura T, Silvia CB. Clonidine after renal ischemia to lessen acute renal failure and microvascular damage. Kidney Int. 1980;18:309–322. doi: 10.1038/ki.1980.141. [DOI] [PubMed] [Google Scholar]
- Tsutsui H, Sugiura T, Hayashi K. Moxonidine prevents ischemia/reperfusion-induced renal injury in rats. Eur J Pharmacol. 2009;603:73–78. doi: 10.1016/j.ejphar.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Arslan M, Comu FM, Isik B. Effect of dexmedetomidine on erythrocyte deformability during ischemia-reperfusion injury of liver in diabetic rats. Bratisl Lek Listy. 2012;113:687–691. doi: 10.4149/bll_2012_156. [DOI] [PubMed] [Google Scholar]
- Bostan H, Kalkan Y, Tomak Y. Reversal of rocuronium-induced neuromuscular block with sugammadex and resulting histopathological effects in rat kidneys. Ren Fail. 2011;33(10):1019–1024. doi: 10.3109/0886022X.2011.618972. [DOI] [PubMed] [Google Scholar]
- Durak I, Canbolat O, Kavutçu M. Activities of total, cytoplasmic, and mitochondrial superoxide dismutase enzymes in sera and pleural fluids from patients with lung cancer. J Clin Lab Anal. 1996;10:17–20. doi: 10.1002/(SICI)1098-2825(1996)10:1<17::AID-JCLA4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Aebi H. Catalase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. New York (NY): Academic Press; 1974. pp. 673–677. [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Durak I, Kavutcu M, Kacmaz M. Effects of isoflurane on nitric oxide metabolism and oxidant status of guinea pig myocardium. Acta Anaesthesiol Scand. 2001;45:119–122. doi: 10.1034/j.1399-6576.2001.450118.x. [DOI] [PubMed] [Google Scholar]
- Van Ye TM, Roza AM, Pieper GM. Inhibition of intestinal lipid peroxidation does not minimize morphologic damage. J Surg Res. 1993;55:553–558. doi: 10.1006/jsre.1993.1183. [DOI] [PubMed] [Google Scholar]
- Vaghasiya J, Sheth N, Bhalodia Y. Sitagliptin protects renal ischemia reperfusion induced renal damage in diabetes. Regul Pept. 2011;166:48–54. doi: 10.1016/j.regpep.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Wan X, Hou L-J, Zhang L-Y. IKKα is involved in kidney recovery and regeneration ofacute ischemia/reperfusion injury in mice through IL-10-producing regulatory T cells. Dis Model Mech. 2015;8:733–742. doi: 10.1242/dmm.018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon PJ, Stafford-Smith M, White WD. Acute renal failure following cardiac surgery. Nephrol Dial Transplant. 1999;14:1158–1162. doi: 10.1093/ndt/14.5.1158. [DOI] [PubMed] [Google Scholar]
- Khajuria A, Tay C, Shi J. Anesthetics attenuate ischemia-reperfusion induced renal injury: effects and mechanisms. Acta Anaesthesiol Taiwan. 2014;52:176–184. doi: 10.1016/j.aat.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Collino M, Benetti E, Miglio G. Peroxisome proliferator activated receptor β/δ agonism protects the kidney against ischemia/reperfusion injury in diabetic rats. Free Radic Biol Med. 2011;50:345–353. doi: 10.1016/j.freeradbiomed.2010.11.018. [DOI] [PubMed] [Google Scholar]
- Melin J, Hellberg O, Larsson E. Protective effect of insulin on ischemic renal injury in diabetes mellitus. Kidney Int. 2002;61:1383–1392. doi: 10.1046/j.1523-1755.2002.00284.x. [DOI] [PubMed] [Google Scholar]
- Kurcer Z, Parlakpinar H, Vardi N. Protective effects of chronic melatonin treatment against renal ischemia/ reperfusion injury in streptozotocin-induced diabetic rats. Exp Clin Endocrinol Diabetes. 2007;115:365–371. doi: 10.1055/s-2007-971056. [DOI] [PubMed] [Google Scholar]
- Baltalarli A, Ozcan V, Bir F. Ascorbic acid (vitamin C) and iloprost attenuate the lung injury caused by ischemia/reperfusion of the lower extremities of rats. Ann Vasc Surg. 2006;20(1):49–55. doi: 10.1007/s10016-005-9284-0. [DOI] [PubMed] [Google Scholar]
