Abstract
Introduction
Previous studies have suggested cerebrospinal fluid (CSF) levels of neurofilament light (NFL) and total tau are elevated in Huntington’s disease (HD) and may be used as markers of disease stage. Biomarkers are needed due to the slow disease progression and the limitations of clinical assessment. This study aims to validate the role of NFL and tau as biomarkers in HD.
Methods
CSF was obtained from a cohort of HD patients and premanifest HD-mutation carriers. Unified Huntington’s Disease Rating Scale (UHDRS) testing was performed on all subjects at the time of sampling. NFL and tau concentrations were determined by ELISA. Spearman correlations were calculated with R version 3.2.3.
Results
11 premanifest HD and 12 manifest HD subjects were enrolled. NFL and tau levels were correlated. NFL showed strong correlations with all items included in the clinical assessment (for example the total functional capacity (TFC) (r = - 0.70 p < 0.01) and total motor score (TMS) (r = 0.83p < 0.01). Tau showed slightly weaker correlations (eg. TMS (r = 0.67 p < 0.01); TFC (r = - 0.59 p < 0.01)). NFL was significantly correlated with 5-year probability of disease onset, whereas tau was not.
Conclusion
This study strengthens the case for NFL as a useful biomarker of disease stage. NFL was strongly correlated to all evaluated items in the UHDRS assessment. Tau also has a potential for use as a biomarker but correlations to clinical tests are weaker in this study. We suggest that NFL and possibly tau be used in clinical drug trials as biomarkers of disease progression that are potentially influenced by future disease-modifying therapies.
1. Introduction
Huntington’s disease (HD) is an autosomal dominant inherited neurodegenerative disease caused by a CAG expansion of the HTT-gene. The triplet expansion gives rise to a cascade of downstream pathophysiological events that are still subject to intense research. The leading hypothesis is that mutant huntingtin (mtHTT) interacts with other proteins and alters their function, resulting in impaired axonal transport, disturbed energy metabolism, dysregulation of gene transcription, protein aggregation [1], and decreased neurotrophic support in the striatum. The last offers an explanation of why the striatum is severely affected [2]. Surprisingly, due to a process called RAN-translation, mtHTT is not the only disease-causing protein that is expressed from an expanded HTT-gene, and this new finding warrants further investigation [3].
The symptoms of HD include motor impairment and chorea, cognitive decline and a variety of psychiatric symptoms. Current treatment options are purely symptomatic, as there are no disease-modifying drugs. However, several promising clinical trials are underway [4].
One difficulty in performing HD trials is the lack of `wet`biomarkers for disease progression, and currently trials rely on clinical evaluation susceptible to symptom fluctuations and inter-rater variability. Some promising candidate biomarkers recently suggested remain to be validated [5]. In the search for biomarkers, cerebrospinal fluid (CSF) has an advantage over blood because of its proximity to the neurodegenerative process. Biomarkers are needed in clinical trials due to the slow disease progression and the limitations of clinical assessment [6].
Neurofilament triplet is a family of structural proteins of neurons especially found in their axons present in the white matter of the brain. Neurofilament light subunit (NFL) is hypothesized mainly to be a biomarker of white matter lesions [7]. Its elevated concentrations are linked to axonal damage in neurological diseases like multiple sclerosis [8] and amyotrophic lateral sclerosis [9]. Disease-modifying therapies have already been shown to lower NFL levels in multiple sclerosis which was associated with improved clinical and radiological outcome [10, 11]. Neuroimaging studies show pathological white matter changes already in premanifest and early HD [12].
Total tau (hereafter referred to as tau) is primarily an axonal protein with microtubule stabilizing function which has gained use as a biomarker in neurodegenerative diseases, most notably in Alzheimer´s disease [13]. Accumulating evidence from studies with different methodologies suggest HD is a secondary tauopathy, where neurofibrillary tangles are overrepresented in later stages as well as in a phenotype with prominent dementia (for a review see [14]). CSF studies have found elevated levels of NFL [15–17] and tau [16–19] in HD and that these are possible biomarkers for disease stage [16, 17, 19].
