Abstract
We introduce a new biochemically responsive Mn-based MRI contrast agent that provides a 9-fold change in relaxivity via switching between the Mn3+ and Mn2+ oxidation states. Interchange between oxidation states is promoted by a “Janus” ligand that isomerizes between binding modes that favor Mn3+ or Mn2+. It is the only ligand that supports stable complexes of Mn3+ and Mn2+ in biological milieu. Rapid interconversion between oxidation states is mediated by peroxidase activity (oxidation) and L-cysteine (reduction). This Janus system provides a new paradigm for the design of biochemically responsive MRI contrast agents.
Molecular magnetic resonance imaging (MRI) adds a dimension of biochemical specificity to the rich anatomic and physiological data attainable through MRI.1,2 Biochemical specificity is achieved either by conjugating an MRI contrast agent to a targeting vector, or engineering the contrast agent to undergo change in magnetic relaxation, or “activation,” in the presence of a biochemical stimulus. Innovative chemistry has generated molecular MRI contrast agents capable of detecting protein targets,3 pH change,4 redox activity,5 hypoxia,6 ion flux,7 neurotransmitters,8 necrosis,9 and enzyme activity.10
Sensitivity, dynamic range, and rate of response are the three main challenges to developing molecular MRI contrast agents. T1-relaxation agents are detected at ≥10 μM (metal ion), thus only a handful of cellular and protein targets are candidates for imaging with targeted agents.1 Activatable agents suffer from poor dynamic range. With a few notable exceptions,3,11,12 activatable Gd-based agents rarely achieve >2-fold r1 change in the presence of physiologically relevant levels of biochemical stimuli. Often, activatable contrast agents require prolonged incubation times before measurable relaxation change is observed.
Elegant examples of activatable agents detected via changes in the chemical exchange saturation transfer (CEST) effect have been reported,4 but these agents are typically detected with far lower sensitivity than T1-agents.1
Although comparatively underexplored, coordination complexes that undergo a biochemically mediated change in paramagnetism offer a promising strategy to expand the dynamic range of activatable probes.13–15 We, and others, have pursued biochemically activated MR contrast agents that utilize the Mn3+/2+ couple as the activation mechanism.16–20 The Mn3+/2+ couple is physiologically tenable and can be tuned through ligand modifications.21 High spin Mn2+ is a potent relaxation agent, whereas Mn3+ is a much less effective relaxation agent.22
Rational design of redox activated Mn-based contrast agents is challenging. Most ligand systems support a single oxidation state. Polyaminocarboxylate chelators like BPED, Chart 1, and EDTA bind Mn2+ with high affinity, Table S1,23 but the redox potential of the corresponding Mn3+ complex is very high and the Mn3+ chelates decompose within seconds in aqueous media.24 Mn3+ is stabilized by strongly electron releasing ligands like HBED, Chart 1, and TPPS, Fig S1. Coordination of Mn2+ by these ligands triggers spontaneous oxidation to Mn3+.18,25 The HBET ligand, Fig S1, stabilizes both oxidation states but the Mn3+/2+ redox potential is >0.50 V more positive than that of tissues and cells.26,27 Although Mn3+-HBET can be prepared and is stable in aqueous solution, it does not persist in blood plasma, Figs S2–3.
Chart 1.
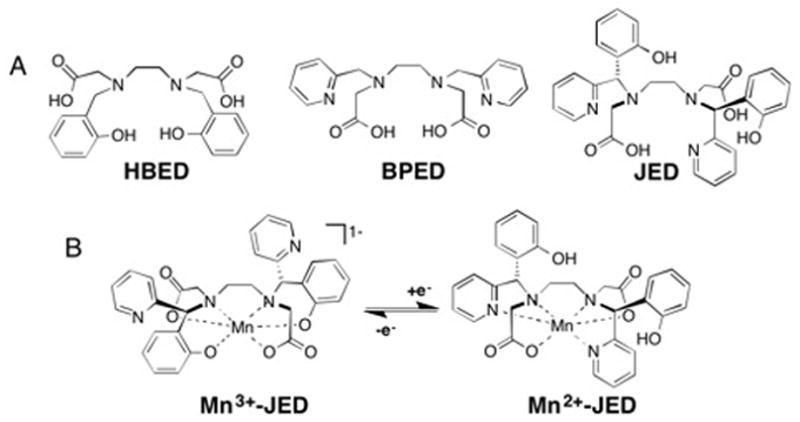
(A) Mn3+ and Mn2+ selective chelators, HBED and BPED, respectively, and the Janus chelate, JED, designed to support both Mn3+- and Mn2+. (B) Redox triggered isomerization of Mn3+- and Mn2+-JED.
