Abstract
We estimated the prevalence of obesity, overweight, and underweight among US adolescents with and without autism and other learning and behavioral developmental disabilities (DDs) and assessed the health consequences of obesity among adolescents with DDs. From the 2008 to 2010 National Health Interview Survey, we selected 9,619 adolescents ages 12–17 years. Parent respondents reported weight, height, presence of DDs and health conditions. We calculated body mass index (BMI) and defined obesity, overweight, and underweight as ≥95th, ≥85th to <95th, and <5th percentiles, respectively, using established criteria. We created mutually-exclusive DD subgroups using the following order of precedence: autism; intellectual disability; attention-deficit-hyper-activity-disorder; learning disorder/other developmental delay. We compared BMI outcomes among adolescents in each DD group versus adolescents without DDs using multivariable logistic regression. Socio-demographic factors and birthweight were included as confounders. Estimates were weighted to reflect the US population. Both obesity and underweight prevalences were higher among adolescents with than without DDs [adjusted prevalence ratios (aPR) 1.5 (1.25–1.75) and 1.5 (1.01–2.20), respectively]. Obesity was elevated among adolescents with all DD types, and was highest among the autism subgroup [aPR 2.1 (1.44–3.16)]. Adolescents with either a DD or obesity had higher prevalences of common respiratory, gastrointestinal, dermatological and neurological conditions/symptoms than nonobese adolescents without DDs. Adolescents with both DDs and obesity had the highest estimates for most conditions. Obesity is high among adolescents with autism and other DDs and poses added chronic health risks. Obesity prevention and management approaches for this vulnerable population subgroup need further consideration.
Keywords: Obesity, Overweight, Underweight, Developmental disability, Children, Concurrent medical conditions, Autism
Introduction
Obesity is estimated as 20 % among US children aged 6–17 years [1]. While some studies suggest even higher estimates in children with developmental disabilities (DDs) [2–5], they were limited in that DDs were either generally defined (i.e. by combining DDs with differing functional impacts as a single entity) [2, 3, 6–8] or the focus was limited to a single condition such as autism [5] or attention deficit disorder/attention deficit hyperactivity disorder (ADD/ADHD) [4]. Moreover, none accounted for the fact that many children are diagnosed with multiple DDs.
Additionally, the prevalence of underweight has not been well studied among children with DDs; nor have studies investigated the full body mass index (BMI) range more generally among children with DDs. While only 3.7 % of US children are underweight [9], children with DDs are potentially at higher risk for underweight because of dietary restrictions imposed in response to concurrent food allergies or as a potential treatment for behavioral symptoms, dietary aversions among children with some DDs, the potential for appetite suppression associated with some psychoactive medications, and gastrointestinal disturbances associated with DDs.
In this study, we provide nationally-representative estimates of obesity, overweight, and underweight among adolescent children with and without several specific learning and behavioral DDs. We examine both the risk factors for obesity and the health consequences of having obesity for adolescents with DDs compared to adolescents without DDs.
Methods
Data Source
The NHIS is an annual in-person health survey of the civilian non-institutionalized US population [10]. The multistage sample design is based on household as the primary sampling unit. Within sampled households, one randomly-selected adult is asked to complete a family demographic survey and an individual adult health survey. In households with children, one child is additionally randomly sampled and a knowledgeable adult household member, usually a parent (92 %), is asked to complete a health survey about the child. For the current study, we used data from the 2008 to 2010 child and family surveys.
Study Population
Our analysis included 9,619 adolescents aged 12–17 years. We necessarily limited our sample to this age range because height and weight data were not collected for younger children [11].
Developmental Disability Definitions
We analyzed children with and without DDs associated with learning and behavior: autism, intellectual disability (ID), ADD/ADHD, learning disability (LD), and other developmental delay. Autism, ID, ADD/ADHD, and other developmental delay were ascertained with the following question format: “Has a doctor or health professional ever told you that [CHILD] had [CONDITION]?” LD was ascertained from a slightly different question: “Has a representative from a school or a health professional ever told you that [CHILD] had a learning disability?” Although the NHIS uses the term “mental retardation”, we synonymously use the more current term “intellectual disability” in this paper. As a subtype of the overarching disorder of ADHD, ADD was subsumed under the term “ADHD” for ease of presentation.
We created two general analytic groups: (1) adolescents without learning and behavioral DDs as our comparison group and (2) adolescents with any of the aforementioned DDs. We also sub-divided the DD group into four mutually-exclusive groups using the following order of precedence for adolescents with more than one DD diagnosis: (1) autism, (2) ID without autism, (3) ADHD without autism or ID (4) LD or other developmental delay without autism, ID, or ADHD. This order of precedence was chosen such that ADHD and LD could be examined without the co-occurring effects of the generally more pervasive DDs, autism and ID. Sample size constraints precluded further subdivisions of the autism and ID groups.
BMI Outcomes
Parents reported adolescents’ current height and weight. These data were used to compute BMI and BMI national percentile rankings for sex and age groups based on established CDC criteria [12, 13]. Obesity was defined as BMI ≥95th percentile; overweight was BMI of 85th to <95th percentile; healthy weight was BMI of 5th to<85th percentile; and underweight was BMI <5th percentile.
Demographics
We assessed the following as potential confounders and obesity risk factors: child sex, low birth weight (LBW) (<2,500 g), age (12–14 vs. 15–17 years), race-ethnicity (non-Hispanic White (NHW), non-Hispanic Black (NHB), Hispanic, Asian, American Indians/Native American, and other), health insurance (insured vs. uninsured), poverty-to-income ratio (derived based on family income in relation to US Census Bureau poverty thresholds, <200 vs. ≥200 %), and maternal education (≤high school vs. >high school) [11, 14, 15].
Concurrent Medical Conditions
We reviewed current literature on medical conditions associated with either childhood obesity or the presence of a DD [6, 8, 16–27]. Based on that review and our sample size, we examined parent report of the general occurrence of asthma; the recent (past 12 months) occurrence of respiratory allergy, food allergy, eczema/skin allergy, and headaches/migraines; and the very recent (past 2 weeks) occurrence of stomach/intestinal illness and head/chest cold.
Statistical Analysis
We estimated percentage distributions for demographic characteristics among adolescents with and without any of the aforementioned DDs and adolescents within each of the DD-specific subgroups and assessed differences with Chi square tests.