This study aims to compare NFL and tau, head-to-head, in an HD-cohort to validate their role as biomarkers for disease progression in HD.
2. Materials and methods
2.1 Definition of participants and clinical assessment
The participants were recruited from the HD clinic at Uppsala University Hospital and were either premanifest gene expansion carriers or manifest HD subjects. Premanifest gene expansion carriers were defined as individuals with a CAG-expansion (>35 repeats) of the HD-gene and with a diagnostic confidence level (DCL) below 4 [20]. Manifest HD subjects were defined as individuals with a CAG-expansion (>35 repeats) in the HD gene and a DCL of 4 [20].
Clinical assessment included the Unified HD Rating Scale ´99 (UHDRS) total motor score (TMS) [21], total functional capacity (TFC) [22], stroop word matching task, stroop color matching task, stroop interference, symbol-digit modality test (SDMT) [21], letter verbal fluency test [23], category fluency test (animals) [24]. Disease burden was calculated according to CAG-repeat number and age, using the formula (CAG-35.5) x age [25]. 5-year probability of disease onset was determined by age and CAG-repeat number [26]. Clinical assessment took place on the day of CSF collection by experienced clinical HD raters.
The study was conducted in accordance with the declaration of Helsinki and was approved by the regional ethical review board in Uppsala, Sweden (DNR 2012/274). All participants signed an informed consent before study entry.
2.2 CSF sample collection and handling
CSF was collected by lumbar puncture according to a standardized protocol at Uppsala University Hospital. The CSF was put on ice before centrifugation at 1300 G for 10 minutes at 4 degrees Celsius. The acellular proportion was stored at -70 degrees Celsius until the time of analysis. Polypropylene tubes (Sarstedt) were used throughout the procedures of collecting and storage of CSF to avoid protein adsorption. The time of day for sampling varied and there was no standard of fasting before the procedure.
2.3 Biochemical analyses
CSF NFL levels were measured using a sandwich ELISA method (NF-light® ELISA, Uman Diagnostics, Umeå, Sweden) as described previously in detail [27], while CSF tau levels were measured using the INNOTEST ELISA method (hTau Ag, Fujirebio Europe, Belgium) as described previously [28]. All samples were analyzed in one batch by board-certified laboratory technicians who were blinded to clinical information.
2.4 Statistical analyses
Tests for normality of distribution included Shapiro-Wilk in conjunction with inspection of histograms and the skewness statistic. Age was normally distributed but NFL and tau levels were not. A Mann-Whitney U test of the effect of sex category on tau and NFL concentrations was performed.
The correlations between tau and NFL versus clinical test scores, were evaluated using Spearman rank correlation coefficients. In addition to univariate correlation the Spearman correlation coefficients were adjusted for age and disease burden, respectively. Statistical significance was defined by a p-value of less than 0.05.
In order to evaluate the difference in NFL and tau between manifest and premanifest HD two separate analyses of covariance models were calculated, adjusted for age.
The p-values for the age-adjusted correlations between NFL and the independent factors were adjusted for multiplicity using the Bonferroni-Holm method, and all other reported p-values are unadjusted.
Statistical analyses were performed using R version 3.2.3. Graphs were created with GraphPad Prism version 7.
3. Results
The study enrolled 23 participants (mean age 42.7; standard deviation [SD] 14.4 range 19–72 years; Table 1). Of these, 11 (47.8%) were premanifest gene expansion carriers—12 (52.2%) were manifest HD subjects. The characteristics of both subgroups are described in Table 1.
Table 1. Characteristics of the study population.
| Group | N | Age Mean (SD) | Male: female ratio | CAG Mean (SD) | Disease burden Mean (SD) | TFC Mean (SD) | TMS Mean (SD) |
|---|---|---|---|---|---|---|---|
| Total | 23 | 42.7 (14.4) | 13:10 | 43.7 (3.3) | 326.3 (94.7) | 11.4 (2.8) | 17.3 (20.4) |
| Premanifest HD | 11 | 33 (9.6) | 6:5 | 44.1 (4.1) | 261.2 (78.7) | 13 (0) | 1.3 (1.3) |
| Manifest HD | 12 | 51.5 (12.4) | 8:5 | 43.5 (2.6) | 385.6 (65.6) | 9.9 (3.2) | 32.1 (18.4) |
HD, Huntington’s Disease; SD, Standard deviation; CAG, CAG expansion length; TFC, Total Functional Capacity; TMS, Total Motor Score; Disease burden, (CAG-35.5) x age.
