SYNOPSIS
Best practices that translate the evidence for high dose HM feeding for preterm infants during the NICU hospitalization have been described in multiple studies but their implementation has been compromised largely due to economic and ideologic concerns. Although the rates of “any” HM feeding have increased over the last decade, efforts to help mothers maintain human milk provision through to NICU discharge have remained problematic throughout the world. Special emphasis should be placed on prioritizing the early lactation period of coming to volume so that mothers have sufficient HM volume to achieve their personal HM feeding goals. Finally, donor HM does not provide the same risk reduction as own mothers’ HM for multiple morbidities in preterm infants, providing needed evidence for channeling of limited resources into NICU programs that promote the use of mothers’ own HM.
Keywords: human milk, neonatal intensive care unit, breast pump, preterm infants, lactation initiation and maintenance
Human milk (HM; milk from the infant’s own mother) feedings during the neonatal intensive care unit (NICU) hospitalization represent a cost-effective strategy to reduce disease burden and associated costs in preterm infants.1–7 However, this evidence must be translated into NICU best practices that target barriers to high-dose HM feedings if preterm infants and their mothers are to receive the benefits of this knowledge. Although multiple studies have revealed effective interventions for modifying barriers to maternal lactation and HM feeding in this population, economic and ideologic concerns have limited their wide-scale adaptation.8 As a result, many mothers of preterm infants fail to achieve their HM feeding goals, and infants receive either donor human milk or formula, neither of which achieves similar reduction in disease burden and cost.9 This chapter reviews data on the initiation and maintenance of lactation for mothers of preterm infants; summarizes best practices for protecting maternal HM volume during the NICU hospitalization; delineates predictable, preventable problems in the feeding of HM, and details quality indicators that measure the effectiveness of NICU HM feeding programs.
Methodology
The literature used to create this review spans multiple specialties, including preterm infants, nutrition, human milk science, lactation physiology, breast pump dependency, NICU lactation support and the economics of human milk feeding for very low birthweight (VLBW; <1500 grams birthweight) infants. These citations were accumulated over a number of years by the authors, who are primary researchers in this field. Thus, this expert review reflects current evidence, controversies and implications for research and practice.
Initiation and Maintenance of Lactation in Mothers of Preterm Infants
Initiation of Lactation
The past decade is characterized by an increasing proportion of mothers who initiate lactation (begin providing HM) for their preterm infants,3,10,11 many because they change the decision from formula to HM due to information they received from NICU health care providers.12–14 Studies have confirmed that NICU messaging about the superiority of HM does not make mothers feel guilty or coerced, but instead is interpreted as needed information to make the best feeding decision for their infants.12,13 Although black and/or low-income mothers have been especially likely to change from formula to HM after speaking with their infant’s care providers,12–14 black preterm infants in the United States remain less likely than their Caucasian counterparts to receive any HM, especially if their mothers are low-income.5,15–17 Specific talking points for sharing the science of HM with families of preterm infants have been published, and can standardize evidence-based messaging about providing HM within the NICU.8,18,19
Maintenance of Lactation
The maintenance of lactation, usually measured by whether the infant is still receiving partial or exclusive HM at the time of NICU discharge, (HM continuation through NICU discharge), remains a global problem with only a handful of best practices demonstrated to be effective.20–27 In a prospective cohort study, Hoban et al reported that mothers of very low birthweight (VLBW; <1500 grams birthweight) infants changed their HM feeding goals over the course of the NICU hospitalization, and became increasingly unlikely to achieve their goals for exclusive or partial HM as the hospitalization progressed.14 It has been proposed that the profound dislike and inconvenience of long-term HM expression, maternal stress and fatigue, insufficient encouragement and assistance from family and friends, and inconsistent advice in the NICU all play a role in mothers’ discontinuation of HM provision prior to NICU discharge.25–28 Furthermore, it is likely that some mothers, especially those whose initial pre-birth intent was to formula-feed, revert back to their pre-birth feeding goals, especially as the infant’s condition improves and the mother perceives that “HM has done its job” of protecting from acquired morbidities.8,14
Maintenance of lactation is related to maternal HM volume
The failure to maintain HM provision through to NICU discharge is related to insufficient pumped HM to meet the infant’s nutritional requirements. However, it is not clear whether insufficient HM volume precedes less intensive HM expression efforts or vice versa. For example, does the mother see that despite her best efforts, her pumped HM volume decreases, and she becomes discouraged and pumps less frequently, eventually discontinuing HM provision? Or, is the primary catalyst the dislike and inconvenience of pumping, such that the mother pumps less frequently, notes decreased HM volume and then decides that her growing preterm infant is doing well with partial supplements of formula or donor milk, ceasing HM provision altogether?8,14,26 These distinctions are important because they require different interventions to prevent early cessation of HM provision.
Successful initiatives to improve the maintenance of lactation
A handful of multi-institutional quality initiatives have demonstrated higher rates of HM provision at NICU discharge by adopting multidisciplinary infant nutrition and lactation teams that incorporate clear protocols for premature infants.24 Other initiatives have focused on developing a NICU nursing lactation team, increasing availability of hospital grade breast pumps, and implementing lactation rounds.23 Larger state-wide initiatives that report increased rates of HM feedings at NICU discharge have included multidisciplinary teams to provide education and advocacy for HM provision, to support establishing and maintaining HM supply, and to provide a consistent and comprehensive nutritional monitoring program.22 Many of the evidence-based interventions to maintain lactation in this population such as timely access to effective and efficient breast pumps, freezer storage space and proactive NICU-specific lactation care, have not been adopted due to the upstart economic investments that would be required. (Table 1). In many instances it is easier to acquire institutional approval for donor human milk infrastructure than for programs that facilitate mothers’ providing their own HM, despite the fact that own mothers’ HM is more economical to provide and acquire and provides greater protection from acquired morbidities.9,29,30
Table 1. Economic Barriers to the Initiation and Maintenance of Lactation: Estimated Average Cost of Required Services per Infant.