Within each analytic DD group, we estimated the prevalence of each BMI outcome outside the healthy range. We computed prevalence ratios and 95 % confidence intervals that compared BMI estimates for adolescents with each DD type to estimates for adolescents without DDs. We additionally conducted stratum-specific assessments based on child’s sex, age, race-ethnicity, and birthweight. The stratum-specific data informed the creation of logistic regression models to estimate adjusted prevalence ratios (PRs) for associations between each DD type and each BMI outcome. In these models, we included each variable assessed in stratified analyses and health insurance and maternal education as potential confounders. We did not include interaction terms because stratum-specific variation was fairly minimal.
Based on our initial findings, we conducted additional analyses for the obesity outcome. We assessed risk factors for obesity within each of the DD and DD subgroups of adolescents using multivariable logistic regression models. Potential risk factors were the same factors considered as potential confounders in the previous models.
Finally, we examined the prevalence of concurrent health conditions among four groups of adolescents: non-obese/no DDs; obese/no DDs; non-obese/one or more DDs; obese/one or more DDs. We compared adolescents in each of these groups and thus generated 4 sets of adjusted PRs. A previous analysis of these data indicated that children with these DDs were at increased risk for many concurrent health conditions compared to children without DDs [27]. Here we examined whether obesity was associated with an added health impact beyond the impact from obesity alone or having a DD alone.
All but one set of estimates in this study were weighted to adjust for non-response and unequal selection probabilities and thus, produce estimates reflective of the US non-institutionalized population of children ages 12–17 years; additionally, analyses were conducted using SUDAAN (version 9.0) software to account for the complex sampling design in variance estimation [28]. We assessed the total BMI distributions for adolescents with and without DDs using an unweighted sample and statistically compared these distributions with the Kolmogorov–Smirnov test for equality of distribution functions.
Human subjects review was not required for this study since this was a secondary analysis of de-identified datasets.
Results
Characteristics of Study Population
Our final study population included 1,478 adolescents who had one or more learning/behavioral DDs and 8,141 adolescents without any of these DDs. We estimated the prevalence of any DDs among US adolescents as 15.4 %. DD-specific prevalence estimates were 0.6 % for ID without autism, 1 % for autism, 3.9 % for LD and other DDs without ADHD, autism, and ID, and 8.9 % for ADHD without autism and ID.
Adolescents with any DD were more likely to be male than adolescents without DDs; the proportion ranged among specific DDs from 59 % (ID group) to 80 % (autism group) (Table 1). Age distributions were similar for adolescents with and without DDs. Adolescents with DDs were more likely to be NHW and have health insurance than adolescents without DDs, but they were also more likely to be from a low-income family. While the proportion of adolescents whose mothers were educated beyond high school was similar for adolescents with and without DDs (61 % for both), advanced maternal education varied widely for specific DDs, from 46 % (ID group) to 71 % (autism group). History of LBW was higher in adolescents with than without DDs, with the exception of autism.
Table 1.
Demographics of children with developmental disabilities among US adolescents 12–17 years of age, NHIS, 2008–2010
| Children without developmental disabilities (N = 8,141) % (SE) |
Children with any learning and behavioral disability (N = 1,478) % (SE) |
Children with autism (N = 93) % (SE) |
Children with intellectual disability (ID) without autism (N = 60) % (SE) |
Children with ADHD without autism/ID (N = 845) % (SE) |
Children with LD/other DD without ADHD/autism/ID (N = 376) % (SE) |
|
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 47.0 (0.7) | 67.5 (1.4)a | 79.7 (1.5)a | 58.7 (3.5) | 71.4 (1.8)a | 70.1 (2.2)a |
| Female | 53.0 (0.7) | 32.5 (1.4) | 20.3 (1.5) | 41.3 (3.5) | 28.6 (1.8) | 29.9 (2.2) |
| Age (years) | ||||||
| 12–14 | 50.2 (0.7) | 48.2 (1.5) | 45.5 (1.8) | 30.5 (3.0)b | 48 (1.8) | 47.7 (2.2) |
| 15–17 | 49.8 (0.7) | 51.8 (1.5) | 54.5 (1.8) | 69.5 (3.0) | 52 (1.8) | 52.3 (2.2) |
| Race/ethnicity | ||||||
| Non-Hispanic White | 56.9 (0.8) | 68.9 (1.3)a | 71.3 (1.3) | 61.3 (4.4) | 73.7 (1.4)a | 71.6(1.8)a |
| Non-Hispanic Black | 13.6 (0.7) | 14.1 (1.0) | 13.0 (0.8) | 11.4 (2.1) | 14.3 (1.1) | 15.8 (1.3) |
| American Indian/Native American | 0.6 (0.2) | 0.8 (0.2) | 0 | 1.1(0.1) | 0.6 (0.2) | 0.3 (0.4)c |
| Asian | 4.5 (0.3) | 0.8 (0.3) | 2.5 (0.3) | 1.8 (0.1) | 0.4 (0.1) | 0.3 (0.1) |