We hypothesized that we could stabilize both oxidation states using a ligand that isomerizes between Mn3+ or Mn2+ selective chelators. Our prototype ligand to test this strategy, JED (“Janus HBED/BPED”), Chart 1, is designed to present a HBED-type donor set to Mn3+ and a BPED-type donor set to Mn2+. Upon oxidation or reduction, the opposite Janus face will capture the otherwise unstable oxidation state. By analogy with previously characterized Mn2+ complexes with acyclic hexadentate chelators, we anticipated the Mn2+ complex would form a 7-coordinate, ternary complex with a rapidly exchanging water co-ligand, a requisite to high relaxivity.28,29 The smaller Mn3+ ion is expected to be 6-coordinate with no inner-sphere water ligand and low relaxivity, analogous to the related [Mn(EHPG)]− complex, Fig S1.30
Diasteromerically pure JED (R,R/S,S) was isolated following a 7 step synthesis, Scheme 1. Aldol reaction of phenol and 2-pyrdinecarboxaldyde, 1, followed by SeO2 oxidation yielded the synthon for the Janus pyridyl-N/phenlato-O donor, 2. Double condensation of 2 with ethylenediamine yielded 3, which was reduced with NaBH4 to a diastereomeric mixture of diamine 4. Preparative separation of the diastereomers of 4 proved difficult, but facile separation was achieved after Zn chelation, Figs S4–5. Zn was stripped from the diastereomerically pure 4 with excess DTPA at pH 5.0. Introduction of the acetate-O donors via reductive amination of (R,R/S,S) 4 with glyoxylic acid yielded JED. The Mn2+ and Mn3+ complexes were independently synthesized via reaction of JED with MnCl2 or MnF3, respectively.
Scheme 1. Synthesis of JEDa,b.
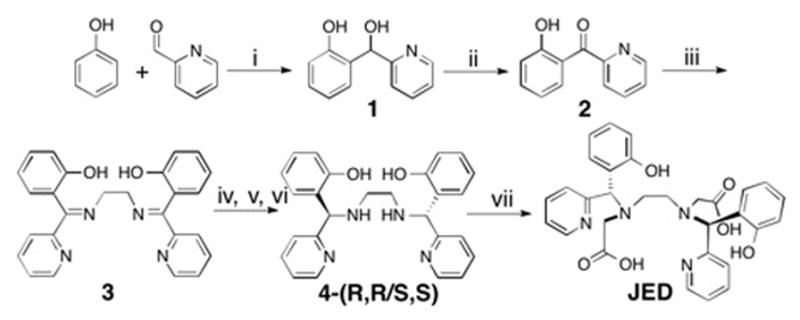
a(i) MgCl2, Me2NEt, CH2Cl2, RT; (ii) SeO2, dioxane, 100 °C; (iii) 0.5 mol. equiv. ethylenediamine, MeOH, RT; (iv) NaBH4, (v) Zn(OTf)2, 1:1 MeCN:H2O, RT – diastereomers of Zn•4 separated by RP-HPLC; (vi) excess DTPA, pH 5.0; (vii) glyoxylic acid, NaBH3CN, NaHCO3, MeOH. bJED was prepared from the (R,R/S,S) diastereomer of 4; (S,S) 4 and JED are depicted here.