| Barrier | Cost per Infant | Low | High | Assumptions |
|---|---|---|---|---|
| 1 Provision of professionally-produced, evidence-based materials targeting the importance of HM for preterm infants + monitoring of HM volume and goals for HM feeding | Brochure + education sheets = $10; Mother’s Milk Volumes (MMV) record = $5.50; Maternal Goals record = $0.10; |
$15.60 | $15.60 | 1 set of materials per infant |
| 2 Hospital grade electric breast pump rental for 3 months (through term, corrected age) | $40 to $70 per month | $120.00 | $210.00 | 3 month rental; low estimate assumes $40 per month, wholesale cost; high estimate assumes $70/month retail cost |
| 3 Provision of pump kit | $32.91 cost per kit | $32.91 | $32.91 | 1 kit per infant |
| 4 Access to custom-fitted breast shields for effective, efficient and comfortable HM removal | $7 retail cost | $7.00 | $7.00 | 1 set per infant |
| 5 Provision of sufficient numbers of hospital-grade HM storage containers for pumped HM | $0.21 cost per container (Jegier 2013) | $89.46 | $134.19 | Low estimate assumes 6 containers per day (350 mL/120 * 1.2 rounded to next multiple of 3); high estimate assumes 9 containers/day (700 mL/120 *1.2 rounded to next multiple of 3); for 71-day NICU stay |
| 6 Access to NICU-specific lactation support from NICU-based certified breastfeeding peer counselors (BPC) during coming to volume, weekly group forums and on an individual basis, as needed | $18/hour + 26% fringe benefits | $417.11 | $539.79 | 71-day NICU stay; $18/hour + 26% fringe benefits; low estimate assumes 2 BPC for 44 infants per day; high estimate assumes 2 BPC for 33 infants per day (75%) |
| 7 Availability of NICU freezers for safe storage of all pumped HM (e.g., not telling mothers to store HM in the home and bring it in as needed) | $8,000 cost per freezer | $6.92 | $10.37 | Storage for 15 infants per freezer; low estimate assumes 15 year life; high estimate assumes 10 year life; for 71 day length of NICU stay |
| 8 Use of HM waterless warmers to avoid HM contamination with waterborne pathogens | $773 cost per warmer | $30.07 | $50.12 | 1 warmer per infant for 71-day NICU stay; low estimate assumes 5 year life; high estimate assumes 3 year life |
| 9 Use of liners for waterless warmer | $3.25 cost per liner | $230.75 | $230.75 | 1 liner per day for 71-day NICU stay |
| 10 Basic creamatocrit and/or other HM analysis technology to individualize HM feedings and HM collection strategies | $1,500 per creamatocrit | $0.38 | $0.63 | 1 creamatocrit per NICU; assumes 800 NICU admissions per year; low estimate assumes 5 year life; high estimate assumes 3 year life |
| 11 Availability of infant scales for measuring HM intake during breastfeeding | $1,500 per scale | $3.89 | $6.48 | 1 scale per 15 infants for NICU stay; low estimate assumes 5 year life; high estimate assumes 3 year life |
|
| ||||
| Total | $954.08 | $1,237.84 | ||
Costs reported in 2015 US dollars.
Based on Average length of NICU stay for VLBW infants of 71 days from RUMC data
Research Priorities to Improve the Initiation and Maintenance of Lactation in Mothers of Preterm Infants
Most previous studies addressing barriers to the initiation and maintenance of lactation in mothers of preterm infants have focused primarily on motivational and behavioral interventions such as skin-to-skin care, patterns of breast pump use and models of support.23–25,27,31–34 However, many breast pump-dependent mothers of preterm infants have chronic health problems or pregnancy and birth complications that impact lactation outcomes and that may be unresponsive to current behavioral and motivational interventions.8,18,35 These complications, which include pre-pregnancy body-mass-index (BMI) >25, preterm birth, Cesarean delivery and preeclampsia, as well as prolonged bedrest and medications to treat these complications, impact the hormonal processes that regulate secretory differentiation and early lactation.36–42 However, because preterm infants require so little HM volume in the early post-birth period, these maternal HM volume problems can easily go unrecognized for days or weeks, making the problems more difficult to diagnose and manage. Thus, a research priority is understanding the role of maternal health complications that impact lactation outcomes for breast pump dependent mothers of preterm infants, who are often ill themselves.
Another research priority is addressing the mothers’ consistent reports about the dislike and inconvenience of breast pump use. Mothers of preterm infants are completely breast pump dependent, meaning that the breast pump regulates the lactation processes of HM removal and mammary gland stimulation, which are critical to continued HM production.35 Despite the reality that breast pump-dependency will continue for weeks or months, surprisingly few rigorous studies have examined features of breast pumps, breast pump suction patterns, breast shield-sizing and other product-related considerations such as the ability to warm breast shields.35,43,44 Whereas it is well-known that breast pump evaluations in this population should include objective outcomes that include effectiveness, efficiency, comfort and convenience of the breast pump, the primary outcome measures in most studies continues to be pumped HM volume and maternal “preferences,” both of which lack rigor and are affected by multiple extraneous variables. 35 Thus, a critical research priority for the maintenance of lactation in mothers of preterm infants is the improvement in the design of breast pumps and breast pump supplies so that they optimize efficiency and convenience, consistent with mothers’ concerns.35,45 Unfortunately, ideological barriers to breast pump research affect the study and dissemination of findings from industry-funded trials as well as the selection of breast pumps for clinical NICU use based on data versus compliance with WHO code marketing interpretation and other ideologic initiatives.45,46
Protecting Maternal HM Volume in Breast Pump Dependent Mothers of Preterm Infants
Breast pump-dependent mothers of preterm infants have specific, predictable barriers to the initiation and maintenance of lactation, which do not affect mothers of healthy term infants. These lactation processes and barriers have been delineated in two recent review papers,8,35 and are summarized in Table 2 according to the stage of lactation: initiation, coming to volume and the maintenance of established lactation. Of particular concern are initiation and coming to volume because the first two weeks post-birth is a critical period for transition of the mammary gland from secretory differentiation to secretory activation, and little is known about these processes in breast pump-dependent mothers of preterm infants.38,41,43,47 Furthermore, several studies indicate that daily pumped HM volume at either week 1 or week 2 post-birth in this population predicts HM continuation at NICU discharge.35,48–50 In one recently completed study, mothers of VLBW infants who successfully experienced coming to volume, e.g., achieving HM volume ≥ 500 mL/day by day 14 post-birth, were over 3 times more likely to provide HM at NICU discharge than mothers who did not achieve this threshold.50
Table 2. Critical Periods for Protecting Maternal Milk Volume in Breast Pump.