| Other | 2.9 (0.2) | 3.4 (0.5) | 4.6 (0.8) | 2.4 (2.4) | 3.2 (0.7) | 3.1 (0.7) |
| Hispanic | 21.5 (0.6) | 12.0 (0.9) | 8.6 (0.8) | 22.0 (1.8) | 7.8 (0.7) | 8.9 (0.9) |
| Poverty-to-income ratio | ||||||
| At or above 200 % | 62.3 (0.8) | 54.7 (1.6)a | 57.5 (1.9) | 44.1 (1.9)b | 56.9 (2.0)b | 51.2 (2.2)b |
| Below 200 % | 37.7 (0.8) | 45.3 (1.6) | 42.5 (1.9) | 55.9 (1.9) | 43.1 (2.0) | 48.8 (2.2) |
| Maternal education | ||||||
| ≤High school | 39.2 (0.8) | 39.4 (1.8) | 29.4 (2.4) | 54.2 (3.0) | 37.0 (2.3) | 44.3 (2.3) |
| >High school | 60.8 (0.8) | 60.6 (1.8) | 70.6 (2.4) | 45.8 (3.0) | 63.0 (2.3) | 55.7 (2.3) |
| Healthcare insurance | ||||||
| Uninsured | 10.5 (0.7) | 6.8 (1.2)b | 14.2 (0.1) | 0.6 (0.01)a | 6.1 (1.1)b | 4.5 (0.5)b |
| Insured | 89.5 (0.7) | 93.2(1.2) | 85.8 (0.1) | 99.4 (0.01) | 93.9 (1.1) | 95.5 (0.5) |
| Birthweight (g) | ||||||
| Less than 2,500 | 8.8 (0.4) | 12.1 (1.0)a | 8.4 (1.0) | 21.7 (3.3)b | 9.9 (1.0) | 11.1 (1.2) |
| Greater than or equal to 2,500 | 91.2 (0.4) | 87.9 (1.0) | 91.6 (1.0) | 78.3 (3.3) | 90.1 (1.0) | 88.9 (1.2) |
| Disability | ||||||
| ADHD | 0 | 65.0(1.5) | 60.7 (2.2) | 48.7 (3.6) | 100 (0) | 0 |
| Autism | 0 | 6.0 (0.7) | 100 (0) | 0 | 0 | 0 |
| LD | 0 | 56.4 (1.6) | 73.3(1.7) | 89.3 (0.7) | 42.3 (2.1) | 95.7(0.8) |
| ID | 0 | 5.2 (0.7) | 23.4(1.9) | 100 (0) | 0 | 0 |
| Other DD | 0 | 25.4 (1.3) | 72.6(1.1) | 64.7 (2.1) | 11.4 (1.4) | 25.8(2.3) |
Weighted % presented; Standard error (SE); Statistical significance of a P < .001; b P <.05; cRelative standard error (RSE) greater than 50 % and should be considered too unreliable for most purposes; Percent missing: maternal education (1 %); poverty-to-income ratio (12.5 %); healthcare insurance (<1 %); birthweight (7.3 %); child BMI (6 %)
Prevalence of Obesity, Overweight, and Underweight
The prevalence of obesity in adolescents with any DD was 20.4 % compared to 13.1 % of adolescents without DDs (Table 2). This 60 % differential was statistically significant and remained significant after adjustment for confounders. Among adolescents with DDs, those with autism had the highest obesity prevalence (31.8 %), while those with in the ADHD group had the lowest prevalence (17.6 %).
Table 2.
Prevalence and unadjusted and adjusted prevalence ratios for adolescents 12–17 years of age with developmental disabilities, by BMI-status, NHIS 2008–2010
| Children without developmental disabilities | Children with any learning and behavioral disability | Children with autism | Children with intellectual disability (ID) without autism | Children with ADHD without autism/ID | Children with LD/other DD without ADHD/autism/ID | |
|---|---|---|---|---|---|---|
| Underweight (< 5th percentile) | ||||||
| Prevalence (SE) | 3.5 (0.3) | 5.6 (1)b | 4.5 (1.0) | 14.8 (0.9)b | 5.0 (1.3) | 6.0 (1.8) |
| Unadjusted PR (95 % CI) | Ref | 1.6 (1.07–2.33) | 1.2 (0.41–3.55) | 4.3 (1.21–15.15) | 1.3 (0.77–2.27) | 1.6 (0.72–3.43) |
| aPR (95 % CI) | Ref | 1.5 (1.01–2.27) | 1.1 (0.31–4.05) | 1.5 (0.35–6.61) | 1.2 (0.71–2.18) | 1.4 (0.63–2.93) |
| Overweight (85th to < 95th percentile) | ||||||
| Prevalence (SE) | 18.2 (0.6) | 17.5 (1.2) | 20.9 (1.4) | 15.7 (3.8) | 18.0 (1.6) | 20.7 (2.1) |
| Unadjusted PR (95 % CI) | Ref | 1.0 (0.83–1.15) | 1.2 (0.65–2.06) | 1.0 (0.42–2.52) | 1.0 (0.81–1.23) | 1.2 (0.89–1.53) |
| aPR (95 % CI) | Ref | 1.0 (0.82–1.15) | 1.2 (0.62–2.12) | 1.1 (0.49–2.58) | 1.0 (0.80–1.23) | 1.2 (0.88–1.51) |
| Obese (95th percentile or above) | ||||||
| Prevalence (SE) | 13.1 (0.5) | 20.4 (1.3)a | 31.8 (1.5)a | 19.8 (5.0) | 17.6 (1.4)b | 20.3(1.9)a |
| Unadjusted PR (95 % CI) | Ref | 1.6 (1.34–1.82) | 2.2 (1.50–3.24) | 1.5 (0.71–2.98) | 1.3 (1.04–1.53) | 1.5 (1.14–1.83) |
| aPR (95 % CI) | Ref | 1.5 (1.25–1.75) | 2.1 (1.44–3.16) | 1.4 (0.69–2.98) | 1.2 (0.98–1.51) | 1.3 (0.99–1.70) |
Adjusted for child’s age, sex, race/ethnicity, mother’s education, poverty-to-income ratio, and birthweight
Ref: The referent group for this analysis is all children not in the BMI group of interest. Supplemental analysis (data not shown) in which the referent group is normal weight children showed similar results
SE Standard error, CI confidence interval, aPR Adjusted prevalence ratio
P < .001;
P <.05
The prevalence of overweight for adolescents with any DD was 17.5 % which was similar to the prevalence in adolescents without DDs. Variation across DDs was minimal with no significant differences.
The prevalence of underweight for adolescents with any DD was 5.6 % compared to 3.5 % for adolescents without DDs. This 60 % differential remained significant after adjustment for potential confounders. While the prevalence of underweight was elevated for adolescents in all four DD groups, the increase was particularly notable among adolescents in the ID group who had a fourfold higher prevalence. However, this difference was greatly reduced after adjustment for confounders; LBW was found to be a particularly impactful confounder.