“BPED-type” binding of the Mn2+ was confirmed by UV-vis spectroscopy. Prior work with HBET and Mn2+-HBET complexes showed that phenol ionization is accompanied by a significant red-shift that can be used to track phenolato-O coordination.17 JED exhibits a similar red shift (268 to 308 nm) at pH > 10, Fig S9. The absorbance profile of Mn2+-JED shows no evidence of phenol ionization out to pH 8, Fig S10. The Mn3+ complex can only exist if the Janus switch to HBED-type binding occurs. HBED-type binding to Mn3+ is further evidenced by UV-vis spectroscopy. The Mn3+-JED LMCT transitions, Fig S12, reflect those of known Mn3+ complexes of ligands similar to HBED.30
Cyclic voltammetry measurements performed on Mn-JED reveal irreversible oxidation and reduction events, Fig S11. From this data we estimate the oxidation and reduction potentials occur at 0.91 V and 0.01 V vs. NHE, respectively. The Mn-JED oxidation and reduction events mirror the Mn2+-BPED oxidation event, 1.11 V, and Mn3+-HBED reduction event, −0.17 V, Table S1.
The thermodynamic stability of Mn2+-JED was evaluated by monitoring the direct competition reaction with BPED (log KpH 7.4 = 11.2, Table S1) using HPLC (0.1 M KNO3, RT) and yielded a log KpH 7.4 = 10.8±0.2 for Mn2+-JED. Mn2+-JED is among the most stable Mn2+ complexes reported, Table S1. Direct measurement of Mn3+ formation constants is challenging, given the fact that the Mn3+ aqua ion spontaneously disproportionates to Mn2+ and Mn4+ in water. Instead, the Mn3+-JED formation constant, log KpH 7.4 = 28.6, was estimated from the Mn2+ formation constant and redox potentials,31 Eq S3. A log KpH 7.4 = 29.4 is estimated for Mn3+-HBED. The congruence between the Mn2+- and Mn3+-JED formation constants with those of Mn2+-BPED and Mn3+-HBED, respectively, provides additional evidence of BPED- and HBED-type JED coordination.
The Mn2+ complex is a much stronger relaxation agent than its Mn3+ sister complex. Relaxivity values were recorded in water and human blood plasma at 1.4 T, 4.7 T and 11.7 T, 37 °C, Table 1. The Mn2+ vs. Mn3+ r1 ratios are amongst the largest measured for any reported activatable Gd- or Mn-based relaxation agent, with a 9-fold change measured in human plasma at 1.4T. For Gd-based agents, the largest dynamic ranges reported are achieved via reactions that result in products that constrain Gd rotational dynamics.11,12 Altering rotational dynamics provides a profound effect at field strengths ≤1.5 T, but is nearly obsolete by 3.0T.32 Mn-JED maintains a 3.3 to 5.0-fold r1 change at 4.7T and 11.7T. The high relaxivity of the Mn2+ complex is consistent with a tertiary complex with a rapidly exchanging water co-ligand. The higher relaxivity of the Mn2+ complex is consistent with a tertiary complex with a rapidly exchanging water co-ligand, while the lower relaxivity of the Mn3+ complex is consistent with a coordinatively saturated complex that precludes water coordination. The increase in relaxivity observed in blood plasma at 1.4T suggests some degree of protein binding.
Table 1.
Relaxivity values, r1, of Mn3+- and Mn2+-JED recorded in water or human plasma (in parenthesis) at 1.41T, 4.7Ta or 11.7T, 37 °C. Relaxivity of Mn2+-JED is much higher regardless of applied field strength.
| Mn3+ | Mn2+ | r1 Mn2+/ r1 Mn3+ | |
|---|---|---|---|
| 1.4T | 0.5±0.01 (0.9±0.01) | 3.3±0.06 (8.0±0.39) | 6.6 (8.9) |
| 4.7Ta | 0.9±0.02 (1.1±0.05) | 4.3±0.32 (3.6±0.21) | 4.8 (3.3) |
| 11.7T | 0.5±0.01 (0.5±0.02) | 2.5±0.08 (1.9±0.08) | 5.0 (4.8) |
4.7T measurements performed at RT.
Independently isolated Mn3+- and Mn2+-JED persist in human blood plasma at 37 °C with little inter-conversion over the course of 24 h. Mn speciation was evaluated by HPLC interfaced to an ICP for Mn detection, Fig 1. Some inter-conversion between oxidation states was observed, but the reaction proceeds slowly. Comparison of the resultant 24 h HPLC traces indicates that equilibrium had not yet been achieved. Both complexes are very stable in plasma; >95% of Mn is present as JED complexes at 24 h.