Dependent Mothers of Preterm Infants
| Critical Period |
Problem | Impact | Best Practice(s) | References |
|---|---|---|---|---|
| Initiation |
|
|
|
35,41–43, 45,51–54,81–86 |
| Coming to Volume |
|
|
|
18,35,81 |
| Maintenance of Established Lactation |
|
|
|
14,26,27,33, 77,81,87 |
Early Lactation: Initiation and Coming to Volume
The early post-birth lactation stages of initiation and coming to volume warrant further detail because they pose predictable problems for breast pump-dependent mothers of preterm infants and should be monitored and addressed proactively.35 The initiation of lactation coincides with the closure of tight junctions in the mammary epithelium,38,47 a process that is disrupted and/or delayed by preterm and/or complicated birth,41 lack of exposure to human infant-specific sucking patterns,43 delayed breast pump use,51,52 early hormonal contraception,53 and prolonged hand expression in the absence of breast pump use.54
Coming to volume refers to the lactation stage between the onset of lactogenesis II and the establishment of a threshold HM volume, typically ≥500 mL/day.35 This transition heralds the autocrine control of lactation55 via the suckling-induced prolactin surge56 and feedback inhibition of lactation.57 Coming to volume in a breast pump-dependent mother with a NICU infant is impaired by easily-overlooked conditions that lead to HM stasis, thereby triggering feedback inhibition of lactation. These conditions include using an ineffective breast pump that does not empty the breasts thoroughly, improperly fitted breast shields that obstruct the outflow of HM from the ducts, inappropriate breast pump suction pressures, short pumping sessions and long intervals between breast pump use.35 Furthermore, several lines of evidence suggests that the early post-birth stages of initiation and coming to volume are critical periods for the programming of lactation structures and functions, making it difficult or impossible for mothers with low HM volume to ‘catch up’ after these critical periods have passed.35,43,54
Clinical Tools to Monitor Coming to Volume and Maternal HM Feeding Goals
All available evidence indicates that the NICU staff should prioritize the first 14 days post-birth using proactive interventions to achieve maternal HM volume measures that are ≥500 mL/day.18,35 A NICU toolkit for managing these early lactation phases has been described and includes a user-friendly pumping diary (My Mom Pumps for Me!), the Coming to Volume Assessment Tool and a weekly maternal feeding goals interview tool (My Plans for Feeding my Baby at NICU discharge) that assures mothers’ individual HM feeding goals are monitored and supported.18 Whereas the costs for these interventions are often assumed to be unaffordable, in reality they are quite economical (Table 1), especially considering that the cost savings afforded to each additional fed mL of HM during the first 14 days post-birth is valued at $534 toward reduction in NICU costs of care, exclusive of costs specifically attributed to necrotizing enterocolitis.2
Improving the Use of HM for Preterm Infants
Considerable global variation exists in the storage, handling, fortification and feeding of HM in the NICU, as detailed in two recent review papers.3,8 Barriers to the integration of evidence-based practices to improve the use of HM for preterm infants include lack of HM/lactation specialists with NICU expertise, cost of investment in resources and ideologic objections to the use of technology that makes breastfeeding “unnatural”.
Safe Handling of Pumped HM in the NICU
When HM is pumped, transferred among containers, stored, warmed, fortified and fed via gavage infusion, there are multiple avenues for compromising the nutritional and bioactive components that actually reach the infant.3,8 Furthermore, HM is easily contaminated during these processes and can serve as an excellent medium for bacterial growth, especially if HM has been previously frozen. These concerns are the most comprehensively addressed by feeding freshly pumped HM that has not been either refrigerated or frozen (e.g., directly from mother to infant), and this strategy should be prioritized to the greatest possible extent.3,8 Table 3 summarizes best practices for safe handling of pumped HM in the NICU.
Table 3. Safe Handling of Pumped HM for Preterm Infant Feeding in the NICU.
Data from Meier PP, Patel AL, Bigger HR, et al. Human milk feedings in the neonatal intensive care unit. In: Rajendram R, Preedy VR, Patel VB, eds. Diet and nutrition in critical care. New York: Springer-Verlag; 2015:807–822 and Meier PP, Rossman B, Patel AL, Johnson TJ, Engstrom JL, Hoban R, Patra K, Bigger HR. Human milk in the neonatal intensive care unit. In: A state-of-the-art view about human milk and lactation. Stuttgart: Thieme; in press.
| Objective | Best Practices |
|---|---|
| A. Maximize nutritive and bioactive components |
|
| B. Optimize nutrient delivery and utilization |
|
| C. Minimize bacterial contaminants and bacterial growth |
|
| D. Eliminate errors in HM fed to the wrong infant |
|
New data about changes in the integrity of HM with storage have significant clinical implications for conserving pumped HM.58–60 Slutzah et al reported that freshly pumped, unfortified HM is safely fed after refrigeration for 96 hours.58 Separate studies suggest that pumped HM can be thawed and refrozen at least one time61 (allowing aliquoting of HM from large containers into smaller ones for smaller volume feeds), and that HM from serial pumpings can be safely added to previously pumped HM over a 24-hour period.62
There is no evidence to inform whether HM storage and preparation should be centralized within a milk bank area or prepared at the bedside by the NICU nurse. A centralized service is potentially safer with respect to misallocation of HM (e.g., infant receiving another mother’s HM), although this assumption has not been tested. Centralized preparation may also be more efficient and convenient, especially if donor HM is used to supplement mothers’ own HM. In contrast, HM preparation at the bedside by a NICU nurse enables more individualization of the feeding that potentially impacts infant outcome. For example, the NICU nurse can prioritize fresh versus frozen HM, colostrum versus mature HM and other strategies that are impossible when a centralized service has already prepared 12 or 24 hours of HM feedings in advance. The pros and cons of each practice have been reviewed recently.8 One single-center study has demonstrated a reduction in the rate of HM misallocation in the NICU with the adoption of an electronic HM tracking/scanning system, while others have implemented non-electronic measures to reduce errors.63,64
Within- and Between- Mother Variability in Pumped HM and Impact on Infant Growth
The within- and between- mother variability in pumped HM fed in the NICU has been documented for decades.3,8 Of all HM components, lipid which contributes 50–60% of HM calories, is the most variable with one study of pumped HM specimens provided by NICU mothers revealing minimum and maximum values for caloric density of 604 kCal/L and 1098 kCal/L respectively.65 Multiple modifiable factors contribute to lipid variability, which can be quickly identified and managed using the creamatocrit or other more costly HM analysis technologies.3,65 Table 4 summarizes common NICU scenarios that result in low-lipid, high lactose HM being fed with resultant slow infant weight gain and potential feed intolerance. Recent review papers on this topic provide extensive clinical examples of the impact of low-lipid HM on infant growth and feed intolerance.3,8 Although the adequacy of protein impacts infant growth, HM protein varies little after the first month of lactation, during which time protective and growth proteins (e.g., secretory IgA, lactoferrin, epidermal growth factor) are more concentrated that nutritive protein.66,67
Table 4. Factors Influencing the HM Lipid Received by the Preterm Infant.