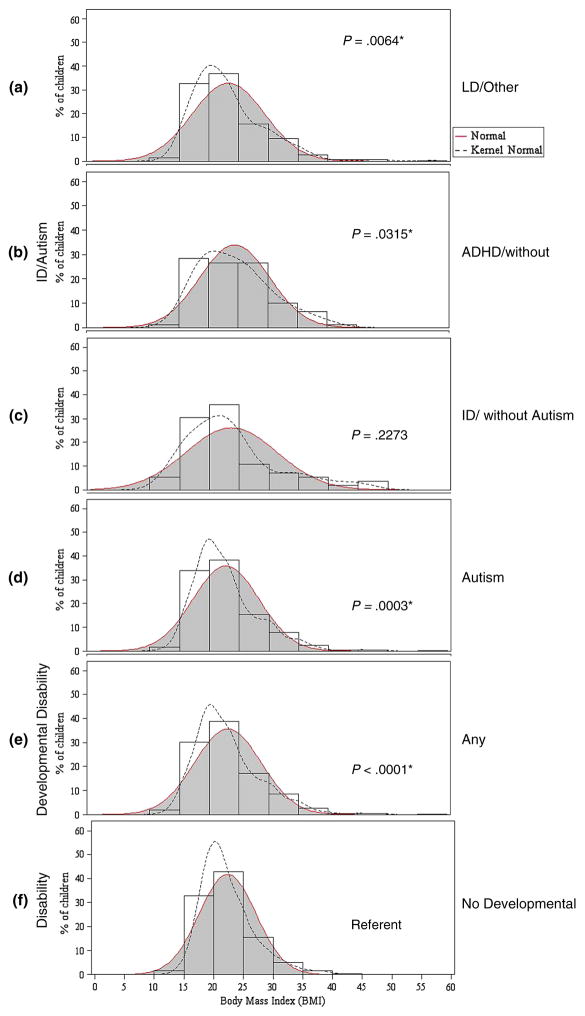
The overall BMI distribution for adolescents in each of the four DD groups was wider than that for adolescents without DDs (Fig. 1). These differences were statistically significant, with the exception of the ID group (which was limited by small sample size). While the median BMIs varied only slightly across groups of adolescents with and without DDs, adolescents with DDs were less likely to have a BMI near their group’s median.
Fig. 1.
Body mass index distributions of adolescents 12–17 years of age with and without developmental disabilities, NHIS 2008–2010. *Statistical significance is based on comparison of distribution curves (a–e) to distribution curve (f) using the Kolmogorov–Smirnov test P value
Supplemental Analyses of Underweight and Obesity Status by Prescription Medication Use
Because many psychoactive medications might be associated with child appetite and metabolism, we repeated prevalence analyses for adolescents with the two most commonly reported DDs, ADHD and LD/other developmental delay, after stratifying by recent prescription medication use. Among adolescents with both DD types, obesity was significantly increased only for those adolescents NOT taking prescription medications [aPR 1.6 (1.2–2.1) for the ADHD group and aPR 1.9 (1.3–2.7) for the LD/other developmental delay group]. Additionally, adolescents with these DDs who were taking prescription medications tended to have a higher prevalence of underweight than adolescents without DDs; however, these findings did not reach statistical significance (data not shown). We were unable to study these medication subsets in children with autism or ID because of sample size constraints.
Risk Factors for Obesity
The obesity risk factor profile among adolescents without DDs was different than the profile for adolescents with DDs (Table 3). Among adolescents without DDs, obesity was higher in boys than girls and among adolescents 12–14 versus 15–17 years of age. Neither sex nor age were risk factors for obesity among adolescents with DDs. Additionally, LBW was associated with a twofold increase in obesity among adolescents in two DD groups (autism and ID without autism) but was not associated with obesity among adolescents without DDs. (However the association for the ID group approached but did not reach statistical significance.)
Table 3.
Risk factors for obesity within adolescents 12–17 years of age without and with developmental disabilities, by developmental disability type, NHIS, 2008–2010
| Model included the following factors | Children without developmental disabilities aPR (95 % CI) |
Children with any learning and behavioral disability aPR (95 % CI) |
Children with autism aPR (95 % CI) |
Children with intellectual disability (ID) without autism aPR (95 % CI) |
Children with ADHD without autism/ID aPR (95 % CI) |
Children with LD/other DD without ADHD/autism/ID aPR (95 % CI) |
|---|---|---|---|---|---|---|
| Sexa | ||||||
| Male | 1.5 (1.30–1.77) | 1.0 (0.78–1.39) | 1.1 (0.36–3.10) | 1.1 (0.40–3.23) | 1.0 (0.61–1.51) | 1.2 (0.64–2.29) |
| Female | Ref | Ref | Ref | Ref | Ref | Ref |
| Age (years)a | ||||||
| 12–14 | 1.4 (1.19–1.63) | 1.0 (0.73–1.24) | 0.5 (0.25–1.10) | 0.5 (0.18–1.42) | 1.0 (0.66–1.44) | 0.7 (0.43–1.25) |
| 15–17 | Ref | Ref | Ref | Ref | Ref | Ref |
| Race/ethnicitya | ||||||
| Non-Hispanic black | 1.8 (1.38–2.26) | 1.3 (0.89–1.79) | 2.3 (1.27–4.13) | 0.5 (0.62–2.20) | 1.3 (0.78–2.01) | 0.8 (0.41–1.64) |
| American Indian/Native American | 1.9 (0.89–4.02) | 2.6 (1.83–3.70) | n/a | 1.8 (0.36–8.55) | 3.0 (1.82–4.98) | 2.4 (1.01–5.70) |
| Asian | 0.8 (0.53–1.23) | 0.9 (0.29–2.74) | 1.6 (0.66–4.08) | 1.8 (0.35–8.78) | 3.0 (2.00–4.53) | 2.4 (1.23–4.65) |
| Other | 1.6 (1.04–2.39) | 1.5 (0.82–2.57) | 0.4 (0.06–2.46) | 1.8 (0.36–8.55) | 2.0 (0.99–3.93) | 0.7 (0.19–2.59) |
| Hispanic | 1.6 (1.26–1.90) | 1.2 (0.85–1.66) | 0.5 (0.11–2.40) | 1.2 (0.62–2.20) | 1.2 (0.70–1.96) | 1.2 (0.64–2.30) |
| Non-Hispanic White | Ref | Ref | Ref | Ref | Ref | Ref |
| Maternal Educationa | ||||||
| High school or less | 0.7 (0.57–0.83) | 1.0 (0.78–1.37) | 0.5 (0.29–1.00) | 1.9 (0.74–4.90) | 1.1 (0.76–1.60) | 1.4 (0.85–2.43) |
| Beyond high school | Ref | Ref | Ref | Ref | Ref | Ref |
| Poverty-to-income ratioa | ||||||
| Below 200 % | 1.2 (0.98–1.45) | 1.4 (1.08–1.94) | 0.6 (0.30–1.24) | 3.7 (1.12–12.25) | 1.5 (1.01–2.28) | 2.5 (1.46–4.42) |
| At or above 200 % | Ref | Ref | Ref | Ref | Ref | Ref |
| Birthweighta | ||||||
| Less than 2,500 g | 1.0 (0.76–1.26) | 1.4 (0.97–1.96) | 2.7 (1.54–4.76) | 2.4 (0.95–6.03) | 1.0 (0.55–1.92) | 1.6 (0.77–3.53) |
| Greater than or equal to 2,500 | Ref | Ref | Ref | Ref | Ref | Ref |
aPR Adjusted prevalence ratio, CI confidence interval
Prevalence ratio is adjusted for child’s age, sex, race/ethnicity, mother’s education, poverty-to-income ratio, and birthweight
The three risk factors related to socioeconomic status, race-ethnicity, maternal education, and poverty-to-income ratio, were associated with obesity among adolescents both with and without DDs, but the specific factors associated with obesity within each group and subgroup varied.