Figure 1.
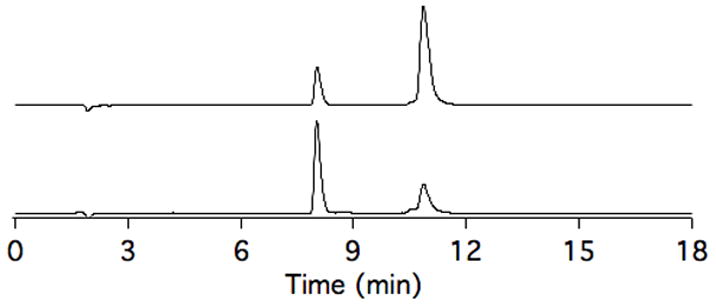
HPLC-ICP-MS traces showing Mn speciation of solutions of Mn2+-JED (top trace) and Mn3+-JED (bottom trace) incubated for 24 h, 37 °C, in human blood plasma. Mn3+- and Mn2+-JED elute at 8.0 and 10.9 min. Inter-conversion between the Mn3+- and Mn2+- complexes is slow, not reaching equilibrium within 24h.
Peroxidase enzymes amplify the reactivity of reactive oxygen species. High peroxidase activity is a salient feature of the acute inflammatory response and molecular imaging of peroxidase has been pursued in animal models of vasculitis, stroke, aneurism, and myocardial infarction.33–36 Up to 250 U/mg (~250,000 U/mL) peroxidase activity has been measured in atherosclerotic lesions,37 while here we show that Mn2+-JED to Mn3+-JED conversion is rapidly mediated by peroxidase activity 4 orders of magnitude lower than what is observed in vivo. Fig 2A shows HPLC traces recorded pre- and post- H2O2/ peroxidase incubation and confirms that conversion to Mn3+-JED occurs without byproducts. Fig 2B depicts rapid peroxidase mediated oxidation of Mn2+-JED, kobs = 19.1±4.75 s−1, in human blood plasma supplied with a steady state of H2O2 via the glucose/glucose oxidase reaction. Peroxidase mediated oxidation to Mn3+-JED occurs on the order of seconds while in blood plasma inter-conversion between oxidation states occurs on the order of days. H2O2 alone triggers negligible conversion, further underscoring a selectively for peroxidase mediated oxidation. The rate measured using r1 change tracks with the rate measured by optical absorbance at 450 nm (ε = 1180 M−1cm−1 for Mn3+-JED), Figs S12–13.
Figure 2.
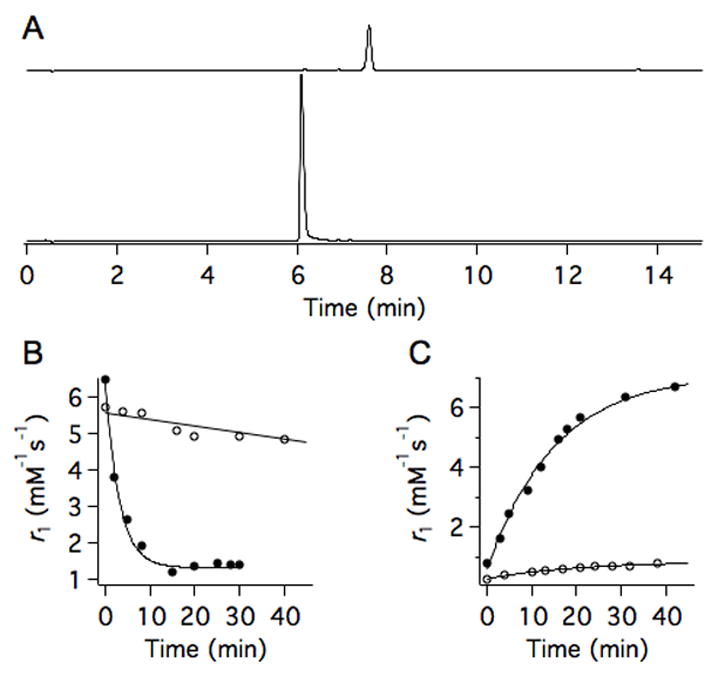
Biochemically mediated interconversion of Mn-JED oxidation states by peroxidase and thiols. (A) Conversion of Mn2+-JED (top trace, 7.6 min) to Mn3+-JED (bottom trace, 6.1 min) triggered by H2O2/ peroxidase (25 U/mL) in PBS buffer monitored by HPLC with 254 nm detection. The difference in peak height between the pre- and post-peroxidase treated samples results from the 2-fold higher extinction co-efficient of Mn3+-JED. (B) Oxidation of Mn2+-JED in the presence of H2O2 (open circles) or H2O2/peroxidase (15 U/mL, closed circles) in human blood plasma monitored by NMR. H2O2 was generated in situ by the glucose/ glucose oxidase reaction. (C) Reduction of Mn3+-JED in human blood plasma without (open circles) and with 5 mol. equiv. L-Cys (filled circles) added. Relaxometry measurements were performed at 1.4T, 37 °C.