3.1 NFL and tau concentrations
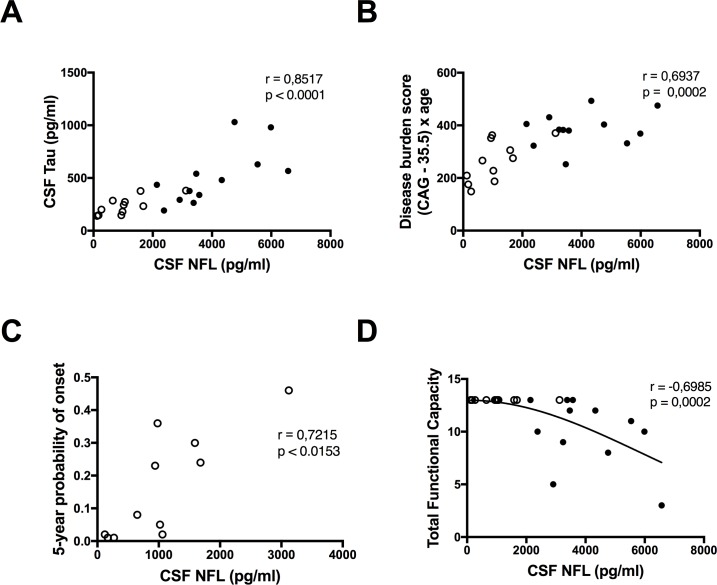
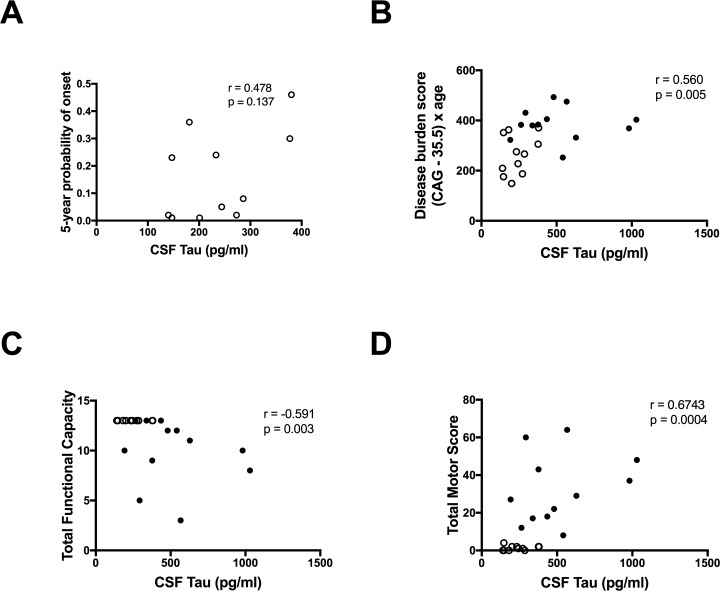
The concentrations of NFL were significantly higher in the manifest HD patients compared with the premanifest gene expansion carriers after adjustment for age (p = 0.003) but there was no significant difference between the two groups in the concentrations of tau after adjustment for age (p = 0.416). The concentrations of NFL and tau were significantly correlated (r = 0.85 p, < 0.0001) (Fig 1A). There was a significant correlation with disease burden for NFL (r = 0.69, p < 0.01) (Fig 1B) and tau (r = 0.56, p < 0.01) (Fig 2B). NFL correlated with 5-year probability of disease onset in the premanifest gene expansion carriers (n = 11 r = 0.72 p = 0.0153) (Fig 1C) but the correlation for tau was not significant (n = 11 r = 0.48, p = 0.1373) (Fig 2A).