Concurrent Health Conditions
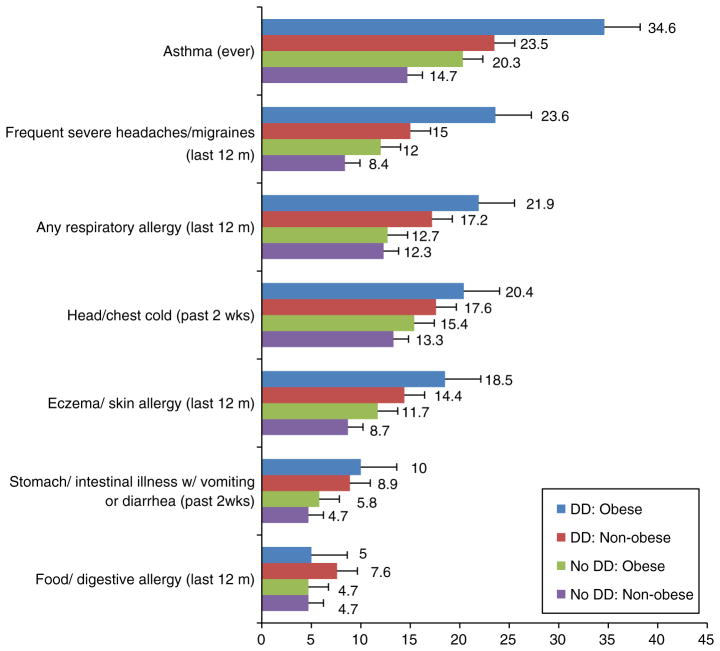
Non-obese adolescents without DDs had the lowest prevalence estimates for most health conditions assessed, while obese adolescents with DDs had the highest estimates (Fig. 2). Obesity in the absence of a DD was associated with asthma, eczema, and frequent severe headaches/migraines (Table 4). Having a DD in the absence of obesity was associated with all of the aforementioned conditions and also respiratory allergy and very recent intestinal illness. Additionally, among adolescents with DDs, being obese conferred an additional risk for several conditions. Obese adolescents with DDs had a 50 % higher asthma prevalence than non-obese adolescents with DDs. Likewise, adolescents with both a DD and obesity had 30–40 % higher prevalences of respiratory allergy, eczema/skin allergy, and frequent severe headaches/migraines than adolescents with a DD but not obesity; however, the confidence intervals for the adjusted PRs slightly overlapped 1.0.
Fig. 2.
Percentages of concurrent medical conditions among non-obese and obese adolescents 12–17 years of age with and without developmental disabilities, NHIS 2008–2010. ┤: Displays error bars for the percent using standard error
Table 4.
Concurrent medical conditions among non-obese and obese adolescents 12–17 years of age with developmental disabilities, NHIS 2008–2010
| Medical conditions | Obese children without DDs versus non-obese children without DDs (Risk associated with obesity in the absence of DDs) aPR (95 % CI) |
Non-obese children with DDs versus non-obese children without DDs (Risk associated with having a DD in the absence of obesity) aPR (95 % CI) |
Obese children with DDs versus non-obese children with DDs (Additional risk associated with obesity among children who have a DD) aPR (95 % CI) |
Obese children with DDs versus obese children without DDs (Additional risk associated with having a DD among children who are obese) aPR (95 % CI) |
|---|---|---|---|---|
| Asthma (ever) | 1.27 (1.07–1.50) | 1.56 (1.21–2.01) | 1.50 (1.12–2.02) | 1.36 (1.13–1.63) |
| Any respiratory allergy (last 12 m) | 1.07 (0.85–1.35) | 1.53 (1.12–2.08) | 1.29 (0.94–1.76) | 1.28 (1.06–1.55) |
| Head/chest cold (past 2 weeks) | 1.22 (0.98–1.52) | 1.22 (0.87–1.70) | 1.16 (0.84–1.61) | 1.34 (1.10–1.63) |
| Eczema/skin allergy (last 12 m) | 1.41 (1.14–1.74) | 1.41 (1.05–1.91) | 1.32 (0.96–1.80) | 1.56 (1.29–1.88) |
| Food/digestive allergy (last 12 m) | 1.04 (0.74–1.47) | 1.10 (0.67–1.82) | 0.68 (0.37–1.25) | 1.51 (1.14–2.00) |
| Stomach/intestinal illness w/vomiting or diarrhea (past 2 weeks) | 1.19 (0.86–1.64) | 1.40 (0.92–2.12) | 1.08 (0.70–1.67) | 1.64 (1.29–2.08) |
| Frequent severe headaches/migraines (last 12 m) | 1.37 (1.10–1.69) | 1.71 (1.33–2.20) | 1.38 (1.00–1.90) | 1.60 (1.32–1.94) |
Prevalence ratio is adjusted for child’s age, sex, race/ethnicity, mother’s education, poverty-to-income ratio, and birthweight
aPR Adjusted prevalence ratio, CI confidence interval
Discussion
Underweight and Obesity Prevalence
This population-based study indicates that US adolescents with learning and behavioral DDs are 60 % more likely to be obese and also 60 % more likely to be underweight than adolescents without DDs.