Data from Meier PP, Patel AL, Bigger HR, et al. Human milk feedings in the neonatal intensive care unit. In: Rajendram R, Preedy VR, Patel VB, eds. Diet and nutrition in critical care. New York: Springer-Verlag; 2015:807–822 and Meier PP, Rossman B, Patel AL, Johnson TJ, Engstrom JL, Hoban R, Patra K, Bigger HR. Human milk in the neonatal intensive care unit. In: A state-of-the-art view about human milk and lactation. Stuttgart: Thieme; in press.
| Factor | Impact | Best Practices |
|---|---|---|
| Pumping | ||
|
|
|
|
|
|
|
|
|
| Storage | ||
|
|
|
| Handling | ||
|
|
|
| Feeding | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Feeding at Breast in the NICU
Feeding at breast for preterm infants can be conceptualized as a series of steps, including: breast pump use at the infant’s bedside; skin-to-skin holding; tasting HM (suckling after breast pump use to remove all or some of the HM); and finally consuming full feedings at the breast.18,68–71 There are no data to indicate that infants must attain a threshold weight or gestational age to begin tasting HM, and several studies reveal that preterm infants remain more physiologically stable during breast than bottle feeding.8 However, a myriad of international studies suggest that preterm infants are prone to underconsumption of HM during exclusive at-breast feeding until they reach approximately term, corrected age despite the fact that the mother has more than enough HM and can remove it effectively with a breast pump.3,8
HM Transfer during Breastfeeding Requires Mature Infant Suction Pressures
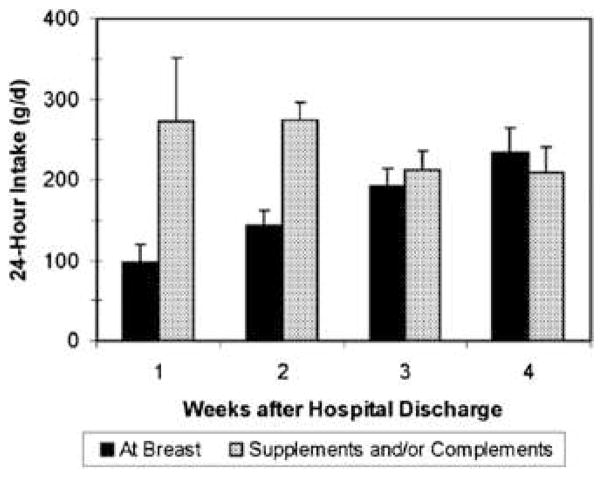
This ineffective and inefficient HM removal by preterm infants is due to weak intraoral suction pressures that are critical to breastfeeding but not bottle feeding. Suction pressures strengthen as the infant matures, as does the ability to stay awake and alert during the feeding and not slip off the breast repeatedly.8 Figure 1 depicts HM intake by breast and bottle during the first 4 weeks post-discharge in VLBW infants whose mothers had adequate HM for their requirements.72 As demonstrated in Figure 1, mothers have adequate volumes of HM and can remove it with a breast pump, but the infant cannot remove the available HM during exclusive breastfeeding. Figure 1 can be used to help families understand that NICU discharge does not mean that breastfeeding will magically “work” because the mother and infant are no longer separated. In fact, the breast continues to synthesize HM only because additional breast pump use removes HM effectively and efficiently. Early discontinuation of breast pump use during this transition to exclusive at-breast feedings predisposes to low HM volume and inadequate infant intake.8
Figure 1.
Volume of milk consumed at the breast and as extra milk (supplements and complements of pumped mothers’ milk) during the first 4 weeks at home in premature infants discharged from the neonatal intensive care unit. (Courtesy of N. Hurst, PhD, Houston, TX; P. Meier, PhD, Chicago, IL; and J. Engstrom, PhD, Chicago, IL.)
Use of Evidence-Based Lactation Technologies to Facilitate Breastfeeding
The first month post-discharge can be extremely difficult for breastfeeding mothers with preterm infants in part because lactation technologies that can guide this transition are not commonly employed. First, test-weights performed during the last week or two in the NICU using accurate scales can help individualize breastfeeding strategies for use in the home.8 For example, if serial test-weights reveal that the infant consumes only 5–10 mLs at breast, more bottle supplement of HM is required than if the infant consumes 80% of the prescribed volume. Multiple studies of test-weights to measure HM intake during breastfeeding reveal that they are accurate, acceptable by mothers, and cannot be replaced by clinical indices such as counting swallows or checking for milk in the infant’s mouth.45,73,74 One randomized study revealed that mothers of preterm infants can use of test-weights in the home after NICU discharge to manage supplements and complements of their pumped HM until infants can consume exclusive breastfeeds.75
A second lactation aid that can be useful during this transition is the ultra-thin silicone nipple shield, which partially compensates for weak suction pressures by creating and maintaining a nipple shape for the infant to latch onto.8,72,76 Although not originally designed as a milk transfer device, evidence indicates that use of the nipple shield increases HM transfer during breastfeeding in preterm infants for whom maintaining sufficient suction pressure to extract HM is suboptimal or impossible.8 Multiple ideological objections to nipple shield use in this population abound, including that it decreases HM transfer, decreases maternal HM volume, looks like a bottle nipple, and is addictive. In contrast, data indicate that the nipple shield can serve as a short-term lactation aid in this population until suction pressures mature sufficiently to allow effective and efficient transfer of HM during exclusive breastfeeding.8,72,76
Evidence-Based Quality Indicators that Target High-Dose HM Feedings
Given the link between high-dose HM feedings and improved short- and long-term health and cost outcomes, many NICUs and have established quality improvement initiatives for the use of HM. The most commonly used metrics are the proportion of preterm infants who ever receive HM and the proportion who are still receiving any or exclusive HM at the time of NICU discharge. However, Bigger et al., using data from a prospective cohort study, revealed that significant proportions of VLBW infants who were discharged as “no HM” had received very high-dose HM feedings through the first 14 and 28 days of life.77 These data, which emphasize collecting measures of “dose” (in mL/kg/d or as a proportion of total enteral feeding), in addition to the “ever received” and “still receiving” HM quality indicators, are consistent with findings that link early high-dose HM to the reduction in the risk of necrotizing enterocolitis and sepsis and associated increased costs.77
Another concern in developing quality improvement initiatives for the use of HM is the increasing tendency to combine own mother’s HM and donor HM into the same metric, which is often called human milk-fed or breast milk-fed.9 The distinction between own mother’s HM and donor HM is critical when measuring quality outcomes because donor HM does not provide similar risk reduction from sepsis, BPD and neurodevelopmental problems when compared to mother’s own HM.9,78 Many of the bioactive components in HM are mother-specific such as probiotic bacteria (HM microbiome) and accompanying prebiotic oligosaccharides,79,80 and multiple other HM components are reduced or eradicated due to longitudinal changes in lactation (e.g., early HM versus later HM), preterm versus term HM, storage, freeze-thaw cycle and pasteurization, all of which impact donor HM.9 Furthermore, the processes involved in achieving high rates of mothers’ own HM feedings in the NICU are completely different from acquiring DHM, and scarce funds are often invested into establishing a donor HM infrastructure rather than in acquiring HM from the infants’ own mothers.9
Summary
Although the evidence for high dose HM feeding for preterm infants during the NICU hospitalization is widely accepted, best practices that translate and implement this evidence into daily clinical NICU care have been slow to follow. These best practices have been delineated and model programs for improving the use of HM during the NICU hospitalization have been described. However, increasing the rates of high-dose HM feedings for this population requires an economic investment in personnel, equipment and supplies as well as a commitment to select best practices based on evidence rather than ideology. Special emphasis should be placed on prioritizing the early lactation period of coming to volume so that mothers have sufficient HM volume to achieve their personal HM feeding goals. Finally, it is important to recognize that donor HM does not provide the same risk reduction as own mothers’ HM for multiple morbidities in preterm infants, providing evidence for the channeling of limited resources into NICU programs that promote the use of mothers’ own HM.