Proliferating tumors are characterized by regions of hypoxia,38 and thiol-rich microenvironments.39,40 Mn3+-JED is readily reduced to Mn2+-JED in the presence of thiols. Fig 2C depicts the cysteine mediated reduction of Mn3+-JED by incubation with 5 mol. equiv. L-Cys in blood plasma with kobs = 3.60±0.50 s−1. L-Cys reduction results in a remarkable 8.5-fold increase in r1.
The MRI contrast between equimolar solutions of Mn2+-JED and Mn3+-JED is profound. Fig 3A shows a conventional T1-weighted image at 4.7 T of phantoms containing 0.5 mM Mn2+-JED before (−) and after (+) peroxidase mediated oxidation. In this image the higher relaxivity Mn2+-JED solution is much brighter than the low relaxivity Mn3+-JED solution. Gd-bis-5HT-DTPA is a state of the art peroxidase sensing Gd-based agent whose relaxivity change is mediated by a change in rotational correlation time upon oxidation, Fig S14.41 However at 4.7 T oxidation only results in a 15% r1 change and this small difference is highlighted in the image in Fig 3B. By using an inversion pre-pulse we can generate positive contrast in the oxidized Mn3+-JED complex. Fig 3C shows the same phantoms as in 3A imaged with a 325 ms inversion pre-pulse. The inversion time was chosen to null signal in the untreated sample, generating large positive contrast in the oxidized sample. Fig 3D shows the same inversion pre-pulse sequence applied to the Gd-bis-5HT-DTPA samples from Fig 3B. Regardless of the scanning protocol, the contrast generated following oxidation of the Mn-based agent is much larger than that possible with the Gd-based agent. Fig 3E compares percentage r1 change observed afer peroxidase oxidation of Mn-JED and Gd-bis-5HT-DTPA at 4.7 T. The Mn agent undergoes 380% r1 change whereas the activatable Gd-based agents experiences <15% r1 change. At 1.4 T, the r1 change is also much greater for the Mn-JED system (790%) than for Gd-bis-5HT-DTPA (60%). Images of phantoms prepared in human blood plasma exhibit comparable contrast, Fig S15.
Figure 3.
MR phantom images at 4.7 T, RT, of water with 0.5 mM Mn2+-JED (A and C) or Gd-bis-5HT-DTPA (B and D) before (−) and after (+) incubation with H2O2/ peroxidase (45 U/mL). Images (A and C) acquired using a T1-weighted FLASH sequence, or (B and D) with a 325 ms inversion pre-pulse to generate large positive contrast in the oxidized sample; see SI for image acquisition details. Note the high contrast between the samples containing Mn2+- vs. Mn3+-JED, but low contrast for the Gd-based system. (E) Percentage r1 change after peroxidase mediated oxidation of the Mn- and Gd- based agents. A detailed description of the scanning parameters is in the SI.
To our knowledge, JED is the only chelator capable of stabilizing both Mn3+ and Mn2+ in the physiological milieu, and enables rapid and reversible biochemically mediated interconversion between Mn3+ (low r1) and Mn2+ (high r1). The biochemically mediated r1 change observed with Mn-JED is the largest of any activatable Gd- or Mn-based contrast agent. Peroxidase mediated oxidation of Mn2+- to Mn3+-JED provides over an order of magnitude greater r1 change than the state of the art Gd-based peroxidase sensor, and is achieved in minutes at peroxidase concentrations 1000-fold below what is seen in vivo. Mn-JED offers a promising new paradigm for activatable MRI contrast agent development.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Heart, Lung, and Blood Institute (K25HL128899), the National Institute of Biomedical Imaging and Bioengineering (R01EB009062, R21EB022804) and instrumentation funded by the National Center for Research Resources and the Office of the Director (P41RR14075, S10RR023385, S10OD010650).