We found that 31.8 % of adolescents with autism were obese, more than twice the prevalence observed for adolescents without DDs. Two previous studies of US children with autism that assessed the 2003 National Survey of Children’s Health (NSCH) reported obesity estimates of 30.4 % for children 3–17 years of age and 21.1 % for children 10–17 years of age [3, 5]. The differences in estimates from our study and previous studies might partially reflect differences in the survey time periods.
The explanation for the autism-obesity association cannot be examined in this study. Previous studies reported that children with autism have overly selective or idiosyncratic food preferences [7, 29, 30] and that some children with autism tend to solely eat highly caloric foods and foods that are high in sugar content [31, 32]. A recent study reported that adolescents with autism have decreased levels of moderate to vigorous physical activity and have increased sedentary activity compared to their peers without autism [33]. While we observed a twofold-higher obesity prevalence among adolescents with autism than without DDs, we did not observe a prevalence differential for overweight. In fact, in contrast to our sample of children without DDs, children with autism had a higher prevalence of obesity (31.8 %) than overweight (20.9 %). A similar pattern was observed for children with ID, but not for children in the other DD groups. While we can only speculate on the reason for this unusual pattern of obesity versus overweight, the increased obesity risk among children with autism might be driven by marked differences in dietary and physical activity patterns among the subset with the most severe developmental functional limitations. Additionally, previous studies indicate a substantial proportion of individuals with autism and ID receive various psychotropic medications, with weight gain and increased appetites as common untoward side effects [34, 35]. These effects might be more likely to lead to obesity than overweight. We were unable to evaluate the impact of prescription drug use on BMI in our samples of children with autism and ID.
Our findings for adolescents with ID without autism must be interpreted cautiously due to sample size constraints. Nonetheless, our estimates generally inform the sparse literature for this subgroup and suggest that prevalence of both obesity and underweight might be elevated among children with ID. To date, most studies examining obesity and ID have been in adult samples comparing institutionalized and non-institutionalized persons and suggest that low income and associated environmental factors have considerable impacts on caloric intake and physical activity among persons with ID [31, 36]. Studies also report that children with ID have significantly higher rates of poverty, are more physically inactive, and play less often with other children than children without DDs [3, 32, 37, 38].
Our initial finding of a 20–30 % increased prevalence of obesity among adolescents with ADHD and LD/other developmental delay was limited to adolescents who were NOT taking prescription medications. In this subset, obesity was 60–90 % higher than the rate observed among adolescents without DDs. These findings are comparable to the findings reported by previous studies [3, 39]. Studies have also reported that children with ADHD have difficulty with self-regulation, low physical activity, low gross motor performance, and delayed motor development, which might contribute to their obesity risk [40].
One previous study suggested an increase in underweight among those children with ADHD who were taking prescription medications [39]. While we also observed a higher prevalence of underweight in this subset of children with ADHD, the estimate was imprecise.
In addition to increased obesity prevalence among adolescents with DDs, we also observed variation in obesity risk factors among adolescents with and without DDs. While race-ethnicity and SES indicators were associated with obesity in both adolescents with and without DDs, the specific race-ethnicity groups associated with higher obesity risk varied across DDs. Additionally, age and sex were associated with obesity only among adolescents without DDs. LBW was associated with obesity only among adolescents with DDs. We lacked data to explore this DD-specific LBW association further.
Data on obesity risk factors among children with DDs has been limited. Our findings among adolescents without DDs generally match previous studies that report higher levels of obesity in NHB and Hispanic adolescents than NHW adolescents [41–44], in adolescent males than females [41], and in children living in families with lower than higher household incomes [7]. Additionally, a recent meta-analysis indicated no association between obesity and LBW in children generally [45].
Obesity and DD Co-morbidity and Health Consequences
Our assessment of concurrent health conditions in relation to both obesity and DD statuses, expands on a previous report by our group that documented higher prevalence of common respiratory, gastrointestinal, dermatological and neurological health conditions among children with DDs than children without DDs [27]. Here, we document that the prevalence of these concurrent medical conditions is additionally impacted by obesity such that adolescents who had either a learning or behavioral DD or were obese had higher estimates for nearly every health condition included in this analysis than non-obese adolescents without DDs. For nearly all conditions assessed, the highest estimates were observed for adolescents with both obesity and a DD.
These findings are supported by previous studies of health consequences of obesity among children with DDs [3, 6, 8, 17], although Yamaki et al. [8] did not distinguish in their study between obesity and overweight. Studies have also shown associations between increased BMI and asthma risk among children generally [23, 46, 47]. Obesity not only increases risk of asthma but has been observed to also interfere with optimal asthma management [23].
Limitations and Strengths
Interpretation of our findings should include consideration of potential limitations. Because NHIS data are cross-sectional, our ability to assess temporality and infer causal pathways between DDs, BMI, and various health conditions was limited. All health conditions and DDs were based on parent report and not verified clinically. BMI estimates were not based on direct measurement and thus should not be considered the US population gold standard. Because children with DDs may use more health care, they might be weighed and measured slightly more frequently than children without DDs, which could possibly impact parental recall. We have no other reason to believe that parental height and weight reporting was differential between adolescents with and without DDs. A previous study comparing parent-reported weight and height estimates from national surveys, including NHIS, to measured estimates from NHANES found that the relative differences in parent-reported versus measured estimates of mean height were very small (<2 %) among all adolescents as were the relative differences in mean weight for adolescent males; relative differences in mean weight for adolescent females were slightly larger (4–6 % depending on age) and suggestive of under-reporting [44]. Thus, obesity rates reported here might have been slightly attenuated among females. We lacked data on dietary intake, physical activity levels, and family history of obesity. However for a portion of our sample (33 %), maternal BMI was available from a supplemental adult health survey. Limited analyses of this subsample suggests that our findings of associations between DD status and obesity and underweight prevalence were independent of maternal BMI, as were our findings about obesity risk factors (data not shown). While we were able to examine the DD-BMI outcome relationships by prescription medication use generally, we lacked data on specific psychoactive medications. Lastly, NHIS samples do not include institutionalized children, and thus a small percentage of children with the most severe DDs might not be represented in our analysis.
Despite the limitations, this analysis includes a recent and comprehensive assessment of BMI status, risk factors, and potential health consequences of obesity among US adolescents with learning and behavioral DDs. To our knowledge, no other US study specifically examined the full range of the BMI distribution in children with these DDs; nor did other studies to date consider the co-occurrence of DDs. The NHIS sample afforded us the opportunity to create mutually-exclusive DD groups, and thus examine DDs such as ADHD and LD independently from autism and ID, which are typically more pervasive.