KEY POINTS.
The evidence for the use of human milk feedings during the NICU hospitalization for preterm infants has been slowly adopted into clinical best practices.
In multiple instances, these best practices have been identified and tested, but are not adopted due to economical and ideological constraints.
The early post-birth periods of maternal secretory activation and coming to volume appear to comprise a critical window for the protection of maternal human milk provision through to NICU discharge.
Lactation technologies that improve the use of human milk during the NICU hospitalization have been detailed in the scientific literature, but not widely implemented.
Donor HM feeding infrastructure costs can compete with costs for the acquisition of mothers’ own HM in the NICU, with implications for the cost-effective prioritization of limited resources.
Footnotes
DISCLOSURE STATEMENT: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Paula P Meier, Director, Clinical Research and Lactation, Neonatal Intensive Care Unit; Professor, Women, Children, and Family Nursing, Rush University; Professor, Pediatrics, Rush Medical College, Chicago, IL.
Tricia J Johnson, Professor, Health Systems Management, Rush University Medical Center, Chicago, IL.
Aloka L Patel, Associate Professor, Department of Pediatrics-Neonatology, Rush University Medical Center, Chicago, IL.
Beverly Rossman, Assistant Professor, Department of Women, Children, and Family Nursing, Rush University, Chicago, IL.
References
- 1.Patel A, Johnson T, Engstrom J, et al. Impact of early human milk on sepsis and health care costs in very low birthweight infants. J Perinatol. 2013;33(7):514–9. doi: 10.1038/jp.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson TJ, Patel AL, Bigger HR, Engstrom JL, Meier PP. Cost savings of human milk as a strategy to reduce the incidence of necrotizing enterocolitis in very low birth weight infants. Neonatology. 2015;107(4):271–6. doi: 10.1159/000370058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meier PP, Patel AL, Bigger HR, et al. Human milk feedings in the neonatal intensive care unit. In: Rajendram R, Preedy VR, Patel VB, editors. Diet and nutrition in critical care. New York: Springer-Verlag; 2015. pp. 807–822. [Google Scholar]
- 4.Patel AL, Johnson TJ, Robin B, et al. The direct and indirect influence of own Mother’s milk on bronchopulmonary dysplasia and costs. Arch Dis Child Fetal Neonatal Ed. doi: 10.1136/archdischild-2016-310898. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vohr BR, Poindexter BB, Dusick AM, et al. Beneficial effects of breast milk in the neonatal intensive care unit on the developmental outcome of extremely low birth weight infants at 18 months of age. Pediatrics. 2006;118(1):e115–23. doi: 10.1542/peds.2005-2382. [DOI] [PubMed] [Google Scholar]
- 6.Vohr BR, Poindexter BB, Dusick AM, et al. Persistent beneficial effects of breast milk ingested in the neonatal intensive care unit on outcomes of extremely low birth weight infants at 30 months of age. Pediatrics. 2007;120(4):e953–9. doi: 10.1542/peds.2006-3227. [DOI] [PubMed] [Google Scholar]
- 7.Belfort MB, Anderson PJ, Nowak VA, et al. Breast milk feeding, brain development, and neurocognitive outcomes: A 7-year longitudinal study in infants born at less than 30 weeks’ gestation. J Pediatr. 2016 doi: 10.1016/j.jpeds.2016.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meier PP, Rossman B, Patel AL, Johnson TJ, Engstrom JL, Hoban R, Patra K, Bigger HR. A state-of-the-art view about human milk and lactation. Stuttgart: Thieme; Human milk in the neonatal intensive care unit. in press. [Google Scholar]
- 9.Meier PP, Patel AL, Esquerra-Zwiers A. Donor human milk update 2016: Evidence, mechanisms and priorities for research and practice. J Pediatr. doi: 10.1016/j.jpeds.2016.09.027. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colaizy TT, Morriss FH. Positive effect of NICU admission on breastfeeding of preterm US infants in 2000 to 2003. J Perinatol. 2008;28(7):505–510. doi: 10.1038/jp.2008.32. [DOI] [PubMed] [Google Scholar]
- 11.Colaizy TT, Saftlas AF, Morriss FH., Jr Maternal intention to breast-feed and breast-feeding outcomes in term and preterm infants: Pregnancy risk assessment monitoring system (PRAMS), 2000–2003. Public Health Nutr. 2012;15(4):702–710. doi: 10.1017/S1368980011002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sisk PM, Lovelady CA, Dillard RG. Effect of education and lactation support on maternal decision to provide human milk for very-low-birth-weight infants. Adv Exp Med Biol. 2004;554:307–311. doi: 10.1007/978-1-4757-4242-8_28. [DOI] [PubMed] [Google Scholar]
- 13.Miracle DJ, Meier PP, Bennett PA. Mothers’ decisions to change from formula to mothers’ milk for very-low-birth-weight infants. J Obstet Gynecol Neonatal Nurs. 2004;33(6):692–703. doi: 10.1177/0884217504270665. [DOI] [PubMed] [Google Scholar]
- 14.Hoban R, Bigger H, Patel AL, Rossman B, Fogg LF, Meier P. Goals for human milk feeding in mothers of very low birth weight infants: How do goals change and are they achieved during the NICU hospitalization? Breastfeed Med. 2015;10(6):305–311. doi: 10.1089/bfm.2015.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee HC, Gould JB. Factors influencing breast milk versus formula feeding at discharge for very low birth weight infants in california. J Pediatr. 2009;155(5):657–62. e1–2. doi: 10.1016/j.jpeds.2009.04.064. [DOI] [PubMed] [Google Scholar]
- 16.Sisk PM, Lovelady CA, Dillard RG, Gruber KJ, O’Shea TM. Maternal and infant characteristics associated with human milk feeding in very low birth weight infants. J Hum Lact. 2009;25(4):412–419. doi: 10.1177/0890334409340776. [DOI] [PubMed] [Google Scholar]