- Basel H, Kavak S, Demir H. Effect of levosimendan injection on oxidative stress of rat myocardium. Toxicol Ind Health. 2013;29(5):435–440. doi: 10.1177/0748233712436643. [DOI] [PubMed] [Google Scholar]
- Yang C-L, Chen C-H, Tsai P-S. Protective effects of dexmedetomidine-ketamine combination against ventilator-induced lung injury in endotoxemia rats. J Surg Res. 2011;167(2):e273–e281. doi: 10.1016/j.jss.2010.02.020. [DOI] [PubMed] [Google Scholar]
- Yoshitomi O, Hara T, Akiyama D. Direct protective effects of dexmedetomidine against myocardial ischemia reperfusion injury in anesthetized pigs. Anesthesiology. 2004;101:A633. doi: 10.1097/SHK.0b013e318254d3fb. [DOI] [PubMed] [Google Scholar]
- Engelhard K, Werner C, Eberspächer E. The effect of the alpha 2-agonist dexmedetomidine and the N-methyl-D-aspartate antagonist S(+)-ketamine on the expression of apoptosis-regulating proteins after incomplete cerebral ischemia and reperfusion in rats. Anesth Analg. 2003;96:524–531. doi: 10.1097/00000539-200302000-00041. [DOI] [PubMed] [Google Scholar]
- Lai Y-C, Tsai P-S, Huang C-J. Effects of dexmedetomidine on regulating endotoxin-induced up-regulation of inflammatory molecules in murine macrophages. J Surg Res. 2009;154:212–219. doi: 10.1016/j.jss.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Dong X, Xing Q, Li Y. Dexmedetomidine protects against ischemia-reperfusion injury in rat skeletal muscle. J Surg Res. 2014;186(1):240–245. doi: 10.1016/j.jss.2013.07.052. [DOI] [PubMed] [Google Scholar]
- Kucuk A, Yaylak F, Cavunt-Bayraktar A. The protective effects of dexmedetomidine on hepatic ischemia reperfusion injury. Bratisl Med J. 2014;115(11):680–684. doi: 10.4149/BLL_2014_132. [DOI] [PubMed] [Google Scholar]
- Hanci V, Erol B, Bektaş S. Effect of dexmedetomidine on testicular torsion/detorsion damage in rats. Urol Int. 2010;84(1):105–111. doi: 10.1159/000273476. [DOI] [PubMed] [Google Scholar]
- Bektaş H, Özzeybek D, Günenç F. The effects of two different doses of dexmedetomidine in a testicular ıschemia-reperfusion model in rats. Turkiye Klinikleri J Med Sci. 2012;32(3):766–774. doi: 10.5336/medsci.2011-26915. [DOI] [Google Scholar]
- Cakir M, Polat A, Tekin S. The effect of dexmedetomidine against oxidative and tubular damage induced by renal ischemia reperfusion in rats. Ren Fail. 2015;37(4):704–708. doi: 10.3109/0886022X.2015.1011550. [DOI] [PubMed] [Google Scholar]
- Göksedef D, Balkanay OO, Ömeroğlu SN. The effects of dexmedetomidine infusion on renal functions after coronary artery bypass graft surgery-a randomized, double-blind, placebo-controlled study. Türk Göğüs Kalp Damar Cerrahisi Dergisi. 2013;21:594–602. [Google Scholar]
- Hoffman WE, Kochs E, Werner C. Dexmedetomidine improves neurologic outcome from incomplete ischemia in the rat. Reversal by the alpha 2- adrenergic antagonist atipamezole. Anesthesiology. 1991;75:328–332. doi: 10.1097/00000542-199108000-00022. [DOI] [PubMed] [Google Scholar]
- Kocoglu H, Ozturk H, Ozturk H. Effect of dexmedetomidine on ischemia-reperfusion injury in rat kidney: a histopathologic study. Ren Fail. 2009;31:70–74. doi: 10.1080/08860220802546487. [DOI] [PubMed] [Google Scholar]
- Gonullu E, Ozkardesler S, Kume T. Comparison of the effects of dexmedetomidine administered at two different times on renal ischemia/reperfusion injury in rats. Braz J Anesthesiol. 2014;64(3):152–158. doi: 10.1016/j.bjan.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Liu G, Song H, Qiu L. Dexmedetomidine preconditioning inhibits the long term inflammation induced by renal ischemia/reperfusion injury in rats. Acta Cir Bras. 2016;31(1):8–14. doi: 10.1590/S0102-865020160010000002. [DOI] [PubMed] [Google Scholar]