Footnotes
The authors declare no competing financial interests.
Supporting Information. Experimental details, synthetic procedures, compound characterization, structures not depicted in manuscript text, additional spectra, HPLC traces, and MRI acquisition parameters. The Supporting Information is available free of charge on the ACS Publications website. (PDF)
References
- 1.Boros E, Gale EM, Caravan P. Dalton Trans. 2015;44:4804. doi: 10.1039/c4dt02958e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelovski G. Angew Chem Int Ed. 2016;55:7038. doi: 10.1002/anie.201510956. [DOI] [PubMed] [Google Scholar]
- 3.Caravan P. Acc Chem Res. 2009;42:851. doi: 10.1021/ar800220p. [DOI] [PubMed] [Google Scholar]
- 4.De Leon-Rodriguez LM, Lubag AJM, Malloy CR, Martinez GV, Gillies RJ, Sherry AD. Acc Chem Res. 2009;42:948. doi: 10.1021/ar800237f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsitovich PB, Burns PJ, McKay AM, Morrow JR. J Inorg Biochem. 2014;133:143. doi: 10.1016/j.jinorgbio.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Do QN, Ratnakar SJ, Kovács Z, Sherry AD. ChemMedChem. 2014;9:1116. doi: 10.1002/cmdc.201402034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lubag AJM, De León-Rodríguez LM, Burgess SC, Sherry AD. Proc Natl Acad Sci, USA. 2011;108:18400. doi: 10.1073/pnas.1109649108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee TY, Cai LX, Lelyveld VS, Hai A, Jasanoff A. Science. 2014;344:533. doi: 10.1126/science.1249380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang S, Chen HH, Yuan H, Dai G, Schühle DT, Mekkaoui C, Ngoy S, Liao R, Caravan P, Josephson L, Sosnovik DE. Circ Cardiovasc Imaging. 2011;4:729. doi: 10.1161/CIRCIMAGING.111.966374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hingorani DV, Bernstein AS, Pagel MD. Contrast Media Mol Imaging. 2014;10:245. doi: 10.1002/cmmi.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu J, Martins AF, Preihs C, Jordan VC, Chirayil S, Zhao P, Wu Y, Nasr K, Kiefer GE, Sherry AD. J Am Chem Soc. 2015;137:14173. doi: 10.1021/jacs.5b09158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nivorozhkin AL, Kolodziej AF, Caravan P, Greenfield MT, Lauffer RB, McMurry TJ. Angew Chem Int Ed. 2001;40:2903. [PubMed] [Google Scholar]
- 13.Ekanger LA, Ali MM, Allen MJ. Chem Commun. 2014;50:14835. doi: 10.1039/c4cc07027e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ekanger LA, Polin LA, Shen Y, Haacke EM, Martin PD, Allen MJ. Angew Chem Int Ed. 2015;54:14398. doi: 10.1002/anie.201507227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsitovich PB, Spernyak JA, Morrow JR. Angew Chem Int Ed. 2013;52:13997. doi: 10.1002/anie.201306394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loving GS, Mukherjee S, Caravan P. J Am Chem Soc. 2013;135:4623. doi: 10.1021/ja312610j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gale EM, Mukherjee S, Liu C, Loving GS, Caravan P. Inorg Chem. 2014;53:10748. doi: 10.1021/ic502005u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aime S, Botta M, Gianolio E, Terreno E. Angew Chem Int Ed. 2000;39:747. doi: 10.1002/(sici)1521-3773(20000218)39:4<747::aid-anie747>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Yu M, Ambrose SL, Whaley ZL, Fan S, Gorden JD, Beyers RJ, Schwartz DD, Goldsmith CR. J Am Chem Soc. 2014;136:12836. doi: 10.1021/ja507034d. [DOI] [PubMed] [Google Scholar]