Fig 1. Correlations between NFL and disease progression in Huntington’s disease.
(A) Neurofilament light (NFL) and tau levels are significantly correlated. NFL correlates positively with (B) disease burden and (C) 5-year probability of disease onset but negatively with (D) total functional capacity. ○ Premanifest gene expansion carrier • Manifest Huntington’s disease.
Fig 2. Correlations between tau and disease progression in Huntington’s disease.
Tau does not correlate with (A) 5-year probalility of disease onset but correlations with (B) disease burden, (C) total functional capacity, and (D) total motor score were significant. ○ Premanifest gene expansion carrier • Manifest Huntington’s disease.
Three young presymptomatic gene expansion carriers, who were among those farthest from predicted disease onset, had normal NFL levels according to the laboratory’s age-stratified reference range [29]. There was no association between sex and concentrations of tau or NFL (p = 0.74).
3.2 NFL and tau correlations with clinical testing
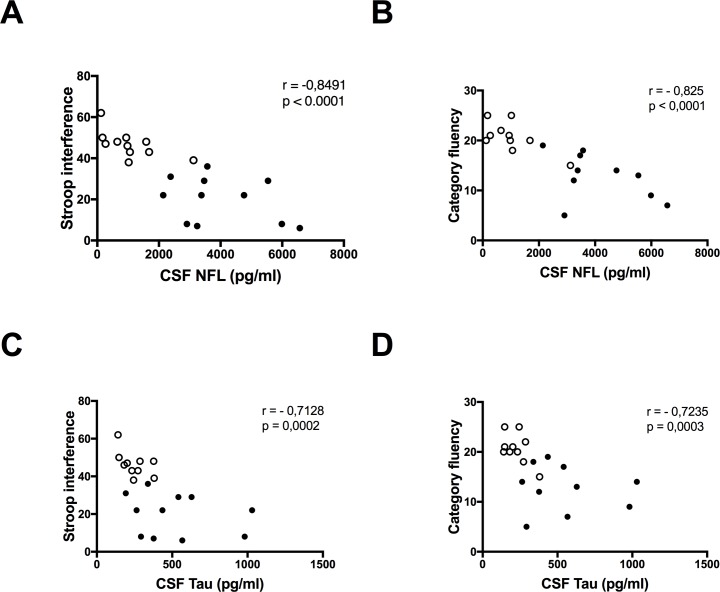
NFL showed strong correlations with all tests in the clinical assessment. After adjustment for age correlations for 6 out of 8 tests scores remained significant–correlations with total functional capacity (Fig 1D) and symbol digit modality were no longer significant. Correlations with total motor score, stroop color, stroop interference (Fig 3A) and letter verbal fluency remained significant after adjustment for multiplicity using the Bonferroni-Holm method. None of the NFL correlations survived adjustment for disease burden (Table 2).
Fig 3. Comparing the correlations of neurofilament light and tau with cognitive test scores.
Neurofilament light (NFL) has stronger correlations with clinical scores like (A) stroop interferens, and (B) category fluency, compared with (C and D) tau. ○ Premanifest gene expansion carrier • Manifest Huntington’s disease.
Table 2. Spearman correlations between NFL and clinical test scores adjusted for age and adjusted for disease burden compared with the direct correlations.
| Partial Correlation (age) | p-value | Partial Correlation (disease burden) | p-value | Direct correlation | p-value | |
|---|---|---|---|---|---|---|
| Total motor score | 0.63 | <0.01* | 0.27 | 0.23 | 0.83 | <0.01 |
| Stroop color | -0.56 | 0.01* | 0.02 | 0.93 | -0.82 | <0.01 |
| Stroop word Reading | -0.46 | 0.04 | 0.10 | 0.67 | -0.75 | <0.01 |
| Stroop interference | -0.64 | <0.01* | -0.22 | 0.35 | -0.85 | <0.01 |
| Category fluency | -0.56 | 0.01 | -0.21 | 0.40 | -0.83 | <0.01 |
| Verbal fluency—letters | -0.60 | <0.01* | -0.27 | 0.25 | -0.75 | <0.01 |
| Symbol-digit modality test | -0.34 | 0.14 | 0.11 | 0.65 | -0.76 | <0.01 |
| Total functional capacity | -0.31 | 0.17 | -0.05 | 0.84 | -0.70 | <0.01 |
* significant after adjustment for multiplicity using the Bonferroni-Holm method.