- 17.Pineda RG. Predictors of breastfeeding and breastmilk feeding among very low birth weight infants. Breastfeed Med. 2011;6(1):15–19. doi: 10.1089/bfm.2010.0010. [DOI] [PubMed] [Google Scholar]
- 18.Meier PP, Patel AL, Bigger HR, Rossman B, Engstrom JL. Supporting breastfeeding in the neonatal intensive care unit: Rush mother’s milk club as a case study of evidence-based care. Pediatr Clin North Am. 2013;60(1):209–226. doi: 10.1016/j.pcl.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez NA, Miracle DJ, Meier PP. Sharing the science on human milk feedings with mothers of very-low-birth-weight infants. J Obstet Gynecol Neonatal Nurs. 2005;34(1):109–119. doi: 10.1177/0884217504272807. [DOI] [PubMed] [Google Scholar]
- 20.Davanzo R, Monasta L, Ronfani L, Brovedani P, Demarini S Breastfeeding in Neonatal Intensive Care Unit Study Group. Breastfeeding at NICU discharge: A multicenter italian study. J Hum Lact. 2013;29(3):374–380. doi: 10.1177/0890334412451055. [DOI] [PubMed] [Google Scholar]
- 21.Riley B, Schoeny M, Rogers L, et al. Barriers to human milk feeding at discharge of very low-birthweight infants: Evaluation of neighborhood structural factors. Breastfeed Med. 2016 doi: 10.1089/bfm.2015.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee HC, Kurtin PS, Wight NE, et al. A quality improvement project to increase breast milk use in very low birth weight infants. Pediatrics. 2012;130(6):e1679–87. doi: 10.1542/peds.2012-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bixby C, Baker-Fox C, Deming C, Dhar V, Steele C. A multidisciplinary quality improvement approach increases breastmilk availability at discharge from the neonatal intensive care unit for the very-low-birth-weight infant. Breastfeed Med. 2016;11:75–79. doi: 10.1089/bfm.2015.0141. [DOI] [PubMed] [Google Scholar]
- 24.University of California San Diego Health. [Accessed May 10, 2016];Supporting Premature Infant Nutrition (SPIN) Web site. https://health.ucsd.edu/specialties/obgyn/maternity/newborn/nicu/spin/about/Pages/default.aspx. Updated 2016.
- 25.Hurst N, Engebretson J, Mahoney JS. Providing mother’s own milk in the context of the NICU: A paradoxical experience. J Hum Lact. 2013;29(3):366–373. doi: 10.1177/0890334413485640. [DOI] [PubMed] [Google Scholar]
- 26.Rossman B, Kratovil AL, Greene MM, Engstrom JL, Meier PP. “I have faith in my milk”: The meaning of milk for mothers of very low birth weight infants hospitalized in the neonatal intensive care unit. J Hum Lact. 2013;29(3):359–365. doi: 10.1177/0890334413484552. [DOI] [PubMed] [Google Scholar]
- 27.Rossman B, Engstrom JL, Meier PP, Vonderheid SC, Norr KF, Hill PD. “They’ve walked in my shoes”: Mothers of very low birth weight infants and their experiences with breastfeeding peer counselors in the neonatal intensive care unit. J Hum Lact. 2011;27(1):14–24. doi: 10.1177/0890334410390046. [DOI] [PubMed] [Google Scholar]
- 28.Fleurant E, Schoeny M, Hoban R, et al. Barriers to human milk feeding at discharge of VLBW infants: Maternal goal setting as a key social factor. Breastfeed Med. doi: 10.1089/bfm.2016.0105. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jegier BJ, Johnson TJ, Engstrom JL, Patel AL, Loera F, Meier P. The institutional cost of acquiring 100 mL of human milk for very low birth weight infants in the neonatal intensive care unit. J Hum Lact. 2013;29(3):390–399. doi: 10.1177/0890334413491629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jegier BJ, Meier P, Engstrom JL, McBride T. The initial maternal cost of providing 100 mL of human milk for very low birth weight infants in the neonatal intensive care unit. Breastfeed Med. 2010;5(2):71–77. doi: 10.1089/bfm.2009.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossman B, Kratovil AL, Greene MM, Engstrom JL, Meier PP. “I have faith in my milk”: The meaning of milk for mothers of very low birth weight infants hospitalized in the neonatal intensive care unit. J Hum Lact. 2013 doi: 10.1177/0890334413484552. [DOI] [PubMed] [Google Scholar]
- 32.Rossman B, Engstrom JL, Meier PP. Healthcare providers’ perceptions of breastfeeding peer counselors in the neonatal intensive care unit. Res Nurs Health. 2012;35(5):460–474. doi: 10.1002/nur.21496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossman B, Greene MM, Meier PP. The role of peer support in the development of maternal identity for “NICU moms”. J Obstet Gynecol Neonatal Nurs. 2015;44(1):3–16. doi: 10.1111/1552-6909.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strand H, Blomqvist YT, Gradin M, Nyqvist KH. Kangaroo mother care in the neonatal intensive care unit: Staff attitudes and beliefs and opportunities for parents. Acta Paediatr. 2014;103(4):373–378. doi: 10.1111/apa.12527. [DOI] [PubMed] [Google Scholar]
- 35.Meier PP, Patel AL, Hoban R, Engstrom JL. Which breast pump for which mother: An evidence-based approach to individualizing breast pump technology. J Perinatol. 2016;36(7):493–499. doi: 10.1038/jp.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hernandez LL, Grayson BE, Yadav E, Seeley RJ, Horseman ND. High fat diet alters lactation outcomes: Possible involvement of inflammatory and serotonergic pathways. PLoS One. 2012;7(3):e32598. doi: 10.1371/journal.pone.0032598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neville MC, Webb P, Ramanathan P, et al. The insulin receptor plays an important role in secretory differentiation in the mammary gland. Am J Physiol Endocrinol Metab. 2013;305(9):E1103–14. doi: 10.1152/ajpendo.00337.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neville MC. Introduction: Tight junctions and secretory activation in the mammary gland. J Mammary Gland Biol Neoplasia. 2009;14(3):269–270. doi: 10.1007/s10911-009-9150-8. [DOI] [PubMed] [Google Scholar]