Conclusion
Obesity poses significant chronic health risks within an already vulnerable population of adolescents with DDs. Current prevention strategies to combat childhood obesity may not be appropriate for all families of children with DDs. Most reports emphasize the importance of physical activity and recommend activities [17] which may or may not be appropriate for children and adolescents with DDs. Population-specific approaches have been proposed for subgroups such as infants, toddlers, school-age children and adolescents, and children in ethnic minority populations, but currently, there are no specific recommendations for children or adolescents with DDs [17]. Obesity prevention and management approaches for this at-risk group thus needs further consideration. Although the prevalence of underweight was much lower than obesity, our finding of a potential increased risk for underweight among children with DDs, is also in need of further investigation to understand the underlying causes and possible prevention strategies.
Contributor Information
Keydra L. Phillips, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, 1600 Clifton Rd, Mailstop E-86, Atlanta, GA 30333, USA
Laura A. Schieve, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, 1600 Clifton Rd, Mailstop E-86, Atlanta, GA 30333, USA
Susanna Visser, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, 1600 Clifton Rd, Mailstop E-86, Atlanta, GA 30333, USA.
Sheree Boulet, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, 1600 Clifton Rd, Mailstop E-86, Atlanta, GA 30333, USA.
Andrea J. Sharma, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, GA, USA
Michael D. Kogan, Maternal and Child Health Bureau, Health Resources and Services Administration, Rockville, MD, USA
Coleen A. Boyle, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, 1600 Clifton Rd, Mailstop E-86, Atlanta, GA 30333, USA
Marshalyn Yeargin-Allsopp, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, 1600 Clifton Rd, Mailstop E-86, Atlanta, GA 30333, USA.
References
- 1.Singh GK, Kogan MD. A 75th Anniversary Publication. Health Resources and Services Administration, Maternal and Child Health Bureau. Rockville, MD: U.S. Department of Health and Human Services; 2010. Childhood obesity in the United States, 1976–2008: Trends and current racial/ethnic, socioeconomic, and geographic disparities; pp. 1–8. [Google Scholar]
- 2.Bandini LG, Curtin C, Hamad C, Tybor DJ, Must A. Prevalence of overweight in children with developmental disorders in the continuous national health and nutrition examination survey (NHANES) 1999–2002. The Journal of Pediatrics. 2005;146:738–743. doi: 10.1016/j.jpeds.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 3.Chen AY, Kim SE, Houtrow AJ, Newacheck PW. Prevalence of obesity among children with chronic conditions. Obesity. 2009;18:210–213. doi: 10.1038/oby.2009.185. [DOI] [PubMed] [Google Scholar]
- 4.Curtin C, Bandini LG, Perrin EC, Tybor DJ, Must A. Prevalence of overweight in children and adolescents with attention deficit hyperactivity disorder and autism spectrum disorders: A chart review. BMC Pediatrics. 2005;5:48–54. doi: 10.1186/1471-2431-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curtin C, Anderson SE, Must A, Bandini L. The prevalence of obesity in children with autism: A secondary data analysis using nationally representative data from the National Survey of Children’s Health. BMC Pediatrics. 2010;10:11–15. doi: 10.1186/1471-2431-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rimmer J, Yamaki K, Davis B, Wang E, Vogel LC. Obesity and obesity-related secondary conditions in adolescents with intellectual/developmental disabilities. Journal of Intellectual Disability Research. 2010;54(9):787–794. doi: 10.1111/j.1365-2788.2010.01305.x. [DOI] [PubMed] [Google Scholar]
- 7.Van Cleave J, Gortmaker SL, Perrin JM. Dynamics of obesity and chronic health conditions among children and youth. JAMA. 2010;303(7):623–630. doi: 10.1001/jama.2010.104. [DOI] [PubMed] [Google Scholar]
- 8.Yamaki K, Rimmer J, Lowry BM, Vogel LC. Prevalence of obesity-related chronic health conditions in overweight adolescents with disabilities. Research in Developmental Disabilities. 2011;32:280–288. doi: 10.1016/j.ridd.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Fryar CD, Ogden CL. Prevalence of underweight among children and adolescents aged 2–19 years: United States, 1963–1965 through 2007–2010. Division of Health and Nutrition Examination Surveys. Health E-Stat. 2012:1–3. [Google Scholar]
- 10.Centers for Disease Control and Prevention. Division of Health Interview Statistics NCHS. National Health Interview Survey (NHIS) public use data release: NHIS survey description. National Center for Health Statistics; 2013. [Accessed October 30, 2013]. http://www.cdc.gov/nchs/nhis/about_nhis.htm. [Google Scholar]
- 11.Bloom B, Cohen RA, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2008. National Center for Health Statistics Vital Health Statistics. 2009;10(244):1–81. [PubMed] [Google Scholar]
- 12.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC growth charts for the United States: Methods and development. Vital Health Statistics. 2002;11:1–190. [PubMed] [Google Scholar]
- 13.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: Improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 14.Bloom B, Cohen RA, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2009. National Center for Health Statistics Vital Health Statistics. 2010;10(247):1–82. [PubMed] [Google Scholar]
- 15.Bloom B, Cohen RA, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2010. National Center for Health Statistics Vital Health Statistics. 2011;10(250):1–80. [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. [Accessed October 30, 2013];Basics about childhood obesity. 2013 http://www.cdc.gov/obesity/childhood/basics.html.