Moderate to strong correlations were found between tau and all the clinical scores included (Figs 2 and 3), but the magnitudes were slightly lower compared to those of NFL (Fig 3). None of the tau correlations survived adjustment for age, or disease burden score (Table 3).
Table 3. Spearman correlations between tau and clinical test scores adjusted for age and adjusted for disease burden compared with the direct correlations.
| Partial Correlation (age) | p-value | Partial Correlation (disease burden) | p-value | Direct correlation | p-value | |
|---|---|---|---|---|---|---|
| Total motor score | 0.31 | 0.15 | 0.03 | 0.88 | 0.67 | <0.01 |
| Stroop color | -0.27 | 0.24 | 0.12 | 0.60 | -0.67 | <0.01 |
| Stroop word Reading | -0.25 | 0.27 | 0.09 | 0.71 | -0.63 | <0.01 |
| Stroop interference | -0.38 | 0.09 | -0.08 | 0.73 | -0.71 | <0.01 |
| Category fluency | -0.35 | 0.14 | -0.08 | 0.76 | -0.72 | <0.01 |
| Verbal fluency—Letters | -0.29 | 0.20 | -0.02 | 0.92 | -0.59 | <0.01 |
| Symbol-digit modality test | -0.21 | 0.37 | 0.04 | 0.87 | -0.65 | <0.01 |
| Total functional capacity | -0.14 | 0.55 | 0.03 | 0.89 | -0.59 | <0.01 |
4. Discussion
This study found significantly increased levels of NFL in manifest HD compared to premanifest gene expansion carriers. Tau levels had a similar tendency, although not significant after adjustment for age.
The following two sections will discuss the ability of NFL and tau to predict the clinical phenotype in HD, and compare these results with previous findings to analyze their relevance.
4.1 Previous findings
The first study on NFL enrolled only manifest HD patients (n = 35) and found that they had significantly higher levels of NFL in CSF compared with age and gender matched controls [15]. Out of 14 items in the clinical test battery, only the total functional capacity was significantly correlated with NFL levels after adjustment for age. Another study focused on evaluating a novel assay for quantifying mutant huntingtin (mtHTT) in CSF but also performed analyses of NFL (n = 14) and tau (n = 24) including both premanifest gene expansion carriers and manifest HD patients. Interestingly, mtHTT had a positive linear association with NFL and tau respectively (perhaps indicating that mtHTT was leaking out of degenerating neurons). NFL had correlations with cognitive test scores of similar magnitude as those of mtHTT but the correlations of NFL did not survive adjustment for disease burden, perhaps due to fewer available samples. Even though the sample size for tau was the same as for mtHTT, correlations with clinical test scores did not survive adjustment for disease burden, indicating tau may not have the same predictive ability on phenotype as mtHTT [16]. A recent study of tau included a larger sample (n = 52) and found significant correlations between tau concentrations, total functional capacity, total motor score and cognitive test scores after adjustment for age and in the majority of these tests, the correlations remained significant after adjustment for disease burden as well [19]. Recent findings in a study by Vinther-Jensen et al [17] (n = 80) support NFL as a correlate for disease burden, with levels rising already in the premanifest stage, where tau levels were only elevated in the premanifest gene expansion carriers with psychiatric symptoms compared to those without. Total motor score was correlated with NFL levels after adjustment for disease burden.
4.2 Neurofilament light subunit correlates stronger with disease progression compared with tau
This study strengthens the case for NFL as a biomarker of disease stage which was correlated to 6/8 items in the UHDRS assessment after adjustment for age. Total motor score, stroop color, stroop interference and letter verbal fluency remained significant after adjustment for multiplicity. The correlations with clinical scores were stronger than in the first study published that used an older ELISA with lower precision [15], and of the same magnitude as in a more recent study [16].