- 39.Hurst NM. Recognizing and treating delayed or failed lactogenesis II. J Midwifery Womens Health. 2007;52(6):588–594. doi: 10.1016/j.jmwh.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Rasmussen KM. Association of maternal obesity before conception with poor lactation performance. Annu Rev Nutr. 2007;27:103–121. doi: 10.1146/annurev.nutr.27.061406.093738. [DOI] [PubMed] [Google Scholar]
- 41.Cregan MD, De Mello TR, Kershaw D, McDougall K, Hartmann PE. Initiation of lactation in women after preterm delivery. Acta Obstet Gynecol Scand. 2002;81(9):870–877. doi: 10.1034/j.1600-0412.2002.810913.x. [DOI] [PubMed] [Google Scholar]
- 42.Hartmann PE, Cregan MD, Ramsay DT, Simmer K, Kent JC. Physiology of lactation in preterm mothers: Initiation and maintenance. Pediatr Ann. 2003;32(5):351–355. doi: 10.3928/0090-4481-20030501-11. [DOI] [PubMed] [Google Scholar]
- 43.Meier PP, Engstrom JL, Janes JE, Jegier BJ, Loera F. Breast pump suction patterns that mimic the human infant during breastfeeding: Greater milk output in less time spent pumping for breast pump-dependent mothers with premature infants. J Perinatol. 2012;32(2):103–110. doi: 10.1038/jp.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meier PP, Engstrom JL, Hurst NM, et al. A comparison of the efficiency, efficacy, comfort, and convenience of two hospital-grade electric breast pumps for mothers of very low birthweight infants. Breastfeed Med. 2008;3(3):141–150. doi: 10.1089/bfm.2007.0021. [DOI] [PubMed] [Google Scholar]
- 45.Meier PP, Engstrom JL, Patel AL, Jegier BJ, Bruns N. Improving the use of human milk during and after the NICU stay. Clin Perinatol. 2010;37(1):217–45. doi: 10.1016/j.clp.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meier PP. Concerns regarding industry-funded trials: letter to the editor. Journal of human lactation: official journal of International Lactation Consultant Association. 2005;21(2):121–123. [PubMed] [Google Scholar]
- 47.Pang WW, Hartmann PE. Initiation of human lactation: Secretory differentiation and secretory activation. J Mammary Gland Biol Neoplasia. 2007;12(4):211–221. doi: 10.1007/s10911-007-9054-4. [DOI] [PubMed] [Google Scholar]
- 48.Hill PD, Aldag JC, Chatterton RT, Zinaman M. Comparison of milk output between mothers of preterm and term infants: The first 6 weeks after birth. J Hum Lact. 2005;21(1):22–30. doi: 10.1177/0890334404272407. [DOI] [PubMed] [Google Scholar]
- 49.Wilson E, Christensson K, Brandt L, Altman M, Bonamy AK. Early provision of mother’s own milk and other predictors of successful breast milk feeding after very preterm birth: A regional observational study. J Hum Lact. 2015;31(3):393–400. doi: 10.1177/0890334415581164. [DOI] [PubMed] [Google Scholar]
- 50.Patel AL. Barriers to continued provision of human milk for mothers of VLBW infants. 2016 Sep 6; [Google Scholar]
- 51.Parker LA, Sullivan S, Krueger C, Mueller M. Association of timing of initiation of breastmilk expression on milk volume and timing of lactogenesis stage II among mothers of very low-birth-weight infants. Breastfeed Med. 2015 doi: 10.1089/bfm.2014.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parker LA, Sullivan S, Krueger C, Kelechi T, Mueller M. Effect of early breast milk expression on milk volume and timing of lactogenesis stage II among mothers of very low birth weight infants: A pilot study. J Perinatol. 2012;32(3):205–209. doi: 10.1038/jp.2011.78. [DOI] [PubMed] [Google Scholar]
- 53.Berens P, Labbok M Academy of Breastfeeding Medicine. ABM clinical protocol #13: Contraception during breastfeeding, revised 2015. Breastfeed Med. 2015;10:3–12. doi: 10.1089/bfm.2015.9999. [DOI] [PubMed] [Google Scholar]
- 54.Lussier MM, Brownell EA, Proulx TA, et al. Daily breastmilk volume in mothers of very low birth weight neonates: A repeated-measures randomized trial of hand expression versus electric breast pump expression. Breastfeed Med. 2015;10:312–317. doi: 10.1089/bfm.2015.0014. [DOI] [PubMed] [Google Scholar]
- 55.Knight CH, Peaker M, Wilde CJ. Local control of mammary development and function. Rev Reprod. 1998;3(2):104–112. doi: 10.1530/ror.0.0030104. [DOI] [PubMed] [Google Scholar]
- 56.Glasier A, McNeilly AS, Howie PW. The prolactin response to suckling. Clin Endocrinol (Oxf) 1984;21(2):109–116. doi: 10.1111/j.1365-2265.1984.tb03449.x. [DOI] [PubMed] [Google Scholar]
- 57.Blatchford DR, Hendry KA, Wilde CJ. Autocrine regulation of protein secretion in mouse mammary epithelial cells. Biochem Biophys Res Commun. 1998;248(3):761–766. doi: 10.1006/bbrc.1998.9057. [DOI] [PubMed] [Google Scholar]
- 58.Slutzah M, Codipilly CN, Potak D, Clark RM, Schanler RJ. Refrigerator storage of expressed human milk in the neonatal intensive care unit. J Hum Lact. 2010;26(3):233–4. doi: 10.1016/j.jpeds.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 59.Schanler RJ, Fraley JK, Lau C, Hurst NM, Horvath L, Rossmann SN. Breastmilk cultures and infection in extremely premature infants. J Perinatol. 2011;31(5):335–338. doi: 10.1038/jp.2011.13. [DOI] [PubMed] [Google Scholar]
- 60.Handa D, Ahrabi AF, Codipilly CN, et al. Do thawing and warming affect the integrity of human milk? J Perinatol. 2014 doi: 10.1038/jp.2014.113. [DOI] [PubMed] [Google Scholar]
- 61.Rechtman DJ, Lee ML, Berg H. Effect of environmental conditions on unpasteurized donor human milk. Breastfeed Med. 2006;1(1):24–26. doi: 10.1089/bfm.2006.1.24. [DOI] [PubMed] [Google Scholar]
- 62.Stellwagen LM, Vaucher YE, Chan CS, Montminy TD, Kim JH. Pooling expressed breastmilk to provide a consistent feeding composition for premature infants. Breastfeed Med. 2013;8:205–209. doi: 10.1089/bfm.2012.0007. [DOI] [PubMed] [Google Scholar]