- 17.Daniels SR. The consequences of childhood overweight and obesity. The Future of Children. 2006;16:47–67. doi: 10.1353/foc.2006.0004. [DOI] [PubMed] [Google Scholar]
- 18.Daniels SR, Jacobson MS, McCrindle BW, Eckel RH, Sanner BM. American Heart Association childhood obesity research summit report. Circulation. 2009;119:e489–e517. doi: 10.1161/CIRCULATIONAHA.109.192216. [DOI] [PubMed] [Google Scholar]
- 19.Dietz WH, Robinson TN. Assessment and treatment of childhood obesity. Pediatrics in Review. 1993;14:337–343. doi: 10.1542/pir.14-9-337. [DOI] [PubMed] [Google Scholar]
- 20.Dietz WH. Health consequences of obesity in youth: Childhood predictors of adult disease. Pediatrics. 1998;101(Suppl):518–525. [PubMed] [Google Scholar]
- 21.Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: The Bogalusa Heart Study. The Journal of Pediatrics. 2007;150(1):12–17. doi: 10.1016/j.jpeds.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 22.Han JC, Lawlor DA, Kimm SY. Childhood obesity. Lancet. 2010;375(9727):1737–1748. doi: 10.1016/S0140-6736(10)60171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutherland ER. Obesity and asthma. Immunology and Allergy Clinics of North America. 2008;28(3):589–602. doi: 10.1016/j.iac.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swartz MB, Puhl R. Childhood obesity: A societal problem to solve. Obesity Reviews. 2003;4(1):57–71. doi: 10.1046/j.1467-789x.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 25.Taylor ED, Theim KR, Mirch MC, Ghorbani S, Tanofsky-Kraff M, Adler-Wailes DC, et al. Orthopedic complications of overweight in children and adolescents. Pediatrics. 2006;117(6):2167–2174. doi: 10.1542/peds.2005-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitlock EP, Williams SB, Gold R, Smith PR, Shipman SA. Screening and interventions for childhood overweight: A summary of evidence for the US Preventive Services Task Force. Pediatrics. 2005;116(1):e125–e144. doi: 10.1542/peds.2005-0242. [DOI] [PubMed] [Google Scholar]
- 27.Schieve LA, Gonzalez V, Boulet SL, Visser SN, Ric CE, Braun KV, et al. Concurrent medical conditions and health care use and needs among children with learning and behavioral developmental disabilities, National Health Interview Survey, 2006–2010. Research in Developmental Disabilities. 2012;33(2):467–476. doi: 10.1016/j.ridd.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Bieler GS, Brown GG, Williams RL, Brogan DJ. Estimating model-adjusted risks, risk differences, and risk ratios from complex survey data. American Journal of Epidemiology. 2010;171(5):618–623. doi: 10.1093/aje/kwp440. [DOI] [PubMed] [Google Scholar]
- 29.Schreck KA, Williams K, Smith AF. A comparison of eating behaviors between children with and without autism. Journal of Autism and Developmental Disorders. 2004;34(4):433–438. doi: 10.1023/b:jadd.0000037419.78531.86. [DOI] [PubMed] [Google Scholar]
- 30.Schreck KA, Williams K. Food preferences and factors influencing food selectivity for children with autism spectrum disorders. Research in Developmental Disabilities. 2006;27:353–363. doi: 10.1016/j.ridd.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Yamaki K, Fujiura GT. Employment and income status of adults with developmental disabilities living in the community. Mental Retardation. 2002;40(2):132–141. doi: 10.1352/0047-6765(2002)040<0132:EAISOA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 32.Rurangirwa J, Van Naarden Braun K, Schendel D, Year-gin-Allsopp M. Healthy behaviors and lifestyles in young adults with a history of developmental disabilities. Research in Developmental Disabilities. 2006;27(4):381–399. doi: 10.1016/j.ridd.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 33.MacDonald M, Esposito P, Ulrich D. The physical activity patterns of children with autism. BMC Research Notes. 2011;4:422–426. doi: 10.1186/1756-0500-4-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weeden M, Ehrhardt K, Poling A. Psychotropic drug treatments for people with autism and other developmental disorders: A primer for practicing behavior analysts. Behavior Analysis in Practice. 2010;3(1):4–12. doi: 10.1007/BF03391753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doyle CA, McDougle CJ. Pharmacologic treatments for the behavioral symptoms associated with autism spectrum disorders across the lifespan. Dialogues in Clinical Neuroscience. 2012;14(3):263–279. doi: 10.31887/DCNS.2012.14.3/cdoyle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rimmer JH, Yamaki Y. Obesity and intellectual disability. Mental Retardation and Developmental Disabilities Research Reviews. 2006;12:22–27. doi: 10.1002/mrdd.20091. [DOI] [PubMed] [Google Scholar]
- 37.Reinehr T, Dobe M, Winkel K, Schaefer A, Hoffmann D. Obesity in disabled children and adolescents. An overlooked group of patients. Deutsches Ärzteblatt International. 2010;107(15):268–275. doi: 10.3238/arztebl.2010.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rurangirwa J, Van Naarden Braun K, Schendel D, Year-gin-Allsopp M. Healthy behaviors and lifestyles in young adults with a history of developmental disabilities. Research in Developmental Disabilities. 2006;27(4):381–399. doi: 10.1016/j.ridd.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Waring ME, Lapane KL. Overweight in children and adolescents in relation to attention-deficit/hyperactivity disorder: Results from a national sample. Pediatrics. 2008;122(1):e1–e6. doi: 10.1542/peds.2007-1955. [DOI] [PubMed] [Google Scholar]
- 40.Kim J, Mutyala B, Agiovlasitis S, Fernhall B. Health behaviors and obesity among US children with attention deficit hyperactivity disorder by gender and medication use. Preventive Medicine. 2011;52(3–4):218–222. doi: 10.1016/j.ypmed.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303(3):242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 42.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 43.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 44.Akinbami LJ, Ogden CL. Childhood overweight prevalence in the United States: The impact of parent-reported height and weight. Obesity (Silver Spring) 2009;17(8):1574–1580. doi: 10.1038/oby.2009.1. [DOI] [PubMed] [Google Scholar]
- 45.Yu ZB, Han SP, Zhu GZ, Zhu C, Wan XJ, Cao XG, et al. Birth weight and subsequent risk of obesity: A systematic review and meta-analysis. Obesity Reviews. 2011;12(7):525–542. doi: 10.1111/j.1467-789X.2011.00867.x. [DOI] [PubMed] [Google Scholar]
- 46.Gilliland FD, Berhane K, Islam T, McConnell R, Gauderman WJ, Gilliland SS, et al. Obesity and the risk of newly diagnosed asthma in schoolage children. American Journal of Epidemiology. 2003;158:406–415. doi: 10.1093/aje/kwg175. [DOI] [PubMed] [Google Scholar]
- 47.Gold DR, Damokosh AI, Dockery DW, Berkey CS. Body-mass index as a predictor of incident asthma in a prospective cohort of children. Pediatric Pulmonology. 2003;36(6):514–521. doi: 10.1002/ppul.10376. [DOI] [PubMed] [Google Scholar]