Our study, in agreement with two previous studies [16, 17], found NFL levels in premanifest gene expansion carriers that increase in the prodromal stage as the 5-year probability of disease onset rises, which indicates ongoing axonal damage in the white matter. This is in agreement with previous knowledge about early involvement of white matter before clinical onset of HD. However, we note that those individuals farthest from predicted clinical onset had normal NFL values (according to published reference values [29]). In agreement with a recent study [17], we found no correlation between tau and 5-year probability of disease onset, which indicates that tau is less suitable than NFL in the premanifest stage.
The magnitude of correlations between tau and disease stage were similar in our study compared to Rodrigues et al. [19], but correlations did not survive adjustment for age or disease burden.
Overall, these results suggest that, compared to tau, NFL is superior in the ability to predict the clinical phenotype in HD.
The most important limitation to this study is the small sample. Some trends suggested herein may prove significant in larger materials. Timing of meals and the time of day for sampling varied, but this is not likely to influence the results, at least regarding tau [30]. To avoid such limitations, we would like to recommend the HD Clarity project which is a new initiative for a multicenter collection of HD CSF which aims to enroll a large number of participants allowing statistical power for multiple analyses while offering a standardized protocol for CSF collection.
Disease burden is likely a confounding factor for the direct correlation between any adequate biomarker and disease stage in HD. It is therefore no surprise that adjustment for disease burden weakens the correlations of tau and NFL with clinical scores sizably. After all, the natural history of HD is governed by disease burden to a large extent [26]. Keeping in mind that disease burden is unchangeable, the correlations seen in this study are strong and suggest that especially NFL could be valuable for monitoring disease activity. While measurement of CSF mtHTT may offer proof of concept for huntingtin lowering drugs, it does not directly reflect neurodegeneration like the biomarkers in this study. Therefore, we suggest that NFL and possibly tau be used in clinical drug trials as biomarkers of disease progression that are potentially influenced by future disease-modifying therapies.
Supporting information
(XLSX)
Acknowledgments
The authors thank Marcus Thuresson, Statisticon AB for statistical advice and Dag Nyholm for careful review of the manuscript. Finally, we thank Marie-Louise Göller, Camilla Ekwall, Jenny Grundström and all members of the clinical HD-team at the Dept. of Neurology, Uppsala University Hospital that contributed to the clinical data collection.
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
JS received funding by The Selander Foundation and Åland Cultural Foundation for this study. The funding sources had no influence on the design or conduct of the study, nor the decision to publish.
References
- 1.Ross CA, Aylward EH, Wild EJ, Langbehn DR, Long JD, Warner JH, et al. Huntington disease: natural history, biomarkers and prospects for therapeutics. Nature reviews Neurology. 2014;10(4):204–16. Epub 2014/03/13. 10.1038/nrneurol.2014.24 [DOI] [PubMed] [Google Scholar]