- 63.Dougherty D, Nash A. Bar coding from breast to baby: A comprehensive breast milk management system for the NICU. Neonatal Netw. 2009;28(5):321–328. doi: 10.1891/0730-0832.28.5.321. [DOI] [PubMed] [Google Scholar]
- 64.Drenckpohl D, Bowers L, Cooper H. Use of the six sigma methodology to reduce incidence of breast milk administration errors in the NICU. Neonatal Netw. 2007;26(3):161–166. doi: 10.1891/0730-0832.26.3.161. [DOI] [PubMed] [Google Scholar]
- 65.Meier PP, Engstrom JL, Zuleger JL, et al. Accuracy of a user-friendly centrifuge for measuring creamatocrits on mothers’ milk in the clinical setting. Breastfeed Med. 2006;1(2):79–87. doi: 10.1089/bfm.2006.1.79. [DOI] [PubMed] [Google Scholar]
- 66.Lonnerdal B. Bioactive proteins in human milk: Mechanisms of action. J Pediatr. 2010;156(2 Suppl):S26–30. doi: 10.1016/j.jpeds.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 67.Molinari CE, Casadio YS, Hartmann BT, et al. Proteome mapping of human skim milk proteins in term and preterm milk. J Proteome Res. 2012;11(3):1696–1714. doi: 10.1021/pr2008797. [DOI] [PubMed] [Google Scholar]
- 68.Nyqvist KH, Anderson GC, Bergman N, et al. State of the art and recommendations. kangaroo mother care: Application in a high-tech environment. Breastfeed Rev. 2010;18(3):21–28. [PubMed] [Google Scholar]
- 69.Spatz DL. Ten steps for promoting and protecting breastfeeding for vulnerable infants. J Perinat Neonatal Nurs. 2004;18(4):385–396. doi: 10.1097/00005237-200410000-00009. [DOI] [PubMed] [Google Scholar]
- 70.Hurst NM, Valentine CJ, Renfro L, Burns P, Ferlic L. Skin-to-skin holding in the neonatal intensive care unit influences maternal milk volume. J Perinatol. 1997;17:213–217. [PubMed] [Google Scholar]
- 71.Davanzo R, Brovedani P, Travan L, et al. Intermittent kangaroo mother care: A NICU protocol. J Hum Lact. 2013;29(3):332–338. doi: 10.1177/0890334413489375. [DOI] [PubMed] [Google Scholar]
- 72.Meier P, Patel AL, Wright K, Engstrom JL. Management of breastfeeding during and after the maternity hospitalization for late preterm infants. Clin Perinatol. 2013;40(4):689–705. doi: 10.1016/j.clp.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meier PP, Engstrom JL. Test weighing for term and premature infants is an accurate procedure. Arch Dis Child Fetal Neonatal Ed. 2007;92(2):F155–6. doi: 10.1136/adc.2006.113480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haase B, Barreira J, Murphy PK, Mueller M, Rhodes J. The development of an accurate test weighing technique for preterm and high-risk hospitalized infants. Breastfeed Med. 2009;4(3):151–156. doi: 10.1089/bfm.2008.0125. [DOI] [PubMed] [Google Scholar]
- 75.Hurst NM, Meier PP, Engstrom JL, Myatt A. Mothers performing in-home measurement of milk intake during breastfeeding of their preterm infants: Maternal reactions and feeding outcomes. J Hum Lact. 2004;20(2):178–187. doi: 10.1177/0890334404264168. [DOI] [PubMed] [Google Scholar]
- 76.Meier PP, Brown LP, Hurst NM, et al. Nipple shields for preterm infants: Effect on milk transfer and duration of breastfeeding. Journal of Human Lactation. 2000;16(2):106–114. doi: 10.1177/089033440001600205. [DOI] [PubMed] [Google Scholar]
- 77.Bigger HR, Fogg LJ, Patel A, Johnson T, Engstrom JL, Meier PP. Quality indicators for human milk use in very low-birthweight infants: Are we measuring what we should be measuring? J Perinatol. 2014;34(4):287–91. doi: 10.1038/jp.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Quigley M, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2014;4:CD002971. doi: 10.1002/14651858.CD002971.pub3. [DOI] [PubMed] [Google Scholar]
- 79.Collado MC, Cernada M, Neu J, Perez-Martinez G, Gormaz M, Vento M. Factors influencing gastrointestinal tract and microbiota immune interaction in preterm infants. Pediatr Res. 2015;77(6):726–731. doi: 10.1038/pr.2015.54. [DOI] [PubMed] [Google Scholar]
- 80.Underwood MA, Gaerlan S, De Leoz ML, et al. Human milk oligosaccharides in premature infants: Absorption, excretion, and influence on the intestinal microbiota. Pediatr Res. 2015 doi: 10.1038/pr.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meier PP, Engstrom JL, Rossman B. Breastfeeding peer counselors as direct lactation care providers in the neonatal intensive care unit. J Hum Lact. 2013;29(3):313–322. doi: 10.1177/0890334413482184. [DOI] [PubMed] [Google Scholar]
- 82.Lopez LM, Grey TW, Stuebe AM, Chen M, Truitt ST, Gallo MF. Combined hormonal versus nonhormonal versus progestin-only contraception in lactation. Cochrane Database Syst Rev. 2015;3:CD003988. doi: 10.1002/14651858.CD003988.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cregan MD, de Mello TR, Hartmann PE. Pre-term delivery and breast expression: Consequences for initiating lactation. Adv Exp Med Biol. 2000;478:427–428. doi: 10.1007/0-306-46830-1_60. [DOI] [PubMed] [Google Scholar]
- 84.Hartmann P, Cregan M. Lactogenesis and the effects of insulin-dependent diabetes mellitus and prematurity. J Nutr. 2001;131(11):3016S–20S. doi: 10.1093/jn/131.11.3016S. [DOI] [PubMed] [Google Scholar]
- 85.Mulready-Ward C, Sackoff J. Outcomes and factors associated with breastfeeding for <8 weeks among preterm infants: Findings from 6 states and NYC, 2004–2007. Matern Child Health J. 2012 doi: 10.1007/s10995-012-1178-5. [DOI] [PubMed] [Google Scholar]
- 86.Dewey KG. Maternal and fetal stress are associated with impaired lactogenesis in humans. J Nutr. 2001;131(11):3012S–5S. doi: 10.1093/jn/131.11.3012S. [DOI] [PubMed] [Google Scholar]
- 87.Esquerra-Zwiers A, Rossman B, Meier P, Engstrom J, Janes J, Patel A. “It’s somebody else’s milk”: Unraveling the tension in mothers of preterm infants who provide consent for pasteurized donor human milk. J Hum Lact. 2016;32(1):95–102. doi: 10.1177/0890334415617939. [DOI] [PMC free article] [PubMed] [Google Scholar]