- 20.Yu M, Beyers RJ, Gorden JD, Cross JN, Goldsmith CR. Inorg Chem. 2012;51:9153. doi: 10.1021/ic3012603. [DOI] [PubMed] [Google Scholar]
- 21.Jackson TA, Karapetian A, Miller AF, Brunold TC. J Am Chem Soc. 2004;126:12477. doi: 10.1021/ja0482583. [DOI] [PubMed] [Google Scholar]
- 22.Lauffer RB. Chem Rev. 1987;87:901. [Google Scholar]
- 23.Lacoste RG, Christoffers GV, Martell AE. J Am Chem Soc. 1965;87:2385. [Google Scholar]
- 24.Hamm RE, Suwyn MA. Inorg Chem. 1967;6:139. [Google Scholar]
- 25.Frost AE, Freedman HH, Westerback SJ, Martell AE. J Am Chem Soc. 1958;80:530. [Google Scholar]
- 26.Jones DP, Carlson JL, Mody VC, Jr, Cai J, Lynn MJ, Sternberg P., Jr Free Rad Biol Med. 2000;28:625. doi: 10.1016/s0891-5849(99)00275-0. [DOI] [PubMed] [Google Scholar]
- 27.Kirlin WG, Cai J, Thompson SA, Diaz D, Kavanagh TJ, Jones DP. Free Rad Biol Med. 1999;27:1208. doi: 10.1016/s0891-5849(99)00145-8. [DOI] [PubMed] [Google Scholar]
- 28.Drahoš B, Lukeš I, Tóth E. Eur J Inorg Chem. 2012;2012:1975. [Google Scholar]
- 29.Gale EM, Atanasova I, Blasi F, Ay I, Caravan P. J Am Chem Soc. 2015;137:15548. doi: 10.1021/jacs.5b10748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bihari S, Smith PA, Parsons S, Sadler PJ. Inorg Chim Acta. 2001;331:310. [Google Scholar]
- 31.Rorabacher DB. Chem Rev. 2004;104:651. doi: 10.1021/cr020630e. [DOI] [PubMed] [Google Scholar]
- 32.Caravan P, Farrar CT, Frullano L, Uppal R. Contrast Media Mol Imag. 2009:89. doi: 10.1002/cmmi.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su HS, Nahrendorf M, Panizzi P, Breckwoldt MO, Rodriguez E, Iwamoto Y, Aikawa E, Weissleder R, Chen JW. Radiology. 2012;262:181. doi: 10.1148/radiol.11110040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nahrendorf M, Sosnovik D, Chen JW, Panizzi P, Figueiredo JL, Aikawa E, Libby P, Swirski FK, Weissleder R. Circulation. 2008;117:1153. doi: 10.1161/CIRCULATIONAHA.107.756510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Breckwoldt MO, Chen JW, Stangenberg L, Aikawa E, Rodriguez E, Qiu S, Moskowitz MA, Weissleder R. Proc Natl Acad Sci, USA. 2008;105:18584. doi: 10.1073/pnas.0803945105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeLeo MJ, III, Gounis MJ, Hong B, Ford JC, Wakhloo AK, Bogdanov AA., Jr Radiology. 2009;252:696. doi: 10.1148/radiol.2523081426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. J Clin Invest. 1994;94:437. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bourgeois M, Rajerison H, Guerard F, Mougin-Degraef M, Barbet J, Michel N, Cherel M, Faivre-Chauvet A, Gestin JF. Nucl Med Rev. 2011;14:90. doi: 10.5603/nmr.2011.00022. [DOI] [PubMed] [Google Scholar]
- 39.Kuppusamy P, Li H, Ilangovan G, Cardounel AJ, Zweier JL, Yamada K, Krishna MC, Mitchell JB. Cancer Res. 2002;62:307. [PubMed] [Google Scholar]
- 40.Jorgenson TC, Zhong W, Oberley TD. Cancer Res. 2013;73:6118. doi: 10.1158/0008-5472.CAN-13-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodríguez E, Nilges M, Weissleder R, Chen JW. J Am Chem Soc. 2010;132:168. doi: 10.1021/ja905274f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