- 2.Nopoulos PC. Huntington disease: a single-gene degenerative disorder of the striatum. Dialogues in Clinical Neuroscience. 2016;18(1):91–8. PubMed Central PMCID: PMCPMC4826775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banez-Coronel M, Ayhan F, Tarabochia AD, Zu T, Perez BA, Tusi SK, et al. RAN Translation in Huntington Disease. Neuron. 2015;88(4):667–77. Epub 2015/11/22. PubMed Central PMCID: PMCPMC4684947. 10.1016/j.neuron.2015.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zielonka D, Mielcarek M, Landwehrmeyer GB. Update on Huntington's disease: advances in care and emerging therapeutic options. Parkinsonism & related disorders. 2015;21(3):169–78. Epub 2015/01/13. [DOI] [PubMed] [Google Scholar]
- 5.Byrne LM, Wild EJ. Cerebrospinal Fluid Biomarkers for Huntington's Disease. Journal of Huntington's disease. 2016;5(1):1–13. Epub 2016/04/01. 10.3233/JHD-160196 [DOI] [PubMed] [Google Scholar]
- 6.Scahill RI, Wild EJ, Tabrizi SJ. Biomarkers for Huntington's disease: an update. Expert opinion on medical diagnostics. 2012;6(5):371–5. Epub 2013/03/14. 10.1517/17530059.2012.701205 [DOI] [PubMed] [Google Scholar]
- 7.Jonsson M, Zetterberg H, van Straaten E, Lind K, Syversen S, Edman A, et al. Cerebrospinal fluid biomarkers of white matter lesions—cross-sectional results from the LADIS study. European journal of neurology. 2010;17(3):377–82. Epub 2009/10/23. 10.1111/j.1468-1331.2009.02808.x [DOI] [PubMed] [Google Scholar]
- 8.Teunissen CE, Iacobaeus E, Khademi M, Brundin L, Norgren N, Koel-Simmelink MJ, et al. Combination of CSF N-acetylaspartate and neurofilaments in multiple sclerosis. Neurology. 2009;72(15):1322–9. Epub 2009/04/15. 10.1212/WNL.0b013e3181a0fe3f [DOI] [PubMed] [Google Scholar]
- 9.Rosengren LE, Karlsson JE, Karlsson JO, Persson LI, Wikkelso C. Patients with amyotrophic lateral sclerosis and other neurodegenerative diseases have increased levels of neurofilament protein in CSF. Journal of neurochemistry. 1996;67(5):2013–8. Epub 1996/11/01. [DOI] [PubMed] [Google Scholar]
- 10.Axelsson M, Malmestrom C, Gunnarsson M, Zetterberg H, Sundstrom P, Lycke J, et al. Immunosuppressive therapy reduces axonal damage in progressive multiple sclerosis. Multiple sclerosis (Houndmills, Basingstoke, England). 2014;20(1):43–50. Epub 2013/05/25. [DOI] [PubMed] [Google Scholar]
- 11.Kuhle J, Disanto G, Lorscheider J, Stites T, Chen Y, Dahlke F, et al. Fingolimod and CSF neurofilament light chain levels in relapsing-remitting multiple sclerosis. Neurology. 2015;84(16):1639–43. Epub 2015/03/27. PubMed Central PMCID: PMCPMC4409586. 10.1212/WNL.0000000000001491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosas HD, Tuch DS, Hevelone ND, Zaleta AK, Vangel M, Hersch SM, et al. Diffusion tensor imaging in presymptomatic and early Huntington's disease: Selective white matter pathology and its relationship to clinical measures. Movement disorders: official journal of the Movement Disorder Society. 2006;21(9):1317–25. Epub 2006/06/07. [DOI] [PubMed] [Google Scholar]
- 13.Spillantini MG, Goedert M. Tau pathology and neurodegeneration. The Lancet Neurology. 2013;12(6):609–22. Epub 2013/05/21. 10.1016/S1474-4422(13)70090-5 [DOI] [PubMed] [Google Scholar]
- 14.Gratuze M, Cisbani G, Cicchetti F, Planel E. Is Huntington's disease a tauopathy? Brain: a journal of neurology. 2016;139(Pt 4):1014–25. Epub 2016/03/13. [DOI] [PubMed] [Google Scholar]
- 15.Constantinescu R, Romer M, Oakes D, Rosengren L, Kieburtz K. Levels of the light subunit of neurofilament triplet protein in cerebrospinal fluid in Huntington's disease. Parkinsonism & related disorders. 2009;15(3):245–8. Epub 2008/12/06. [DOI] [PubMed] [Google Scholar]
- 16.Wild EJ, Boggio R, Langbehn D, Robertson N, Haider S, Miller JR, et al. Quantification of mutant huntingtin protein in cerebrospinal fluid from Huntington's disease patients. The Journal of clinical investigation. 2015;125(5):1979–86. Epub 2015/04/07. PubMed Central PMCID: PMCPMC4463213. 10.1172/JCI80743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vinther-Jensen T, Bornsen L, Budtz-Jorgensen E, Ammitzboll C, Larsen IU, Hjermind LE, et al. Selected CSF biomarkers indicate no evidence of early neuroinflammation in Huntington disease. Neurology(R) neuroimmunology & neuroinflammation. 2016;3(6):e287. Epub 2016/10/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Constantinescu R, Romer M, Zetterberg H, Rosengren L, Kieburtz K. Increased levels of total tau protein in the cerebrospinal fluid in Huntington's disease. Parkinsonism & related disorders. 2011;17(9):714–5. Epub 2011/07/08. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigues FB, Byrne L, McColgan P, Robertson N, Tabrizi SJ, Leavitt BR, et al. Cerebrospinal fluid total tau concentration predicts clinical phenotype in Huntington's disease. Journal of neurochemistry. 2016. Epub 2016/06/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reilmann R, Leavitt BR, Ross CA. Diagnostic criteria for Huntington's disease based on natural history. Movement disorders: official journal of the Movement Disorder Society. 2014;29(11):1335–41. Epub 2014/08/29. [DOI] [PubMed] [Google Scholar]
- 21.Unified Huntington's Disease Rating Scale: reliability and consistency. Huntington Study Group. Movement disorders: official journal of the Movement Disorder Society. 1996;11(2):136–42. Epub 1996/03/01. [DOI] [PubMed] [Google Scholar]
- 22.Shoulson I, Fahn S. Huntington disease: clinical care and evaluation. Neurology. 1979;29(1):1–3. Epub 1979/01/01. [DOI] [PubMed] [Google Scholar]
- 23.Benton AL. Differential behavioural effects in frontal lobe disease. Neuropsychologia. 1968;6:53–60. [Google Scholar]
- 24.Butters N, Granholm E., Salmon D.P., Grant I., Wolfe J. Episodic and semantic memory: a comparison of amnesic and demented patients. Journal of clinical and experimental neuropsychology: official journal of the International Neuropsychological Society. 1987;9(5):479–97. [DOI] [PubMed] [Google Scholar]
- 25.Penney JB Jr., Vonsattel JP, MacDonald ME, Gusella JF, Myers RH. CAG repeat number governs the development rate of pathology in Huntington's disease. Annals of neurology. 1997;41(5):689–92. Epub 1997/05/01. 10.1002/ana.410410521 [DOI] [PubMed] [Google Scholar]
- 26.Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR. A new model for prediction of the age of onset and penetrance for Huntington's disease based on CAG length. Clinical genetics. 2004;65(4):267–77. Epub 2004/03/18. 10.1111/j.1399-0004.2004.00241.x [DOI] [PubMed] [Google Scholar]
- 27.Norgren N, Rosengren L, Stigbrand T. Elevated neurofilament levels in neurological diseases. Brain research. 2003;987(1):25–31. Epub 2003/09/23. [DOI] [PubMed] [Google Scholar]
- 28.Blennow K, Wallin A, Agren H, Spenger C, Siegfried J, Vanmechelen E. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer disease? Molecular and chemical neuropathology / sponsored by the International Society for Neurochemistry and the World Federation of Neurology and research groups on neurochemistry and cerebrospinal fluid. 1995;26(3):231–45. Epub 1995/12/01. [DOI] [PubMed] [Google Scholar]
- 29.Vagberg M, Norgren N, Dring A, Lindqvist T, Birgander R, Zetterberg H, et al. Levels and Age Dependency of Neurofilament Light and Glial Fibrillary Acidic Protein in Healthy Individuals and Their Relation to the Brain Parenchymal Fraction. PloS one. 2015;10(8):e0135886 Epub 2015/09/01. PubMed Central PMCID: PMCPMC4552591. 10.1371/journal.pone.0135886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cicognola C, Chiasserini D, Eusebi P, Andreasson U, Vanderstichele H, Zetterberg H, et al. No diurnal variation of classical and candidate biomarkers of Alzheimer's disease in CSF. Molecular neurodegeneration. 2016;11(1):65 Epub 2016/09/09. PubMed Central PMCID: PMCPMC5013624. 10.1186/s13024-016-0130-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.